TAT-PBX1 Reverses Hyperglycemia Through β-Cell Regeneration and Functional Restoration in an STZ-Induced Diabetic Model
Abstract
1. Introduction
2. Results
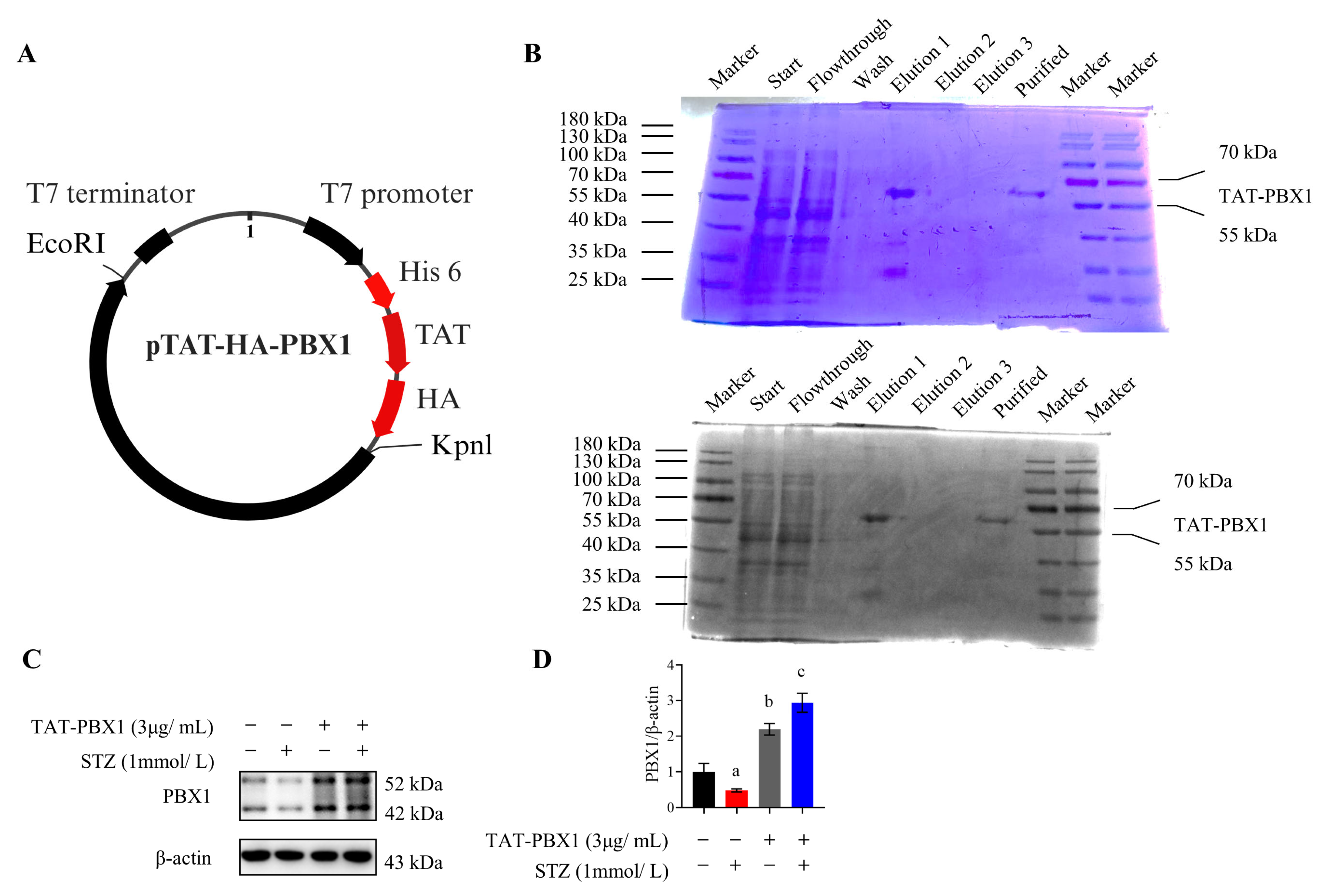
2.1. Purification of TAT-PBX1
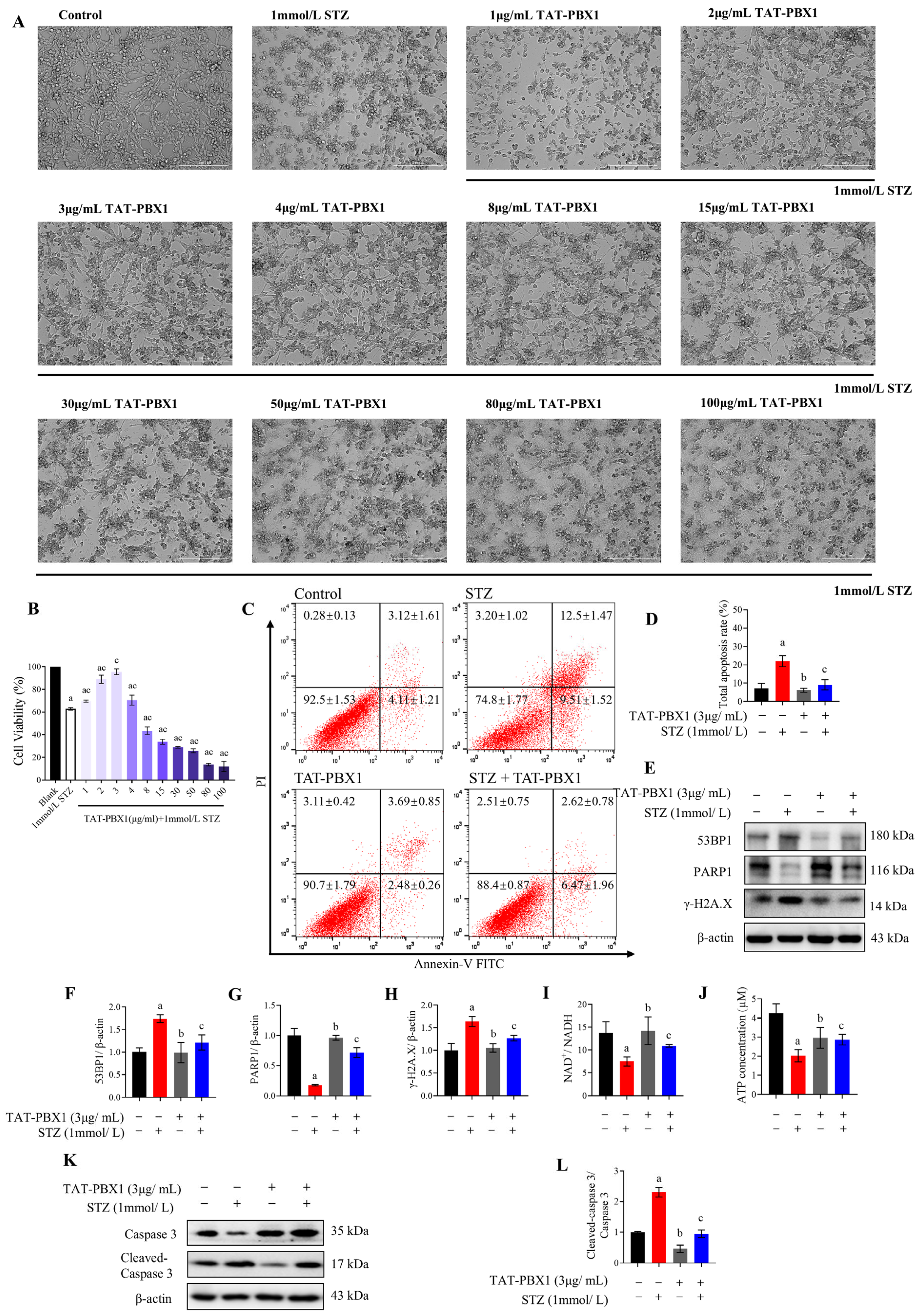
2.2. TAT-PBX1 Reduces STZ-Induced DNA Damage and Apoptosis in MIN6 Cells and Partially Restores Energy Supply
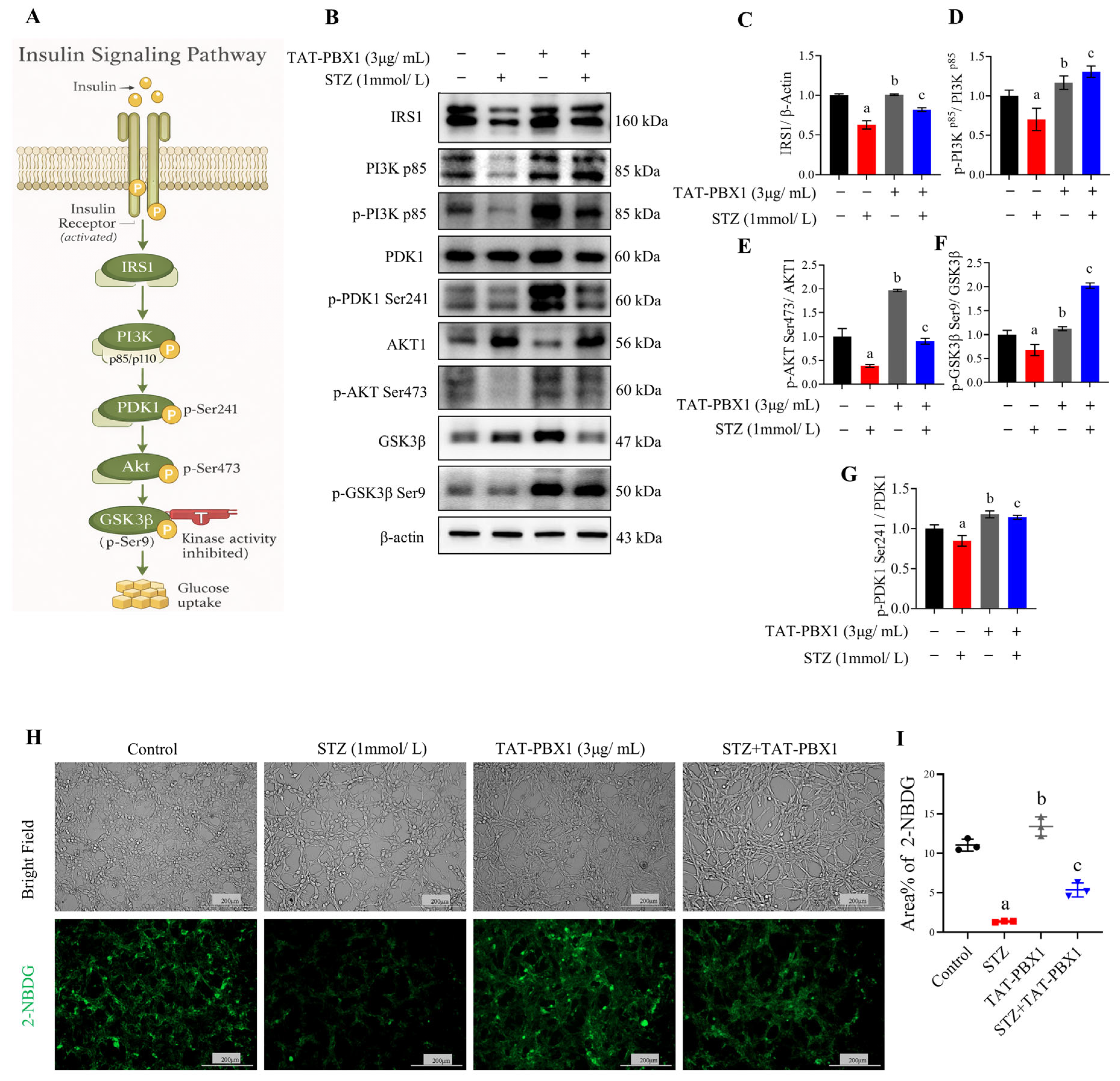
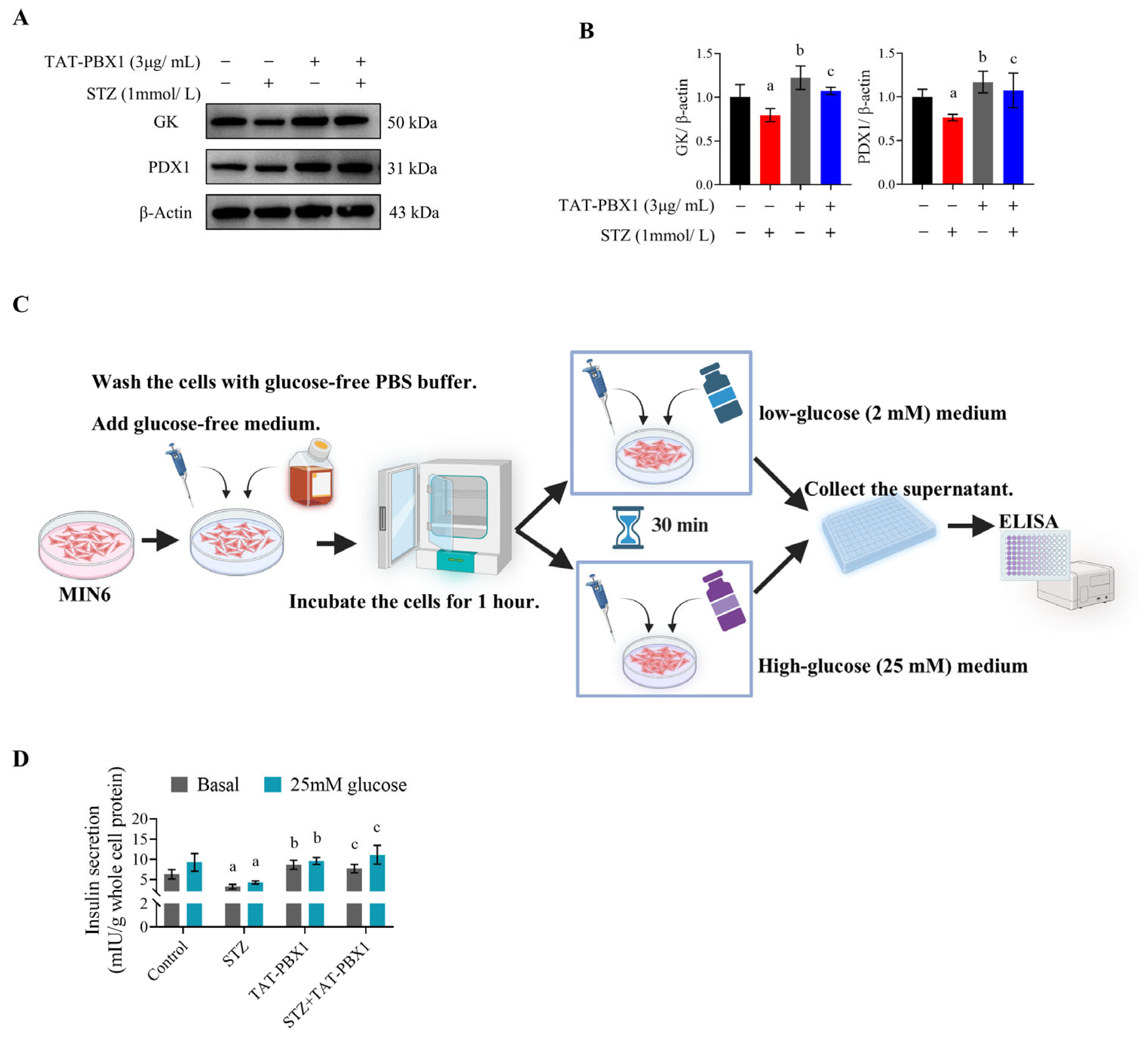
2.3. TAT-PBX1 Restores Insulin Signaling in STZ-Injured MIN6 Cells and Improves Insulin Secretory Function
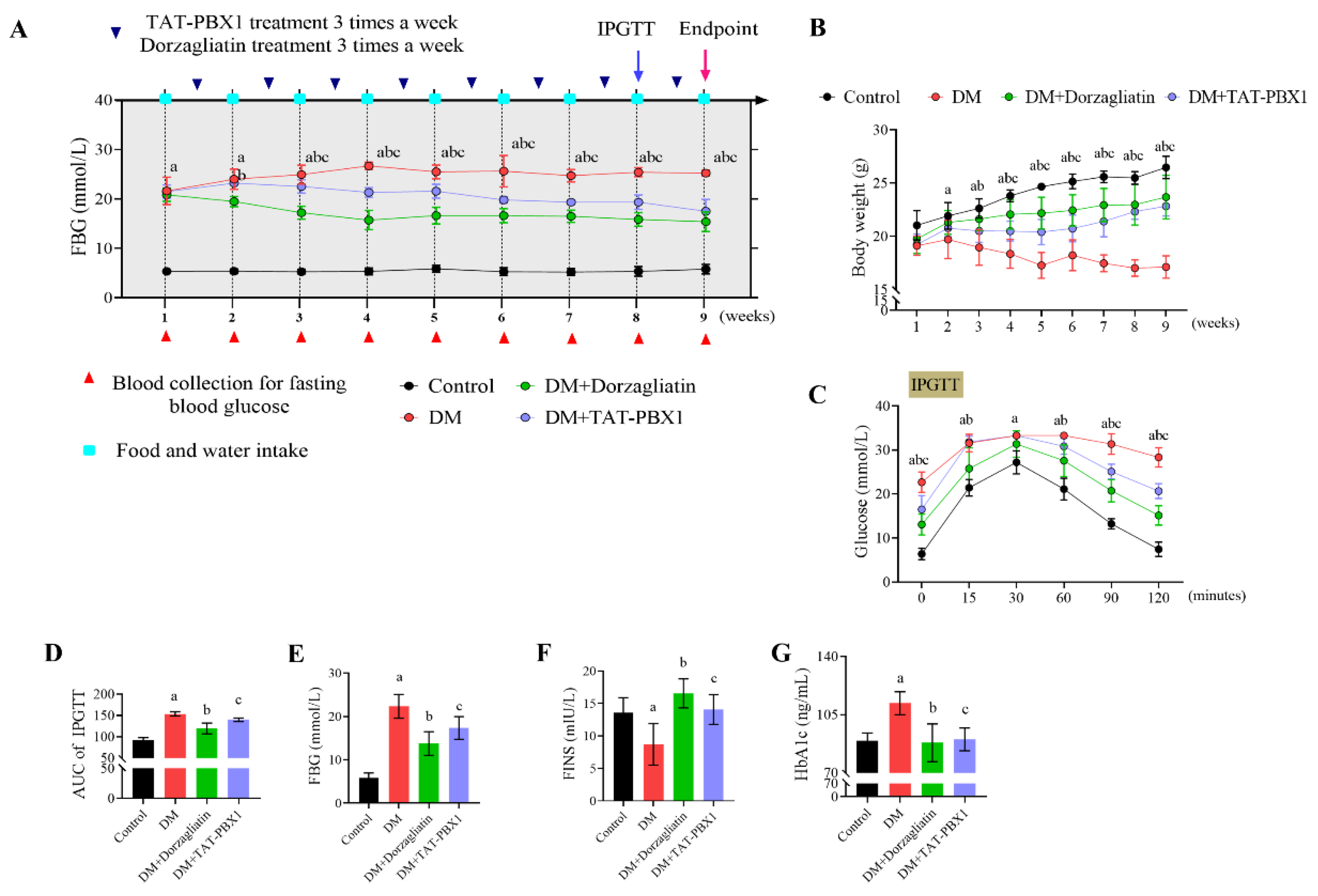
2.4. Therapeutic Evaluation of TAT-PBX1 in an STZ-Induced Diabetic Mouse Model
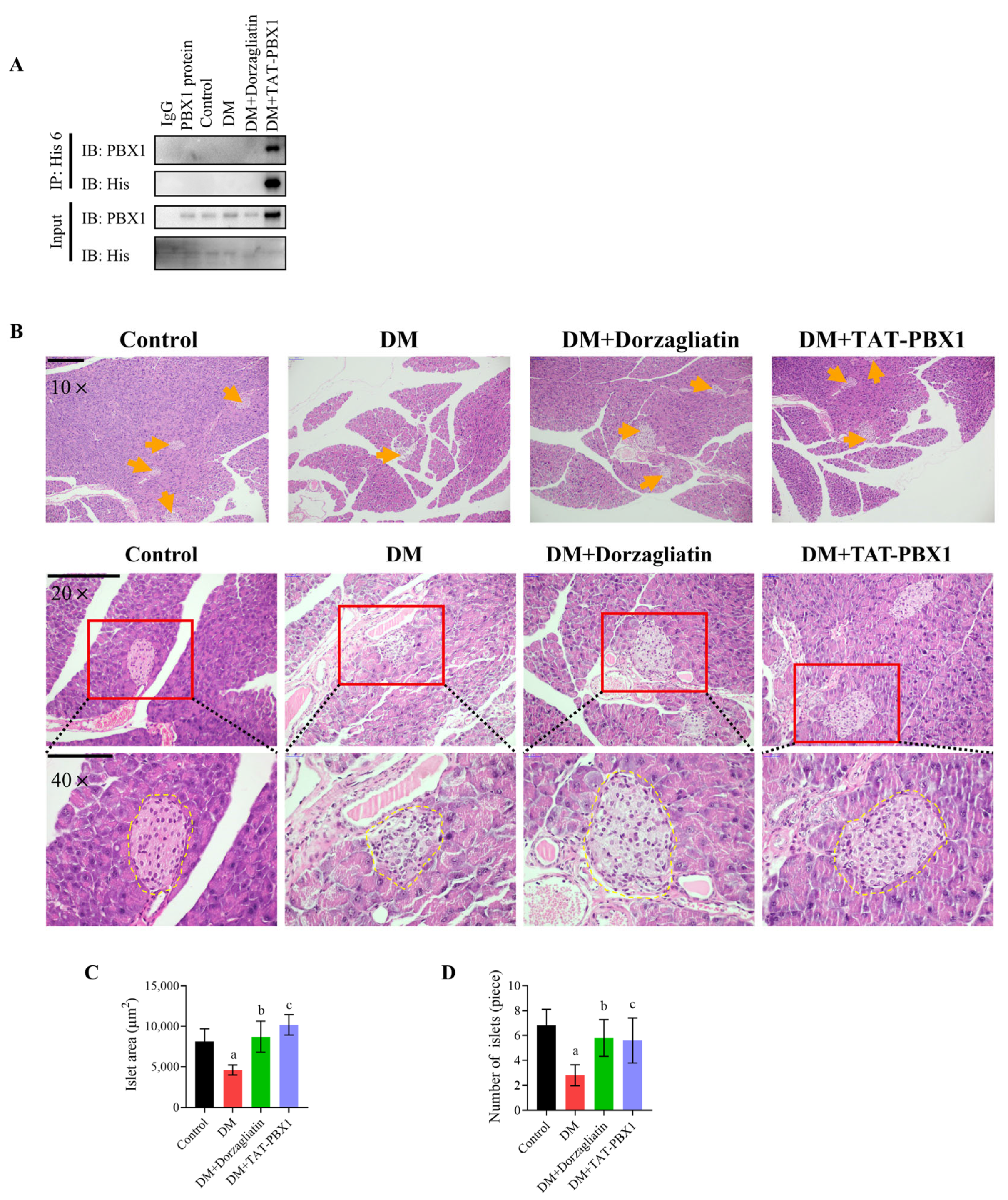
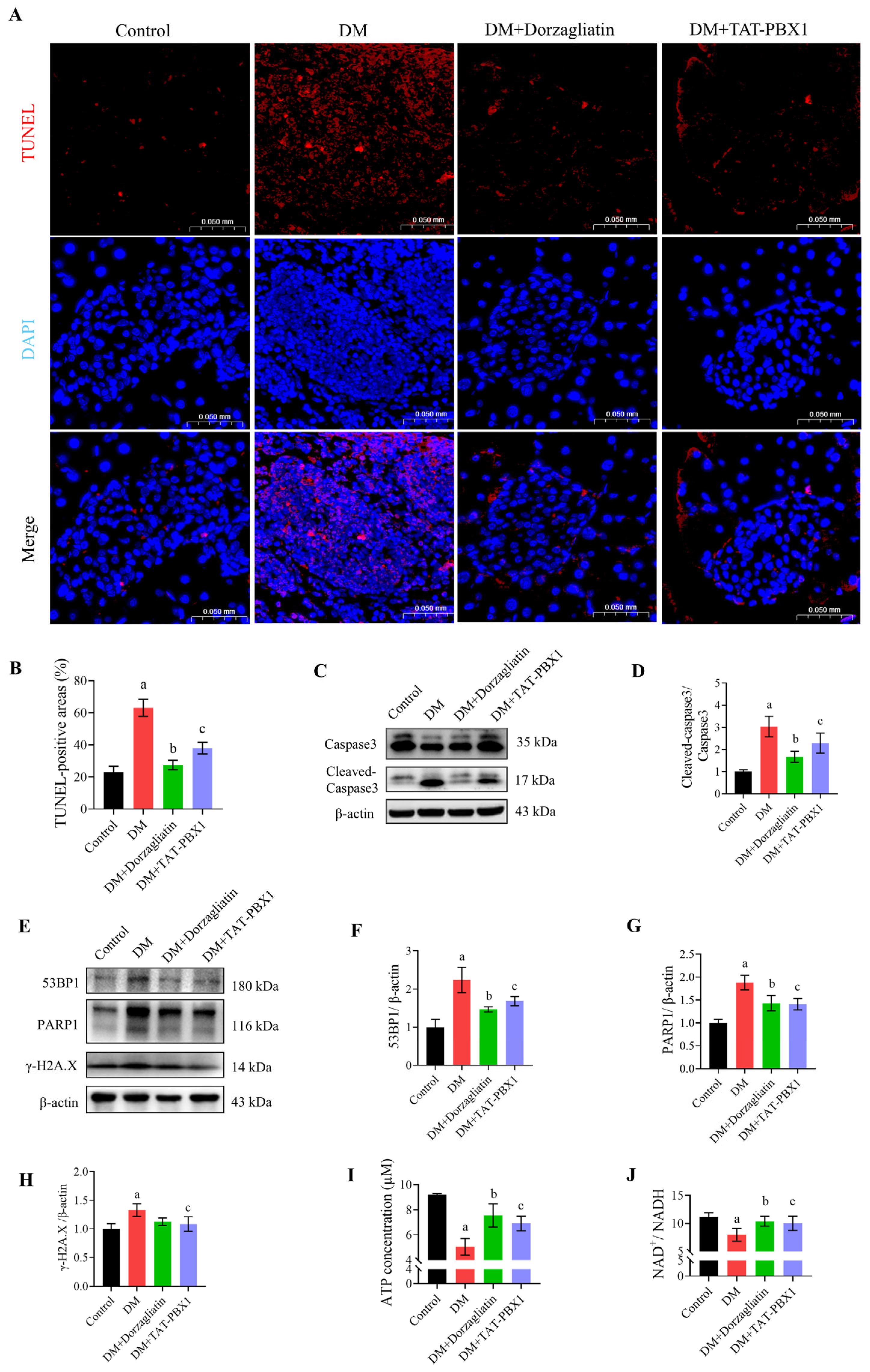
2.5. TAT-PBX1 Improves Islet Pathology and Reduces Apoptosis In Vivo
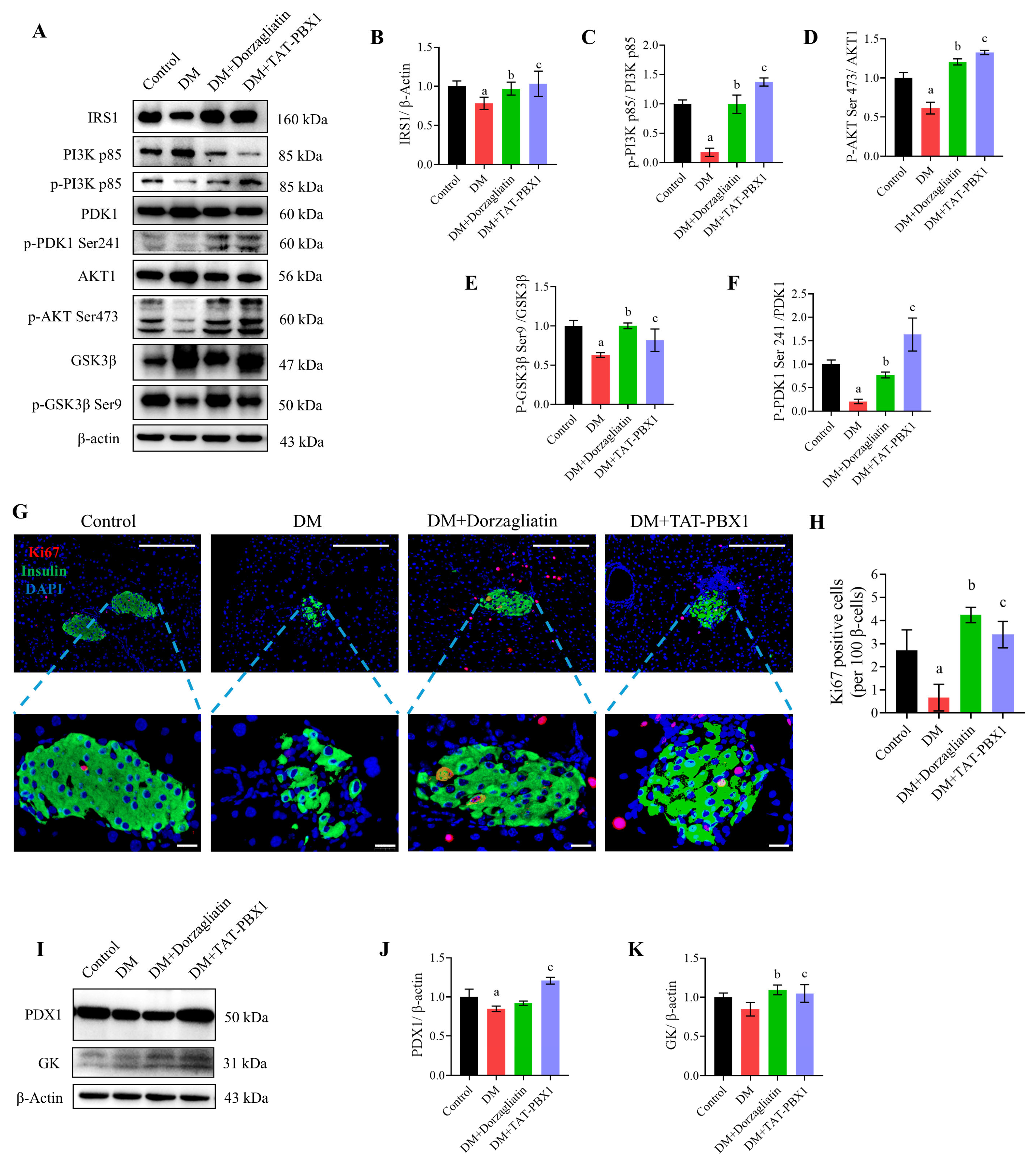
2.6. TAT-PBX1 Restores Insulin Signaling and Increases β-Cell Proliferative Markers In Vivo
3. Discussion
4. Materials and Methods
4.1. Construction, Purification, and Endotoxin Removal of TAT-PBX1
4.2. Cell Culture
4.3. STZ-Induced MIN6 Cell Injury Model
4.4. CCK-8 Cell Viability Assay
4.5. Annexin V/PI Flow Cytometry
4.6. 2-NBDG Glucose Uptake Assay
4.7. GSIS
4.8. Animals and Experimental Design
4.8.1. Experimental Animals
4.8.2. STZ-Induced Insulin-Deficient (T1DM-like) Mouse Model
4.8.3. Experimental Groups and Treatment
4.8.4. IPGTT
4.9. ELISA
4.10. ATP Measurement
4.11. NAD+/NADH Ratio
4.12. Western Blot
4.13. Immunoprecipitation
4.14. H&E Staining
4.15. TUNEL Staining
4.16. Immunofluorescence Staining
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Bai, L.; Xiao, W.; Zhong, L.; Shi, J. Advances in targeting glycogen synthase kinase 3β for diabetes therapy. Bioorganic Chem. 2025, 166, 109051. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Ramkumar, K.M. Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic β-cells in diabetes. Apoptosis Int. J. Program. Cell Death 2023, 28, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yi, X.; Zhang, J.; Yao, Y.; Panichayupakaranant, P.; Chen, H. Recent Advances in the Drugs and Glucose-Responsive Drug Delivery Systems for the Treatment of Diabetes: A Systematic Review. Pharmaceutics 2024, 16, 1343. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J.; Li, Y.; Wang, R.; Dai, C.; Zhang, B.; Zhang, X.; Xu, L.; Tao, Y.; Han, M.; et al. DRAK2 suppresses autophagy by phosphorylating ULK1 at Ser(56) to diminish pancreatic β cell function upon overnutrition. Sci. Transl. Med. 2024, 16, eade8647. [Google Scholar] [CrossRef]
- Castelblanco, E.; Shyr, Z.A.; Ramirez-Sotero, I.; Yan, Z.; Chen, S.X.; Diwan, A.; Remedi, M.S. Restoration of pancreatic beta cell identity and autophagy in K(ATP)-induced diabetes by intermittent fasting. Diabetologia 2025, 68, 2781–2794. [Google Scholar] [CrossRef]
- Tsuura, Y.; Ishida, H.; Okamoto, Y.; Kato, S.; Horie, M.; Ikeda, H.; Seino, Y. Reduced sensitivity of dihydroxyacetone on ATP-sensitive K+ channels of pancreatic beta cells in GK rats. Diabetologia 1994, 37, 1082–1087. [Google Scholar] [CrossRef]
- Rönn, T.; Ofori, J.K.; Perfilyev, A.; Hamilton, A.; Pircs, K.; Eichelmann, F.; Garcia-Calzon, S.; Karagiannopoulos, A.; Stenlund, H.; Wendt, A.; et al. Genes with epigenetic alterations in human pancreatic islets impact mitochondrial function, insulin secretion, and type 2 diabetes. Nat. Commun. 2023, 14, 8040. [Google Scholar] [CrossRef]
- Long, X.; Liu, S.; Yang, X.; Zhao, Y.; Yang, S.; Wei, Y.; Pan, C.; Chen, S.; Jiang, P.; Qi, B.; et al. The Hypoglycemic Activity of Gracilaria lemaneiformis Polysaccharide Gels Based on IR/IRS-2/PI3k/Akt/Glut4 and Glycometabolism Signaling Pathways in HepG2 Cells. Gels 2025, 11, 366. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, C.; Wan, L.; Peng, L.; Wang, K.; Zhou, F.; Fang, W.; Wen, S.; Bai, Q.; Yang, X.; et al. Epigallocatechin gallate prevents and alleviates type 2 diabetes mellitus (T2DM) through gut microbiota and multi-organ interactions in Wistar healthy rats and GK T2DM rats. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Oleson, B.J.; Corbett, J.A. Can insulin secreting pancreatic β-cells provide novel insights into the metabolic regulation of the DNA damage response? Biochem. Pharmacol. 2020, 176, 113907. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lokuge, S.D.; Huang, M.; Wang, S.; Liu, S.; Liang, J.; Katturajan, R.; Marakovits, C.; Yang, Z.; Wan, J.; et al. SIRT6 Is a Key Regulator of Pancreatic β-Cell Survival and Function During Aging. Diabetes 2025, 74, 1976–1991. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Umehara, M.; Minamida, A.; Yamauchi-Sawada, H.; Sunahara, Y.; Matoba, Y.; Okuno-Ozeki, N.; Nakamura, I.; Nakata, T.; Yagi-Tomita, A.; et al. Streptozotocin induces renal proximal tubular injury through p53 signaling activation. Sci. Rep. 2023, 13, 8705. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Abdellatif, A.; Bahria, K.; Slama, N.; Oukrif, D.; Shalaby, A.; Birkmayer, G.; Oumouna, M.; Benachour, K. NADH intraperitoneal injection prevents massive pancreatic beta cell destruction in a streptozotocin-induced diabetes in rats. Histochem. Cell Biol. 2024, 161, 239–253. [Google Scholar] [CrossRef]
- Kao, T.W.; Chen, H.H.; Lin, J.; Wang, T.L.; Shen, Y.A. PBX1 as a novel master regulator in cancer: Its regulation, molecular biology, and therapeutic applications. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189085. [Google Scholar] [CrossRef]
- Duesing, K.; Charpentier, G.; Marre, M.; Tichet, J.; Hercberg, S.; Balkau, B.; Froguel, P.; Gibson, F. Evaluating the association of common PBX1 variants with type 2 diabetes. BMC Med. Genet. 2008, 9, 14. [Google Scholar] [CrossRef]
- Ban, J.Y.; Kang, S.A.; Jung, K.H.; Kim, H.J.; Uhm, Y.K.; Kim, S.K.; Yim, S.V.; Choe, B.K.; Hong, S.J.; Seong, Y.H.; et al. The association of PBX1 polymorphisms with overweight/obesity and metabolic alterations in the Korean population. Nutr. Res. Pract. 2008, 2, 289–294. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Wang, X.; Zhang, Z.; Elbein, S.C. Evaluation of sequence variants in the pre-B cell leukemia transcription factor 1 gene: A positional and functional candidate for type 2 diabetes and impaired insulin secretion. Mol. Genet. Metab. 2005, 86, 384–391. [Google Scholar] [CrossRef]
- Oriente, F.; Perruolo, G.; Cimmino, I.; Cabaro, S.; Liotti, A.; Longo, M.; Miele, C.; Formisano, P.; Beguinot, F. Prep1, A Homeodomain Transcription Factor Involved in Glucose and Lipid Metabolism. Front. Endocrinol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Kim, S.K.; Selleri, L.; Lee, J.S.; Zhang, A.Y.; Gu, X.; Jacobs, Y.; Cleary, M.L. Pbx1 inactivation disrupts pancreas development and in Ipf1-deficient mice promotes diabetes mellitus. Nat. Genet. 2002, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Birnbaum, M.J.; Stoffers, D.A. Three-amino-acid-loop-extension homeodomain factor Meis3 regulates cell survival via PDK1. Proc. Natl. Acad. Sci. USA 2010, 107, 20494–20499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, F.; Zou, F.; Zhang, Y.; Wang, B.; Zhang, Y.; Lian, A.; Han, X.; Liu, Z.; Liu, X.; et al. PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation and reprogramming through activation of the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Stem Cell Res. Ther. 2019, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sui, Y.; Lian, A.; Han, X.; Liu, F.; Zuo, K.; Liu, M.; Sun, W.; Wang, Z.; Liu, Z.; et al. PBX1 Attenuates Hair Follicle-Derived Mesenchymal Stem Cell Senescence and Apoptosis by Alleviating Reactive Oxygen Species-Mediated DNA Damage Instead of Enhancing DNA Damage Repair. Front. Cell Dev. Biol. 2021, 9, 739868. [Google Scholar] [CrossRef]
- Li, P.; Zhu, D. Clinical investigation of glucokinase activators for the restoration of glucose homeostasis in diabetes. J. Diabetes 2024, 16, e13544. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Fenske, R.J.; Kimple, M.E. Targeting dysfunctional beta-cell signaling for the potential treatment of type 1 diabetes mellitus. Exp. Biol. Med. 2018, 243, 586–591. [Google Scholar] [CrossRef]
- Lenzen, S.; Jörns, A. Therapy concepts in type 1 diabetes mellitus treatment: Disease modifying versus curative approaches. J. Mol. Med. 2024, 102, 1451–1455. [Google Scholar] [CrossRef]
- Judder, M.I.; Islam, M.; Kaur, H.; Rahman, R.; Deka, K.; Kapil, M.J.; Pathak, B.J.; Sarma, T.; Moidul Islam, M. Therapeutic Trends in Diabetes Management: A Review on Oral Hypoglycemic Agents (OHAs) Utilization in Tertiary Care. Cardiovasc. Hematol. Disord. Drug Targets, 2025; in press. [Google Scholar] [CrossRef]
- Shi, Q.; Nong, K.; Vandvik, P.O.; Guyatt, G.H.; Schnell, O.; Rydén, L.; Marx, N.; Brosius, F.C., 3rd; Mustafa, R.A.; Agarwal, A.; et al. Benefits and harms of drug treatment for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ (Clin. Res. Ed.) 2023, 381, e074068. [Google Scholar] [CrossRef]
- Foster, T.P.; Bruggeman, B.S.; Haller, M.J. Emerging Immunotherapies for Disease Modification of Type 1 Diabetes. Drugs 2025, 85, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Prabath, I.; Chandrasekaran, I.; Varadarajan, S. Dorzagliatin: A Breakthrough Glucokinase Activator Coming on Board to Treat Diabetes Mellitus. Cureus 2024, 16, e65708. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Piaggi, S.; Federico, G.; Scarpato, R. High levels of γ-H2AX foci and cell membrane oxidation in adolescents with type 1 diabetes. Mutat. Res. 2014, 770, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.; Chugunova, E.; Kolesnikov, S.; Semenova, N.; Michalevich, I.; Nikitina, O.; Lesnaya, A.; Kolesnikova, L. Receiver Operator Characteristic (ROC) Analysis of Lipids, Proteins, DNA Oxidative Damage, and Antioxidant Defense in Plasma and Erythrocytes of Young Reproductive-Age Men with Early Stages of Type 1 Diabetes Mellitus (T1DM) Nephropathy in the Irkutsk Region, Russia. Metabolites 2022, 12, 1282. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Huerta Guevara, A.P.; Postmus, A.C.; Flores, R.R.; Sano, T.; Jurdzinski, A.; Angelini, L.; McGowan, S.J.; O’Kelly, R.D.; Wade, E.A.; et al. Failure to repair endogenous DNA damage in β-cells causes adult-onset diabetes in mice. Aging Biol. 2023, 1, e20230015. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Maegawa, T.; Nagura, T.; Kobayashi, M.; Babaya, N.; Ikegami, H.; Horio, F.; Ohno, T. DNA repair protein RAD50 is involved in the streptozotocin-induced diabetes susceptibility of mice. Exp. Anim. 2025, 74, 264–275. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD(+) metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Fukaya, M.; Tamura, Y.; Chiba, Y.; Tanioka, T.; Mao, J.; Inoue, Y.; Yamada, M.; Waeber, C.; Ido-Kitamura, Y.; Kitamura, T.; et al. Protective effects of a nicotinamide derivative, isonicotinamide, against streptozotocin-induced β-cell damage and diabetes in mice. Biochem. Biophys. Res. Commun. 2013, 442, 92–98. [Google Scholar] [CrossRef]
- Wilk, A.; Hayat, F.; Cunningham, R.; Li, J.; Garavaglia, S.; Zamani, L.; Ferraris, D.M.; Sykora, P.; Andrews, J.; Clark, J.; et al. Extracellular NAD(+) enhances PARP-dependent DNA repair capacity independently of CD73 activity. Sci. Rep. 2020, 10, 651. [Google Scholar] [CrossRef]
- Fu, S.; Zou, P.; Fang, Z.; Zhou, X.; Chen, J.; Gong, C.; Quan, L.; Lin, B.; Chen, Q.; Lang, J.; et al. Incidence and risk of endocrine and metabolic abnormalities linked to PARP inhibitors in solid tumors: A meta-analysis. BMC Cancer 2025, 25, 183. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules 2020, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, M.; Yang, L.; Xie, Q.; Fan, R.; Lu, X.; Huang, X.; Tong, N.; Duan, Z. Pancreatic Islet Cell Hormones: Secretion, Function, and Diabetes Therapy. MedComm 2025, 6, e70359. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.R.; Freeman, J.L.R.; Dunn, I.; Dvergsten, C.; Kirkman, M.S.; Buse, J.B.; Valcarce, C. The SimpliciT1 Study: A Randomized, Double-Blind, Placebo-Controlled Phase 1b/2 Adaptive Study of TTP399, a Hepatoselective Glucokinase Activator, for Adjunctive Treatment of Type 1 Diabetes. Diabetes Care 2021, 44, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Chen, X.; Liu, J.; Wen, Z.; Wen, S.; Zeng, W.; Lin, S.; Chen, Y.; Shi, G.; Zeng, L. Glucokinase activator improves glucose tolerance and induces hepatic lipid accumulation in mice with diet-induced obesity. Liver Res. 2023, 7, 124–135. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, D.; Gan, S.; Dong, X.; Su, J.; Li, W.; Jiang, H.; Zhao, W.; Yao, M.; Song, W.; et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2022, 28, 974–981. [Google Scholar] [CrossRef]
- Liang, T.T.; Cao, M.J.; Wang, Q.; Zou, J.S.; Yang, X.M.; Gu, L.F.; Shi, F.H. Evaluating the Overall Safety of Glucokinase Activators in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 4539–4552. [Google Scholar] [CrossRef]
- Ford, B.E.; Chachra, S.S.; Alshawi, A.; Brennan, A.; Harnor, S.; Cano, C.; Baker, D.J.; Smith, D.M.; Fairclough, R.J.; Agius, L. Chronic glucokinase activator treatment activates liver Carbohydrate response element binding protein and improves hepatocyte ATP homeostasis during substrate challenge. Diabetes Obes. Metab. 2020, 22, 1985–1994. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Liu, Z.; Han, X.; Lian, A.; Zhang, Y.; Zuo, K.; Wang, Y.; Liu, M.; Zou, F.; et al. Internalization of the TAT-PBX1 fusion protein significantly enhances the proliferation of human hair follicle-derived mesenchymal stem cells and delays their senescence. Biotechnol. Lett. 2020, 42, 1877–1885. [Google Scholar] [CrossRef]
- Jin, G.; Zheng, K.; Liu, S.; Yi, H.; Wei, W.; Xu, C.; Xiang, X.; Kang, Y. In Vivo PK-PD and Drug-Drug Interaction Study of Dorzagliatin for the Management of PI3Kα Inhibitor-Induced Hyperglycemia. Pharmaceuticals 2025, 18, 927. [Google Scholar] [CrossRef]
- Jin, G.; Liu, S.; Zheng, K.; Cheng, X.; Chai, R.; Ye, W.; Wei, W.; Li, Y.; Huang, A.; Li, G.; et al. Therapeutic management of PI3Kα inhibitor-induced hyperglycemia with a novel glucokinase activator: Advancing the Frontier of PI3Kα inhibitor therapy. Mol. Metab. 2025, 96, 102151. [Google Scholar] [CrossRef]
- He, X.; Pei, S.; Meng, X.; Hua, Q.; Zhang, T.; Wang, Y.; Zhang, Z.; Zhu, X.; Liu, R.; Guo, Y.; et al. Punicalagin Attenuates Neuronal Apoptosis by Upregulating 5-Hydroxymethylcytosine in the Diabetic Mouse Brain. J. Agric. Food Chem. 2022, 70, 4995–5004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Meng, X.; Zhao, Z.; Zhang, X.; Guo, R.; Yang, S.; Mao, S.; Zong, Z.; Liu, J. TAT-PBX1 Reverses Hyperglycemia Through β-Cell Regeneration and Functional Restoration in an STZ-Induced Diabetic Model. Pharmaceuticals 2026, 19, 85. https://doi.org/10.3390/ph19010085
Meng X, Zhao Z, Zhang X, Guo R, Yang S, Mao S, Zong Z, Liu J. TAT-PBX1 Reverses Hyperglycemia Through β-Cell Regeneration and Functional Restoration in an STZ-Induced Diabetic Model. Pharmaceuticals. 2026; 19(1):85. https://doi.org/10.3390/ph19010085
Chicago/Turabian StyleMeng, Xiangyuan, Zhenhu Zhao, Xin Zhang, Ruihan Guo, Shuran Yang, Shuhua Mao, Ziyu Zong, and Jinyu Liu. 2026. "TAT-PBX1 Reverses Hyperglycemia Through β-Cell Regeneration and Functional Restoration in an STZ-Induced Diabetic Model" Pharmaceuticals 19, no. 1: 85. https://doi.org/10.3390/ph19010085
APA StyleMeng, X., Zhao, Z., Zhang, X., Guo, R., Yang, S., Mao, S., Zong, Z., & Liu, J. (2026). TAT-PBX1 Reverses Hyperglycemia Through β-Cell Regeneration and Functional Restoration in an STZ-Induced Diabetic Model. Pharmaceuticals, 19(1), 85. https://doi.org/10.3390/ph19010085





