Eupatorium lindleyanum DC Ameliorates Carbon Tetrachloride-Induced Hepatic Inflammation and Fibrotic Response in Mice
Abstract
1. Introduction
2. Results
2.1. Chemical Profiling of Eup Extract
2.2. Bioinformatics Analysis of mRNA-Seq Gene
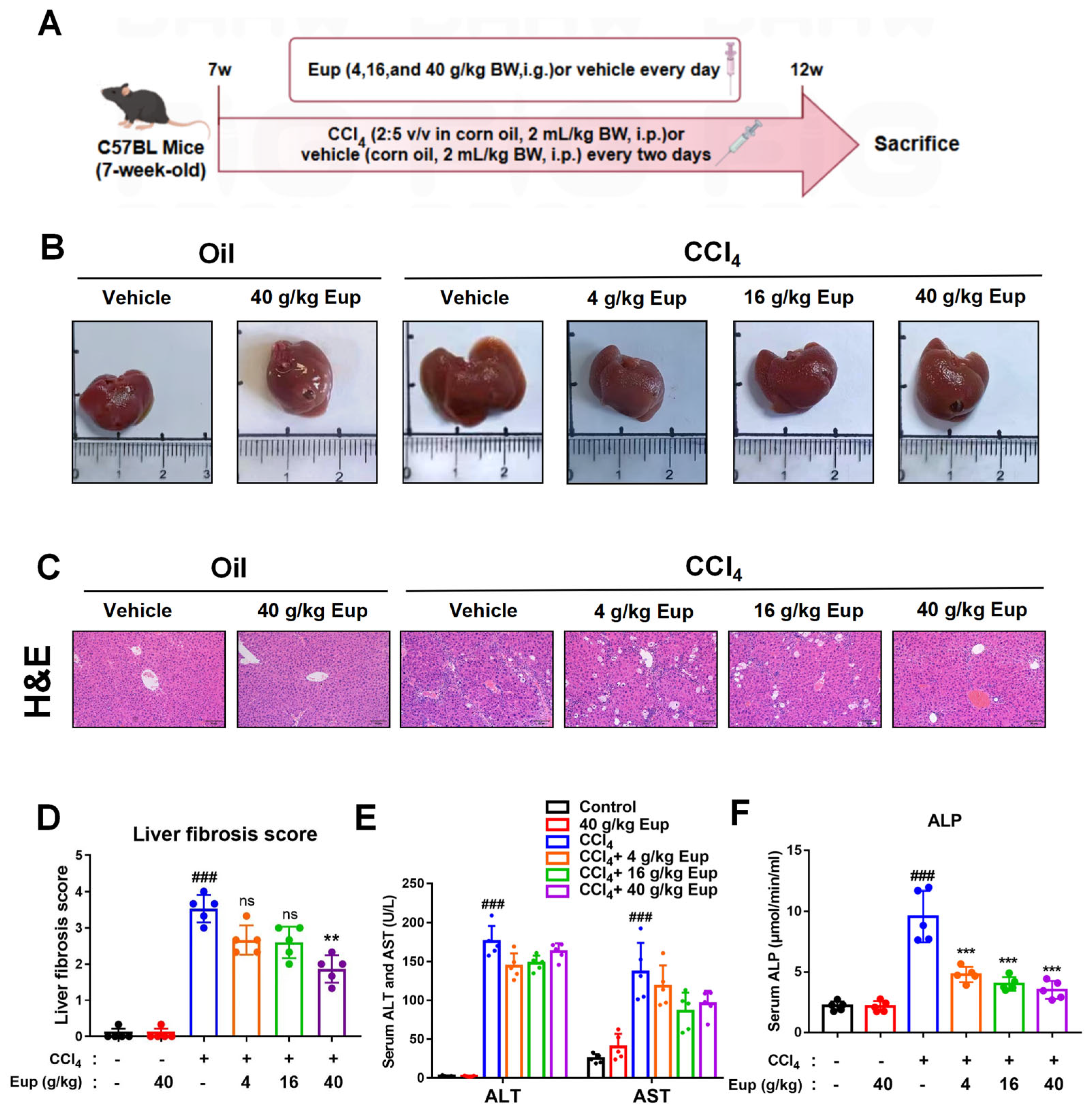
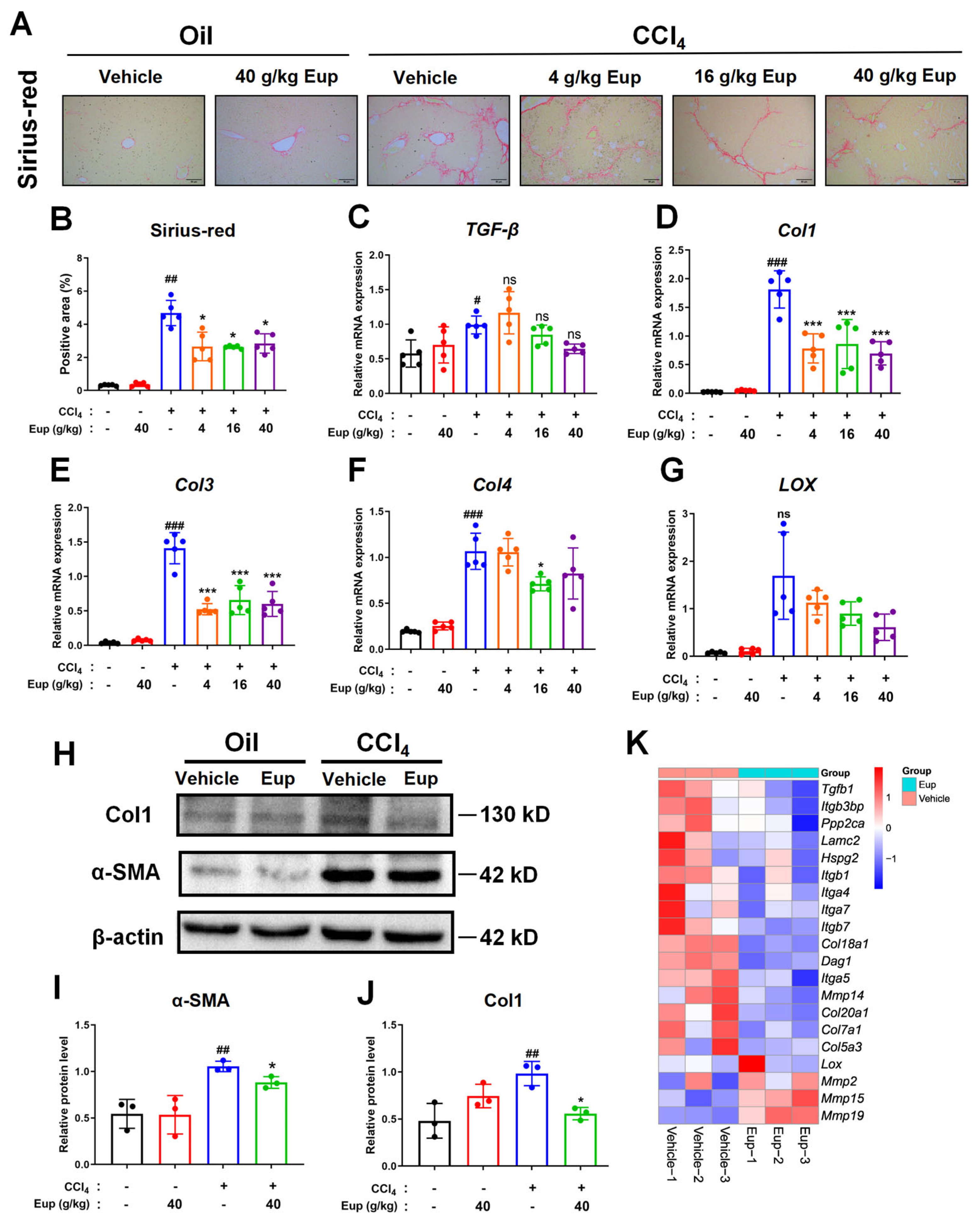
2.3. Eup Attenuates CCl4-Induced Liver Fibrosis in Mice
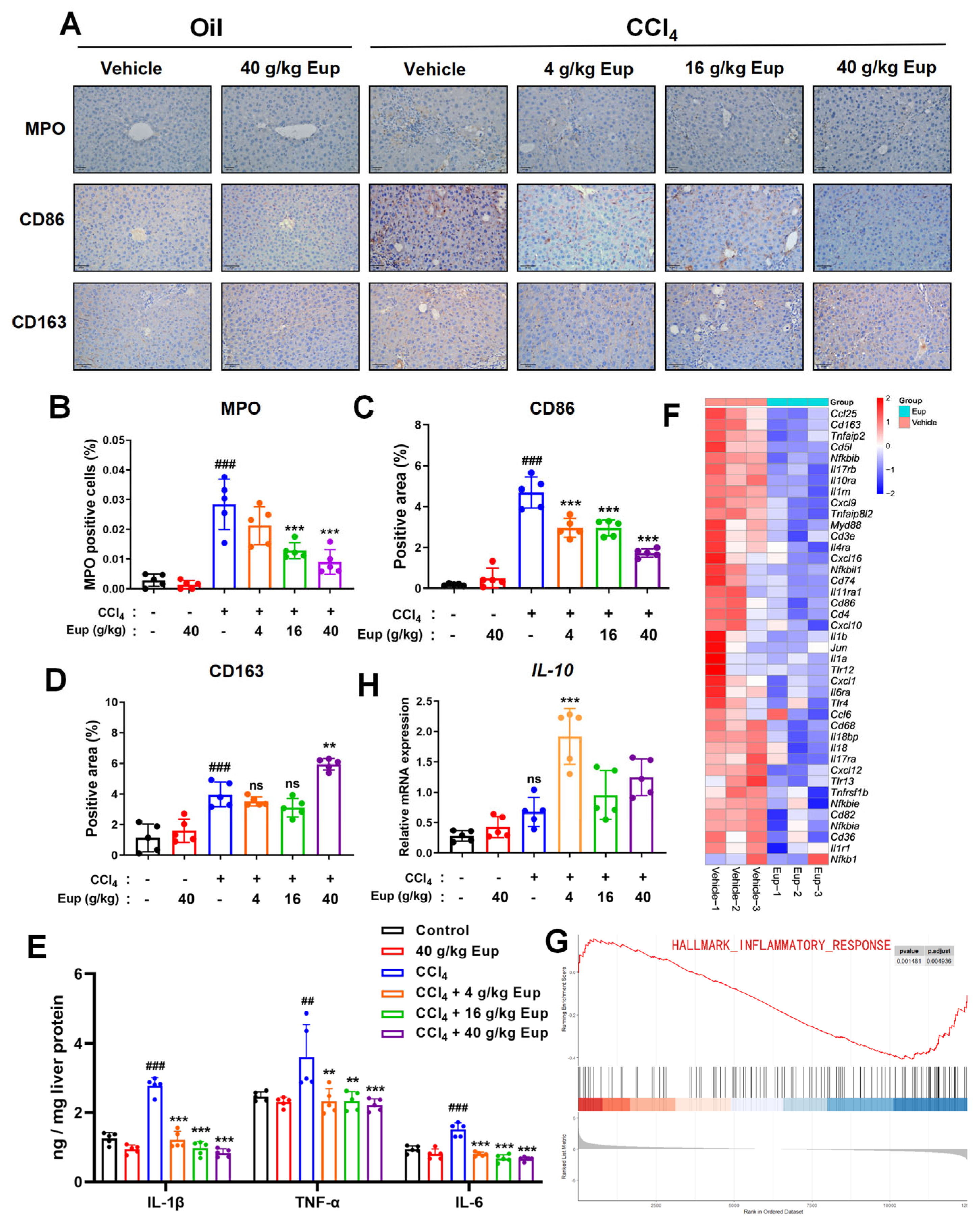
2.4. Eup Treatment Therapeutically Mitigates the Inflammation Progression of CCl4-Evoked Hepatic Fibrosis
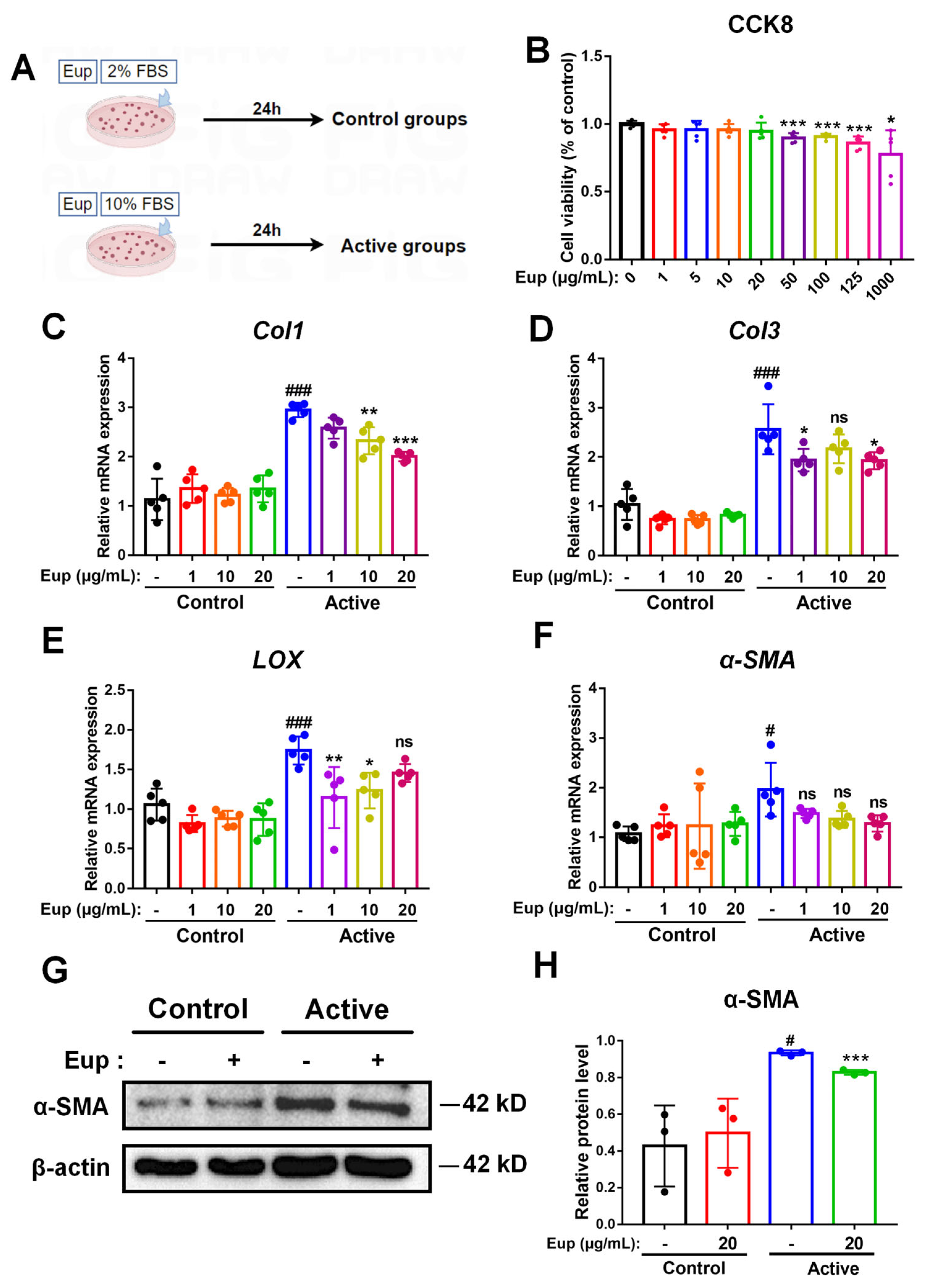
2.5. Eup Treatment Inhibits HSC Activation In Vitro
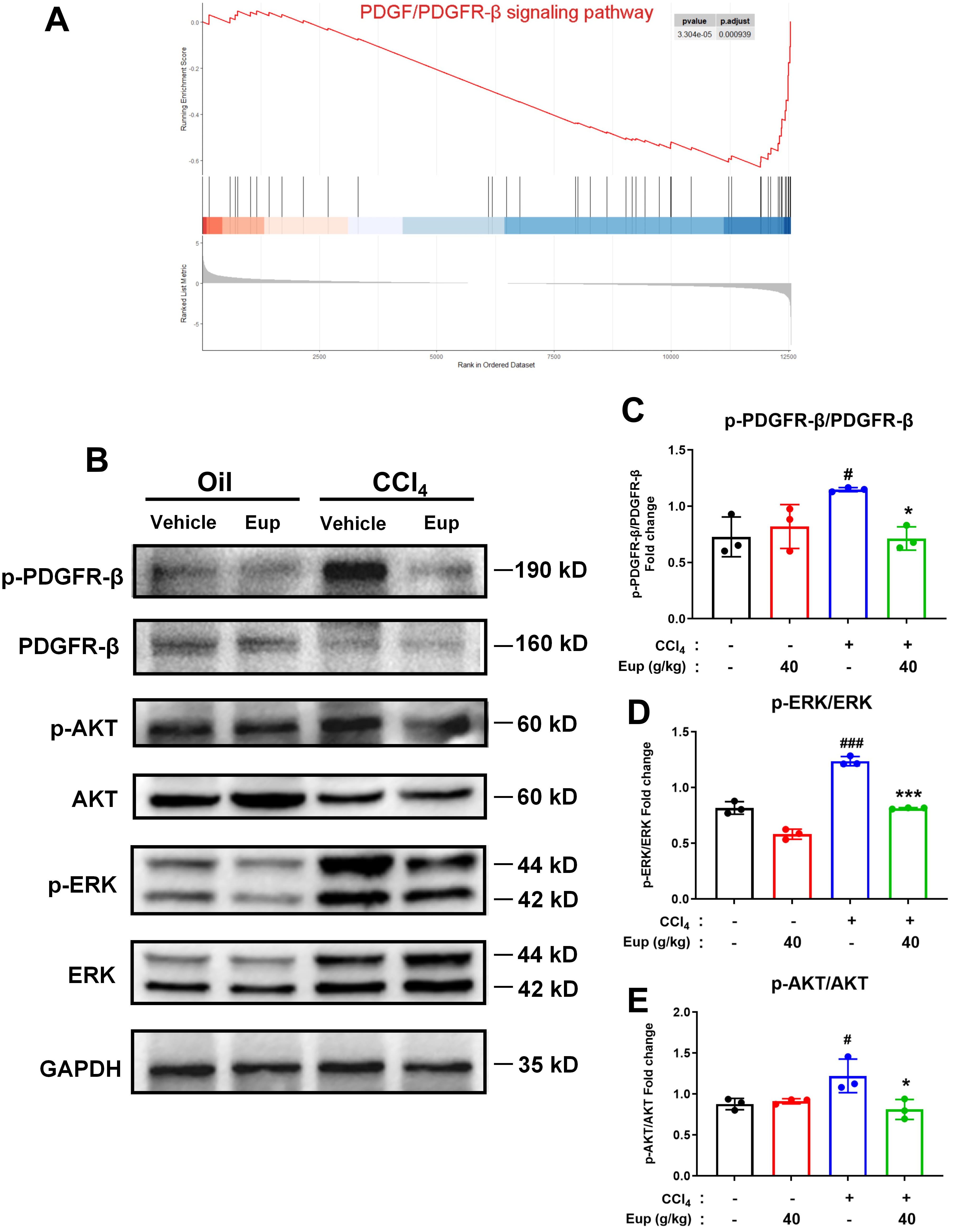
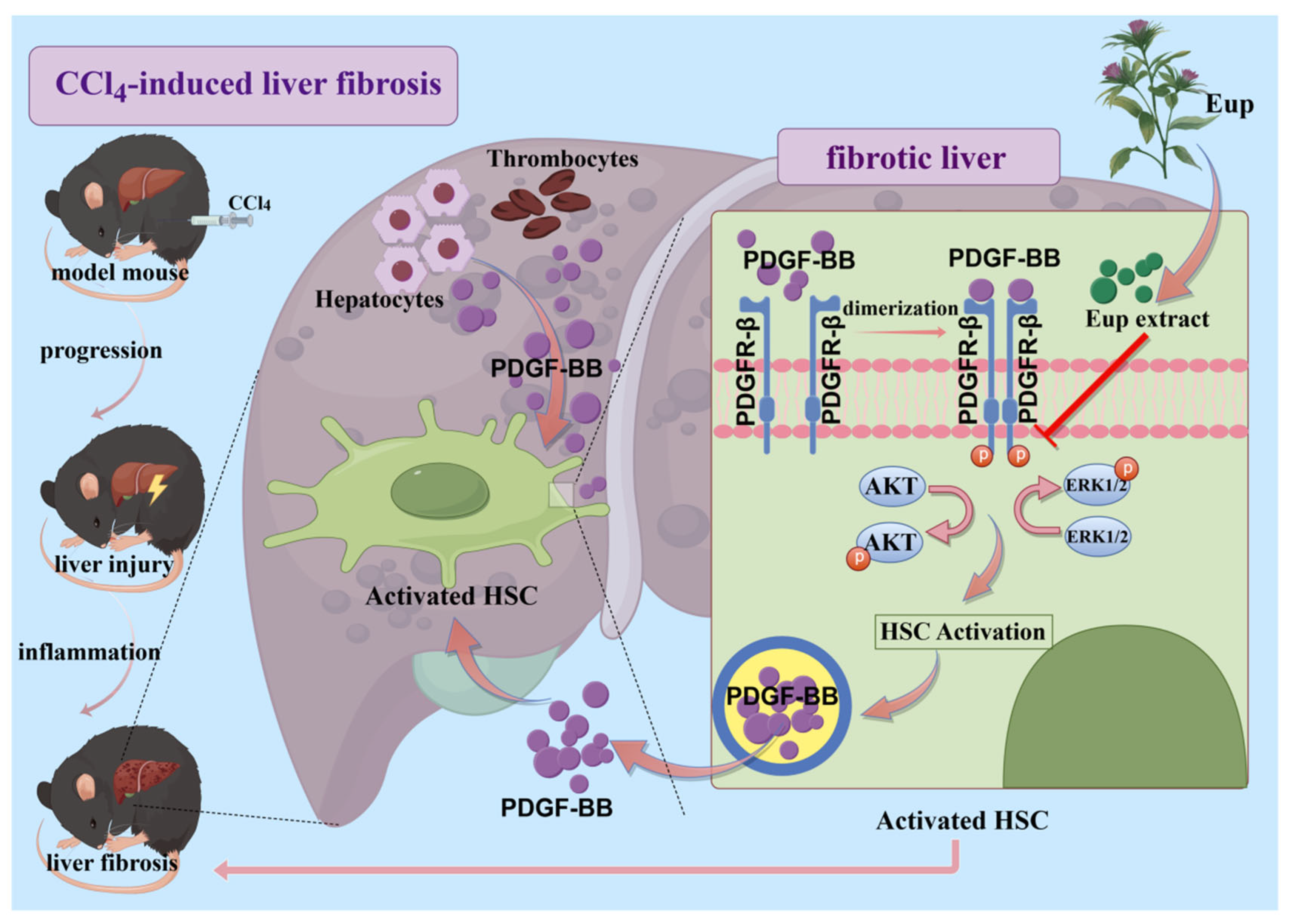
2.6. Eup Improves CCl4-Mediated Murine Liver Fibrosis by Modulating the PDGF/PDGFR-β Signaling Pathway
2.7. Eup Suppresses PDGF-BB-Induced HSCs Activation by Inhibiting the PDGF-BB/PDGFR-β Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation of Eup
4.2. Animals and Experimental Design
4.3. Histological Analysis: H&E Staining, Sirius-Red Staining, and Fibrotic Score
4.4. Immunohistochemistry (IHC) Staining
4.5. Serum Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Alkaline Phosphatase (ALP) Detection
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Quantification of Inflammatory Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blot
4.9. Cell Treatments
4.10. RNA-Sequencing Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Eup | Eupatorium lindleyanum DC |
| PCA | Principal Component Analysis |
| GSEA | Gene Set Enrichment Analysis |
| HSC | Hepatic stellate cells |
| CLD | Chronic liver disease |
| ECM | Extracellular matrix |
| ROS | Reactive oxygen species |
| TGF-β1 | Transforming growth factor-beta 1 |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| BW | Body weight |
| i.g. | Intragastrically |
| i.p. | Intraperitoneally |
| H&E staining | Haematoxylin and eosin (H&E) staining |
| IHC | Immunohistochemistry staining |
| HRP | Horseradish peroxidase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| RIPA | Radioimmunoprecipitation assay buffer |
| TBS | Tris-buffered saline |
| SDS-PAGE | Sodium dodecyl sulphatepolyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
| BSA | Bovine serum albumin |
| ECL | Enhanced chemiluminescence |
| FBS | Fetal Bovine Serum |
| DEGs | Differentially expressed genes |
| FDR | False discovery rate |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| UPLC-Q/TOF-LC/MS | Ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry |
| α-SMA | α-smooth muscle actin |
| Col1 | Collagen type I |
| Col3 | Collagen type III |
| LOX | Lysyl oxidase |
| ERK | PI3K/AKT and MAPK pathways |
| CSF | Clinically Significant Fibrosis |
| TCM | Traditional Chinese medicine |
| DMN | Dimethylnitrosamine |
| NF-κB | Nuclear factor kappa B |
| iNOS | Inducible nitric oxide synthase |
| MAPK | Mitogen-activated protein kinase |
References
- Qi, J.; Li, L.; Yan, X.; Hua, W.; Zhou, Z. Sappanone A Alleviates the Severity of Carbon Tetrachloride-Induced Liver Fibrosis in Mice. Antioxidants 2023, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Yan, X.; Chen, M.; Lin, Y.; Li, L.; Wang, Y.; Huang, J.; He, J.; Yang, M.; Hua, W. Sappanone A alleviates metabolic dysfunction-associated steatohepatitis by decreasing hepatocyte lipotoxicity via targeting Mup3 in mice. Phytomedicine 2025, 136, 156341. [Google Scholar] [CrossRef] [PubMed]
- Nasr, P.; Jönsson, C.; Ekstedt, M.; Kechagias, S. Non-metabolic causes of steatotic liver disease. Metab. Target Organ Damage 2023, 3, 19. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Marks, L.M.; Jensen, T.; DeGrado, T.R. Noninvasive testing in the diagnosis of metabolic dysfunction-associated steatohepatitis. Metab. Target Organ Damage 2024, 4, 28. [Google Scholar] [CrossRef]
- Czochra, P.; Klopcic, B.; Meyer, E.; Herkel, J.; Garcia-Lazaro, J.F.; Thieringer, F.; Schirmacher, P.; Biesterfeld, S.; Galle, P.R.; Lohse, A.W.; et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J. Hepatol. 2006, 45, 419–428. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Liu, W.; Zong, C.; Wei, L.; Shi, Y.; Han, Z. Mesenchymal stromal cells in hepatic fibrosis/cirrhosis: From pathogenesis to treatment. Cell. Mol. Immunol. 2023, 20, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Sun, B. The Molecular Mechanisms of Liver Fibrosis and Its Potential Therapy in Application. Int. J. Mol. Sci. 2022, 23, 12572. [Google Scholar] [CrossRef]
- Pei, Q.; Yi, Q.; Tang, L. Liver Fibrosis Resolution: From Molecular Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 9671. [Google Scholar] [CrossRef]
- Seifert, R.A.; Hart, C.E.; Phillips, P.E.; Forstrom, J.W.; Ross, R.; Murray, M.J.; Bowen-Pope, D.F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J. Biol. Chem. 1989, 264, 8771–8778. [Google Scholar] [CrossRef]
- Lambrecht, J.; Verhulst, S.; Mannaerts, I.; Sowa, J.P.; Best, J.; Canbay, A.; Reynaert, H.; van Grunsven, L.A. A PDGFRβ-based score predicts significant liver fibrosis in patients with chronic alcohol abuse, NAFLD and viral liver disease. EBioMedicine 2019, 43, 501–512. [Google Scholar] [CrossRef]
- Wu, L.; Huang, X.Q.; Li, N.; Xie, C.; Rao, S.X.; Chen, S.Y.; Li, F. A magnetic resonance imaging modality for non-invasively distinguishing progression of liver fibrosis by visualizing hepatic platelet-derived growth factor receptor-beta expression in mice. J. Gastroenterol. Hepatol. 2021, 36, 3448–3456. [Google Scholar] [CrossRef]
- Contreras, O.; Córdova-Casanova, A.; Brandan, E. PDGF-PDGFR network differentially regulates the fate, migration, proliferation, and cell cycle progression of myogenic cells. Cell. Signal. 2021, 84, 110036. [Google Scholar] [CrossRef]
- Shen, H.; Fan, J.; Minuk, G.; Gong, Y. Apoptotic and survival signals in hepatic stellate cells. Zhong Nan Da Xue Xue Bao. Yi Xue Ban = J. Cent. South University. Med. Sci. 2007, 32, 726–734. [Google Scholar]
- Campbell, J.S.; Hughes, S.D.; Gilbertson, D.G.; Palmer, T.E.; Holdren, M.S.; Haran, A.C.; Odell, M.M.; Bauer, R.L.; Ren, H.P.; Haugen, H.S.; et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Kocabayoglu, P.; Lade, A.; Lee, Y.A.; Dragomir, A.C.; Sun, X.; Fiel, M.I.; Thung, S.; Aloman, C.; Soriano, P.; Hoshida, Y.; et al. β-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 2015, 63, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Yu, Y.; Huang, E.; Yao, Y.; Guo, D.; Peng, H.; Tian, B.; Zheng, Q.; Jia, M.; et al. Hepatocyte Ninjurin2 promotes hepatic stellate cell activation and liver fibrosis through the IGF1R/EGR1/PDGF-BB signaling pathway. Metab. Clin. Exp. 2023, 140, 155380. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Chen, P.; Gu, Y.; Wang, S.; Wang, L.; Chen, C.; Wang, R.; Yuan, Y. Salvianolic acid B inhibits hepatic stellate cell activation and liver fibrosis by targeting PDGFRβ. Int. Immunopharmacol. 2023, 122, 110550. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Li, Y.; Huang, Y.; Zhu, Y.; Lv, W.; Wang, R.; Gou, L.; Cheng, C.; Feng, Z.; et al. Roseotoxin B alleviates cholestatic liver fibrosis through inhibiting PDGF-B/PDGFR-β pathway in hepatic stellate cells. Cell Death Dis. 2020, 11, 458. [Google Scholar] [CrossRef]
- Wang, R.; Liu, F.; Chen, P.; Li, S.; Gu, Y.; Wang, L.; Chen, C.; Yuan, Y. Gomisin D alleviates liver fibrosis through targeting PDGFRβ in hepatic stellate cells. Int. J. Biol. Macromol. 2023, 235, 123639. [Google Scholar] [CrossRef]
- Xiuying, L. Advances on the Lindley Eupatorium lindleyanum. Chin. Wild Plant Resour. 2021, 40, 27–31. [Google Scholar]
- Mengyuan, L.; Shasha, T. Advances in Research on Chemical Components and Pharmacological Effects of Eupatorium lindleyanum DC. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 37–42. [Google Scholar]
- Chu, C.; Ren, H.; Xu, N.; Xia, L.; Chen, D.; Zhang, J. Eupatorium lindleyanum DC. sesquiterpenes fraction attenuates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2016, 185, 263–271. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Lai, F.; Wang, Y.; Lou, C. Traditional Applications, Phytochemistry, and Pharmacological Activities of Eupatorium lindleyanum DC.: A Comprehensive Review. Front. Pharmacol. 2020, 8, 577124. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, C.; Xue, S.; Gao, Y.; Chen, G.; Shan, C.; Ding, N.; Peng, G.; Li, C.; Zheng, Y. Comprehensive Comparison of Three Different Medicinal Parts of Eupatorium lindleyanum DC. Using the RRLC-Q-TOF-MS-Based Metabolic Profile and In Vitro Anti-Inflammatory Activity. Molecules 2024, 29, 3551. [Google Scholar] [CrossRef]
- Yuanda, Z. Study on the Antibacterial, Antit ussive and Antiasthmatic Effects of Yemazhui. China Pharm. 2001, 12, 12–14. [Google Scholar]
- Xianlun, L.; Chunjun, C.; Xiaochen, W.; Long, X.; Jian, Z. Protective Effects of Various Chemical Fractions of YeMaZhui on Acute Lung Injury in Mice. West. J. Tradit. Chin. Med. 2017, 30, 9–15. [Google Scholar]
- Wu, Z.; Xu, X.; Dai, L.; Wang, Y.; Yang, B.; Zhao, H.; Lou, C. Eupalinolide J induces apoptosis, cell cycle arrest, mitochondrial membrane potential disruption and DNA damage in human prostate cancer cells. J. Toxicol. Sci. 2020, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H. The Anticancer Mechanism of Ibrutinib and Eupalinolide A Targeting Thioredoxin Reductase; Lanzhou University: Lanzhou, China, 2019. [Google Scholar]
- Weidong, W.; Naixin, W.; Jiaojiao, L.; Tongtian, Z.; Chao, L. Ultrasonic Extraction and Antioxidant Activity Evaluation of Flavonoids from the Whole Plant of Eupatorium lindleyanum DC. Food Sci. 2010, 31, 50–54. [Google Scholar]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.; Omara, E.A.; Sleem, A.A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Wang, Y.; Yang, L.; Wang, D. Embelin can protect mice from thioacetamide-induced acute liver injury. Biomed. Pharmacother. 2019, 118, 109360. [Google Scholar] [CrossRef]
- Pahutyan, N.; Navoyan, Q.; Arajyan, G.; Harutyunyan, S.; Pogosyan, A.; Gasparyan, H. The impact of diamide derivatives of oxalic acid on free radical lipid oxidation in white rat brain and liver. Georgian Med. News 2024, 349, 25–30. [Google Scholar] [PubMed]
- Caligiuri, A.; Gentilini, A.; Pastore, M.; Gitto, S.; Marra, F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells 2021, 10, 2759. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, X.; Huang, J.; Chen, H.; Tang, H. Prevalence of steatotic liver disease and associated fibrosis in the United States: Results from NHANES 2017-March 2020. J. Hepatol. 2024, 80, e70–e71. [Google Scholar] [CrossRef]
- Suzuki, A.; Henao, R.; Reed, M.C.; Nijhout, H.F.; Tripathi, M.; Singh, B.K.; Yen, P.M.; Diehl, A.M.; Abdelmalek, M.F. Lower hepatic CBS and PEMT expression in advanced NAFLD: Inferencing strategies to lower homocysteine with a mathematical model. Metab. Target Organ Damage 2024, 4, 21. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Nian, M.; Sheng, Q.; Zhang, C.; Han, C.; Dou, X.; Ding, Y. Therapeutic potential and mechanism of Chinese herbal medicines in treating fibrotic liver disease. Chin. J. Nat. Med. 2023, 21, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sinica. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.Y.; Yang, X.M.; Chen, W.C.; Zhang, M.J.; Zhong, X.D.; Chen, W.G.; Zhong, B.L.; He, S.Q.; Sun, H.T. Paeoniflorin Coordinates Macrophage Polarization and Mitigates Liver Inflammation and Fibrogenesis by Targeting the NF-[Formula: See text]B/HIF-1α Pathway in CCl(4)-Induced Liver Fibrosis. Am. J. Chin. Med. 2023, 51, 1249–1267. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Shan, L.; Cao, S.; Li, K.; Wu, Y.; Zhang, Q. Notoginsenoside R1, An Active Compound from Panax notoginseng, Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis via MAPK Signaling Pathway. Am. J. Chin. Med. 2022, 50, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Lu, Y.; Zhang, W.; Zhang, Z. The Application of Berberine in Fibrosis and the Related Diseases. Am. J. Chin. Med. 2024, 52, 753–773. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, F.; Cao, Z.; Wang, T.; Pan, L.; Luo, W.; Ding, W.; Li, J.; Jin, L.; Liu, H.; et al. Eupalinolide A induces autophagy via the ROS/ERK signaling pathway in hepatocellular carcinoma cells in vitro and in vivo. Int. J. Oncol. 2022, 61, 131. [Google Scholar] [CrossRef]
- Tian, S.; Chen, Y.; Yang, B.; Lou, C.; Zhu, R.; Zhao, Y.; Zhao, H. F1012-2 inhibits the growth of triple negative breast cancer through induction of cell cycle arrest, apoptosis, and autophagy. Phytother. Res. PTR 2018, 32, 908–922. [Google Scholar] [CrossRef]
- Bingül, İ.; Başaran-Küçükgergin, C.; Aydın, A.F.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, H.; Shen, T.; Tang, X.; Yang, Y.; Ling, W. Cyanidin-3-O-β-glucoside Purified from Black Rice Protects Mice against Hepatic Fibrosis Induced by Carbon Tetrachloride via Inhibiting Hepatic Stellate Cell Activation. J. Agric. Food Chem. 2015, 63, 6221–6230. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology 2010, 272, 1–10. [Google Scholar] [CrossRef]
- Zhou, Z.; Kim, J.W.; Qi, J.; Eo, S.K.; Lim, C.W.; Kim, B. Toll-Like Receptor 5 Signaling Ameliorates Liver Fibrosis by Inducing Interferon β-Modulated IL-1 Receptor Antagonist in Mice. Am. J. Pathol. 2020, 190, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Caiado, F.; Pietras, E.M.; Manz, M.G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 2021, 218, e20201541. [Google Scholar] [CrossRef] [PubMed]
- Pradere, J.P.; Kluwe, J.; De Minicis, S.; Jiao, J.J.; Gwak, G.Y.; Dapito, D.H.; Jang, M.K.; Guenther, N.D.; Mederacke, I.; Friedman, R.; et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology (Baltim. Md.) 2013, 58, 1461–1473. [Google Scholar] [CrossRef]
- Costa, D.; Simbrunner, B.; Jachs, M.; Hartl, L.; Bauer, D.; Paternostro, R.; Schwabl, P.; Scheiner, B.; Stättermayer, A.F.; Pinter, M.; et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 2021, 74, 819–828. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, H.; Dai, R.; Shang, L.; Zhang, X.; Wen, H. Macrophage polarization in tissue fibrosis. PeerJ 2023, 11, e16092. [Google Scholar] [CrossRef]
- Rao, J.; Wang, H.; Ni, M.; Wang, Z.; Wang, Z.; Wei, S.; Liu, M.; Wang, P.; Qiu, J.; Zhang, L.; et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022, 71, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, X.Q.; Li, W.X.; Huang, H.M.; Li, H.D.; Pan, X.Y.; Li, X.F.; Huang, C.; Meng, X.M.; Zhang, L.; et al. PSTPIP2 connects DNA methylation to macrophage polarization in CCL4-induced mouse model of hepatic fibrosis. Oncogene 2018, 37, 6119–6135. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.W.; Chung, J.; Sverdlov, D.Y.; Miyamoto, M.; Kim, Y.O.; Ogawa, S.; Arch, R.H.; et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014, 147, 1378–1392. [Google Scholar] [CrossRef]
- Tangkijvanich, P.; Santiskulvong, C.; Melton, A.C.; Rozengurt, E.; Yee, H.F., Jr. p38 MAP kinase mediates platelet-derived growth factor-stimulated migration of hepatic myofibroblasts. J. Cell. Physiol. 2002, 191, 351–361. [Google Scholar] [CrossRef]
- Fang, L.; Zhan, S.; Huang, C.; Cheng, X.; Lv, X.; Si, H.; Li, J. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol. Appl. Pharmacol. 2013, 272, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.C.; Kim, J.W.; Zhu, J.; Li, S.; Yan, J.; Liu, Q.; Koo, I.; Koshkin, S.A.; Hao, F.; Zhong, G.; et al. Inhibition of heme-thiolate monooxygenase CYP1B1 prevents hepatic stellate cell activation and liver fibrosis by accumulating trehalose. Sci. Transl. Med. 2024, 16, eadk8446. [Google Scholar] [CrossRef]
- Erdenebileg, S.; Kim, M.; Nam, Y.; Cha, K.H.; Le, T.T.; Jung, S.H.; Nho, C.W. Artemisia argyi ethanol extract ameliorates nonalcoholic steatohepatitis-induced liver fibrosis by modulating gut microbiota and hepatic signaling. J. Ethnopharmacol. 2024, 333, 118415. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yan, X.; Li, L.; Qiu, K.; Huang, W.; Zhou, Z. CXCL5 promotes lipotoxicity of hepatocytes through upregulating NLRP3/Caspase-1/IL-1β signaling in Kupffer cells and exacerbates nonalcoholic steatohepatitis in mice. Int. Immunopharmacol. 2023, 123, 110752. [Google Scholar] [CrossRef] [PubMed]


| NO. | Retention Time (min) | Molecular Formula | (m/z) (Mass Error) (ppm) | Identification |
|---|---|---|---|---|
| 1 | 0.344 | C6H8O7 | 192.027 (−2.55) | Citric acid |
| 2 | 1.107 | C4H6O6 | 150.0164 (−0.57) | Tartaric acid |
| 3 | 1.107 | C9H9NO4 | 195.0532 (−0.88) | Peristrophamide |
| 4 | 1.107 | C8H7NO3 | 165.0426 (−5.22) | Coixol |
| 5 | 1.202 | C5H10O5 | 150.0528 (−8.36) | Apiose |
| 6 | 1.202 | C17H21N4O9P | 456.1046 (−0.49) | Flavin mononucleotide |
| 7 | 1.297 | C4H6O5 | 134.0215 (−2.59) | Malic acid |
| 8 | 1.393 | C25H28O11 | 504.1632 (−2.63) | Shakuchirin |
| 9 | 1.488 | C3H6O3 | 90.0317 (−7.45) | Dihydroxyacetone |
| 10 | 1.583 | C5H4O3 | 112.016 (−0.53) | Pyromeconic acid |
| 11 | 1.583 | C6H8O7 | 192.027 (−0.68) | Citric acid |
| 12 | 1.869 | C24H42O21 | 666.2219 (−1.13) | Isolychnose |
| 13 | 1.869 | C6H6O3 | 126.0317 (−6.48) | 4-Hydroxymethyl-2-furaldehyde |
| 14 | 2.155 | C4H6O2 | 86.0368 (0.04) | Crotonic acid |
| 15 | 2.155 | C5H6O4 | 130.0266 (−1.72) | Mesaconic acid |
| 16 | 2.346 | C30H52O26 | 828.2747 (−1.76) | Verbascose |
| 17 | 2.441 | C6H8O7 | 192.027 (0.33) | Citric acid |
| 18 | 2.441 | C5H4O3 | 112.016 (−3.93) | Pyromeconic acid |
| 19 | 2.441 | C6H10O5 | 162.0528 (−3.47) | 3,6-Anhydrogalactose |
| 20 | 2.727 | C7H12O6 | 192.0634 (6.06) | Cordycepic acid |
| 21 | 3.013 | C7H6O5 | 170.0215 (1.32) | Gallic acid |
| 22 | 3.013 | C12H17N3O8 | 331.1016 (0.96) | Tetrodonic acid |
| 23 | 3.395 | C6H8O7 | 192.027 (−0.67) | Citric acid |
| 24 | 3.490 | C7H14O4 | 162.0892 (0.91) | Cymarose |
| 25 | 3.776 | C7H6O3 | 138.0317 (−1.85) | 3,4-Dihydroxybenzyl aldehyde |
| 26 | 3.871 | C6H6O | 94.0419 (−3.5) | Phenol |
| 27 | 4.157 | C8H8O2 | 136.0524 (−2.94) | 4-Methyl salicylaldehyde |
| 28 | 4.157 | C9H8O4 | 180.0423 (−3.49) | Caffeic acid |
| 29 | 4.253 | C6H10O8 | 210.0376 (0.13) | Mucic acid |
| 30 | 4.539 | C7H6O4 | 154.0266 (−4.48) | 3,5-Dihydroxybenzoic acid |
| 31 | 4.825 | C25H24O12 | 516.1268 (−2.14) | 1,4-Dicaffeoylquinic acid |
| 32 | 4.920 | C21H20O11 | 448.1006 (−3.68) | 3,3’4’,5,7- Pentahydroxyvone-3-L-rhamnoside |
| 33 | 5.015 | C11H20O5 | 232.1311 (−4.49) | Jioglutin E |
| 34 | 5.015 | C2H2O4 | 89.9953 (−6.64) | Oxalic acid |
| 35 | 5.015 | C15H10O6 | 286.0477 (0.29) | 5,7,2’,3’- Tetrahydroxyflavone |
| 36 | 5.015 | C9H8O5 | 196.0372 (−1.59) | Meconic acid |
| 37 | 5.397 | C20H26O6 | 362.1729 (0.84) | Tetrahydroxy-ent-Kaur-16-en-6,15-dione |
| 38 | 5.492 | C16H12O7 | 316.0583 (0.59) | 3-Methoxy quercetin |
| 39 | 5.873 | C5H10O2 | 102.0681 (−3.97) | 2-Methyl butyric acid |
| 40 | 5.873 | C18H34O5 | 330.2406 (−3.27) | Sanleng acid |
| 41 | 5.873 | C15H10O6 | 286.0477 (−4.67) | 5,7,2’,3’-Tetrahydroxyflavone |
| 42 | 5.873 | C16H12O7 | 316.0583 (−3.52) | 3-Methoxy quercetin |
| 43 | 6.350 | C6H8O7 | 192.027 (−0.98) | Citric acid |
| 44 | 6.350 | C5H8O4 | 132.0423 (−2.05) | 2,3-Dihydroxyl-2-methyl-butyrolactone |
| 45 | 6.350 | C20H24O6 | 360.1573 (−1.2) | 3-(alpha,4-Dihydroxy-3-methoxybenzyl)-4-(hydroxy-3-methoxybenzyl) tetrahydrofuran |
| 46 | 6.922 | C17H26O4 | 294.1831 (−3.36) | Embelin |
| 47 | 6.922 | C13H16O4 | 236.1049 (−0.75) | Asarumin B |
| 48 | 6.922 | C12H19N3O | 221.1528 (−5.49) | Alchorneine |
| 49 | 7.113 | C15H16O2 | 228.115 (−6.07) | 7-Hydroxycadalenal |
| NO. | Retention Time (min) | Molecular Formula | (m/z) (Mass Error) (ppm) | Identification |
|---|---|---|---|---|
| 1 | 1.008 | C5H11NO3 | 133.0739 (−1.51) | 1,4-Dideoxy-1,4-imino-arabinitol |
| 2 | 1.103 | C6H8O7 | 192.0270 (−1.56) | Citric acid |
| 3 | 1.199 | C5H11NO2 | 117.0790 (−1.00) | Betaine |
| 4 | 1.199 | C7H7NO2 | 137.0477 (0.05) | Trigonelline |
| 5 | 1.389 | C6H11NO2 | 129.0790 (1.24) | 6xi-Methoxypiperidin-2-one |
| 6 | 1.484 | C8H13NO2 | 155.0946 (−0.36) | Arecolidine |
| 7 | 1.58 | C6H7NO2 | 125.0477 (−2.7) | 5-Hydroxy-2-pyridine methanol |
| 8 | 1.58 | C9H15NO2 | 169.1103 (−0.36) | Homoarecoline |
| 9 | 1.58 | C8H13NO3 | 171.0895 (0.9) | Desmodilactone |
| 10 | 1.675 | C5H11N3O2 | 145.0851 (−1.92) | gamma-Guanidinobutyric acid |
| 11 | 1.675 | C8H15NO | 141.1154 (−1.35) | Hygrine |
| 12 | 1.675 | C11H15NO7 | 273.0849 (−1.13) | Brachystemoside A |
| 13 | 1.961 | C5H7NO3 | 129.0426 (−1.48) | Pyroglutamic acid |
| 14 | 2.152 | C5H7NO2 | 113.0477 (−2.05) | 1-Cyano-2-hydroxymethyl prop-1-ene-3-ol |
| 15 | 2.152 | C11H17NO7 | 275.1005 (−2.05) | Cardiospermin |
| 16 | 2.342 | C12H16O8 | 288.0845 (−0.38) | Phlorin |
| 17 | 2.628 | C12H17NO6 | 271.1056 (0.84) | Deidaclin |
| 18 | 2.628 | C9H17NO8 | 267.0954 (5.33) | Miserotoxin |
| 19 | 2.628 | C10H13N5O4 | 267.0968 (0.65) | Adenosine |
| 20 | 2.819 | C6H6O3 | 126.0317 (2.56) | 4-Hydroxymethyl-2-furaldehyde |
| 21 | 3.105 | C7H8O4 | 156.0423 (−1.09) | Doederleinic acid |
| 22 | 3.296 | C8H7NO | 133.0528 (−2.28) | Mandelonitrile |
| 23 | 3.296 | C16H27NO5 | 313.1889 (−1.36) | Heliotrine |
| 24 | 3.296 | C15H25NO5 | 299.1733 (3.33) | Echinatine |
| 25 | 3.296 | C15H27NO5 | 301.1889 (−5.66) | Floridinine |
| 26 | 3.391 | C8H5NO2 | 147.0320 (−0.92) | Isatin |
| 27 | 3.582 | C16H18O9 | 354.0951 (0.88) | 4-O-Caffeoyl-D-quinic acid |
| 28 | 3.677 | C8H15NO | 141.1154 (−2.59) | Hygrine |
| 29 | 3.772 | C8H8O2 | 136.0524 (0.38) | 4-Methyl salicylaldehyde |
| 30 | 3.868 | C8H10O4 | 170.0579 (−1.44) | Dictafolin B |
| 31 | 3.963 | C16H24O10 | 376.1370 (3.37) | 6-O-Methyl catalpol |
| 32 | 3.963 | C17H20N4O6 | 376.1383 (0.04) | Vitamin B2 |
| 33 | 3.963 | C13H18O2 | 206.1307 (−0.18) | Arteamisinine I |
| 34 | 4.058 | C20H26O7 | 378.1679 (−0.14) | 1-(4-Hydroxy-3-methoxyphenyl-2-[4-(omega-hydroxypropyl)-2-methoxyphenoxy]propane-1,3-diol |
| 35 | 4.058 | C17H27NO6 | 341.1838 (−0.18) | Acetylindicine |
| 36 | 4.154 | C21H26O10 | 438.1526 (−0.76) | Bruceolide |
| 37 | 4.154 | C26H28O14 | 564.1479 (−1.35) | 5,7,4’-Trihydroxy-6-C-arabinoside-8-C-glucoside flavone |
| 38 | 4.249 | C17H20O9 | 368.1107 (0.79) | Methyl chlorogenate |
| 39 | 4.440 | C25H26O13 | 534.1373 (−2.42) | 6-beta-C-(2’-Galloylglucopyranosyl)-5,7-dihydroxy-2-isopropyl chromone |
| 40 | 4.535 | C14H23NO6 | 301.1525 (−1.49) | Intermediate |
| 41 | 4.535 | C15H10O7 | 302.0427 (−1.03) | 3,5,7,2’,6’-Pentahydroxy flavonol |
| 42 | 4.535 | C15H11O7 | 303.0505 (−1.04) | Delphinidin |
| 43 | 4.535 | C20H31NO8 | 413.2050 (0.01) | Heliosupine N-oxide |
| 44 | 4.726 | C15H11O6 | 287.0556 (−1.06) | Cyanidin |
| 45 | 4.726 | C15H10O6 | 286.0477 (−1.06) | 5,7,2’,3’-Tetrahydroxyflavone |
| 46 | 4.726 | C21H20O12 | 464.0955 (2.55) | 6-Hydroxykaempferol-7-O-glucoside |
| 47 | 4.726 | C27H31O15 | 595.1663 (−1.20) | Pelargonidin-3,5-diglucoside |
| 48 | 4.726 | C27H30O15 | 594.1585 (−1.19) | 6,8-Bis(C-glucosyl)-apigenin |
| 49 | 4.821 | C21H20O11 | 448.1006 (−0.72) | 3,3’4’,5,7-Pentahydroxyvone-3-L-rhamnoside |
| 50 | 4.821 | C21H21O11 | 449.1084 (−0.54) | Cyanidin 3-O-beta-D-galactoside |
| 51 | 5.012 | C15H24O | 220.1827 (−4.78) | (-)-1,10-Epoxy-guaia-11-ene |
| 52 | 5.393 | C22H28O8 | 420.1784 (−0.32) | Caesalmin A |
| 53 | 5.393 | C13H12O2 | 200.0837 (−1.29) | 4,4’-Dihydroxydiphenyl methane |
| 54 | 5.488 | C30H46O3 | 454.3447 (−2.93) | (24Z)-27-Hydroxy-3-oxo-7,24-tirucalladien-21-al |
| 55 | 5.488 | C15H16O2 | 228.1150 (0.28) | 7-Hydroxycadalenal |
| 56 | 5.488 | C16H12O7 | 316.0583 (−1.74) | 3-Methoxy quercetin |
| 57 | 5.488 | C14H14O2 | 214.0994 (−4.68) | Lunularin |
| 58 | 5.584 | C15H22 | 202.1722 (−0.16) | 1,2,9,10-Tetradehydroaristolane |
| 59 | 5.584 | C15H24O | 220.1827 (−2.36) | (-)-1,10-Epoxy-guaia-11-ene |
| 60 | 5.679 | C15H24N2O | 248.1889 (−1.14) | Aphylline |
| 61 | 6.251 | C14H22O2 | 222.1620 (−5.78) | [Z, E]-4,8,12-Trimethyl-3,7,11-tridecatrienoate |
| 62 | 6.346 | C24H30O9 | 462.1890 (−1.85) | 1,1’-Dibenzene-6’,8’,9’-trihydroxy-3-allyl-4-O-beta-D-glucopyranoside |
| 63 | 6.346 | C20H24O6 | 360.1573 (−1.65) | 3-(alpha,4-Dihydroxy-3-methoxybenzyl)-4-(hydroxy-3-methoxybenzyl) tetrahydrofuran |
| 64 | 6.346 | C15H16O2 | 228.1150 (−0.52) | 7-Hydroxycadalenal |
| 65 | 6.346 | C15H14O | 210.1045 (−1.36) | Linderazulene |
| 66 | 6.346 | C17H25NO2 | 275.1885 (−0.53) | Hydroxy-gamma-Sanshool |
| 67 | 6.537 | C12H22O | 182.1671 (−1.91) | Cyclododecanone |
| 68 | 6.537 | C13H20O | 192.1514 (−1.48) | beta-Ionone |
| 69 | 6.537 | C21H27NO7 | 405.1788 (−0.51) | Clivorine |
| 70 | 6.632 | C17H26O4 | 294.1831 (0.02) | Embelin |
| 71 | 6.632 | C17H24O3 | 276.1725 (−1.36) | 6-Shogaol |
| 72 | 7.014 | C17H26O5 | 310.1780 (−1.27) | 2-(1-Ethoxy-2-hydroxy)propyl-4-methoxyphenyl-2-methyl-butyrate |
| 73 | 7.109 | C20H27NO5 | 361.1889 (−2.09) | Cephalofortuneine |
| 74 | 7.204 | C27H28N2O4 | 444.2049 (−1.58) | Trichosanatine |
| 75 | 7.204 | C21H24O6 | 372.1573 (−1.97) | Fargesone A |
| 76 | 7.204 | C21H34O10 | 446.2152 (−0.09) | (Z)-(IS,5R)-beta-Pinen-10-yl-beta-vicianoside |
| 77 | 7.3 | C9H10 | 118.0783 (−0.20) | Isoallylbenzene |
| 78 | 7.3 | C24H30O6 | 414.2042 (−1.24) | Armilliaripin |
| 79 | 7.3 | C19H18O4 | 310.1205 (−1.45) | 3alpha-Hydroxytanshinone IIA |
| 80 | 7.49 | C14H22 | 190.1722 (−3.27) | 4-(1,5-Dimethyl-1,4-hexadienyl)-1-methyl-cyclohexene |
| 81 | 8.539 | C17H30O | 250.2297 (−0.73) | Civetone |
| 82 | 8.539 | C24H41NO7 | 455.2883 (−0.91) | 10-Hydroxynudicaulidine |
| 83 | 8.539 | C18H33NO2 | 295.2511 (−0.39) | Tetrahydrobungeanool |
| 84 | 8.539 | C18H30O2 | 278.2246 (−1.58) | (Z,Z,Z)-9,12,15-Octadecatrienoic acid |
| 85 | 9.016 | C8H4O3 | 148.0160 (−1.83) | Phthalic anhydride |
| 86 | 10.446 | C35H42O12 | 654.2676 (−0.88) | 13-Deacetoxy-13,15-epoxy-11(15- > 1)-abeo-13-epi-baccatin VI |
| 87 | 11.78 | C24H38O4 | 390.2770 (−1.92) | 3alpha-Hydroxy-6-oxo-5alpha-cholanic acid |
| 88 | 14.736 | C6H15N | 101.1205 (−4.14) | Hexyl amine-1 |
| 89 | 17.786 | C5H5NO2 | 111.0320 (−3.51) | 2-Minaline |
| Time (min) | Flow (mL/min) | Phase A (%) | Phase B (%) |
|---|---|---|---|
| 0 | 0.3 | 97 | 3 |
| 3 | 0.3 | 75 | 25 |
| 4 | 0.3 | 55 | 45 |
| 10 | 0.3 | 5 | 95 |
| 13 | 0.3 | 5 | 95 |
| 17 | 0.3 | 97 | 3 |
| 20 | 0.3 | 97 | 3 |
| Gene Symbol | Forward 5’-3’ | Reverse 5’-3’ |
|---|---|---|
| Mice | ||
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
| Col1 | GCTCCTCTTAGGGGCCACT | ATTGGGGACCCTTAGGCCAT |
| Col3 | CTGTAACATGGAAACTGGGGAAA | CCATAGCTGAACTGAAAACCACC |
| Col4 | CCTGGCACAAAAGGGACGA | ACGTGGCCGAGAATTTCACC |
| LOX | CAGCCACATAGATCGCATGGT | GCCGTATCCAGGTCGGTTC |
| TGF-β | CCACCTGCAAGACCATCGAC | CTGGCGAGCCTTAGTTTGGAC |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| Human | ||
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| Col1 | GAGGGCCAAGACGAAGACATC | CAGATCACGTCATCGCACAAC |
| Col3 | GGAGCTGGCTACTTCTCGC | GGGAACATCCTCCTTCAACAG |
| LOX | CGGCGGAGGAAAACTGTCT | TCGGCTGGGTAAGAAATCTGA |
| α-SMA | AAAAGACAGCTACGTGGGTGA | GCCATGTTCTATCGGGTACTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, Y.; Zhuo, L.; Lu, G.; Zhang, M.; Huang, J.; Li, Y.; Liu, W.; Qi, J.; Zhu, A.; et al. Eupatorium lindleyanum DC Ameliorates Carbon Tetrachloride-Induced Hepatic Inflammation and Fibrotic Response in Mice. Pharmaceuticals 2025, 18, 1228. https://doi.org/10.3390/ph18081228
Yang J, Wang Y, Zhuo L, Lu G, Zhang M, Huang J, Li Y, Liu W, Qi J, Zhu A, et al. Eupatorium lindleyanum DC Ameliorates Carbon Tetrachloride-Induced Hepatic Inflammation and Fibrotic Response in Mice. Pharmaceuticals. 2025; 18(8):1228. https://doi.org/10.3390/ph18081228
Chicago/Turabian StyleYang, Jinbao, Yufei Wang, Lijuan Zhuo, Guijun Lu, Meiting Zhang, Jiabin Huang, Yehaomin Li, Wenwen Liu, Jing Qi, An Zhu, and et al. 2025. "Eupatorium lindleyanum DC Ameliorates Carbon Tetrachloride-Induced Hepatic Inflammation and Fibrotic Response in Mice" Pharmaceuticals 18, no. 8: 1228. https://doi.org/10.3390/ph18081228
APA StyleYang, J., Wang, Y., Zhuo, L., Lu, G., Zhang, M., Huang, J., Li, Y., Liu, W., Qi, J., Zhu, A., & Zhou, Z. (2025). Eupatorium lindleyanum DC Ameliorates Carbon Tetrachloride-Induced Hepatic Inflammation and Fibrotic Response in Mice. Pharmaceuticals, 18(8), 1228. https://doi.org/10.3390/ph18081228







