Exploring the Impact of Ultrasound-Assisted Extraction on the Phytochemical Composition and Bioactivity of Tamus communis L. Fruits
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Composition
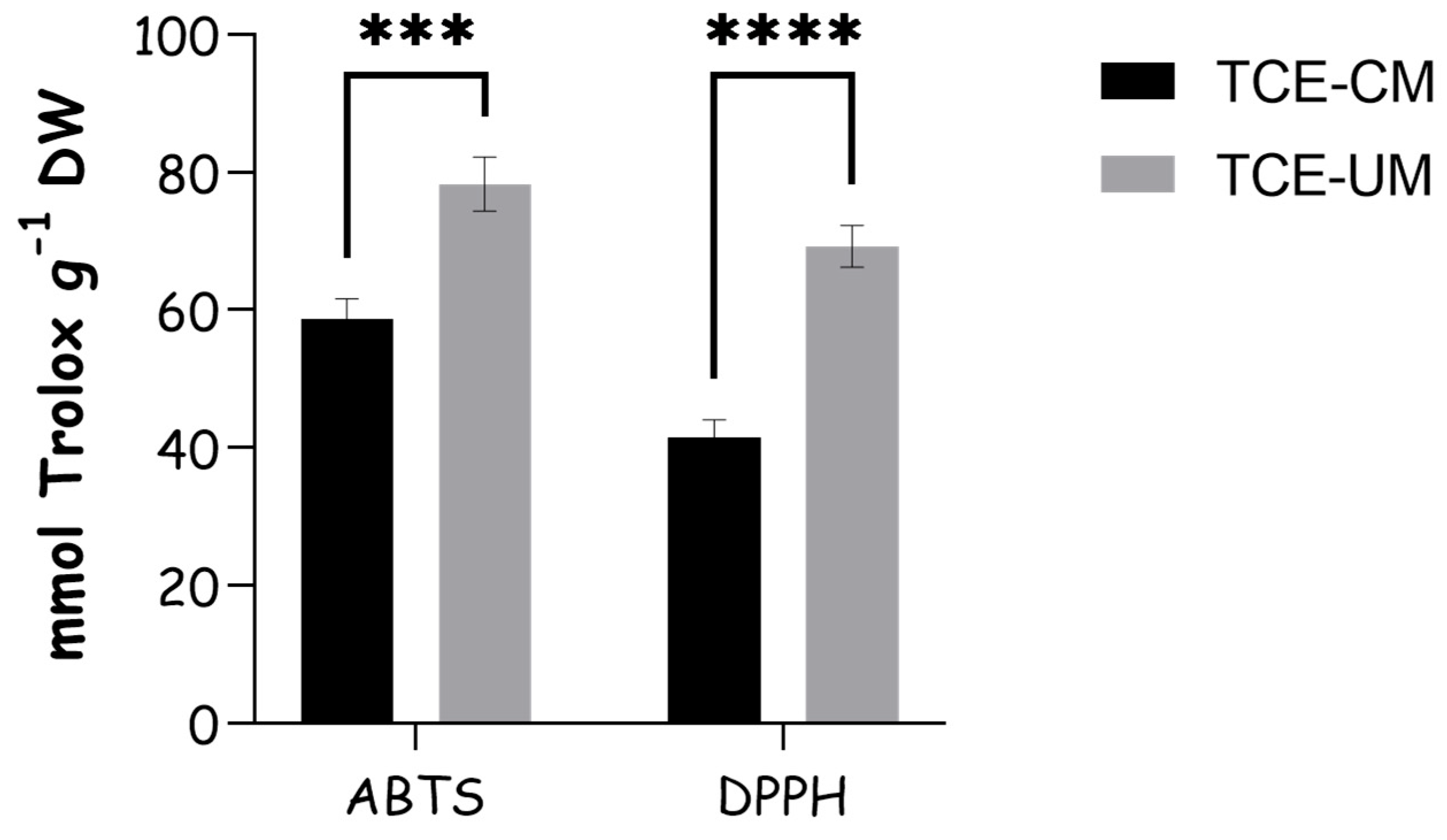
2.2. Antioxidant Capacity Analysis
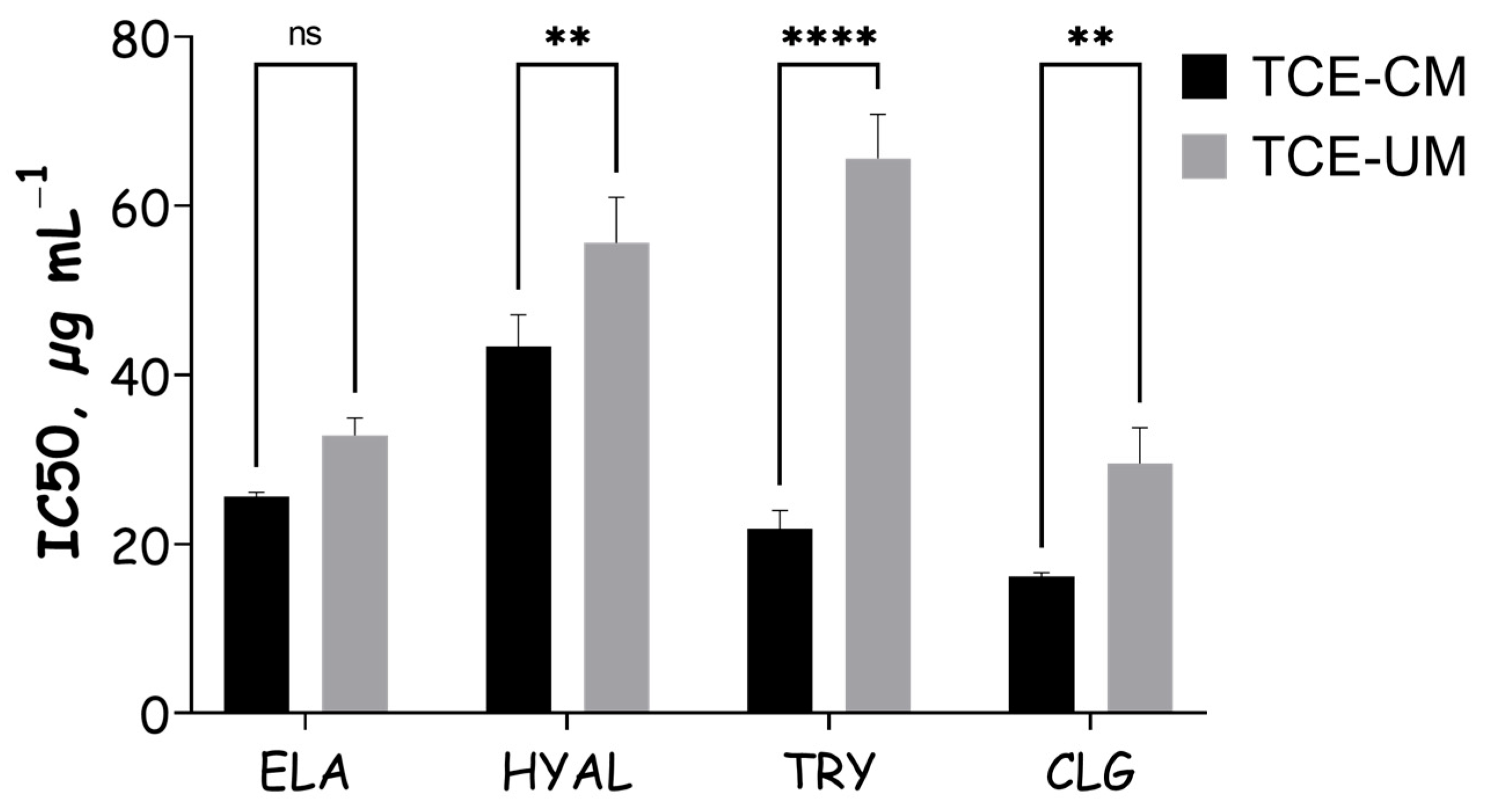
2.3. Skin-Related Enzyme’s Inhibitory Effects
2.4. Antimicrobial Activity
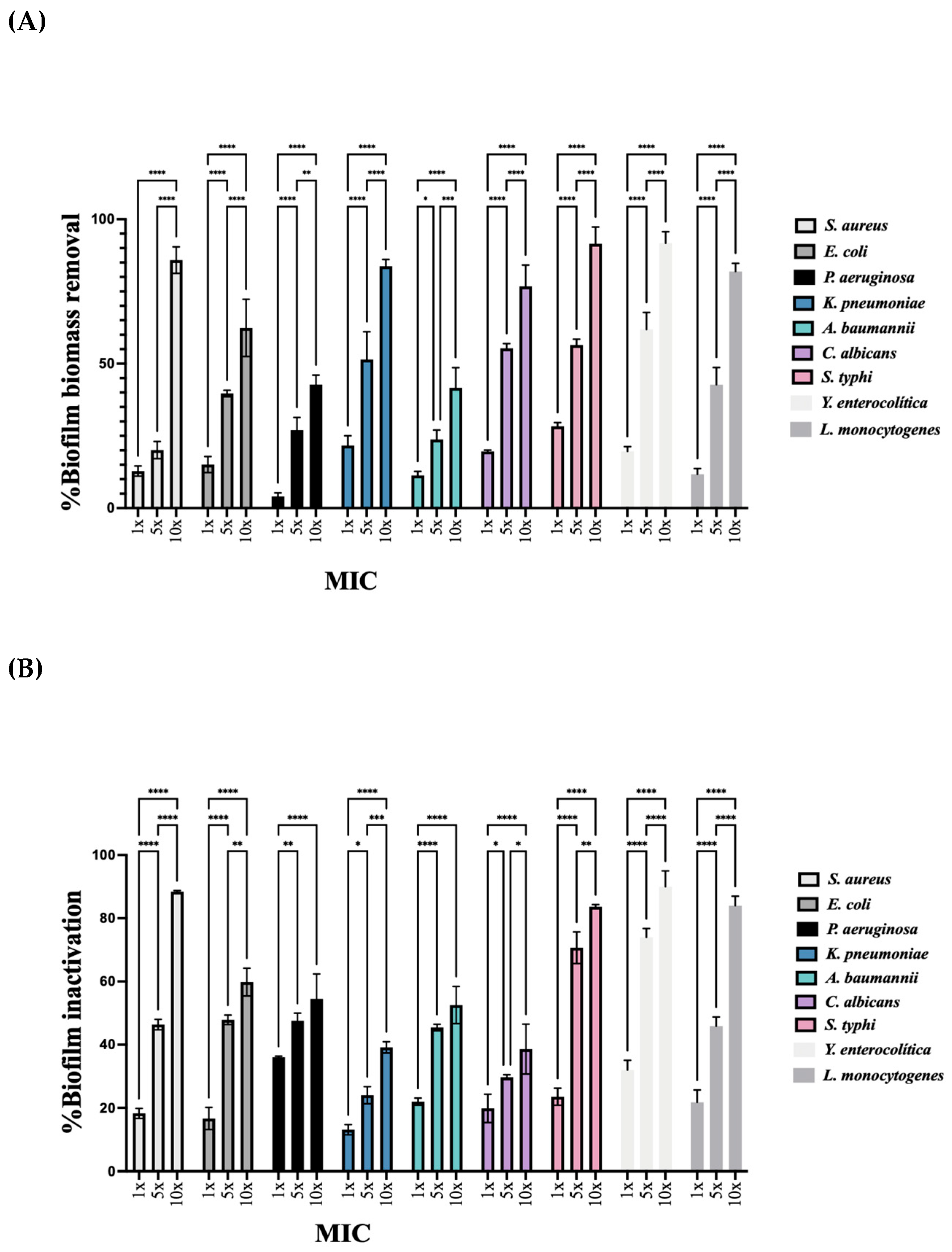
2.5. Biofilm Removal
2.6. Metabolic Inactivation Results
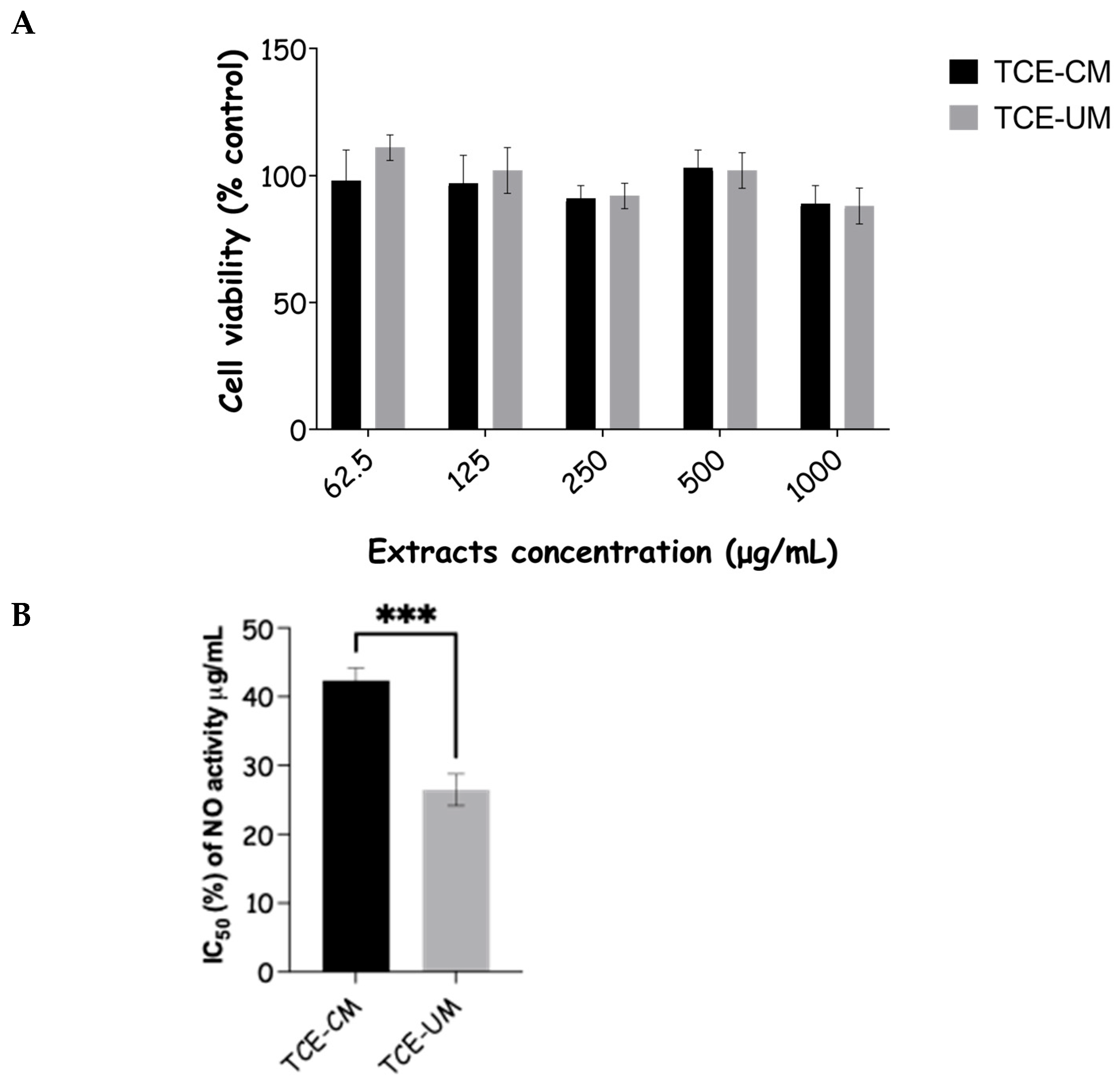
2.7. Cell Toxicity and Anti-Inflammatory Activity of Tamus communis Extracts
3. Materials and Methods
3.1. Chemicals
3.2. Sampling
3.3. Tamus communis Extracts Preparation
3.3.1. Tamus communis Extract-Conventional Method (TCE-CM)
3.3.2. Tamus communis Extract—Ultrasound-Assisted Method (TCE-UM)
3.4. Phenolic Composition
3.5. Antioxidant Capacity
3.6. Anti-Aging Capacity
3.7. Depigmenting and Lightening Properties
3.8. Antimicrobial Capacity Screening
Microorganisms and Culture Media
3.9. Antimicrobial Activity
3.9.1. Bacterial Adhesion/Biofilm Formation and Exposure to Extracts
3.9.2. Biomass Quantification
3.9.3. Metabolic Activity Quantification
3.10. Anti-Inflammatory Activity In Vitro
3.11. Determination of Nitric Oxide Production by Griess Assay
3.12. Cell Culture and Cell Viability Assay
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janjić, V. Plant-Caused Skin Disorders; Cambridge Scholars Publishing, Lady Stephenson Library: Newcastle upon Tyne, UK, 2021. [Google Scholar]
- Slavova, I.; Tomova, T.; Kusovska, S.; Chukova, Y.; Argirova, M. Phytochemical Constituents and Pharmacological Potential of Tamus communis Rhizomes. Molecules 2022, 27, 1851. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Barreira, J.C.; Pereira, E.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Bryonia dioica, Tamus communis and Lonicera periclymenum fruits: Characterization in phenolic compounds and incorporation of their extracts in hydrogel formulations for topical application. Ind. Crops Prod. 2013, 49, 169–176. [Google Scholar] [CrossRef]
- Rafael, M.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Topical anti-inflammatory plant species: Bioactivity of Bryonia dioica, Tamus communis and Lonicera periclymenum fruits. Ind. Crops Prod. 2011, 34, 1447–1454. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Hanula, M.; Pogorzelski, G. Extraction of polyphenols and essential oils from herbs with green extraction methods—An insightful review. Food Chem. 2024, 460, 140456. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Raghunath, S.; Budaraju, S.; Gharibzahedi, S.M.T.; Koubaa, M.; Roohinejad, S.; Mallikarjunan, K. Processing Technologies for the Extraction of Value-Added Bioactive Compounds from Tea. Food Eng. Rev. 2023, 15, 276–308. [Google Scholar] [CrossRef]
- Shrivastav, G.; Prava Jyoti, T.; Chandel, S.; Singh, R. Eco-Friendly Extraction: Innovations, Principles, and Comparison with Traditional Methods. Sep. Purif. Rev. 2024, 54, 241–257. [Google Scholar] [CrossRef]
- Boumerfeg, S.; Baghiani, A.; Messaoudi, D.; Khennouf, S.; Arrar, L. Antioxidant properties and xanthine oxidase inhibitory effects of Tamus communis L. root extracts. Phytother. Res. 2009, 23, 283–288. [Google Scholar] [CrossRef]
- Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z.; Kočevar Glavač, N.; Barlič-Maganja, D. A Review and Evaluation of the Data Supporting Internal Use of Helichrysum italicum. Plants 2021, 10, 1738. [Google Scholar] [CrossRef]
- Abraão, A.S.; Fernandes, N.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus lusitanica L. Fruits as a Novel Source of Bioactive Compounds with Antioxidant Potential: Exploring the Unknown. Antioxidants 2022, 11, 1738. [Google Scholar] [CrossRef]
- Yassir, M.; Tir, M.; Mufti, A.; Feriani, A.; Faidi, B.; Tlili, N.; Sobeh, M. Millettia ferruginea extract attenuates cisplatin-induced alterations in kidney functioning, DNA damage, oxidative stress, and renal tissue morphology. Arab. J. Chem. 2022, 15, 104037. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Zengin, G.; Fernández-de Córdova, M.L.; Bender, O.; Atalay, A.; Ceylan, R.; Mollica, A.; Mocan, A.; Uysal, S.; Guler, G.O.; et al. Traditionally Used Lathyrus Species: Phytochemical Composition, Antioxidant Activity, Enzyme Inhibitory Properties, Cytotoxic Effects, and in silico Studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 2017, 8, 83. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MS(n) and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Maria Carvalho, A.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Al-Qahtani, A.; Hassan, W.; Fantoukh, O.; El-Sayed, M.; Al-Youssef, H. Phytochemical profile, antioxidant and cytotoxic potential of Parkinsonia aculeata L. growing in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1129–1137. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, Additive, and Antagonistic Effects of Food Mixtures on Total Antioxidant Capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Pecyna, A.; Buczaj, A. Process Efficiency and Energy Consumption during the Ultrasound-Assisted Extraction of Bioactive Substances from Hawthorn Berries. Energies 2021, 14, 7638. [Google Scholar] [CrossRef]
- Bodea, I.M.; Garre Pérez, A.; Cătunescu, G.M.; Palop, A. A Review on Microwave and Ultrasound-Assisted Extractions of Essential Oil from Orange Peel Waste. Food Bioprocess Technol. 2025, 18, 7060–7082. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current state of the art on the antioxidant activity of sage (Salvia spp.) and its bioactive components. Planta Medica 2020, 86, 224–238. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Xie, L.-P.; Chen, Q.-X.; Huang, H.; Wang, H.-Z.; Zhang, R.-Q. Inhibitory Effects of Some Flavonoids on the Activity of Mushroom Tyrosinase. Biochemistry 2003, 68, 487–491. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, H.-T.; Cheng, R.-R.; Wang, D.; Yang, C.-R.; Tanaka, T.; Kouno, I.; Zhang, Y.-J. Antioxidant and hyaluronidase inhibitory activities of diverse phenolics in Phyllanthus emblica. Nat. Prod. Res. 2016, 30, 2726–2729. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Kırmızıbekmez, H.; İnan, Y.; Reis, R.; Sipahi, H.; Gören, A.C.; Yeşilada, E. Phenolic compounds from the aerial parts of Clematis viticella L. and their in vitro anti-inflammatory activities*. Nat. Prod. Res. 2019, 33, 2541–2544. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Garcia, J.; Granato, D.; Barros, A. Seed Phytochemical Profiling of Three Olive Cultivars, Antioxidant Capacity, Enzymatic Inhibition, and Effects on Human Neuroblastoma Cells (SH-SY5Y). Molecules 2022, 27, 5057. [Google Scholar] [CrossRef]
- Silva, A.M.; Garcia, J.; Dall'Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Eco-friendly insights on kiwiberry leaves valorization through in-vitro and in-vivo studies. Ind. Crops Prod. 2022, 184, 115090. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Castro, F.; Aires, A.; Marques, G.; Saavedra, M.J. Antimicrobial, Antibiofilm, and Antioxidant Properties of Boletus edulis and Neoboletus luridiformis Against Multidrug-Resistant ESKAPE Pathogens. Front. Nutr. 2021, 8, 773346. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Matkowski, A.; Ślusarczyk, S.; Magné, C.; Poleze, T.; Pereira, C.; Custódio, L. Sea knotgrass (Polygonum maritimum L.) as a potential source of innovative industrial products for skincare applications. Ind. Crops Prod. 2019, 128, 391–398. [Google Scholar] [CrossRef]

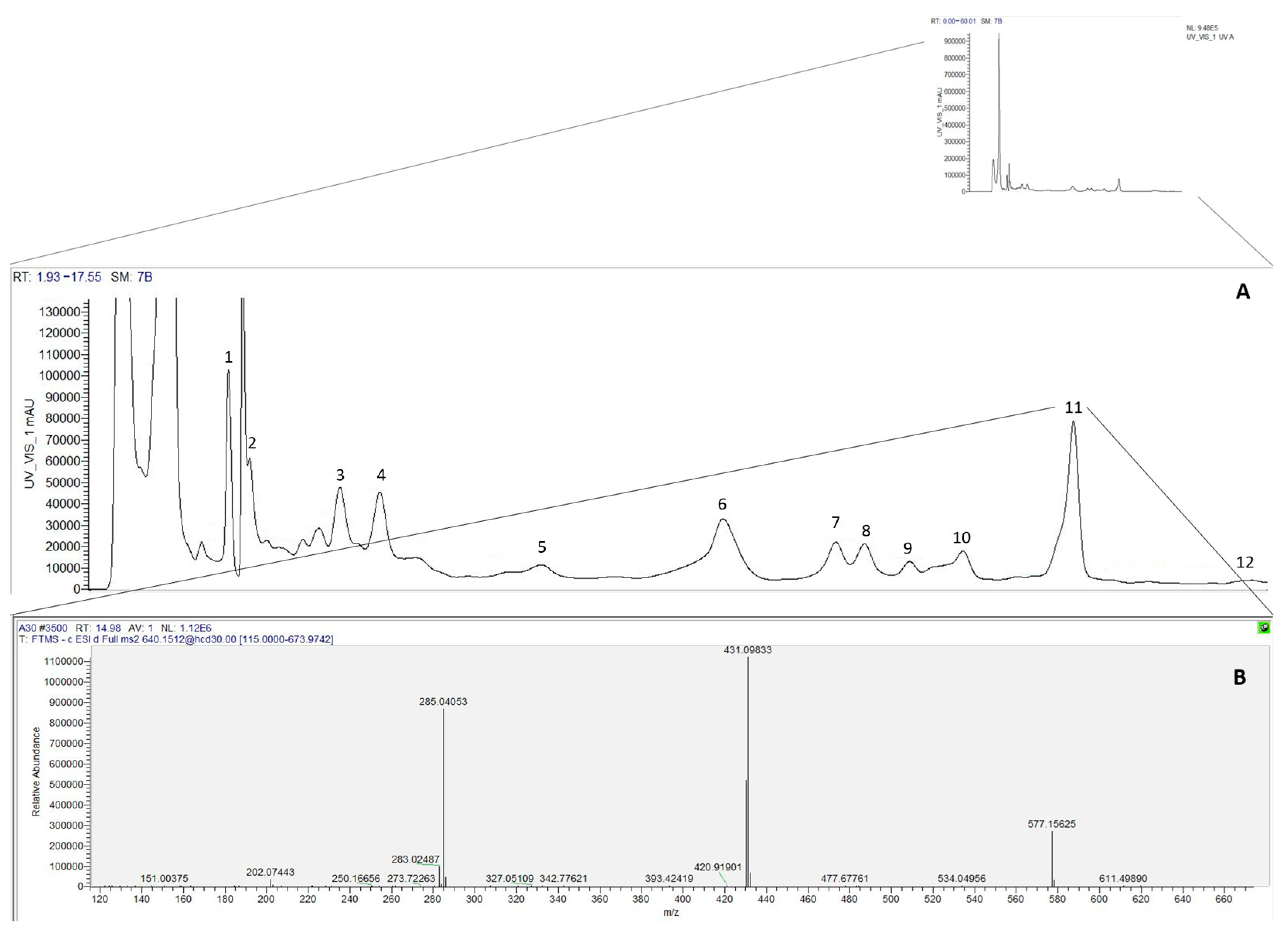
| Peak. | Rt (min) | λ max | [M − H]− m/z | MS2 | Tentative Identification | References | Concentration (mg mL−1) | |
|---|---|---|---|---|---|---|---|---|
| TCE-CM | TCE-UM | |||||||
| 1 | 3.79 | 306 | 341 | 179(100) | Caffeic acid hexoside | [11] | 0.11 ± 0.00 b | 0.31 ± 0.01 a |
| 2 | 4.07 | 321 | 353 | 191(100),179(61),173(4),161(8),135(17) | 3-O-Caffeoylquinic acid | [12] | 0.23 ± 0.02 b | 0.52 ± 0.08 a |
| 3 | 5.27 | 313 | 369 | 223(100) | Sinapic acid rhamnoside | [13] | 0.87 ± 0.02 b | 1.17 ± 0.04 a |
| 4 | 5.81 | 323 | 593 | 505(14),473(24),383(18),353(29),325(11) | Apigenin-6,8-C-diglucoside | [14] | 0.90 ± 0.03 b | 0.96 ± 0.02 a |
| 5 | 7.96 | 348 | 609 | 301(100) | Quercetin-O-neohesperoside | [15] | 0.46 ± 0.03 b | 0.51 ± 0.02 a |
| 6 | 10.34 | 280 | 351 | 163(100) | p-Coumaric acid derivative | [15] | 0.67 ± 0.01 b | 0.90 ± 0.10 a |
| 7 | 11.84 | 346 | 593 | 431(21),285(100) | Kaempferol-3-O-neohesperidoside | [16] | 0.06 ± 0.00 a | 0.07 ± 0.01 a |
| 8 | 12.14 | 335 | 563 | 431(10),285(33) | Kaempferol-O-rhamnosyl-O-pentoside | [17] | 0.02 ± 0.00 b | 0.03 ± 0.00 a |
| 9 | 12.81 | 335 | 431 | 285(100) | Kaempferol-O-rhamnoside | [17] | 0.03 ± 0.00 b | 0.06 ± 0.00 a |
| 10 | 13.52 | 354 | 463 | 301(100) | Quercetin-3-O-glucoside | [12] | 0.01 ± 0.00 b | 0.01 ± 0.00 a |
| 11 | 14.98 | 343 | 577 | 431(100),285(50) | Kaempferol-3,7-di-O-rhamnoside | [17] | 0.21 ± 0.00 b | 0.38 ± 0.03 a |
| 12 | 17.34 | 348 | 447 | 285(100) | Kaempferol-3-O-glucoside | [18] | tr | 0.01 ± 0.00 |
| Total phenolic acids | 1.88 ± 0.04 | 2.90 ± 0.24 | ||||||
| Total flavonoids | 1.75 ± 0.07 | 2.12 ± 0.10 | ||||||
| Total phenolic compounds | 3.64 ± 0.12 | 5.02 ± 0.34 | ||||||
| TCE-CM | TCE-UM | |||
|---|---|---|---|---|
| ORGANISM | MIC | MBC | MIC | MBC |
| Multidrug-resistant clinical isolates | ||||
| Staphylococcus aureus | 62.5 | >62.5 | 31.25 | >31.25 |
| Escherichia coli | 500 | >500 | 125 | >125 |
| Pseudomonas aeruginosa | >1000 | >1000 | 1000 | >1000 |
| Klebsiella pneumoniae | 500 | >500 | 62.5 | >62.5 |
| Acinetobacter baumannii | 1000 | >1000 | 500 | >500 |
| Candida albicans | 250 | 250 | 62.5 | 62.5 |
| Foodborne isolates | ||||
| Salmonella typhi | >1000 | >1000 | >1000 | >1000 |
| Yersinia enterocolitica | 500 | 500 | 125 | 125 |
| Listeria monocytogenes | >1000 | >1000 | 1000 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouvinhas, I.; Saavedra, M.J.; Alves, M.J.; Garcia, J. Exploring the Impact of Ultrasound-Assisted Extraction on the Phytochemical Composition and Bioactivity of Tamus communis L. Fruits. Pharmaceuticals 2025, 18, 1342. https://doi.org/10.3390/ph18091342
Gouvinhas I, Saavedra MJ, Alves MJ, Garcia J. Exploring the Impact of Ultrasound-Assisted Extraction on the Phytochemical Composition and Bioactivity of Tamus communis L. Fruits. Pharmaceuticals. 2025; 18(9):1342. https://doi.org/10.3390/ph18091342
Chicago/Turabian StyleGouvinhas, Irene, Maria José Saavedra, Maria José Alves, and Juliana Garcia. 2025. "Exploring the Impact of Ultrasound-Assisted Extraction on the Phytochemical Composition and Bioactivity of Tamus communis L. Fruits" Pharmaceuticals 18, no. 9: 1342. https://doi.org/10.3390/ph18091342
APA StyleGouvinhas, I., Saavedra, M. J., Alves, M. J., & Garcia, J. (2025). Exploring the Impact of Ultrasound-Assisted Extraction on the Phytochemical Composition and Bioactivity of Tamus communis L. Fruits. Pharmaceuticals, 18(9), 1342. https://doi.org/10.3390/ph18091342









