Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease
Abstract
:1. Introduction
2. Vascular Changes and Their Influence in the Pathology Seen in AD
3. Features That Influence the Development and Prognosis of AD during the Interactions between Cerebrovascular Diseases and Dementia
4. The Influence of Oxidative Stress on the Function of Brain Microvessels in AD
5. The Role of Mitochondrial Abnormalities during the Development of AD
6. Co–Factors for Oxidative Stress-Induced Cerebrovascular Lesions
6.1. Hypoperfusion-Induced Oxidative Stress as a Key Factor for the Development of AD
6.2. Cerebrovascular Lesions Observed During Ischemia/Reperfusion Induced Oxidative Stress
7. The Potential Role of Vasoactive Substances in the Endothelial Content during Ischemia/ Reperfusion
7.1. eNOS Involvement in the Cerebrovascular Tone
7.2. nNOS Expression and Regulation
7.3. iNOS as a Mediator of Oxidation During AD
8. Subcellular Mechanisms Involved in the Development and Maturation of Human AD
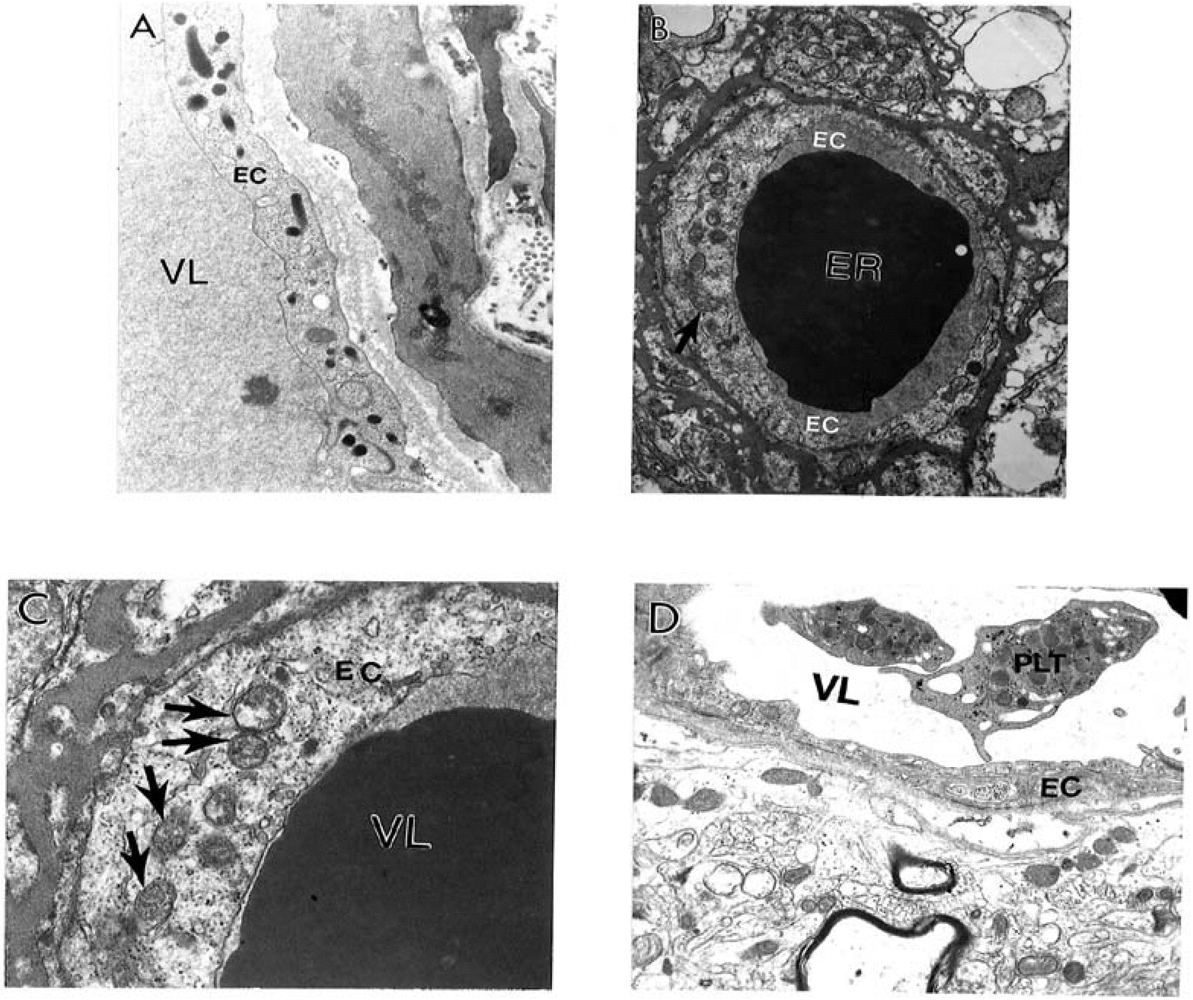
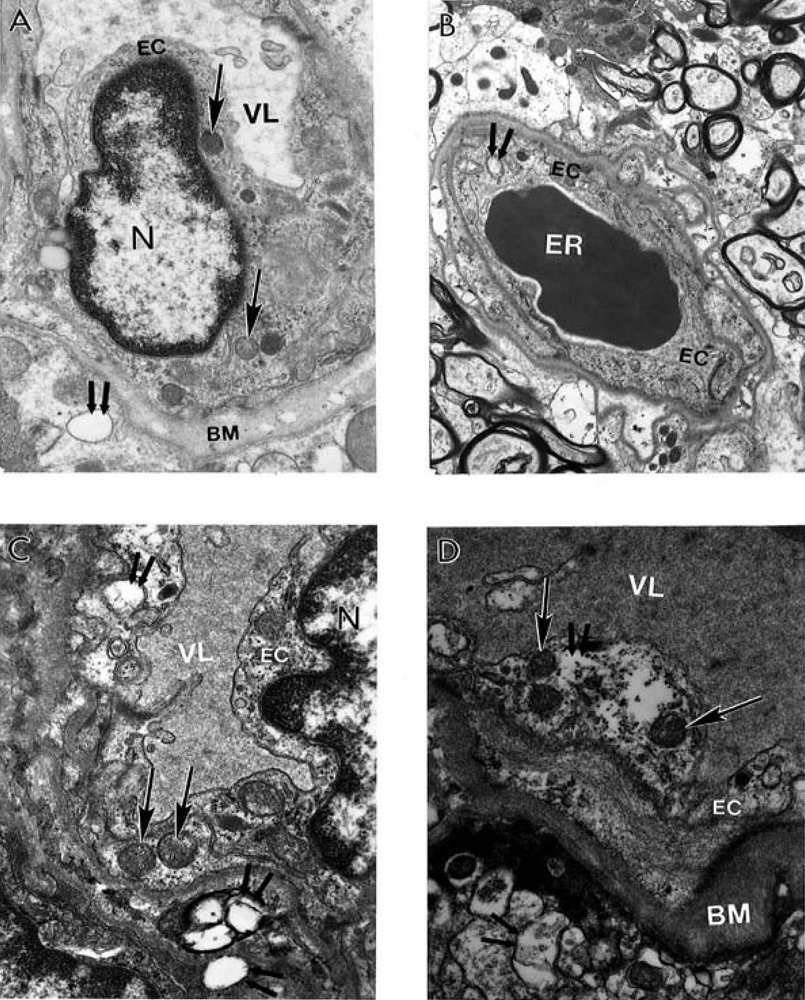
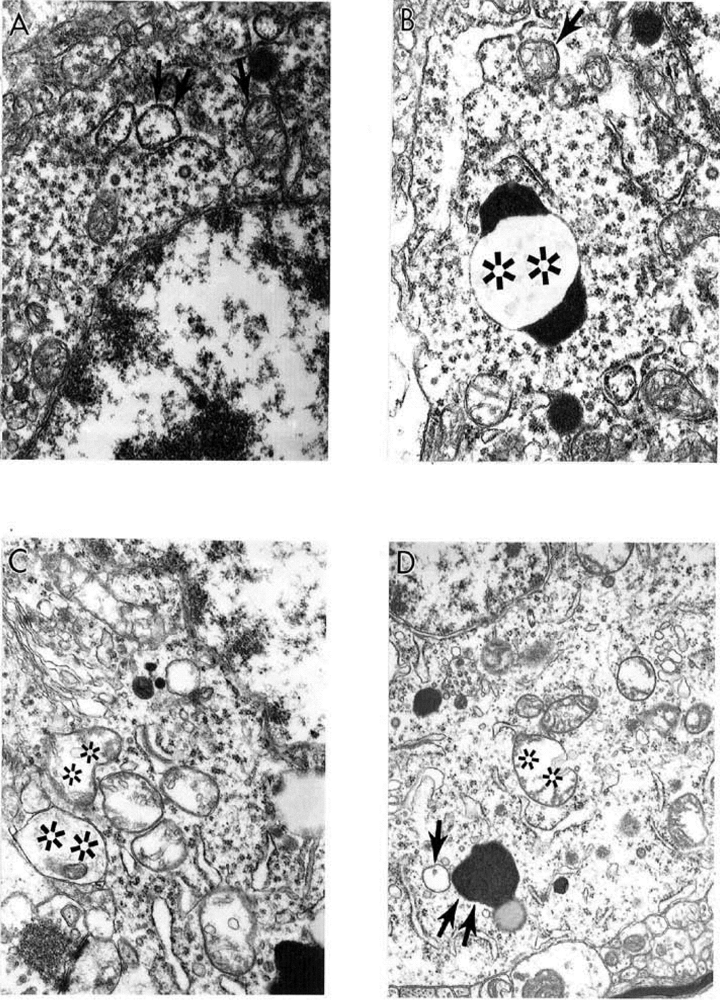
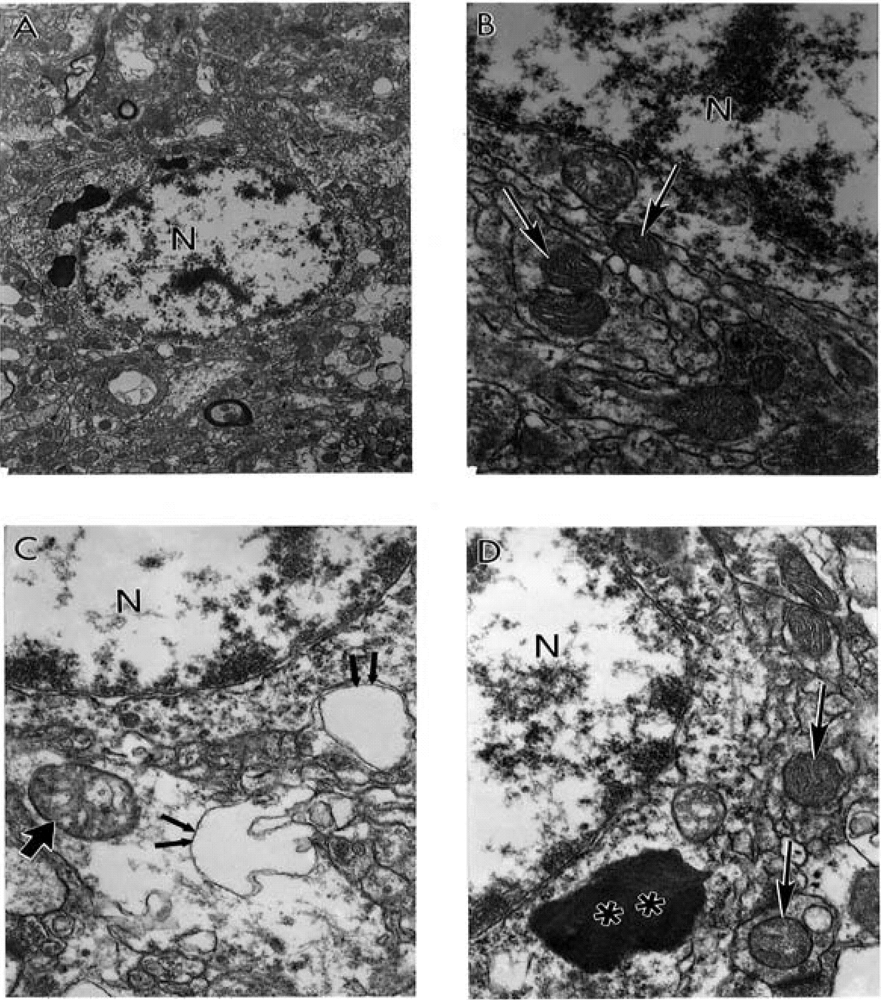
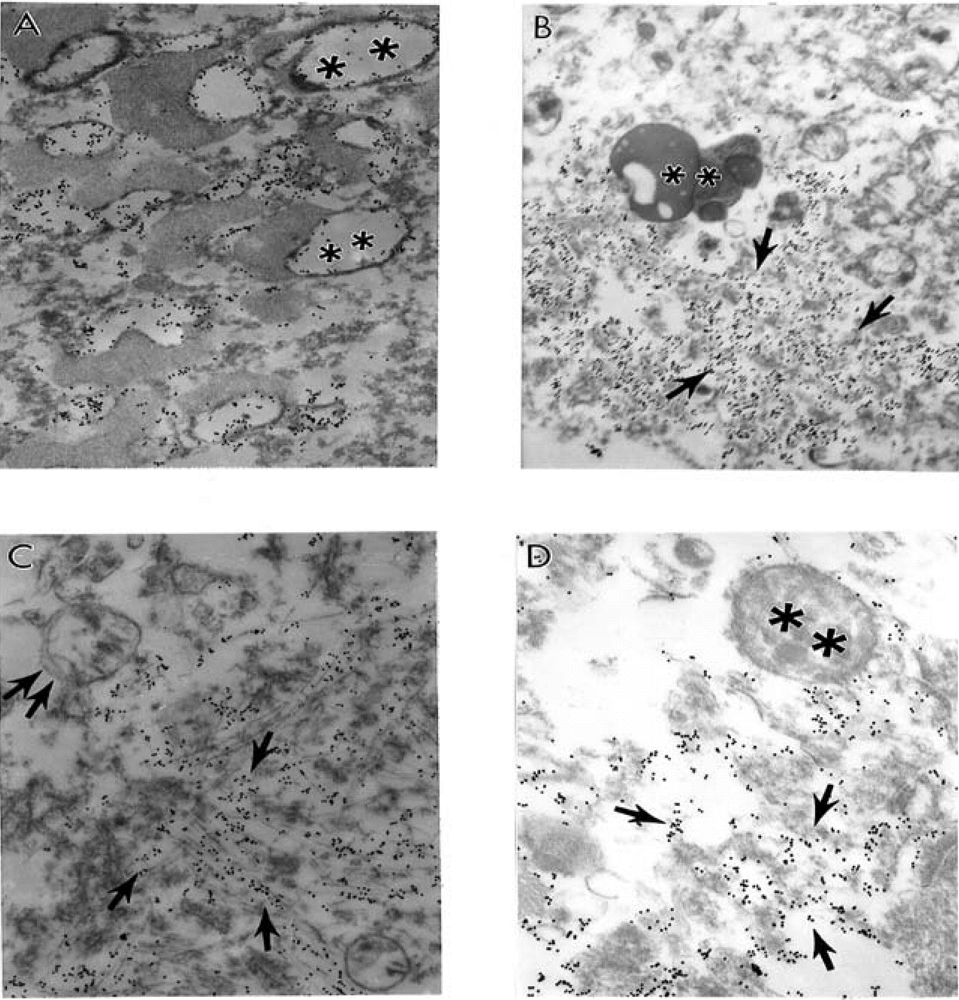
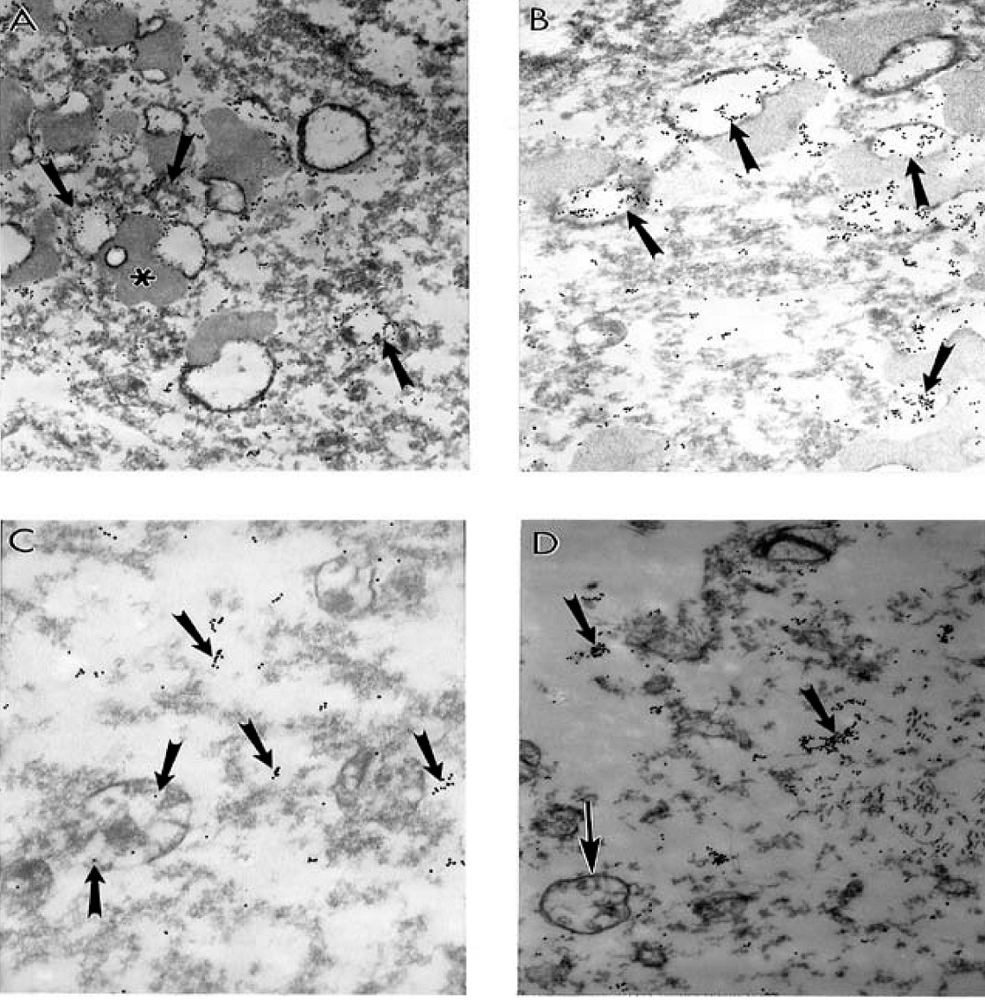
9. Antioxidant Application for the Treatment of AD
10. Conclusions
References
- Aliev, G.; Smith, M.A.; Seyidov, D.; Neal, M.L.; Lamb, B.T.; Nunomura, A.; Gasimov, E.K.; Vinters, H.V.; Perry, G.; LaManna, J.C.; Friedland, R.P. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer's disease. Brain Pathol. 2002, 12, 21–35. [Google Scholar] [PubMed]
- Aliev, G.; Gasimov, E.; Obrenovich, M.E.; Fischbach, K.; Shenk, J.C.; Smith, M.A.; Perry, G. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels: implication in the pathogenesis of Alzheimer's disease. Vasc. Health Risk Manag. 2008, 4, 721–730. [Google Scholar] [PubMed]
- Aliev, G.; Palacios, H.H.; Walrafen, B.; Lipsitt, A.E.; Obrenovich, M.E.; Morales, L. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int. J. Biochem. Cell Biol. 2009, 41, 1989–2004. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Cerebrovascular and cardiovascular pathology in Alzheimer's disease. Int. Rev. Neurobiol. 2009, 84, 35–48. [Google Scholar] [PubMed]
- Kalaria, R.N. The blood–brain barrier and cerebrovascular pathology in Alzheimer's disease. Ann. N. Y. Acad. Sci. 1999, 893, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The role of cerebral ischemia in Alzheimer's disease. Neurobiol. Aging 2000, 21, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Palacios, H.H.; Lipsitt, A.E.; Fischbach, K.; Lamb, B.T.; Obrenovich, M.E.; Morales, L.; Gasimov, E.; Bragin, V. Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox. Res. 2009, 16, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G. Is non-genetic Alzheimer's disease a vascular disorder with neurodegenerative consequences? J. Alzheimer’s Dis. 2002, 4, 513–516. [Google Scholar]
- Aliev, G.; Cirillo, R.; Salvatico, E.; Paro, M.; Prosdocimi, M. Changes in vessel ultrastructure during ischemia and reperfusion of rabbit hindlimb: implications for therapeutic intervention. Microvasc. Res. 1993, 46, 65–76. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Alzheimer disease as a vascular disorder: nosological evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Obrenovich, M.E.; Reddy, V.P.; Shenk, J.C.; Moreira, P.I.; Nunomura, A.; Zhu, X.; Smith, M.A.; Perry, G. Antioxidant therapy in Alzheimer's disease: theory and practice. Mini Rev. Med. Chem. 2008, 8, 1395–1406. [Google Scholar]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction . Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [PubMed]
- Perry, G.; Smith, M.A.; McCann, C.E.; Siedlak, S.L.; Jones, P.K.; Friedland, R.P. Cerebrovascular muscle atrophy is a feature of Alzheimer's disease. Brain Res. 1998, 791, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer's. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Petot, G.J.; Perry, G. Commentary: Diet and Oxidative Stress: A Novel Synthesis of Epidemiological Data on Alzheimer's Disease. Alzheimer's Dis. Rev. 1997, 2, 58–60. [Google Scholar]
- Smith, M.A.; Richey Harris, P.L.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite–mediated damage in Alzheimer's disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [PubMed]
- Smith, M.A.; Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Perry, G. Oxidative stress in Alzheimer's disease. Biochim. Biophys. Acta 2000, 1502, 139–144. [Google Scholar] [PubMed]
- Smith, M.A.; Sayre, L.M.; Perry, G. Is Alzheimer's a Disease of Oxidative Stress? Alzheimer's Dis. Rev. 1996, 1, 63–67. [Google Scholar]
- Smith, M.A.; Vasak, M.; Knipp, M.; Castellani, R.J.; Perry, G. Dimethylargininase, a nitric oxide regulatory protein, in Alzheimer disease. Free Radic. Biol. Med. 1998, 25, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology 1998, 51, 1562–1566. [Google Scholar] [PubMed]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Prelli, F.; Castano, E.M.; van Duinen, S.G.; Bots, G.T.; Luyendijk, W.; Frangione, B. Different processing of Alzheimer's beta–protein precursor in the vessel wall of patients with hereditary cerebral hemorrhage with amyloidosis–Dutch type. Biochem. Biophys. Res. Commun. 1988, 151, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Carney, J.M. Oxidative alterations in Alzheimer's disease. Brain Pathol. 1999, 9, 133–146. [Google Scholar] [PubMed]
- Mecocci, P.; Beal, M.F.; Cecchetti, R.; Polidori, M.C.; Cherubini, A.; Chionne, F.; Avellini, L.; Romano, G.; Senin, U. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol. Chem. Neuropathol. 1997, 31, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Kaufman, A.E.; Koontz, D.; Shoffner, J.M.; Wallace, D.C.; Beal, M.F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; Chiba, S.; Atwood, C.S.; Petersen, R.B.; Smith, M.A. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [PubMed]
- Nunomura, A.; Perry, G.; Hirai, K.; Aliev, G.; Takeda, A.; Chiba, S.; Smith, M.A. Neuronal RNA oxidation in Alzheimer's disease and Down's syndrome. Ann. N. Y. Acad. Sci. 1999, 893, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Pappolla, M.A.; Wade, R.; Hirai, K.; Chiba, S.; Smith, M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [PubMed]
- Perry, G.; Nunomura, A.; Hirai, K.; Takeda, A.; Aliev, G.; Smith, M.A. Oxidative damage in Alzheimer's disease: the metabolic dimension. Int. J. Dev. Neurosci. 2000, 18, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.; Perry, G.; Salomon, R.G.; Smith, M.A. 4–Hydroxynonenal–derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [PubMed]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yan, S.D.; Stern, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard reaction end products are associated with Alzheimer’s disease pathology. Proc. Natl. Acad. Sci, USA 1994, 91, 5710–5714. [Google Scholar] [CrossRef]
- Cirillo, R.; Aliev, G.; Hornby, E.J.; Prosdocimi, M. Endothelium as a therapeutical target in peripheral occlusive arterial diseases: consideration for pharmacological interventions. Pharmacol. Res. 1994, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, R.; Salvatico, E.; Aliev, G.; Prosdocimi, M. Effect of cloricromene during ischemia and reperfusion of rabbit hindlimb: evidence for an involvement of leukocytes in reperfusion–mediated tissue and vascular injury. J. Cardiovasc. Pharmacol. 1992, 20, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Benoit, J.N.; Suzuki, M.; Grisham, M.B. Leukocyte adherence to venular endothelium during ischemia–reperfusion . Am. J. Physiol. 1989, 257, G683–G688. [Google Scholar] [PubMed]
- Sala, A.; Aliev, G.M.; Rossoni, G.; Berti, F.; Buccellati, C.; Burnstock, G.; Folco, G.; Maclouf, J. Morphological and functional changes of coronary vasculature caused by transcellular biosynthesis of sulfidopeptide leukotrienes in isolated heart of rabbit. Blood 1996, 87, 1824–1832. [Google Scholar] [PubMed]
- Salvatico, E.; Aliev, G.M.; Novello, D.; Prosdocimi, M. Functional depression of isolated perfused rat heart mediated by activated leukocytes: protective effect of cloricromene. J. Cardiovasc. Pharmacol. 1994, 24, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Matz, R.L.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium–derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res. 2000, 49, 11–18. [Google Scholar] [PubMed]
- Aliev, G.; Burnstock, G. Watanabe rabbits with heritable hypercholesterolaemia: a model of atherosclerosis. Histol. Histopathol. 1998, 13, 797–817. [Google Scholar] [PubMed]
- Stewart–Lee, A.L.; Ferns, G.A.; Anggard, E.E. Differences in onset of impaired endothelial responses and in effects of vitamin E in the hypercholesterolemic rabbit carotid and renal arteries. J. Cardiovasc. Pharmacol. 1995, 25, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Price, J.M.; Sutton, E.T.; Hellermann, A.; Thomas, T. beta–Amyloid induces cerebrovascular endothelial dysfunction in the rat brain. Neurol. Res. 1997, 19, 534–538. [Google Scholar] [PubMed]
- Iadecola, C.; Zhang, F.; Niwa, K.; Eckman, C.; Turner, S.K.; Fischer, E.; Younkin, S.; Borchelt, D.R.; Hsiao, K.K.; Carlson, G.A. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 1999, 2, 157–161. [Google Scholar] [CrossRef]
- Niwa, K.; Carlson, G.A.; Iadecola, C. Exogenous a beta1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J. Cereb. Blood Flow Metab. 2000, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Moore, P.; Weigel, P.H. Microvessels from Alzheimer's disease brains kill neurons in vitro. Am. J. Pathol. 1999, 154, 337–342. [Google Scholar] [PubMed]
- Mao, P.; Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochim. Biophys. Acta 2009, 1802, 66–79. [Google Scholar] [PubMed]
- Acuna–Castroviejo, D.; Martin, M.; Macias, M.; Escames, G.; Leon, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001, 30, 65–74. [Google Scholar] [PubMed]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer's disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Fiskum, G.; Murphy, A.N.; Beal, M.F. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J. Cereb. Blood Flow Metab. 1999, 19, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Matthews, R.T.; Klockgether, T.; Dichgans, J.; Beal, M.F. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol. Cell Biochem. 1997, 174, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Vancova, O.; Baciak, L.; Kasparova, S.; Kucharska, J.; Palacios, H.H.; Horecky, J.; Aliev, G. In vivo and in vitro assessment of brain bioenergetics in aging rats . JCMM 2009. [Google Scholar]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; Shimohama, S.; Cash, A.D.; Siedlak, S.L.; Harris, P.L.; Jones, P.K.; Petersen, R.B.; Perry, G.; Smith, M.A. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [PubMed]
- Horecky, J.; Baciak, L.; Kasparova, S.; Pacheco, G.; Aliev, G.; Vancova, O. Minimally invasive surgical approach for three–vessel occlusion as a model of vascular dementia in the rat–brain bioenergetics assay. J. Neurol. Sci. 2009, 283, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [PubMed]
- Beckman, K.B.; Ames, B.N. Mitochondrial aging: open questions. Ann. N.Y. Acad. Sci. 1998, 854, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. Endogenous oxidative damage of mtDNA. Mutat. Res. 1999, 424, 51–58. [Google Scholar] [PubMed]
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science 1999, 283, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Smith, M.A.; Vinters, H.; Johnson, A.B.; Nunomura, A.; Perry, G. Mitochondria abnormalities mark vulnerable neurons in Alzheimer's disease. J. Neuropathol. Exper. Neurol. 1999, 58, 511. [Google Scholar] [CrossRef]
- Beal, M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995, 38, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, E.; Tanji, K.; Hirano, M.; Vu, T.H.; DiMauro, S.; Schon, E.A. Mitochondrial involvement in Alzheimer's disease. Biochim. Biophys. Acta 1999, 1410, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Smith, M.A.; Obrenovich, M.E.; de la Torre, J.C.; Perry, G. Role of vascular Hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Alzheimer’s disease. Neurotox. Res. 2003, 5, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Al–Abdulla, N.A.; Martin, L.J. Apoptosis of retrogradely degenerating neurons occurs in association with the accumulation of perikaryal mitochondria and oxidative damage to the nucleus. Am. J. Pathol. 1998, 153, 447–456. [Google Scholar] [PubMed]
- Wallace, D.C. Mitochondrial DNA in aging and disease. Sci. Am. 1997, 277, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cormier, A.; Morin, C.; Zini, R.; Tillement, J.P.; Lagrue, G. In vitro effects of nicotine on mitochondrial respiration and superoxide anion generation. Brain Res. 2001, 900, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer's disease . C.N.S. Spectr. 2009, 14, 8–18. [Google Scholar]
- Aliyev, A.; Chen, S.G.; Seyidova, D.; Smith, M.A.; Perry, G.; de la Torre, J.; Aliev, G. Mitochondria DNA deletions in atherosclerotic hypoperfused brain microvessels as a primary target for the development of Alzheimer's disease . J. Neurol. Sci. 2005, 229-230, 285–292. [Google Scholar] [CrossRef] [PubMed]
- De Jong, G.I.; De Vos, R.A.; Steur, E.N.; Luiten, P.G. Cerebrovascular hypoperfusion: a risk factor for Alzheimer's disease? Animal model and postmortem human studies. Ann. N.Y. Acad. Sci. 1997, 826, 56–74. [Google Scholar]
- De la Torre, J.C. Hemodynamic consequences of deformed microvessels in the brain in Alzheimer's disease. Ann. N.Y. Acad. Sci. 1997, 826, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Frackowiak, R.S. Cerebral function in aging and Alzheimer's disease: the role of PET. Electroencephalogr. Clin. Neurophysiol. Suppl. 1991, 42, 355–365. [Google Scholar] [PubMed]
- Kumar, A.; Schapiro, M.B.; Haxby, J.V.; Grady, C.L.; Friedland, R.P. Cerebral metabolic and cognitive studies in dementia with frontal lobe behavioral features. J. Psychiatr. Res. 1990, 24, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Bengen, J.; Schollmeyer, P.; Wanner, C. Impairment of endothelium–dependent dilation in rabbit renal arteries by oxidized lipoprotein(a). Role of oxygen–derived radicals. Circulation 1995, 92, 1582–1589. [Google Scholar] [PubMed]
- de la Torre, J.C.; Stefano, G.B. Evidence that Alzheimer's disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res. Brain Res. Rev. 2000, 34, 119–136. [Google Scholar] [CrossRef]
- Meguro, K.; Blaizot, X.; Kondoh, Y.; Le Mestric, C.; Baron, J.C.; Chavoix, C. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non–human primate as shown by PET. Implications for Alzheimer's disease . Brain 1999, 122 (Pt. 8), 1519–1531. [Google Scholar] [PubMed]
- Jagust, W.J.; Friedland, R.P.; Budinger, T.F.; Koss, E.; Ober, B. Longitudinal studies of regional cerebral metabolism in Alzheimer's disease. Neurology 1988, 38, 909–912. [Google Scholar] [PubMed]
- de la Torre, J.C. Pathophysiology of neuronal energy crisis in Alzheimer's disease. Neurodegener. Dis. 2008, 5, 126–132. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. Alzheimer disease as a vascular disorder: nosological evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Critically attained threshold of cerebral hypoperfusion: can it cause Alzheimer's disease? Ann. N.Y. Acad. Sci. 2000, 903, 424–436. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Alzheimer's disease prevalence can be lowered with non–invasive testing. J. Alzheimer's Dis. 2008, 14, 353–359. [Google Scholar]
- Hofman, A.; Ott, A.; Breteler, M.M.; Bots, M.L.; Slooter, A.J.; van Harskamp, F.; van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Weinbrecht, P.; Longmuir, I.; Knopp, J.; Mills, M. Cerebral microcirculatory changes during exposure to hypoxia. Adv. Exp. Med. Biol. 1987, 215, 259–263. [Google Scholar] [PubMed]
- Mironov, V.; Hritz, M.A.; LaManna, J.C.; Hudetz, A.G.; Harik, S.I. Architectural alterations in rat cerebral microvessels after hypobaric hypoxia. Brain Res. 1994, 660, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.C.; Agani, F.; Pichiule, P.; LaManna, J.C. Expression of hypoxia–inducible factor–1 alpha in the brain of rats during chronic hypoxia. J. Appl. Physiol. 2000, 89, 1937–1942. [Google Scholar] [PubMed]
- Stewart, P.A.; Isaacs, H.; LaManna, J.C.; Harik, S.I. Ultrastructural concomitants of hypoxia–induced angiogenesis. Acta Neuropathol. (Berl.) 1997, 93, 579–584. [Google Scholar] [CrossRef]
- LaManna, J.C.; Boehm, K.D.; Mironov, V.; Hudetz, A.G.; Hritz, M.A.; Yun, J.K.; Harik, S.I. Increased basic fibroblastic growth factor mRNA in the brains of rats exposed to hypobaric hypoxia. Adv. Exp. Med. Biol. 1994, 361, 497–502. [Google Scholar] [PubMed]
- Harik, N.; Harik, S.I.; Kuo, N.T.; Sakai, K.; Przybylski, R.J.; LaManna, J.C. Time–course and reversibility of the hypoxia–induced alterations in cerebral vascularity and cerebral capillary glucose transporter density. Brain Res. 1996, 737, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Smith, M.A.; Perry, G.; Aliev, G. Mitochondrial failures in Alzheimer's disease. Am. J. Alzheimer’s Dis. Other Demen. 2004, 19, 345–352. [Google Scholar] [CrossRef]
- Aliev, G.; Smith, M.A.; Turmaine, M.; Neal, M.L.; Zimina, T.V.; Friedland, R.P.; Perry, G.; LaManna, J.C.; Burnstock, G. Atherosclerotic lesions are associated with increased immunoreactivity for inducible nitric oxide synthase and endothelin–1 in thoracic aortic intimal cells of hyperlipidemic Watanabe rabbits. Exp. Mol. Pathol. 2001, 71, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Perry, G.; Berridge, M.S.; Aliev, G.; Siedlak, S.L.; Smith, M.A.; LaManna, J.C.; Friedland, R.P. Labeling of cerebral amyloid beta deposits in vivo using intranasal basic fibroblast growth factor and serum amyloid P component in mice. JNM 2002, 43, 1044–1051. [Google Scholar] [PubMed]
- Bednar, M.M.; Raymond, S.; McAuliffe, T.; Lodge, P.A.; Gross, C.E. The role of neutrophils and platelets in a rabbit model of thromboembolic stroke. Stroke 1991, 22, 44–50. [Google Scholar] [PubMed]
- Chen, H.; Chopp, M.; Zhang, Z.G.; Garcia, J.H. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1992, 12, 621–628. [Google Scholar] [PubMed]
- Olah, L.; Wecker, S.; Hoehn, M. Relation of apparent diffusion coefficient changes and metabolic disturbances after 1 hour of focal cerebral ischemia and at different reperfusion phases in rats. J. Cereb. Blood Flow Metab. 2001, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Busto, R.; Ginsberg, M.D. Cerebral endothelial microvilli: formation following global forebrain ischemia. J. Neuropathol. Exp. Neurol. 1984, 43, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.G.; Ames 3d, A. Studies on mechanisms of impairment of cerebral circulation following ischemia: effect of hemodilution and perfusion pressure. Stroke 1972, 3, 538–542. [Google Scholar] [PubMed]
- Wade, J.G.; Amtorp, O.; Sorensen, S.C. No–flow state following cerebral ischemia.Role of increase in potassium concentration in brain interstitial fluid. Arch. Neurol. 1975, 32, 381–384. [Google Scholar] [PubMed]
- Cada, A.; de la Torre, J.C.; Gonzalez–Lima, F. Chronic cerebrovascular ischemia in aged rats: effects on brain metabolic capacity and behavior. Neurobiol. Aging 2000, 21, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995, 9, 1319–1330. [Google Scholar] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Crow, J.P.; Ye, Y.Z.; Strong, M.; Kirk, M.; Barnes, S.; Beckman, J.S. Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament L. J. Neurochem. 1997, 69, 1945–1953. [Google Scholar] [PubMed]
- Dawson, V.L.; Brahmbhatt, H.P.; Mong, J.A.; Dawson, T.M. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal–glial cortical cultures. Neuropharmacology 1994, 33, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.L.; Dawson, T.M. Nitric oxide in neuronal degeneration. Proc. Soc. Exp. Biol. Med. 1996, 211, 33–40. [Google Scholar] [PubMed]
- Dawson, V.L.; Dawson, T.M.; London, E.D.; Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 1991, 88, 6368–6371. [Google Scholar] [CrossRef]
- Michel, T.; Feron, O. Nitric oxide synthases: which, where, how, and why? J. Clin. Invest. 1997, 100, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.M.; Luscher, T.F.; Cosentino, F.; Rabelink, T.J. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation 1998, 97, 108–112. [Google Scholar] [PubMed]
- Xia, Y.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H.; Zweier, J.L. Nitric oxide synthase generates superoxide and nitric oxide in arginine–depleted cells leading to peroxynitrite–mediated cellular injury. Proc. Natl. Acad. Sci. USA 1996, 93, 6770–6774. [Google Scholar] [CrossRef]
- Ignarro, L.J. Biosynthesis and metabolism of endothelium–derived nitric oxide. Annu. Rev . Pharmacol. Toxicol. 1990, 30, 535–560. [Google Scholar] [PubMed]
- Stamler, J.S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Weremowicz, S.; Morton, C.C.; Michel, T. Isolation and chromosomal localization of the human endothelial nitric oxide synthase (NOS3) gene. Genomics 1994, 19, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sessa, W.C. The nitric oxide synthase family of proteins. J. Vasc. Res. 1994, 31, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J. Structure–function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Morris Jr., S.M.; Billiar, T.R. New insights into the regulation of inducible nitric oxide synthesis . Am. J. Physiol. 1994, 266, E829–E839. [Google Scholar] [PubMed]
- Bates, T.E.; Loesch, A.; Burnstock, G.; Clark, J.B. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem. Biophys. Res. Commun. 1995, 213, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.E.; Loesch, A.; Burnstock, G.; Clark, J.B. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem. Biophys. Res. Commun. 1996, 218, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.A. Nitric oxide and focal cerebral ischemia: multiplicity of actions and diverse outcome. Cerebrovasc. Brain Metab. Rev. 1994, 6, 299–324. [Google Scholar] [PubMed]
- Bredt, D.S.; Snyder, S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994, 63, 175–195. [Google Scholar] [PubMed]
- Faraci, F.M.; Brian Jr., J.E. Nitric oxide and the cerebral circulation . Stroke 1994, 25, 692–703. [Google Scholar] [PubMed]
- Almeida, A.; Bolanos, J.P.; Medina, J.M. Nitric oxide mediates glutamate–induced mitochondrial depolarization in rat cortical neurons. Brain Res. 1999, 816, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S. The double–edged role of nitric oxide in brain function and superoxide–mediated injury. J. Dev. Physiol. 1991, 15, 53–59. [Google Scholar] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Beckman, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994, 233, 229–240. [Google Scholar] [PubMed]
- Cazevieille, C.; Muller, A.; Meynier, F.; Bonne, C. Superoxide and nitric oxide cooperation in hypoxia/reoxygenation–induced neuron injury. Free Radic. Biol. Med. 1993, 14, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [PubMed]
- Lafon–Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA–dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M. Role of endothelium–derived relaxing factor in cerebral circulation: large arteries vs. microcirculation . Am. J. Physiol. 1991, 261, H1038–H1042. [Google Scholar] [PubMed]
- Fabricius, M.; Lauritzen, M. Examination of the role of nitric oxide for the hypercapnic rise of cerebral blood flow in rats . Am. J. Physiol. 1994, 266, H1457–H1464. [Google Scholar] [PubMed]
- Iadecola, C. Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia? Proc. Natl. Acad. Sci. USA 1992, 89, 3913–3916. [Google Scholar] [CrossRef]
- Buisson, A.; Margaill, I.; Callebert, J.; Plotkine, M.; Boulu, R.G. Mechanisms involved in the neuroprotective activity of a nitric oxide synthase inhibitor during focal cerebral ischemia. J. Neurochem. 1993, 61, 690–696. [Google Scholar] [PubMed]
- Buisson, A.; Plotkine, M.; Boulu, R.G. The neuroprotective effect of a nitric oxide inhibitor in a rat model of focal cerebral ischaemia. Br. J. Pharmacol. 1992, 106, 766–767. [Google Scholar] [PubMed]
- Samadani, A.F.; Dawson, T.M.; Dawson, V.L. Nitric oxide synthase in model of focal ischemia. Stroke 1997, 28, 1283–1288. [Google Scholar] [PubMed]
- Hamada, Y.; Hayakawa, T.; Hattori, H.; Mikawa, H. Inhibitor of nitric oxide synthesis reduces hypoxic–ischemic brain damage in the neonatal rat. Pediatr. Res. 1994, 35, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Li, J.; Ebner, T.J.; Xu, X. Nitric oxide contributes to functional hyperemia in cerebellar cortex . Am. J. Physiol. 1995, 268, R1153–R1162. [Google Scholar] [PubMed]
- Iadecola, C.; Zhang, F.; Casey, R.; Clark, H.B.; Ross, M.E. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke 1996, 27, 1373–1380. [Google Scholar] [PubMed]
- Iadecola, C.; Zhang, F.; Xu, S.; Casey, R.; Ross, M.E. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J. Cereb. Blood Flow Metab. 1995, 15, 378–384. [Google Scholar] [PubMed]
- Nakashima, M.N.; Yamashita, K.; Kataoka, Y.; Yamashita, Y.S.; Niwa, M. Time course of nitric oxide synthase activity in neuronal, glial, and endothelial cells of rat striatum following focal cerebral ischemia. Cell Mol. Neurobiol. 1995, 15, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pelligrino, D.A.; Baughman, V.L.; Koenig, H.M.; Albrecht, R.F. The role of neuronal nitric oxide synthase in regulation of cerebral blood flow in normocapnia and hypercapnia in rats. J Cereb. Blood Flow Metab. 1995, 15, 774–778. [Google Scholar] [PubMed]
- Garthwaite, J.; Beaumont, P.S. Excitatory amino acid receptors in the parallel fibre pathway in rat cerebellar slices. Neurosci. Lett. 1989, 107, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Huang, P.L.; Panahian, N.; Fishman, M.C.; Moskowitz, M.A. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 1996, 16, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, P.L.; Panahian, N.; Dalkara, T.; Fishman, M.C.; Moskowitz, M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994, 265, 1883–1885. [Google Scholar] [PubMed]
- Eliasson, M.J.; Sampei, K.; Mandir, A.S.; Hurn, P.D.; Traystman, R.J.; Bao, J.; Pieper, A.; Wang, Z.Q.; Dawson, T.M.; Snyder, S.H.; Dawson, V.L. Poly(ADP–ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997, 3, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Thorns, V.; Hansen, L.; Masliah, E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp. Neurol. 1998, 150, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bogumil, R.; Knipp, M.; Fundel, S.M.; Vasak, M. Characterization of dimethylargininase from bovine brain: evidence for a zinc binding site. Biochemistry 1998, 37, 4791–4798. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, M.; Tsuji, H.; Ogawa, T.; Sasaoka, K. Detection of NG,NG–dimethylarginine dimethylaminohydrolase in the nitric oxide–generating systems of rats using monoclonal antibody. Arch. Biochem. Biophys. 1993, 300, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, M.; Whitley, G.S.; Tsuji, H.; Ogawa, T. Detection of NG,NG–dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J. Biochem. (Tokyo) 1995, 117, 237–238. [Google Scholar]
- MacAllister, R.J.; Parry, H.; Kimoto, M.; Ogawa, T.; Russell, R.J.; Hodson, H.; Whitley, G.S.; Vallance, P. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br. J. Pharmacol. 1996, 119, 1533–1540. [Google Scholar] [PubMed]
- Beckman, J.S.; Carson, M.; Smith, C.D.; Koppenol, W.H. ALS, SOD and peroxynitrite. Nature 1993, 364, 584. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Liu, J.; Shenk, J.C.; Fischbach, K.; Pacheco, G.J.; Chen, S.G.; Obrenovich, M.E.; Ward, W.F.; Richardson, A.G.; Smith, M.A.; Gasimov, E.; Perry, G.; Ames, B.N. Neuronal mitochondrial amelioration by feeding acetyl–L–carnitine and lipoic acid to aged rats. JCMM 2009, 2, 320–333. [Google Scholar]
- Ames, B.N. Delaying the mitochondrial decay of aging. Ann. N.Y. Acad. Sci. 2004, 1019, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Mitochondrial decay, a major cause of aging, can be delayed. J. Alzheimer's Dis. 2004, 6, 117–121. [Google Scholar]
- Ames, B.N.; Liu, J. Delaying the mitochondrial decay of aging with acetyl L-Carnitine. Ann. N.Y. Acad. Sci. 2004, 1033, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl–L–carnitine and/or R–alpha –lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Kuratsune, H.; Cotman, C.W.; Ames, B.N. Comparison of the effects of L–carnitine and acetyl–L–carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N.Y. Acad. Sci. 2004, 1033, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Milgram, N.W.; Araujo, J.A.; Hagen, T.M.; Treadwell, B.V.; Ames, B.N. Acetyl–L–Carnitine and alpha–Lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007, 21, 3756–3762. [Google Scholar] [CrossRef] [PubMed]
- Shenk, J.C.; Liu, J.; Fischbach, K; Xu, K.; Puchowicz, M.; Obrenovich, M.E; Gasimov, E.; Alvarez, L,M.; Ames, B.N.; LaManna, J.C.; Aliev, G. The effect of acetyl–L–Carnitine and R–Lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer's disease . J. Neurol. Sci. 2009, 283, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez–Pinilla, F. Brain foods: the effects of nutrients on brain function . Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp. Neurol. 2009, 218, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Killilea, D.W.; Ames, B.N. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate–binding affinity and activity in brain by feeding old rats acetyl–L–Carnitine and/or R–alpha–Lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 1876–1881. [Google Scholar] [CrossRef]
- Long, J.; Gao, F.; Tong, L.; Cotman, C.W.; Ames, B.N.; Liu, J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha–Lipoic acid and acetyl–L–Carnitine. Neurochem. Res. 2009, 34, 755–63. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008, 10, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Amyloid precursor protein–mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer's disease. J. Neurochem. 2006, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Atamna, H.; Kuratsune, H.; Ames, B.N. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann. N.Y. Acad. Sci. 2002, 959, 133–166. [Google Scholar] [PubMed]
- Reid, P.C.; Urano, Y.; Kodama, T.; Hamakubo, T. Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. JCMM 2007, 11, 383–392. [Google Scholar] [PubMed]
- Aliev, G.; Shenk, J.C.; Fischbach, K.; Perry, G. Stem cell niches as clinical targets: the future of anti–ischemic therapy? Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Cedazo–Minguez, A. Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. JCMM 2007, 11, 1227–1238. [Google Scholar] [PubMed]
- Mas, E.; Dupuy, A.M.; Artero, S.; Portet, F.; Cristol, J.P.; Ritchie, K.; Touchon, J. Functional Vitamin E deficiency in ApoE4 patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2006, 21, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sanchez–Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce–Levie, K.; Cheng, I.H.; Gan, L.; Palop, J.J.; Bonventre, J.V.; Mucke, L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, T.S.; Reddy, P.H. Can herbs provide a new generation of drugs for treating Alzheimer's disease? Brain Res. Brain Res. Rev. 2005, 50, 361–376. [Google Scholar] [PubMed]
- Aliev, G.; Castellani, R.J.; Petersen, R.B.; Burnstock, G.; Perry, G.; Smith, M.A. Pathobiology of familial hypercholesterolemic atherosclerosis. J. Submicrosc. Cytol. Pathol. 2004, 36, 225–240. [Google Scholar] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5644–5651. [Google Scholar] [CrossRef]
- Reiman, E.M.; Caselli, R.J.; Chen, K.; Alexander, G.E.; Bandy, D.; Frost, J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. USA 2001, 98, 3334–3339. [Google Scholar] [CrossRef]
- Aliev, G.; Smith, M.A.; de la Torre, J.C.; Perry, G. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer's disease. Mitochondrion 2004, 4, 649–663. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aliev, G.; Palacios, H.H.; Gasimov, E.; Obrenovich, M.E.; Morales, L.; Leszek, J.; Bragin, V.; Solís Herrera, A.; Gokhman, D. Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease. Pharmaceuticals 2010, 3, 158-187. https://doi.org/10.3390/ph3010158
Aliev G, Palacios HH, Gasimov E, Obrenovich ME, Morales L, Leszek J, Bragin V, Solís Herrera A, Gokhman D. Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease. Pharmaceuticals. 2010; 3(1):158-187. https://doi.org/10.3390/ph3010158
Chicago/Turabian StyleAliev, Gjumrakch, Hector H. Palacios, Eldar Gasimov, Mark E. Obrenovich, Ludis Morales, Jerzy Leszek, Valentin Bragin, Arturo Solís Herrera, and Dmitry Gokhman. 2010. "Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease" Pharmaceuticals 3, no. 1: 158-187. https://doi.org/10.3390/ph3010158
APA StyleAliev, G., Palacios, H. H., Gasimov, E., Obrenovich, M. E., Morales, L., Leszek, J., Bragin, V., Solís Herrera, A., & Gokhman, D. (2010). Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease. Pharmaceuticals, 3(1), 158-187. https://doi.org/10.3390/ph3010158



