Impact of Glycosylation on Effector Functions of Therapeutic IgG †
Abstract
:1. Introduction
2. IgG Fc Glycosylation and Its Impact on IgG/FcγR Interactions
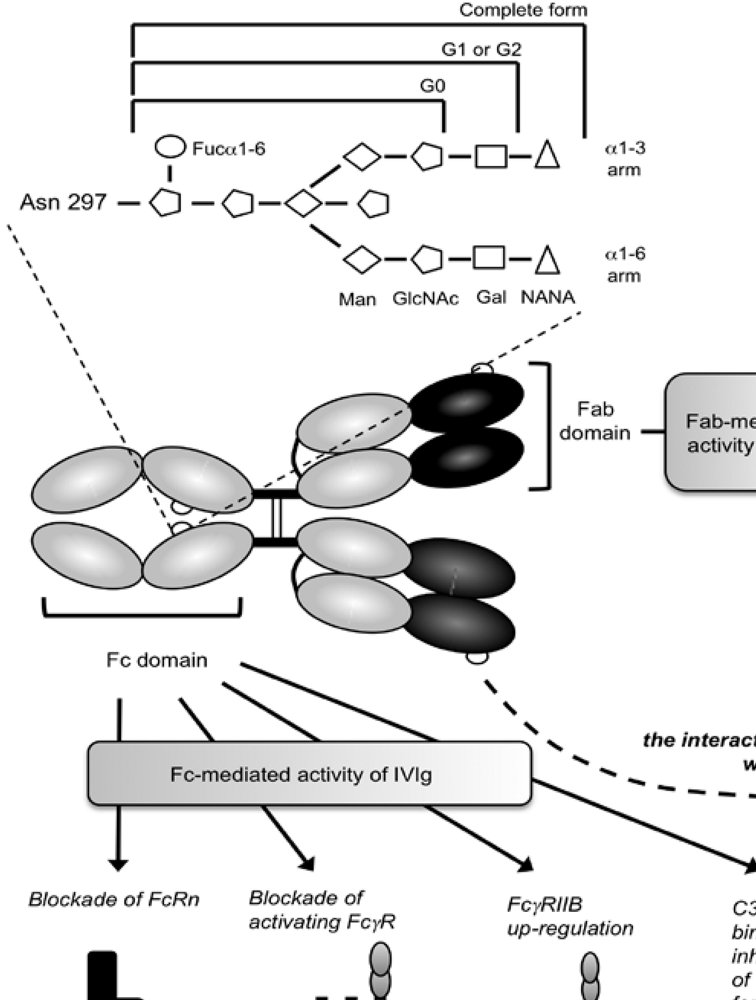
3. IgG Fc Glycosylation and Immunological Diseases
4. Engineering Fc Glycosylation for Optimizing mAb Efficacy
| GA101 | LFB-R603 | BLX-301 | |
|---|---|---|---|
| Company | Roche [Glycart] | LFB Biotechnologies [GTC Biotherapeutics] | Biolex |
| Format | Humanized | Chimæric | Humanized |
| Type of anti-CD20 | Type II | Type I | NA |
| Glyco-engineering | Low fucose | Low fucose | No fucose / G0 |
| CDC* | ↘ | = | ↘ |
| ADCC** | ↗ | ↗ | ↗ |
| PCD° | ↗ | = | NA°° |
| Phase Development | Phase II | Phase I/II | Pre-clinical |
| Indication | NHL / CLL+ | CLL | NHL |
| Reference | [51] | [34] | NA |
5. Role of Glycosylation in the Inflammatory Activity of IVIg
6. Conclusion
Acknowledgements
References
- Huhn, C.; Selman, M.H.J.; Ruhaak, L.R.; Deelder, A.M.; Wuhrer, M. IgG glycosylation analysis. Proteomics 2009, 9, 882–913. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kuroki, Y.; Ohtsubo, K.; Taniguchi, N. Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: Their functions and target proteins. Carbohydr. Res. 2009, 344, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Sibéril, S.; Dutertre, C.A.; Fridman, W.H.; Teillaud, J.L. FcgammaR: The key to optimize therapeutic antibodies? Crit. Rev. Oncol. Hematol. 2007, 62, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Abès, R.; Dutertre, C.A.; Agnelli, L.; Teillaud, J.L. Activating and inhibitory Fcgamma receptors in immunotherapy: Being the actor or being the target. Exp. Rev. Clin. Immunol. 2009, 5, 735–747. [Google Scholar] [CrossRef]
- Sutton, B.J.; Phillips, D.C. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem. Soc. Trans. 1983, 11, 130–132. [Google Scholar] [PubMed]
- Labeta, M.O.; Margni, R.A.; Leoni, J.; Binaghi, R.A. Structure of asymmetric non-precipitating antibody: Presence of a carbohydrate residue in only one Fab region of the molecule. Immunology 1986, 57, 311–317. [Google Scholar] [PubMed]
- Rademacher, T.W.; Homans, S.W.; Parekh, R.B.; Dwek, R.A. Immunoglobulin G as a glycoprotein. Biochem. Soc. Symp. 1985, 51, 1331–1348. [Google Scholar]
- Rademacher, T.W. Monitoring and control of glycosylation. Anim. Cell Biotechnol. 1994, 6, 5–19. [Google Scholar]
- Wallick, S.C.; Kabat, E.A.; Morrison, S.L. Glycosylation of a VH residue of a monoclonal antibody against alpha (1–6) dextran increases its affinity for antigen . J. Exp. Med. 1988, 168, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Tao, M.H.; Kabat, E.A.; Morrison, S.L. Antibody variable region glycosylation: Position effects on antigen binding and carbohydrate structure. EMBO. J. 1991, 10, 2717–2723. [Google Scholar] [PubMed]
- Jacquemin, M.; Radcliffe, C.M.; Lavend'homme, R.; Wormald, M.R.; Vanderelst, L.; Wallays, G.; Dewaele, J.; Collen, D.; Vermylen, J.; Dwek, R.A. Variable region heavy chain glycosylation determines the anticoagulant activity of a factor VIII antibody. J. Thromb. Haemost. 2006, 4, 1047–1055. [Google Scholar] [CrossRef]
- Rademacher, T.W.; Williams, P.; Dwek, R.A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 6123–6127. [Google Scholar] [CrossRef]
- Kumpel, B.M.; Rademacher, T.W.; Rook, G.A.W.; Williams, P.; Wilson, I.B.H. Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Human Antib. Hybridomas. 1994, 5, 143–151. [Google Scholar]
- Lund, J.; Takahashi, N.; Nakagawa, H.; Goodall, M.; Bentley, T.; Hindley, S.A.; Tyler, R.; Jefferis, R. Control of IgG/Fc glycosylation: A comparison of oligosaccharides from chimeric human/mouse and mouse subclass IgGs. Mol. Immunol. 1993, 30, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, T.W.; Jones, R.H.V.; Williams, P. Significance and molecular basis for IgG glycosylation changes in rheumatoid arthritis . Alavi, A., Axford, J, Eds.; Plenum: New York, NY, USA, 1995. [Google Scholar]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulin’s. Ann. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Nose, M.; Wigzell, H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1983, 80, 6632–6636. [Google Scholar] [CrossRef]
- Wright, A.; Morrison, S.L. Effect of altered CH2-associated carbohydrate structure in the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J. Exp. Med. 1994, 180, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Mullinax, F. Abnormality of IgG structure in RA and SLE. Arthritis Rheum. 1975, 18, 417. [Google Scholar]
- Rook, G.A.W.; Steele, J.; Brealey, R.; Whyte, A.; Isenberg, D.; Sumar, N.; Nelson, J.L.; Bodman, K.B.; Young, A.; Roitt, I.M. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 1991, 4, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Sumar, N.; Bodman, K.; Goyal, S.; Sinclair, H.; Roitt, I.; Isenberg, D. Agalactosyl IgG: An aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991, 34, 1425–1429. [Google Scholar] [PubMed]
- Tsai, C.M.; Zopf, D.A.; Yu, R.K.; Wistar, R.; Ginsberg, V.A. Waldenström macroglobulin that is both a cold agglutinin and a cryoglobulin because it binds N-acetyl-neuraminosyl residues. Proc. Natl. Acad. Sci. USA. 1977, 74, 4591–4596. [Google Scholar] [CrossRef]
- Middaugh, C.S.; Litman, G.W. Atypical glycosylation of an IgG monoclonal cryoimmunoglobulin. J. Biol. Chem. 1987, 262, 3671–3673. [Google Scholar] [PubMed]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Cartron, G.; Salles, G.; Solal-Céligny, P.; Bardos, P.; Colombat, P.; Watier, H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor Fcgamma RIIIa gene . Blood 2002, 99, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R.; Takechi, Y.; Moroi, Y.; Houghton, A.; Ravetch, J.V. Fc receptors are required in passive and active immunity to melanoma. Proc. Natl. Acad. Sci. USA 1998, 95, 652–656. [Google Scholar] [CrossRef]
- Sibéril, S.; de Romeuf, C.; Bihoreau, N.; Fernandez, N.; Meterreau, J.L.; Regenman, A.; Nony, E.; Gaucher, C.; Glacet, A.; Jorieux, S. Selection of a human anti-RhD monoclonal antibody for therapeutic use: Impact of IgG glycosylation on activating and inhibitory Fc gamma R functions. Clin. Immunol. 2006, 118, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Jiang, L.; Pan, L.Z.; LaBarre, M.J.; Anderson, D.; Reff, M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for Fcgamma RIII. Biotechnol. Bioeng. 2001, 74, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Yamane-Ohnuki, N.; Kinoshita, S.; Inoue-Urakubo, M.; Kusunoki, M.; Iida, S.; Nakano, R.; Wakitani, M.; Niwa, R.; Sakurada, M.; Uchida, K. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 2004, 87, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Umana, P.; Jean-Mairet, J.; Moudry, R.; Amstutz, H.; Bailey, J.E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 1999, 17, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O'Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.P. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Sakurada, M.; Kobayashi, Y.; Uehara, A.; Matsushima, K.; Ueda, R.; Nakamura, K.; Shitara, K. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin. Cancer Res. 2005, 11, 2327–2336. [Google Scholar] [CrossRef]
- de Romeuf, C.; Dutertre, C.A.; Le Garff-Tavernier, M.; Gaucher, C.; Glacet, A.; Jorieux, S.; Bihoreau, N.; Behrens, C.K.; Béliard, R. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of Fcgamma RIIIA/CD16 . Br. J. Haematol. 2008, 140, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.;Kaveri. Intravenous immunoglobulins in autoimmune and inflammatory disorders: Beyond a simple substitution. Transfus. Clin. Biol. 2009, 16, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulins. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Sibéril, S.; Dutertre, C.A.; Fridman, W.H.; Teillaud, J.L. FcgammaR: The key to optimize therapeutic antibodies? Crit. Rev. Oncol. Hematol. 2007, 62, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Debré, M.; Bonnet, M.C. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 1993, 342, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.; Towers, T.L.; Ravetch, J.V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001, 291, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Samuelsson, A.; Pollard, J.W.; Ravetch, J.V. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity 2003, 18, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Madaio, M.P.; Ravetch, J.V. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J. Exp. Med. 2006, 203, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Park-Min, K.H.; Serbina, N.V.; Yang, W.; Ma, X.; Krystal, G.; Neel, B.J.; Nutt, S.L.; Hu, X.; Ivashkiv, L.B. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulins. Immunity 2007, 26, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, S.; Petkova, S.; Sproule, T.J.; Shaffer, D.J.; Christianson, G.J.; Roopenian, D. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J. Clin. Invest. 2004, 113, 1328–1333. [Google Scholar] [PubMed]
- Hamerman, J.A.; Lanier, L.L. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006, 320, 1. [Google Scholar]
- Siragam, V.; Crow, A.R.; Brinc, D.; Song, S. Intravenous immunoglobulin ameliorates ITP via activating Fc gamma receptors on dendritic cells. Nat. Med. 2006, 12, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. The antiinflammatory activity of IgG: The intravenous IgG paradox. J. Exp. Med. 2007, 204, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Anthony, R.M.; Wermeling, F.; Karlsson, M.C.; Ravetch, J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 2008, 105, 19571–19578. [Google Scholar] [CrossRef]
- Bayry, J.; Bansal, K.; Kazatchkine, M.D.; Kaveri, S.V. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 24. [Google Scholar] [CrossRef]
- Wierda, W.; Kipps, T.; Mayer, J.; Stilgenbauer, S.; Williams, C.D.; Hellmann, A.; Robak, T.; Furman, R.R.; Hillmen, P.; Trneny, M. Ofatumumab, a novel CD20 monoclonal antibody, is active in patients with Fludarabine- and Alemtuzumab-refractory or bulky Fludarabine-refractory Chronic Lymphocytic Leukemia irrespective of prior Rituximab. Clin. Lymphoma Myeloma 2009, 9, 36. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abès, R.; Teillaud, J.-L. Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals 2010, 3, 146-157. https://doi.org/10.3390/ph3010146
Abès R, Teillaud J-L. Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals. 2010; 3(1):146-157. https://doi.org/10.3390/ph3010146
Chicago/Turabian StyleAbès, Riad, and Jean-Luc Teillaud. 2010. "Impact of Glycosylation on Effector Functions of Therapeutic IgG" Pharmaceuticals 3, no. 1: 146-157. https://doi.org/10.3390/ph3010146
APA StyleAbès, R., & Teillaud, J.-L. (2010). Impact of Glycosylation on Effector Functions of Therapeutic IgG. Pharmaceuticals, 3(1), 146-157. https://doi.org/10.3390/ph3010146




