Pan-Amyloid Reactive Peptides p5+14 and p5R Exhibit Specific Charge-Dependent Binding to Glycosaminoglycans
Abstract
1. Introduction
2. Results
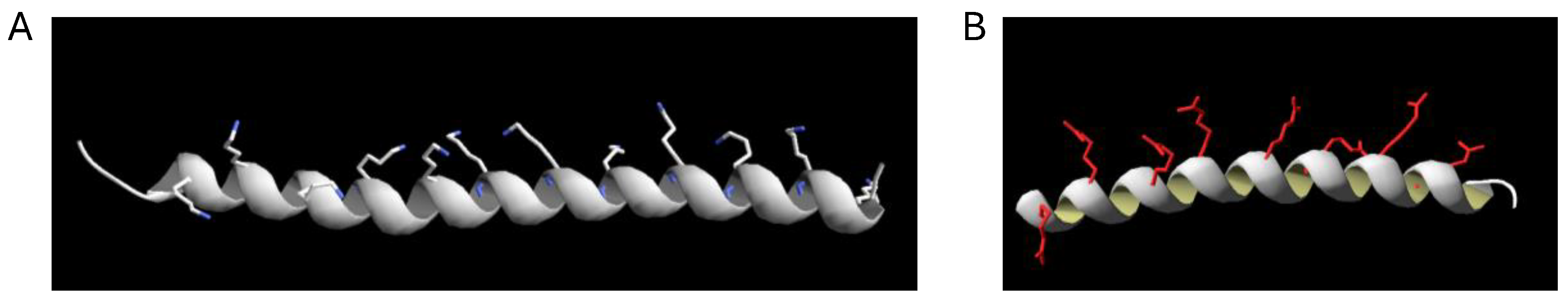
2.1. Structural Models of Peptides p5+14 and p5R
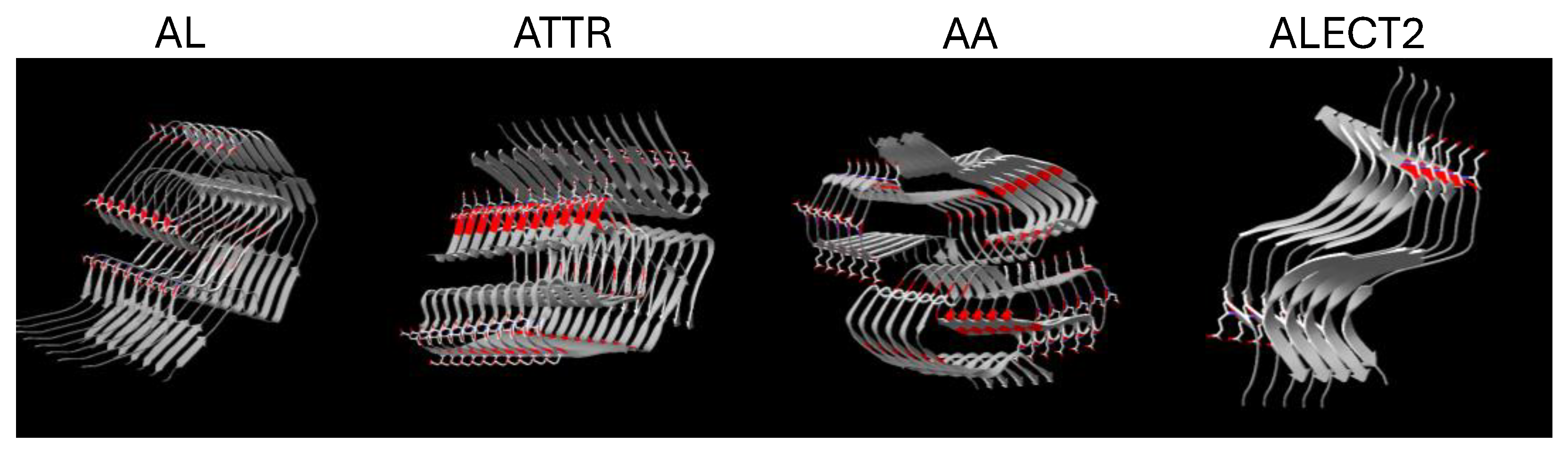
2.2. Polybasic Peptides p5+14 and p5R Bind to Systemic Amyloid Deposits
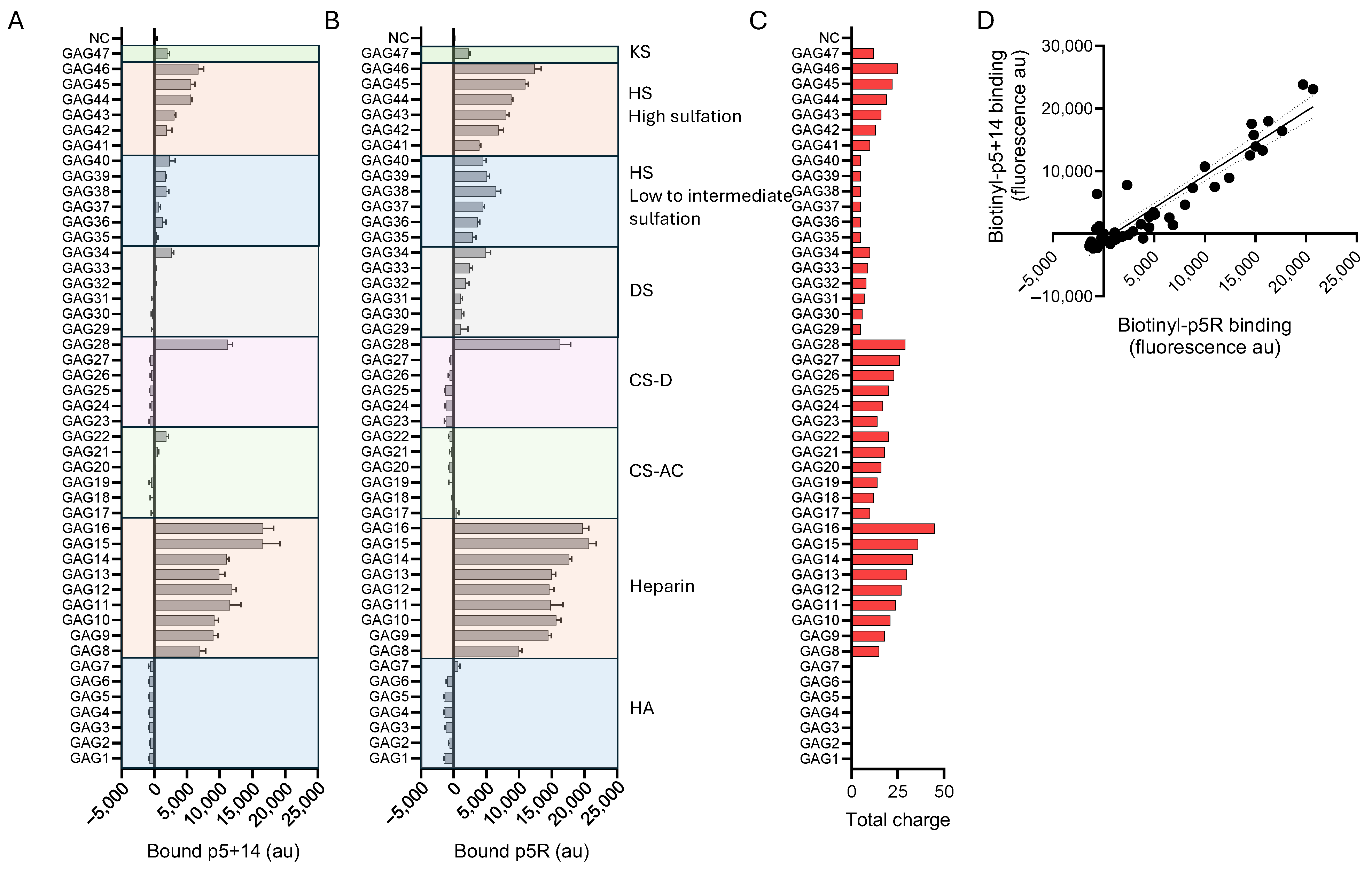
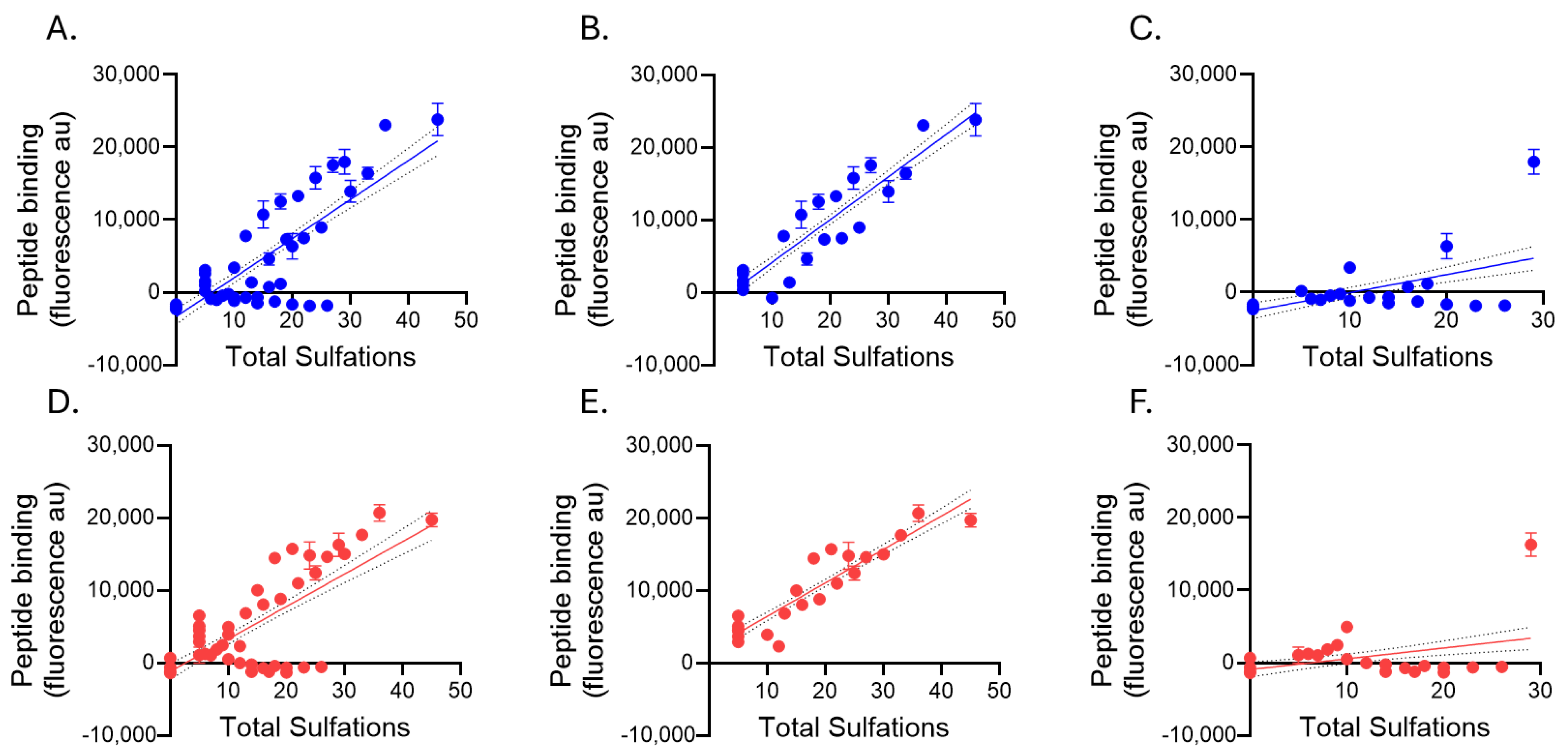
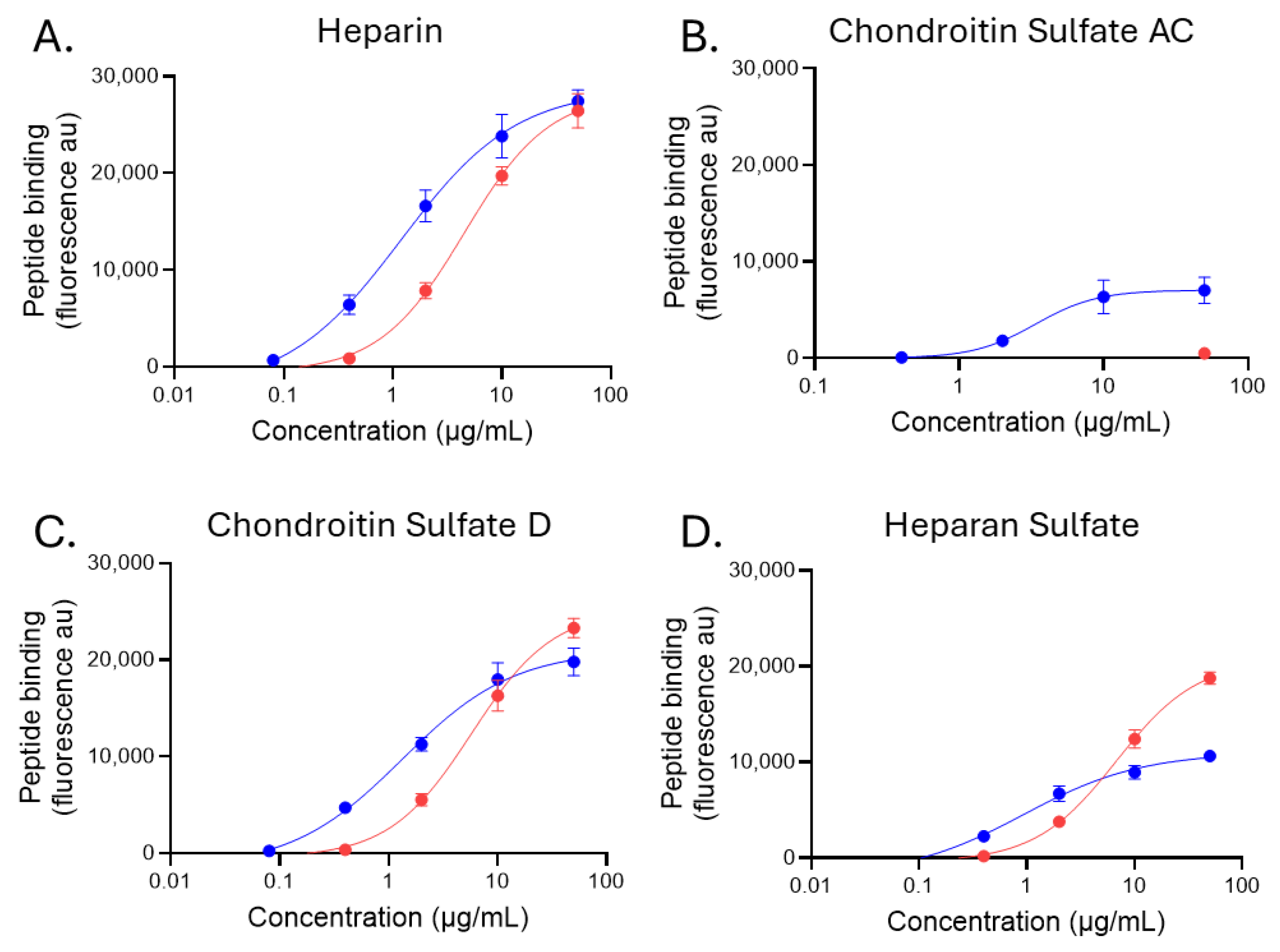
2.3. Glycosaminoglycan Binding of Biotinylated Peptides p5+14 and p5R
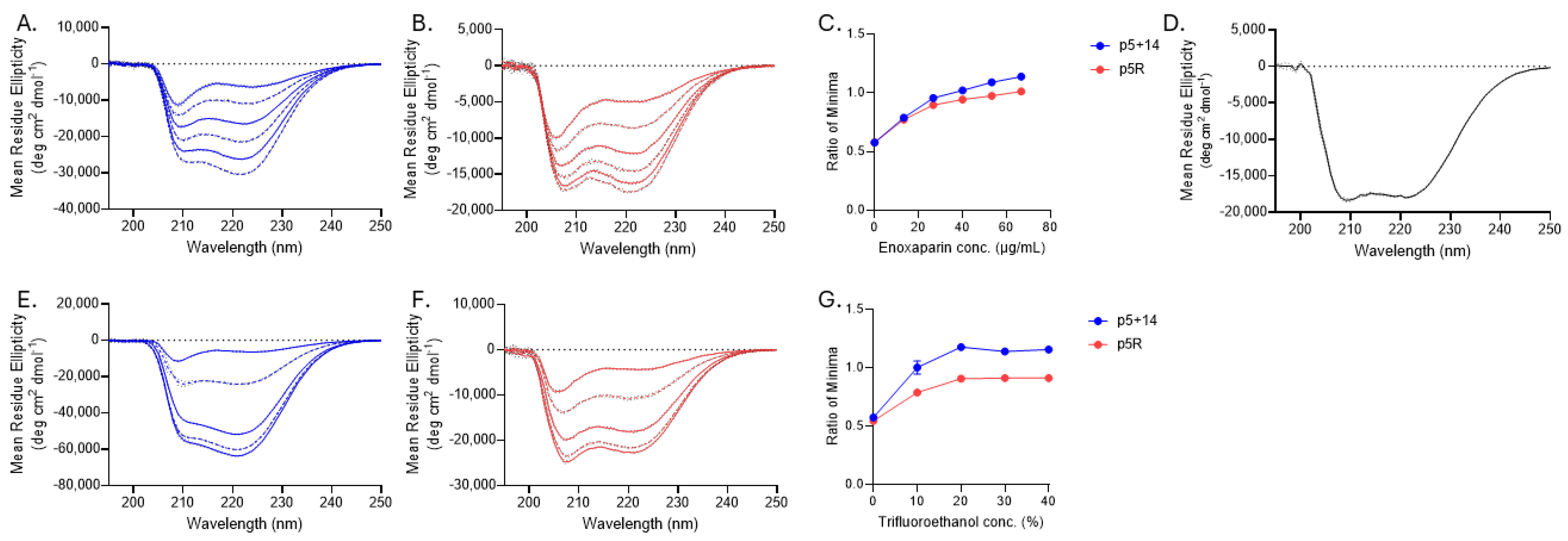
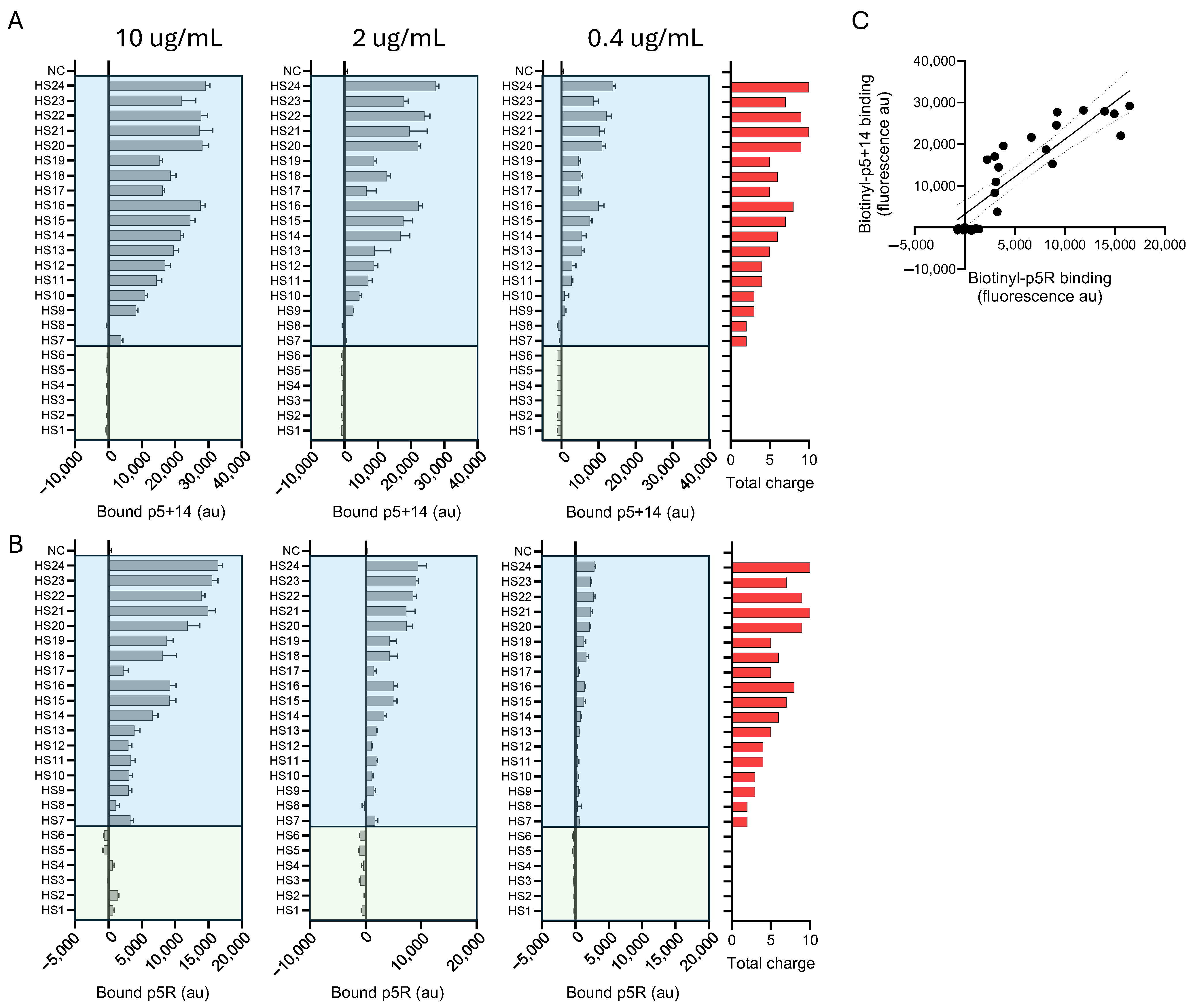
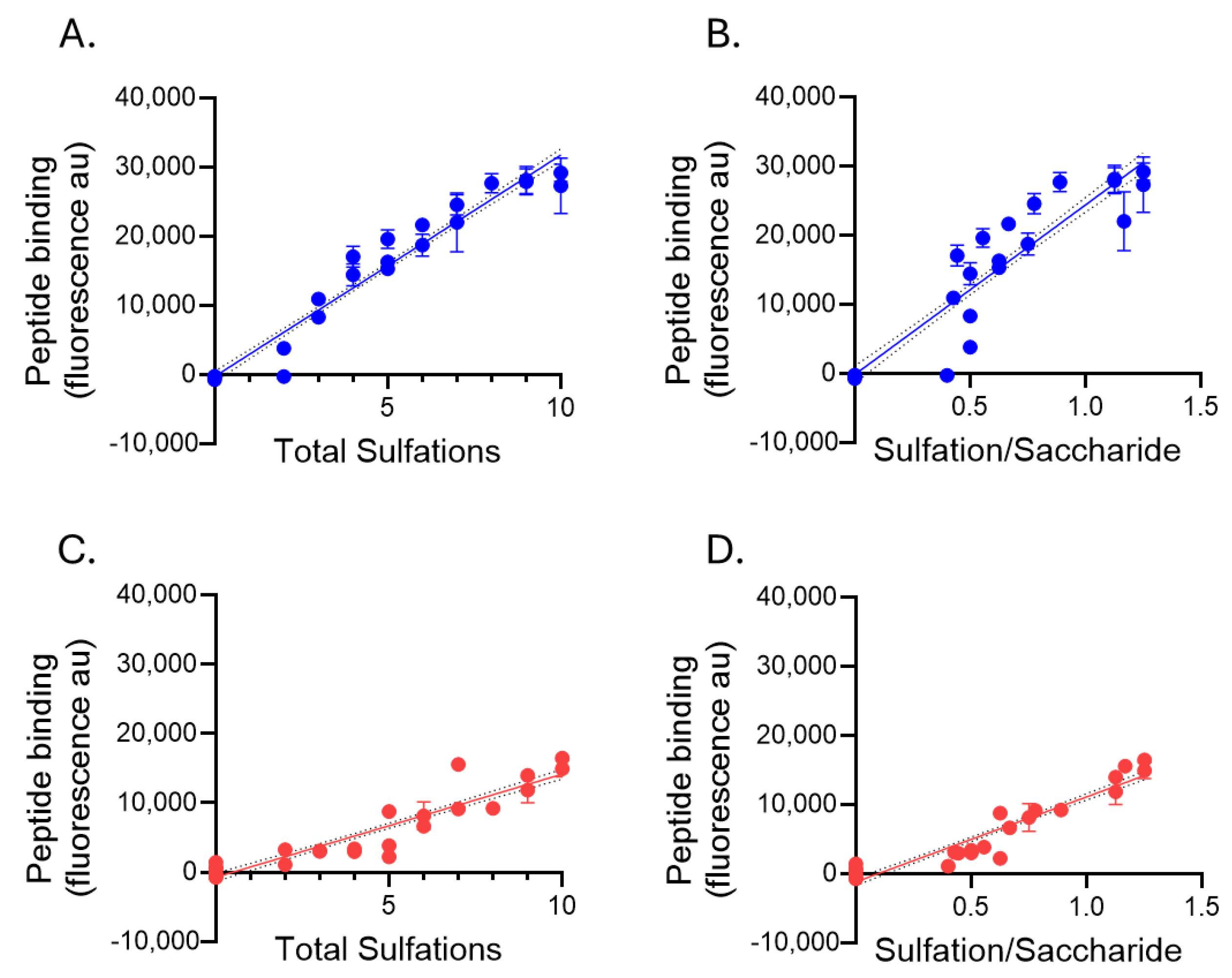
2.4. Heparan Sulfate Binding of Biotinylated Peptides p5+14 and p5R
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Peptide Structure Modeling
4.3. Circular Dichroism
4.4. Glycosaminoglycan and Heparan Sulfate Arrays
4.5. Radiolabeling of Peptide p5+14 and PET/CT Imaging
4.6. Data Visualization and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2018: Recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2018, 25, 215–219. [Google Scholar] [CrossRef]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Eisenberg, D.S.; Fändrich, M.; McPhail, E.D.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2024: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2024, 31, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Vrana, J.A.; Gamez, J.D.; Madden, B.J.; Theis, J.D.; Bergen, H.R.; Dogan, A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009, 114, 4957–4959. [Google Scholar] [CrossRef]
- Vrana, J.A.; Theis, J.D.; Dasari, S.; Mereuta, O.M.; Dispenzieri, A.; Zeldenrust, S.R.; Gertz, M.A.; Kurtin, P.J.; Grogg, K.L.; Dogan, A. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 2014, 99, 1239–1247. [Google Scholar] [CrossRef]
- Tennent, G.A.; Lovat, L.B.; Pepys, M.B. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc. Natl. Acad. Sci. USA 1995, 92, 4299–4303. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Herbert, J.; Hutchinson, W.L.; Tennent, G.A.; Lachmann, H.J.; Gallimore, J.R.; Lovat, L.B.; Bartfai, T.; Alanine, A.; Hertel, C.; et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 2002, 417, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Tennent, G.A.; Hutchinson, W.L.; Gallimore, J.R.; Lachmann, H.J.; Goodman, H.J.; Offer, M.; Millar, D.J.; Petrie, A.; Hawkins, P.N.; et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br. J. Haematol. 2010, 148, 760–767. [Google Scholar] [CrossRef]
- Schulte, T.; Chaves-Sanjuan, A.; Speranzini, V.; Sicking, K.; Milazzo, M.; Mazzini, G.; Rognoni, P.; Caminito, S.; Milani, P.; Marabelli, C.; et al. Helical superstructures between amyloid and collagen in cardiac fibrils from a patient with AL amyloidosis. Nat. Commun. 2024, 15, 6359. [Google Scholar] [CrossRef]
- Hancock, T.J.; Vlasyuk, M.; Foster, J.S.; Macy, S.; Wooliver, D.C.; Balachandran, M.; Williams, A.D.; Martin, E.B.; Kennel, S.J.; Heidel, E.R.; et al. Neutrophils enhance the clearance of systemic amyloid deposits in a murine amyloidoma model. Front. Immunol. 2024, 15, 1487250. [Google Scholar] [CrossRef]
- Noborn, F.; O’CAllaghan, P.; Hermansson, E.; Zhang, X.; Ancsin, J.B.; Damas, A.M.; Dacklin, I.; Presto, J.; Johansson, J.; Saraiva, M.J.; et al. Heparan sulfate/heparin promotes transthyretin fibrillization through selective binding to a basic motif in the protein. Proc. Natl. Acad. Sci. USA 2011, 108, 5584–5589. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, J.; Franklin, T.; Zhang, X.; Deng, J.; Fraser, P.E. Interactions of Alzheimer amyloid-beta peptides with glycosaminoglycans effects on fibril nucleation and growth. Eur. J. Biochem. 1999, 266, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.M.; Cummings, J.A.; Yang, W.; Judge, M.E.; Sheardown, M.J.; Rimvall, K.; Hansen, J.B.; Snow, A.D. Sulfate content and specific glycosaminoglycan backbone of perlecan are critical for perlecan’s enhancement of islet amyloid polypeptide (amylin) fibril formation. Diabetes 1998, 47, 612–620. [Google Scholar] [CrossRef]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Suda, H.; Tsuzuike, N.; Seto, K.; Seki, M.; Yamaguchi, Y.; Hasegawa, K.; Takahashi, N.; Yamamoto, S.; Gejyo, F.; et al. Glycosaminoglycan and proteoglycan inhibit the depolymerization of beta2-microglobulin amyloid fibrils in vitro. Kidney Int. 2003, 64, 1080–1088. [Google Scholar] [CrossRef]
- Suk, J.Y.; Zhang, F.; Balch, W.E.; Linhardt, R.J.; Kelly, J.W. Heparin accelerates gelsolin amyloidogenesis. Biochemistry 2006, 45, 2234–2242. [Google Scholar] [CrossRef]
- Buell, A.K. Stability matters, too—The thermodynamics of amyloid fibril formation. Chem. Sci. 2022, 13, 10177–10192. [Google Scholar] [CrossRef]
- Snow, A.D.; Bramson, R.; Mar, H.; Wight, T.N.; Kisilevsky, R. A temporal and ultrastructural relationship between heparan sulfate proteoglycans and AA amyloid in experimental amyloidosis. J. Histochem. Cytochem. 1991, 39, 1321–1330. [Google Scholar] [CrossRef]
- Li, J.P.; Galvis, M.L.E.; Gong, F.; Zhang, X.; Zcharia, E.; Metzger, S.; Vlodavsky, I.; Kisilevsky, R.; Lindahl, U. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proc. Natl. Acad. Sci. USA 2005, 102, 6473–6477. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.B.; Lewkowicz, E.; Gursky, O.; Straub, J.E. Elucidating the Mechanism of Recognition and Binding of Heparin to Amyloid Fibrils of Serum Amyloid A. Biochemistry 2025, 64, 266–276. [Google Scholar] [CrossRef]
- Lindahl, B.; Lindahl, U. Amyloid-specific heparan sulfate from human liver and spleen. J. Biol. Chem. 1997, 272, 26091–26094. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K. Heparan sulfate S-domains and extracellular sulfatases (Sulfs): Their possible roles in protein aggregation diseases. Glycoconj. J. 2018, 35, 387–396. [Google Scholar] [CrossRef]
- Nuvolone, M.; Nevone, A.; Merlini, G. Targeting Amyloid Fibrils by Passive Immunotherapy in Systemic Amyloidosis. BioDrugs 2022, 36, 591–608. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Judge, D.P.; Cappelli, F.; Fontana, M.; Garcia-Pavia, P.; Gibbs, S.; Grogan, M.; Hanna, M.; Hoffman, J.; Masri, A.; et al. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2024, 390, 132–142. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.-C.; Sekijima, Y.; et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: A randomized clinical trial. Amyloid 2023, 30, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef]
- Coelho, T.; Marques, W.; Dasgupta, N.R.; Chao, C.-C.; Parman, Y.; França, M.C.; Guo, Y.-C.; Wixner, J.; Ro, L.-S.; Calandra, C.R.; et al. Eplontersen for Hereditary Transthyretin Amyloidosis With Polyneuropathy. JAMA 2023, 330, 1448–1458. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Siepen, F.A.D.; Donal, E.; Lairez, O.; van der Meer, P.; Kristen, A.V.; Mercuri, M.F.; Michalon, A.; Frost, R.J.; Grimm, J.; et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. N. Engl. J. Med. 2023, 389, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Suhr, O.B.; Grogan, M.; da Silva, A.M.; Karam, C.; Garcia-Pavia, P.; Drachman, B.; Zago, W.; Tripuraneni, R.; Kinney, G.G. PRX004 in variant amyloid transthyretin (ATTRv) amyloidosis: Results of a phase 1, open-label, dose-escalation study. Amyloid 2025, 32, 14–21. [Google Scholar] [CrossRef]
- Edwards, C.V.; Rao, N.; Bhutani, D.; Mapara, M.; Radhakrishnan, J.; Shames, S.; Maurer, M.S.; Leng, S.; Solomon, A.; Lentzsch, S.; et al. Phase 1a/b study of monoclonal antibody CAEL-101 (11-1F4) in patients with AL amyloidosis. Blood 2021, 138, 2632–2641. [Google Scholar] [CrossRef]
- Wall, J.S.; Kennel, S.J.; Stuckey, A.C.; Long, M.J.; Townsend, D.W.; Smith, G.T.; Wells, K.J.; Fu, Y.; Stabin, M.G.; Weiss, D.T.; et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood 2010, 116, 2241–2244. [Google Scholar] [CrossRef]
- Lousada, I.; Comenzo, R.L.; Landau, H.; Guthrie, S.; Merlini, G. Light Chain Amyloidosis: Patient Experience Survey from the Amyloidosis Research Consortium. Adv. Ther. 2015, 32, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Rintell, D.; Heath, D.; Mendendez, F.B.; Cross, E.; Cross, T.; Knobel, V.; Gagnon, B.; Turtle, C.; Cohen, A.; Kalmykov, E.; et al. Patient and family experience with transthyretin amyloid cardiomyopathy (ATTR-CM) and polyneuropathy (ATTR-PN) amyloidosis: Results of two focus groups. Orphanet J. Rare Dis. 2021, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Gundapaneni, B.; Sultan, M.B.; Ines, M.; Garcia-Pavia, P. Improved long-term survival with tafamidis treatment in patients with transthyretin amyloid cardiomyopathy and severe heart failure symptoms. Eur. J. Heart Fail. 2023, 25, 2060–2064. [Google Scholar] [CrossRef]
- Wall, J.S.; Richey, T.; Stuckey, A.; Donnell, R.; Macy, S.; Martin, E.B.; Williams, A.; Higuchi, K.; Kennel, S.J. In vivo molecular imaging of peripheral amyloidosis using heparin-binding peptides. Proc. Natl. Acad. Sci. USA 2011, 108, E586–E594. [Google Scholar] [CrossRef]
- Wall, J.S.; Richey, T.; Williams, A.; Stuckey, A.; Osborne, D.; Martin, E.; Kennel, S.J. Comparative analysis of peptide p5 and serum amyloid P component for imaging AA amyloid in mice using dual-isotope SPECT. Mol. Imaging Biol. 2012, 14, 402–407. [Google Scholar] [CrossRef]
- Martin, E.B.; Williams, A.; Heidel, E.; Macy, S.; Kennel, S.J.; Wall, J.S. Peptide p5 binds both heparinase-sensitive glycosaminoglycans and fibrils in patient-derived AL amyloid extracts. Biochem. Biophys. Res. Commun. 2013, 436, 85–89. [Google Scholar] [CrossRef]
- Wall, J.S.; Richey, T.; Macy, S.; Heidel, E.; Wooliver, C.; Kennel, S.J. A novel method for quantifying peripheral tissue amyloid load by using the radiolabeled amyloidophilic peptide, p5. Amyloid 2013, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Martin, E.B.; Richey, T.; Stuckey, A.C.; Macy, S.; Wooliver, C.; Williams, A.; Foster, J.S.; McWilliams-Koeppen, P.; Uberbacher, E.; et al. Preclinical Validation of the Heparin-Reactive Peptide p5+14 as a Molecular Imaging Agent for Visceral Amyloidosis. Molecules 2015, 20, 7657–7682. [Google Scholar] [CrossRef]
- Martin, E.B.; Williams, A.; Richey, T.; Stuckey, A.; Heidel, R.E.; Kennel, S.J.; Wall, J.S. Comparative evaluation of p5+14 with SAP and peptide p5 by dual-energy SPECT imaging of mice with AA amyloidosis. Sci. Rep. 2016, 6, 22695. [Google Scholar] [CrossRef]
- Wall, J.S.; Martin, E.B.; Lands, R.; Ramchandren, R.; Stuckey, A.; Heidel, R.E.; Whittle, B.; Powell, D.; Richey, T.; Williams, A.D.; et al. Cardiac Amyloid Detection by PET/CT Imaging of Iodine ((124)I) Evuzamitide ((124)I-p5+14): A Phase 1/2 Study. JACC Cardiovasc. Imaging 2023, 16, 1433–1448. [Google Scholar] [CrossRef]
- Clerc, O.F.; Cuddy, S.A.; Robertson, M.; Vijayakumar, S.; Neri, J.C.; Chemburkar, V.; Kijewski, M.F.; Di Carli, M.F.; Bianchi, G.; Falk, R.H.; et al. Cardiac Amyloid Quantification Using (124)I-Evuzamitide ((124)I-P5+14) Versus (18)F-Florbetapir: A Pilot PET/CT Study. JACC Cardiovasc. Imaging 2023, 16, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Smiley, D.A.; Einstein, A.J.; O’gOrman, K.J.; Santana, D.; Teruya, S.; Chan, N.; Nalbandian, A.; Poterucha, T.J.; Helmke, S.T.; Mintz, A.; et al. Early Detection of Transthyretin Cardiac Amyloidosis Using (124)I-Evuzamitide Positron Emission Tomography/Computed Tomography. JACC Cardiovasc. Imaging 2025, 18, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.B.; Kennel, S.J.; Richey, T.; Wooliver, C.; Osborne, D.; Williams, A.; Stuckey, A.; Wall, J.S. Dynamic PET and SPECT imaging with radioiodinated, amyloid-reactive peptide p5 in mice: A positive role for peptide dehalogenation. Peptides 2014, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kennel, S.J.; Stuckey, A.; McWilliams-Koeppen, H.P.; Richey, T.; Wall, J.S. Tc-99m Radiolabeled Peptide p5 + 14 is an Effective Probe for SPECT Imaging of Systemic Amyloidosis. Mol. Imaging Biol. 2016, 18, 483–489. [Google Scholar] [CrossRef]
- Wall, J.S.; Kennel, S.J.; Martin, E.B. Dual-Energy SPECT and the Development of Peptide p5+14 for Imaging Amyloidosis. Mol. Imaging 2017, 16, 1536012117708705. [Google Scholar] [CrossRef]
- Wall, J.S.; Martin, E.B.; Endsley, A.; Stuckey, A.C.; Williams, A.D.; Powell, D.; Whittle, B.; Hall, S.; Lambeth, T.R.; Julian, R.R.; et al. First in Human Evaluation and Dosimetry Calculations for Peptide (124)I-p5+14-a Novel Radiotracer for the Detection of Systemic Amyloidosis Using PET/CT Imaging. Mol. Imaging Biol. 2022, 24, 479–488. [Google Scholar] [CrossRef]
- Martin, E.B.; Stuckey, A.; Powell, D.; Lands, R.; Whittle, B.; Wooliver, C.; Macy, S.; Foster, J.S.; Guthrie, S.; Kennel, S.J.; et al. Clinical Confirmation of Pan-Amyloid Reactivity of Radioiodinated Peptide (124)I-p5+14 (AT-01) in Patients with Diverse Types of Systemic Amyloidosis Demonstrated by PET/CT Imaging. Pharmaceuticals 2023, 16, 629. [Google Scholar] [CrossRef]
- Kennel, S.J.; Jackson, J.W.; Stuckey, A.; Richey, T.; Foster, J.S.; Wall, J.S.; Hohn, A. Preclinical evaluation of Tc-99m p5+14 peptide for SPECT detection of cardiac amyloidosis. PLoS ONE 2024, 19, e0301756. [Google Scholar] [CrossRef]
- Wall, J.; Martin, E.; Heidel, R.E.; Stuckey, A.; Whittle, B.; Jackson, J.; Williams, A.; Mehmood, M.; Kassira, A.K.; Hung, R.R.; et al. DETECTION OF AMYLOID CARDIOMYOPATHY IN PATIENTS WITH SYSTEMIC ATTR AND AL AMYLOIDOSIS USING SPECT/CT AND PLANAR IMAGING OF A TECHNETIUM-99M-LABELED PEPTIDE—TECHNETIUM-99M (99MTC) EVUZAMITIDE. JACC 2024, 83 (Suppl. S13), 1369. [Google Scholar] [CrossRef]
- Wall, J.S.; Williams, A.; Richey, T.; Stuckey, A.; Huang, Y.; Wooliver, C.; Macy, S.; Heidel, E.; Gupta, N.; Lee, A.; et al. A binding-site barrier affects imaging efficiency of high affinity amyloid-reactive peptide radiotracers in vivo. PLoS ONE 2013, 8, e66181. [Google Scholar] [CrossRef]
- Martin, E.B.; Donnell, R.; Richey, T.; Stuckey, A.; Kennel, S.J.; Wall, J.S. Discrete binding patterns of two heparin-reactive proteins, basic fibroblast growth factor and peptide p5R, in amyloid-laden and healthy mice. Biochem. Biophys. Res. Commun. 2021, 552, 136–141. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Liu, M.; Kennel, S.J.; Wall, J.S.; Cheng, X. Computational investigation of the binding of a designed peptide to lambda light chain amyloid fibril. Phys. Chem. Chem. Phys. 2021, 23, 20634–20644. [Google Scholar] [CrossRef]
- Wall, J.S.; Williams, A.; Wooliver, C.; Martin, E.B.; Cheng, X.; Heidel, R.E.; Kennel, S.J. Secondary structure propensity and chirality of the amyloidophilic peptide p5 and its analogues impacts ligand binding—In vitro characterization. Biochem. Biophys. Rep. 2016, 8, 89–99. [Google Scholar] [CrossRef][Green Version]
- Roccatano, D.; Colombo, G.; Fioroni, M.; Mark, A.E. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. Proc. Natl. Acad. Sci. USA 2002, 99, 12179–12184. [Google Scholar] [CrossRef]
- Foster, J.S.; Williams, A.D.; Macy, S.; Richey, T.; Stuckey, A.; Wooliver, D.C.; Koul-Tiwari, R.; Martin, E.B.; Kennel, S.J.; Wall, J.S. A Peptide-Fc Opsonin with Pan-Amyloid Reactivity. Front. Immunol. 2017, 8, 1082. [Google Scholar] [CrossRef]
- Wall, J.S.; Williams, A.; Foster, J.S.; Martin, E.B.; Richey, T.; Stuckey, A.; Macy, S.; Zago, W.; Kinney, G.G.; Kennel, S.J. A bifunctional peptide, “peptope”, for pre-targeting antibody 7D8 to systemic amyloid deposits. Amyloid 2017, 24 (Suppl. S1), S22–S23. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.S.; Balachandran, M.; Hancock, T.J.; Martin, E.B.; Macy, S.; Wooliver, C.; Richey, T.; Stuckey, A.; Williams, A.D.; Jackson, J.W.; et al. Development and characterization of a prototypic pan-amyloid clearing agent—A novel murine peptide-immunoglobulin fusion. Front. Immunol. 2023, 14, 1275372. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.B.; Williams, A.; Richey, T.; Wooliver, C.; Stuckey, A.; Foster, J.S.; Kennel, S.J.; Wall, J.S. Evaluation of the effect of D-amino acid incorporation into amyloid-reactive peptides. J. Transl. Med. 2017, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Williams, A.; Richey, T.; Stuckey, A.; Wooliver, C.; Scott, J.C.; Donnell, R.; Martin, E.B.; Kennel, S.J. Specific Amyloid Binding of Polybasic Peptides In Vivo Is Retained by beta-Sheet Conformers but Lost in the Disrupted Coil and All D-Amino Acid Variants. Mol. Imaging Biol. 2017, 19, 714–722. [Google Scholar] [CrossRef]
- Foster, J.S.; Koul-Tiwari, R.; Williams, A.; Martin, E.B.; Richey, T.; Stuckey, A.; Macy, S.; Kennel, S.J.; Wall, J.S. Preliminary characterization of a novel peptide-Fc-fusion construct for targeting amyloid deposits. Amyloid 2017, 24 (Suppl. S1), S26–S27. [Google Scholar] [CrossRef]
- Wall, J.S.; Williams, A.D.; Foster, J.S.; Richey, T.; Stuckey, A.; Macy, S.; Wooliver, C.; Campagna, S.R.; Tague, E.D.; Farmer, A.T.; et al. Bifunctional amyloid-reactive peptide promotes binding of antibody 11-1F4 to diverse amyloid types and enhances therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2018, 115, E10839–E10848. [Google Scholar] [CrossRef]
- Smits, N.C.; Kurup, S.; Rops, A.L.; Dam, G.B.T.; Massuger, L.F.; Hafmans, T.; Turnbull, J.E.; Spillmann, D.; Li, J.-P.; Kennel, S.J.; et al. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. J. Biol. Chem. 2010, 285, 41143–41151. [Google Scholar] [CrossRef]
- Fromm, J.R.; Hileman, R.E.; Caldwell, E.E.; Weiler, J.M.; Linhardt, R.J. Differences in the interaction of heparin with arginine and lysine and the importance of these basic amino acids in the binding of heparin to acidic fibroblast growth factor. Arch. Biochem. Biophys. 1995, 323, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.B.; Ainsworth, C.F.; Stevens, F.J.; Schiffer, M. Three quaternary structures for a single protein. Proc. Natl. Acad. Sci. USA 1996, 93, 7017–7021. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Wiese, S.; Adak, V.; Engler, J.; Agarwal, S.; Fritz, G.; Westermark, P.; Zacharias, M.; Fändrich, M. Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis. Nat. Commun. 2019, 10, 5008. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.S.; Flores, M.D.; Zink, S.; Schibrowsky, N.A.; Sawaya, M.R.; Rodriguez, J.A. Cryo-EM structure of a human LECT2 amyloid fibril reveals a network of polar ladders at its core. Structure 2023, 31, 1386–1393.e3. [Google Scholar] [CrossRef]
- Liberta, F.; Loerch, S.; Rennegarbe, M.; Schierhorn, A.; Westermark, P.; Westermark, G.T.; Hazenberg, B.P.C.; Grigorieff, N.; Fändrich, M.; Schmidt, M. Cryo-EM fibril structures from systemic AA amyloidosis reveal the species complementarity of pathological amyloids. Nat. Commun. 2019, 10, 1104. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Nelissen, N.; Van Laere, K.; Thurfjell, L.; Owenius, R.; Vandenbulcke, M.; Koole, M.; Bormans, G.; Brooks, D.J.; Vandenberghe, R. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J. Nucl. Med. 2009, 50, 1251–1259. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Dyck, R.F.; De Beer, F.C.; Skinner, M.; Cohen, A.S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin. Exp. Immunol. 1979, 38, 284–293. [Google Scholar] [PubMed]
- Habicht, G.; Haupt, C.; Friedrich, R.P.; Hortschansky, P.; Sachse, C.; Meinhardt, J.; Wieligmann, K.; Gellermann, G.P.; Brodhun, M.; Götz, J.; et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Abeta protofibrils. Proc. Natl. Acad. Sci. USA 2007, 104, 19232–19237. [Google Scholar] [CrossRef] [PubMed]
- Haupt, C.; Morgado, I.; Kumar, S.T.; Parthier, C.; Bereza, M.; Hortschansky, P.; Stubbs, M.T.; Horn, U.; Fändrich, M. Amyloid fibril recognition with the conformational B10 antibody fragment depends on electrostatic interactions. J. Mol. Biol. 2011, 405, 341–348. [Google Scholar] [CrossRef]
- Zhang, Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins 2007, 69 (Suppl. S8), 108–117. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]



| Peptide | Sequence | Molecular Weight 1 | Amino Acid Length | Basic Residues | Average Charge per Residue |
|---|---|---|---|---|---|
| p5+14 | GGGYS KAQKA QAKQA KQAQK AQKAQ AKQAK QAQKA QKAQA KQAKQ | 4766.5 | 45 | 12 | 0.267 |
| p5R | GGGYS RAQRA QARQA RQAQR AQRAQ ARQAR Q | 3481.8 | 31 | 8 | 0.258 |
| Biotinyl-Peptide | Sulfations (All GAGs) | Sulfations (heparin and HS Only) | Sulfations (Non-Heparin and HS) | |
|---|---|---|---|---|
| p5+14 | Pearson r | 0.7925 | 0.9181 | 0.5309 |
| p-value | <0.0001 | <0.0001 | 0.0063 | |
| p5R | Pearson r | 0.7215 | 0.9223 | 0.3714 |
| p-value | <0.0001 | <0.0001 | 0.0676 |
| Apparent EC50: µg/mL (Molarity) | ||||
|---|---|---|---|---|
| Biotinyl-Peptide | Heparin (GAG16) | Chondroitin Sulfate AC (GAG22) | Chondroitin Sulfate D (GAG28) | Heparan Sulfate (GAG46) |
| p5+14 | 1.198 (251.3 nM) | 3.376 (708.2 nM) | 1.334 (279.9 nM) | 0.932 (195.5 nM) |
| p5R | 4.537 (1.303 µM) | nd * | 5.758 (1.65 µM) | 6.792 (1.95 µM) |
| Biotinyl-Peptide | HS Total Sulfations (Net Charge) | HS Sulfations/Saccharide (Charge Density) | |
|---|---|---|---|
| p5+14 | Pearson r | 0.9764 | 0.9250 |
| p-values | <0.0001 | <0.0001 | |
| p5R | Pearson r | 0.924 | 0.9446 |
| p-values | <0.0001 | <0.0001 |
| Apparent Ec50: µg/mL (Molarity) | ||||
|---|---|---|---|---|
| Biotinyl-Peptide | HS13 | HS16 | HS22 | HS24 |
| p5+14 | - | 0.6685 (140.2 nM) | 0.4734 (99.31 nM) | 0.4482 (94.03 nM) |
| p5R | 2.338 (671.5 nM) | 1.930 (554.3 nM) | 1.241 (356.4 nM) | 1.358 (390.0 nM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hancock, T.J.; Williams, A.D.; Foster, J.S.; Wall, J.S.; Martin, E.B. Pan-Amyloid Reactive Peptides p5+14 and p5R Exhibit Specific Charge-Dependent Binding to Glycosaminoglycans. Pharmaceuticals 2025, 18, 1340. https://doi.org/10.3390/ph18091340
Hancock TJ, Williams AD, Foster JS, Wall JS, Martin EB. Pan-Amyloid Reactive Peptides p5+14 and p5R Exhibit Specific Charge-Dependent Binding to Glycosaminoglycans. Pharmaceuticals. 2025; 18(9):1340. https://doi.org/10.3390/ph18091340
Chicago/Turabian StyleHancock, Trevor J., Angela D. Williams, James S. Foster, Jonathan S. Wall, and Emily B. Martin. 2025. "Pan-Amyloid Reactive Peptides p5+14 and p5R Exhibit Specific Charge-Dependent Binding to Glycosaminoglycans" Pharmaceuticals 18, no. 9: 1340. https://doi.org/10.3390/ph18091340
APA StyleHancock, T. J., Williams, A. D., Foster, J. S., Wall, J. S., & Martin, E. B. (2025). Pan-Amyloid Reactive Peptides p5+14 and p5R Exhibit Specific Charge-Dependent Binding to Glycosaminoglycans. Pharmaceuticals, 18(9), 1340. https://doi.org/10.3390/ph18091340









