Loss of Response to Long-Term Infliximab Therapy in Children with Crohn’s Disease
Abstract
:1. Introduction
2. Experimental Section
2.1. Design and Inclusion
2.2. Data Acquisition
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
| Demographics | |||
|---|---|---|---|
| Female | 23 (32.4%) | ||
| Age (years) | 14.4 (3.95–20.1) | ||
| History of disease | |||
| Duration (weeks) | Pre-2007 | 2007-Onwards | |
| 135 (12–578) | 54 (5–128) | p < 0.001 | |
| Disease classification (Montréal) | |||
| Age at Diagnosis | <16 years | >16 years | |
| 65 (91.5%) | 6 (8.45%) | ||
| Location | Ileocolonic | Colonic | Ileal |
| 45 (63.4%) | 22 (31.0%) | 4 (5.63%) | |
| + upper GI | 41 (57.7%) | ||
| Behaviour | Inflammatory | Fibrostenotic | Penetrating |
| 60 (83%) | 4 (7%) | 7 (10%) | |
| + perianal | 28 (39.4%) | ||
| Disease severity (PCDAI †; n = 59) | |||
| Severe | 33 (55.9%) | PCDAI ≥ 40 | |
| Moderate | 26 (44.1%) | PCDAI 30–37.5 | |
| Medications | |||
| IM * | Thiopurines | Methotrexate | None |
| 46 (64.8%) | 10 (14.1%) | 15 (21.1%) | |
| Corticosteroids | ≥1 mg/kg | <1 mg/kg | None |
| 3 (4.23%) | 10 (14.1%) | 58 (81.7%) | |
| Budesonide | 3 (4.23%) | ||
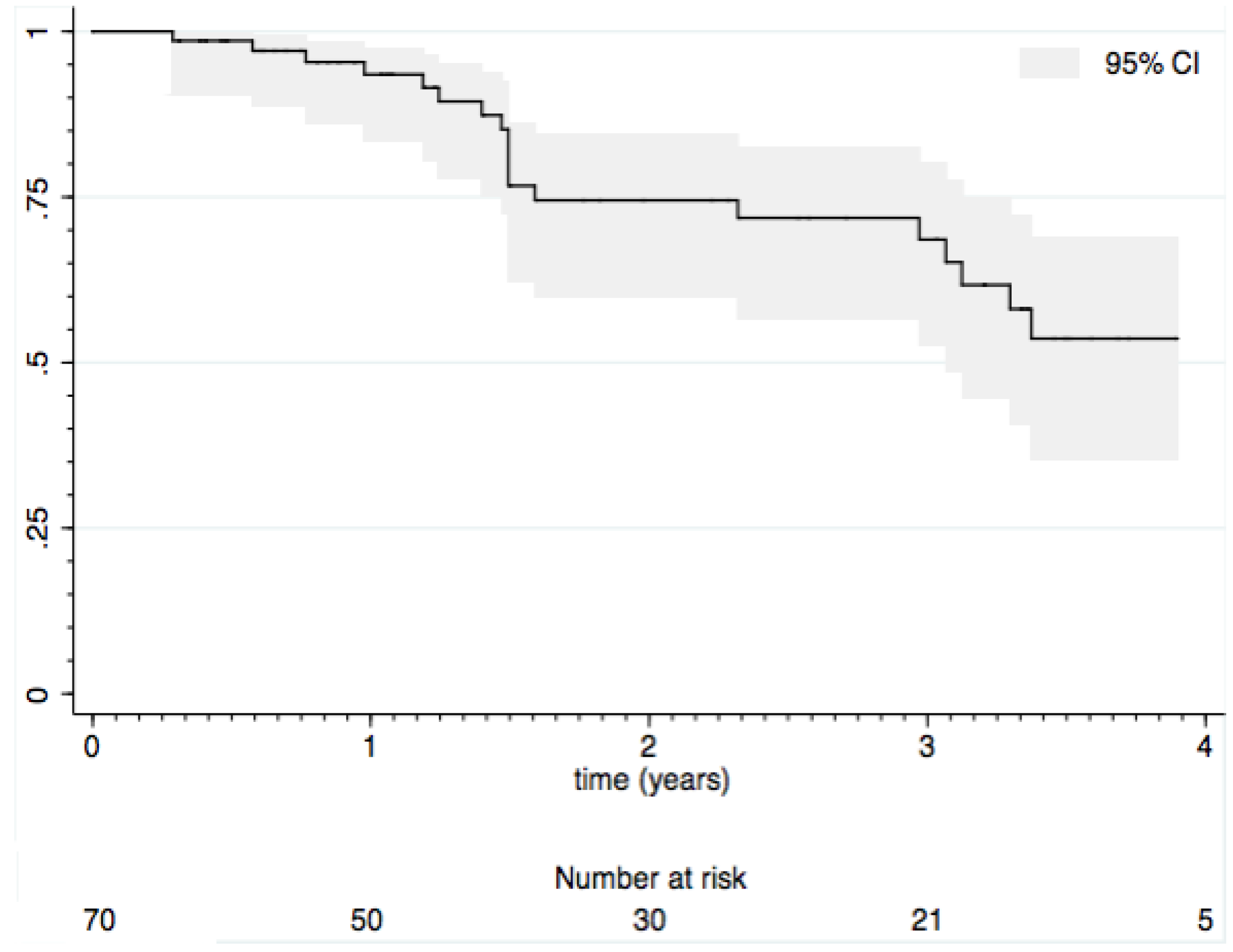
3.2. Loss of Response

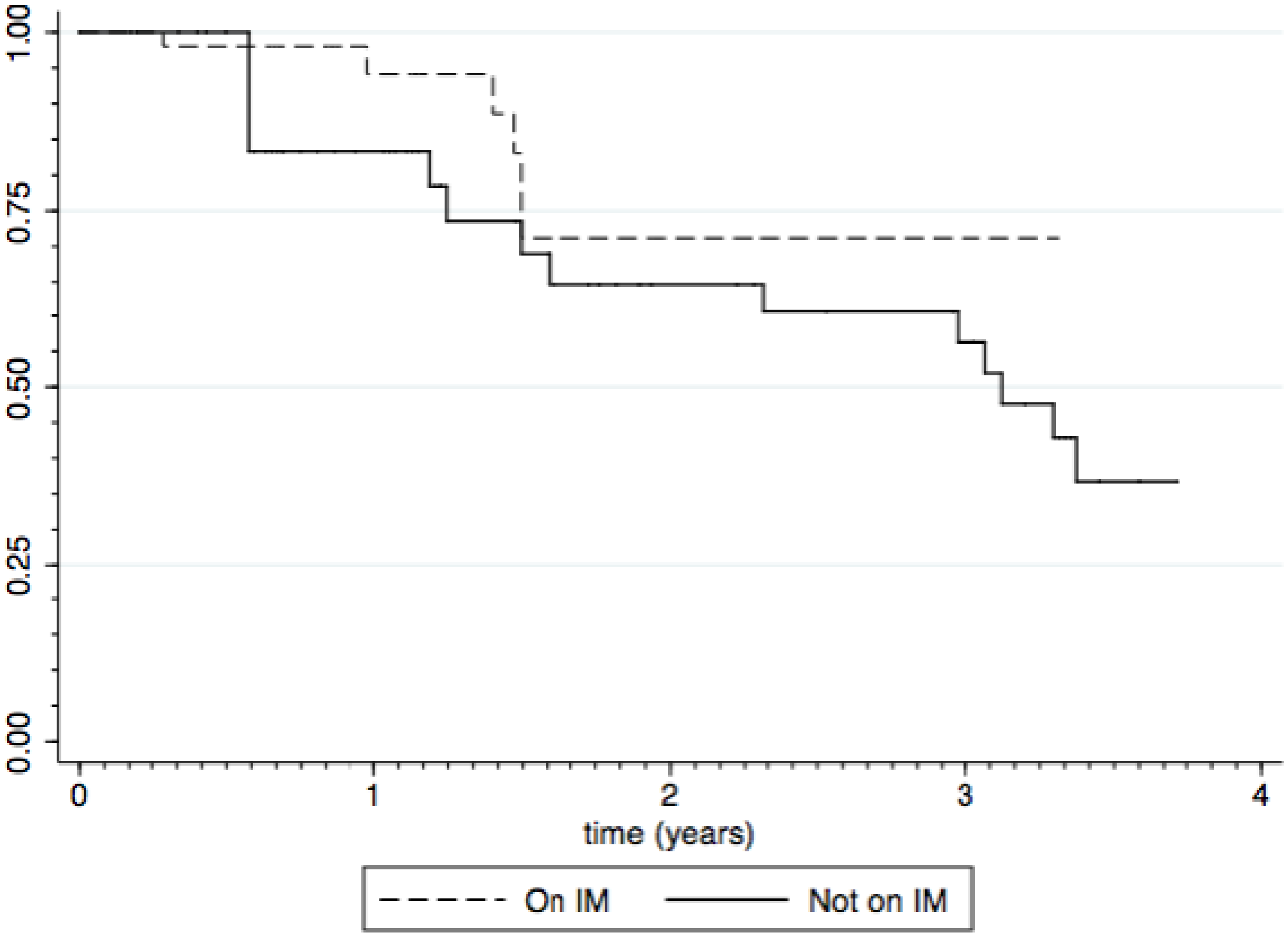
3.3. Impact of Immunomodulators
| Variable | Coefficient | Std. error | p value |
|---|---|---|---|
| Remission post induction | −1.31 | 0.593 | 0.027 |
| Ileal location | 1.68 | 0.906 | 0.064 |
| Induction age | 62.0 | 42.4 | 0.144 |
| Disease duration | −1.19 | 0.814 | 0.144 |
| Diagnosis age | −61.9 | 42.4 | 0.145 |
| Post-IM status | 0.668 | 0.618 | 0.280 |
| Female gender | 0.069 | 0.770 | 0.929 |
3.4. Growth
| Diagnosis | Baseline | Change from baseline (weeks on infliximab) | ||||||
|---|---|---|---|---|---|---|---|---|
| 34 | 58 | 82 | 106 | 130 | 154 | |||
| n | 41 | 67 | 51 | 43 | 36 | 29 | 20 | 16 |
| Height SDS | −0.061 | −0.33 | 0.058 * | 0.32 * | 0.40 * | 0.65 * | 0.74 * | 0.86 * |
| Weight SDS | NA | −0.77 | 0.52 * | 0.62 * | 0.46 * | 0.61 * | 0.69 * | 0.48 * |
3.5. Adverse Events
4. Discussion
5. Conclusions
Abbreviations
| IFX: | Infliximab |
| IM: | Immunomodulator |
| LoR: | Loss of Response (to infliximab) |
| PCDAI: | Paediatric Crohn’s disease activity index |
| REACH: | A Randomized; multicenter; open-label study to Evaluate the safety and efficacy of Anti-TNF-α Chimeric monoclonal antibody in pediatric subjects with moderate to severe Crohn’s disease |
| SDS: | Standard Deviation Score |
| SONIC: | Study of Immunomodulator Naive Patients in Crohn’s Disease |
| TNF: | Tumor Necrosis Factor |
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Type | Clinical information | Time on IFX at event | Total time on IFX | Secondary loss of response |
|---|---|---|---|---|---|
| 10 | Surgery | Drainage of perianal abscess | 0.851 | 1.40 | Yes |
| 12 | Infection | Pneumonia (community-acquired) | 0.175 | 0.767 | Yes |
| 30 | Infection | Herpes zoster | 2.38 | 3.49 | No |
| 31 | Surgery | Ileocaecal resection | 0.104 | 2.53 | No |
| 31 | Exacerbation | IV steroids | 0.350 | 2.53 | No |
| 36 | Surgery | Drainage of perianal abscess with seton placement | 0.361 | 2.57 | No |
| 51 | Exacerbation | IV steroids | 0.329 | 0.594 | No |
| 54 | Infection | Pneumonia (community-acquired) | 1.13 | 3.12 | Yes |
| 56 | Surgery | Sigmoid dilatation complicated by perforation | 0.638 | 3.35 | No |
| 64 | Infection | Pneumonia (community acquired) | 0.222 | 1.25 | Yes |
| 67 | Surgery | Drainage of perianal abscess | 1.49 | 1.49 | Yes |
| 68 | Surgery | Drainage of perianal Abscess | 1.59 | 1.59 | Yes |
| 68 | Exacerbation | IV antibiotics | 0.704 | 1.59 | Yes |
| 68 | Infection | Pneumonia (community acquired) | 1.28 | 1.59 | Yes |
| 75 | Exacerbation | IV steroids | 0.287 | 0.287 | Yes |
| 86 | Exacerbation | IV steroids | 2.32 | 2.32 | Yes |
| 87 | Infection | Herpes zoster | 1.10 | 1.49 | Yes |
| 95 | Exacerbation (Erythema nodosum, episcleritis) | IV steroids | 0.137 | 3.20 | No |
| 95 | Exacerbation | IV steroids | 1.57 | 3.20 | No |
References
- Yang, L.S.; Alex, G.; Catto-Smith, A.G. The use of biological agents in pediatric inflammatory bowel disease. Curr. Opin. Peds. 2012, 24, 609–614. [Google Scholar] [CrossRef]
- Gouldthorpe, O.; Catto-Smith, A.G.; Alex, G. Biologics in paediatric Crohn’s Disease. Gastro Res. Prac 2011, 2011, 287574. [Google Scholar]
- Yanai, H.; Hanauer, S.B. Assessing Response and Loss of Response to Biological Therapies in IBD. Am. J. Gastroenterol. 2011, 106, 685–98. [Google Scholar] [CrossRef]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J.; Liu, G.; Travers, S.; Heuschkel, R.; Markowitz, J.; et al. Induction and Maintenance Infliximab Therapy for the Treatment of Moderate-to-Severe Crohn’s Disease in Children. Gastroenterology 2007, 132, 863–873. [Google Scholar] [CrossRef]
- Hyams, J.S.; Lerer, T.; Griffiths, A.; Pfefferkorn, M.; Kugathasan, S.; Evans, J.; Otley, A.; Carvalho, R.; Mack, D.; Bousvaros, A.; et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 816–822. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Lachaux, A.; Cézard, J.P.; Morali, A.; Maurage, C.; Giniès, J.L.; Viola, S.; Goulet, O.; Lamireau, T.; Scaillon, M.; et al. Efficacy of infliximab in pediatric Crohn’s disease: A randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm. Bowel Dis. 2009, 15, 388–394. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Kotlyar, D.S.; Osterman, M.T.; Diamond, R.H.; Porter, D.; Blonski, W.C.; Wasik, M.; Sampat, S.; Mendizabal, M.; Lin, M.V.; Lichtenstein, G.R. A systematic review of factors that contribute to Hepatosplenic T-Cell Lymphoma in patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 36–41. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Steenholdt, C.; Brynskov, J.; Thomsen, O.O.; Munck, L.K.; Fallingborg, J.; Christensen, L.A.; Pedersen, G.; Kjeldsen, J.; Jacobsen, B.A.; Oxholm, A.S.; et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut 2013. [Google Scholar] [CrossRef]
- Connell, W.; Andrews, J.M.; Brown, S.; Sparrow, M. Practical guidelines for treating inflammatory bowel disease safely with anti-tumour necrosis factor therapy in Australia. Intern. Med. J. 2010, 40, 139–149. [Google Scholar] [CrossRef]
- Motil, K.J.; Grand, R.J.; Davis-Kraft, L.; Ferlic, L.L.; Smith, E.O. Growth failure in children with inflammatory bowel disease: A prospective study. Gastroenterology 1993, 105, 681–691. [Google Scholar]
- Sawczenko, A.; Sandhu, B.K. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch. Dis. Child. 2003, 88, 995–1000. [Google Scholar] [CrossRef]
- Sawczenko, A.; Ballinger, A.B.; Savage, M.O.; Sanderson, I.R. Clinical features affecting final adult height in patients with pediatric-onset Crohn’s disease. Pediatrics 2006, 118, 124–129. [Google Scholar] [CrossRef]
- MacRae, V.E.; Wong, S.C.; Farquharson, C.; Ahmed, S.F. Cytokine actions in growth disorders associated with pediatric chronic inflammatory diseases (review). Int. J. Mol. Med. 2006, 18, 1011–1018. [Google Scholar] [Green Version]
- Wong, S.C.; Smyth, A.; McNeill, E.; Galloway, P.J.; Hassan, K.; McGrogan, P.; Ahmed, S.F. The growth hormone insulin-like growth factor 1 axis in children and adolescents with inflammatory bowel disease and growth retardation. Clin. Endocrinol. (Oxf.) 2010, 73, 220–228. [Google Scholar] [Green Version]
- MacRae, V.E.; Farquharson, C.; Ahmed, S.F. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J. Endocrinol. 2006, 189, 319–328. [Google Scholar] [CrossRef]
- Pfefferkorn, M.; Burke, G.; Griffiths, A.; Markowitz, J.; Rosh, J.; Mack, D.; Otley, A.; Kugathasan, S.; Evans, J.; Bousvaros, A.; et al. Growth abnormalities persist in newly diagnosed children with Crohn disease despite current treatment paradigms. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 168–174. [Google Scholar] [CrossRef]
- Walters, T.D.; Gilman, A.R.; Griffiths, A.M. Linear growth improves during infliximab therapy in children with chronically active severe Crohn’s disease. Inflamm. Bowel Dis. 2007, 13, 424–430. [Google Scholar] [CrossRef]
- Van Assche, G.; Dignass, A.; Panes, J.; Beaugerie, L.; Karagiannis, J.; Allez, M.; Ochsenkuhn, T.; Orchard, T.; Rogler, G.; Louis, E.; et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J. Crohns. Colitis 2010, 4, 7–27. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Available online: http://pbs.gov.au/medicine/item/4284L-5753T-5754W-5755X-5756Y-5757B-5758C-6397Q-6448J-6496X-9612X-9613Y-9617E-9654D-9674E (accessed on 2 October 2013).
- Van Assche, G.; Magdelaine-Beuzelin, C.; D’Haens, G.; Baert, F.; Noman, M.; Vermeire, S.; Ternant, D.; Watier, H.; Paintaud, G.; Rutgeerts, P. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology 2008, 134, 1861–1868. [Google Scholar] [CrossRef]
- Turner, D.; Griffiths, A.M.; Walters, T.D.; Seah, T.; Markowitz, J.; Pfefferkorn, M.; Keljo, D.; Otley, A.; Leleiko, N.S.; Mack, D.; et al. Appraisal of the pediatric Crohn’s disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am. J. Gastroenterol. 2010, 105, 2085–2092. [Google Scholar] [CrossRef]
- Rosh, J.R.; Lerer, T.; Markowitz, J.; Goli, S.R.; Mamula, P.; Noe, J.D.; Pfefferkorn, M.D.; Kelleher, K.T.; Griffiths, A.M.; Kugathasan, S.; et al. Retrospective evaluation of the safety and effect of adalimumab therapy (RESEAT) in pediatric Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 3042–3049. [Google Scholar] [CrossRef]
- Russell, R.K.; Wilson, M.L.; Loganathan, S.; Bourke, B.; Kiparissi, F.; Mahdi, G.; Torrente, F.; Rodrigues, A.; Davies, I.; Thomas, A.; et al. A British Society of Paediatric Gastroenterology, Hepatology and Nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2011, 33, 946–953. [Google Scholar] [CrossRef]
- Malik, S.; Wong, S.; Bishop, J.; Hassan, K.; McGrogan, P.; Ahmed, S.; Russell, R. Improvement in growth of children with crohn disease following anti-TNF-α therapy can be independent of pubertal progress and glucocorticoid reduction. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 31–37. [Google Scholar] [CrossRef]
- Van Limbergen, J.; Russell, R.K.; Drummond, H.E.; Aldhous, M.C.; Round, N.K.; Nimmo, E.R.; Smith, L.; Gillett, P.M.; McGrogan, P.; Weaver, L.T.; et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008, 135, 1114–1122. [Google Scholar] [CrossRef]
- Vernier-Massouille, G.; Balde, M.; Salleron, J.; Turck, D.; Dupas, J.L.; Mouterde, O.; Merle, V.; Salomez, J.L.; Branche, J.; Marti, R.; et al. Natural history of pediatric Crohn’s disease: A population-based cohort study. Gastroenterology 2008, 135, 1106–1113. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gouldthorpe, O.; Catto-Smith, A.G.; Alex, G.; Simpson, D. Loss of Response to Long-Term Infliximab Therapy in Children with Crohn’s Disease. Pharmaceuticals 2013, 6, 1322-1334. https://doi.org/10.3390/ph6101322
Gouldthorpe O, Catto-Smith AG, Alex G, Simpson D. Loss of Response to Long-Term Infliximab Therapy in Children with Crohn’s Disease. Pharmaceuticals. 2013; 6(10):1322-1334. https://doi.org/10.3390/ph6101322
Chicago/Turabian StyleGouldthorpe, Oliver, Anthony G. Catto-Smith, George Alex, and Di Simpson. 2013. "Loss of Response to Long-Term Infliximab Therapy in Children with Crohn’s Disease" Pharmaceuticals 6, no. 10: 1322-1334. https://doi.org/10.3390/ph6101322
APA StyleGouldthorpe, O., Catto-Smith, A. G., Alex, G., & Simpson, D. (2013). Loss of Response to Long-Term Infliximab Therapy in Children with Crohn’s Disease. Pharmaceuticals, 6(10), 1322-1334. https://doi.org/10.3390/ph6101322




