Phytochemical Profile, Antioxidant Capacity, and Photoprotective Potential of Brazilian Humulus Lupulus
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Field Conditions
3.2. Extract Obtention
3.3. Chemical Identification of the Crude Extracts of H. Lupulus Cones and Leaves by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry (UHPLC-MS/MS)
3.4. Evaluation of the Total Phenol Content (TP) of the Crude Extracts of H. Lupulus Cones and Leaves
3.5. Evaluation of the Flavonoid Content of the Crude Extracts of H. Lupulus Cones and Leaves
3.6. Antioxidant Activity
3.6.1. Scavenging of 2.2-Diphenyl-1-Picrylhydrazyl (DPPH•) Free Radicals
3.6.2. Ferric Reducing Antioxidant Power (FRAP)
3.6.3. ABTS•⁺ Radical Method (2.2′azinobis-(3ethylbenzthiazoline-6-Sulfonic Acid))
3.6.4. In Vitro Determination of Sun Protection Factor (SPF)
3.7. Determination of the UVA/UVB Ratio and Critical Wavelength (λc)
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galaverni, M.; Ganino, T.; Rodolfi, M. Evaluation of the Stability of Hop Pellets Subjected to Different Storage Conditions During Three Years. J. Stored Prod. Res. 2024, 107, 102353. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the Potential of Microwaves and Ultrasounds in the Green Extraction of Bioactive Compounds from Humulus lupulus for the Food and Pharmaceutical Industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a Novel Multipurpose Crop for the Mediterranean Region of Europe: Challenges and Opportunities of Their Cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Da Rosa Almeida, A.; de Conto, L.C. Hops in Brazil: A Promising and Emerging Crop. Food Sci. Today 2024, 3, 1–6. [Google Scholar] [CrossRef]
- Gonsaga, R.F. Development of Hops Hybrids Adapted to Tropical Conditions. Ph.D. Thesis, Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil, 2021. [Google Scholar]
- Contin, D.R.; Habermann, E.; de Souza, B.C.; de Oliveira, E.A.; Martinez, C.A.; Vieira, P.C.; Da Costa, F.B. Exploring the Tropical Acclimation of European and American Hop Cultivars (Humulus lupulus L.): Focus on Physiology, Productivity, and Chemical Composition. Eur. J. Agron. 2023, 151, 126990. [Google Scholar] [CrossRef]
- Aquino, A.M.; Teixeira, A.J.; Assis, R.L. Technical Reference for Agricultural Attractiveness—Hops; Embrapa: Nova Friburgo, Brazil, 2019. [Google Scholar]
- Almeida, A.D.R.; Maciel, M.V.D.O.B.; Machado, M.H.; Bazzo, G.C.; de Armas, R.D.; Vitorino, V.B.; Barreto, P.L.M. Bioactive Compounds and Antioxidant Activities of Brazilian Hop (Humulus lupulus L.) Extracts. Int. J. Food Sci. Technol. 2020, 55, 340–347. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Livestock and Supply; Secretariat of Family Agriculture and Cooperatives; Creuz, A.; Kretzer, S.G. Hops in Brazil: Perspectives and Realities; MAPA/SAF: Brasília, Brazil, 2022; p. 175. ISBN 978-65-86803-89-1. [Google Scholar]
- Da Rosa Almeida, A.; De Oliveira Brisola Maciel, M.V.; Gandolpho, B.C.G.; Machado, M.H.; Teixeira, G.L.; Bertoldi, F.C.; Noronha, C.M.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Brazilian Grown Cascade Hop (Humulus lupulus L.): LC-ESI-MS-MS and GC-MS Analysis of Chemical Composition and Antioxidant Activity of Extracts and Essential Oils. J. Am. Soc. Brew. Chem. 2021, 79, 156–166. [Google Scholar] [CrossRef]
- Sabino, B.C.C.; Bonfim, F.P.G.; Cabral, M.N.F.; Viriato, V.; Pak Campos, O.; Neves, C.S.; Marques, M.O.M. Phytochemical Characterization of Humulus lupulus L. Varieties Cultivated in Brazil: Agricultural Zoning for the Crop in Tropical Areas. Horticulturae 2025, 11, 470. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and In Vitro Effects of Cultivars of Humulus lupulus L. Hops on Cholinesterase Activity and Microbial Growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef]
- Kellner, V.; Jurková, M.; Čulík, J.; Horák, T.; Čejka, P. Some phenolic compounds in Czech hops and beer of Pilsner type. Brew. Sci. 2007, 60, 31–37. [Google Scholar]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, P.; Lozano, T.; Gvaladze, A.; Guzman, B.; Siomyk, H.; Haas, G. Effects of Humulus lupulus extract or its Components on Viability, Lipid Peroxidation, and expression of Vascular Endothelial Growth Factor in Melanoma Cells and Fibroblasts. Madridge J. Clin. Res. 2017, 1, 15–19. [Google Scholar] [CrossRef]
- Pereira, M.C.; Moreira, J.V.; Souza, S.C.; de Souza Coutinho, H.P.; Soares, K.L.; Kerr, H.K.A.; Ribeiro, J.S.; Silva, Q.M.d.; Pires, M.C.; Porto, M.L. Evaluation of the Chemical, Antioxidant, and Antimicrobial Profile of Humulus lupulus L. var. Teamaker Extract for Use as a Cosmetic Ingredient. Cuad. Educ. Desarro. 2025, 17, e8099. [Google Scholar] [CrossRef]
- Kurzawa, M.; Wilczyńska, E.; Brudzyńska, P.; Sionkowska, A. Total Phenolic Conten, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucus carota), Tomato (Solanum lycopersicum) and Hop (Humulus lupulus) Extracts. Cosmetics 2022, 9, 134. [Google Scholar] [CrossRef]
- Lela, L.; Ponticelli, M.; Vassallo, A.; Ostuni, A.; Sinisgalli, C.; Faraone, I.; Mirella, L.; Caddeo, C.; Santoro, V.; De Tommasi, N. Nanotechnological Exploitation of the Antioxidant Potential of Humulus lupulus L. Extract. Food Chem. 2022, 373, 131493. [Google Scholar] [CrossRef]
- Vergun, O.; Shymanska, O.; Ivanišová, E.; Fishchenko, V. Antioxidant activity of extracts of wild Humulus lupulus L. Agrobiodivers. Improv. Nutr. Health Life Qual. 2021, 5, 47–54. [Google Scholar] [CrossRef]
- Santarelli, V.; Neri, L.; Carbone, K.; Macchioni, V.; Pittia, P. Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants 2021, 11, 41. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant Activity of Selected Phenols Estimated by ABTS and FRAP Methods. Adv. Hyg. Exp. Med. 2013, 67, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Toniolo, C.; Martinoli, L.; Nencioni, L. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxid. Med. Cell. Longev. 2018, 2018, 5919237. [Google Scholar] [CrossRef] [PubMed]
- Keskin, Ş.; Şirin, Y.; Çakir, H.E.; Keskin, M. An Investigation of Humulus lupulus L.: Phenolic Composition, Antioxidant Capacity and Inhibition Properties of Clinically Important Enzymes. S. Afr. J. Bot. 2019, 120, 170–174. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Altinterim, B. Citrus, Rutin and on Their Vein Permeability Effects. Res. J. Agric. Environ. Manag. 2014, 3, 80–81. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on In Vivo and In Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Bessada, S.M.; Barreira, J.C.; Oliveira, M.B.P. Asteraceae Species with Most Prominent Bioactivity and Their Potential Applications: A Review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A.; Schüll, F.; Bertazzoni, J. The Impact of Climatic Conditions on the Biogenesis of Various Compounds in Hops. BrewingScience 2021, 74, 160–171. [Google Scholar]
- Thomaz, E.L.; Fidalski, J. Interrill erodibility of different sandy soils increases along a catena in the Caiuá Sandstone Formation. Rev. Bras. Ciênc. Solo 2020, 44, e0190064. [Google Scholar] [CrossRef]
- Spósito, M.B.; Ismael, R.V.; Barbosa, C.M.A.; Tagliaferro, A.L. Hop cultivation. In Série Prod Rural; ESALQ: Piracicaba, Brazil, 2019; Volume 68, pp. 1–82. ISSN 1414-4530. Available online: https://www.researchgate.net/publication/334672293_A_Cultura_do_Lupulo (accessed on 12 December 2024). (In Portuguese)
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–Antioxidant Activity Relationships of o-Hydroxyl, o-Methoxy, and Alkyl Ester Derivatives of p-Hydroxybenzoic Acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef]
- Karamac, M.; Kosińska, A.; Pegg, R.B. Comparison of Radical-Scavenging Activities for Selected Phenolic Acids. Pol. J. Food Nutr. Sci. 2005, 55, 165–170. [Google Scholar]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.; Seo, K.S.; Kang, K.Y.; Park, S.H.; Ha, T.H.; Kang, S.Y. Comparative Study on Phenolic Compounds and Antioxidant Activities of Hop (Humulus lupulus L.) Strobile Extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A new perspective of a well-recognized raw material: Phenolic content, antioxidant and antimicrobial activities and α-and β-acids profile of Brazilian hop (Humulus lupulus L.) extracts. LWT 2021, 41, 110905. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, S.; Shi, J.; Li, H.; Li, Z. Millet Bran Polyphenol Enhances the Sensitivity of Colorectal Cancer Cells to Oxaliplatin by Blocking GM3 Ganglioside Catabolism. Food Funct. 2021, 12, 291–301. [Google Scholar] [CrossRef]
- Dare, R.G.; Bontempo, P.; Ambrosio, P.; Cacciapuoti, G.; Fogliano, V.; Ritieni, A.; d’Acierno, A.; Ferranti, P.; Picariello, G. Abilities of Protocatechuic Acid and Its Alkyl Esters, Ethyl and Heptyl Protocatechuates, to Counteract UVB-Induced Oxidative Injuries and Photoaging in Fibroblasts L929 Cell Line. J. Photochem. Photobiol. B Biol. 2020, 203, 111771. [Google Scholar] [CrossRef]
- Shanmugasundaram, B.U.; Stanely, S.P.; Ponnian, S.M.P. Protocatechuic Acid Attenuates Isoproterenol-Induced Heart Failure by Modulating Cardiac Oxidative Stress, LDL-R/SREBP-2/PPAR-α, and Bax/Bcl-2/Bcl-xL/Cyt, c/Caspase-9 and Caspase-3 Pathways. Eur. J. Pharmacol. 2025, 998, 177492. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Iseki, K. In Vitro and In Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Research. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic Acid Exhibits Cholesterol Lowering and Fatty Liver Attenuating Properties by Up-Regulating the Gene Expression of PPAR-α in Hypercholesterolemic Rats Induced with a High-Cholesterol Diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Karcheva-Bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and α-Glucosidase Activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef]
- Lukitasari, M.; Nugroho, D.A.; Widodo, N. Chlorogenic Acid: The Conceivable Chemosensitizer Leading to Cancer Growth Suppression. J. Evid. Based Integr. Med. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Tian, L.; Su, C.P.; Wang, Q.; Wu, F.J.; Bai, R.; Zhang, H.M.; Liu, J.Y.; Lu, W.J.; Wang, W.; Lan, F.; et al. Chlorogenic Acid: A Potent Molecule That Protects Cardiomyocytes from TNF-α–Induced Injury via Inhibiting NF-κB and JNK Signals. J. Cell. Mol. Med. 2019, 23, 4666–4678. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic Studies on the Antioxidant Activity of p-Coumaric Acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Kaiser, S.; Warth, J.; Galaverna, R.S.; Dariva, C.; Silva, C.M.; Maróstica Júnior, M.R.; Meireles, M.A.A. Extraction Optimization of Polyphenols, Oxindole Alkaloids and Quinovic Acid Glycosides from Cat’s Claw Bark by Box–Behnken Design. Ind. Crops Prod. 2013, 48, 153–161. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Dispõe sobre protetores solares e produtos multifuncionais em cosméticos. In Proceedings of the Resolução da Diretoria Colegiada—RDC nº 629; Brasília, Brasil, 10 March 2022, Ministério da Saúde: Brasília, Brasil, 2022. Available online: https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-629-de-10-de-marco-de-2022-388083342 (accessed on 16 June 2025).
- Boots UK Limited. Measurement of UVA:UVB Ratio According to the Boots Star Rating System (2008 Revision); Boots UK Limited: Nottingham, UK, 2008; Available online: https://cdnmedia.eurofins.com/apac/media/601375/boots-star.pdf (accessed on 10 August 2024).
- Wang, S.Q.; Stanfield, J.W.; Osterwalder, U. In vitro assessments of UVA protection by popular sunscreens available in the United States. J. Am. Acad. Dermatol. 2008, 59, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Photoprotection of Natural Flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar] [CrossRef]
- Da Cruz Gomes, J.; de Souza Silva, A.; Reis, M.Y.D.F.A.; do Nascimento, Y.M.; de Sousa, A.P.; Silva, L.H.A.C. Phytochemical Determination and Sun Protection Factor Evaluation of the Species Acmella oleracea and Cipura paludosa. Braz. J. Dev. 2022, 8, 2137–2154. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Menezes, I.R.A.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv. Pharmacol. Pharm. Sci. 2018, 28, 5341487. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of Some Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047. [Google Scholar]
- Sharma, S.H.; Kumar, J.S.; Chellappan, D.R.; Nagarajan, S. Molecular Chemoprevention by Morin–A Plant Flavonoid That Targets Nuclear Factor Kappa B in Experimental Colon Cancer. Biomed. Pharmacother. 2018, 100, 367–373. [Google Scholar] [CrossRef]
- Yong, H.J.; Ahn, J.J. Antioxidant and Skin Protection Effect of Morin upon UVA Exposure. Biomed. Dermatol. 2018, 2, 12. [Google Scholar] [CrossRef]
- Scali, S.; Mezzena, M. Photostabilization Effect of Quercetin on the UV Filter Combination, Butyl Methoxydibenzoylmethane–Octyl Methoxycinnamate. Photochem. Photobiol. 2010, 86, 273–278. [Google Scholar] [CrossRef]
- Vicentini, F.T.; Georgetti, S.R.; Jabor, J.R.; Caris, J.A.; Bentley, M.V.L.; Fonseca, M.J. Photostability of Quercetin under Exposure to UV Irradiation. J. Photochem. Photobiol. B Biol. 2007, 88, 66–72. [Google Scholar]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Pereira, O.R.; Santos, G.; Sousa, M.J. Hop by-products: Pharmacological activities and potential application as cosmetics. Cosmetics 2022, 9, 139. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Borgohain, R.; Handique, J.G.; Guha, A.K.; Pratihar, S. A theoretical study of antioxidant activity of some Schiff bases derived from biologically important phenolic aldehydes and phenylenediamines. J. Phys. Org. Chem. 2018, 31, e3757. [Google Scholar] [CrossRef]
- Kumar, S.; Priyadarsini, K.I.; Sainis, K.B. Free Radical Scavenging Activity of Vanillin and o-Vanillin Using 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical. Redox Rep. 2002, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Jacquin, J.; Moureu, S.; Deweer, C.; Hakem, A.; Paguet, A.-S.; Bonneau, N.; Bordage, S.; Dermont, C.; Sahpaz, S.; Muchembled, J.; et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy 2022, 12, 2826. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Sá, P.G.S.; Guimarães, A.L.; Oliveira, A.P.; Siqueira Filho, J.A.; Fontana, A.P.; Damasceno, P.K.F.; Cardoso Branco, C.R.; Branco, A.; Guedes da Silva Almeida, J.R. Total phenols. total flavonoids and antioxidant activity of Selaginella convoluta (Arn.) Spring (Selaginellaceae). Rev. Ciênc. Farm. Básica Apl. 2012, 33, 561–566. (In Portuguese) [Google Scholar]
- Alves, E.; Kubota, E.H. Phenolic content. total flavonoid content and antioxidant activity of commercial propolis samples. Rev. Ciênc. Farm. Básica Apl. 2013, 34, 37–41. (In Portuguese) [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Scientific Methodology: Determination of Total Antioxidant Activity in Fruits by Free-Radical DPPH Capture. Embrapa Agroindústria Tropical. Comun. Técn. 127, 2007, 4p. ISSN: 1679-6535. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/426953/metodologia-cientifica-determinacao-da-atividade-antioxidante-total-em-frutas-pela-captura-do-radical-livre-dpph (accessed on 6 May 2025). (In Portuguese).
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.S.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Scientific Methodology: Determination of Total Antioxidant Activity in Fruits by the Ferric Reducing Antioxidant Power (FRAP) Method. Embrapa Agroindústria Tropical: Fortaleza. CE. Brazil. Comunicado Técnico Online 125, 2006, 4 p. ISSN: 1679-6535. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/664098/metodologia-cientifica-determinacao-da-atividade-antioxidante-total-em-frutas-pelo-metodo-de-reducao-do-ferro-frap (accessed on 6 May 2025). (In Portuguese).
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.S.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Scientific Methodology: Determination of Total Antioxidant Activity in Fruits by Free-Radical ABTS+ Capture. Embrapa Agroindústria Tropical: Fortaleza. CE. Brazil. Comunicado Técnico 1258. 2007. 6 p. ISSN: 1679-6535. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/426954/metodologia-cientifica-determinacao-da-atividade-antioxidante-total-em-frutas-pela-captura-do-radical-livre-abts (accessed on 6 May 2025). (In Portuguese).
- Oliveira, M.B.S.; Valentim, I.B.; Santos, T.R.; Xavier, J.A.; Ferro, J.N.S.; Barreto, E.O.; Santana, A.E.G.; Melo, L.V.; Bottoli, C.B.G.; Goulart, M.O.F. Phtoprotective and antiglycation activities of non-toxic Cocos nucifera Linn. (Arecaceae) husk fiber ethanol extract and its phenol chemical composition. Ind. Crops Prod. 2021, 162, 113246. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.d’A.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Wu, Y.; Liu, X.; Chen, Z.; Li, T.; Zhan, X.; Lu, C. Light Color Dihydroxybenzophenone Grafted Lignin with High UVA/UVB Absorbance Ratio for Efficient and Safe Natural Sunscreen. Ind. Eng. Chem. Res. 2020, 59, 17057–17068. [Google Scholar] [CrossRef]
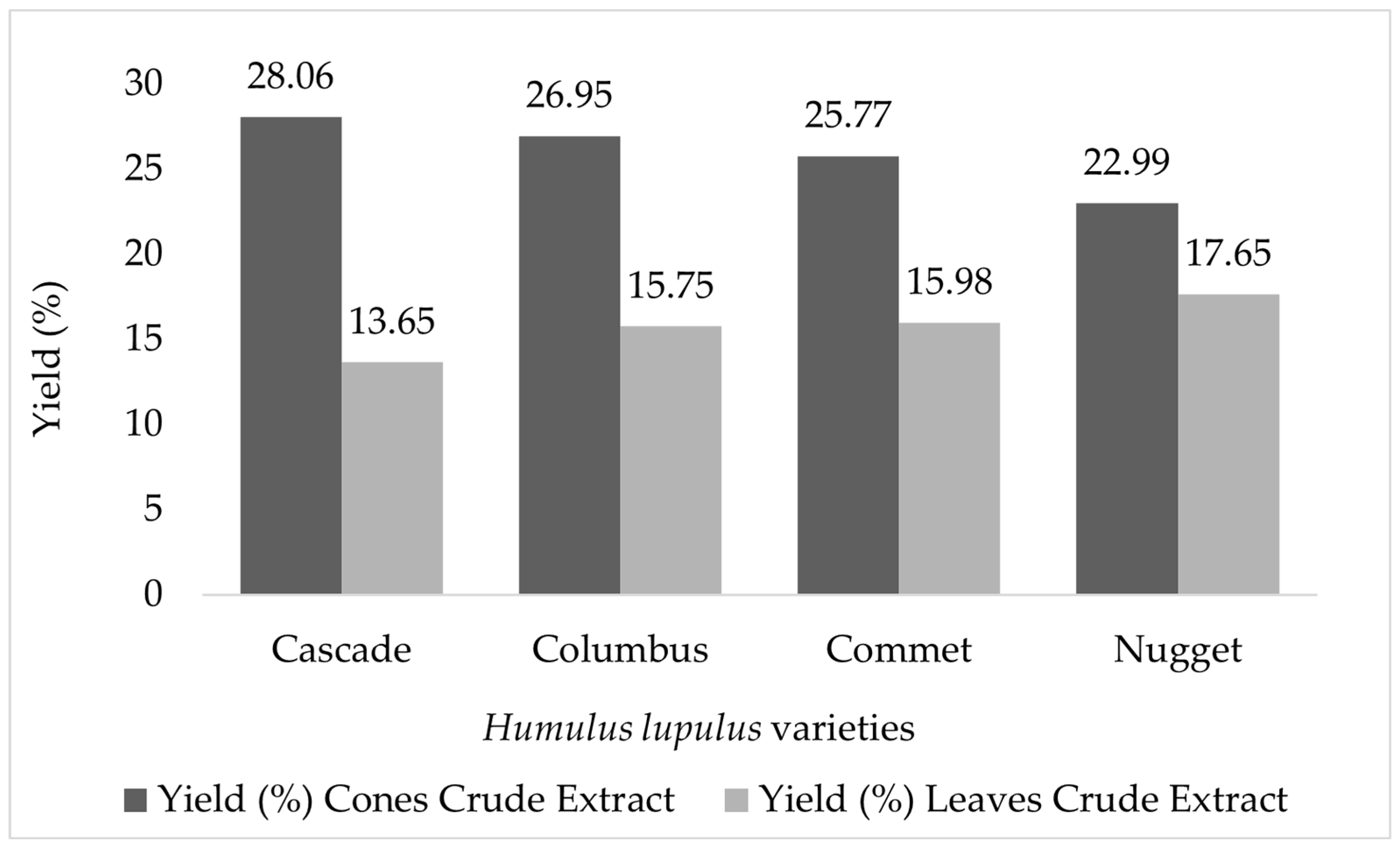
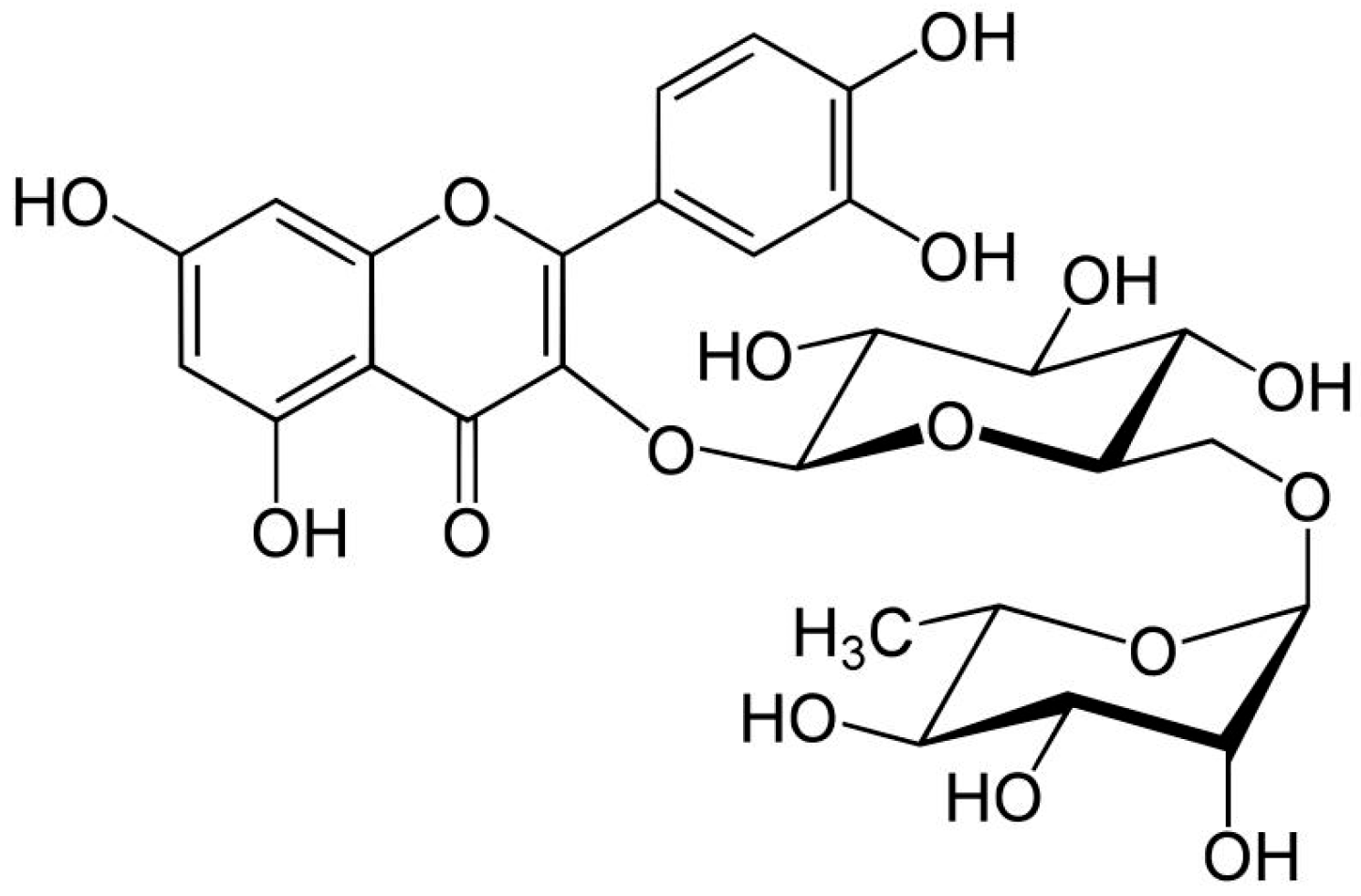
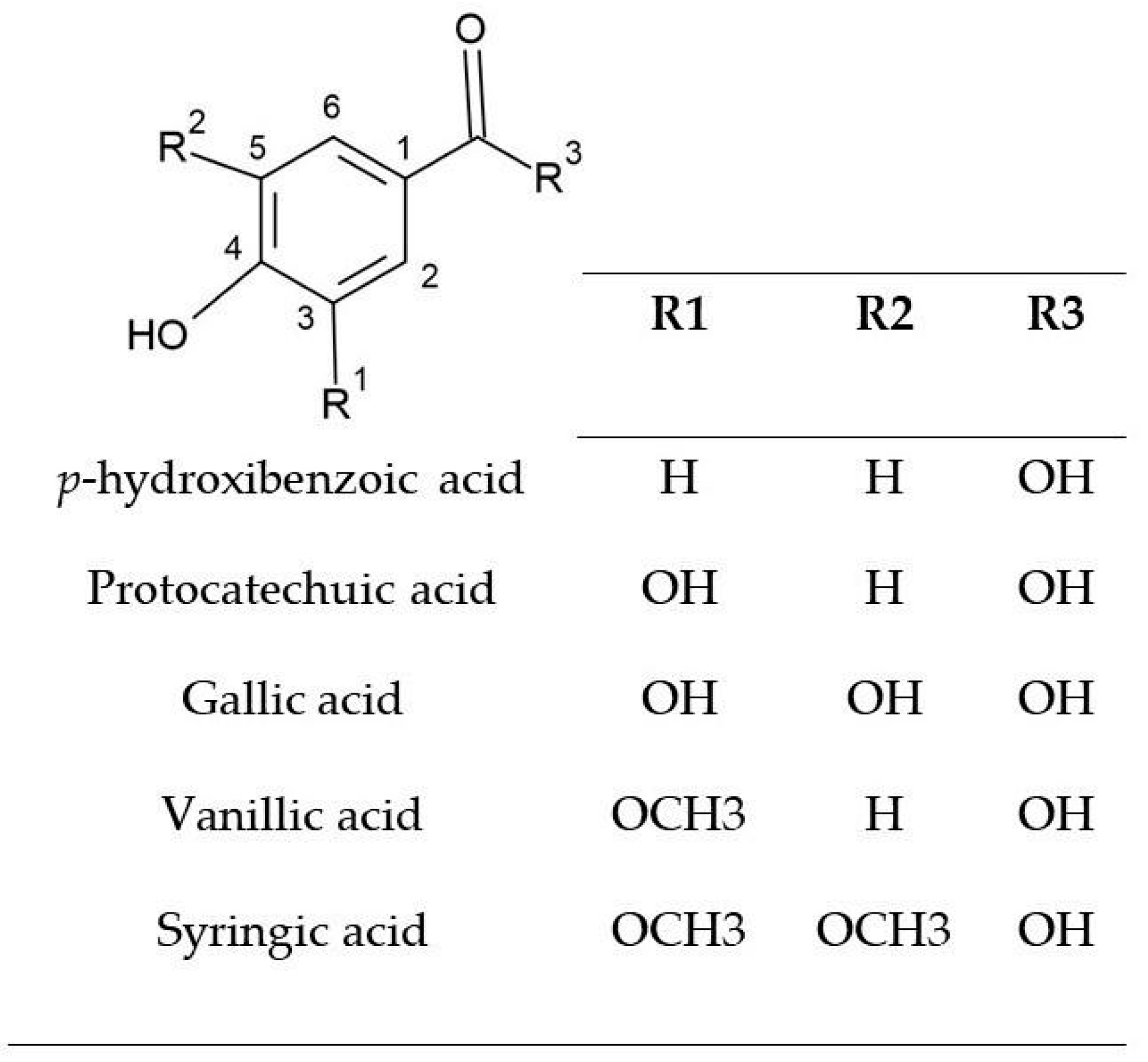
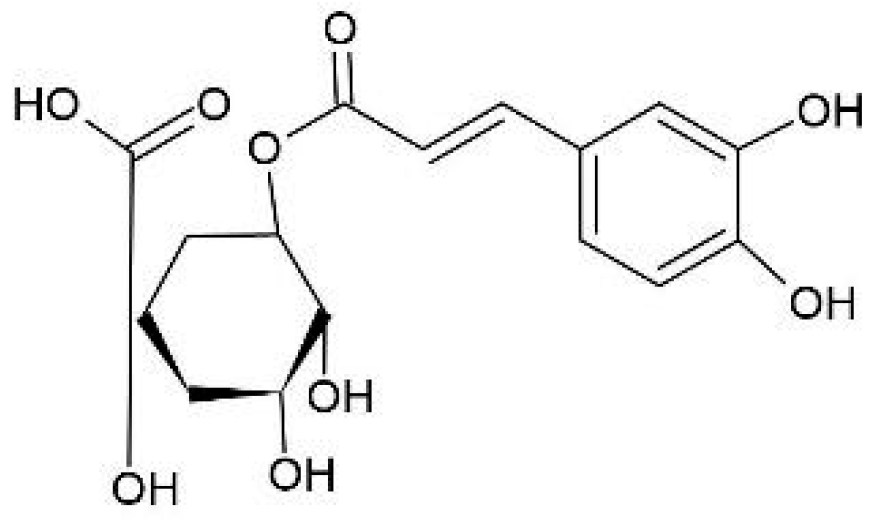
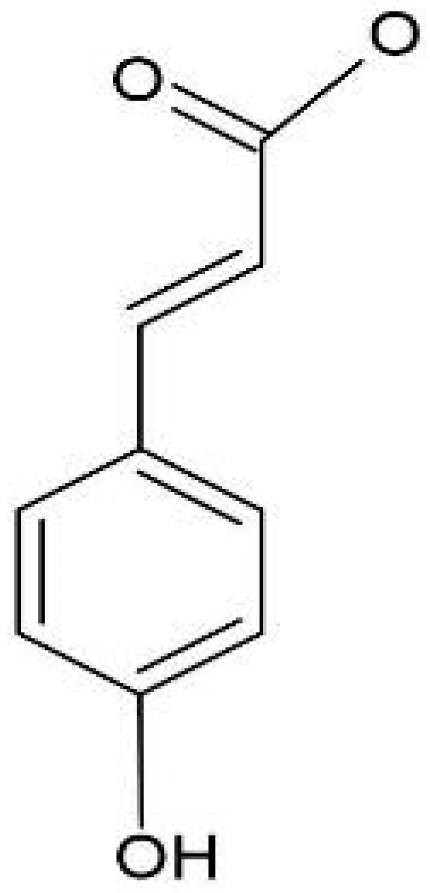
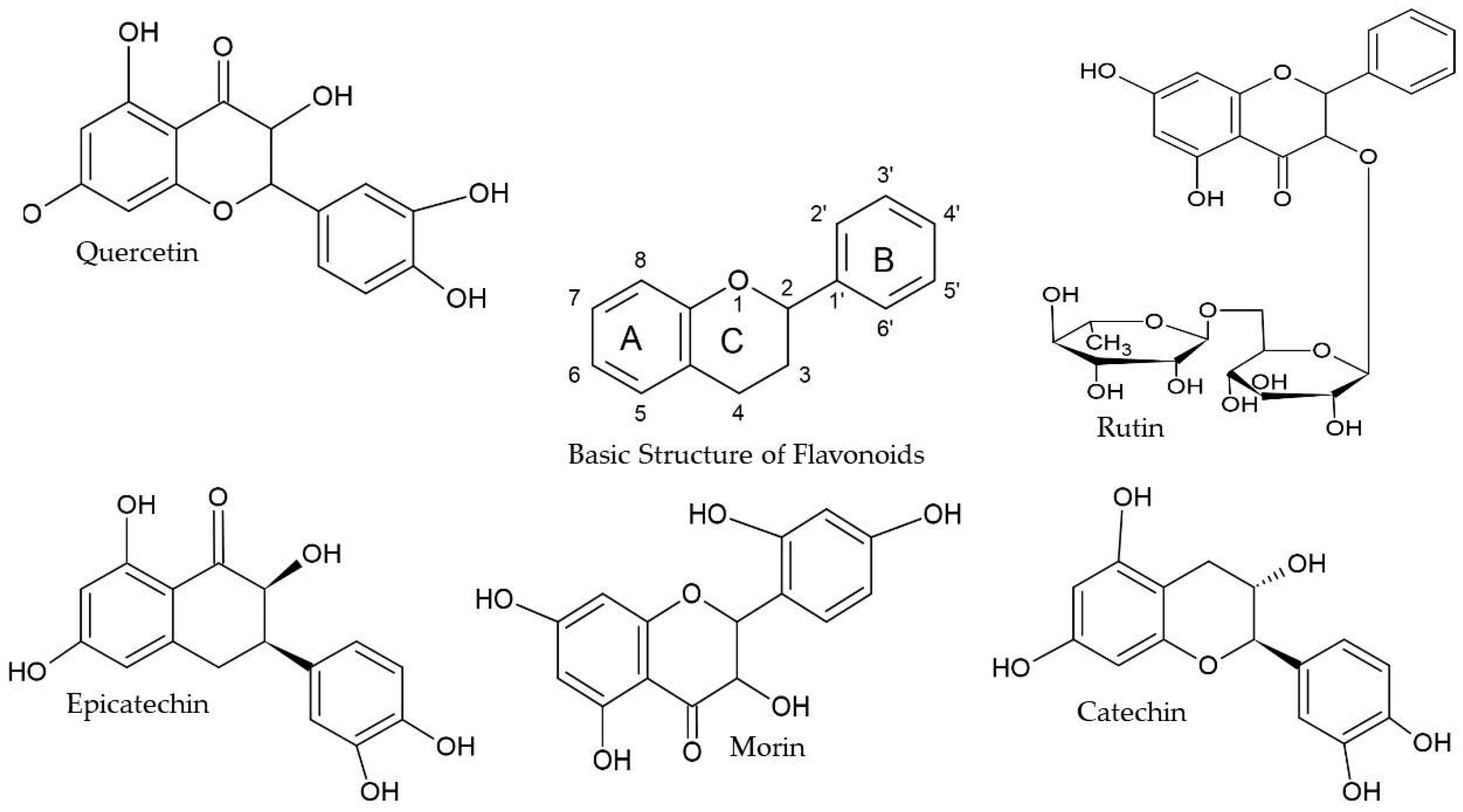
| Compound | Precursor and Productions m/z | Cascade | Columbus | Comet | Nugget |
|---|---|---|---|---|---|
| [M-H] | |||||
| Phenolic Acid | |||||
| Chlorogenic acid | 354.80 > 162.95 | 107. 21 ± 5.42 B | 185.41 ±15.80 A | 59.12 ± 1.34 C | 127.99 ± 2.17 B |
| Sinapic acid | 225.00 > 206.95 | 66.45 ± 0.58 B | 37.28 ± 0.41 C | 31.40 ± 0.45 D | 118.62 ± 1.60 A |
| Gallic acid | 169.20 > 124.90 | 92.62 ± 3.75 B | 106.38 ± 1.89 A | 32.43 ± 1.45 D | 76.81 ± 2.15 C |
| Protocatechuic acid | 153.20 > 108.95 | 272.16 ± 15.78 BC | 335.85 ± 20.23 B | 211.05 ± 9.62 C | 416.96 ± 23.06 A |
| Syringic acid | 197.20 > 182.05 | 12.35 ± 0.29 BC | 15.63 ± 1.29 B | 10.29 ± 0.77 C | 23.22 ± 1.55 A |
| Vanillic acid | 167.40 > 152.00 | 59.59 ± 4.30 A | 35.78 ± 3.07 B | 35.17 ± 0.74 B | 63.44 ± 0.94 A |
| p-Hydroxybenzoic acid | 137.20 > 92.90 | 226.60 ± 7.66 B | 129.27 ± 1.71 D | 175.01 ± 10.26 C | 330.61 ± 14.33 A |
| p-Coumaric acid | 163.20 > 119.00 | 109.67 ± 5.55 B | 85.87 ± 3.41 B | 202.33 ± 11.66 A | 176.68 ± 2.28 A |
| Caffeic acid | 179.20 > 135.00 | 14.08 ± 1.13 B | 17.34 ± 1.40 B | 13.58 ± 1.26 B | 27.26 ± 0.64 A |
| Ferulic acid | 193.20 > 133.95 | 122.55 ± 7.80 B | 92.77 ± 2.15 C | 120.25 ± 2.27 B | 143.96 ± 0.04 A |
| Quinic acid | 191.20 > 84.80 | 64.25 ± 1.51 C | 125.29 ± 4.21 A | 104.75 ± 3.12 B | 61.99 ± 0.80 C |
| Organic Acids | |||||
| Malic acid | 133.20 > 114.90 | 191.73 ± 0.14 D | 223.21 ± 8.87 C | 354.37 ± 10.89 A | 309.66 ± 2.55 B |
| Fumaric acid | 115.20 > 71.00 | 193.09 ± 3.64 B | 120.08 ± 8.85 C | 82.05 ± 7.82 D | 193.09 ± 3.64 A |
| Nicotinic acid | 122.40 > 77.90 | 46.52 ± 1.95 A | 19.98 ± 1.59 C | 14.56 ± 0.19 C | 37.84 ± 2.82 B |
| Flavonoids | |||||
| Catechin | 289.10 > 245.10 | 54.83 ± 0.17 B | 51.30 ± 4.85 B | 53.42 ± 4.94 B | 177.18 ± 0.66 A |
| Morin | 302.80 > 152.90 | 150.76 ± 9.48 B | 86.94 ± 2.78 C | 141.04 ± 1.58 B | 265.37 ± 10.28 A |
| Epicatechin | 289.10 > 245.10 | 53.38 ± 4.89 B | 56.32 ± 1.15 B | 71.29 ± 6.68 B | 174.77 ± 13.28 A |
| Quercetin | 301.10 > 151.00 | 117.35 ± 11.65 B | 101.97 ± 7.70 B | 159.57 ± 0.01 A | 164.32 ± 12.59 A |
| Rutin | 609.00 > 300.00 | >500 µg/g A | >500 µg/g A | >500 µg/g A | >500 µg/g A |
| Luteolin | 286.90 > 152.85 | <1 µg/g A | <1 µg/g A | <1 µg/g A | <1 µg/g A |
| Naringenin | 271.10 > 151.00 | 13.19 ± 1.18 B | 14.42 ± 0.33 B | 4.44 ± 0.10 C | 35.77 ± 2.57 A |
| Phenolic Aldehydes | |||||
| Hydroxybenzaldehyde | 123.10 > 76.9500 | 116.78 ± 7.10 B | 77.74 ± 5.29 C | 183.84 ± 9.81 A | 143.94 ± 6.33 B |
| Vanillin | 151.40 > 136.05 | 101.14 ± 1.64 A | 45.22 ± 2.15 B | 21.98 ± 1.47 C | 92.16 ± 3.75 A |
| Isovaniline | 153.10 > 65.00 | 21.21 ± 0.07 B | 11.21 ± 0.15 C | 11.72 ± 0.94 C | 38.48 ± 0.34 A |
| Syringaldehyde | 183.00 > 94.95 | 27.75 ± 0.56 A | 11.20 ± 0.82 C | 16.10 ± 0.10 B | 27.06 ± 0.26 A |
| Alkaloids | |||||
| Caffeine | 194.90 > 137.95 | 6.71 ± 0.28 A | 3.55 ± 0.20 B | 5.80 ± 0.30 A | 5.99 ± 0.02 A |
| Lactones | |||||
| Coumarin | 146.80 > 90.95 | <1 µg/g A | <1 µg/g A | <1 µg/gA | <1 µg/gA |
| Compound | Precursor and Productions | Cascade | Columbus | Comet | Nugget | |
|---|---|---|---|---|---|---|
| m/z | ||||||
| [M-H] | ||||||
| Phenolic Acids | ||||||
| Clorogenic acid | 354.80 > 162.95 | 120.079 ± 2.589 B | 116.728 ± 1.919 A | 88.510 ± 7.101 B | 114.786 ± 0.465 B | |
| Sinapic acid | 225.00 > 206.95 | 13.588 ± 0.159 B | 68.604 ± 4.498 A | 15.899 ± 0.880 B | 60.870 ± 4.385 A | |
| Protocatechuic acid | 153.20 > 108.95 | 150.327 ± 7.818 C | 440.340 ± 1.069 A | 198.009 ± 3.340 B | 154.820 ± 12.153 C | |
| p-Hydroxybenzoic acid | 137.20 > 92.90 | 160.596 ± 10.599 C | 402.108 ± 335 A | 259.665 ± 13.260 B | 261.192 ± 13.661 B | |
| p-Coumaric acid | 163.20 > 119.00 | 121.808 ± 8.819 B | 324.489 ± 12.106 A | 316.636 ± 11.398 A | 351.902 ± 2.624 A | |
| Caffeic acid | 179.20 > 135.00 | 57.132 ± 2.264 B | 89.278 ± 2.750 A | 57.931 ± 3.287 B | 67.170 ± 5.059 B | |
| Ferulic acid | 193.20 > 133.95 | 124.179 ± 6.323 B | 33.175 ± 28.197 A | 78.208 ± 2.748 B | 79.097 ± 0.992 B | |
| Organic Acids | ||||||
| Quinic acid | 191.20 > 84.80 | 62.762 ± 2.371 B | 66.513 ± 0.521 B | 49.185 ± 2.577 C | 81.253 ± 3.374 A | |
| Malic acid | 133.20 > 114.90 | 41.851 ± 0.060 A | 42.080 ± 1.511 A | 21.753 ± 1.731 B | 25.632 ± 0.138 B | |
| Nicotinic acid | 122.40 > 77.90 | 113.368 ± 4.824 B | 175.723 ± 8.326 A | 53.480 ± 3.968 D | 76.379 ± 2.059 C | |
| Flavonoids | ||||||
| Catechin | 289.10 > 245.10 | ND | ND | 74.206 ± 5.217 A | 71.339 ± 2.473 A | |
| Epicatechin | 289.10 > 245.10 | ND | ND | 79.518 ± 6.356 A | 81.253 ± 3.374 A | |
| Quercetin | 301.10 > 151.00 | ND | ND | 11.823 ± 0.972 A | 6.845 ± 0.024 B | |
| Rutin | 609.00 > 300.00 | >500 µg/g A | >500 µg/g A | > 500 µg/g A | >500 µg/g A | |
| Luteolin | 286.90 > 152.85 | <1 µg/g A | <1 µg/g A | <1 µg/g A | <1 µg/g A | |
| Naringenin | 271.10 > 151.00 | ND | ND | 8.395 ± 0.635 B | 36.018 ± 1.652 A | |
| Phenolic Aldehydes | ||||||
| Hydroxybenzaldehyde | 123.10 > 76.9500 | 33.898 ± 0.598 B | 117.360 ± 6.586 A | 119.836 ± 8.836 A | 109.823 ± 5.818 A | |
| Vanillin | 151.40 > 136.05 | 102.835 ± 5.839 A | 196.922 ± 10.557 AB | 248.363 ± 22.271 C | 157.535 ± 1.061 B | |
| Isovaniline | 153.10 > 65.00 | 21.776 ± 1.694 B | 28.420 ± 1.958 A | 22.678 ± 0.425 AB | 27.601 ± 1.398 AB | |
| Syringaldehyde | 183.00 > 94.95 | 17.992 ± 0.985 B | 40.758 ± 1.093 A | 22.743 ± 2.206 B | 34.031 ± 2.649 A | |
| Alkaloids | ||||||
| Caffeine | 194.90 > 137.95 | 16.126 ± 0.904 A | 18.973 ± 0.329 A | 102.02 ± 0.865 B | 18.023 ± 1.731 A | |
| Total Phenol Content (µg Gallic Acid Equivalent/mg CE) | ||||
|---|---|---|---|---|
| Cones | ||||
| 1.00 | 0.75 | 0.50 | 0.25 | |
| Cascade | 95.95 ± 2.43 A,a,A | 76.33 ± 3.14 A,b,A | 54.52 ± 1.61 A,c,A | 44.23 ± 0.48 A,d,A |
| Columbus | 88.92 ± 7.51 A,a,A | 76.79 ± 3.34 A,b,A | 47.17 ± 3.04 B,c, B | 23.44 ± 0.5 BC,d,CD |
| Comet | 76.42 ± 0.36 B,a, BC | 52.19 ± 0.73 C,b,C | 37.90 ± 0.77 C, c, C | 19.68 ± 1.30 C, d,CDE |
| Nugget | 87.48 ± 4.79 AB, a, AB | 68.31 ± 0.39 B, b, B | 48.54 ± 3.89 AB, c, AB | 24.96 ± 2.70 B, d,C |
| Leaves | ||||
| Cascade | 53.08 ± 4.17 B, a, DE | 41.62 ± 1.93 B, b, D | 27.61 ± 1.41 B, c, A | 13.98 ± 0.42 B, d, E |
| Columbus | 46.39 ± 4.57 B, a, E | 34.90 ± 2.11 C, b, E | 29.34 ± 2.41 AB, b, B | 13.38 ± 2.03 B,c, E |
| Comet | 64.36 ± 5.46 A, a, CD | 46.32 ± 2.06 AB, b, CD | 35.96 ± 4.14 A, c, CD | 17.69 ± 2.46 B, d, DE |
| Nugget | 69.70 ± 0.87 A, a, C | 49.56 ± 2.23 A, b, C | 35.16 ± 1.07 A, c, CD | 35.66 ± 5.08 A, c, B |
| Flavonoid Content (µg Equivalent of Quercetin/mg of CE) | ||||
|---|---|---|---|---|
| 1.00 | 0.75 | 0.5 | 0.25 | |
| Cones | ||||
| Cascade | 193.82 ± 1.20 A.a.E | 188.30 ± 3.66 A,a,D | 189.33 ± 2.40 A,a, E | 187.34 ± 6.51 A, a, F |
| Columbus | 170.53 ± 2.77 A, a, F | 163.61 ± 3.23 A, a, E | 164.00 ± 3.25 A, a, F | 167.87 ± 5.72 A, a, G |
| Comet | 196.68 ± 2.40 B, b, E | 193.24 ± 2.97 B, b, D | 197.59 ± 2.86 B, b, E | 222.21 ± 1.30 A, a, DE |
| Nugget | 247.15 ± 2.11 AB,ab, D | 247.84 ± 3.00 AB, ab, C | 255.15 ± 2.28 A, a, C | 238.13 ± 6.51 B, b, D |
| Leaves | ||||
| Cascade | 438.05 ± 2.76 A, a, C | 443.25 ± 3.42 A, a, C | 450.28 ± 7.57 A, a, b | 459.93 ± 9.86 A,a,c |
| Columbus | 620.27 ± 10.08 BC, b, B | 610.03 ± 8.99 C,a, A | 627.42 ± 9.44 B, a, a | 649.56 ± 15.96 A, a, a |
| Comet | 696.67 ± 4.03 A, a, A | 602.97 ± 3.42 C, c, A | 620.86 ± 3.53 B, b, a | 627.87 ± 8.63 B, b, b |
| Nugget | 243.13 ± 2.28 A, a, D | 158.53 ± 2.81 C, c, E | 227.85 ± 5.53 B, b, d | 216.97 ± 7.72 B, b, e |
| DPPH• IC50 (mg/mL) | FRAP IC50 (µM Ferrous Sulfate/mg) | ABTS•+ IC50 (mM of Trolox/mg of CE) | |
|---|---|---|---|
| Cones | |||
| Cascade | 1.34 ± 0.16 B,AB | 2.35 ± 0.03 C,BC | 5.11 ± 0.61 B,B |
| Columbus | 1.62 ± 0.23 B,AB | 2.33 ± 0.10 C,BC | 5.40 ± 0.48 BC,B |
| Comet | 2.97 ± 0.59 C,B | 2.52 ± 0.04 B,B | 7.10 ± 0.68 D,B |
| Nugget | 2.25 ± 0.83 BC,AB | 2.42 ± 0.03 BC,B | 6.42 ± 0.12 DC,B |
| Quercetina | 0.01 ± 0.01 A,A | - | - |
| Trolox | - | 9.17 ± 0.01 A | 0.25 ± 0.01 a,A |
| Leaves | |||
| Cascade | 2.87 ± 1.03 b,B | 1.19 ± 0.05 c, D | 22.60 ± 1.56 D,E |
| Columbus | 3.13 ± 1.16 b,B | 2.36 ± 0.15 b,B | 15.19 ± 0.75 C,D |
| Comet | 1.63 ± 1.37 ab, AB | 2.15 ± 0.41 b, BC | 12.50 ± 1.06 B, C |
| Nugget | 2.99 ± 1.12 b, B | 1.92 ± 0.07 b, C | 10.99 ± 0.75 B, C |
| Quercetina | 0.01 ± 0.01 a, A | - | - |
| Trolox | - | 9.17 ± 0.01 a, A | 0.25 ± 0.01 a, A |
| Sun Protection Factor (SPF) | ||||
|---|---|---|---|---|
| 1.00 | 0.75 | 0.50 | 0.25 | |
| Cones | ||||
| Cascade | 39.48 ± 0.09 A,a,A | 27.56± 2.97 A,b,A | 20.86 ± 3.29 A,b,A | 12.40 ± 3.69 A,c,A |
| Columbus | 33.27 ± 0.19 B,a,B | 25.20 ± 0.28 A,b,A | 15.80 ± 0.11 B,c,B | 7.53 ± 0.53 A,d, BCD |
| Comet | 38.88 ± 0.25 A,a,A | 27.66 ± 0.14 A,b,A | 21.33 ± 0.43 A,c,A | 8.84 ± 0.47 A,d,ABC |
| Nugget | 39.07 ± 0.83 A,a,A | 28.00 ± 0.52 A,b,A | 19.41 ± 0.08 AB,c,A | 10.438 ± 0.72 A,d,AB |
| Leaves | ||||
| Cascade | 16.02 ± 0.11 D,a,F | 12.15 ± 0.01 A,b,B | 7.81 ± 0.12 C,c,D | 4.57 ± 0.29 A,d,DE |
| Columbus | 17.90 ± 0.45 C,a,E | 13.71 ± 0.26 A,b,B | 8.40 ± 0.55 C,c,CD | 5.38 ± 0.40 A,d,CDE |
| Comet | 22.37 ± 0.66 A,a,C | 14.90 ± 4.50 A,b,B | 11.27 ± 0.16 A,b,C | 3.12 ± 0.09 B,c,E |
| Nugget | 20.45 ± 0.34 B,a,D | 15.52 ± 0.36 A,b,B | 10.08 ± 0.63 B,c,CD | 4.72 ± 0.49 AD,d,DE |
| UVA/UVB Ratio, Anti-UVA Protection, and Critical Wavelength (λc) | ||||||
|---|---|---|---|---|---|---|
| 1.00 | 0.75 | 0.50 | 0.25 | anti-UVA protection | CW(λc) | |
| Cones | ||||||
| Cascade | 1.95 ± 0.01 A,c,E | 1.93 ± 0.01 A,c,D | 1.98 ± 0.01 A,b,E | 2.02 ± 0.01 A,a,A | Ultra | 370 |
| Columbus | 1.58 ± 0.01 C,c,G | 1.58 ± 0.01 D,c,F | 1.67 ± 0.01 B,b,F | 1.72 ± 0.01 B,a,B | Ultra | 370 |
| Comet | 1.76 ± 0.02 B,a,F | 1.62 ± 0.01 C,c,EF | 1.64 ± 0.01 C,c,F | 1.68 ± 0.01 C,b,C | Ultra | 370 |
| Nugget | 1.73 ± 0.01 B,a,F | 1.65 ± 0.02 B,b,E | 1.67 ± 0.01 B,b,F | 1.72 ± 0.01 B,a,B | Ultra | 370 |
| Leaves | ||||||
| Cascade | 2.29 ± 0.01 D,b,D | 2.55 ± 0.01 B,a,B | 2.22 ± 0.01 D,c,D | 2.12 ± 0.02 C,d,A | Ultra | 375 |
| Columbus | 2.41 ± 0.02 C,a,C | 2.42 ± 0.01 C,a,C | 2.42 ± 0.01 C,a,C | 2.10 ± 0.01 C,b,B | Ultra | 380 |
| Comet | 2.50 ± 0.01 B,a,B | 2.42 ± 0.03 C,b,C | 2.50 ± 0.02 B,a,B | 2.41 ± 0.09 B,c,C | Ultra | 380 |
| Nugget | 3.00 ± 0.01 A,a,A | 3.02 ± 0.02 A,a,A | 3.03 ± 0.01 A,a,A | 3.02 ± 0.03 A,b,B | Ultra | 385 |
| UVA/UVB Limits | ||||||
|---|---|---|---|---|---|---|
| 0–0.2 | 0.21–0.4 | 0.41–0.6 | 0.61–0.8 | 0.81–0.9 | >0.91 | |
| Number of stars | - | * | ** | *** | **** | ***** |
| UVA protection | Very low | Moderate | Good | Superior | Maximum | Ultra |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.C.C.; da Silva, F.P.A.; Beirão, G.A.R.; Severino, J.J.; Machado, M.d.A.; Barbosa, M.P.d.S.B.; Reyes, G.B.; Rickli, M.E.; Lopes, A.D.; Jacomassi, E.; et al. Phytochemical Profile, Antioxidant Capacity, and Photoprotective Potential of Brazilian Humulus Lupulus. Pharmaceuticals 2025, 18, 1229. https://doi.org/10.3390/ph18081229
Silva GCC, da Silva FPA, Beirão GAR, Severino JJ, Machado MdA, Barbosa MPdSB, Reyes GB, Rickli ME, Lopes AD, Jacomassi E, et al. Phytochemical Profile, Antioxidant Capacity, and Photoprotective Potential of Brazilian Humulus Lupulus. Pharmaceuticals. 2025; 18(8):1229. https://doi.org/10.3390/ph18081229
Chicago/Turabian StyleSilva, Gabriela Catuzo Canonico, Fabiana Pereira Alves da Silva, Gabriel Augusto Rodrigues Beirão, José Júnior Severino, Mariane de Almeida Machado, Marina Pereira da Silva Bocchio Barbosa, Giulia Boito Reyes, Max Emerson Rickli, Ana Daniela Lopes, Ezilda Jacomassi, and et al. 2025. "Phytochemical Profile, Antioxidant Capacity, and Photoprotective Potential of Brazilian Humulus Lupulus" Pharmaceuticals 18, no. 8: 1229. https://doi.org/10.3390/ph18081229
APA StyleSilva, G. C. C., da Silva, F. P. A., Beirão, G. A. R., Severino, J. J., Machado, M. d. A., Barbosa, M. P. d. S. B., Reyes, G. B., Rickli, M. E., Lopes, A. D., Jacomassi, E., Nunes, M. G. I. F., Francisco, J. P., Barros, B. C. B., do Valle, J. S., Gonçalves, J. E., & Gazim, Z. C. (2025). Phytochemical Profile, Antioxidant Capacity, and Photoprotective Potential of Brazilian Humulus Lupulus. Pharmaceuticals, 18(8), 1229. https://doi.org/10.3390/ph18081229











