Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia marina
Abstract
1. Introduction
2. Results
2.1. Characterization of Avicennia marina Extracts
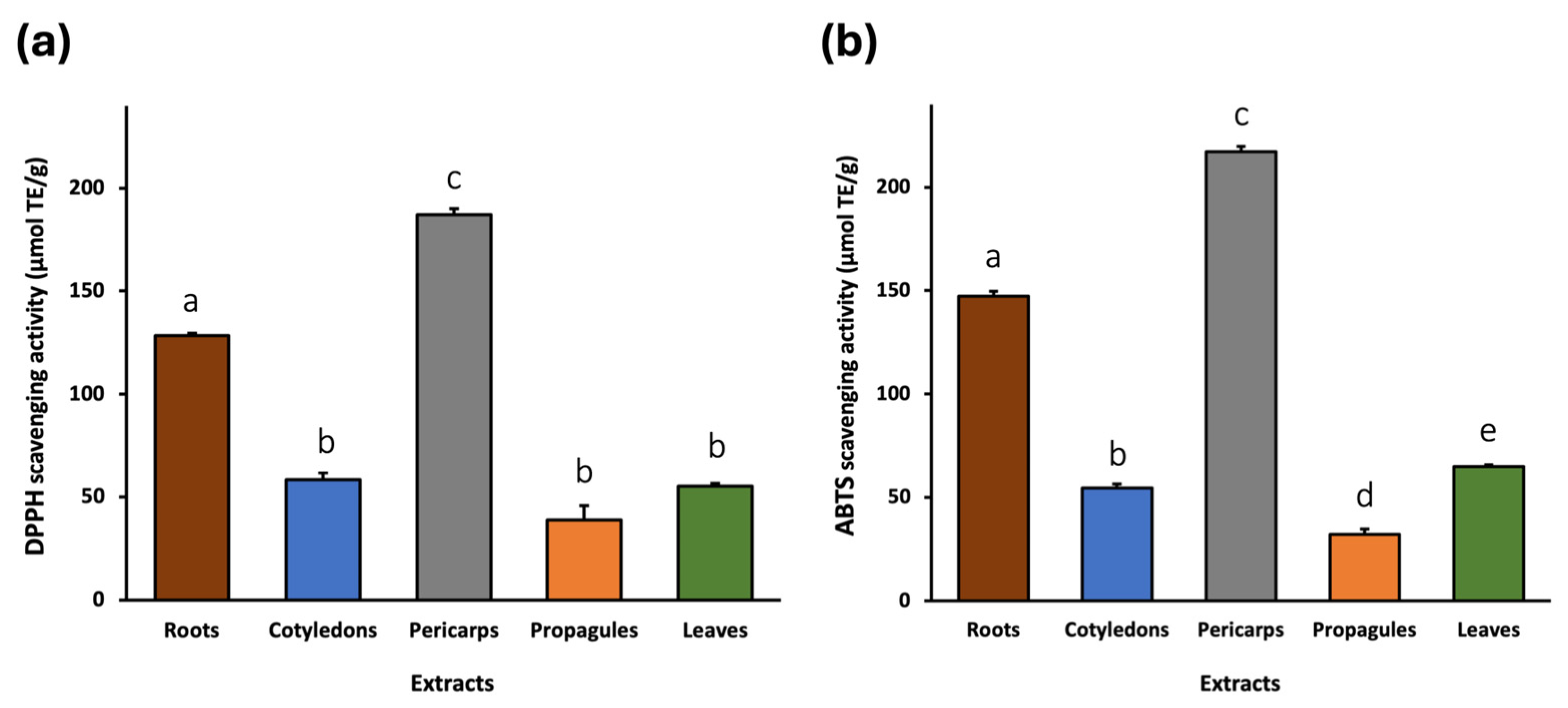
2.2. Antioxidant Activity
2.2.1. DPPH and ABTS Assays
2.2.2. Correlation Between Compound Classes and Antioxidant Activity
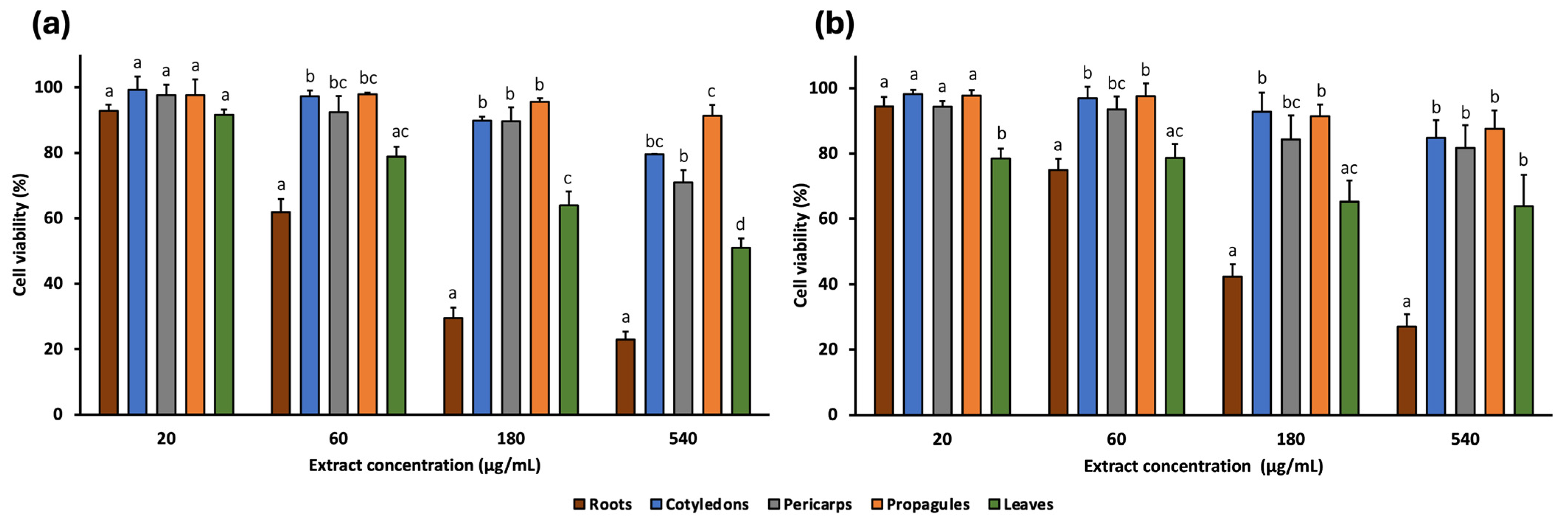
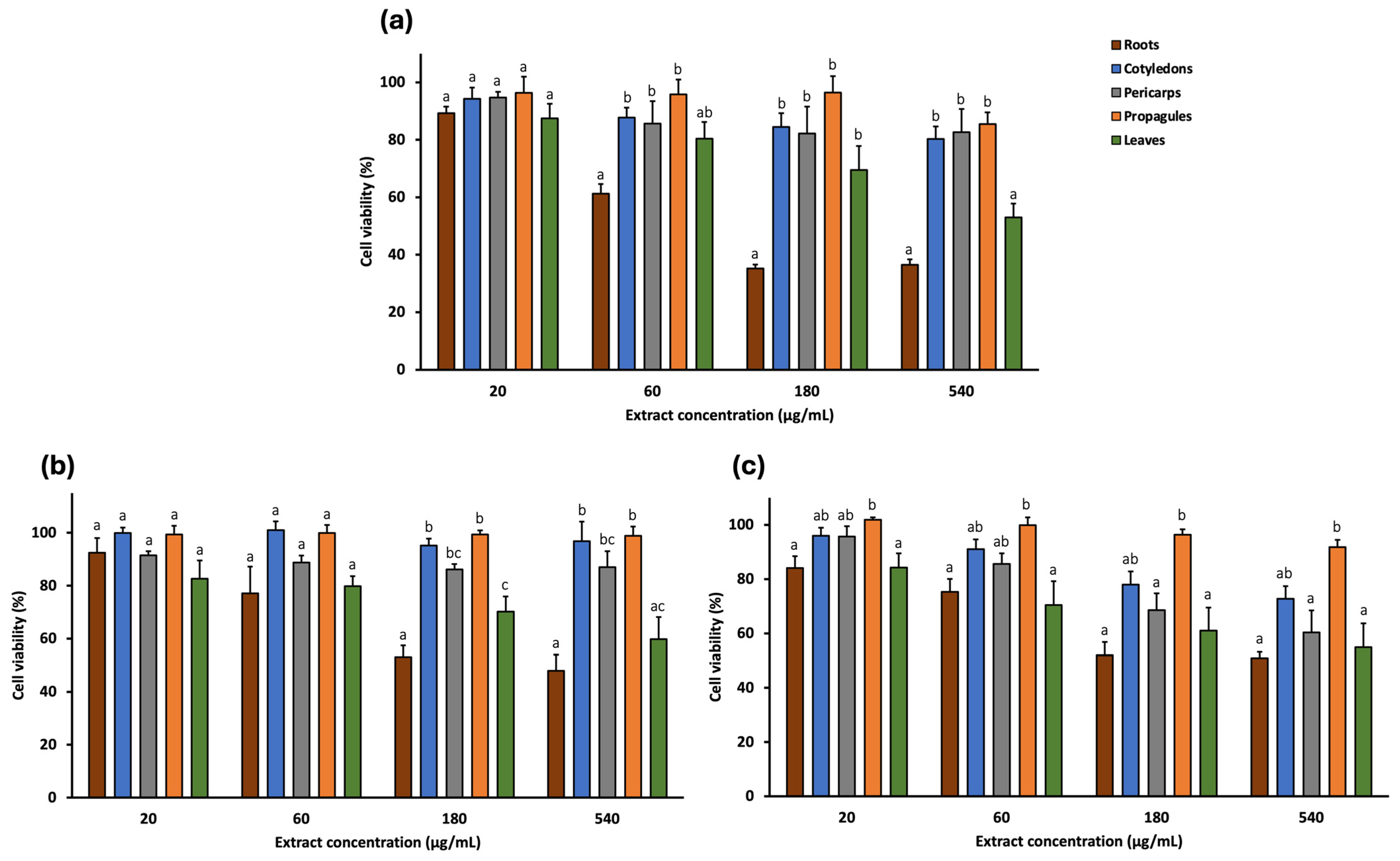
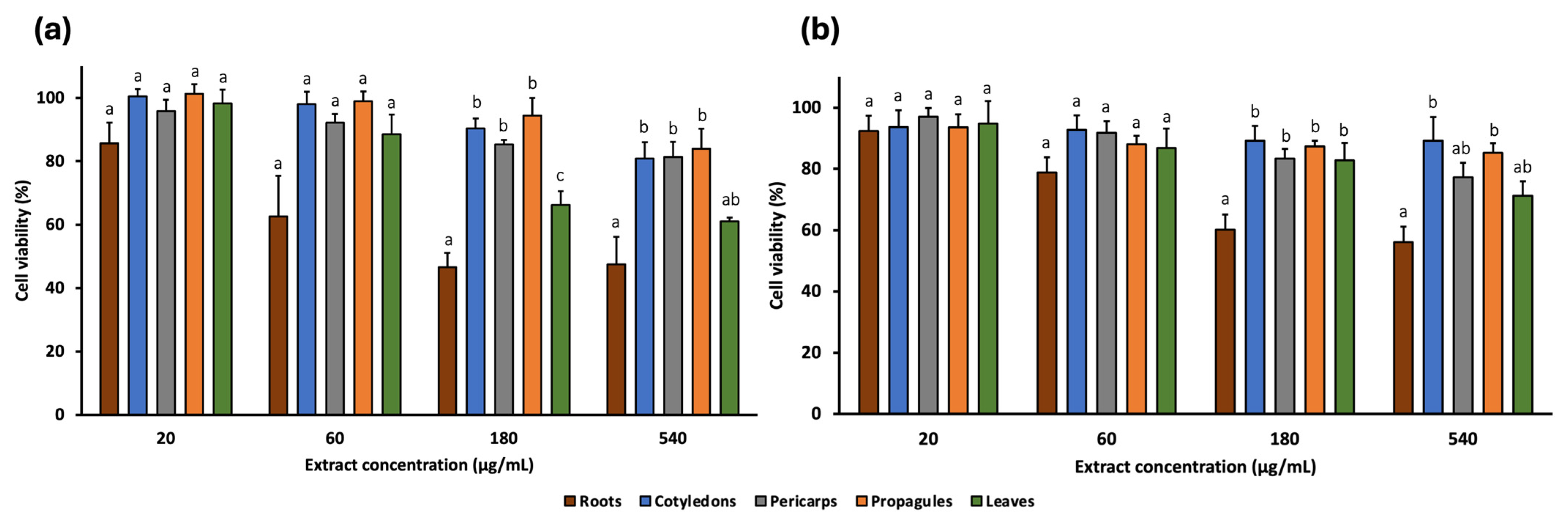
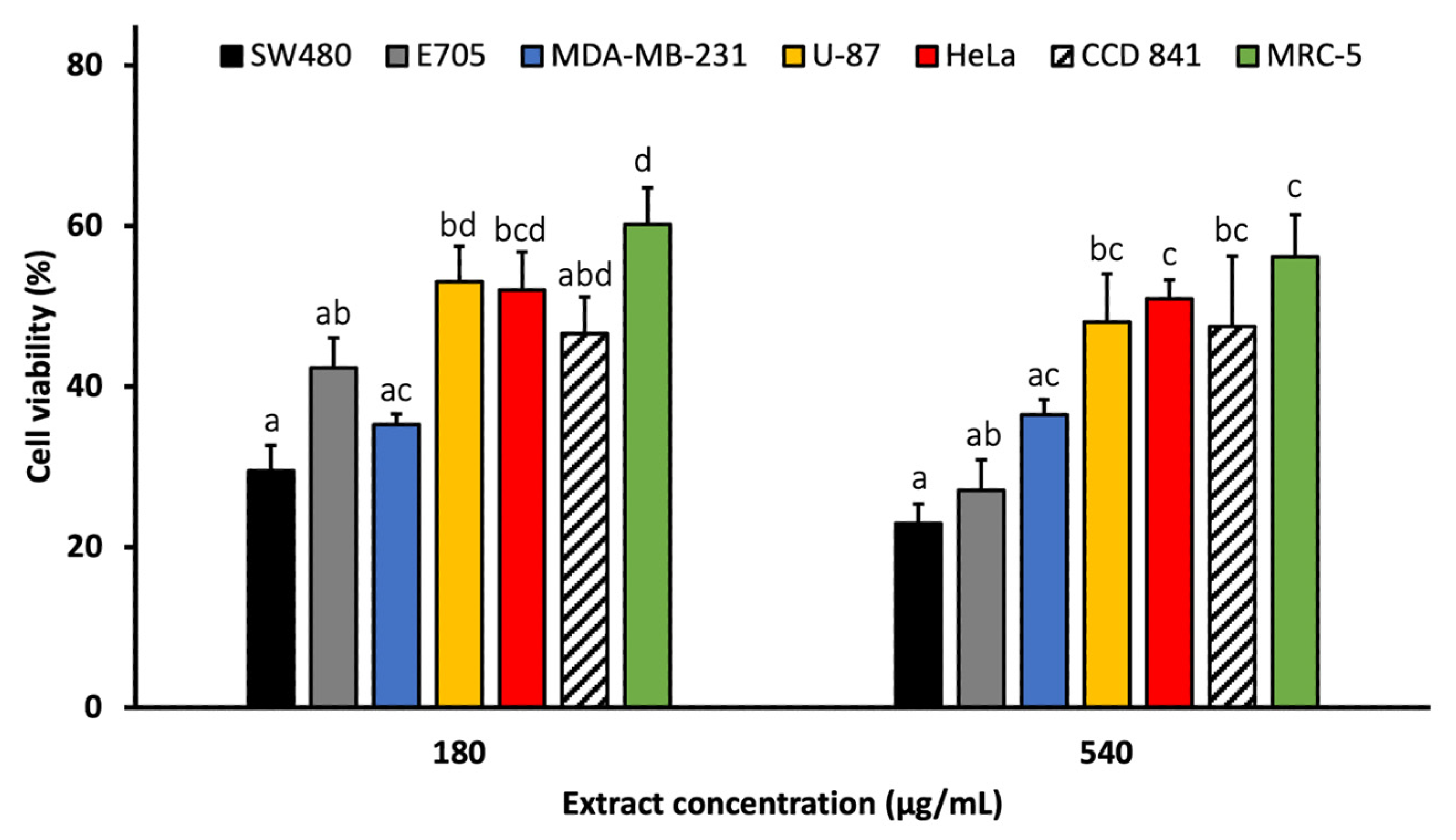
2.3. In Vitro Cytotoxic Activity
2.4. In Silico Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Sample Preparation and Extraction
4.4. Characterization of Extracts
4.5. Determination of Antioxidant Activity
4.6. Cytotoxicity Evaluation
4.6.1. Cell Lines and Culture Conditions
4.6.2. Viability Assay
4.7. In Silico Prediction for Anticancer Activity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kathiresan, K.; Bingham, B.L. Biology of Mangroves and Mangrove Ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Cerri, F.; Louis, Y.D.; Fallati, L.; Siena, F.; Mazumdar, A.; Nicolai, R.; Zitouni, M.S.; Adam, A.S.; Mohamed, S.; Lavorano, S.; et al. Mangroves of the Maldives: A Review of Their Distribution, Diversity, Ecological Importance and Biodiversity of Associated Flora and Fauna. Aquat. Sci. 2024, 86, 44. [Google Scholar] [CrossRef]
- Cerri, F.; Giustra, M.; Anadol, Y.; Tomaino, G.; Galli, P.; Labra, M.; Campone, L.; Colombo, M. Natural Products from Mangroves: An Overview of the Anticancer Potential of Avicennia marina. Pharmaceutics 2022, 14, 2793. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.; Dahse, H.M.; Moellmann, U.; Fu, H.; Grabley, S.; Sattler, I.; Lin, W. Unusual Naphthoquinone Derivatives from the Twigs of Avicennia marina. J. Nat. Prod. 2007, 70, 923–927. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. New Flavonoids from Avicennia marina. Fitoterapia 2000, 71, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.M.; Duan, X.J.; Wang, B.G. Iridoid Glucosides and Flavones from the Aerial Parts of Avicennia marina. Chem. Biodivers. 2006, 3, 799–806. [Google Scholar] [CrossRef]
- Sun, Y.; Ouyang, J.; Deng, Z.; Li, Q.; Lin, W. Structure Elucidation of Five New Iridoid Glucosides from the Leaves of Avicennia marina. Magn. Reson. Chem. 2008, 46, 638–642. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.; Dahse, H.-M.; Moellmann, U.; Grabley, S.; Lin, W.; Sattler, I. New Abietane Diterpenoids from the Mangrove Avicennia marina. Planta Med. 2008, 74, 432–437. [Google Scholar] [CrossRef]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Rengasamy Kannan, R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, H.; Wu, X.; Feng, Z.; Li, X.; Tavakoli, S.; Wu, K.; Deng, L.; Luo, H. Botany, traditional uses, phytochemistry, pharmacological activities, and toxicity of the mangrove plant Avicennia marina: A comprehensive review. Phytochem. Rev. 2025, 1–36. [Google Scholar] [CrossRef]
- ElDohaji, L.M.; Hamoda, A.M.; Hamdy, R.; Soliman, S.S. Avicennia marina a natural reservoir of phytopharmaceuticals: Curative power and platform of medicines. J. Ethnopharmacol. 2020, 263, 113179. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sham, A.; Elbadawi, A.A.; Hassan, A.H.; Alhosani, B.K.K.; El-Esawi, M.A.; AlKhajeh, A.S.; AbuQamar, S.F. A Consortium of Rhizosphere-Competent Actinobacteria Exhibiting Multiple Plant Growth-Promoting Traits Improves the Growth of Avicennia marina in the United Arab Emirates. Front. Mar. Sci. 2021, 8, 715123. [Google Scholar] [CrossRef]
- Department of Health—Abu Dhabi. Encyclopedia of Medicine Plant of UAE. Available online: https://www.medicinalplants.doh.gov.ae/Encyclopedia-of-medicine-plant-of-UAE (accessed on 12 August 2025).
- Friis, G.; Killilea, M.E. Mangrove Ecosystems of the United Arab Emirates. In A Natural History of the Emirates; Burt, J.A., Ed.; Springer: Cham, Switzerland, 2023; pp. 217–240. [Google Scholar]
- Haseeba, K.P.; Aboobacker, V.M.; Vethamony, P.; Al-Khayat, J.A. Significance of Avicennia Marina in the Arabian Gulf Environment: A Review. Wetlands 2025, 45, 16. [Google Scholar] [CrossRef]
- Mitra, S.; Naskar, N.; Lahiri, S.; Chaudhuri, P. A study on phytochemical profiling of Avicennia marina mangrove leaves collected from Indian Sundarbans. Sustain. Chem. Environ. 2023, 4, 100041. [Google Scholar] [CrossRef]
- Khattab, R.A.; Temraz, T.A. Mangrove Avicennia marina of Yanbu, Saudi Arabia: GC-MS constituents and mosquito repellent activities. Egypt. J. Aquat. Biol. Fish. 2017, 21, 45–54. [Google Scholar] [CrossRef]
- Al-Mur, B.A. Biological activities of Avicennia marina roots and leaves regarding their chemical constituents. Arab. J. Sci. Eng. 2021, 46, 5407–5419. [Google Scholar] [CrossRef]
- Mohammed, H.A. Phytochemical Analysis, Antioxidant Potential, and Cytotoxicity Evaluation of Traditionally Used Artemisia Absinthium L. (Wormwood) Growing in the Central Region of Saudi Arabia. Plants 2022, 11, 1028. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Taher, E.A.; Al-Sagheer, N.A.; Al-Khulaidi, A.W.; Azizi, M.; Naguib, D.M. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal. Agric. Biotechnol. 2021, 34, 102020. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemistry and Anticancer Effects of Mangrove (Rhizophora mucronata Lam.) Leaves and Stems Extract against Different Cancer Cell Lines. Pharmaceuticals 2022, 16, 4. [Google Scholar] [CrossRef]
- Botosoa, E.P.; Shahidi, F. Phenolics and polyphenolics in mangrove plants: Antioxidant activity and recent trends in food application—A review. Crit. Rev. Food Sci. Nutr. 2025, 1–35. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; Zhang, J.; Chen, Y.; Lu, S. Simultaneous Determination of 32 Polyphenolic Compounds in Berries via HPLC–MS/MS. Molecules 2025, 30, 2008. [Google Scholar] [CrossRef] [PubMed]
- Kodikara, C.; Netticadan, T.; Bandara, N.; Wijekoon, C.; Sura, S. A new UHPLC-HRMS metabolomics approach for the rapid and comprehensive analysis of phenolic compounds in blueberry, raspberry, blackberry, cranberry and cherry fruits. Food Chem. 2024, 445, 138778. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Choudhary, K.K. Utilizing UHPLC-HRMS-metabolomic profiling to uncover enhanced bioactive potential and health benefits in chili (Capsicum annum L.) under salinity stress. Food Chem. 2025, 483, 144255. [Google Scholar] [CrossRef]
- Lee, I.Y.; Lee, D.H.; Park, J.H.; Joo, N. UHPLC-HRMS/MS–Based Metabolic Profiling and Quantification of Phytochemicals in Different Parts of Coccinia grandis (L.) Voigt. Food Sci. Nutr. 2025, 13, e70004. [Google Scholar] [CrossRef]
- Zanatta, A.C.; Vilegas, W.; Edrada-Ebel, R. UHPLC-(ESI)-HRMS and NMR-Based Metabolomics Approach to Access the Seasonality of Byrsonima intermedia and Serjania marginata From Brazilian Cerrado Flora Diversity. Front. Chem. 2021, 9, 534. [Google Scholar] [CrossRef]
- Mateos-Molina, D.; Ben Lamine, E.; Antonopoulou, M.; Burt, J.A.; Das, H.S.; Javed, S.; Judas, J.; Khan, S.B.; Muzaffar, S.B.; Pilcher, N.; et al. Synthesis and Evaluation of Coastal and Marine Biodiversity Spatial Information in the United Arab Emirates for Ecosystem-Based Management. Mar. Pollut. Bull. 2021, 167, 112319. [Google Scholar] [CrossRef]
- United Arab Emirates—Climatology | Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/country/united-arab-emirates/climate-data-historical#cckp-watershed-map (accessed on 1 April 2025).
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Maganj, J.; Zhang, Y.A.N.; Rowland, I.R.; Wagstaff, C. Analysis of Phytochemical Composition and Chemoprotective Capacity of Rocket (Eruca Sativa and Diplotaxis Tenuifolia) Leafy Salad Following Cultivation in Different Environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef]
- Abd-Elgawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat Affects the Chemical Profile, Allelopathy, and Antioxidant Properties of Essential Oils and Phenolic Enriched Extracts of the Invasive Plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef]
- Rozirwan; Nugroho, R.Y.; Hendri, M.; Fauziyah; Putri, W.A.E.; Agussalim, A. Phytochemical Profile and Toxicity of Extracts from the Leaf of Avicennia marina (Forssk.) Vierh. Collected in Mangrove Areas Affected by Port Activities. S. Afr. J. Bot. 2022, 150, 903–919. [Google Scholar] [CrossRef]
- Neugart, S.; Krumbein, A.; Zrenner, R. Influence of Light and Temperature on Gene Expression Leading to Accumulation of Specific Flavonol Glycosides and Hydroxycinnamic Acid Derivatives in Kale (Brassica oleracea var. sabellica). Front. Plant Sci. 2016, 7, 181383. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Du, F.; Liu, H.Y.; Liang, Z.S. Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J. Med. Plants 2010, 4, 2691–2699. [Google Scholar] [CrossRef]
- Ferdinando, M.D.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Falahi, H.; Sharifi, M.; Maivan, H.Z.; Chashmi, N.A. Phenylethanoid Glycosides Accumulation in Roots of Scrophularia Striata as a Response to Water Stress. Environ. Exp. Bot. 2018, 147, 13–21. [Google Scholar] [CrossRef]
- Franzoni, G.; Trivellini, A.; Bulgari, R.; Cocetta, G.; Ferrante, A. Bioactive molecules as regulatory signals in plant responses to abiotic stresses. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Duxford, UK, 2019; Volume 1, pp. 169–182. [Google Scholar]
- Sarri, E.; Termentzi, A.; Abraham, E.M.; Papadopoulos, G.K.; Baira, E.; Machera, K.; Loukas, V.; Komaitis, F.; Tani, E. Salinity Stress Alters the Secondary Metabolic Profile of M. sativa, M. arborea and Their Hybrid (Alborea). Int. J. Mol. Sci. 2021, 22, 4882. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2015, 101, 3–19. [Google Scholar] [CrossRef]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of Seasonal Variation on Thymus longicaulis C. Presl Chemical Composition and Its Antioxidant and Anti-Inflammatory Properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef]
- Fiori, J.; Amadesi, E.; Fanelli, F.; Tropeano, C.V.; Rugolo, M.; Gotti, R. Cellular and Mitochondrial Determination of Low Molecular Mass Organic Acids by LC–MS/MS. J. Pharm. Biomed. Anal. 2018, 150, 33–38. [Google Scholar] [CrossRef]
- Wang, D.-D.; Liang, J.; Yang, W.-Z.; Hou, J.j.; Yang, M.; Da, J.; Wang, Y.; Jiang, B.h.; Liu, X.; Wu, W.y.; et al. HPLC/QTOF-MS-Oriented Characteristic Components Data Set and Chemometric Analysis for the Holistic Quality Control of Complex TCM Preparations: Niuhuang Shangqing Pill as an Example. J. Pharm. Biomed. Anal. 2014, 89, 130–141. [Google Scholar] [CrossRef]
- Seo, O.N.; Kim, G.S.; Park, S.; Lee, J.H.; Kim, Y.H.; Lee, W.S.; Lee, S.J.; Kim, C.Y.; Jin, J.S.; Choi, S.K.; et al. Determination of Polyphenol Components of Lonicera japonica Thunb. Using Liquid Chromatography–Tandem Mass Spectrometry: Contribution to the Overall Antioxidant Activity. Food Chem. 2012, 134, 572–577. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.M.; Quirantes-Piné, R.; Rodríguez-Pérez, C.; Madani, K.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Tentative Characterisation of Iridoids, Phenylethanoid Glycosides and Flavonoid Derivatives from Globularia alypum L. (Globulariaceae) Leaves by LC-ESI-QTOF-MS. Phytochem. Analy 2014, 25, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Xu, Q.; Li, R.; Shi, L.; Han, Y.; Zhu, Y.; Wu, G.; Qin, M. Chemical Profiles and Quality Evaluation of Buddleja officinalis Flowers by HPLC-DAD and HPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 164, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Michalak, B.; Patyra, A.; Majdan, M. UHPLC-DAD-ESI-MS/MS and HPTLC Profiling of Ash Leaf Samples from Different Commercial and Natural Sources and Their in Vitro Effects on Mediators of Inflammation. Phytochem. Anal. 2020, 31, 57–67. [Google Scholar] [CrossRef]
- García-Villegas, A.; Fernández-Ochoa, Á.; Alañón, M.E.; Rojas-García, A.; Arráez-Román, D.; De La Luz Cádiz-Gurrea, M.; Segura-Carretero, A. Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract. Mar. Drugs 2024, 25, 3723. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Guo, H.; Yang, M.; Wang, B.-R.; Guo, D.-A. Analysis of Multiple Constituents in a Chinese Herbal Preparation Shuang-Huang-Lian Oral Liquid by HPLC-DAD-ESI-MSn. J. Pharm. Biomed. Anal. 2007, 44, 430–438. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, J.; Zhao, Y.; Huang, W.; Jiang, Y.; Li, M.; Wu, X.; Lu, B. Varietal Classification and Antioxidant Activity Prediction of Osmanthus fragrans Lour. Flowers Using UPLC–PDA/QTOF–MS and Multivariable Analysis. Food Chem. 2017, 217, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D.; Huang, X.; Zhao, F.L.; Tang, Y.L.; Yin, L. Study on the Chemical Markers of Caulis Lonicerae japonicae for Quality Control by HPLC-QTOF/MS/MS and Chromatographic Fingerprints Combined with Chemometrics Methods. Anal. Methods 2015, 7, 2064–2076. [Google Scholar] [CrossRef]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Potential Bioactive Phenolics of Macedonian sideritis Species Used for Medicinal “Mountain Tea”. Food Chem. 2011, 125, 13–20. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of Phenolic Compounds in Rose Hip (Rosa canina) Using Liquid Chromatography Coupled to Electrospray Ionisation Tandem Mass Spectrometry and Diode-Array Detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Characterization of Phenolic Acids in Black Carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1331–1340. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF–MS/MS Application to Investigate Chemical Compositions in a Fraction with Protein Tyrosine Phosphatase 1B Inhibitory Activity from Rosa rugosa Flowers. Phytochem. Anal. 2013, 24, 661–670. [Google Scholar] [CrossRef]
- Innocenti, M.; La Marca, G.; Malvagia, S.; Giaccherini, C.; Vincieri, F.F.; Mulinacci, N. Electrospray Ionisation Tandem Mass Spectrometric Investigation of Phenylpropanoids and Secoiridoids from Solid Olive Residue. Rapid Commun. Mass Spectrom. 2006, 20, 2013–2022. [Google Scholar] [CrossRef]
- Matos, P.; Figueirinha, A.; Paranhos, A.; Nunes, F.; Cruz, P.; Geraldes, C.F.G.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Acanthus Mollis—Contribution of Benzoxazinoids and Phenylpropanoids. J. Ethnopharmacol. 2018, 227, 198–205. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of Phenolic Compounds in Strawberries by Liquid Chromatography Electrospray Ionization Mass Spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, P.; Qu, H.; Cheng, Y. Characterization of Phenolic Compounds in Erigeron breviscapus by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2971–2984. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Z.; Wang, J.; Wu, G.; Li, S. Comprehensive Separation and Identification of Chemical Constituents from Apocynum venetum Leaves by High-Performance Counter-Current Chromatography and High Performance Liquid Chromatography Coupled with Mass Spectrometry. J. Chromatogr. B 2010, 878, 3149–3155. [Google Scholar] [CrossRef]
- Nijat, D.; Abdulla, R.; Liu, G.-Y.; Luo, Y.-Q.; Aisa, H.A. Identification and Quantification of Meiguihua Oral Solution Using Liquid Chromatography Combined with Hybrid Quadrupole-Orbitrap and Triple Quadrupole Mass Spectrometers. J. Chromatogr. B 2020, 1139, 121992. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum vulgare) Using Liquid Chromatography-Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Fayez, S.; Eltanany, B.M.; Abu-Elghait, M.; El-Demerdash, A.; Badawy, M.S.E.M.; Pont, L.; Benavente, F.; Saber, F.R. Agro-Byproduct Valorization of Radish and Turnip Leaves and Roots as New Sources of Antibacterial and Antivirulence Agents through Metabolomics and Molecular Networking. Sci. Hortic. 2024, 328, 112924. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Ouyang, H.; He, M.; Zhao, W.; Wei, W.; Cui, Y.; Yang, S.; Zhong, G.; Feng, Y.; et al. Characterization of Chemical Constituents and Metabolites in Rat Plasma after Oral Administration of Ainsliaea fragrans Champ by Using UHPLC-QTOF-MS/MS. J. Chromatogr. B 2024, 1244, 124259. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xin, J.; Lv, Y.; Chen, W.; Xu, X.; Wang, J.; Tian, S.; Xie, B.; Shen, Y.; Zu, X. Effects of Processing on the Efficacy and Metabolites of Cistanche tubulosa Using Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. Biomed. Chromatogr. 2023, 37, e5621. [Google Scholar] [CrossRef]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite Profiling of Mycorrhizal Roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef]
- Huhman, D.V.; Sumner, L.W. Metabolic Profiling of Saponins in Medicago Sativa and Medicago Truncatula Using HPLC Coupled to an Electrospray Ion-Trap Mass Spectrometer. Phytochemistry 2002, 59, 347–360. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Wang, R.; Pang, H.; Wang, J.; Liu, L. Determining the Chemical Profile of Caragana jubata (Pall.) Poir. by UPLC–QTOF–MS Analysis and Evaluating Its Anti-Ischemic Stroke Effects. J. Ethnopharmacol. 2023, 309, 116275. [Google Scholar] [CrossRef] [PubMed]
- Saleri, F.D.; Chen, G.; Li, X.; Guo, M. Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities. Molecules 2017, 22, 1077. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Silva, C.; Holandino, C.; Homsani, F.; Luiz, R.R.; Prodestino, J.; Farah, A.; Lima, J.d.P.; Simas, R.C.; Castilho, C.V.V.; Leitão, S.G.; et al. Homeopathic Medicine of Melissa officinalis Combined or Not with Phytolacca decandra in the Treatment of Possible Sleep Bruxism in Children: A Crossover Randomized Triple-Blinded Controlled Clinical Trial. Phytomedicine 2019, 58, 152869. [Google Scholar] [CrossRef]
- Liu, R.; Cai, Z.; Xu, B. Characterization and Quantification of Flavonoids and Saponins in Adzuki Bean (Vigna angularis L.) by HPLC-DAD-ESI-MSn Analysis. Chem. Cent. J. 2017, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.; Tran, C.A.; Trinh, T.D.; Nguyen Thi, N.L.; Tran Phan, H.N.; Le, V.N.; Le, N.H.; Phung, V.T. UHPLC-Q-TOF-MS/MS Dereplication to Identify Chemical Constituents of Hedera Helix Leaves in Vietnam. J. Anal. Methods Chem. 2022, 8, 1167265. [Google Scholar] [CrossRef]
- Kite, G.C. Characterization of phenylethanoid glycosides by multiple-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8563. [Google Scholar] [CrossRef]
- Qi, M.; Xiong, A.; Geng, F.; Yang, L.; Wang, Z. A Novel Strategy for Target Profiling Analysis of Bioactive Phenylethanoid Glycosides in Plantago Medicinal Plants Using Ultra-Performance Liquid Chromatography Coupled with Tandem Quadrupole Mass Spectrometry. J. Sep. Sci. 2012, 35, 1470–1478. [Google Scholar] [CrossRef]
- Pagliari, S.; Sicari, M.; Pansera, L.; Guidi Nissim, W.; Mhalhel, K.; Rastegar, S.; Germanà, A.; Cicero, N.; Labra, M.; Cannavacciuolo, C.; et al. A comparative metabolomic investigation of different sections of Sicilian Citrus x limon (L.) Osbeck, characterization of bioactive metabolites, and evaluation of in vivo toxicity on zebrafish embryo. J. Food Sci. 2024, 89, 3729–3744. [Google Scholar] [CrossRef]
- Fu, Z.; Xue, R.; Li, Z.; Chen, M.; Sun, Z.; Hu, Y.; Huang, C. Fragmentation patterns study of iridoid glycosides in Fructus Gardeniae by HPLC-Q/TOF-MS/MS. Biomed. Chromatogr. 2014, 28, 1795–1807. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Elmi, A.; Spina, R.; Kordofani, M.A.Y.; Laurain-Mattar, D.; Nour, H.; Abchir, O.; Chtita, S. In Vitro and In Silico Analysis for Elucidation of Antioxidant Potential of Djiboutian Avicennia marina (Forsk.) Vierh. Phytochemicals. J. Biomol. Struct. Dyn. 2024, 42, 3410–3425. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhuang, L.H.; Zhou, J.J.; Song, S.W.; Li, J.; Huang, H.Z.; Chi, B.J.; Zhong, Y.H.; Liu, J.W.; Zheng, H.L.; et al. Combined Metabolome and Transcriptome Analysis Reveals a Critical Role of Lignin Biosynthesis and Lignification in Stem-like Pneumatophore Development of the Mangrove Avicennia marina. Planta 2024, 259, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, Y.; Lin, W.H. Isolation and Identification of Compounds from Marine Mangrove Plant Avicennia marina. Beijing Da Xue Xue Bao Yi Xue Ban J. Peking Univ. Health Sci. 2009, 41, 221–225. [Google Scholar]
- Kartikaningsih, H.; Djamaludin, H.; Fauziyah, J.N.; Audina, N.; Noviyanti, L.; Saputra, D. Green extraction of Avicennia marina leaves by natural deep eutectic solvents: Phytochemical profile, antioxidant activity, molecular docking and admet analysis. Rasayan J. Chem. 1123, 17, 1123–1133. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Xiao, Q.; Zhang, S.; Xiao, Z.; Li, Q.; Long, L.; Huang, L. Complete Assignments of 1H and 13C NMR Data for 10 Phenylethanoid Glycosides. Magn. Reson. Chem. 2004, 42, 659–662. [Google Scholar] [CrossRef]
- Vinh, L.B.; Nguyet, N.T.M.; Yang, S.Y.; Kim, J.H.; Van Thanh, N.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Hwang, I.; Kim, Y.H. Cytotoxic Triterpene Saponins from the Mangrove Aegiceras corniculatum. Nat. Prod. Res. 2019, 33, 628–634. [Google Scholar] [CrossRef]
- Clemente, S.M.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 2020, 25, 5144. [Google Scholar] [CrossRef]
- Kang, J.H.; Uddin, N.; Kim, S.; Zhao, Y.; Yoo, K.C.; Kim, M.J.; Hong, S.A.; Bae, S.; Lee, J.Y.; Shin, I.; et al. Tumor-Intrinsic Role of ICAM-1 in Driving Metastatic Progression of Triple-Negative Breast Cancer through Direct Interaction with EGFR. Mol. Cancer 2024, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 2016; ISBN 1-316-79065-7. [Google Scholar]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and Regulation of Primary Metabolism during Seed Germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Sharif, Y.; Chen, H.; Deng, Y.; Ali, N.; Khan, S.A.; Zhang, C.; Xie, W.; Chen, K.; Cai, T.; Yang, Q.; et al. Cloning and Functional Characterization of a Pericarp Abundant Expression Promoter (AhGLP17-1P) From Peanut (Arachis hypogaea L.). Front. Genet. 2022, 12, 821281. [Google Scholar] [CrossRef] [PubMed]
- Zietz, M.; Weckmuller, A.; Schmidt, S.; Rohn, S.; Schreiner, M.; Krumbein, A.; Kroh, L.W. Genotypic and climatic influence on the antioxidant activity of flavonoids in kale (Brassica oleracea var. sabellica). J. Agric. Food Chem. 2010, 58, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Wong, K.; Ho, K.P.; Zhou, L.G. Enhancement of saponin production in Panax ginseng cell culture by osmotic stress and nutrient feeding. Enzym. Microb. Technol. 2005, 36, 133–138. [Google Scholar] [CrossRef]
- Oku 2003Oku, H.; Baba, S.; Koga, H.; Takara, K.; Iwasaki, H. Lipid composition of mangrove and its relevance to salt tolerance. J. Plant Res. 2003, 116, 37–45. [Google Scholar] [CrossRef]
- Xue, Z.; Yang, B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules 2016, 21, 991. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New Insights on Bioactivities and Biosynthesis of Flavonoid Glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; Zollo, F.; Incollingo, F.; Raimo, G.; Evangelista, G.; Iorizzi, M. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chem. 2012, 133, 21–28. [Google Scholar] [CrossRef]
- Budzianowska, A.; Kikowska, M.; Budzianowski, J. Phenylethanoid glycosides accumulation and antiradical activity of fractionated extracts of Plantago ovata Forssk. callus cultures lines. Plant Cell Tissue Organ Cult. 2024, 156, 54. [Google Scholar] [CrossRef]
- Lu, S.-H.; Zuo, H.-J.; Huang, J.; Li, W.-N.; Huang, J.-L.; Li, X.-X. Chemical constituents from the leaves of Ligustrum robustum and their bioactivities. Molecules 2023, 28, 362. [Google Scholar] [CrossRef]
- Ji, S.L.; Cao, K.K.; Zhao, X.X.; Kang, N.X.; Zhang, Y.; Xu, Q.M.; Yang, S.L.; Liu, Y.L.; Wang, C. Antioxidant activity of phenylethanoid glycosides on glutamate-induced neurotoxicity. Biosci. Biotechnol. Biochem. 2019, 83, 2016–2026. [Google Scholar] [CrossRef]
- Wei, W.; Lan, X.B.; Liu, N.; Yang, J.M.; Du, J.; Ma, L.; Zhang, W.J.; Niu, J.G.; Sun, T.; Yu, J.Q. Echinacoside Alleviates Hypoxic-Ischemic Brain Injury in Neonatal Rat by Enhancing Antioxidant Capacity and Inhibiting Apoptosis. Neurochem. Res. 2019, 44, 1582–1592. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Behbahani, M.; Sadeghi-Aliabadi, H. Antiproliferative Activity and Apoptosis Induction of Crude Extract and Fractions of Avicennia marina. Iran. J. Basic Med. Sci. 2013, 16, 1203. [Google Scholar]
- Tanjung, I.B.; Azizah, N.N.; Arsianti, A.; Anisa, A.S.; Audah, K.A. Evaluation of the Ethyl Acetate Extract of the Roots of Avicennia marina as Potential Anticancer Drug. In Proceedings of the 6th International Conference of Food, Agriculture, and Natural Resource (IC-FANRES 2021), Tangerang, Indonesia, 4–5 August 2021; Atlantis Press: Dordrecht, The Netherlands, 2022; Volume 16, pp. 75–81. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; McDonald, M.M. Protocols for Screening Chemical Agents and Natural Products against Animal Tumors and Other Biological Systems. Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]
- Addy, B.S.; Firempong, C.K.; Komlaga, G.; Addo-Fordjour, P.; Domfeh, S.A.; Afolayan, O.; Emikpe, B.O. In vitro antiproliferative activities of some Ghanaian medicinal plants. Clin. Phytosci 2024, 10, 19. [Google Scholar] [CrossRef]
- Du, J.R.; Long, F.Y.; Chen, C. Research Progress on Natural Triterpenoid Saponins in the Chemoprevention and Chemotherapy of Cancer. Enzymes 2014, 36, 95–130. [Google Scholar] [CrossRef]
- da Silva Magedans, Y.V.; Phillips, M.A.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: From Metabolites to Genes and Back. Phytochem. Rev. 2020, 20, 461–482. [Google Scholar] [CrossRef]
- Yang, X.W.; Dai, Z.; Wang, B.; Liu, Y.P.; Zhao, X.D.; Luo, X.D. Antitumor Triterpenoid Saponin from the Fruits of Avicennia marina. Nat. Prod. Bioprospect 2018, 8, 347–353. [Google Scholar] [CrossRef]
- Duke, N.C. A Systematic Revision of the Mangrove Genus Avicennia (Avicenniaceae) in Australasia. Aust. Syst. Bot. 1991, 4, 299–324. [Google Scholar] [CrossRef]
- Friis, G.; Vizueta, J.; Smith, E.G.; Nelson, D.R.; Khraiwesh, B.; Qudeimat, E.; Salehi-Ashtiani, K.; Ortega, A.; Marshell, A.; Duarte, C.M.; et al. A high-quality genome assembly and annotation of the gray mangrove, Avicennia marina. G3 Genes Genomes Genet. 2021, 11, jkaa025. [Google Scholar] [CrossRef]
- Friis, G.; Smith, E.G.; Lovelock, C.E.; Ortega, A.; Marshell, A.; Duarte, C.M.; Burt, J.A. Rapid diversification of grey mangroves (Avicennia marina) driven by geographic isolation and extreme environmental conditions in the Arabian Peninsula. Mol. Ecol. 2024, 33, e17260. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of Temperature, Time, and Solvent Ratio on the Extraction of Phenolic Compounds and the Anti-Radical Activity of Clinacanthus nutans Lindau Leaves by Response Surface Methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of Bioactive Compounds from Mango (Mangifera indica L. var. Carabao) Seed Kernel with Ethanol–Water Binary Solvent Systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Díaz Huamaní, K.S.; Palomino Villegas, Y.C.; Allcca-Alca, E.E.; León-Calvo, N.C.; Colque Ayma, E.J.; Zirena Vilca, F.; Mariotti-Celis, M.S. Exploring a Sustainable Process for Polyphenol Extraction from Olive Leaves. Foods 2024, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive Solvent Extraction of Polyphenols and Flavonoids from Cistus creticus L. Leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Giustra, C.M.; Carabetta, S.; Guidi Nissim, W.; Russo, M.; Branduardi, P.; Labra, M.; Campone, L. LC-MS and GC-MS Data Fusion Metabolomics Profiling Coupled with Multivariate Analysis for the Discrimination of Different Parts of Faustrime Fruit and Evaluation of Their Antioxidant Activity. Antioxidants 2023, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Verbanac, D.; Jain, S.C.; Jain, N.; Chand, M.; Paljetak, H.C.; Matijašic, M.; Peric, M.; Stepanic, V.; Saso, L. An efficient and convenient microwave-assisted chemical synthesis of (thio)xanthones with additional in vitro and in silico characterization. Bioorg. Med. Chem. 2012, 20, 3180–3185. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the PASS Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Desai, T.H.; Joshi, S.V. In Silico Evaluation of Apoptogenic Potential and Toxicological Profile of Triterpenoids. Indian J. Pharmacol. 2019, 51, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of Activity Spectra for Biologically Active Substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
| No. | RT (min) | [M − H]− | Formula | Δ ppm | MS/MS | Name | Class | Part | IL | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.58 | 701.1893 [M + Cl]− | C24H42O21 | −2.1677 | 665.2134, 485.1499, 443.1393, 383.1182, 341.1075, 179.0549 | Stachyose | Tetrasaccharides | Cotyledons/pericarps/propagules/roots | IL2 | [41] |
| 2 | 0.99 | 191.0188 | C6H8O7 | 6.3850 | 111.0073 | Citric acid | Tricarboxylic acids | Cotyledons/pericarps | IL2 | [42] |
| 3 | 3.87 | 373.1139 | C16H22O10 | 0.3221 | 211.0605, 167.0700, 149.0597, 123.0440, 105.0333 | Geniposidic acid | Iridoid glycosides | Leaves/cotyledons/pericarps/propagules/roots | IL2 | [43] |
| 4 | 4.03 | 353.0875 | C16H18O9 | 0.8633 | 191.0551, 179.0339, 161.0233, 135.0439 | Caffeoylquinic acid isomer | Hydroxycinnamic acids and derivatives | Roots | IL2 | [44] |
| 5 | 4.05 | 375.1291 | C16H24O10 | 1.5167 | 213.0756, 169.0857, 151.0753, 133.0644, 125.0595, 107.0490 | Mussaenosidic acid | Iridoid glycosides | Leaves/cotyledons/pericarps/propagules | IL2 | [45] |
| 6 | 4.33 | 375.1285 | C16H24O10 | 3.1119 | 213.0747, 169.0854, 151.0748, 133.0644, 125.0591, 113.0230, 107.0484 | (Epi)loganic acid | Iridoid glycosides | Leaves | IL2 | [45] |
| 7 | 4.53 | 487.1451 | C21H28O13 | 1.2588 | 179.0334, 161.0228, 135.0435 | Cistanoside F | Phenylethanoid glycosides | Pericarps | IL2 | [46] |
| 8 | 4.80 | 327.0715 | C14H16O9 | 1.9983 | 179.0335, 165.0389, 147.0283, 135.0434, 105.0178 | Unidentified | - | Leaves | ||
| 9 | 5.09 | 353.0871 | C16H18O9 | −3.0904 | 191.0550, 179.0337, 173.0442, 161.0232, 135.0439, | Caffeoylquinic acid isomer | Hydroxycinnamic acids and derivatives | Leaves/cotyledons/pericarps/propagules/roots | IL2 | [44] |
| 10 | 5.47 | 371.0982 | C15H16O11 | −8.7858 | 209.0635, 179.0337, 161.0228, 135.0435, 129.0178 | Caffeoyl hexaric acid | Hydroxycinnamic acids and derivatives | Leaves | IL2 | [47] |
| 11 | 5.60 | 443.0655 | C18H20O11S | −0.3243 | 275.0218, 167.0338, 152.0105, 123.0440, 108.0204 | Unidentified | - | Roots | ||
| 12 | 5.65 | 415.1603 | C19H28O10 | 1.6114 | 235.0963, 191.1062, 173.0958, 149.0953, 137.0590, 101.0226 | Icariside D1 | Flavonoid glycosides | Leaves | IL2 | [48] |
| 13 | 5.93 | 639.1964 | C29H36O16 | −5.2193 | 621.1807, 529.1554, 459.1488, 251.0549, 179.0337, 161.0232, 151.0387 | Suspensaside isomer | Phenylethanoid glycosides | Pericarps/roots | IL2 | [49,50] |
| 14 | 5.95 | 639.1964 | C29H36O16 | −5.2193 | 621.1807, 529.1554, 459.1488, 251.0549, 179.0337, 161.0232, 151.0387 | Suspensaside isomer | Phenylethanoid glycosides | Pericarps/roots | IL2 | [49,50] |
| 15 | 6.14 | 537.1628 | C25H30O13 | −2.6672 | 493.1708, 375.1275, 323.0758, 213.0752, 179.0334, 169.0854, 161.0230, 151.0750, 135.0435, 125.0593, 107.0486 | Grandifloroside | Hydroxycinnamic acid and derivatives | Leaves | IL2 | [51] |
| 16 | 6.33 | 619.1644 | C29H32O15 | 3.9407 | 383.0758, 311.0549, 267.0646 | Unidentified | - | Pericarps/roots | ||
| 17 | 6.41 | 639.1929 | C29H36O16 | 0.2477 | 621.1817, 529.1554, 459.1493, 251.0549 179.0338, 161.0236, 151.0385 | Suspensaside isomer | Phenylethanoid glycosides | Roots | IL2 | [49,50] |
| 18 | 6.65 | 521.1658 | C25H30O12 | 1.2446 | 357.1176, 169.0854, 163.0385, 151.0749, 145.0280, 125.0591, 119.0486, 117.0329, 107.0486 | Marinoid C | Iridoid glycosides | Leaves/cotyledons/pericarps | IL3 | [7] |
| 19 | 6.65 | 653.2091 | C29H34O17 | 9.55333 | 621.1822, 459.1499, 179.0338, 161.0234, 151.0388, 135.0437 | Suspensaside methyl ether | Phenylethanoid glycosides | Roots | IL2 | [49,50] |
| 20 | 6.79 | 623.1981 | C29H36O15 | 0.0705 | 461.1657, 161.0233, 113.0283 | Verbascoside (acteoside) isomer | Phenylethanoid glycosides | Leaves/pericarps/roots | IL2 | [52] |
| 21 | 6.9 | 463.0874 | C21H20O12 | 1.7229 | 301.0324, 300.0264, 271.0235, 255.0285 | Quercetin 3-O-hexoside | Flavonoid glycosides | Roots | IL2 | [53,54,55] |
| 22 | 7.02 | 667.2239 | C31H40O16 | 0.6864 | 621.1824, 459.1499, 179.0338, 161.0235, 151.0386, 135.0436 | β-ethyl-OH-verbascoside | Phenylethanoid glycosides | Pericarps | IL2 | [56,57] |
| 23 | 7.11 | 623.2001 | C29H36O15 | −3.1336 | 461.1661, 161.0235, | Verbascoside (acteoside) isomer | Phenylethanoid glycosides | Leaves/pericarps/roots | IL2 | [52] |
| 24 | 7.21 | 621.1838 | C29H34O15 | −2.0992 | 461.1652, 179.0337, 161.0233, 151.0387 | Suspensaside A | Phenylethanoid glycosides | Roots | IL2 | [49,50] |
| 25 | 7.21 | 461.0718 | C21H18O12 | 1.6222 | 285.0391 | Kaempferol-3-O-glucuronide | Flavonoid glycosides | Leaves | IL2 | [58] |
| 26 | 7.21 | 681.2063 | C31H38O17 | −3.9236 | 519.1708, 490.1321, 181.0129, 179.0334, 161.0230 | Unidentified | - | Pericarps | ||
| 27 | 7.3 | 447.0926 | C21H20O11 | 1.5287 | 327.0494, 285.0648, 284.0315, 255.0288, 227.0338, 151.0013 | Kaempferol 3-O-glucoside | Flavonoid glycosides | Roots | IL2 | [55,59,60,61] |
| 28 | 7.40 | 623.1658 | C28H32O16 | −6.4750 | 315.0494, 314.0421, 300.0258, 299.0187, 271.0234 | Isorhamnetin-3-O-rutinoside | Flavonoid glycosides | Leaves/pericarps | IL2 | [62] |
| 29 | 7.40 | 491.0828 | C22H20O13 | 0.6385 | 315.0499, 300.0264 | Isorhamnetin glucuronide | Flavonoid glycosides | Leaves | IL2 | [63] |
| 30 | 7.51 | 535.1477 | C25H28O13 | −3.7032 | 329.1021, 179.0338, 161.0232, 149.0595, 135.0438 | Unidentified | - | Leaves/Pericarps | ||
| 31 | 7.51 | 477.1036 | C22H22O12 | −5.1250 | 315.0467, 314.0420, 285.0392, 271.0236, 257.0441, 243.0286, | Isorhamnetin 7-glucoside | Flavonoid glycosides | Leaves | IL2 | [63] |
| 32 | 7.61 | 471.1874 | C22H32O11 | −0.4545 | 287.1273, 263.1278, 219.1379, 201.1273, 186.1036, 147.1166 | Unidentified | - | Pericarps | ||
| 33 | 7.86 | 519.1143 | C24H24O13 | 0.2199 | 315.0472, 314.0423, 299.0186, 285.0383, 271.0236, 257.0443, 243.0286 | Unidentified | - | Leaves | ||
| 34 | 7.90 | 553.1556 | C25H30O14 | 1.2256 | 329.1021, 197.0445, 182.0206, 153.0454, 149.0596, 131.0489, | Marinoid D | Iridoid glycosides | Cotyledons/pericarps/propagules/roots | IL3 | [7] |
| 35 | 7.95 | 505.1757 | C25H29O11 | 0.2000 | 357.1184, 213.0757, 195.0650, 169.0857, 151.0753, 147.0439, 125.0596, 113.0230, 107.0487, 103.0539 | Marinoid A | Iridoid glycosides | Leaves | IL3 | [7] |
| 36 | 8.03 | 519.1505 | C25H28O12 | 0.5766 | 313.1072, 295.0961, 163.0388, 149.0596, 145.0282, 131.0490, 119.0487 | Unidentified | - | Leaves/cotyledons/pericarps/roots | ||
| 37 | 8.03 | 475.0887 | C22H20O12 | −1.0510 | 300.0589, 299.0554, 285.0358, 284.0318. | Diosmetin 7-glucuronide | Flavonoid glycosides | Leaves | IL2 | [64] |
| 38 | 8.24 | 549.1616 | C26H30O13 | −0.4279 | 343.1176, 325.1064, 193.0495, 175.0387, 149.0595, 134.0360, 131.0489 | Unidentified | - | Leaves/cotyledons/pericarps/roots | ||
| 39 | 8.36 | 591.2119 | C29H36O13 | −6.0539 | 179.0333, 161.0234, 133.0282, 113.0228 | Jionoside C | Phenylethanoid glycosides | Pericarps | IL2 | [65] |
| 40 | 8.62 | 825.4276 | C44H66O16 | 0.2536 | 663.3744, 601.3735 | Unknown triterpene saponin | Triterpene saponins | Roots | IL3 | [66,67] |
| 41 | 8.70 | 539.2152 | C26H36O12 | −3.3318 | 193.0485, 183.1010, 175.0382, 149.0591, 131.0485, 121.0642 | Unidentified | - | Leaves | ||
| 42 | 8.80 | 541.2285 | C26H38O12 | 1.0147 | 193.0485, 185.1166, 175.0382, 149.0591, 131.0485, 121.0642 | Unidentified | - | Leaves | ||
| 43 | 8.88 | 825.4285 | C42H66O16 | −0.8354 | 663.3744, 601.3735, 487.3421 | Unknown triterpene saponin | Triterpene saponins | Roots | IL3 | [66,67] |
| 44 | 8.97 | 299.0546 | C16H12O6 | 5.0379 | 285.0345, 284.0313, 256.0363, 227.0334 | Trihydroxy-methoxyflavone | Flavones | Leaves | IL2 | [68] |
| 45 | 8.99 | 825.4273 | C42H66O16 | 0.6166 | 663.3744, 601.3735, | Medicoside G (medicagenic acid 3,28-di-glucoside) | Triterpene saponins | Roots | IL2 | [66,67] |
| 46 | 9.08 | 809.4316 | C42H66O15 | 1.5979 | 689.3884, 647.3788, 629.3680, 585.3786 | Esculentoside C (phycolaccoside D) | Triterpene saponins | Cotyledons/pericarps/propagules/roots | IL2 | [69,70] |
| 47 | 9.30 | 505.1711 | C25H30O11 | 0.8600 | 281.1170, 195.0649, 151.0750, 147.0438, 133.0645, 107.0486 | Unidentified | - | Leaves | ||
| 48 | 9.38 | 503.1572 | C25H28O11 | −2.6077 | 279.1010, 253,0854, 209.0954, 195.0647, 147.0437, 131.0486, 103.0536 | Unidentified | - | Leaves/pericarps | ||
| 49 | 9.54 | 809.4342 | C42H66O15 | −1.6102 | 647.3797, 471.3469 | Azukisaponin III | Triterpene saponins | Roots | IL2 | [71] |
| Compound Class | DPPH | ABTS | ||
|---|---|---|---|---|
| ρ-Value | p-Value | p-Value | p-Value | |
| Iridoid glycosides | −0.103 | 0.870 | 0.510 | 0.935 |
| Hydroxycinnamic acid and derivatives | −0.112 | 0.858 | 0.224 | 0.718 |
| Phenylethanoid glycosides | 0.791 | 0.111 | 0.949 | 0.014 |
| Flavonoid glycosides | 0.205 | 0.741 | 0.574 | 0.322 |
| Triterpene saponins | 0.447 | 0.450 | 0.224 | 0.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerri, F.; De Santes, B.; Spena, F.; Salvioni, L.; Forcella, M.; Fusi, P.; Pagliari, S.; Stahl, H.; Galli, P.; Colombo, M.; et al. Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia marina. Pharmaceuticals 2025, 18, 1308. https://doi.org/10.3390/ph18091308
Cerri F, De Santes B, Spena F, Salvioni L, Forcella M, Fusi P, Pagliari S, Stahl H, Galli P, Colombo M, et al. Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia marina. Pharmaceuticals. 2025; 18(9):1308. https://doi.org/10.3390/ph18091308
Chicago/Turabian StyleCerri, Federico, Beatrice De Santes, Francesca Spena, Lucia Salvioni, Matilde Forcella, Paola Fusi, Stefania Pagliari, Henrik Stahl, Paolo Galli, Miriam Colombo, and et al. 2025. "Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia marina" Pharmaceuticals 18, no. 9: 1308. https://doi.org/10.3390/ph18091308
APA StyleCerri, F., De Santes, B., Spena, F., Salvioni, L., Forcella, M., Fusi, P., Pagliari, S., Stahl, H., Galli, P., Colombo, M., Giustra, M., & Campone, L. (2025). Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia marina. Pharmaceuticals, 18(9), 1308. https://doi.org/10.3390/ph18091308











