A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011
Abstract
:1. Introduction
2. Materials and Methods
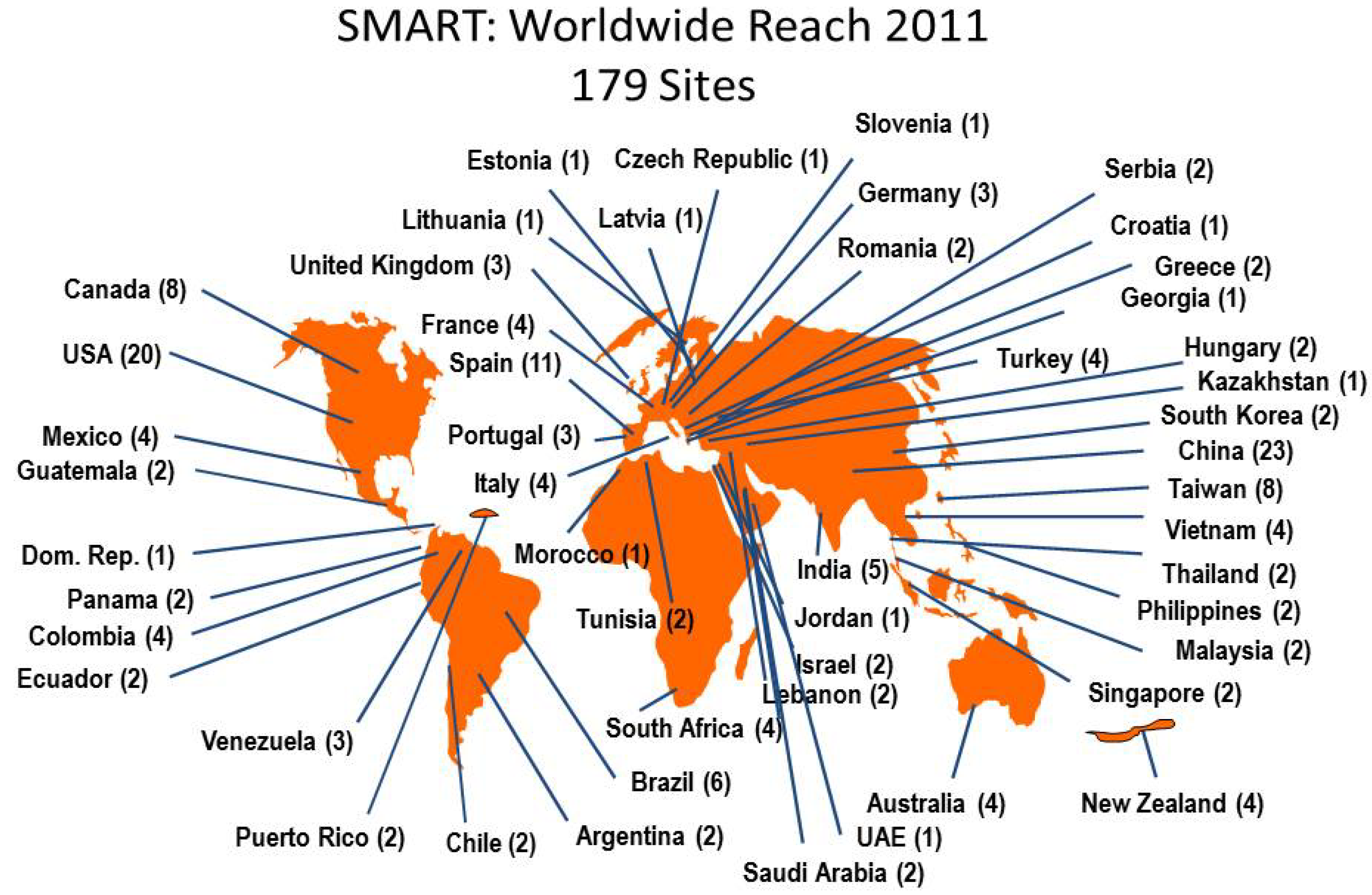
2.1. Isolate Collection and Sites
2.2. Susceptibility Testing
- ertapenem 0.03–4,
- imipenem 0.06–8,
- cefepime 0.5–32,
- ceftazidime 0.5–128,
- ceftazidime-clavulanic acid 0.12/4–16/4,
- cefoxitin 2–16,
- ciprofloxacin 0.25–2,
- amikacin 4–32,
- levofloxacin 0.5–4,
- cefotaxime 0.5–128,
- cefotaxime-clavulanic acid 0.12/4–16/4,
- piperacillin-tazobactam 2/4–64/4,
- ampicillin-sulbactam 2/2–16/2, and
- ceftriaxone 1–32.
2.3. Quality Control
3. Results and Discussion
| IAI Pathogen | N | % |
|---|---|---|
| Escherichia coli | 43,973 | 47.8 |
| Klebsiella pneumoniae | 13,385 | 14.5 |
| Pseudomonas aeruginosa | 8,674 | 9.4 |
| Enterobacter cloacae | 5,564 | 6.0 |
| Proteus mirabilis | 3,282 | 3.6 |
| Other | 17,208 | 18.7 |
| Total | 92,086 | 100.0 |
| UTI Pathogen | N | % |
|---|---|---|
| Escherichia coli | 10,956 | 44.3 |
| Klebsiella pneumoniae | 2,906 | 11.8 |
| Pseudomonas aeruginosa | 1,331 | 5.4 |
| Proteus mirabilis | 1,137 | 4.6 |
| Enterobacter cloacae | 617 | 2.5 |
| Other | 7,758 | 31.4 |
| Total | 24,705 | 100.0 |
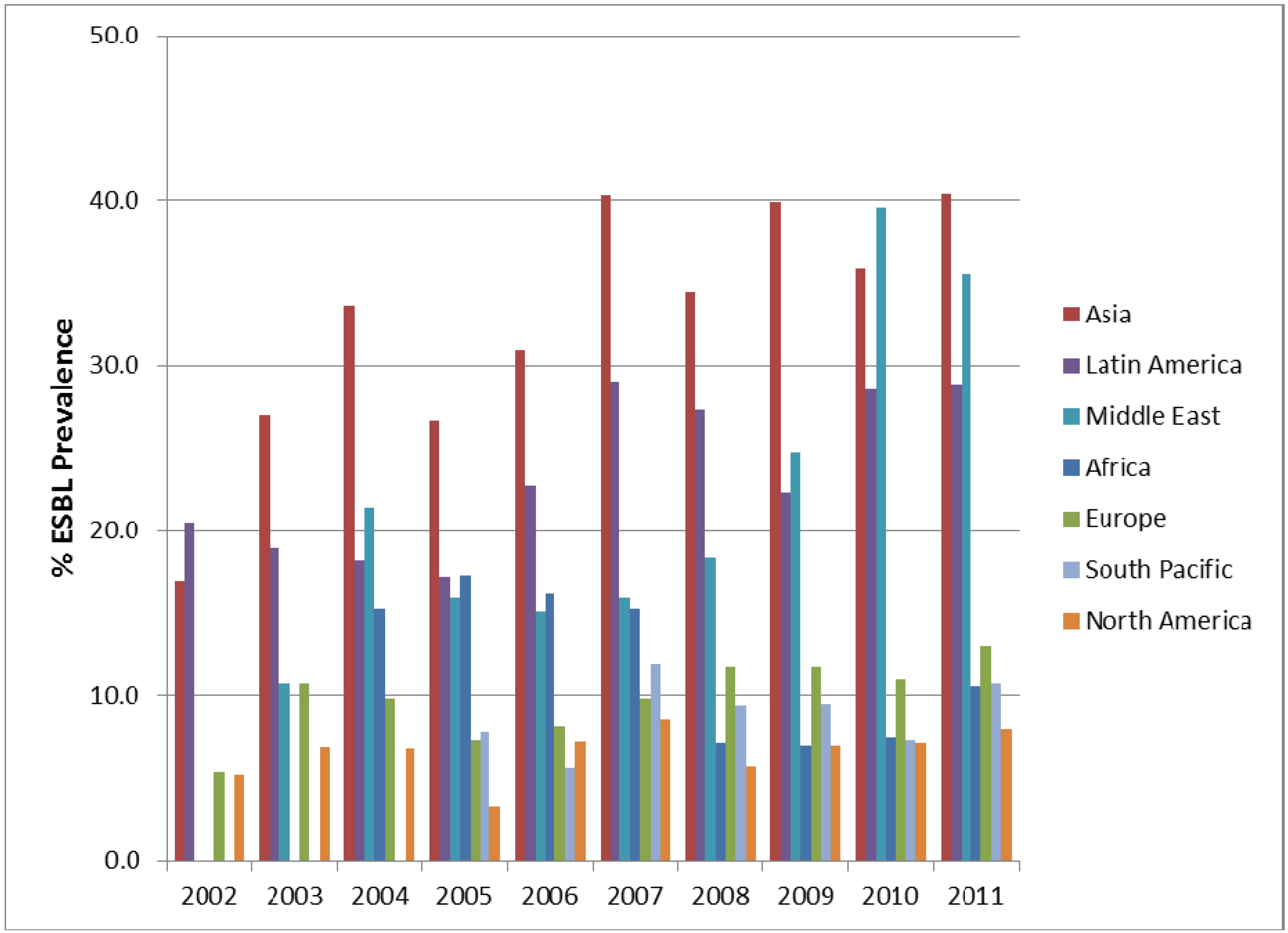
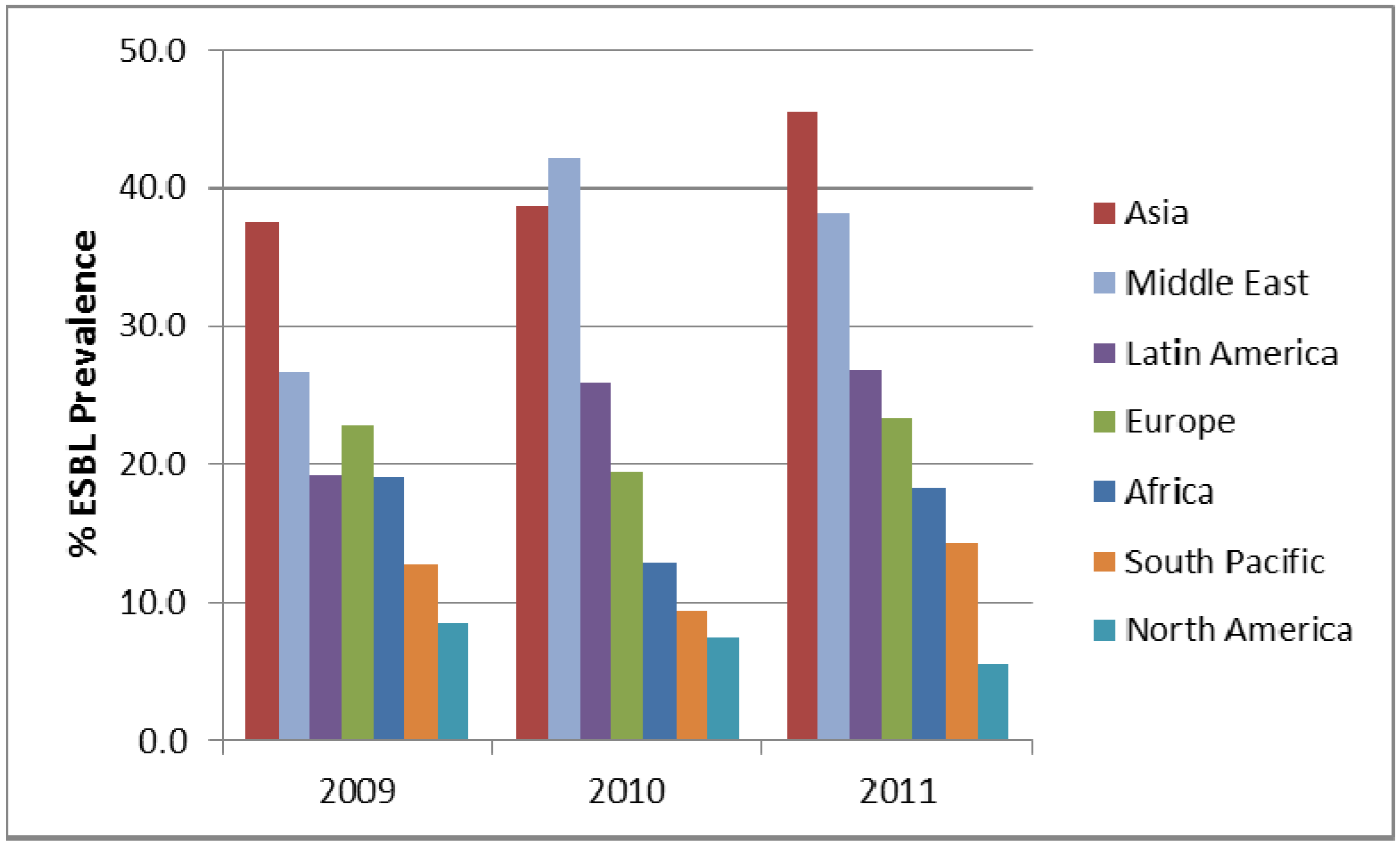
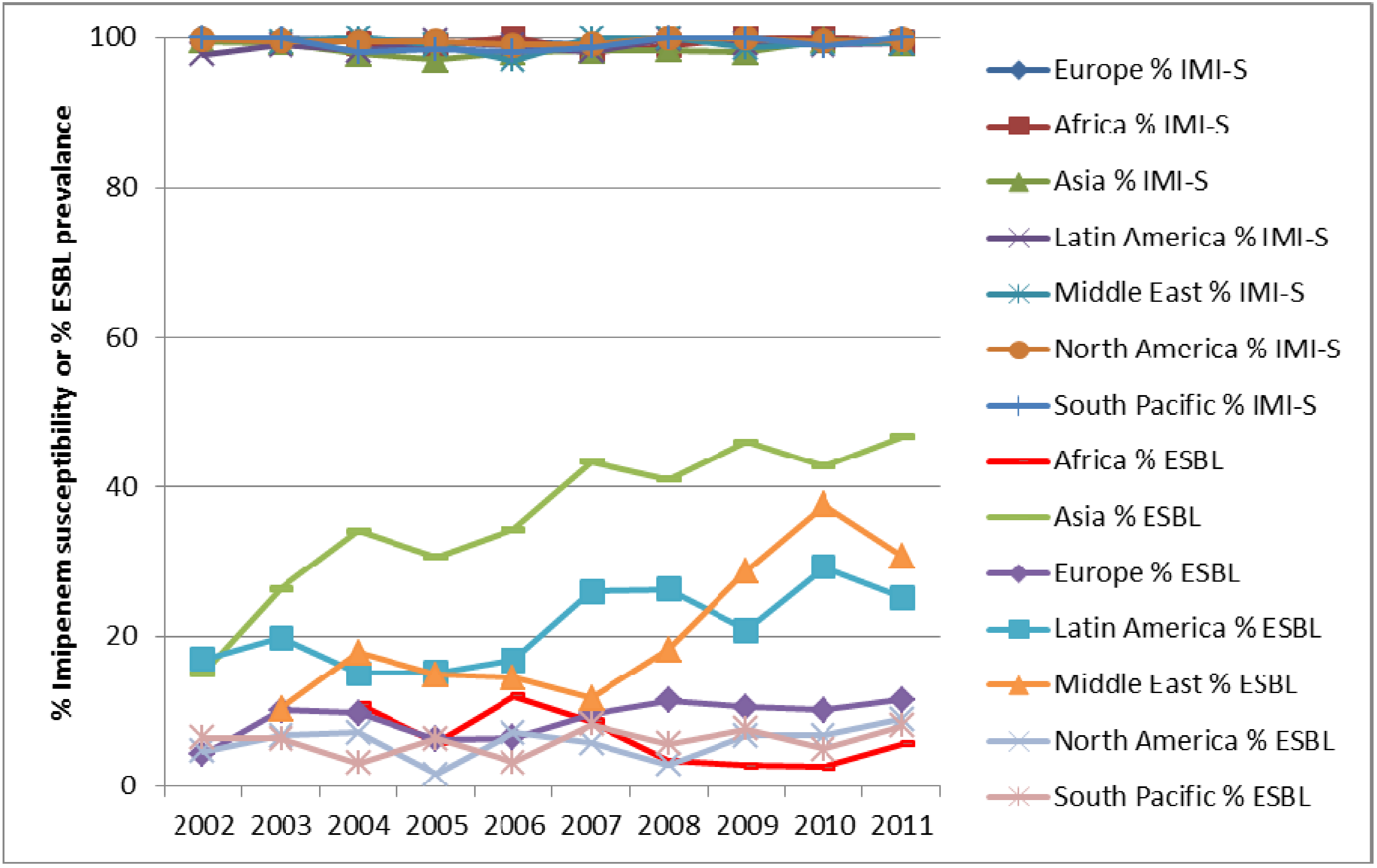
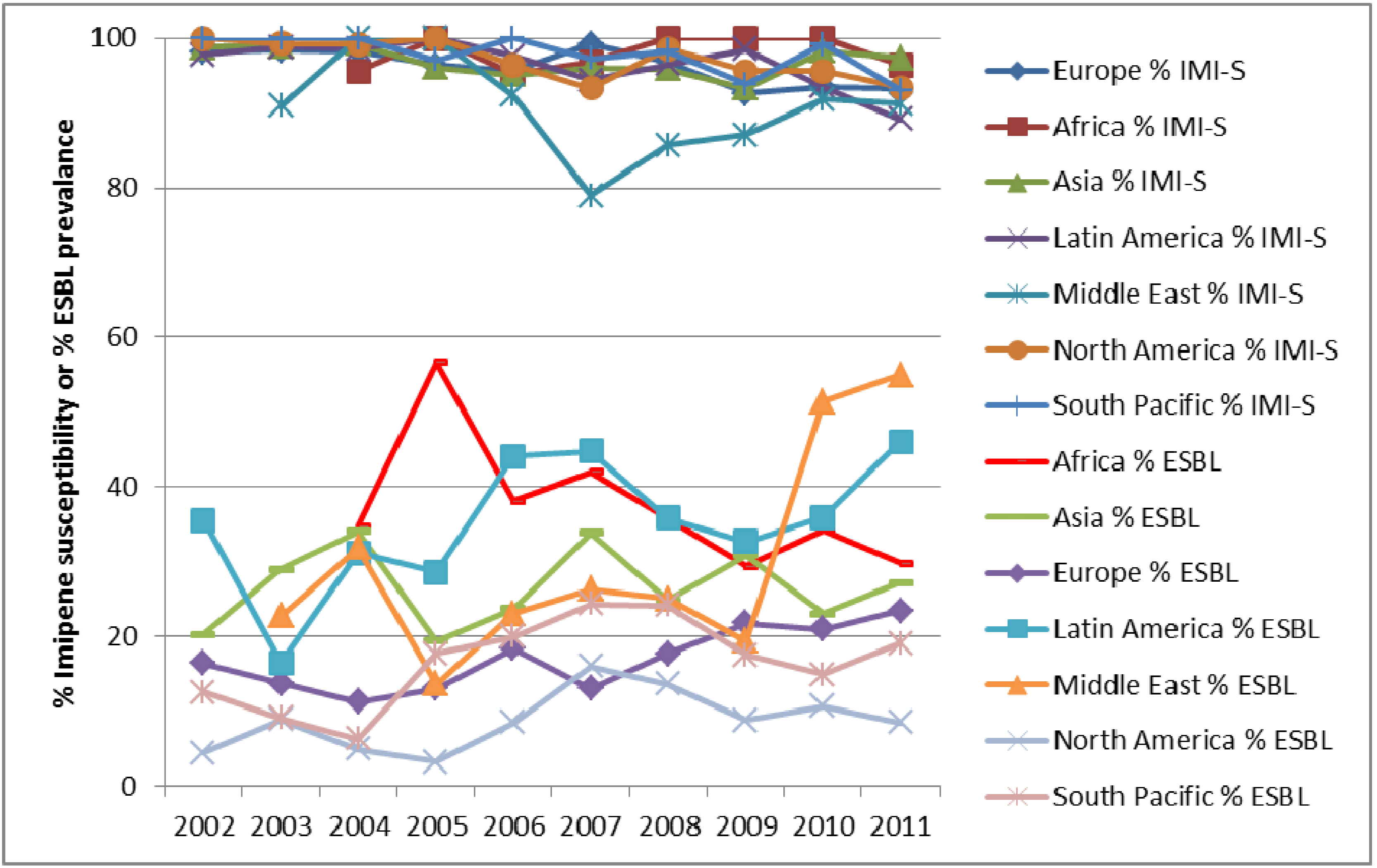
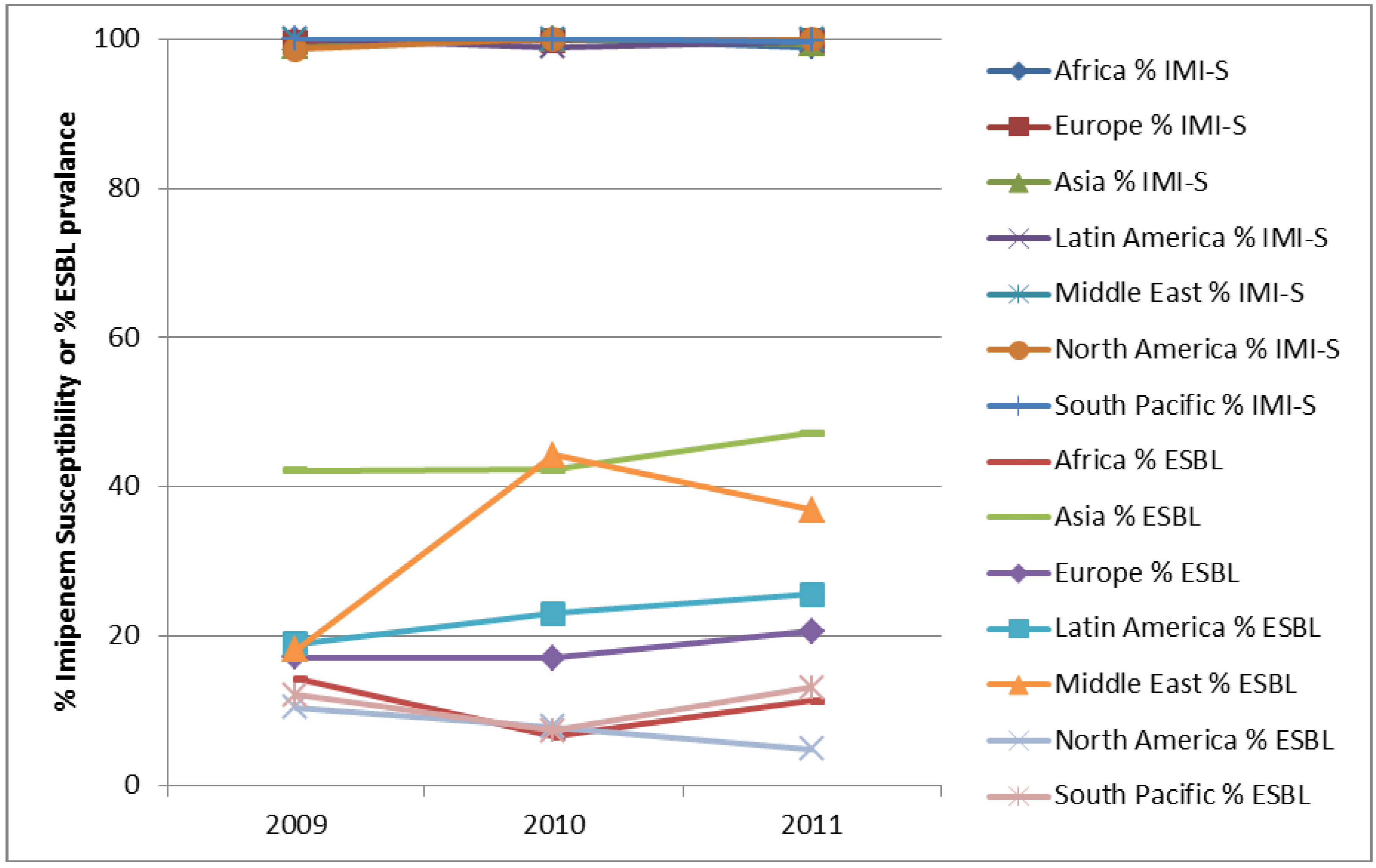
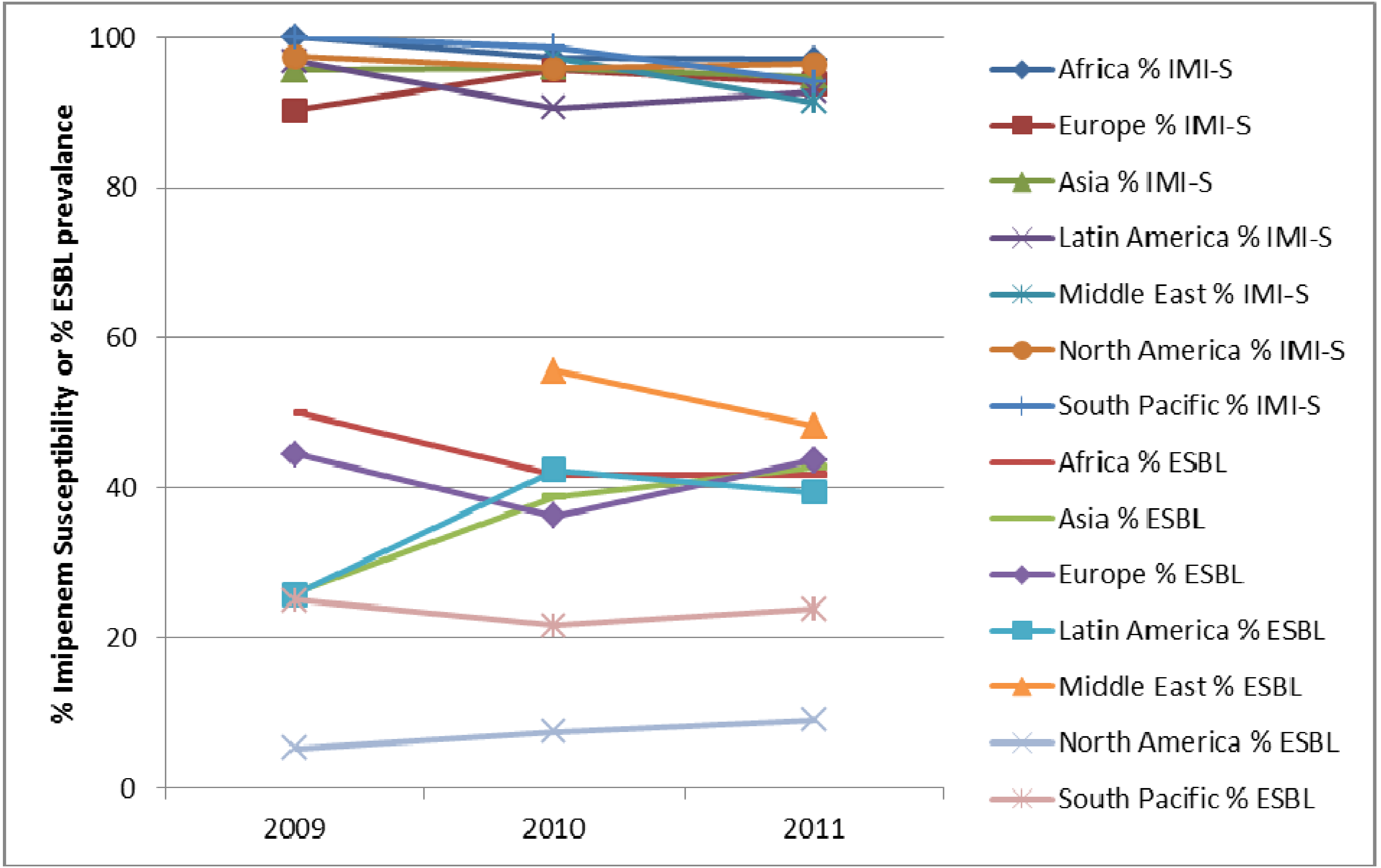

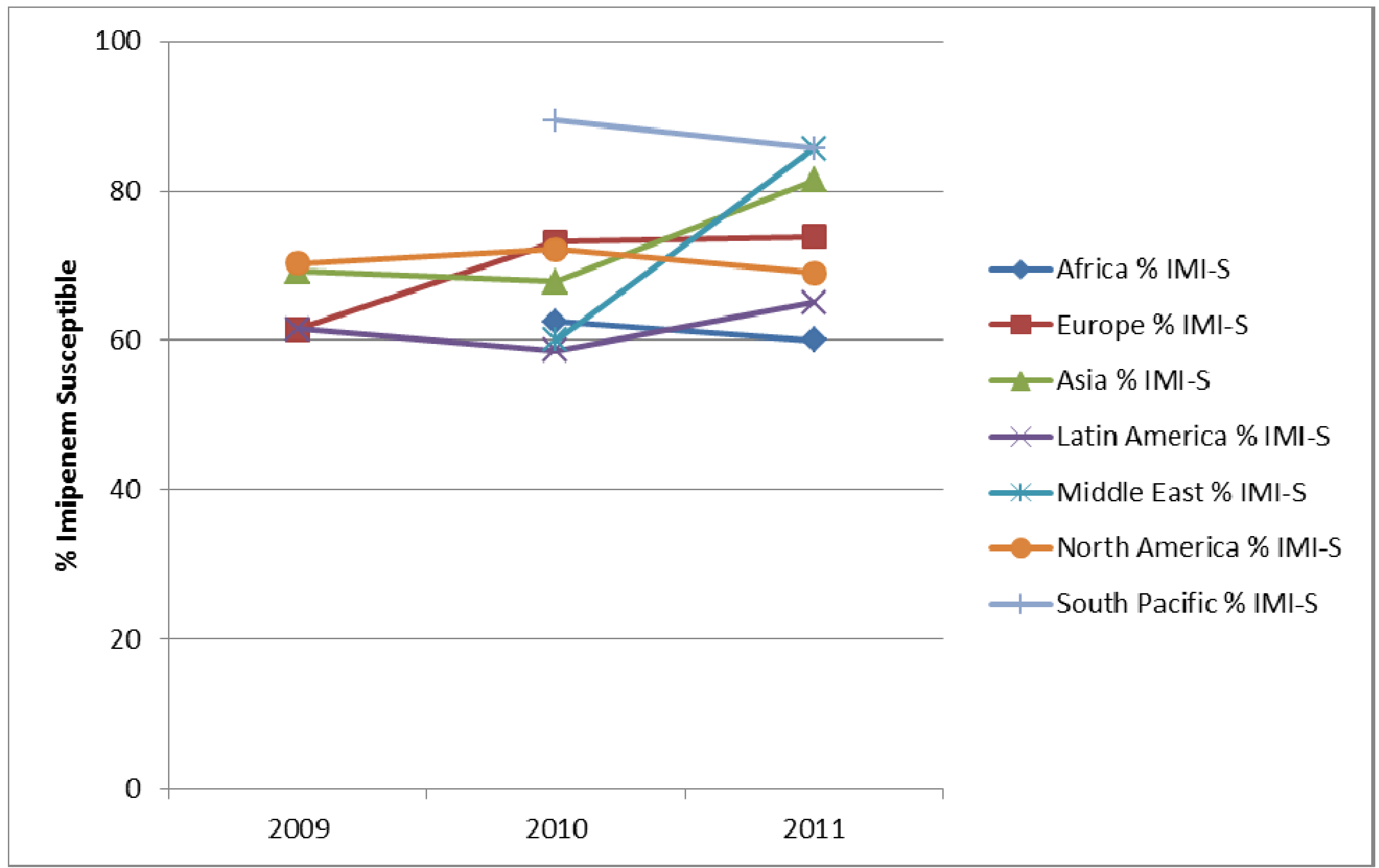
4. Conclusions
Acknowedgements
Conflicts of Interest
References
- Hawser, S. Surveillance programmes and antibiotic resistance: Worldwide and regional monitoring of antibiotic resistance trends. Antibiotic Resist. 2012, 211, 31–43. [Google Scholar]
- Felmingham, D.; White, A.R.; Jacobs, M.R.; Appelbaum, P.C.; Poupard, J.; Miller, L.A.; Grüneberg, R.N. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 2005, 56, ii3–ii21. [Google Scholar]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standards—Ninth Edition; CLSI document M07-A9; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Performance Standards for Antimicrobial Susceptibility Testing, Twenty-second Informational Supplement; CLSI document M100-S23; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2013.
- Hawser, S.P.; Bouchillon, S.K.; Hoban, D.J.; Badal, R.E. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli from patients with intra-abdominal infections worldwide from 2005–2007: Results from the SMART study. Int. J. Antimicrob. Agents. 2009, 34, 585–588. [Google Scholar]
- Lascols, C.; Hackel, M.; Hujer, A.M.; Marshall, S.H.; Bouchillon, S.K.; Hoban, D.J.; Hawser, S.P.; Badal, R.E.; Bonomo, R.A. Using nucleic acid microarrays to perform molecular epidemiology and detect novel β-lactamases: A snapshot of extended-spectrum β-lactamases throughout the world. J. Clin. Microbiol. 2012, 50, 1632–1639. [Google Scholar] [CrossRef]
- Hawser, S.P.; Bouchillon, S.K.; Lascols, C.; Hackel, M.; Hoban, D.J.; Badal, R.E.; Woodford, N.; Livermore, D.M. Susceptibility of Klebsiella pneumoniae isolates from intra-abdominal infections and molecular characterization of ertapenem-resistant isolates. Antimicrob. Agents Chemother. 2011, 55, 3917–3921. [Google Scholar]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-β-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar]
- Castanheira, M.; Deshpande, L.M.; Mathai, D. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: Report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob. Agents Chemother. 2010, 55, 1274–1278. [Google Scholar]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Lascols, C.; Hackel, M.; Marshall, S.H.; Hujer, A.M.; Bouchillon, S.; Badal, R.; Hoban, D.; Bonomo, R.A. Increasing prevalence and dissemination of NDM-1 metallo-β-lactamase in India: Data from the SMART study (2009). J. Antimicrob. Chemother. 2011, 66, 1992–1997. [Google Scholar]
- Sheng, W.H.; Badal, R.E.; Hsueh, P.R.; SMART Program. Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: Results of the study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob. Agents Chemother. 2013, 57, 2981–2988. [Google Scholar]
- Yang, Q.; Wang, H.; Chen, M.; Ni, Y.; Yu, Y.; Hu, B.; Sun, Z.; Huang, W.; Hu, Y.; Ye, H.; et al. Surveillance of antimicrobial susceptibility of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in China: the 2002–2009 Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 2010, 36, 507–512. [Google Scholar]
- Hawser, S.P.; Badal, R.E.; Bouchillon, S.K.; Hoban, D.J.; SMART India Working Group. Antibiotic susceptibility of intra-abdominal infection isolates from Indian hospitals during 2008. J. Med. Microbiol. 2010, 59, 1050–1054. [Google Scholar] [CrossRef]
- Hawser, S.P.; Badal, R.E.; Bouchillon, S.K.; Hoban, D.J. Trending eight years of in vitro activity of ertapenem and comparators against Escherichia coli from intra-abdominal infections in North America—SMART 2002–2009. J. Chemother. 2011, 23, 266–272. [Google Scholar]
- Brink, A.J.; Botha, R.F.; Poswa, X.; Senekal, M.; Badal, R.E.; Grolman, D.C.; Richards, G.A.; Feldman, C.; Boffard, K.D.; Veller, M.; et al. Antimicrobial susceptibility of gram-negative pathogens isolated from patients with complicated intra-abdominal infections in South African hospitals (SMART Study 2004–2009): Impact of the new carbapenem breakpoints. Surg. Infect. 2012, 13, 43–49. [Google Scholar]
- Yang, Q.; Zhang, H.; Wang, Y.; Xu, Y.; Chen, M.; Badal, R.E.; Wang, H.; Ni, Y.; Yu, Y.; Hu, B.; et al. A ten-year surveillance for antimicrobial susceptibility of Escherichia coli and Klebsiella pneumoniae in community- and hospital-associated intra-abdominal infections in China. J. Med. Microbiol. 2013, 62, 1343–1349. [Google Scholar]
- Hawser, S.P.; Badal, R.E.; Bouchillon, S.K.; Hoban, D.J.; Biedenbach, D.J.; Cantón, R.; Paterson, D.L. Monitoring the global in vitro activity of ertapenem against Escherichia coli from intra-abdominal infections: SMART 2002–2010. Int. J. Antimicrob. Agents 2013, 41, 224–228. [Google Scholar] [CrossRef]
- Hoban, D.J.; Lascols, C.; Nicolle, L.E.; Badal, R.; Bouchillon, S.; Hackel, M.; Hawser, S. Antimicrobial susceptibility of Enterobacteriaceae, including molecular characterization of extended-spectrum β-lactamase-producing species, in urinary tract isolates from hospitalized patients in North America and Europe: Results from the SMART study 2009–2010. Diagn. Microbiol. Infect. Dis. 2012, 74, 62–67. [Google Scholar] [CrossRef]
- Bouchillon, S.K.; Badal, R.E.; Hoban, D.J.; Hawser, S.P. Antimicrobial Susceptibility of Inpatient Urinary Tract Isolates of Gram-Negative Bacilli in the United States: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) Program: 2009–2011. Clin. Ther. 2013, 35, 872–877. [Google Scholar] [CrossRef]
- Hawser, S.P.; Bouchillon, S.K.; Hoban, D.J.; Badal, R.E. Epidemiologic trends, occurrence of extended-spectrum β-lactamase production, and performance of ertapenem and comparators in patients with intra-abdominal infections: Analysis of global trend data from 2002–2007 from the SMART study. Surg. Infect. 2010, 11, 371–378. [Google Scholar] [CrossRef]
- Babinchak, T.; Badal, R.; Hoban, D.; Hackel, M.; Hawser, S.; Lob, S.; Bouchillon, S. Trends in susceptibility of selected gram-negative bacilli isolated from intra-abdominal infections in North America: SMART 2005–2010. Diagn. Microbiol. Infect. Dis. 2013, 76, 379–381. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing, Twenty-second Informational Supplement; CLSI document M100-S20U; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2010.
- Badal, R.E.; Lewis, J.S., II; Motyl, M.R. Changes in the CLSI Interpretive Criteria for Enterobacteriaceae and Ertapenem. Clin. Microbiol. Newsl. 2011, 33, 185–186. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing, Twenty-second Informational Supplement; CLSI document M100-S22; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morrissey, I.; Hackel, M.; Badal, R.; Bouchillon, S.; Hawser, S.; Biedenbach, D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals 2013, 6, 1335-1346. https://doi.org/10.3390/ph6111335
Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals. 2013; 6(11):1335-1346. https://doi.org/10.3390/ph6111335
Chicago/Turabian StyleMorrissey, Ian, Meredith Hackel, Robert Badal, Sam Bouchillon, Stephen Hawser, and Douglas Biedenbach. 2013. "A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011" Pharmaceuticals 6, no. 11: 1335-1346. https://doi.org/10.3390/ph6111335
APA StyleMorrissey, I., Hackel, M., Badal, R., Bouchillon, S., Hawser, S., & Biedenbach, D. (2013). A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals, 6(11), 1335-1346. https://doi.org/10.3390/ph6111335




