Abstract
Background/Objectives: RMC-113, a 3-alkynyl-6-aryl-disubstituted isothiazolo[4,3-b]pyridine, is a dual inhibitor of the lipid kinases PIKfyve and PIP4K2C with broad-spectrum antiviral activity. The aim was to study the structure–activity relationship (SAR) of isothiazolo[4,3-b]pyridines as dual PIKfyve/PIP4K2C inhibitors. Methods: A series of isothiazolo[4,3-b]pyridines was synthesized by introducing structural variety at positions 3 and 6 of the central scaffold. The primary assay to guide the synthetic chemistry was a biochemical PIKfyve assay, with a number of analogues also tested for PIP4K2C binding affinity. Finally, isothiazolo[4,3-b]pyridines were also evaluated for antiviral and antitumoral activity in cell-based assays. Results: PIKfyve inhibition tolerated a wide variety of substituents on the aryl ring at position 6 of the isothiazolo[4,3-b]pyridine scaffold, with the 4-carboxamide analogue emerging as the most potent (IC50 = 1 nM). The SAR at position 3 was more restricted, although the introduction of electron-donating groups (such as a methyl and methoxy) on the pyridinyl ring yielded potent PIKfyve inhibitors, with IC50 values in the low nM range. The acetylenic moiety was essential for PIKfyve inhibition, and only the saturated ethyl linker displayed potent PIKfyve inhibition, albeit less active than the acetylene counterpart. The compounds were 2- to 5-fold less potent on PIP4K2C relative to PIKfyve. These dual PIKfyve/PIP4K2C inhibitors displayed antiviral activity against both the venezuelan equine encephalitis virus (VEEV) and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A screening against a panel of cancer cell lines revealed antitumoral activity, although some of the potent PIKfyve/PIP5K2C inhibitors lacked antitumoral activity. Conclusions: Isothiazolo[4,3-b]pyridines are dual PIKfyve/PIP4K2C inhibitors displaying broad-spectrum antiviral, as well as antitumoral, activity.
1. Introduction
PIKfyve (named so after its function and domain structure phospho-inositide kinase for five position containing a Fyve finger) is a lipid kinase that phosphorylates the hydroxyl group at position 5 of the inositol ring of phosphatidylinositol-3-phosphate (PI(3)P), thereby yielding PI(3,5)P2 [1]. PI(3,5)P2 is involved in intracellular trafficking and lysosomal acidification and is thus crucial for the regulation of autophagy [2]. Hence, PIKfyve is involved in the regulation of endomembrane homeostasis and affects several aspects of endosome processing in the course of endocytic cargo transport [3]. PI(3,5)P2 is the biochemical precursor for PI(5)P, which is generated via dephosphorylation catalysed by a 3-phosphatase. It has been linked to intracellular membrane trafficking, autophagy regulation, peroxisome function and cholesterol homeostasis [4].
Several small molecule inhibitors of PIKfyve are known (Figure 1). YM201636 was the first reported inhibitor [5], with an IC50 of 33 nM. Since then, several other PIKfyve inhibitors, such as APY0201 [6], apilimod [7] and vacuolin-1 [8] have been described. More recently, different chemotypes were reported as PIKfyve inhibitors. For example, a series of indolyl pyrimidinamines [9] has potent PIKfyve activity with excellent kinome-wide selectivity. Pyrrolo[2,3-d]pyrimidine L22 is another very potent PIKfyve inhibitor (KD = 0.47 nM) [10].
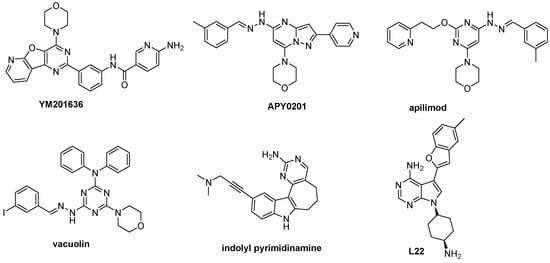
Figure 1.
Existing PIKfyve inhibitors.
Recent studies indicate the potential of PIKfyve as therapeutic target in multiple disease areas, including viral infections, neurodegenerative disorders and cancers [11]. Apilimod display a strong antiproliferative effect in a large panel of non-Hodgkin lymphoma (NHL) B-cell lines, displaying IC50 values of less than 200 nM [12]. Oral administration of apilimod inhibits the growth of subcutaneous Burkitt lymphoma xenografts in mice. Apilimod is currently being evaluated in clinical trials for patients with B-cell malignancies [12]. Perturbation of endosomal trafficking is the mechanism by which PIKfyve inhibitors suppress the replication of various viruses, including Ebola virus [13], Marburg virus [14] and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [9,13]. Lastly, PIKfyve inhibition was shown to reduce both the trafficking of tau seeds into lysosomes and the induction of tau aggregation and to promote secretion of neurotoxic aggregates in Amyotrophic Lateral Sclerosis (ALS) patient iPSC-derived induced motor neurons, proposing a candidate strategy for the treatment of tauopathies [15,16].
PI-5-phosphate 4-kinases (PI5P4Ks) phosphorylate position 4 of PI-5-P to make PI(4,5)P2, which is an important precursor for second messengers inositol-1,4,5-triphosphate, diacylgycerol and phosphatidylinositol-3,4,5-trisphosphate [17]. In mammals, the PI5P4K family consists of three isoforms (α, β and γ), and the genes encoding the PI5P4Ks are called PIP4K2A, PIP4K2B and PIP4K2C. At the protein level, PIP4K2A and PIP4K2B are 83% identical, whereas PIP4K2C is approximately 60% identical to PIP4K2A and PIP4K2B. PIP4K2A is the most catalytically active, displaying significantly more activity compared to PIP4K2B, whereas PIP4K2C has very little inherent activity [18]. PIP4K2’s are important for cancer cell proliferation [19] and it is generally accepted that the PIP4K2’s are oncogenic. This spurred the search for small molecule inhibitors (Figure 2). NIH-12848 is a selective PIP4K2C inhibitor, with only moderate affinity (KD = 2.8 µM) [20]. Subsequent optimization yielded a pyrrolo[3,2-d]pyrimidine analogue with stronger affinity for PIP4K2C (KD = 68 nM) [21]. A series of thieno[2,3-d]pyrimidines (e.g., ARUK2001791) was recently disclosed that display low nM PIP4K2C potency, excellent kinase selectivity (including versus the other PIP4K2 isoforms) and appropriate pharmacokinetic properties to be used for in vivo experiments [22]. THZ-P1-2 is a covalent pan-PIP4K2 inhibitor, with PIP4K2C being the main target (KD = 4.8 nM) and lower activity on PIP4K2A and PIP4K2B (IC50 values of 0.95 µM and 5.9 µM, respectively) [23]. Selective small molecule PIP4K2C inhibitors have not been studied in depth for their antiviral and/or antitumoral properties.
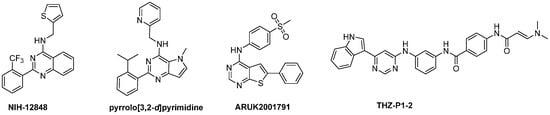
Figure 2.
Reported PIP4K2C inhibitors.
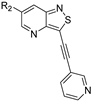
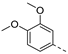
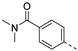
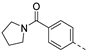
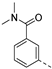
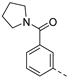
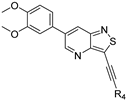
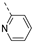
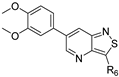
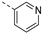
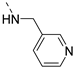
We recently reported the discovery of a dual PIKfyve/PIP4K2C inhibitor, called RMC-113, based on an isothiazolo[4,3-b]pyridine scaffold [24]. RMC-113 displayed strong binding affinity for PIKfyve (KD = 370 nM) and PIP4K2C (KD = 46 nM) and was also a potent inhibitor of PIKfyve in an enzymatic assay (IC50 = 8 nM). Using RMC-113 as a chemical probe, this allowed us to validate PIP4K2C, beyond PIKfyve, as a druggable antiviral target with RMC-113 displaying broad-spectrum antiviral activity against SARS-CoV-2, the vaccine strain of VEEV (TC-83), dengue virus 2, Ebola virus and Marburg virus. However, this previous, mechanistic study did not include a structure–activity relationship (SAR) study, as only RMC-113 was investigated. Therefore, in order to demonstrate that RMC-113 is not a singleton hit and to study the structural features that were required for dual PIKfyve and PIP4K2C inhibition, we embarked upon the synthesis and SAR study of 3,6-disubstituted isothiazolo[4,3-b]pyridines (Figure 3). Three different regions of RMC-113 were subjected to structural modifications: (1) the dimethoxyphenyl moiety; (2) the alkynyl linker and (3) the pyridinyl moiety, whereas the central isothiazolo[4,3-b]pyridine scaffold was kept intact.
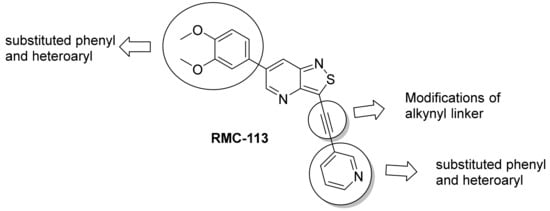
Figure 3.
Hit compound RMC-113 and medicinal chemistry strategy.
2. Results and Discussion
2.1. Chemistry
The synthesis of 3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridines with structural variation at position 6 started from the key intermediates 3-bromo- and 3,6-dibromoisothiazolo[4,3-b]pyridine 1a and 1b, respectively, which were synthesized following known procedures [25] (Scheme 1). A regioselective Sonogashira cross-coupling reaction with 3-ethynylpyridine, using standard reaction conditions (copper iodide, Pd(PPh3)2Cl2 as catalyst, triethylamine as base and tetrahydrofuran as solvent), afforded the 3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridines 2a-b in a good yield. It has been shown before that the presence of an external oxidant such as atmospheric oxygen can contribute to the undesirable homocoupling of acetylenes, also known as the Glaser coupling [26]. In order to avoid this copper-catalyzed oxidative homocoupling and to reduce as much as possible the amount of oxygen in the solvent, a continuous flow of argon was passed through the reaction mixture prior to the addition of the catalyst and also during the dropwise addition (over a period of 30 min) of a solution of the acetylene in THF. Both the continuous flow of argon and the slow addition of the acetylenes were crucial to allow easy purifications and hence to obtain a good yield. The subsequent Suzuki cross-coupling reaction with a wide range of boronic acids and boronic pinacol esters (using Pd(PPh3)4 as catalyst, potassium carbonate as base in a mixture of dioxane and water) furnished compounds 3a-s in moderate to good yields.
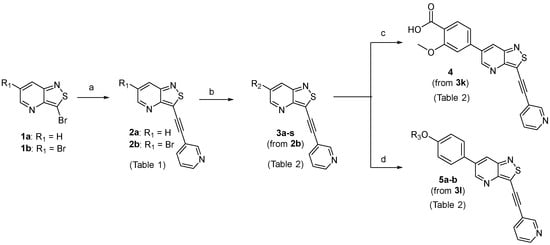
Scheme 1.
Synthesis of 3-(pyridin-3-ylethynyl)-6-aryl-isothiazolo[4,3-b]pyridines. Reagents and conditions: (a) 3-ethynylpyridine, CuI, Pd(PPh3)2Cl2, Et3N, THF, 30 °C; (b) from 2b: boronic acid or ester, Pd(PPh3)4, K2CO3, dioxane/H2O (9:1), 90 °C, overnight; (c) from 3k: LiOH, THF/H2O, 50 °C, overnight; (d) from 3l: appropriate chloride, K2CO3, KI, DMF at 100 °C (for 5a) or acetone at reflux (for 5b).
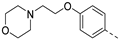
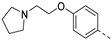
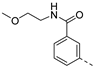
The saponification of the methyl ester group of compound 3k using lithium hydroxide in a mixture of tetrahydrofuran and water afforded the carboxylic acid 4 in excellent yield (94%). Compound 3l, bearing a 4-hydroxyphenyl moiety, was subjected to alkylation reactions with 1-(2-chloroethyl)pyrrolidine and 4-(2-chloroethyl)morpholine yielding compounds 5a (25%) and 5b (48%), respectively.
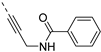
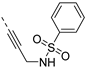
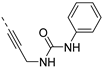
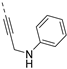
The synthesis of isothiazolo[4,3-b]pyridines with a 3,4-dimethoxyphenyl residue at position 6 and with structural modifications at position 3 is depicted in Scheme 2. Using similar reaction conditions as in Scheme 1, different alkynyl groups were introduced at position 3 of the scaffold, yielding a variety of 3-alkynyl substituted isothiazolo[4,3-b]pyridines 6a-y. Intermediate 8 was obtained using N-Boc-propargylamine as coupling partner in the Sonogashira reaction. The synthesis of compound 12 with an anilinopropargyl moiety at position 3 of the scaffold, was achieved by a Sonogashira coupling with N-(propargyl)aniline (which was prepared by alkylation of aniline with propargyl bromide, using potassium carbonate as base). Applying a regioselective nucleophilic aromatic substitution (with 3-(aminomethyl)pyridine) and Suzuki coupling (using 3-pyridylboronic acid) on compound 1b afforded the 3-substituted isothiazolo[4,3-b]pyridines 14 and 16, respectively. The 3-substituted-6-bromo-isothiazolo[4,3-b]pyridines 6a-y, 12, 14 and 16 were subjected to a Suzuki coupling with 3,4-dimethoxyphenylboronic acid yielding final compounds 7a-y, 13, 15 and 17. Intermediate 9 was obtained in a similar way. Deprotection of the tert-butoxycarbonyl (Boc) group using a 4M hydrogen chloride solution in 1,4-dioxane [27] afforded the propargylamino derivative 10. Coupling with benzoyl chloride, phenyl isocyanate and benzenesulfonyl chloride, using triethylamine as base, gave the corresponding amide 11a, urea 11b and sulfonamide 11c, respectively.
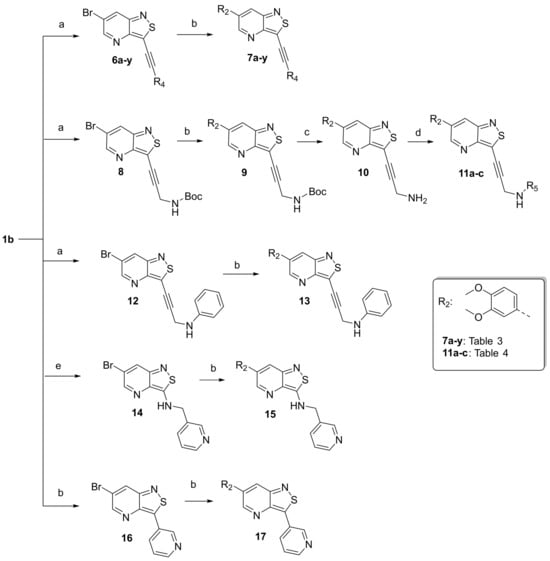
Scheme 2.
Synthesis of 3-substituted-6-(3,4-dimethoxyphenyl)-isothiazolo[4,3-b]pyridines. Reagents and conditions: (a) alkyne, CuI, Pd(PPh3)2Cl2, Et3N, THF, 30 °C; (b) boronic acid or ester, Pd(PPh3)4, K2CO3, dioxane/H2O (9:1), 90 °C, overnight; (c) HCl, dioxane, rt; (d) acid chloride or sulfonyl chloride or isocyanate, TEA, DCM, rt; (e) 3-(aminomethyl)pyridine, K2CO3, acetonitrile, reflux.
The exocyclic amino group of 3-amino-6-bromo-isothiazolo[4,3-b]pyridine 18 (synthesized according to a literature procedure) [25] was coupled with nicotinoyl chloride yielding amide 19. A Suzuki reaction furnished target compound 20 (Scheme 3).
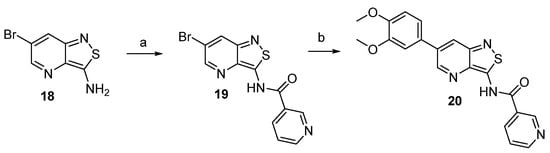
Scheme 3.
Synthesis of a 3-N-acyl-isothiazolo[4,3-b]pyridine. Reagents and conditions: (a) nicotinoyl chloride, Et3N, DCM, rt; (b) boronic acid, Pd(PPh3)4, K2CO3, dioxane/H2O, 90 °C, overnight.
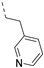
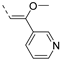
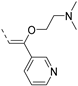
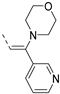
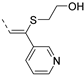
The insertion of structural modifications on the acetylenic moiety is shown in Scheme 4. The alkyne functionality of the known compound 21 (RMC-113) [28] was reduced by catalytic hydrogenation using palladium hydroxide and hydrogen gas at atmospheric pressure, yielding the corresponding alkane 22 in low yield (20%). The addition of various nucleophiles to the alkynyl moiety of compound 21 yielded compounds 23a-d. Theoretically, four possible isomers can be formed, arising from the attack of the nucleophile to one of the ethynyl carbons and the possible formation of (E)- or (Z)-diastereomers. However, one major product was isolated. In order to unequivocally identify the structure (and especially the regio- and stereochemistry of the alkenyl moiety), the information from a combination of one-dimensional (1H- and 13C-NMR) and two-dimensional (COSY, HSQC, HMBC and NOESY) NMR spectra was applied (Figure 3). Compound 23a was selected as a representative example, with the structures of the remaining compounds 23b-d being interpreted in analogy. From 1H-NMR, 13C-NMR, COSY and HSQC spectra, the formation of the ethenyl moiety was evident. The 1H-NMR spectrum of compound 23a showed a vinylic proton at δH = 7.37 ppm, whereas the 13C-NMR spectrum showed olefinic carbons at δC 104.07 ppm and 157.26 ppm. The exact position of the methoxy group on the double bond was confirmed by HMBC. The vinylic proton (δH = 7.37 ppm) showed long-range HMBC correlations to C-3 (δC = 156.32 ppm) and C-3a (δC = 144.29 ppm). Finally, in the NOESY spectrum of compound 23a, the signal at δH 7.37 ppm (assigned to the vinylic proton) showed correlations with δH 7.97 and 8.95 (both protons on the 3-pyridyl moiety), and hence the stereochemistry of the double bond was assigned as being the (Z)-diastereomer (Figure 4).
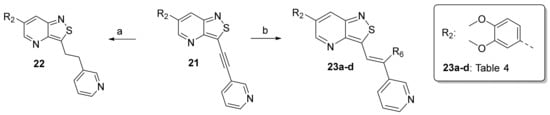
Scheme 4.
Structural modifications of the alkynyl linker. Reagents and conditions: (a) Pd(OH)2/C 20 wt%, EtOH/THF, 60 °C, 3 days; (b) methanol (for 23a), 2-(dimethylamino)ethan-1-ol (for 23b), t-BuOK, 80 °C or morpholine, 80 °C (for 23c) or 2-mercaptoethanol, K2CO3, dioxane, 80 °C (for 23d).
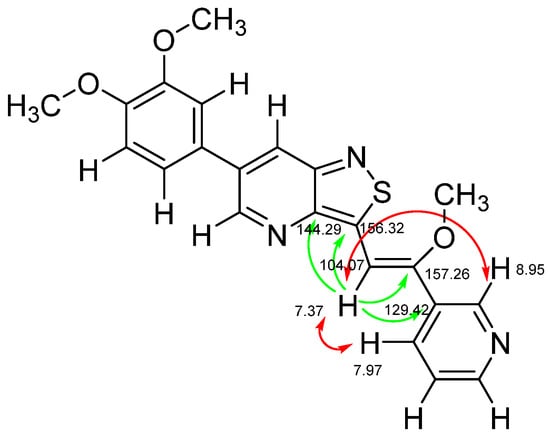
Figure 4.
Key chemical shifts, HMBC couplings and NOESY correlations in compound 23a (green arrows: HMBC couplings; red arrows: NOE correlation).
2.2. Enzymatic Assays
2.2.1. PIKfyve Inhibition
The primary assay guiding the medicinal chemistry was a biochemical PIKfyve assay, in which apilimod was included as positive control, yielding very low IC50 values (IC50 = 0.000786 µM), in agreement with literature values. RMC-113 (compound 21), the lead compound, was included as a reference molecule. At the start of the project, no information was available with respect to the importance of the 3,4-dimethoxyphenyl and pyridyl moiety for PIKfyve inhibition and, therefore, a first round of SAR focused on these two groups.
The 6-unsubstituted analogue 2a displayed greatly diminished potency on PIKfyve (IC50 = 1.15 µM), relative to RMC-113. Similarly, replacing the 3-pyridinyl moiety by an aliphatic n-propyl side chain afforded derivative 7a, with 100-fold reduced activity on PIKfyve (IC50 = 0.85 µM), relative to RMC-113 (Table 1). Consequently, the subsequent SAR study focused on the insertion of various (hetero)aryl groups at position 6 and various bulky groups at position 3.

Table 1.
Initial SAR of RMC-113.
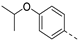
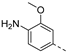
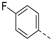
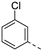
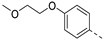
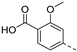
In a first round of SAR (Table 2), the focus was on the replacement of both dimethoxy groups by other electron-donating substituents, including a single methoxy group at various positions, such as in derivatives 3a and 3b, both exhibiting equipotency on PIKfyve as RMC-113. Similarly, the 2-methyl (compound 3c), is as active as RMC-113 as PIKfyve inhibitor (both having an IC50 value of 8 nM), whereas the 4-dimethylamino (compound 3d), and the 4-amino-3-methoxy (compound 3f) congeners are more potent PIKfyve inhibition than RMC-113. In contrast, the 4-isopropoxy analogue (compound 3e) was slightly less active (IC50 = 20 nM) than RMC-113 (IC50 = 8 nM). Since the presence of methoxy groups may impact metabolic stability, various halogens were introduced on the phenyl moiety. In addition, these halogens are also electron-withdrawing, allowing the study the importance of the electronics of the phenyl ring. The presence of a fluorine (compounds 3g and 3h) or a chlorine (compound 3i) was well tolerated, as evidenced by the good PIKfyve potency of the respective analogs. In order to address potential aqueous solubility issues for future in vivo animal studies, water solubilizing groups were introduced. The presence of a carboxylic acid on the phenyl group (compound 4) led to decreased PIKfyve inhibition (IC50 = 49 nM versus 8 nM for RMC-113). In contrast, insertion of an ethylene glycol side chain or basic amines yielded compounds 3i and 5a-b, respectively, that were even more potent than RMC-113, making this position ideally suited to manipulate physicochemical properties.

Table 2.
PIKfyve inhibition of 6-aryl-3-(pyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridines.
A carboxylic acid functions as a chemical handle that allows for easy introduction of structural variety and the convenient exploration of the SAR by the synthesis of a series of amides. Indeed, various carboxamides, either at the meta (compounds 3p-s) or para (compounds 3m-o) position demonstrated potent PIKfyve inhibition. Especially a carboxamide at position 4 (compound 3m) or a methoxyethylaminocarbonyl at position 3 (compound 3s) of the phenyl ring gave rise to very potent PIKfyve inhibitors.
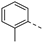
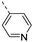
An aliphatic chain on the ethynyl group did not lead to potent PIKfyve inhibition (Table 1), and hence, we focused on various aromatic derivatives instead of the 3-pyridinyl group. The 3,4-dimethoxyphenyl group was selected as substituent at position 6 of the scaffold, since RMC-113 (compound 21) was already extensively biologically profiled [24], which allowed then for easier comparison. The synthesis of a phenyl analogue (compound 7b), halogenated phenyl derivatives (compounds 7c-e) as well as an anisole (compound 7f) was effected (Table 3). These compounds are 2-8-fold less potent on PIKfyve relative to RMC-113, pointing towards an essential role of the 3-pyridinyl group.

Table 3.
SAR of the pyridinyl moiety.
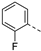
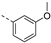
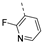
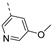
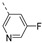
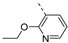
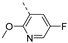
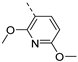
Therefore, subsequent efforts focused on the introduction of various small substituents (methyl, methoxy, ethoxy, fluorine, cyano, trifluoromethyl and COOCH3) on the 3-pyridinyl moiety, affording compounds 7g-v. The substitution pattern had a profound impact on PIKfyve inhibition. The presence of electron-withdrawing groups on the pyridine ring (at various positions) had a detrimental impact on PIKfyve inhibition (when compared to the unsubstituted analogue RMC-113), exemplified by the methylester analogue 7m (IC50 = 0.12 µM), the trifluoromethyl derivative 7n (IC50 = 0.68 µM), and especially the picolinonitrile analogue 7q which was completely devoid of PIKfyve inhibition (IC50 > 10 µM). Similarly, the insertion of a fluorine at different positions of the pyridinyl ring afforded compounds 7j, 7k and 7r that were less active as PIKfyve inhibitors than lead compound RMC-113.
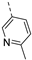
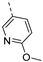
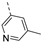
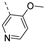
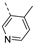
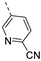
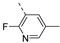
Compounds 7i and 7l emerged as the most potent PIKfyve inhibitors in this series (IC50 values of 2 and 3 nM, respectively), suggesting that the presence of an electron-donating group (methyl or methoxy) is beneficial for PIKfyve inhibition. The position of this substituent on the 3-pyridinyl ring is of paramount importance for PIKfyve inhibition. The 5-methoxy-3-pyridinyl analogue 7l (IC50 = 3 nM) is 200-fold more potent than the 4-methoxy-3-pyridinyl analogue 7h (IC50 = 0.59 µM) and 6-fold more potent than the 6-methoxy-3-pyridinyl analogue 7o (IC50 = 0.019 µM). Similarly, the 5-methyl-3-pyridinyl analogue 7i (IC50 = 0.002 µM) is more potent than the 4-methyl-3-pyridinyl and 6-methyl-3-pyridinyl congeners 7g and 7p, displaying IC50 values of 0.36 and 0.033 µM, respectively. In general, the insertion of substituents at position 2 of the 3-pyridinyl ring (yielding compounds 7s-u) was detrimental for PIKfyve inhibition, with IC50 values exceeding 100 nM. Only the 2-fluoro-5-methyl-3-pyridinyl analogue 7v displayed potent PIKfyve inhibitory activity with an IC50 value of 42 nM.
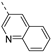
Fusing the pyridinyl group with a phenyl ring yielded the quinoline analogue 7w (IC50 = 5.69 µM), with 700-fold less potency on PIKfyve relative to RMC-113. This suggested that increasing the steric bulk at this position was not tolerated for PIKfyve inhibition. Moving the nitrogen around in the aromatic ring yielded the 4-pyridinyl analogue 7x and the 2-pyridinyl analogue 7y that both displayed potent PIKfyve inhibition, albeit less active than the corresponding 3-pyridinyl congener.
To probe the importance of the alkynyl linker for PIKfyve inhibition, various modifications were made. The acetylene moiety is a rigid linker, which might negatively impact aqueous solubility. Also, since this triple bond reacts with various nucleophiles (Scheme 4), it might lead to irreversible inhibition of proteins. Altogether, this spurred us to explore the SAR of the alkynyl moiety (Table 4). Deletion of the triple bond yielded the 3-(pyridin-3-yl)-isothiazolo[4,3-b]pyridine analogue 17, having greatly reduced PIKfyve potency (IC50 = 0.602 µM), relative to RMC-113. Replacing the triple bond with an aminomethylene or amide linker furnished compounds 15 and 20, respectively, that were practically inactive. The presence of a fully saturated ethyl linker yielded compound 22 that maintained PIKfyve inhibition, albeit less potent than its acetylenic congener RMC-113, with an IC50 value of 33 nM. Furthermore, the addition of oxygen-, nitrogen- and sulfur-containing nucleophiles to the triple bond afforded alkenyl analogues (compounds 23a-d) with diminished PIKfyve inhibition, relative to the alkynyl derivative RMC-113. Overall, these data revealed a clear preference for an ethynyl linker for PIKfvye inhibition. Therefore, a propargylamine was introduced, since it retained the alkynyl linker, but allowed for easy structural variation of the terminal amino functionality. Conversion of this amino group into a carboxamide (compound 11a), a sulfonamide (compound 11b), a urea (compound 11c) or an aniline (compound 13) yielded derivatives that were less potent on PIKfvye than RMC-113, with IC50 values between 0.18 and 10 µM.

Table 4.
SAR of the alkynyl linker.
2.2.2. PIP5K2C Binding Assay
Due the low enzymatic activity of PIP4K2C [29], a classical biochemical enzymatic assay to screen for inhibitors is not trivial, and therefore a binding assay was used. Compound 21 (RMC-113), known for its dual activity as PIP4K2C binder (KD = 0.046 µM) and PIKfyve inhibitor (IC50 = 0.008 µM) was included as a reference (Table 5) [24]. From the newly synthesized isothiazolo[4,3-b]pyridines, various potent PIKfvye inhibitors were selected with structural variation of the dimethoxyphenyl moiety, with either electron-donating (compounds 3a and 3f) or electron-withdrawing (compounds 3i, 3m, 4 and 3s) groups. In addition, a few representatives from the analogues that were prepared to probe the SAR of the pyridinyl ring were also selected. Since in this series, there is more variation in the SAR, compounds were selected that are potent PIKfyve inhibitors (compounds 7e, 7k, 7l, 7o and 7r), have intermediate PIKfyve inhibitory activity (compound 7w) or completely lack PIKfyve inhibition (compound 7q).

Table 5.
PIP4K2C affinity and PIKfyve inhibition of selected isothiazolo[4,3-b]pyridines.
Compounds that lacked (compound 7q) or showed moderate (compound 7w) PIKfyve inhibition, were also devoid of PIP4K2C binding affinity (KD values of 30 µM and 2 µM, respectively). All other compounds evaluated were potent PIKfyve inhibitors (with IC50 values in the range of 1.6 to 98 nM) that showed a 2- to 5-fold loss in potency in the PIP4K2C assay (when compared to the PIKfyve data), with KD values in range of 30 to 110 nM.
2.3. Cellular Assays
PIKfyve emerged as a promising drug target in various diseases, including virology and oncology. In addition, we recently validated PIP4K2C as a cellular target for the development of antiviral agents [24], although its role as drug target in oncology is less clear. Therefore, a representative selection of the newly synthesized PIKfyve inhibitors was evaluated for antiviral and antitumoral activity.
2.3.1. Antiviral Activity
PIKfyve inhibitors decrease endocytic trafficking of various viruses (such as filoviruses and coronaviruses) and hence PIKfyve inhibition has been proposed as a broad-spectrum antiviral strategy [13]. PIP4K2C regulates viral entry, viral RNA replication and viral assembly/egress [24]. The lead compound RMC-113 has been shown to display antiviral activity against SARS-CoV-2, the vaccine strain of VEEV (TC-83), dengue virus 2, Ebola virus and Marburg virus [24]. As part of the current SAR study, the antiviral profiling was limited to two unrelated viruses: SARS-CoV-2, a beta-coronavirus, and the venezuelan equine encephalitis virus (VEEV), an alphavirus belonging to the family of Togaviridae. In order to demonstrate a selective antiviral effect, compounds were also evaluated for potential cytotoxicity against U-87 MG and VeroE6/TMPRS2 cells, that were used for VEEV and SARS-CoV-2 screening, respectively. RMC-113 showed potent antiviral activity against SARS-CoV-2 and VEEV (vaccine strain, TC-83) with low µM EC50 values for both viruses and without apparent cytotoxicity, in agreement with previous studies (Table 6) [24]. Although apilimod is a very potent PIKfyve inhibitor, this did not translate in improved antiviral efficacy, when compared to RMC-113. A selection of the newly synthesized isothiazolo[4,3-b]pyridines, with either structural variations on the phenyl ring at position 6 (compounds 3a, 3h, 3f, 3m, 4, 5a-b and 3s) or on the pyridinyl ring (compounds 7e-f, 7k-m, 7o-p, 7r and 7w) was investigated for antiviral activity. The majority of the compounds demonstrated a comparable antiviral profile (i.e., active against both viruses without displaying cytotoxicity) as RMC-113. Although compounds 3m, 3s and 7l are more potent PIKfyve inhibitors than RMC-113, they still have a similar antiviral activity, compared to RMC-113. Compound 7w was the least active PIKfvye inhibitor (IC50 = 5.69 µM) that was studied for its antiviral activity. It was less potent against VEEV (EC50 = 2.08 µM) than RMC-113, whereas in the SARS-CoV-2 screening, only cytotoxicity was observed. Overall, there is no clear correlation between enzymatic potency and antiviral activity. This might be due to the fact that a cellular antiviral effect is the result of various factors, including solubility, permeability, efflux, stability, and interaction with various targets.

Table 6.
Antiviral activity of selected isothiazolo[4,3-b]pyridines.
RMC-113 has been shown to have activity against viruses from different families (i.e., Coronaviridae, Togaviridae, Filoviridae, Flaviviridae) and, hence, can be designated as a broad-spectrum antiviral agent. The newly synthesized analogues are very close analogues of RMC-113 and therefore, most likely, these are also candidates for the development of broad-spectrum antiviral agents.
2.3.2. Antitumoral Activity
PIKfyve inhibitors are known to inhibit the proliferation of various types of cancer cells [30]. The role of PIP5K2C in cellular proliferation has not been investigated yet. Therefore, a selection of dual PIKfyve/PIP5K2C inhibitors was screened for antitumoral activity against a panel of cancer cell lines, representing solid (Capan-1: pancreatic ductal adenocarcinoma cell line; HCT-116: colon cancer cell line; LN-229: human glioblastoma cell line; NCI-H460: human non-small-cell lung cell line) and hematological (DND-41: T-cell leukemia cell line; HL-60: acute myeloid leukaemia cell line; K-562: chronic myelogenous leukemia cell line; Z-138: B-cell acute lymphoblastic leukemia cell line) cancers. Various known PIKfyve inhibitors (apilimod, vacuolin-1 and YM-201636) were included as controls (Table 7). In line with prior reports, apilimod shows potent antiproliferative activity against all cell lines investigated [12], whereas vacuolin-1 and YM-201636 showed reduced activity [31]. The lead compound 21 (RMC-113) showed low micromolar activity against all cancer cell lines tested. Potent PIKfyve inhibitors with structural modifications of the dimethoxyphenyl moiety (such as compounds 3g, 3b and 3m) showed a comparable antitumoral effect to RMC-113. In contrast, potent PIKfyve inhibitors harboring structural variations on the pyridinyl moiety showed lower (compounds 7l) or no (compounds 7i and 7p) antitumoral activity. These findings suggest that other factors, besides PIKfvye and/or PIP5K2C, also play a role in the anticancer activity of this compound class.

Table 7.
Antiproliferative activity of selected isothiazolo[4,3-b]pyridines.
3. Materials and Methods
- General
For all reactions, analytical grade solvents were used. Argon was used to carry out reactions under an inert atmosphere. Melting points were recorded with a Stuart SMP20 melting point apparatus. 1H and 13C NMR spectra were recorded on a Bruker Avance 300 MHz instrument (1H NMR, 300 MHz; 13C NMR, 75 MHz), 500 MHz instrument (1H NMR, 500 MHz; 13C NMR, 125 MHz) or a 600 MHz instrument (1H NMR, 600 MHz; 13C NMR, 150 MHz), using tetramethylsilane as internal standard for 1H NMR spectra and DMSO-d6 (39.5 ppm) or CDCl3 (77.2 ppm) for 13C NMR spectra. Abbreviations used are s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, b = broad. Coupling constants are expressed in Hz. High-resolution mass spectra were acquired on a quadrupole orthogonal acceleration time-of-flight mass spectrometer (Synapt G2 HDMS, Waters, Milford, MA, USA). Samples were infused at 3 mL/min and spectra were obtained in positive or negative ionization mode with a resolution of 15,000 (FWHM) using leucine enkephalin as lock mass. Precoated aluminum sheets (Fluka silica gel/TLC-cards, 254 nm) were used for TLC. Column chromatography was performed on silica gel 0.060–0.200 mm, 60 (Acros Organics). The ratio or percentage of solvents in the mobile phase is indicated as (v/v) or percentage (%), respectively. The elution procedure is indicated as percentage at starting point, percentage of end point and running time when a gradient is applied.
- 3-Amino-2-cyanopyridine
- This compound was prepared from 3-nitro-2-cyanopyridine (436 mg, 2.9 mmol, 1.0 eq.) and iron powder (810 mg, 14.5 mmol, 5 eq.) in acetic acid (10 mL) using a previously described protocol [25]. The title compound was isolated without further purification as a light-brown solid in 90% yield (313 mg, 2.63 mmol).
- 1H NMR (400 MHz, DMSO-d6) δ: 7.86 (dd, J = 4.3, 1.4 Hz, 1H), 7.31 (dd, J = 8.6, 4.3 Hz, 1H), 7.21 (dd, J = 8.6, 1.5 Hz, 1H), 6.25 (s, 2H).
- 13C NMR (101 MHz, DMSO-d6) δ: 148.8, 139.0, 128.1, 123.1, 117.0, 114.5.
- HRMS m/z [M+H]+ calcd for C6H5N3 120.0556, found 120.0563.
- 3-Aminopyridine-2-carbothioamide
- This compound was prepared from 3-amino-2-cyanopyridine (300 mg, 2.5 mmol, 1.0 eq.) and phosphorus pentasulfide (2.22 g, 5 mmol, 2 eq.) in ethanol (10 mL) using a previously described protocol [25]. The crude mixture was purified by silica gel chromatography (PE/EtOAc, 8:2), yielding the title compound as a light-brown solid in 65% yield (252 mg, 1.64 mmol).
- 1H NMR (400 MHz, CDCl3) δ: 9.57 (s, 1H), 7.87 (dd, J = 4.1, 1.5 Hz, 1H), 7.26 (s, 1H), 7.19 (dd, J = 8.4, 4.1 Hz, 1H), 7.07 (dd, J = 8.4, 1.4 Hz, 1H), 6.86 (s, 2H).
- 13C NMR (101 MHz, CDCl3) δ: 195.2, 146.8, 136.2, 129.9, 128.1, 126.9.
- HRMS m/z [M+H]+ calcd for C6H7N3S 154.0433, found 154.0439.
- Isothiazolo[4,3-b]pyridin-3-amine
- This compound was prepared from 3-aminopyridine-2-carbothioamide (237 mg, 1.55 mmol, 1.0 eq.) and an 30% aqueous hydrogenperoxide solution (310 μL, 3.1 mmol, 2 eq.) in methanol (5 mL) using a previously described protocol [25]. The title compound was isolated without further purification as a dark-yellow solid in 82% yield (192 mg, 1.27 mmol).
- 1H NMR (400 MHz, DMSO-d6) δ: 8.28 (dd, J = 3.8, 1.4 Hz, 1H), 7.83 (s, 2H), 7.69 (dd, J = 9.0, 1.4 Hz, 1H), 7.24 (dd, J = 9.0, 3.8 Hz, 1H).
- 13C NMR (101 MHz, DMSO-d6) δ: 172.4, 153.6, 143.4, 134.8, 127.9, 123.6.
- HRMS m/z [M+H]+ calcd for C6H5N3S 152.0277, found 152.0278.
- 3-Bromoisothiazolo[4,3-b]pyridine (1a)
- Isothiazolo[4,3-b]pyridin-3-amine (170 mg, 1.12 mmol, 1.0 eq.) was dissolved in 48% aqueous HBr (17 mL) and stirred for 10 min at room temperature. CuBr (323 mg, 2.25 mmol, 2 eq.) was added and the mixture was cooled to 0 °C. An aqueous solution (8.5 mL) of NaNO2 (233 mg, 3.37 mmol, 3 eq.) was added dropwise (0.5 mL min−1). The reaction mixture was stirred for 2 h at 0 °C then overnight at room temperature. The mixture was cooled to 0 °C, neutralized with a 2M NaOH solution and extracted with EtOAc (30 mL) three times. The combined organic layers were washed with brine (30mL) and dried over Na2SO4. The solvent was removed in vacuo and the crude was purified by silica gel chromatography (PE/EtOAc, 8:2) yielding the title compound as a yellow solid in 61% yield (147 mg, 0.68 mmol).
- 1H NMR (400 MHz, CDCl3) δ: 8.87 (dd, J = 3.9, 1.5 Hz, 1H), 8.12 (dd, J = 9.0, 1.5 Hz, 1H), 7.40 (dd, J = 9.0, 3.8 Hz, 1H).
- 13C NMR (101 MHz, CDCl3) δ: 154.9, 152.2, 146.5, 135.8, 130.0, 123.6.
- HRMS m/z [M+H]+ calcd for C6H3BrN2S 214.9274, found 214.9285.
- Sonogashira coupling at position 3 of the isothiazolo[4,3-b]pyridine scaffold
- General procedure
- A solution of 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (1 eq.) [25] and triethylamine (3 eq.) in THF, was degassed with a flow of argon for 5 min. Then, Pd(PPh3)2Cl2 (0.02 eq.) and CuI (0.01 eq.) were added and the reaction mixture was allowed to reach 30 °C. Subsequently, a solution of the appropriate acetylene in THF was added slowly over a period of 30 min. The reaction was degassed a second time, filled with argon and stirred at 30 °C overnight. After disappearance of the starting material as monitored by TLC, the volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography. Compounds 2a-b and 6a-y were made according to this procedure.
- 3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (2a)
- This compound was prepared from 3-bromoisothiazolo[4,3-b]pyridine 1a (60 mg, 0.28 mmol, 1.0 eq.) and 3-ethynylpyridine (86 mg, 0.84 mmol, 3 eq.), Pd(PPh3)2Cl2 (4 mg, 0.007 mmol, 0.025 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude mixture was purified by silica gel chromatography (PE/EtOAc, 7:3), yielding the title compound as a brown solid in 80% yield (53 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 8.91 (s, 1H), 8.89 (dd, J = 3.9, 1.5 Hz, 1H), 8.64 (dd, J = 4.9, 1.7 Hz, 1H), 8.18 (dd, J = 8.9, 1.5 Hz, 1H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H), 7.43 (dd, J = 9.0, 3.8 Hz, 1H), 7.36 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H).
- 13C NMR (75 MHz, CDCl3) δ: 154.93, 152.29, 151.92, 149.77, 148.84, 143.61, 138.71, 130.07, 123.44, 123.14, 119.24, 104.15, 80.01.
- HRMS m/z [M+H]+ calcd for C13H7N3S 238.0433, found 238.0440.
- 6-Bromo-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (2b)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light-yellow solid in 67% yield (72 mg, 0.23 mmol). Spectral data are in agreement with literature [28].
- 1H NMR (600 MHz, CDCl3) δ: 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.94–7.98 (m, 1H, arom H), 8.36 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.84 (d, J = 2.1 Hz, 1H, arom H), 8.90 (d, J = 1.5 Hz, 1H, arom H).
- HR-MS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9533.
- 6-Bromo-3-(pent-1-yn-1-yl)isothiazolo[4,3-b]pyridine (6a)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), pent-1-yne (69 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 71% yield (67.7 mg, 0.24 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 1.10 (t, J = 7.4 Hz, 3H, CH3), 1.75 (h, J = 7.2 Hz, 2H, CH2), 2.65 (t, J = 7.1 Hz, 2H, CH2), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.77 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C11H9BrN2S 280.9743, found 280.9744.
- 6-Bromo-3-(phenylethynyl)isothiazolo[4,3-b]pyridine (6b)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), ethynylbenzene (104 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 81% (87 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 8.82 (d, J = 2.0 Hz, 1H), 8.33 (d, J = 2.0 Hz, 1H), 7.67 (m, 2H), 7.43 (m, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 154.90, 152.47, 146.71, 145.93, 131.96, 131.28, 129.89, 128.54, 121.68, 120.83, 103.01 ppm.
- 6-Bromo-3-((4-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6c)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-4-fluorobenzene (122 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 89% yield (100.5 mg, 0.3 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.07–7.16 (m, 2H, arom H), 7.62–7.71 (m, 2H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.82 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrFN2S 332.9492, found 332.9483.
- 6-Bromo-3-((3-chlorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6d)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-3-chlorobenzene (139 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a light-yellow solid in 83% yield (98.6 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.31–7.44 (m, 2H, arom H) 7.52–7.58 (m, 1H, arom H), 7.65–7.68 (m, 1H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrClN2S 348.9197, found 348.9206.
- 6-Bromo-3-((2-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6e)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-2-fluorobenzene (123 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 78% yield (88.3 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.12–7.24 (m, 2H, arom H), 7.37–7.48 (m, 1H, arom H), 7.59–7.70 (m, 1H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrFN2S 332.9492, found 332.9483.
- 6-Bromo-3-((3-methoxyphenyl)ethynyl)isothiazolo[4,3-b]pyridine (6f)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylanisole (135 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general p Sonogashira coupling rocedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a yellow solid in 77% yield (90.4 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.85 (s, 3H, OCH3), 6.99 (ddd, J = 8.0, 2.5, 1.4 Hz, 1H, arom H), 7.17–7.23 (m, 1H, arom H), 7.25–7.35 (m, 2H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H).
- HRMS m/z [M+H]+ calcd for C15H9BrN2OS 344.9693, found 344.9693.
- 6-Bromo-3-((6-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6g)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-methylpyridine (120 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and diethyl ether (in a ratio of 10:0.3) as mobile phase, affording the title compound as a yellow solid in 83% yield (93.2 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.61 (s, 3H, CH3), 7.22 (d, J = 8.1 Hz, 1H, arom H), 7.84 (dd, J = 8.0, 2.1 Hz, 1H, arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.78 (d, J = 1.5 Hz 1H, arom H), 8.83 (d, J = 2.0 Hz, 1H, arom H) ppm.HRMS m/z [M+H]+ calcd for C14H8BrN3S 329.9696, found 329.9696.
- 6-Bromo-3-((6-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6h)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 64% yield (75.3 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.99 (s, 3H, OCH3), 6.79 (d, J = 8.6 Hz, 1H arom H), 7.82 (dd, J = 2.3, 8.6 Hz, 1H, arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.49 (d, J = 2.0 Hz, 1H, arom H), 8.82 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3OS 345.9645, found 345.9651.
- 6-Bromo-3-((5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6i)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-methylpyridine (120 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 79% yield (88.7 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.39 (s, 3H, CH3), 7.77 (s, 1H arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.47 (s, 1H, arom H), 8.71 (s, 1H, arom H), 8.83 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3S 329.9696, found 329.9694.
- 6-Bromo-3-((6-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6j)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 68% yield (77.3 mg, 0.23 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.02 (dd, J = 2.9, 8.5 Hz, 1H arom H), 8.06 (dt, J = 2.3, 8.0 Hz, 1H, arom H), 8.36 (d, J = 2.0 Hz, 1H, arom H), 8.55 (d, J = 1.5 Hz, 1H, arom H), 8.84 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9446.
- 6-Bromo-3-((2-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6k)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), methyl 3-ethynyl-2-fluoropyridine (138 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a beige solid in 81% yield (92.1 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.26–7.31 (m, 1H, arom H), 8.03–8.12 (m, 1H, arom H), 8.28 (d, J = 4.4 Hz, 1H, arom H), 8.37 (d, J = 2.0 Hz, 1H, arom H), 8.85 (d, J = 1.9 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9445.
- 6-Bromo-3-((5-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6l)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 73% yield (85.9 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.91 (s, 3H, OCH3). 7.44 (bs, 1H, arom H), 8.36 (bs, 2H, arom H), 8.50 (s, 1H, arom H), 8.85 (d, J = 1.7 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3OS 345.9645, found 345.9646.
- Methyl 5-((6-bromoisothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (6m)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), methyl 5-ethynylnicotinate (165 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a beige solid in 65% yield (82.7 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 4.00 (s, 3H, COOCH3), 8.38 (d, J = 1.9 Hz, 1H, arom H), 8.57 (t, J = 1.8 Hz, 1H, arom H), 8.86 (d, J = 1.9 Hz, 1H, arom H), 9.04 (d, J = 1.8 Hz, 1H, arom H), 9.23 (d, J = 1.8 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C15H8BrN3O2S 373.9594, found 373.9589.
- 6-Bromo-3-((6-(trifluoromethyl)pyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6n)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-(trifluoromethyl)pyridine (175 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a light-yellow solid in 75% yield (98.0 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.76 (d, J = 8.1 Hz, 1H, arom H), 8.14 (d, J = 7.9 Hz, 1H, arom H), 8.38 (d, J = 1.8 Hz, 1H, arom H), 8.86 (d, J = 1.7 Hz, 1H, arom H), 8.98 (s, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H5BrF3N3S 383.9413, found 383.9413.
- 6-Bromo-3-((4-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6o)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-4-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as an orange solid in 71% yield (79 mg, 0.23 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.83 (d, J = 2.1 Hz, 1H), 8.75 (s, 1H), 8.55 (s, 1H), 8.34 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 5.4 Hz, 1H), 4.00 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 165.7, 154.9, 154.0, 152.8, 152.1, 147.0, 145.4, 131.4, 121.0, 102.1, 82.7, 56.1 ppm.
- 6-Bromo-3-((4-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6p)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynylpicolinonitrile (131 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 55% yield (59 mg, 0.18 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.84 (d, J = 2.1 Hz, 1H), 8.81 (s, 1H), 8.50 (d, J = 5.1 Hz, 1H), 8.35 (d, J = 2.1 Hz, 1H), 7.23 (d, J = 5.0 Hz, 1H), 2.59 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.0, 152.5, 149.9, 149.5, 147.1, 145.1, 131.4, 124.6, 121.1, 119.5, 104.1, 82.9, 20.5 ppm.
- 5-((6-Bromoisothiazolo[4,3-b]pyridin-3-yl)ethynyl)picolinonitrile (6q)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a light-brown solid in 53% yield (58 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.96 (d, J = 1.5 Hz, 1H), 8.87 (d, J = 1.9 Hz, 1H), 8.39 (d, J = 2.1 Hz, 1H), 8.09 (dd, J = 8.1, 2.1 Hz, 1H), 7.76 (dd, J = 8.1, 0.9 Hz, 1H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.5, 153.3, 147.3, 143.5, 139.6, 133.3, 131.6, 128.0, 122.6, 121.3, 116.8, 102.7, 83.4 ppm.
- 6-Bromo-3-((5-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6r)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as an off-white solid in 56% yield (60 mg, 0.18 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.85 (d, J = 2.1 Hz, 1H), 8.72 (s, 1H), 8.53 (d, J = 2.7 Hz, 1H), 8.37 (d, J = 2.1 Hz, 1H), 7.69 (ddd, J = 8.7, 2.8, 1.6 Hz, 1H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.2, 148.3 (d, J = 4.1 Hz), 147.2, 144.2, 139.1, 138.9, 131.6, 125.5, 125.3, 121.2, 103.2 (d, J = 2.5 Hz), 80.5 ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9454.
- 6 -Bromo-3-((2-ethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6s)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 2-ethoxy-3-ethynylpyridine (150 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 82% yield (101 mg, 0.28 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.82 (d, J = 2.1 Hz, 1H), 8.34 (d, J = 2.1 Hz, 1H), 8.20 (dd, J = 5.0, 2.0 Hz, 1H), 7.88 (dd, J = 7.4, 2.0 Hz, 1H), 6.92 (dd, J = 7.4, 5.0 Hz, 1H), 4.50 (q, J = 7.1 Hz, 2H), 1.48 (t, J = 7.1 Hz, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 163.5, 155.0, 152.7, 148.3, 147.0, 145.9, 142.0, 131.4, 121.0, 116.3, 106.2, 104.2, 81.4, 62.8, 14.7 ppm.
- HRMS m/z [M+H]+ calcd for C15H10BrN3OS 359.9801, found 359.9799.
- 6-Bromo-3-((5-fluoro-2-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6t)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-5-fluoro-2-methoxypyridine (290 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 78% yield (183 mg, 0.50 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.84 (d, J = 2.1 Hz, 1H), 8.35 (d, J = 2.1 Hz, 1H), 8.06 (d, J = 3.0 Hz, 1H), 7.66 (dd, J = 7.7, 3.0 Hz, 1H), 4.04 (s, 3H).
- 13C NMR (101 MHz, CDCl3) δ 159.9, 155.6, 154.9, 153.1, 152.9, 147.0, 144.8, 135.1, 134.8, 131.3, 129.2, 129.0, 120.9, 106.5, 106.4, 102.0, 101.9, 82.0, 54.7 ppm.
- HRMS m/z [M+H]+ calcd for C14H7BrFN3OS 363.9550, found 363.9571.
- 6-Bromo-3-((2,6-dimethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6u)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-2,6-dimethoxypyridine (313 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. This compound was obtained using 3-ethynyl-2,6-dimethoxypyridine. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as orange solid in 82% yield (200 mg, 0.53 mmol).
- 1H NMR (400 MHz, Chloroform-d) 8.80 (d, J = 2.1 Hz, 1H), 8.31 (d, J = 2.1 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 6.37 (d, J = 8.2 Hz, 1H), 4.06 (s, 3H), 3.98 (s, 3H).
- 13C NMR (101 MHz, CDCl3) 163.9, 163.4, 154.8, 152.2, 146.6, 146.4, 144.3, 131.2, 120.7, 105.1, 102.0, 96.4, 80.1, 54.2, 53.9.
- HRMS m/z [M+H]+ calcd for C15H10BrN3O2S 375.9750, found 375.9747.
- 6-Bromo-3-((2-fluoro-5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6v)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-2-fluoro-5-methylpyridine (259 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a light-brown solid in 91% yield (205 mg, 0.59 mmol).
- 1H NMR (400 MHz, Chloroform-d) 8.85 (d, J = 2.1 Hz, 1H), 8.36 (d, J = 2.1 Hz, 1H), 8.07 (dt, J = 2.1, 0.9 Hz, 1H), 7.88 (ddd, J = 8.6, 2.4, 0.8 Hz, 1H), 2.37 (s, 4H).
- 13C NMR (101 MHz, CDCl3) 162.0, 159.6, 154.9, 153.0, 148.3, 148.1, 147.1, 144.4, 144.0, 144.0, 131.4, 130.9, 130.9, 121.0, 105.5, 105.2, 99.9, 99.9, 82.1, 82.1, 17.3 ppm. HRMS m/z [M+H]+ calcd for C14H7BrFN3S 347.9601, found 347.9603.6-Bromo-3-(quinolin-3-ylethynyl)isothiazolo[4,3-b]pyridine (6w)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylquinoline (156 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 81% yield (100 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.62 (t, J = 7.5 Hz, 1H, arom H), 7.75–7.83 (m, 1H, arom H), 7.85 (d, J = 8.1 Hz, 1H, arom H), 8.14 (d, J = 8.5 Hz, 1H, arom H), 8.37 (d, J = 2.0 Hz, 1H, arom H), 8.49 (d, J = 1.9 Hz, 1H, arom H), 8.86 (d, J = 2.0 Hz, 1H, arom H), 9.11 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C17H8BrN3S 365.9696, found 365.9686.
- 6-Bromo-3-(pyridin-4-ylethynyl)isothiazolo[4,3-b]pyridine (6x)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 4-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a light-yellow solid in 59% yield (63 mg, 0.2 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.52 (dd, J = 4.5, 1.5 Hz, 2H, arom H), 8.37 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 5.9 Hz, 2H, arom H), 8.85 (d, J = 2.1 Hz, 1H, arom H) ppm. HRMS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9532.
- 6-Bromo-3-(pyridin-2-ylethynyl)isothiazolo[4,3-b]pyridine (6y)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 2-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light-yellow solid in 74% yield (79 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.30–7.38 (m, 1H, arom H), 7.68–7.80 (m, 2H, arom H), 8.35 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 4.8 Hz, 1H, arom H), 8.84 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9535.
- tert-Butyl (3-(6-bromoisothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)carbamate (8)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), N-Boc-propargylamine (158 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a light-orange solid in 89% yield (111.2 mg, 0.3 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 1.47 (s, 9H, 3 × CH3), 4.40 (d, J = 5.1 Hz, 2H, CH2), 4.93 (bs, 1H, NH), 8.32 (d, J = 2.0 Hz, 1H, arom H), 8.79 (d, J = 2.0 Hz, 1H, arom H) ppm. HR-MS m/z [M+H]+ calcd for C14H14BrN3O2S 368.0063, found 368.0065.
- Suzuki coupling at position 6 of the isothiazolo[4,3-b]pyridine scaffold
- General procedure
- A solution of the appropriate 3-substituted-6-bromoisothiazolo[4,3-b]pyridine analogue (1 eq.) in a mixture of dioxane/water (ratio 9:1) was degassed with argon and subsequently, the corresponding boronic acid or ester (1.2 eq.), Pd(PPh3)4 (0.02 eq.) and K2CO3 (2 eq.) were added. The mixture was degassed a second time, filled with argon and stirred at 90 °C overnight. After completion of the reaction as monitored by TLC, the reaction mixture was cooled down to room temperature and the volatiles were evaporated to dryness. The resulting residue was purified by silica gel flash chromatography and precipitated with diethyl ether yielding the title compounds. Compounds 3a-p, 7a-y were synthesized according to this procedure.
- 6-(3-Methoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3a)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-methoxyphenylboronic acid (29 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 69% yield (37.4 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.91 (s, 3H, OCH3), 7.03 (ddd, J = 8.3, 2.6, 0.8 Hz, 1H, arom H), 7.21–7.23 (m, 1H, arom H), 7.28–7.30 (m, 1H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.45–7.49 (m, 1H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.27 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.7, 1.1 Hz, 1H, arom H), 8.93 (d, J = 0.9 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.41 (OCH3), 80.02 (C triple bond), 104.15 (C triple bond), 113.39 (CH), 114.19 (CH), 119.23 (C), 119.96 (CH), 123.13 (CH), 126.44 (CH), 130.45 (CH), 136.44 (C), 138.12 (C), 138.69 (CH), 143.46 (C), 148.02 (C), 149.81 (CH), 152.33 (CH), 152.44 (CH), 155.12 (C), 160.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H13N3OS 344.0852, found 344.0835.
- 6-(2-Methoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3b)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-methoxyphenylboronic acid (29 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 74% yield (40.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.86 (s, 3H, OCH3), 7.06 (d, J = 8.2 Hz, 1H, arom H), 7.12 (td, J = 7.5, 0.9 Hz, 1H, arom H), 7.36 (ddd, J = 7.9, 5.0, 0.5 Hz, 1H, arom H), 7.41–7.48 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.64 (d, J = 3.7 Hz, 1H, arom H), 8.92 (s, 1H, arom H), 9.09 (d, J = 1.9 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.53 (OCH3), 80.16 (C triple bond), 103.74 (C triple bond), 111.37 (CH), 119.33 (C), 121.32 (CH), 123.11 (CH), 125.95 (C), 128.57 (CH), 130.46 (CH), 130.85 (CH), 134.75 (C), 138.68 (CH), 142.84 (C), 147.40 (C), 149.71 (CH), 152.32 (CH), 154.54 (CH), 155.34 (C), 156.72 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H13N3OS 344.0852, found 344.0875.
- 3-(Pyridin-3-ylethynyl)-6-(2-tolyl)isothiazolo[4,3-b]pyridine (3c)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-tolylboronic acid (26 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 81% yield (41.8 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.35 (s, 3H, OCH3), 7.29–7.42 (m, 5H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.08 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.89 (d, J = 2.0 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H) ppm. 13C NMR (150 MHz, CDCl3) δ: 20.42 (CH3), 79.99 (C triple bond), 104.13 (C triple bond), 119.20 (C), 123.12 (CH), 126.38 (CH), 128.66 (CH), 128.79 (CH), 129.93 (CH), 130.84 (CH), 135.74 (C), 136.82 (C), 137.57 (C), 138.69 (CH), 143.44 (C), 147.60 (C), 149.78 (CH), 152.30 (CH), 153.76 (CH), 154.87 (C) ppm.
- HR-MS m/z [M+H]+ calcd for C20H13N3S 328.0903, found 328.0912.
- N,N-Dimethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)aniline (3d)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(dimethylamino)phenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase and a second time using dichloromethane and methanol (10:0.2), affording the title compound as an orange solid in 74% yield (41.7 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.07 (s, 6H, 2 ࠹NCH3), 6.84–6.91 (m, 2H, arom H), 7.38 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.62–7.68 (m, 2H, arom H), 7.95–8.03 (m, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.2 Hz, 1H, arom H), 8.94 (s, 1H, arom H), 9.19 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 40.28 (CH3), 80.26 (C triple bond), 103.64 (C triple bond), 112.74 (CH), 119.37 (C), 123.11 (CH), 123.63 (CH), 123.71 (C), 128.24 (CH), 136.49 (C), 138.68 (CH), 142.71 (C), 147.28 (C), 149.68 (CH), 150.86 (C), 152.31 (CH), 152.73 (CH), 155.72 (C) ppm.
- HR-MS m/z [M+H]+ calcd for C21H16N4S 357.1168, found 357.1167.
- 6-(4-Isopropoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3e)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-isopropoxyphenylboronic acid (34 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (47.5 mg, 0.13 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 1.40 (s, 3H, CH3), 1.42 (s, 3H, CH3), 4.62–4.70 (hept, J = 6.0 Hz, 1H, CH), 7.06 (d, J = 8.7 Hz, 2H, arom H), 7.38 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.65 (d, J = 8.7 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.7 Hz, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.66 (d, J = 4.8 Hz, 1H, arom H), 8.94 (s, 1H, arom H), 9.16 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 22.03 (CH3), 70.10 (CH), 80.15 (C triple bond), 103.99 (C triple bond), 116.57 (CH), 119.32 (C), 123.18 (CH), 125.16 (CH), 128.61 (C), 128.80 (CH), 136.24 (C), 138.76 (CH), 143.18 (C), 147.62 (C), 149.80 (CH), 152.34 (CH), 152.57 (CH), 155.40 (C), 158.84 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3OS 372.1165, found 372.1155.
- 2-Methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)aniline (3f)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-amino-3-methoxyphenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 10:0.1) as mobile phase and a second time using dichloromethane and methanol (10:0.1), affording the title compound as a light yellow solid in 77% yield (43.6 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 4.08 (bs, 2H, NH2), 6.84 (d, J = 8.0 Hz, 1H, arom H), 7.11 (d, J = 1.9 Hz, 1H, arom H), 7.18 (dd, J = 8.0, 1.9 Hz, 1H, arom H), 7.36 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.59 (OCH3), 80.18 (C triple bond), 103.77 (C triple bond), 109.25 (CH), 114.97 (CH), 119.30 (C), 120.60 (CH), 123.10 (CH), 124.28 (CH), 126.38 (C), 136.77 (C), 137.51 (C), 138.66 (CH), 142.87 (C), 147.41 (C), 147.67 (C), 149.70 (CH), 152.29 (CH), 152.69 (CH), 155.52 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H14N4OS 359.0961, found 359.0965.
- 6-(4-Fluorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3g)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-fluorophenylboronic acid (27 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 81% yield (42.4 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.22–7.28 (m, 2H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.65–7.71 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H), 9.11 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.95 (C triple bond), 104.27 (C triple bond), 116.48 (d, J = 21.8 Hz, CH), 119.20 (C), 123.24 (CH), 126.25 (CH), 129.36 (d, J = 8.3 Hz, CH), 132.85 (C), 135.57 (C), 138.70 (CH), 143.62 (C), 147.90 (C), 149.85 (CH), 152.12 (CH), 152.33 (CH), 155.00 (C), 163.36 (d, J = 249.6 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: -112.426 ppm.
- HRMS m/z [M+H]+ calcd for C19H10FN3S 332.0652, found 332.0652.
- 6-(2-Fluorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3h)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-fluorophenylboronic acid (27 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light-yellow solid in 72% yield (37.6 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.24–7.28 (m, 1H, arom H), 7.33 (td, J = 7.5, 1.1 Hz, 1H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.44–7.49 (m, 1H, arom H), 7.56 (td, J = 7.7, 1.7 Hz, 1H, arom H), 7.96–8.00 (m, 1H, arom H), 8.29 (dd, J = 2.0, 0.9 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92–8.94 (d, J = 1.5 Hz, 1H, arom H), 9.08 (t, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.95 (C triple bond), 104.22 (C triple bond), 116.57 (d, J = 22.2 Hz, CH), 119.21 (C), 123.13 (CH), 124.64 (d, J = 13.5 Hz, C), 125.01 (d, J = 3.3 Hz, CH), 128.87 (d, J = 2.0 Hz, CH), 130.75 (d, J = 1.8 Hz, CH), 130.76 (d, J = 8.2 Hz, CH), 131.69 (C), 138.71 (CH), 143.59 (C), 147.84 (C), 149.82 (CH), 152.32 (CH), 152.98 (d, J = 3.5 Hz, CH), 154.82 (C), 159.96 (d, J = 249.6 Hz, C-F) ppm. 19F NMR (471 MHz, CDCl3) δ: −116.62 ppm.
- HRMS m/z [M+H]+ calcd for C19H10FN3S 332.06521, found 332.0646.
- 6-(3-Chlorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3i)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-chlorophenylboronic acid (30 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 79% yield (43.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.36–7.39 (m, 1H, arom H), 7.44–7.51 (m, 2H, arom H), 7.58 (dt, J = 7.2, 1.6 Hz, 1H, arom H), 7.69 (t, J = 1.6 Hz, 1H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.26 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.10 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.90 (C triple bond), 104.39 (C triple bond), 119.16 (C), 123.15 (CH), 125.75 (CH), 126.78 (CH), 127.67 (CH), 128.98 (CH), 130.62 (CH), 135.19 (C), 135.35 (C), 138.56 (C), 138.71 (CH), 143.79 (C), 148.16 (C), 149.86 (CH), 151.80 (CH), 152.32 (CH), 154.81 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H10ClN3S 348.0357, found 348.0362.
- 6-(4-(2-Methoxyethoxy)phenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3j)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(2-methoxyethoxy)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a light yellow solid in 79% yield (48.4 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.48 (s, 3H, OCH3), 3.76–3.83 (m, 2H, OCH2), 4.18–4.22 (m, 2H, OCH2), 7.10 (d, J = 8.7 Hz, 2H, arom H), 7.36 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.64 (d, J = 8.7 Hz, 2H, arom H), 7.97 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.19 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.4 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.13 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 59.31 (OCH3), 67.48 (OCH2), 70.95 (OCH2), 80.15 (C triple bond), 104.01 (C triple bond), 115.53 (CH), 119.29 (C), 123.17 (CH), 125.27 (CH), 128.74 (CH), 129.16 (C), 136.10 (C), 138.73 (CH), 143.20 (C), 147.64 (C), 149.80 (CH), 152.34 (CH), 152.48 (CH), 155.33 (C), 159.65 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1115.
- Methyl 2-methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzoate (3k)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-methoxy-4-methoxycarbonylphenylboronic acid (40 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure and DMF as solvent. The reaction mixture was stirred at 120 °C overnight. The residue was purified by precipitation using subsequently, methanol, dichloromethane and diethyl ether, affording the title compound as a light yellow solid in 75% yield (47.6 mg, 0.12 mmol).
- 1H NMR (300 MHz, DMSO) δ: 3.83 (s, 1H, OCH3), 3.98 (s, 1H, OCH3), 7.53–7.61 (m, 2H, arom H), 7.65 (s, 1H, arom H), 7.81 (d, J = 8.0 Hz, 1H, arom H), 8.12–8.18 (m, 1H, arom H), 8.70 (d, J = 4.8 Hz, 1H, arom H), 8.73 (d, J = 1.7 Hz, 1H, arom H), 8.91 (s, 1H, arom H), 9.34 (d, J = 1.7 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C22H15N3O3S 402.0907, found 402.0903.
- 4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenol (3l)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-hydroxyphenylboronic acid (26 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (42.1 mg, 0.13 mmol).
- 1H NMR (500 MHz, DMSO) δ: 6.92–6.97 (m, 2H, arom H), 7.55 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.75–7.80 (m, 2H, arom H), 8.14 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.40 (d, J = 2.1 Hz, 1H, arom H), 8.69 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.90 (d, J = 2.0 Hz, 1H, arom H), 9.25 (d, J = 2.0 Hz, 1H, arom H), 9.89 (s, 1H, OH) ppm.
- 13C NMR (126 MHz, DMSO) δ: 80.34 (C triple bond), 103.59 (C triple bond), 116.24 (CH), 118.45 (C), 123.84 (CH), 123.92 (CH), 126.45 (C), 129.04 (CH), 135.75 (C), 138.88 (CH), 142.00 (C), 147.19 (C), 150.24 (CH), 151.67 (CH), 152.61 (CH), 155.16 (C), 158.65 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H11N3OS 330.0696, found 330.0685.
- 4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3m)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-aminocarbonylphenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and methanol (in a ratio of 10:0.3) as mobile phase, affording the title compound as a beige solid in 78% yield (44.3 mg, 0.12 mmol).
- 1H NMR (600 MHz, DMSO) δ: 7.50 (s, 1H, NH), 7.57 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 8.03–8.08 (m, 4H), 8.12 (s, 1H, NH), 8.15–8.18 (m, 1H, arom H), 8.66 (d, J = 2.1 Hz, 1H, arom H), 8.70 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92 (dd, J = 2.1, 0.7 Hz, 1H, arom H), 9.34 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 80.25 (C triple bond), 103.95 (C triple bond), 118.42 (C), 123.96 (CH), 126.40 (CH), 127.64 (CH), 128.42 (CH), 134.47 (C), 134.92 (C), 138.64 (C), 138.93 (CH), 142.54 (C), 147.90 (C), 150.32 (CH), 151.71 (CH), 152.38 (CH), 154.76 (C), 167.35 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H12N4OS 357.0805, found 357.0790.
- N-Ethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3n)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(N-ethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 75% yield (45.5 mg, 0.12 mmol).1H NMR (600 MHz, CDCl3) δ: 1.30 (t, J = 7.3 Hz, 3H, CH3), 3.51–3.56 (m, 2H, CH2), 6.26 (t, J = 5.1 Hz, 1H, NH), 7.38 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.75–7.79 (m, 2H, arom H), 7.93–7.97 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.30 (d, J = 2.1 Hz, 1H, arom H), 8.65 (d, J = 4.0 Hz, 1H, arom H), 8.93 (s, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 14.90 (CH3), 35.06 (CH2), 79.91 (C triple bond), 104.39 (C triple bond), 119.17 (C), 123.19 (CH), 126.82 (CH), 127.71 (CH), 127.91 (CH), 135.07 (C), 135.43 (C), 138.71 (CH), 139.55 (C), 143.77 (C), 148.17 (C), 149.85 (CH), 151.86 (CH), 152.30 (CH), 154.85 (C), 166.59 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1112.
- N,N-Dimethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3o)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(N,N-dimethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 79% yield (48.0 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.06 (s, 3H, CH3), 3.16 (s, 3H, CH3), 7.37 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.61 (d, J = 8.2 Hz, 2H, arom H), 7.75 (d, J = 8.2 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.29 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.39 (CH3), 39.56 (CH3), 79.91 (C triple bond), 104.32 (C triple bond), 119.16 (C), 123.14 (CH), 126.66 (CH), 127.58 (CH), 128.15 (CH), 135.69 (C), 136.82 (C), 137.85 (C), 138.69 (CH), 143.68 (C), 148.09 (C), 149.83 (CH), 151.99 (CH), 152.29 (CH), 154.91 (C), 170.76 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1108.
- (4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenyl)(pyrrolidin-1-yl)methanone (3p)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(pyrrolidine-1-carbonyl)phenylboronic acid (42 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 81% yield (52.5 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ 1.93 (p, J = 6.6 Hz, 2H, CH2), 1.96–2.05 (m, 2H, CH2), 3.51 (t, J = 6.6 Hz, 2H, CH2), 3.70 (t, J = 7.0 Hz, 2H, CH2), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.71 (d, J = 8.4 Hz, 2H, arom H), 7.75 (d, J = 8.4 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 24.41 (CH2), 26.40 (CH2), 46.27 (CH2), 49.61 (CH2), 79.91 (C triple bond), 104.30 (C triple bond), 119.15 (C), 123.13 (CH), 126.65 (CH), 127.46 (CH), 128.19 (CH), 135.69 (C), 137.64 (C), 138.04 (C), 138.69 (CH), 143.66 (C), 148.09 (C), 149.82 (CH), 151.99 (CH), 152.28 (CH), 154.91 (C), 168.80 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H18N4OS 411.1274, found 411.1271.
- N,N-Dimethyl-3-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3q)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(N,N-dimethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 76% yield (46.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.06 (s, 3H, CH3), 3.17 (s, 3H, CH3), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.53 (dt, J = 7.6, 1.1 Hz, 1H, arom H), 7.59 (t, J = 7.7 Hz, 1H, arom H), 7.76–7.72 (m, 1H, arom H), 7.77–7.78 (m, 1H, arom H), 7.99 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.41 (CH3), 39.61 (CH3), 79.93 (C triple bond), 104.30 (C triple bond), 119.17 (C), 123.13 (CH), 126.26 (CH), 126.69 (CH), 127.41 (CH), 128.62 (CH), 129.42 (CH), 135.74 (C), 137.09 (C), 137.57 (C), 138.70 (CH), 143.67 (C), 148.07 (C), 149.82 (CH), 151.99 (CH), 152.29 (CH), 154.90 (C), 170.79 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1118.
- (3-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenyl)(pyrrolidin-1-yl)methanone (3r)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(pyrrolidine-1-carbonyl)phenylboronic acid pinacol ester (61 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a yellow solid in 69% yield (44.8 mg, 0.11 mmol).1H NMR (600 MHz, CDCl3) δ: 1.89–1.96 (m, 2H, CH2), 1.98–2.04 (m, 2H, CH2), 3.51 (t, J = 6.6 Hz, 2H, CH2), 3.70 (t, J = 7.0 Hz, 2H, CH2), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H, arom H), 7.62–7.65 (m, 1H, arom H), 7.74–7.77 (m, 1H, arom H), 7.88 (bs, 1H, arom H), 7.98 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.29 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.13C NMR (150 MHz, CDCl3) δ: 24.40 (CH2), 26.37 (CH2), 46.28 (CH2), 49.65 (CH2), 79.93 (C triple bond), 104.27 (C triple bond), 119.15 (C), 123.12 (CH), 126.36 (CH), 126.64 (CH), 127.43 (CH), 128.81 (CH), 129.32 (CH), 135.79 (C), 136.91 (C), 138.40 (C), 138.69 (CH), 143.62 (C), 148.03 (C), 149.79 (CH), 152.01 (CH), 152.26 (CH), 154.89 (C), 168.85 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H18N4OS 411.1274, found 411.1271.
- N-(2-Methoxyethyl)-3-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3s)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(2-methoxyethylaminocarbonyl)benzeneboronic acid pinacol ester (62 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 75% yield (49.1 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.41 (s, 3H, OCH3), 3.61 (t, J = 5.0 Hz, 2H, CH2), 3.71 (dd, J = 10.3, 5.3 Hz, 2H, CH2), 6.70 (bs, 1H, NH), 7.37 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.62 (t, J = 7.7 Hz, 1H, arom H), 7.83 (d, J = 7.8 Hz, 1H, arom H), 7.86 (d, J = 7.7 Hz, 1H, arom H), 7.96–8.02 (m, 1H, arom H), 8.16 (bs, 1H, arom H), 8.31 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.3 Hz, 1H, arom H), 8.92 (d, J = 1.2 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 39.84 (CH2), 58.86 (OCH3), 71.07 (CH2), 79.94 (C triple bond), 104.33 (C triple bond), 119.18 (C), 123.14 (CH), 126.54 (CH), 126.80 (CH), 127.04 (CH), 129.58 (CH), 130.41 (CH), 135.67 (C), 135.76 (C), 137.28 (C), 138.71 (CH), 143.71 (C), 148.10 (C), 149.83 (CH), 151.98 (CH), 152.30 (CH), 154.88 (C), 166.82 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H18N4O2S 415.1223, found 415.1223.
- 6-(3,4-Dimethoxyphenyl)-3-(pent-1-yn-1-yl)isothiazolo[4,3-b]pyridine (7a)
- This compound was obtained from the precursor 2g (49 mg, 0.17 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (36 mg, 0.20 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 3:2) as mobile phase and a second purification using a mixture of hexane and dichloromethane (in a ratio of 2:3), affording the title compound as a light-yellow solid in 74% yield (44.3 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 1.11 (t, J = 7.4 Hz, 3H, CH3), 1.77 (h, J = 7.3 Hz, 2H, CH2), 2.67 (t, J = 7.1 Hz, 2H, CH2), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 7.02 (d, J = 8.3 Hz, 1H, arom H), 7.18 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.2, 2.2 Hz, 1H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 9.08 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 13.69 (CH3), 21.80 (CH2), 22.59 (CH2), 56.05 (OCH3), 68.81 (C triple bond), 110.47 (CH), 110.93 (C triple bond), 111.76 (CH), 120.19 (CH), 125.38 (CH), 129.62 (C), 136.01 (C), 145.59 (C), 147.56 (C), 149.66 (C), 149.88 (C), 151.74 (CH), 155.15 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H18N2O2S 339.1162, found 339.1162.
- 6-(3,4-Dimethoxyphenyl)-3-(phenylethynyl)isothiazolo[4,3-b]pyridine (7b)
- This compound was prepared according to a procedure described in the literature [28].
- 6-(3,4-Dimethoxyphenyl)-3-((4-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7c)
- This compound was obtained from the precursor 6c (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 83% yield (48.5 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.09–7.14 (m, 2H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.66–7.70 (m, 2H, arom H), 8.19 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.04 (OCH3), 106.85 (C triple bond), 110.43 (CH), 111.76 (CH), 115.93 (d, J = 22.2 Hz, CH), 118.10 (C), 120.20 (CH), 125.35 (CH), 129.44 (C), 133.96 (d, J = 8.5 Hz, CH) 136.17 (C), 144.15 (C), 147.52 (C), 149.68 (C), 149.94 (C), 152.11 (CH), 155.23 (C), 163.28 (d, J = 252.1 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: −108.32 ppm.
- HRMS m/z [M+H]+ calcd for C22H15FN2O2S 391.0911, found 391.0899.
- 6-(3,4-Dimethoxyphenyl)-3-((3-chlorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7d)
- This compound was obtained from the precursor 6d (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 84% yield (49.2 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.33–7.37 (m, 1H, arom H), 7.39–7.42 (m, 1H, arom H), 7.57 (dt, J = 7.5, 1.3 Hz, 1H, arom H), 7.69 (t, J = 1.6 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.04 (OCH3), 78.03 (C triple bond), 106.09 (C triple bond), 110.42 (CH), 111.76 (CH), 120.21 (CH), 123.60 (C), 125.33 (CH), 129.37 (C), 129.72 (CH), 129.83 (CH), 129.93 (CH), 131.63 (CH), 134.40 (C), 136.22 (C), 143.62 (C), 147.64 (C), 149.67 (C), 149.96 (C), 152.28 (CH), 155.23 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H15ClN2O2S 407.0615, found 407.0581.
- 6-(3,4-Dimethoxyphenyl)-3-((2-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7e)
- This compound was obtained from the precursor 6e (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (47.4 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.4 Hz, 1H, arom H), 7.14–7.22 (m, 1H), 7.28 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.39–7.45 (m, 1H, arom H), 7.68 (td, J = 7.3, 1.7 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.05 (OCH3), 81.67 (C triple bond), 101.02 (C triple bond), 110.46 (CH), 110.83 (d, J = 15.5 Hz, C), 111.79 (CH), 115.76 (d, J = 20.5 Hz, CH), 120.22 (CH), 124.12 (d, J = 3.3 Hz, CH), 125.30 (CH), 129.46 (C), 131.52 (d, J = 7.9 Hz, CH), 133.53 (CH), 136.18 (C), 143.73 (C), 147.69 (C), 149.68 (C), 149.95 (C), 152.30 (CH), 155.20 (C), 162.52 (d, J = 253.9 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: −107.77 ppm.
- HRMS m/z [M+H]+ calcd for C22H15FN2O2S 391.0911, found 391.0899.
- 6-(3,4-Dimethoxyphenyl)-3-((3-methoxyphenyl)ethynyl)isothiazolo[4,3-b]pyridine (7f)
- This compound was obtained from the precursor 6e (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. This compound was obtained using the precursor 6f and 3,4-dimethoxyphenylboronic acid. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 72% yield (41.9 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.85 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 6.99 (ddd, J = 8.1, 2.6, 1.3 Hz, 1H, arom H), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.21 (dd, J = 2.6, 1.3 Hz, 1H, arom H), 7.27–7.30 (m, 2H, arom H), 7.32 (t, J = 7.8 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.40 (OCH3), 56.04 (OCH3), 108.04 (C), 110.43 (CH), 111.77 (CH), 116.29 (CH), 116.57 (CH), 120.21 (CH), 122.87 (C), 124.52 (CH), 125.37 (CH), 129.47 (C), 129.57 (CH), 136.15 (C), 144.33 (C), 147.49 (C), 149.67 (C), 149.93 (C), 152.08 (CH), 155.23 (C), 159.36 (C) ppm.HR-MS m/z [M+H]+ calcd for C23H18N2O3S 403.1111, found 403.1093.
- 6-(3,4-Dimethoxyphenyl)-3-((6-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7g)
- This compound was obtained from the precursor 6g (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 79% yield (46.3 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.62 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.23 (d, J = 8.0 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.86 (dd, J = 8.0, 2.2 Hz, 1H, arom H), 8.21 (d, J = 2.1 Hz, 1H, arom H), 8.81 (d, J = 1.6 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 24.67 (CH3), 56.05 (OCH3), 79.43 (C), 104.66 (C), 110.42 (CH), 111.71 (CH), 116.14 (C), 120.22 (CH), 122.85 (CH), 125.38 (CH), 129.37 (C), 136.25 (C), 138.90 (CH), 143.65 (C), 147.57 (C), 149.66 (C), 149.97 (C), 151.71 (CH), 152.30 (CH), 155.23 (C), 159.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1111.
- 6-(3,4-Dimethoxyphenyl)-3-((6-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7h)
- This compound was obtained from the precursor 6h (48 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 8:2) as mobile phase and a second time using dichloromethane and methanol (10:0.1), affording the title compound as a yellow solid in 71% yield (41.3 mg, 0.10 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 6.80 (d, J = 8.6 Hz, 1H, arom H), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 1.9 Hz, 1H, arom H), 7.26–7.32 (m, 1H, arom H), 7.84 (dd, J = 2.3, 8.6 Hz, 1H, arom H), 8.20 (d, J = 1.9 Hz, 1H, arom H), 8.51 (d, J = 2.0 Hz, 1H, arom H), 9.12 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 53.90 (OCH3), 56.08 (OCH3), 78.57 (C), 105.16 (C), 110.47 (CH), 111.01 (CH), 111.81 (CH), 111.92 (C), 120.25 (CH), 125.41 (CH), 129.46 (C), 136.23 (C), 141.27 (CH), 144.16 (C), 147.47 (C), 149.71 (C), 149.98 (C), 150.71 (CH), 152.13 (CH), 155.24 (C), 164.24 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1065.
- 6-(3,4-Dimethoxyphenyl)-3-((5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7i)
- This compound was obtained from the precursor 6i (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 81% yield (47.5 mg, 0.12 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.39 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.20 (d, J = 1.7 Hz, 1 H, arom H), 7.26–7.31 (m, 1H, arom H), 7.80 (s, 1H, arom H), 8.21 (d, J = 1.8 Hz, 1H), 8.47 (s, 1H, arom H), 8.73 (s, 1H, arom H), 9.14 (d, J = 1.8 Hz, 1H, arom H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 18.25 (CH3), 56.08 (OCH3), 79.79 (C), 104.42 (C), 110.47 (CH), 111.82 (CH), 118.68 (C), 120.27 (CH), 125.40 (CH), 129.38 (C), 132.88 (C), 136.32 (C), 139.09 (CH), 143.48 (C), 147.70 (C), 149.48 (CH), 149.73 (C), 150.02 (C), 150.50 (CH), 152.41 (CH), 155.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1115.
- 6-(3,4-Dimethoxyphenyl)-3-((6-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7j)
- This compound was obtained from the precursor 6j (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 75% yield (43.9 mg, 0.11 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.00–7.05 (m, 2H, arom H), 7.19 (d, J = 1.8 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.0 Hz, 1H, arom H), 8.04–8.10 (m, 1H, arom H), 8.22 (d, J = 2.0 Hz, 1H, arom H), 8.56 (s, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm. 19F NMR (282 MHz, CDCl3) δ: −63.82 ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.03 (OCH3), 79.94 (C), 102.56 (C), 109.73 (CH, d, J = 37.9 Hz), 110.34 (CH), 111.72 (CH), 117.13 (C), 120.22 (CH), 125.38 (CH), 129.23 (C), 136.28 (C), 142.98 (C), 143.85 (CH, d, J = 8.3 Hz), 147.67 (C), 149.66 (C), 149.97 (C), 151.03 (CH, d, J = 15.6 Hz), 152.51 (CH), 155.23 (C), 163.22 (CF, d, J = 244.3 Hz) ppm.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0870.
- Methyl 5-((6-(3,4-dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (7k)
- This compound was obtained from the precursor 6k (65 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a light yellow solid in 76% yield (44.5 mg, 0.11 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 1.9 Hz, 1H, arom H), 7.24–7.32 (m, 2H, arom H), 8.04–8.13 (m, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.28 (d, J = 4.3 Hz, 1H, arom H), 9.15 (d, J = 1.9 Hz, 1H, arom H) ppm.
- 19F NMR (282 MHz, CDCl3) δ -61.97 ppm.
- 13C NMR (75 MHz, CDCl3) δ: 56.08 (OCH3), 83.12 (C), 98.68 (d, J = 5.1 Hz, C), 106.54 (d, J = 31.0 Hz, C), 110.45 (CH), 111.81 (CH), 120.27 (CH), 121.2 (d, J = 4.4 Hz, CH), 125.37 (CH), 129.31 (C), 136.36 (C), 142.72 (C), 143.58 (CH), 147.90 (C), 148.15 (d, J = 14.3 Hz, CH), 149.88 (d, J = 23.5 Hz, C), 152.68 (CH), 155.24 (C), 160.34 (d, J = 244.4 Hz, C) ppm.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0859.
- 6-(3,4-Dimethoxyphenyl)-3-((5-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7l)
- This compound was obtained from the precursor 6l (48.5 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 71% yield (41.3 mg, 0.10 mmol).
- 1H NMR (300 MHz, DMSO) δ: 3.84 (s, 3H, OCH3). 3.91 (s, 6H, 2 x OCH3), 7.12 (d, J = 8.8 Hz, 1H, arom H), 7.50 (bs, 2H, arom H), 7.73 (s, 1H, arom H), 8.41 (d, J = 2.4 Hz, 1H, arom H), 8.48 (s, 1H, arom H), 8.54 (s, 1H, arom H), 9.31 (s, 1H, arom H) ppm.
- 13C NMR (75 MHz, DMSO) δ: 56.11 (OCH3), 56.21 (OCH3), 56.34 (OCH3), 80.49 (C), 103.98 (C), 111.51 (CH), 112.68 (CH), 119.08 (C), 120.63 (CH), 122.39 (CH), 125.00 (CH), 128.74 (C), 136.02 (C), 139.67 (CH), 142.41 (C), 144.09 (CH), 147.73 (C), 149.85 (C), 150.25 (C), 153.10 (CH), 155.47 (C), 155.52 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1067.
- Methyl 5-((6-(3,4-dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (7m)
- This compound was obtained from the precursor 6m (49 mg, 0.13 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.3 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2.2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as an orange solid in 69% yield (39.8 mg, 0.09 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 6H, 2 x OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.20 (d, J = 1.8 Hz, 1H, arom H), 7.30 (dd, J = 8.3, 1.9 Hz, 1H, arom H), 8.23 (d, J = 1.9 Hz, 1H, arom H), 8.59 (t, J = 1.8 Hz, 1H, arom H), 9.06 (d, J = 1.8 Hz, 1H, arom H), 9.16 (d, J = 1.9 Hz, 1H, arom H), 9.22 (d, J = 1.8 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 52.70 (OCH3), 56.05 (OCH3), 81.01 (C), 102.68 (C), 110.41 (CH), 111.78 (CH), 119.29 (C), 120.26 (CH), 125.40 (CH), 125.73 (C), 129.25 (C), 136.40 (C), 139.62 (CH), 142.69 (C), 147.82 (C), 149.71 (C), 150.03 (C), 150.51 (CH), 152.66 (CH), 155.27 (C), 155.36 (CH),164.84 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H17N3O4S 432.1012, found 432.1021.
- 6-(3,4-Dimethoxyphenyl)-3-((6-(trifluoromethyl)pyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7n)
- This compound was obtained from the precursor 6n (50 mg, 0.13 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.3 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2.2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 74% yield (42.5 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.76 (dd, J = 8.1, 0.6 Hz, 1H, arom H), 8.16 (dd, J = 8.0, 1.6 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 9.00 (d, J = 1.6 Hz, 1H, arom H), 9.17 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 19F NMR (282 MHz, CDCl3) δ: −68.07 ppm.
- 13C NMR (151 MHz, CDCl3) δ: 56.03 (OCH3), 56.05 (OCH3), 82.19 (C), 102.13 (C), 110.38 (CH), 111.77 (CH), 120.02 (CH), 120.25 (CH), 122.23 (C), 125.39 (CH), 129.14 (C), 136.44 (C), 139.97 (CH), 142.23 (C), 147.61 (q, J = 35.3 Hz, C). 147.85 (C), 149.71 (C), 150.06 (C), 152.16 (CH), 152.80 (CH), 155.27 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H14F3N3O2S 442.0831, found 442.0828.
- 6-(3,4-Dimethoxyphenyl)-3-((4-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7o)
- This compound was obtained from the precursor 6o (62 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as an orange solid in 71% yield (51 mg, 0.13 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.14 (d, J = 2.1 Hz, 1H), 8.76 (s, 1H), 8.52 (d, J = 5.8 Hz, 1H), 8.20 (d, J = 2.1 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.4 Hz, 1H), 6.89 (d, J = 5.8 Hz, 1H), 4.02 (s, 3H), 3.99 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 165.72, 155.35, 154.06, 152.45, 151.98, 150.11, 149.84, 147.84, 143.96, 136.37, 129.62, 125.48, 120.39, 111.94, 110.61, 106.36, 101.14, 83.25, 56.2, 56.1 ppm. HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1058.
- 6-(3,4-Dimethoxyphenyl)-3-((4-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7p)
- This compound was obtained from the precursor 6p (46 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as a brown solid in 95% yield (67 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.15 (d, J = 2.1 Hz, 1H), 8.83 (s, 1H), 8.49 (d, J = 5.1 Hz, 1H), 8.21 (d, J = 2.1 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.24 (d, J = 5.0 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.97 (s, 3H), 2.61 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.42, 152.56, 152.50, 150.12, 149.83, 149.70, 149.38, 147.90, 143.63, 136.40, 129.52, 125.43, 124.54, 120.37, 119.72, 111.93, 110.56, 103.23, 83.51, 56.20, 56.21, 20.57 ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1114.
- 5-((6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)picolinonitrile (7q)
- This compound was obtained from the precursor 6q (48 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as an orange solid in 70% yield (40 mg, 0.10 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.17 (d, J = 2.1 Hz, 1H), 8.98 (dd, J = 2.1, 0.9 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.10 (dd, J = 8.0, 2.1 Hz, 1H), 7.76 (dd, J = 8.1, 0.9 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.46, 153.29, 153.13, 150.27, 149.90, 148.10, 141.98, 139.47, 136.70, 133.12, 129.23, 127.95, 125.57, 122.91, 120.44, 116.90, 119.97, 110.57, 101.93, 84.10, 56.24, 56.22 ppm.
- HRMS m/z [M+H]+ calcd for C22H14N4O2S 399.0910, found 399.0912.
- 6-(3,4-Dimethoxyphenyl)-3-((5-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7r)
- This compound was obtained from the precursor 6r (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 75% yield (44 mg, 0.11 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.16 (d, J = 2.1 Hz, 1H), 8.74 (s, 1H), 8.52 (d, J = 2.8 Hz, 1H), 8.22 (d, J = 2.0 Hz, 1H), 7.70 (ddd, J = 8.7, 2.8, 1.7 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.2 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 154.14 (d, J = 259 Hz), 152.84, 150.20, 149.88, 148.34 (d, J = 4.1 Hz), 147.99, 142.72, 138.89, 138.77(d, J = 23 Hz), 136.56, 129.39, 125.54, 125.34 (d, J= 20 Hz), 120.41, 111.95, 110.58, 102.34 (d, J = 2.6 Hz), 81.11, 56.23, 56.21.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0863.
- 6-(3,4-Dimethoxyphenyl)-3-((2-ethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7s)
- This compound was obtained from the precursor 6s (90 mg, 0.25 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (55 mg, 0.3 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (69 mg, 0.5 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 67% yield (70 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.13 (d, J = 2.0 Hz, 1H), 8.20 (s, 1H), 7.90 (dd, J = 7.4, 1.9 Hz, 1H), 7.29 (dd, J = 8.3, 2.1 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.03 (d, J = 8.3 Hz, 1H), 6.92 (dd, J = 7.4, 5.0 Hz, 1H), 4.52 (q, J = 7.0 Hz, 2H), 3.99 (s, 3H), 3.97 (s, 3H), 1.49 (t, J = 7.0 Hz, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 163.47, 155.34, 152.27, 150.08, 149.83, 148.02, 147.78, 144.37, 142.00, 136.32, 129.66, 125.49, 120.38, 116.33, 111.93, 110.61, 106.47, 103.22, 81.96, 62.79, 56.23, 56.21, 14.72 ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O3S 418.1220, found 418.1208.
- 6-(3,4-Dimethoxyphenyl)-3-((5-fluoro-2-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7t)
- This compound was obtained from the precursor 6s (98 mg, 0.27 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (60 mg, 0.32 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (75 mg, 0.54 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as a yellow solid in 78% yield (90 mg, 0.21 mmol).
- 1H NMR (400 MHz, CDCl3) 9.14 (d, J = 2.1 Hz, 1H), 8.20 (d, J = 2.1 Hz, 1H), 8.06 (d, J = 3.0 Hz, 1H), 7.67 (dd, J = 7.8, 3.0 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 4.05 (s, 3H), 3.99 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 159.88, 155.6, 155.22, 154.36 (d, J = 246 Hz), 152.45, 150.02, 149.73, 147.84, 143.31, 136.32, 134.66 (d, J = 26 Hz), 129.40, 129.05 (d, J = 23 Hz), 125.39, 120.28, 111.81, 110.47, 106.75 (d, J = 6 Hz), 101.13, 82.62, 56.10, 56.08, 54.67 ppm.
- HRMS m/z [M+H]+ calcd for C22H16FN3O3S 422.0969, found 422.0958.
- 6-(3,4-Dimethoxyphenyl)-3-((2,6-dimethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7u)
- This compound was obtained from the precursor 6u (100 mg, 0.27 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (60 mg, 0.32 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (75 mg, 0.54 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 61% yield (70 mg, 0.16 mmol).
- 1H NMR (400 MHz, CDCl3) 9.11 (d, J = 2.1 Hz, 1H), 8.18 (d, J = 2.1 Hz, 1H), 7.80 (d, J = 8.2 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.19 (d, J = 2.1 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 6.38 (d, J = 8.2 Hz, 1H), 4.07 (s, 3H), 3.99 (s, 3H), 3.98 (s, 3H), 3.96 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 163.75, 163.35, 155.16, 151.80, 149.91, 149.69, 147.46, 144.94, 144.33, 136.09, 129.65, 125.35, 120.23, 111.79, 110.50, 104.02, 101.92, 96.76, 80.55, 56.08, 54.15, 53.86 ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O4S 434.1169, found 434.1162.
- 6-(3,4-Dimethoxyphenyl)-3-((2-fluoro-5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7v)
- This compound was obtained from the precursor 6v (101 mg, 0.29 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (63 mg, 0.35 mmol, 1.2 eq.), Pd(PPh3)4 (6.7 mg, 0.0058 mmol, 0.02 eq.) and K2CO3 (80 mg, 0.58 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as an orange solid in 92% yield (107 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3). 9.15 (d, J = 2.0 Hz, 1H), 8.21 (d, J = 2.1 Hz, 1H), 8.06 (s, 1H), 7.90 (d, J = 8.7 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.97 (s, 3H), 2.37 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 160.81 (d, J = 240 Hz), 155.22, 152.59, 150.02, 149.72, 147.94 (d, J = 14 Hz), 147.85, 143.98, 142.87, 136.33, 130.88 (d, J = 5 Hz), 129.33, 125.36, 120.27, 111.81, 110.44, 105.62 (d, J = 32 Hz), 99.06 (d, J = 6 Hz), 82.74, 56.07, 17.30 ppm
- HRMS m/z [M+H]+ calcd for C22H16FN3O2S 406.1020, found 406.1014.
- 6-(3,4-Dimethoxyphenyl)-3-(quinolin-3-ylethynyl)isothiazolo[4,3-b]pyridine (7w)
- This compound was obtained from the precursor 6w (51 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase and a second time using dichloromethane and methanol (in a ratio of 10:0.2), affording the title compound as a yellow solid in 75% yield (43.3 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.21 (d, J = 2.1 Hz, 1H, arom H), 7.30 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.60–7.65 (m, 1H, arom H), 7.77–7.83 (m, 1H, arom H), 7.86 (d, J = 7.9 Hz, 1H, arom H), 8.15 (d, J = 8.5 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.51 (d, J = 1.8 Hz, 1H, arom H), 9.14 (d, J = 1.9 Hz, 1H, arom H), 9.17 (d, J = 2.0 Hz, 1H, arom H) ppm. 13C NMR (150 MHz, CDCl3) δ: 56.1 (OCH3), 56.06 (OCH3), 80.05 (C), 104.87 (C), 110.44 (CH), 111.79 (CH), 116.14 (C), 120.25 (CH), 125.41 (CH), 127.01 (C), 127.61 (CH), 127.92 (CH), 129.36 (C), 129.54 (CH), 130.89 (CH), 136.33 (C), 139.24 (CH), 143.42 (C), 147.36 (C), 147.75 (C), 149.74 (C), 149.71 (C), 151.51 (CH), 152.44 (CH), 155.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H17N3O2S 424.1114, found 424.1113.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-4-ylethynyl)isothiazolo[4,3-b]pyridine (7x)
- This compound was obtained from the precursor 6w (47 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 71% yield (41.8 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.3, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.53–7.55 (m, 2H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 5.8 Hz, 2H, arom H), 9.16 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.08 (OCH3), 80.92 (C triple bond), 104.17 (C triple bond), 110.44 (CH), 111.81 (CH), 120.27 (CH), 125.38 (CH), 125.40 (CH), 129.26 (C), 129.97 (C), 136.42 (C), 142.67 (C), 147.84 (C), 149.74 (C), 149.98 (CH), 150.06 (C), 152.73 (CH), 155.31 (C) ppm. HRMS m/z [M+H]+ calcd for C21H15N3O2S 374.0958, found 374.0948.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-2-ylethynyl)isothiazolo[4,3-b]pyridine (7y)
- This compound was obtained from the precursor 6y (50 mg, 0.16 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (35 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.025 eq.) and K2CO3 (44 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 59% yield (34.6 mg, 0.09 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.32–7.35 (m, 1H, arom H), 7.72–7.78 (m, 2H, arom H), 8.22 (d, J = 2.1 Hz, 1H, arom H), 8.70–8.72 (m, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.06 (OCH3), 56.07 (OCH3), 76.45 (C triple bond), 106.11 (C triple bond), 110.45 (CH), 111.78 (CH), 120.25 (CH), 123.79 (CH), 125.38 (CH), 127.69 (CH), 129.42 (C), 136.30 (C), 136.30 (CH), 142.37 (C), 143.10 (C), 148.04 (C), 149.70 (C), 149.97 (C), 150.39 (CH), 152.46 (CH), 155.19 (C) ppm.
- HRMS m/z [M+H]+ calcd for C21H15N3O2S 374.0958, found 374.0953.
- tert-Butyl (3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)carbamate (9)
- This compound was obtained from the precursor 8 (59 mg, 0.16 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (35 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.025 eq.) and K2CO3 (44 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 87% yield (57.7 mg, 0.13 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 1.48 (s, 9H, 3× CH3), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.42 (d, J = 4.2 Hz, 2H, CH2), 4.98 (bs, 1H, NH), 7.02 (d, J = 8.4 Hz, 1H, arom H), 7.17 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 28.3 (CH3 tBuO), 31.8 (CH2), 56.0 (OCH3), 71.0 (C triple bond), 80.4 (C), 104.8 (C triple bond), 110.5 (CH), 111.8 (CH), 120.2 (CH), 125.4 (CH), 129.4 (C), 136.2 (C), 143.7 (C), 147.8 (C), 149.7 (C), 150.0 (C), 152.3 (CH), 155.1 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H23N3O4S 426.1482, found 426.1489.
- 3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-aminium chloride (10)
- To a solution of the Boc-amino precursor 9 (100 mg, 0.235 mmol, 1.0 eq.) in 1,4-dioxane (5 mL) at 0 °C, hydrogen chloride solution 4.0 M in 1,4-dioxane (10 mL) was added carefully. The resulting mixture was stirred at room temperature for 4–5 h. After completion of the reaction as monitored by TLC, the volatiles were evaporated to dryness and the resulting residue was co-evaporated with dichloromethane and precipitate with a mixture of dichloromethane and diethyl ether (in a ratio 1:4), yielding the title compound as hydrochloric salt (orange solid) in 98% yield (83.3 mg, 0.23 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.90 (s, 2H, CH2), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.18 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C17H15N3O2S 326.0958, found 326.0962.
- Derivatisation of the propargylamino moiety at position 3 of the isothiazolo[4,3-b]pyridine scaffold (11a-c)
- General procedure
- To a solution of the amino precursor 10 (1 eq.) in dry dichloromethane (15 mL), dry triethylamine (2 eq.) was added. The reaction mixture was cooled at 0 °C and the appropriated acid chloride, sulfonyl chloride or isocyanate (1.2 eq.) was added. The resulting mixture was stirred at room temperature overnight. After completion of the reaction as monitored by TLC, the volatiles were evaporated to dryness and the resulting residue was purified by silica gel and precipitated with diethyl ether, yielding the corresponding 3-modified isothiazolo[4,3-b]pyridine. Compounds 11a-c were made according to this procedure.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)benzamide (11a)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0 eq.), benzoyl chloride (24 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 2:3) as mobile phase and a second purification using a mixture of dichloromethane and acetone (in a ratio of 9:1), affording the title compound as a yellow solid in 68% yield (40.3 mg, 0.09 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.75 (d, J = 5.2 Hz, 2H, CH2), 6.80 (bs, 1H, NH), 7.02 (d, J = 8.4 Hz, 1H, arom H), 7.17 (d, J = 2.1 Hz, 1H, arom H), 7.23–7.29 (m, 1H, arom H), 7.44–7.50 (m, 2H, arom H), 7.50–7.56 (m, 1H, arom H), 7.83–7.89 (m, 2H, arom H), 8.19 (d, J = 2.1 Hz, 1H, arom H), 9.08 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 31.16 (CH2), 56.03 (OCH3), 56.06 (OCH3), 71.49 (C), 104.07 (C), 110.43 (CH), 111.77 (CH), 120.22 (CH), 125.50 (CH), 127.10 (CH), 128.63 (CH), 129.27 (C), 131.87 (CH); 136.25 (C), 133.59 (C), 143.38 (C), 147.86 (C), 149.70 (C), 150.00 (C), 152.25 (CH), 155.08 (C), 167.09 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H19N3O3S 430.1220, found 430.1218.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)benzenesulfonamide (11b)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0eq.), benzenesulfonyl chloride (30 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 98:2) as mobile phase, affording the title compound as a yellow solid in 61% yield (39.2 mg, 0.08 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.35 (d, J = 6.1 Hz, 2H, CH2), 5.30 (t, J = 6.0 Hz, 1H, NH), 7.01 (d, J = 8.3 Hz, 1H, arom H), 7.15 (d, J = 2.0 Hz, 1H, arom H), 7.24 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.47–7.58 (m, 3H, arom H), 7.95–7.99 (m, 2H, arom H), 8.16 (d, J = 2.0 Hz, 1H, arom H), 9.05 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 34.33 (CH2), 56.04 (OCH3), 56.07 (OCH3), 72.54 (C triple bond), 102.13 (C triple bond), 110.38 (CH), 111.75 (CH), 120.21 (CH), 125.38 (CH), 127.43 (CH), 129.16 (CH), 133.08 (CH), 136.31 (C), 139.70 (C), 142.55 (C), 147.71 (C), 149.70 (C), 150.04 (C), 152.40 (CH), 155.03 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O4S2 466.0890, found 466.0888.
- 1-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)-3-phenylurea (11c)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0 eq.), phenyl isocyanate (20 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 95:5) as mobile phase, affording the title compound as a light yellow solid in 54% yield (33.1 mg, 0.07 mmol).
- 1H NMR (600 MHz, DMSO) δ: 3.83 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 4.41 (d, J = 5.8 Hz, 2H, CH2), 6.74 (t, J = 5.8 Hz, 1H, NH), 6.89–6.94 (m, 1H, arom H), 7.12 (d, J = 9.0 Hz, 1H, arom H), 7.22–7.26 (m, 2H, arom H), 7.43 (dd, J = 8.6, 1.0 Hz, 2H, arom H), 7.47 (dd, J = 6.9, 2.0 Hz, 2H, arom H), 8.50 (d, J = 2.1 Hz, 1H, arom H), 8.74 (s, 1H, NH), 9.24 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 30.31 (CH2), 55.72 (OCH3), 55.82 (OCH3), 69.73 (C triple bond), 107.47 (C triple bond), 111.13 (CH), 112.30 (CH), 117.96 (CH), 120.20 (CH), 121.51 (CH), 124.61 (CH), 128.43 (C), 128.77 (CH), 135.48 (C), 140.22 (C), 143.19 (C), 147.30 (C), 149.45 (C), 149.82 (C), 152.31 (CH), 154.89 (C), 155.14 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H20N4O3S 445.1329, found 445.1322.
- 2-Methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzoic acid (4)
- To a solution of the precursor 3j (50 mg, 0.13 mmol, 1.0 eq.) in a mixture of THF/H2O (in a ratio of 4:2) (6 mL), LiOH (6.2 mg, 0.26 mmol, 2 eq.) was added and the reaction mixture was stirred at 50 °C overnight. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and a solution of HCl (1 M) was added slowly until pH acid was reached (pH = 2–4). The volatiles were evaporated to dryness and the crude residue was precipitated with methanol and subsequently, with diethyl ether, affording the title compound as a light yellow solid in 94% yield (45.4 mg, 0.12 mmol).
- 1H NMR (600 MHz, DMSO) δ: 3.98 (s, 3H, OCH3), 7.55 (dd, J = 7.9, 1.3 Hz, 1H), 7.60–7.63 (m, 2H, arom H), 7.80 (d, J = 7.9 Hz, 1H, arom H), 8.19–8.23 (m, 1H, arom H), 8.70–8.74 (m, 2H, arom H), 8.94 (d, J = 1.4 Hz, 1H, arom H), 9.34 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 56.18 (OCH3), 80.47 (C), 103.69 (C), 111.91 (CH), 118.64 (C), 119.37 (CH), 121.54 (C), 124.24 (CH), 126.74 (CH), 131.53 (CH), 134.96 (C), 139.61 (CH), 140.60 (C), 142.43 (C), 148.01 (C), 149.70 (CH), 151.15 (CH), 152.46 (CH), 154.74 (C), 158.75 (C), 167.05 (C) ppm.
- HRMS m/z [M+H]+ calcd for C21H13N3O3S 388.0750, found 388.0746.
- 4-(2-(4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenoxy)ethyl)morpholine (5a)
- To a solution of the precursor 3k (99 mg, 0.30 mmol, 1.0 eq.) in acetone, K2CO3 (62 mg, 0.45 mmol, 1.5 eq.) was added and the mixture was stirred at 50 °C for 20 min. Separately, to a second solution of 4-(2-chloroethyl)morpholine hydrochloride (67 mg, 0.36 mmol, 1.2 eq.) in acetone, K2CO3 (62 mg, 0.45 mmol, 1.5 eq.) and KI (100 mg, 0.6 mmol, 2 eq.) was added and the mixture was stirred at 50 °C for 20 min. Then, the second solution was added to the one contained the precursor 3k and the final mixture was stirred at reflux overnight. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase (adding 6 drops of ammonia 25% in 100 mL of solvent), affording the title compound as a light yellow solid in 48% yield (64.5 mg, 0.14 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.58–2.64 (m, 4H, 2 x CH2), 2.86 (t, J = 5.7 Hz, 2H, CH2), 3.74–3.79 (m, 4H, 2 x CH2), 4.20 (t, J = 5.7 Hz, 2H, CH2), 7.06–7.10 (m, 2H, arom H), 7.37 (ddd, J = 7.8, 4.9, 0.8 Hz, 1H, arom H), 7.62–7.67 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92 (dd, J = 2.0, 0.7 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 54.10 (CH2), 57.54 (CH2), 66.01 (CH2), 66.91 (CH2), 80.07 (C), 104.00 (C) 115.49 (CH), 119.25 (C), 123.12 (CH), 125.27 (CH), 128.76 (CH), 129.16 (C), 136.07 (C), 138.68 (CH), 143.23 (C), 147.63 (C), 149.78 (CH), 152.32 (CH), 152.43 (CH), 155.30 (C), 159.53 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H22N4O2S 443.1536, found 443.1531.
- 3-(Pyridin-3-ylethynyl)-6-(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)isothiazolo[4,3-b]pyridine (5b)
- To a solution of the precursor 3k (250 mg, 0.76 mmol, 1.0 eq.) in DMF (10 mL), K2CO3 (157 mg, 1.14 mmol, 1.5 eq.) was added and the mixture was stirred at 50 °C for 20 min. Separately, to a second solution of 1-(2-chloroethyl)pyrrolidine hydrochloride (170 mg, 0.91 mmol, 1.2 eq.) in DMF (10 mL), K2CO3 (157 mg, 1.14 mmol, 1.5 eq.) and KI (252 mg, 1.52 mmol, 2 eq.) was added and the mixture was stirred at 50 °C for 20 min. After this time, the second solution was added to the one contained the precursor 3k and the final mixture was stirred at 100 °C for 5 h. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase (adding 6 drops of ammonia 25% in 100 mL of solvent), affording the title compound as a light yellow solid in 25% yield (80.9 mg, 0.19 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 1.82–1.87 (m, 4H, 2 x CH2), 2.64–2.69 (m, 4H, 2 × CH2), 2.96 (t, J = 5.9 Hz, 2H, CH2), 4.20 (t, J = 5.9 Hz, 2H, CH2), 7.09 (d, J = 8.7 Hz, 2H, arom H), 7.37 (dd, J = 7.6, 5.0 Hz, 1H, arom H), 7.65 (d, J = 8.7 Hz, 2H, arom H), 7.98 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.20 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.14 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 23.48 (CH2), 54.74 (CH2), 54.98 (CH2), 67.28 (CH2), 80.09 (C), 103.96 (C), 115.49 (CH), 119.26 (C), 123.11 (CH), 125.20 (CH), 128.71 (CH), 128.96 (C), 136.13 (C), 138.68 (CH), 143.17 (C), 147.60 (C), 149.76 (CH), 152.31 (CH), 152.49 (CH), 155.33 (C), 159.71 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H22N4OS 427.1587, found 427.1582.
- N-(Propargyl)aniline
- To a suspension of aniline (94 mg, 1.0 mmol, 1.2 eq.) and K2CO3 (174 mg, 1.26mmol, 1.5 eq.) in DMF at 0 °C, propargyl bromide 80% w/w in toluene (125 mg, 0.84 mmol, 1.0 eq.) was added. The reaction mixture was stirred at room temperature overnight. The volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as colourless oil in 87% yield (95.9 mg, 0.73 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.21 (t, J = 2.4 Hz, 1H, CH), 3.92 (d, J = 2.4 Hz, 2H, CH2), 6.65–6.72 (m, 2H, arom H), 6.79 (t, J = 7.3 Hz, 1H, arom H), 7.15–7.29 (m, 2H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C9H9N 132.0808, found 132.0802.
- N-(3-(6-Bromoisothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)aniline (12)
- A solution of 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (100 mg, 0.34 mmol, 1 eq.) in dry THF was degassed with a flow of argon for 5 min. Subsequently, Pd(PPh3)2Cl2 (4.8 mg, 0.0068 mmol, 0.02 eq.), CuI (0.65 mg, 0.0034 mmol, 0.01 eq.) and N-(propargyl)aniline (45 mg, 0.34 mmol, 1.0 eq.) were added and the reaction mixture was filled with argon and stirred at room temperature for 5 h. After disappearance of the starting material as monitored by TLC, the volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 8:2) as mobile phase, affording the title compound as dark oil in 78% yield (91.3 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 4.11 (bs, 1H, NH), 4.40 (s, 2H, CH2), 6.72–6.87 (m, 3H, arom H), 7.19–7.31 (m, 2H, arom H), 8.31 (d, J = 2.1 Hz, 1H, arom H), 8.79 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C15H10BrN3S 343.9852, found 343.9842.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)aniline (13)
- This compound was obtained from the precursor 12 (52 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure.
- The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 8:2) as mobile phase., affording the title compound as a yellow solid in 81% yield (47.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.14 (bs, 1H, NH), 4.42 (s, 2H, CH2), 6.76–6.80 (m, 2H, arom H), 6.82 (tt, J = 7.4, 1.0 Hz, 1H, arom H), 7.02 (d, J = 8.3 Hz, 1H, arom H), 7.16 (d, J = 2.1 Hz, 1H, arom H), 7.23–7.28 (m, 3H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.18 (CH2), 56.03 (OCH3), 56.05 (OCH3), 71.05 (C triple bond), 105.98 (C triple bond), 110.42 (CH), 111.74 (CH), 113.59 (CH), 118.80 (CH), 120.20 (CH), 125.38 (CH), 129.32 (CH), 129.32 (C), 136.15 (C), 143.85 (C), 146.66 (C), 147.76 (C), 149.66 (C), 149.93 (C), 152.16 (CH), 155.07 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O2S 402.1271, found 402.1263.
- 6-Bromo-N-(pyridin-3-ylmethyl)isothiazolo[4,3-b]pyridin-3-amine (14)
- The mixture of 3,6-dibromoisothiazolo[4,3-b]pyridine (147 mg, 0.5 mmol, 1.0 eq.), pyridin-3-ylmethanamine (216 mg, 2.0 mmol, 4.0 eq.) and K2CO3 (276 mg, 2.0 mmol, 4 eq.) in acetonitrile (5 mL) was heated under reflux till the starting material was almost disappeared on TLC. After cooling down to room temoerature, the mixture was concentrated under reduced pressure. The residue was purified with silica gel column (acetone in dichloromethane from 0 to 20% in 15 min.) to yield the title compounds 109 mg (0.34 mmol, 68%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 8.70 (s, 1H), 8.62 (d, J = 4.6 Hz, 1H), 8.31 (s, 1H), 7.98 (s, 1H), 7.75 (d, J = 7.7 Hz, 1H), 7.32 (dd, J = 7.7 & 5.0 Hz, 1H), 6.69 (br., 1H), 4.59 (d, J = 5.5 Hz, 2H), ppm.
- 13C NMR (75 MHz, CDCl3) δ: 154.2, 150.0, 149.5, 145.8, 135.6, 133.1, 131.1, 130.1, 123.8, 121.4, 49.3 ppm.
- 6-(3,4-Dimethoxyphenyl)-N-(pyridin-3-ylmethyl)isothiazolo[4,3-b]pyridin-3-amine (15)
- This compound was obtained from the precursor 14 (55 mg, 0.17 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (36 mg, 0.20 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 50 mg (0.12 mmol, 70%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 8.72 (s, 1H), 8.59 (m, 2H), 7.88 (s, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.30 (m, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.14 (s, 1H), 6.98 (d, J = 8.2 Hz, 1H), 6.86 (t, J = 5.5 Hz, 1H), 4.61 (d, J = 5.5 Hz, 2H), 3.96 (s, 3H), 3.94 (s, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 171.01, 154.50, 149.88, 149.61, 149.51, 145.21, 136.82, 135.52, 133.77, 131.49, 130.35, 124.67, 123.75, 119.99, 111.68, 110.48, 56.04, 49.35 ppm.
- HRMS m/z [M+H]+ calcd for C20H18N4O2S 379.1223, found 379.1224.
- 6-Bromo-3-(pyridin-3-yl)isothiazolo[4,3-b]pyridine (16)
- The title compound was synthesized from 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (294mg, 1.0 mmol, 1.0 eq.) and pyridin-3-ylboronic acid (148 mg, 1.2 mmol, 1.2 eq.) Pd(PPh3)4 (14 mg, 0.02 mmol, 0.02 eq.) and K2CO3 (414 mg, 3.0 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 3 h). The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 248 mg (0.85 mmol, 85%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.28 (s, 1H), 8.82 (s, 1H), 8.72 (d, J = 4.4 Hz, 1H), 8.55 (d, J = 7.6 Hz, 1H), 8.34 (s, 1H), 7.50 (dd, J = 7.6 & 5.2 Hz, 1H) ppm.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-3-yl)isothiazolo[4,3-b]pyridine (17)
- The title compound was synthesized from intemediate 16 (246 mg, 0.5 mmol, 1.0 eq.) and 3,4-dimethoxyphenylboronic acid (109 mg, 0.6 mmol, 1.2 eq.), Pd(PPh3)4 (7 mg, 0.01 mmol, 0.02 eq.) and K2CO3 (212 mg, 1.5 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 4 h). The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 140 mg (0.40 mmol, 80%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.33 (s, 1H), 9.13 (s, 1H), 8.71 (d, J = 3.2 Hz, 1H), 8.62 (d, J = 7.6 Hz, 1H), 8.20 (s, 1H), 7.51 (t, J = 5.3 Hz, 1H), 7.30 (d, J =8.2 Hz, 1H), 7.22 (s, 1H), 7.04 (d, J = 8.2 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 158.24, 156.72, 151.94, 150.60, 149.92, 149.67, 148.86, 143.90, 135.84, 135.34, 129.51, 126.82, 125.05, 123.97, 120.15, 111.79, 110.39, 56.06 ppm.
- HRMS m/z [M+H]+ calcd for C19H15N3O2S 350.0958, found 350.0951.
- N-(6-Bromoisothiazolo[4,3-b]pyridin-3-yl)nicotinamide (19)
- To a mixture of 6-bromoisothiazolo[4,3-b]pyridin-3-amine (115 mg, 0.5 mmol, 1.0 eq.) and nicotinoyl chloride hydrochloride (115 mg, 0.6 mmol, 1.2 eq.) in dichloromethane (5 mL), trimethylamine (0.5 mL) was added. The mixture was stirred at room temperature for 2 h. After concentration under reduced pressure, the residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 110 mg (0.33 mmol, 66%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.18 (d, J = 1.8 Hz, 1H), 9.08 (d, J = 1.8 Hz, 1H), 8.88 (br., 1H), 8.83 (d, J = 4.8 Hz, 1H), 8.53 (d, J = 1.8 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 7.49 (dd, J = 8.0 & 4.8 Hz, 1H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 164.4, 163.7, 148.6, 147.9, 138.7, 135.3, 132.0, 128.7, 125.7, 123.9, 121.9, 114.6 ppm.
- N-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)nicotinamide (20)
- The title compound was synthesized from intemediate 19 (100 mg, 0.3 mmol, 1.0 eq.) and 3,4-dimethoxyphenylboronic acid (66 mg, 0.36 mmol, 1.2 eq.), Pd(PPh3)4 (4.2 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (124 mg, 0.9 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 4 h). The crude residue was purified by silica gel column (acetone in dichloromethane from 0 to 20% in 15 min) to yield the title compounds 82 mg (0.21 mmol, 71%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3+CD3OD) δ: 9.21 (d, J = 2.0 Hz, 1H), 8.79 (s, 2H), 8.43 (m, 2H), 7.61 (dd, J = 8.1 Hz, 5.0 Hz, 1H), 7.31 (dd, J = 8.3 Hz, 1.8 Hz, 1H), 7.23 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 3.98 (s, 3H), 3.96 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3+CD3OD) δ: 165.38, 152.34, 150.55, 149.70, 148.53, 145.77, 140.57, 138.00, 136.38, 130.79, 129.65, 127.94, 125.72, 123.98, 120.69, 115.36, 111.92, 110.33, 55.81, 55.77 ppm. HRMS m/z [M+H]+ calcd for C20H16N4O3S 393.1016, found 393.1019.
- 6-(3,4-Dimethoxyphenyl)-3-(2-(pyridin-3-yl)ethyl)isothiazolo[4,3-b]pyridine (22)
- To a solution of compound 21 (200 mg, 0.54 mmol, 1.0 eq.) in a mixture of EtOH/THF (10 mL, in a ratio of 9:1), palladium hydroxide on carbon 20 wt. % was (100 mg) was added. The air from the flask was evacuated and filled with hydrogen using a balloon. This procedure was repeated 3 times and the reaction mixture was stirred under hydrogen at 60 °C for 3 days. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 9:1) as mobile phase and a second time using hexane and acetone (7:3), affording the title compound as a beige solid in 20% yield (40.4 mg, 0.11 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.27 (t, J = 7.6 Hz, 2H, CH2), 3.79 (t, J = 7.7 Hz, 2H, CH2), 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.23 (dd, J = 7.7, 4.8 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.3 Hz, 1H, arom H), 7.52–7.57 (m, 1H, arom H), 8.13 (d, J = 2.0 Hz, 1H, arom H), 8.54–8.46 (m, 2H, arom H), 9.02 (d, J = 2.0 Hz, 1H, arom H) ppm. 13C NMR (126 MHz, CDCl3) δ: 27.54 (CH2), 33.68 (CH2), 56.05 (OCH3), 110.50 (CH), 111.78 (CH), 120.13 (CH), 123.44 (CH), 125.08 (CH), 129.92 (C), 135.46 (C), 135.83 (C), 135.99 (CH), 145.14 (C), 148.07 (CH), 149.64 (C), 149.79 (C), 149.97 (CH), 150.53 (CH), 155.74 (C), 163.66 (C) ppm. HRMS m/z [M+H]+ calcd for C21H19N3O2S 378.1273, found 378.1271.
- (Z)-6-(3,4-Dimethoxyphenyl)-3-(2-methoxy-2-(pyridin-3-yl)vinyl)isothiazolo[4,3-b]pyridine (23a)
- To the solution of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine 21 (187 mg, 0.5 mmol, 1.0 eq.) in methanol (5 mL) at room temperature, potassium tert-butoxide (112 mg, 1.0 mmol, 2.0 eq.) was added. The resulting mixture was stirred under reflux for 2 h. After cooling down to room temperature, the mixture was concentrated under reduced pressure. The residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (150 mg, 0.37 mmol, 74%).
- 1H NMR (300 MHz,) δ: 8.99 (s, 1H), 8.95 (s, 1H), 8.69 (d, J = 4.5 Hz, 1H), 8.16 (s, 1H), 7.96 (d, J = 7.8 Hz, 1H), 7.42 (dd, J = 7.8 & 4.5 Hz, 1H), 7.36 (s, 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.21 (s, 1H), 7.02 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.96 (s, 3H), 3.90 (s, 3H) ppm.
- 13C NMR (150 MHz, CDCl3 + CD3OD) δ: 157.26, 156.32, 154.34, 150.53, 149.63, 149.63, 148.42, 144.29, 135.96, 134.48, 130.12, 129.42, 124.94, 123.59, 120.11, 111.77, 110.52, 104.07, 58.09, 56.07 ppm. HRMS m/z [M+H]+ calcd for C22H19N3O3S 406.1220, found 406.1208.
- ((Z)-2-((2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)oxy)-N,N-dimethylethan-1-amine (23b)
- To a solution of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (187 mg, 0.5 mmol, 1.0 eq.) in 2-(dimethylamino)ethan-1-ol (4 mL) at room temperature, potassium tert-butoxide (112 g, 1.0 mmol, 2.0 eq.) was added. The resulting mixture was stirred at 80 °C for 2 h. The mixture was cooled down to room temperature and concentrated under reduced pressure. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (167 mg, 0.36 mmol, 72%).
- 1H NMR (300 MHz, CDCl3) δ: 8.99 (s, 1H), 8.97 (d, J = 1.8 Hz, 1H), 8.68 (d, J = 4.0 Hz, 1H), 8.15 (d, J = 1.8 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 7.8 & 4.8 Hz, 1H), 7.31 (m, 2H), 7.20 (d, J = 1.8 Hz, 1H), 7.02 (d, J = 8.3 Hz, 1H), 4.10 (t, J = 6.0 Hz, 2H), 3.99 (s, 3H), 3.96 (s, 3H), 2.89 (t, J = 6.0 Hz, 2H), 2.32 (s, 6H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 156.47, 156.33, 154.25, 150.45, 149.71, 149.6, 148.48, 144.40, 135.94, 134.58, 130.13, 129.92, 124.91, 123.52, 120.10, 111.76, 110.51, 104.11, 68.99, 59.14, 56.06, 45.94 ppm.
- HRMS m/z [M+H]+ calcd for C25H26N4O3S 463.1798, found 463.1795.
- (Z)-4-(2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)morpholine (23c)
- A suspension of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (187 mg, 0.5 mmol, 1.0 eq.) in morpholine (5 mL) was heated at 80 °C for 3h. The mixture was cooled down to room temperature and concentrated under reduced pressure to dryness. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (140 mg, 0.30 mmol, 60%).
- 1H NMR (300 MHz, CDCl3) δ: 8.86 (m, 2H), 8.66 (s, 1H), 7.95 (s, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.53 (dd, J = 7.7 & 5.0 Hz, 1H), 7.23 (d, J = 8.3 Hz, 1H), 7.16 (s, 1H), 7.00 (d, J = 8.3 Hz, 1H), 6.79 (s, 1H), 3.96 (s, 3H), 3.94 (s, 3H), 3.79 (m, 4H), 3.23 (m, 4H) ppm. 13C NMR (75 MHz, CDCl3) δ: 162.4, 154.36, 151.80, 151.42, 150.35, 149.60, 148.00, 144.05, 137.50, 135.94, 131.71, 130.27, 125.10, 124.71, 119.94, 111.71, 110.42, 94.86, 66.62, 66.45, 56.04, 56.02, 48.38 ppm. HRMS m/z [M+H]+ calcd for C25H24N4O3S 461.1642, found 461.1637.
- (Z)-2-((2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)thio)ethan-1-ol (23d)
- The mixture of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine 187 mg, 0.5 mmol, 1.0 eq.), 2-mercaptoethan-1-ol (156 mg, 2.0 mmol, 4.0 eq.) and K2CO3 (280 mg, 2.0 mmol, 4.0 eq.) in THF (5 mL) was heated under reflux stirred for 2 h. The mixture was cooled down to room temperature and concentrated under reduced pressure to dryness. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (167 mg, 0.37 mmol, 74%).
- 1H NMR (300 MHz, CDCl3) δ: 8.93 (s, 1H), 8.80 (d, J = 4.6 Hz, 1H), 8.66 (s, 1H), 7.99 (s, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.64 (s, 1H), 7.49 (dd, J = 7.7 & 5.0 Hz, 1H), 7.20 (d, J = 8.3 Hz, 1H), 7.13 (s, 1H), 6.98 (d, J = 8.3 Hz, 1H), 3.96 (s, 3H), 3.95 (m, 2H), 3.94 (s, 3H), 3.16 (m, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 157.65, 154.50, 151.05, 150.04, 149.83, 149.62, 149.54, 143.95, 141.95, 136.94, 136.21, 133.84, 129.62, 124.86, 120.05, 114.41, 111.73, 110.35, 60.51, 56.05, 35.41 ppm.
- HRMS m/z [M+H]+ calcd for C23H21N3O3S2 452.1097, found 452.1092.
- Kinase assays
Compounds were screened for PIKfyve activity using a mobility shift assay (Nanosyn, Santa Clara, CA, USA). Data are expressed as compound concentration that causes 50% inhibition of the enzymatic activity (IC50 values). Various compounds were also tested for their binding affinity to PIP4K2C, using the DiscoverX/Kinome platform, in which the results are expressed as the dissociation constant value (KD values).
- Antiviral screening
- Cell lines for antiviral and viability assays
Human astrocytes (U-87 MG, ATCC, Manassas, VA, USA) and Vero E6/TMPRSS2 (JCRB cell bank, Osaka, Japan #cat JCRB1819) cells were grown in Dulbecco’s Modified Eagle’s medium DMEM (Gibco, Waltham, MA, USA) supplemented with 10% FCS (Biowest, Bradenton, FL, USA), 1% L-glutamine (Gibco, Waltham, MA, USA), and 1% penicillin-streptomycin (Pen-strep, Gibco, Waltham, MA, USA). Vero E6 (ATCC, Manassas, VA, USA) cells were maintained in DMEM supplemented with 10% FCS, 1% L-glutamine, 1% Pen-strep, 1% nonessential amino acids (Corning, New York, USA), 1% HEPES (Gibco, Waltham, MA, USA), 1% Sodium pyruvate (Thermo Fisher Scientific, Waltham, MA, USA). All cell lines were maintained in a humidified incubator with 5% CO2 at 37 °C and tested negative for mycoplasma by MycoAlert (Lonza, Morristown, NJ, USA). Cells from passages 14–15 were used for this study.
- Virus constructs
The plasmid encoding infectious VEEV (TC-83) with a nanoluciferase reporter (VEEV-TC-83-Cap-nLuc-Tav) used for virus production (VEEV-TC-83-nLuc) was a gift from Dr. William B. Klimstra (Department of Immunology, University of Pittsburgh, Pittsburgh) [32]. Plasmids used to produce SARS-CoV-2 (SARS-CoV-2 expressing nLuc-reporter gene) were a gift from Dr. Luis Martinez-Sobrido [33].
- Virus production
The VEEV-TC-83-nLuc virus was harvested from culture supernatant 24 h post-electroporation. Viral stock for SARS-CoV-2-nLuc was generated as previously described [34]. Virus produced in Vero E6/TMPRSS2 cells and passaged 3–4 times was used for the experiments.
Supernatants were collected, clarified and stored at −80 °C. Viral titers were measured using plaque assays on Vero (VEEV) or Vero E6 cells (SARS-CoV-2).
- Measuring antiviral activity
U-87 MG and Vero E6/TMPRSS2 cells were pretreated with the compounds or DMSO for 1 h prior to infection with VEEV-TC-83-nLuc or SARS-CoV-2-nLuc at a multiplicity of infection (MOI) of 0.01 (VEEV) and 0.5 (SARS-CoV-2) respectively (n = 4). The inhibitors were maintained for the duration of the experiment. Viral infection was measured at 18 h post-infection (VEEV) and 24 h post-infection using a nanoluciferase assay. The relative light units (RLUs) were normalized to DMSO-treated cells (set as 100%).
- Cell viability assays
Cell viability was measured in virus-infected cells using the AlamarBlue® reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s protocol. Fluorescence was detected at 560 nm on GloMax Discover Microplate Reader (Promega, Madison, WI, USA). The raw fluorescence values were normalized to DMSO-treated cells (set as 100%).
- Antitumoral assays
- Cell culture
Cancer cell lines Capan-1, HCT-116, NCI-H460, LN-229, HL-60, K-562 and Z-138 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), and the DND-41 cell line was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ Leibniz-Institut, Braunschweig, Germany). All cell lines were cultured according to the suppliers’ recommendations. Culture media were acquired from Gibco Life Technologies (Waltham, MA, USA) and supplemented with 10% fetal bovine serum (HyClone, GE Healthcare Life Sciences, Boston, MA, USA).
- Cell proliferation assays
Adherent cell lines Capan-1, HCT-116, NCI-H460 and LN-229 were seeded at densities ranging from 500 to 1500 cells per well in 384-well culture plates (Greiner, Vilvoorde, Belgium). After overnight incubation, cells were treated with various concentrations of the test compounds. Suspension cell lines DND-41, HL-60, K-562 and Z-138 were seeded at densities between 2500 to 5500 cells per well in 384-well culture plates containing the test compounds at identical concentration points. After 72 h of incubation with the compounds, cell viability was assessed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) reagent according to the manufacturer’s instructions (Promega, Madison, WI, USA). Absorbance was measured at 490 nm using a SpectraMax Plus 384 (Molecular Devices, San Jose, CA, USA) and the optical density values were utilized to determine the 50% cytotoxic concentration (CC50). Each compound underwent testing in two independent experiments.
4. Conclusions
A previously discovered dual inhibitor of the lipid kinases PIKfyve and PIP4K2C (RMC-113) was subjected to a systematic SAR study by introducing structural modifications at positions 3 and 6 of the central isothiazolo[4,3-b]pyridine scaffold (Figure 5). The SAR study was guided by a biochemical PIKfyve assay. PIKfyve inhibition tolerated a wide variety of substituents (alkyl, alkoxy, halogens and carboxamides) on the aryl ring at position 6, with the 4-carboxamide analogue emerging as the most potent PIKfyve inhibitor (IC50 = 1 nM). In contrast, the SAR at position 3 was more restricted. Replacement of the pyridinyl moiety by (substituted) phenyl rings reduced the enzymatic activity. The introduction of small electron-donating substituents (methyl and methoxy) on the pyridinyl yielded potent PIKfyve inhibitors, with IC50 values in the low nM range. The acetylenic moiety proved to be essential for PIKfyve inhibition, and only the saturated congener containing an ethyl linker displayed potent PIKfyve inhibition, albeit less active than the acetylene counterpart. In general, the SAR against PIKfyve and PIP4K2C ran in parallel, although the compounds were 2- to 5-fold less potent on PIP4K2C relative to PIKfyve. The majority of the dual PIKfyve/PIP4K2C inhibitors displayed low µM antiviral activity against both VEEV and SARS-CoV-2. An antitumoral screening against a variety of cancer cell lines revealed that some of the potent PIKfyve/PIP5K2C inhibitors lack antitumoral activity. Overall, the correlation between enzymatic potency and cellular efficacy is not very clear, which will need further investigation. The most promising antiviral and/or antitumoral compounds will need to be evaluated for their pharmacokinetic properties. Given the fact that some of the isothiazolo[4,3-b]pyridines show antiviral as well as antitumoral activity (e.g., compounds 3m and 7l), priority might be given to antitumoral evaluation.
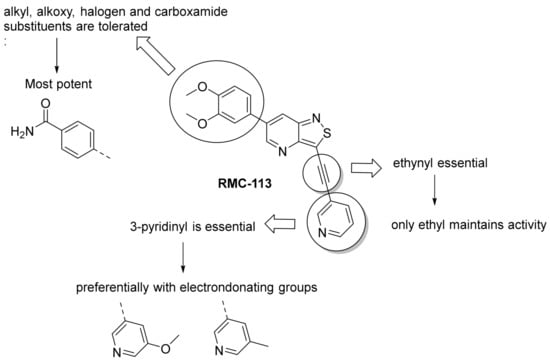
Figure 5.
Summary of the SAR study versus PIKfyve.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18091341/s1, Copies of 1H- and 13C-NMR spectra.
Author Contributions
Conceptualization, D.S., W.D., S.E. and S.D.J.; Data curation, M.K.; Formal analysis, S.E. and S.D.J.; Funding acquisition, D.S., W.D., S.E. and S.D.J.; Investigation, D.K., L.-J.G., B.M.-G., M.K., S.S., D.H.N.T., J.R. and L.P.; Methodology, D.K., L.-J.G., B.M.-G., M.K. and S.S.; Project administration, S.E. and S.D.J.; Resources, D.S., W.D. and S.E.; Supervision, W.D. and S.E.; Validation, D.K., L.-J.G., B.M.-G., M.K., S.S. and L.P.; Writing—original draft, D.K., L.-J.G., B.M.-G., M.K., S.S., D.H.N.T. and S.D.J.; Writing—review and editing, D.S., W.D., S.E. and S.D.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant 1R01AI158569-01 from the NIH, awards HDTRA11810039 from the Defense Threat Reduction Agency/Fundamental Research to Counter Weapons of Mass Destruction, and W81XWH2210283 and W81XWH-16-1-0691 from the Department of Defense/Congressionally Directed Medical Research Programs (to SE and SDJ). MK was supported by a PhRMA Foundation Postdoctoral Fellowship in Translational Medicine and NIH T32 training grant (5T32AI007502-27). Mass spectrometry was made possible by the support of the Hercules Foundation of the Flemish Government (grant 20100225-7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zolov, S.N.; Bridges, D.; Zhang, Y.; Lee, W.-W.; Riehle, E.; Verma, R.; Lenk, G.M.; Converso-Baran, K.; Weide, T.; Albin, R.L.; et al. In Vivo, Pikfyve Generates PI(3,5)P2, Which Serves as Both a Signaling Lipid and the Major Precursor for PI5P. Proc. Natl. Acad. Sci. USA 2012, 109, 17472–17477. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Lang, M.J.; Weisman, L.S. Phosphatidylinositol 3,5-bisphosphate: Regulation of cellular events in space and time. Biochem. Soc. Trans. 2016, 44, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Ríos, P.; Weisman, L.S. Roles of PIKfyve in multiple cellular pathways. Curr. Opin. Cell Biol. 2022, 76, 102086. [Google Scholar] [CrossRef] [PubMed]
- Rameh, L.E.; Blind, R.D. 25 Years of PI5P. Front. Cell. Dev. Biol. 2023, 11, 1272911. [Google Scholar] [CrossRef]
- Jefferies, H.B.; Cooke, F.T.; Jat, P.; Boucheron, C.; Koizumi, T.; Hayakawa, M.; Kaizawa, H.; Ohishi, T.; Workman, P.; Waterfield, M.D.; et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008, 9, 164–170. [Google Scholar] [CrossRef]
- Hayakawa, N.; Noguchi, M.; Takeshita, S.; Eviryanti, A.; Seki, Y.; Nishio, H.; Yokoyama, R.; Noguchi, M.; Shuto, M.; Shima, Y.; et al. Structure-activity relationship study, target identification, and pharmacological characterization of a small molecular IL-12/23 inhibitor, APY0201. Bioorg. Med. Chem. 2014, 22, 3021–3029. [Google Scholar] [CrossRef]
- Cai, X.; Xu, Y.; Cheung, A.K.; Tomlinson, R.C.; Alcázar-Román, A.; Murphy, L.; Billich, A.; Zhang, B.; Feng, Y.; Klumpp, M.; et al. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 2013, 20, 912–921. [Google Scholar] [CrossRef]
- Sano, O.; Kazetani, K.; Funata, M.; Fukuda, Y.; Matsui, J.; Iwata, H. Vacuolin-1 inhibits autophagy by impairing lysosomal maturation via PIKfyve inhibition. FEBS Lett. 2016, 590, 1576–1585. [Google Scholar] [CrossRef]
- Drewry, D.H.; Potjewyd, F.M.; Bayati, A.; Smith, J.L.; Dickmander, R.J.; Howell, S.; Taft-Benz, S.; Min, S.M.; Hossain, M.A.; Heise, M.; et al. Identification and Utilization of a Chemical Probe to Interrogate the Roles of PIKfyve in the Lifecycle of β-Coronaviruses. J. Med. Chem. 2022, 65, 12860–12882. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Wei, Y.; Wei, H.; Yuan, X.; Xiong, B.; Tang, M.; Yang, T.; Yang, Z.; Ye, H.; et al. Discovery of Potent and Selective Phosphatidylinositol 3-Phosphate 5-Kinase (PIKfyve) Inhibitors as Methuosis Inducers. J. Med. Chem. 2024, 67, 165–179. [Google Scholar] [CrossRef]
- Huang, P.; Einav, S.; Asquith, C.R.M. PIKfyve: A lipid kinase target for COVID-19, cancer and neurodegenerative disorders. Nat. Rev. Drug Discov. 2021, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Gayle, S.; Landrette, S.; Beeharry, N.; Conrad, C.; Hernandez, M.; Beckett, P.; Ferguson, S.M.; Mandelkern, T.; Zheng, M.; Xu, T.; et al. Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 2017, 129, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.L.; Chou, Y.Y.; Rothlauf, P.W.; Liu, Z.; Soh, T.K.; Cureton, D.; Case, J.B.; Chen, R.E.; Diamond, M.S.; Whelan, S.P.J.; et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 20803–20813. [Google Scholar] [CrossRef]
- Nelson, E.A.; Dyall, J.; Hoenen, T.; Barnes, A.B.; Zhou, H.; Liang, J.Y.; Michelotti, J.; Dewey, W.H.; DeWald, L.E.; Bennett, R.S.; et al. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Negl. Trop. Dis. 2017, 11, e0005540. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Ferreira, A.; Mariën, J.; Delay, C.; Lee, E.; Trojanowski, J.Q.; Moechars, D.; Annaert, W.; De Muynck, L. PIKfyve activity is required for lysosomal trafficking of tau aggregates and tau seeding. J. Biol. Chem. 2021, 296, 100636. [Google Scholar] [CrossRef]
- Hung, S.T.; Linares, G.R.; Chang, W.H.; Eoh, Y.; Krishnan, G.; Mendonca, S.; Hong, S.; Shi, Y.; Santana, M.; Kueth, C.; et al. PIKFYVE Inhibition Mitigates Disease in Models of Diverse Forms of ALS. Cell 2023, 186, 786–802.e28. [Google Scholar] [CrossRef]
- Rameh, L.E.; Tolias, K.F.; Duckworth, B.C.; Cantley, L.C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 1997, 390, 192–196. [Google Scholar] [CrossRef]
- Bulley, S.J.; Clarke, J.H.; Droubi, A.; Giudici, M.L.; Irvine, R.F. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv. Biol. Regul. 2015, 57, 193–202. [Google Scholar] [CrossRef]
- Arora, G.K.; Palamiuc, L.; Emerling, B.M. Expanding role of PI5P4Ks in cancer: A promising druggable target. FEBS Lett. 2022, 596, 3–16. [Google Scholar] [CrossRef]
- Clarke, J.H.; Giudici, M.L.; Burke, J.E.; Williams, R.L.; Maloney, D.J.; Marugan, J.; Irvine, R.F. The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P-binding site. Biochem. J. 2015, 466, 359–367. [Google Scholar] [CrossRef]
- Boffey, H.K.; Rooney, T.P.C.; Willems, H.M.G.; Edwards, S.; Green, C.; Howard, T.; Ogg, D.; Romero, T.; Scott, D.E.; Winpenny, D.; et al. Development of selective phosphatidylinositol 5-phosphate 4-kinase γ inhibitors with a non-ATP-competitive, allosteric binding mode. J. Med. Chem. 2022, 65, 3359–3370. [Google Scholar] [CrossRef] [PubMed]
- Rooney, T.P.C.; Aldred, G.G.; Boffey, H.K.; Willems, H.M.G.; Edwards, S.; Chawner, S.J.; Scott, D.E.; Green, C.; Winpenny, D.; Skidmore, J.; et al. The Identification of Potent, Selective, and Brain Penetrant PI5P4Kγ Inhibitors as In Vivo-Ready Tool Molecules. J. Med. Chem. 2023, 66, 804–821. [Google Scholar] [CrossRef]
- Sivakumaren, S.C.; Shim, H.; Zhang, T.; Ferguson, F.M.; Lundquist, M.R.; Browne, C.M.; Seo, H.S.; Paddock, M.N.; Manz, T.D.; Jiang, B.; et al. Targeting the PI5P4K Lipid Kinase Family in Cancer Using Covalent Inhibitors. Cell. Chem. Biol. 2020, 27, 525–537. [Google Scholar] [CrossRef]
- Karim, M.; Mishra, M.; Lo, C.W.; Saul, S.; Cagirici, H.; Gourdelier, M.; Ghita, L.; Ojha, A.; Tran, D.H.N.; Agrawal, A.; et al. PIP4K2C inhibition reverses autophagic flux impairment induced by SARS-CoV-2. Nat. Commun. 2025, 16, 6397. [Google Scholar] [CrossRef]
- Kovackova, S.; Chang, L.; Bekerman, E.; Neveu, G.; Barouch-Bentov, R.; Chaikuad, A.; Heroven, C.; Šála, M.; De Jonghe, S.; Knapp, S.; et al. Selective Inhibitors of Cyclin G Associated Kinase (GAK) as Anti-Hepatitis C Agents. J. Med. Chem. 2015, 58, 3393–3410. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, G.; Liu, Q. A mild copper-mediated Glaser-type coupling reaction under the novel CuI/NBS/DIPEA promoting system. Tetrahedron Lett. 2009, 50, 4033–4036. [Google Scholar] [CrossRef]
- Han, G.; Tamaki, M.; Hruby, V.J. Fast, efficient and selective deprotection of the tert-butoxycarbonyl (Boc) group using HCl/dioxane (4 m). J. Pept. Res. 2001, 58, 338–341. [Google Scholar] [CrossRef]
- Wouters, R.; Pu, S.Y.; Froeyen, M.; Lescrinier, E.; Einav, S.; Herdewijn, P.; De Jonghe, S. Cyclin G-associated kinase (GAK) affinity and antiviral activity studies of a series of 3-C-substituted isothiazolo[4,3-b]pyridines. Eur. J. Med. Chem. 2019, 163, 256–265. [Google Scholar] [CrossRef]
- Clarke, J.H.; Irvine, R.F. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem. J. 2013, 454, 49–57. [Google Scholar] [CrossRef]
- Sharma, G.; Guardia, C.M.; Roy, A.; Vassilev, A.; Saric, A.; Griner, L.N.; Marugan, J.; Ferrer, M.; Bonifacino, J.S.; DePamphilis, M.L. A family of PIKFYVE inhibitors with therapeutic potential against autophagy-dependent cancer cells disrupt multiple events in lysosome homeostasis. Autophagy 2019, 15, 1694–1718. [Google Scholar] [CrossRef]
- Hou, J.; Xi, Z.; Niu, J.; Li, W.; Wang, X.; Liang, C.; Sun, H.; Fang, D.; Xie, S. Inhibition of PIKfyve using YM201636 suppresses the growth of liver cancer via the induction of autophagy. Oncol. Rep. 2019, 41, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gardner, C.L.; Watson, A.M.; Ryman, K.D.; Klimstra, W.B. Stable, high-level expression of reporter proteins from improved alphavirus expression vectors to track replication and dissemination during encephalitic and arthritogenic disease. J. Virol. 2014, 88, 2035. [Google Scholar] [CrossRef]
- Chiem, K.; Vasquez, D.M.; Park, J.; Platt, R.N.; Anderson, T.; Walter, M.R.; Kobie, J.J.; Ye, C.; Martinez-Sobrido, L. Generation and Characterization of recombinant SARS-CoV-2 expressing reporter genes. J. Virol. 2021, 95, e02209-20. [Google Scholar] [CrossRef]
- Saul, S.; Karim, M.; Ghita, L.; Huang, P.; Chiu, W.; Durán, V.; Lo, C.; Kumar, S.; Bhalla, N.; Leyssen, P.; et al. Anticancer pan-ErbB inhibitors reduce inflammation and tissue injury and exert broad-spectrum antiviral effects. J. Clin. Investig. 2023, 133, e169510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).