Monoamine Oxidase Inhibitors in Drug Discovery Against Parkinson’s Disease: An Update
Abstract
1. Introduction
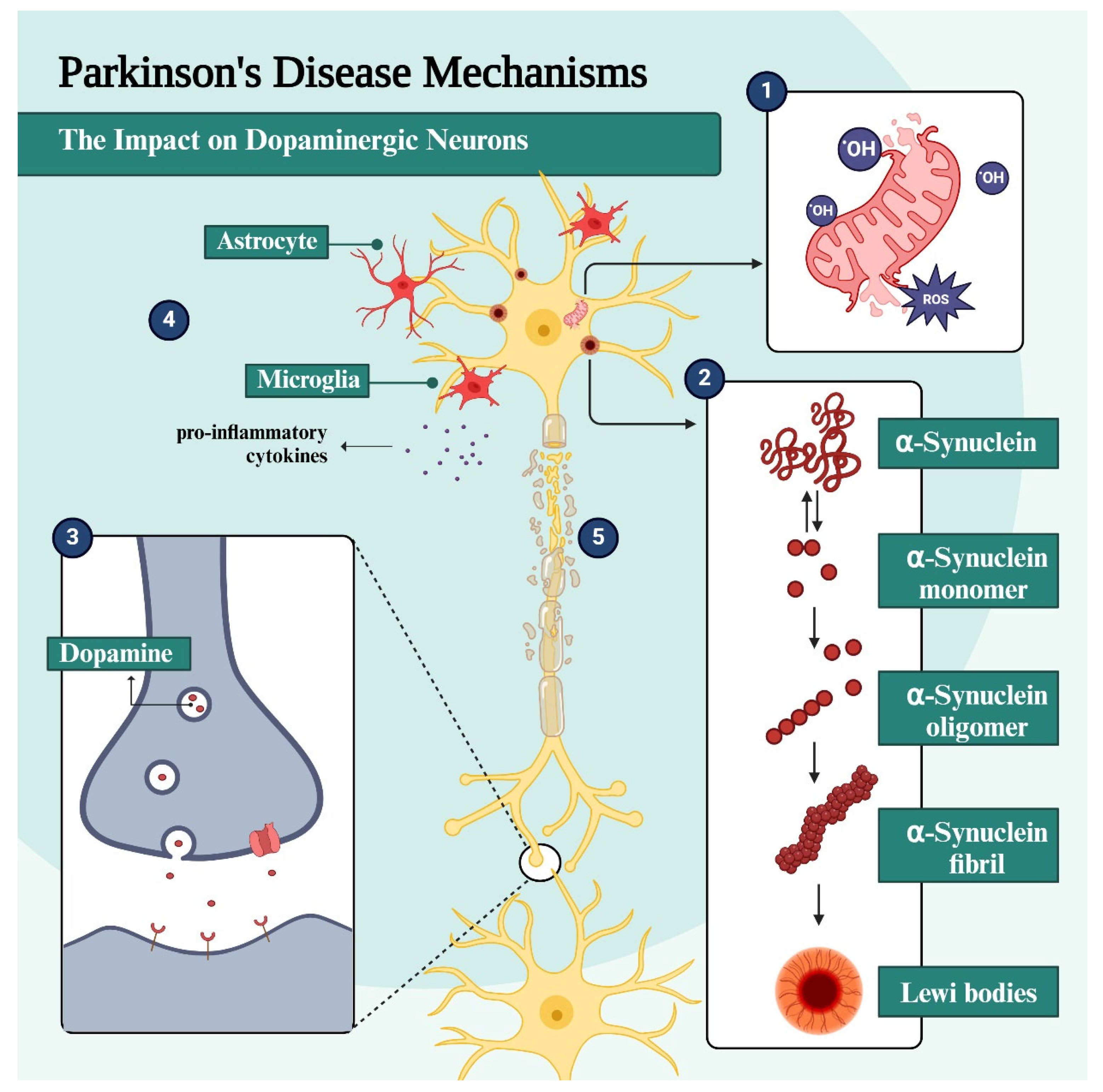
1.1. General Pathophysiological Aspects of Parkinson’s Disease
1.2. The Role of α-Synuclein and Lewy Bodies in PD Pathogenesis
1.3. Oxidative Stress and Neuroinflammation in PD
1.4. Mitochondrial Dysfunction in PD Pathogenesis
1.5. Glutamate Production and Excitotoxicity in PD
1.6. Monoamine Oxidases: Functional Aspects and Its Relevance in the PD Pathogenesis
| Characteristic | MAO-A | MAO-B |
|---|---|---|
| Tissue Location | Mainly expressed in peripheral tissues (liver, kidney, and heart) and brain (cortex and hippocampus) [26] | Mainly expressed in brain (striatum, thalamus, pale globe, and substantia nigra), platelets, and glia [35] |
| Cell distribution | Catecholaminergic neurons, hepatocytes, and cardiomyocytes [26] | Serotoninergic and histaminergic neurons, astrocytes, platelets, and renal cells [36] |
| Primary Substrates | Serotonin, norepinephrine, adrenaline, dopamine (pre-synaptic neurons), and tyramine [25] | Dopamine (synaptic cleft and glial cells), phenylethylamine, benzylamine, and tyramine [35] |
| Physiological Role | Metabolism of peripheral monoamines and regulation of humor and anxiety (via serotonin and noradrenaline) [37] | Control of striatal dopaminergic pathway, motor function modulation, metabolism of phenylamine, and oxidative stress modulation in glial cells [35] |
| Inhibition-related Risk | Tyramine accumulation (Cheese effect) and serotoninergic syndrome [37] | Low risk of cheese effect, which is potentialized by high doses or low selectivity [36] |
| Clinical uses of inhibitors | Antidepressive and anxiolytics [37] | Potential monotherapy for early stages PD or as adjuvant to levodopa on advanced stages [35] |
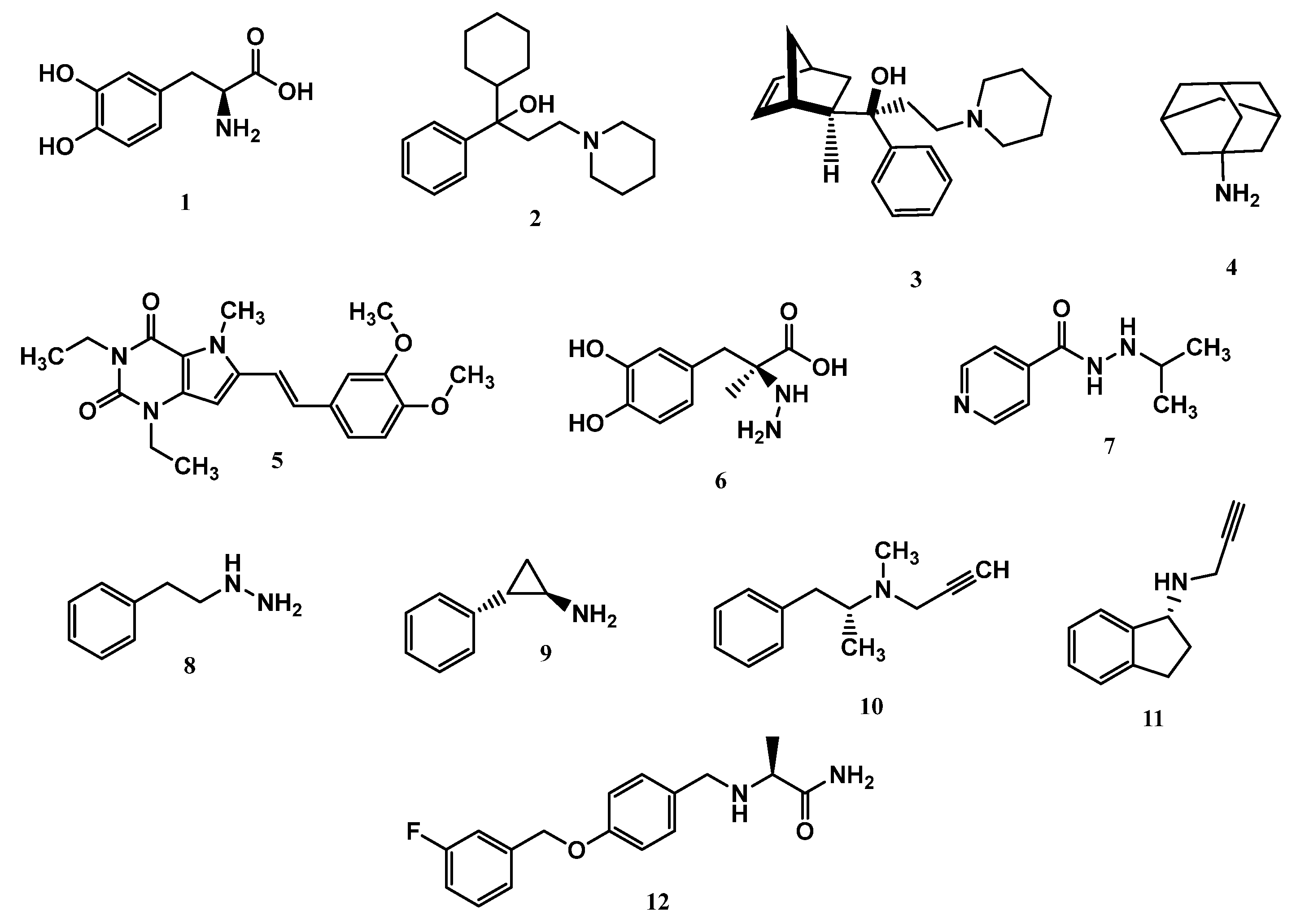
1.7. Current Available Drugs for PD Therapy
2. Methodology
3. Recent Advances in the Search for MAO Inhibitors as Drug Candidates for PD
3.1. Selective MAO Inhibitors
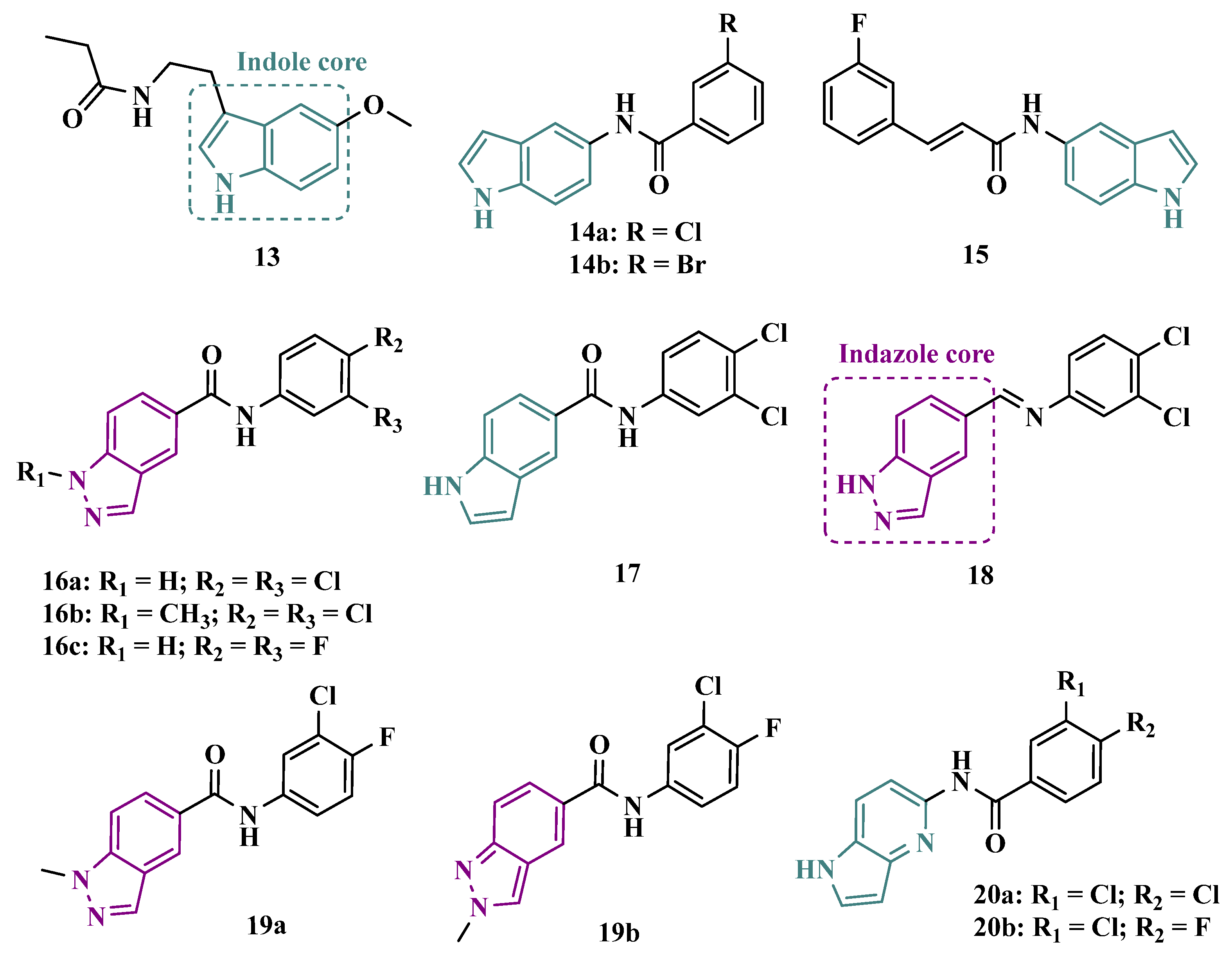
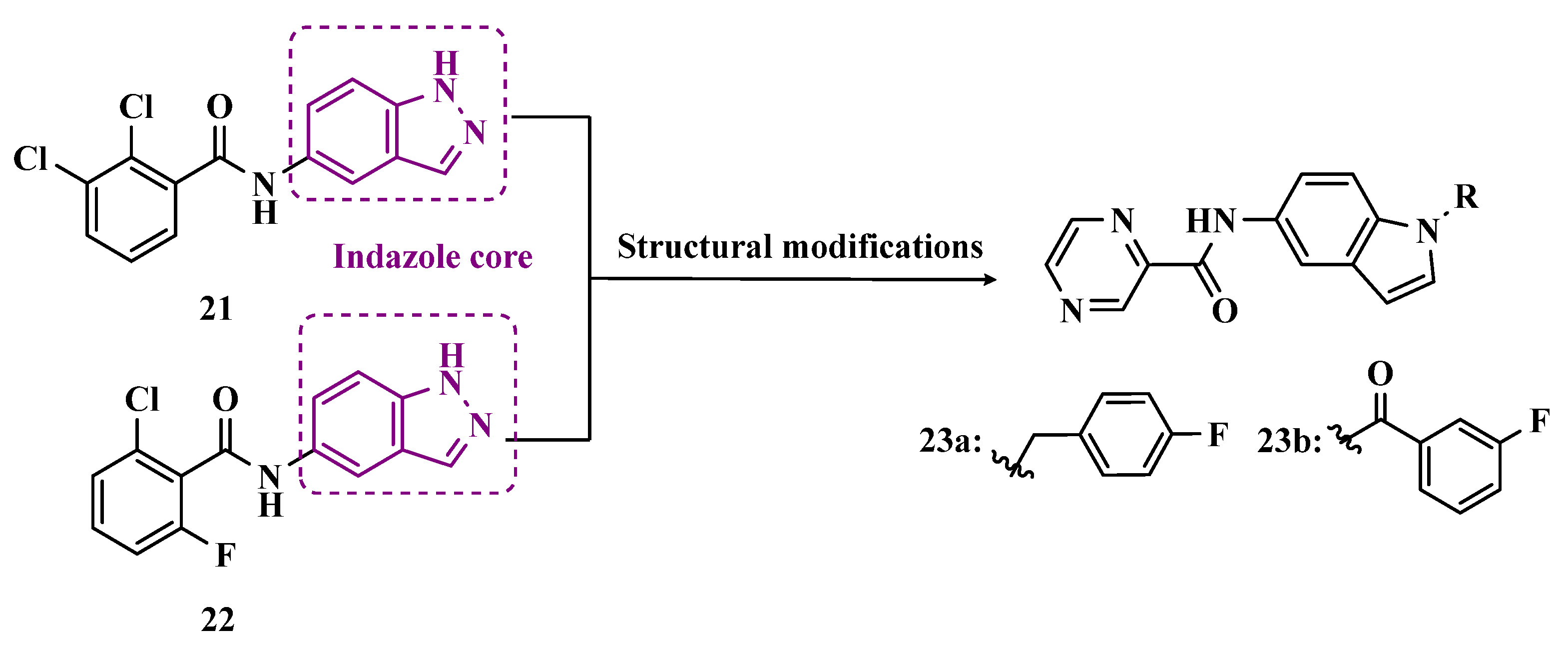
3.1.1. Indazole and Indole/Melatonin-like Inhibitors
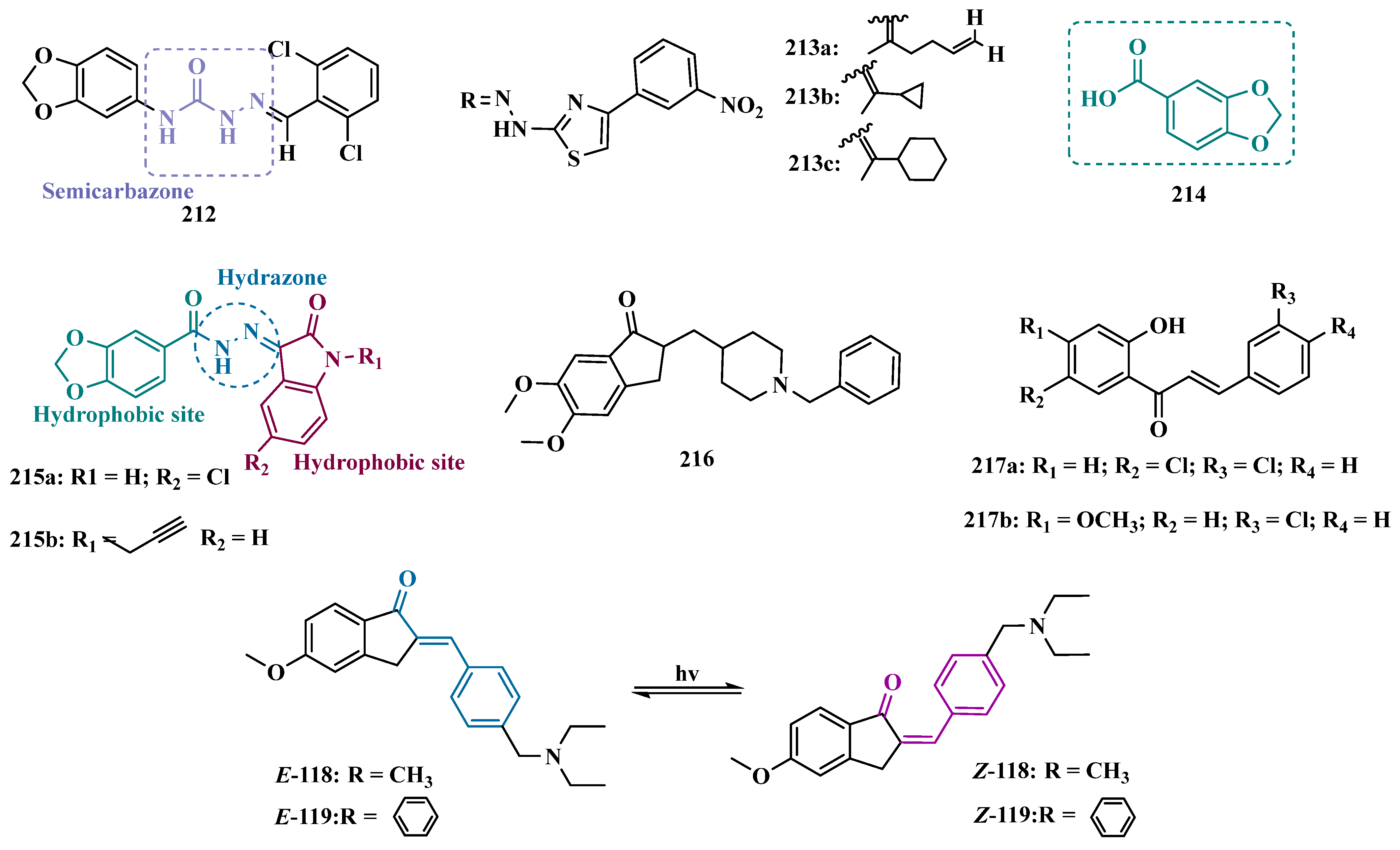
3.1.2. Hydrazide and Hydrazone-Based Analogues
3.1.3. Phthalide, Phthalimide, and Indanone Derivatives
3.1.4. Chalcones
3.1.5. Propargylamine and Phtalonitrile Derivatives
3.1.6. Alkaloids
3.1.7. Benzopyrone Derivatives
3.1.8. Benzyloxy-Based Analogues
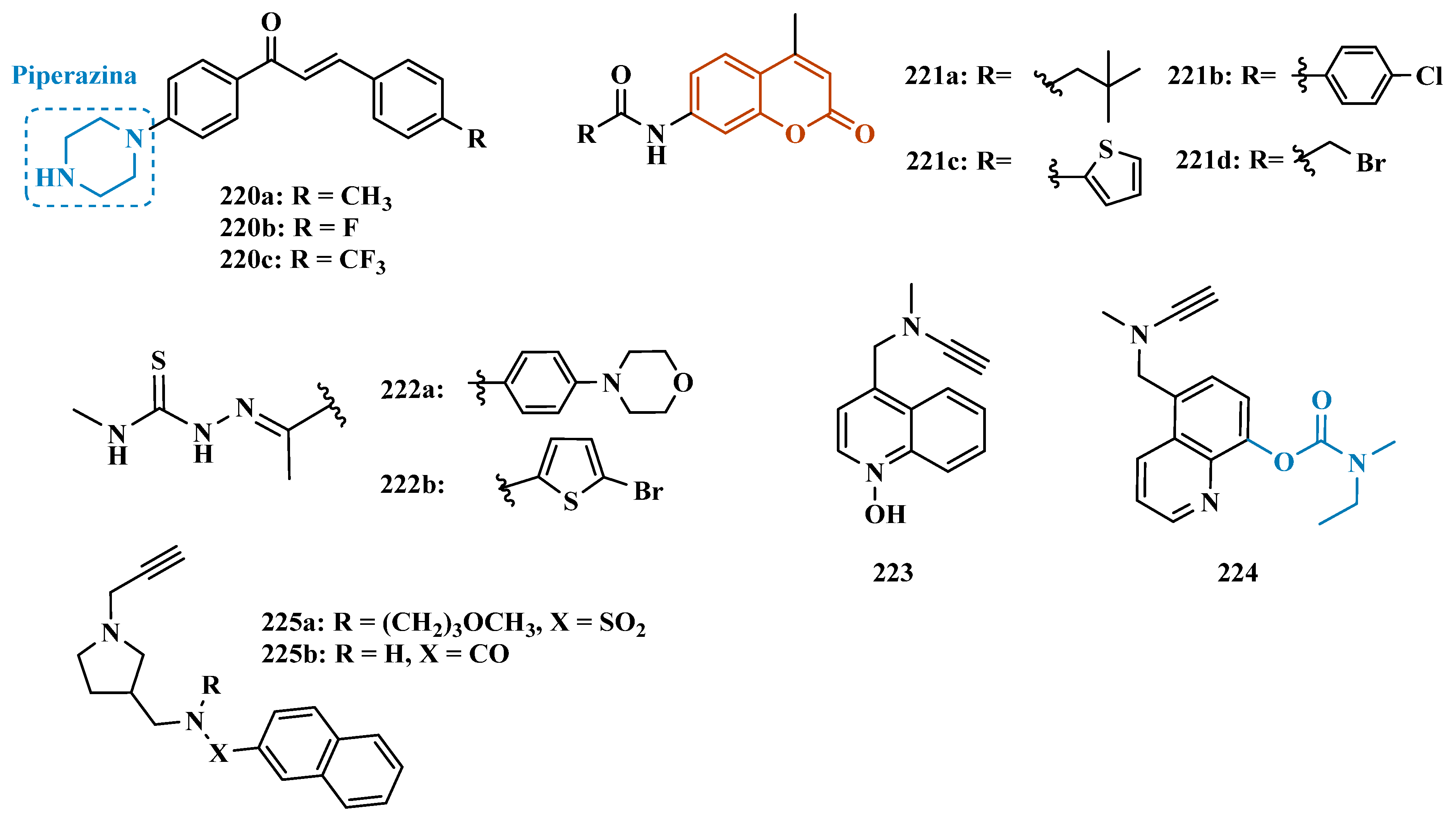
3.1.9. Azole-Based Derivatives
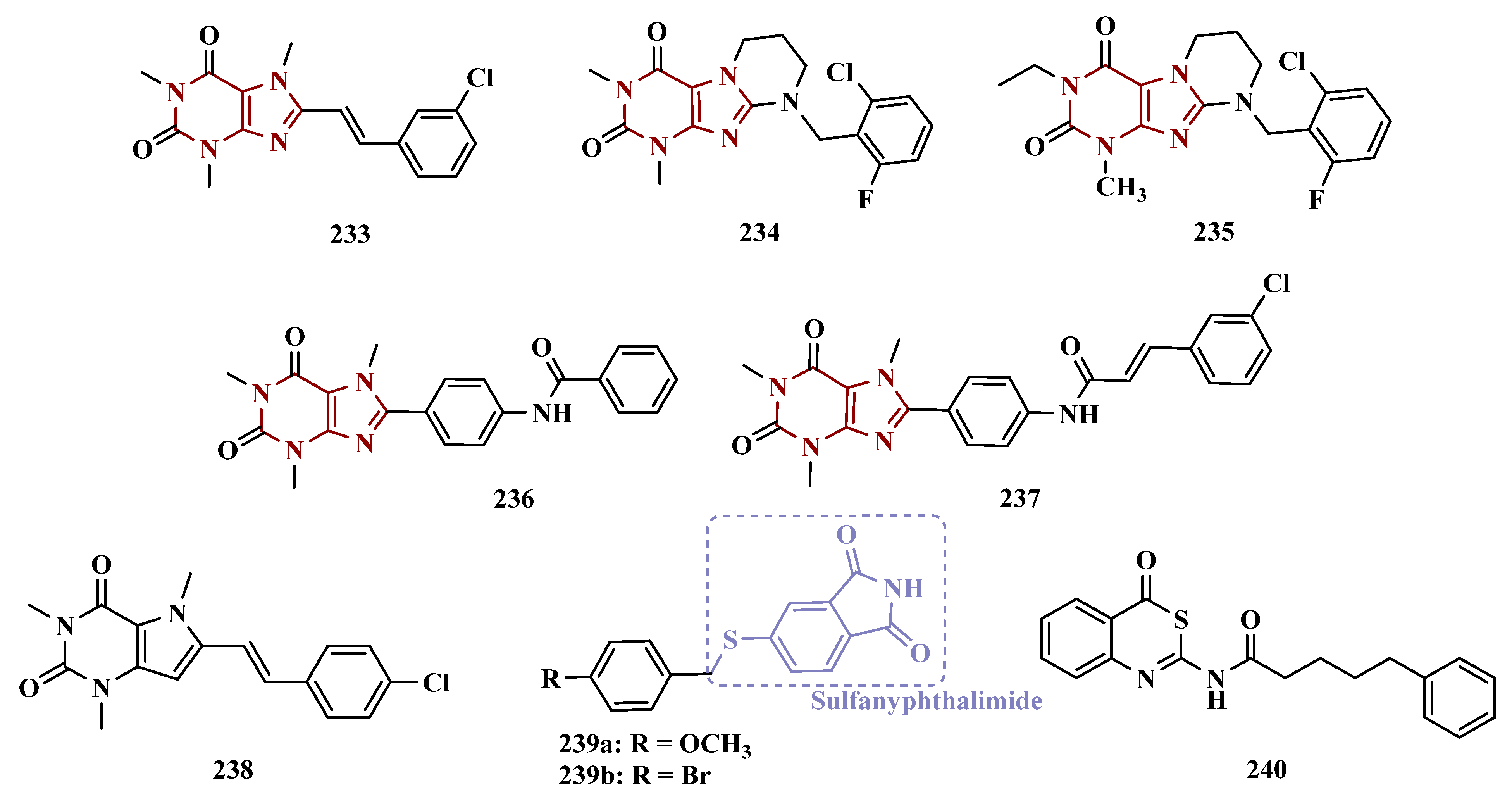
3.1.10. Diverse Molecular Hybrids
3.2. Dual and Multi-Target MAO Inhibitors
3.2.1. Dual Histamine Receptor Modulators and MAO Inhibitors
3.2.2. Dual Cholinesterase and MAO Inhibitors
3.2.3. Dual Adenosine Receptors Antagonists and MAO Inhibitors
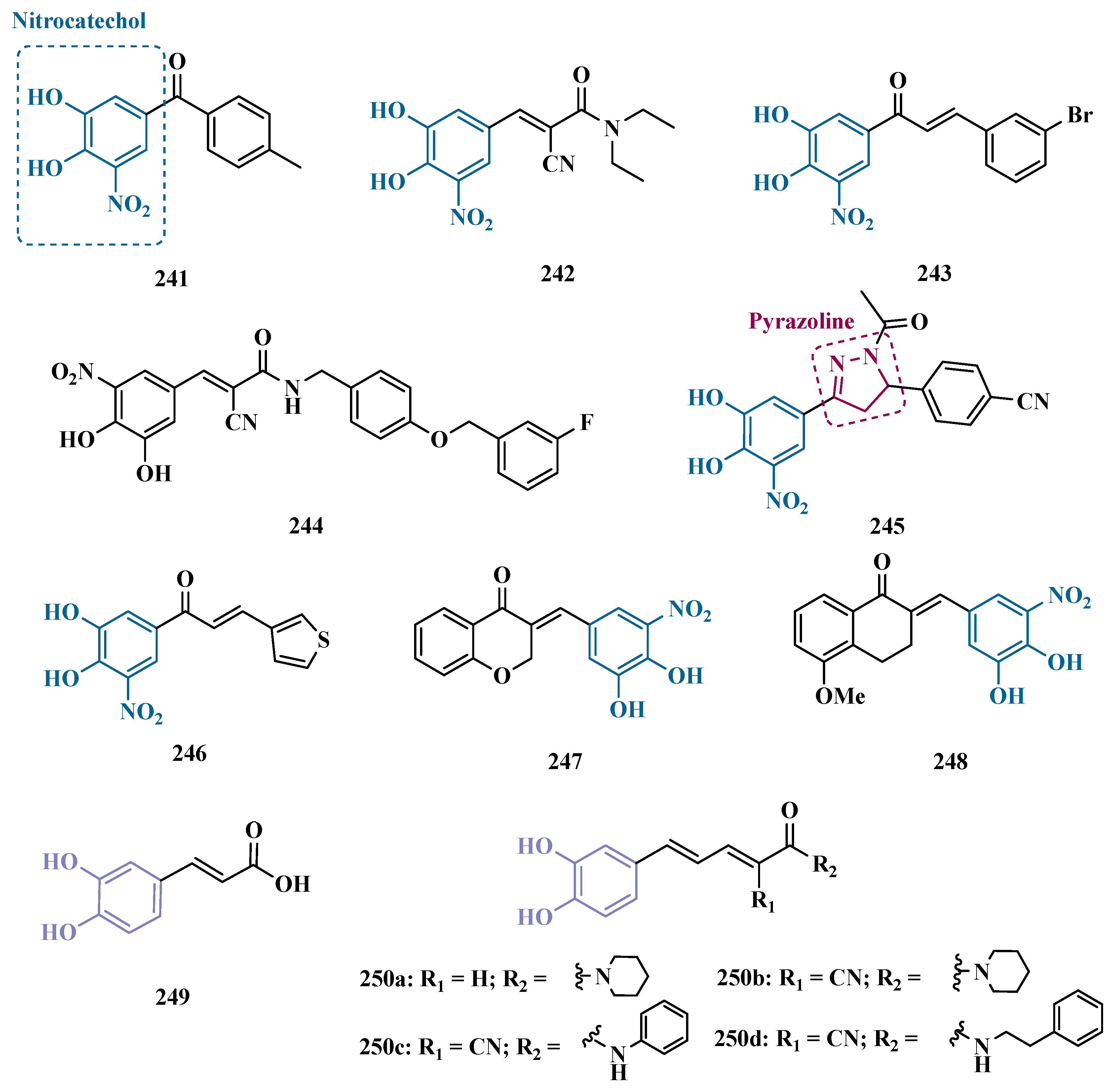
3.2.4. Dual MAO and Catechol O-Methyltransferase Inhibitors
3.2.5. Caspase and MAO-Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| ATP | Adenosine triphosphate |
| ATP synthase | Adenosine triphosphate synthase |
| BBB | Blood–brain barrier |
| CI | Complex I |
| CIII | Complex III |
| LB | Lewy’s bodies |
| COMT | Catechol O-methyltransferase |
| DpA | Dopamine |
| ND | Neurodegenerative disease |
| DNA | Deoxyribonucleic acid |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DOPAL | 3,4-dihydroxyphenylacetaldehyde |
| FAD | Flavine-adenine dinucleotide |
| ROS | Reactive oxygen species |
| OS | Oxidative stress |
| RNS | Reactive nitrogen species |
| GSH | Glutathione |
| LAS | Lysosomal autophagic system |
| LDH | Lactate dehydrogenase |
| CSF | Cerebrospinal fluid |
| MAO | Monoamine oxidase |
| MnSOD | Manganese superoxide dismutase |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NM | Neuromelanin |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| PTP | Permeability transition pore |
| SN | Substantia nigra |
| CNS | Central nervous system |
| SAR | Structure-activity relationship |
| GABA | Gamma-aminobutyric |
| AChEs | Acetylcholinesterases |
| α-SYN | α-synuclein |
References
- Connolly, B.S.; Lang, A.E. Pharmacological Treatment of Parkinson Disease: A Review. JAMA J. Am. Med. Assoc. 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vélez, G.E.; Zoghbi, H.Y. Parkinson’s Disease Genetics and Pathophysiology. Annu. Rev. Neurosci. 2021, 44, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for Prevalence of Parkinson’s Disease and Its Driving Factors in 195 Countries and Territories to 2050: Modelling Study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Wallin, J.; Svenningsson, P. Potential Effects of Leukotriene Receptor Antagonist Montelukast in Treatment of Neuroinflammation in Parkinson’s Disease. Int J Mol Sci 2021, 22, 5606. [Google Scholar] [CrossRef]
- Al-Nuaimi, S.K.; MacKenzie, E.M.; Baker, G.B. Monoamine Oxidase Inhibitors and Neuroprotection: A Review. Am. J. Ther. 2012, 19, 436–448. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 Functions in Oxidative Stress and Neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Alborghetti, M.; Nicoletti, F. Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson’s Disease: From Bench to Bedside. Curr. Neuropharmacol. 2019, 17, 861–873. [Google Scholar] [CrossRef]
- Guglielmi, P.; Carradori, S.; D’Agostino, I.; Campestre, C.; Petzer, J.P. An Updated Patent Review on Monoamine Oxidase (MAO) Inhibitors. Expert Opin. Ther. Pat. 2022, 32, 849–883. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson Disease. Park. Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.L.B.; de Souza, L.R.; Sousa, K.P.A.; Ferreira, J.V.; Padilha, E.C.; da Silva, C.H.T.P.; Taft, C.A.; Hage-Melim, L.I.S. Parkinson’s Disease: A Review from Pathophysiology to Treatment. Mini Rev. Med. Chem. 2019, 20, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, J. Dopamine in Parkinson’s Disease: Precise Supplementation with Motor Planning. Neurosci. Bull. 2018, 34, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Hindeya Gebreyesus, H.; Gebrehiwot Gebremichael, T. The Potential Role of Astrocytes in Parkinson’s Disease (PD). Med. Sci. 2020, 8, 7. [Google Scholar] [CrossRef]
- Berstad, K.; Berstad, J.E.R. Parkinson’s Disease; the Hibernating Spore Hypothesis. Med. Hypotheses 2017, 104, 48–53. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Wang, G. Impact of Dopamine Oxidation on Dopaminergic Neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and Therapeutic Strategies Against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef]
- Pan, L.; Meng, L.; He, M.; Zhang, Z. Tau in the Pathophysiology of Parkinson’s Disease. J. Mol. Neurosci. 2021, 71, 2179–2191. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-Synuclein Oligomers Interact with ATP Synthase and Open the Permeability Transition Pore in Parkinson’s Disease. Nat. Commun. 2018, 9, 2293. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science (1979) 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Keane, P.C.; Kurzawa, M.; Blain, P.G.; Morris, C.M. Mitochondrial Dysfunction in Parkinson’s Disease. Park. Dis. 2011, 2011, 716871. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, L.; Gao, J.; Zhu, M.; Zhang, Y.; Zhang, H.L. Gut Microbial Metabolites in Parkinson’s Disease: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Mol. Neurobiol. 2021, 58, 3745–3758. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Kim, S.; Sim, J.; Yang, S.; An, H.; Nam, M.H.; Jang, D.P.; Lee, C.J. Redefining Differential Roles of MAO-A in Dopamine Degradation and MAO-B in Tonic GABA Synthesis. Exp. Mol. Med. 2021, 53, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Wadood, A.; Sadiq, A.; Ullah, F.; Anichkina, O.; Ghufran, M. In-Silico Evaluations of the Isolated Phytosterols from Polygonum Hydropiper L. Against BACE1 and MAO Drug Targets. J. Biomol. Struct. Dyn. 2022, 40, 10230–10238. [Google Scholar] [CrossRef]
- Naoi, M.; Riederer, P.; Maruyama, W. Modulation of Monoamine Oxidase (MAO) Expression in Neuropsychiatric Disorders: Genetic and Environmental Factors Involved in Type A MAO Expression. J. Neural Transm. 2016, 123, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Upadhyay, S.; Paliwal, S.; Saraf, S.K. Privileged Scaffolds as MAO Inhibitors: Retrospect and Prospects. Eur. J. Med. Chem. 2018, 145, 445–497. [Google Scholar] [CrossRef]
- Edmondson, D.E.; Binda, C. Monoamine Oxidases. Subcell. Biochem. 2018, 87, 117–139. [Google Scholar] [CrossRef]
- Visweswaran, V.; Roshni, P.R. Role of Enzymes in Causing Neurological Disorders. Int. J. Res. Pharm. Sci. 2021, 12, 466–476. [Google Scholar] [CrossRef]
- Ramsay, R.R. Monoamine Oxidases: The Biochemistry of the Proteins as Targets in Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2013, 12, 2189–2209. [Google Scholar] [CrossRef]
- McHugh, P.C.; Buckley, D.A. The Structure and Function of the Dopamine Transporter and Its Role in CNS Diseases, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 98. [Google Scholar]
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine Oxidation Products as Mitochondrial Endotoxins, a Potential Molecular Mechanism for Preferential Neurodegeneration in Parkinson’s Disease. ACS Chem. Neurosci. 2018, 9, 2849–2858. [Google Scholar] [CrossRef]
- Follmer, C. Monoamine Oxidase and α-Synuclein as Targets in Parkinson’s Disease Therapy. Expert Rev. Neurother. 2014, 14, 703–716. [Google Scholar] [CrossRef]
- Anderson, D.G.; Mariappan, S.V.S.; Buettner, G.R.; Doorn, J.A. Oxidation of 3,4-Dihydroxyphenylacetaldehyde, a Toxic Dopaminergic Metabolite, to a Semiquinone Radical and an Ortho-Quinone. J. Biol. Chem. 2011, 286, 26978–26986. [Google Scholar] [CrossRef]
- Tan, Y.-Y.; Jenner, P.; Chen, S.-D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Jaisa-aad, M.; Muñoz-Castro, C.; Healey, M.A.; Hyman, B.T.; Serrano-Pozo, A. Characterization of Monoamine Oxidase-B (MAO-B) as a Biomarker of Reactive Astrogliosis in Alzheimer’s Disease and Related Dementias. Acta Neuropathol. 2024, 147, 66. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 11059. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Yadav, K.S. Parkinson’s Disease: Current Drug Therapy and Unraveling the Prospects of Nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 58, 101790. [Google Scholar] [CrossRef]
- Marsili, L.; Marconi, R.; Colosimo, C. Treatment Strategies in Early Parkinson’s Disease. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 132, pp. 345–360. [Google Scholar]
- Cera, N.; Bifolchetti, S.; Martinotti, G.; Gambi, F.; Sepede, G.; Onofrj, M.; Di Giannantonio, M.; Thomas, A. Amantadine and Cognitive Flexibility: Decision Making in Parkinson’s Patients with Severe Pathological Gambling and Other Impulse Control Disorders. Neuropsychiatr. Dis. Treat. 2014, 10, 1093–1101. [Google Scholar] [CrossRef]
- Borovac, J.A. Side Effects of a Dopamine Agonist Therapy for Parkinson’s Disease: A Mini-Review of Clinical Pharmacology. Yale J. Biol. Med. 2016, 89, 37. [Google Scholar]
- Finberg, J.P.M. Inhibitors of MAO-B and COMT: Their Effects on Brain Dopamine Levels and Uses in Parkinson’s Disease. J. Neural Transm. 2019, 126, 433–448. [Google Scholar] [CrossRef]
- Uchida, S.; Kadowaki-Horita, T.; Kanda, T. Effects of the Adenosine A2A Receptor Antagonist on Cognitive Dysfunction in Parkinson’s Disease. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 119, pp. 169–189. [Google Scholar]
- Hong, R.; Li, X. Discovery of Monoamine Oxidase Inhibitors by Medicinal Chemistry Approaches. MedChemComm 2019, 10, 10–25. [Google Scholar] [CrossRef]
- Schapira, A.H.V. Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s Disease A Review of Symptomatic and Potential Disease-Modifying Effects. CNS Drugs 2011, 25, 1061–1071. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Paik, S.; Park, J.H.; Kim, H.J.; Hassan, A.H.E.; Lee, K.; Park, K.D.; Roh, E.J. Discovery of Novel and Potent Safinamide-Based Derivatives as Highly Selective HMAO-B Inhibitors for Treatment of Parkinson’s Disease (PD): Design, Synthesis, In Vitro, In Vivo and In Silico Biological Studies. Bioorg. Chem. 2021, 115, 105233. [Google Scholar] [CrossRef] [PubMed]
- Elkamhawy, A.; Woo, J.; Gouda, N.A.; Kim, J.; Nada, H.; Roh, E.J.; Park, K.D.; Cho, J.; Lee, K. Melatonin Analogues Potently Inhibit Mao-b and Protect Pc12 Cells Against Oxidative Stress. Antioxidants 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.; Michalska, P.; Crisman, E.; Cuadrado, A.; León, R. Novel Series of Dual NRF2 Inducers and Selective MAO-B Inhibitors for the Treatment of Parkinson’s Disease. Antioxidants 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.; Sanchez-Porro, F.J.; Crisman, E.; Cores, Á.; Jiménez, I.; Cuadrado, A.; Menéndez, J.C.; León, R. Network-Based Drug Optimization Toward the Treatment of Parkinson’s Disease: NRF2, MAO-B, Oxidative Stress, and Chronic Neuroinflammation. J. Med. Chem. 2025, 68, 3495–3517. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Kim, H.J.; Elsherbeny, M.H.; Paik, S.; Park, J.H.; Gotina, L.; Abdellattif, M.H.; Gouda, N.A.; Cho, J.; Lee, K.; et al. Discovery of 3,4-Dichloro-N-(1H-Indol-5-Yl)Benzamide: A Highly Potent, Selective, and Competitive HMAO-B Inhibitor with High BBB Permeability Profile and Neuroprotective Action. Bioorg. Chem. 2021, 116, 105352. [Google Scholar] [CrossRef]
- Elsherbeny, M.H.; Kim, J.; Gouda, N.A.; Gotina, L.; Cho, J.; Pae, A.N.; Lee, K.; Park, K.D.; Elkamhawy, A.; Roh, E.J. Highly Potent, Selective, and Competitive Indole-Based MAO-B Inhibitors Protect PC12 Cells against 6-Hydroxydopamine-and Rotenone-Induced Oxidative Stress. Antioxidants 2021, 10, 1641. [Google Scholar] [CrossRef]
- Chirkova, Z.V.; Kabanova, M.V.; Filimonov, S.I.; Abramov, I.G.; Petzer, A.; Petzer, J.P.; Suponitsky, K.Y. An Evaluation of Synthetic Indole Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2016, 26, 2214–2219. [Google Scholar] [CrossRef]
- Qin, J.; Cheng, W.; Duan, Y.-T.; Yang, H.; Yao, Y. Indazole as a Privileged Scaffold: The Derivatives and Their Therapeutic Applications. Anticancer Agents Med. Chem. 2020, 21, 839–860. [Google Scholar] [CrossRef]
- Tzvetkov, N.T.; Hinz, S.; Küppers, P.; Gastreich, M.; Müller, C.E. Indazole-and Indole-5-Carboxamides: Selective and Reversible Monoamine Oxidase B Inhibitors with Subnanomolar Potency. J. Med. Chem. 2014, 57, 6679–6703. [Google Scholar] [CrossRef]
- Tzvetkov, N.T.; Stammler, H.G.; Neumann, B.; Hristova, S.; Antonov, L.; Gastreich, M. Crystal Structures, Binding Interactions, and ADME Evaluation of Brain Penetrant N-Substituted Indazole-5-Carboxamides as Subnanomolar, Selective Monoamine Oxidase B and Dual MAO-A/B Inhibitors. Eur. J. Med. Chem. 2017, 127, 470–492. [Google Scholar] [CrossRef]
- Tzvetkov, N.T.; Stammler, H.G.; Hristova, S.; Atanasov, A.G.; Antonov, L. (Pyrrolo-Pyridin-5-Yl)Benzamides: BBB Permeable Monoamine Oxidase B Inhibitors with Neuroprotective Effect on Cortical Neurons. Eur. J. Med. Chem. 2019, 162, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Elkamhawy, A.; Paik, S.; Kim, H.J.; Park, J.H.; Londhe, A.M.; Lee, K.; Pae, A.N.; Park, K.D.; Roh, E.J. Discovery of N-(1-(3-Fluorobenzoyl)-1H-Indol-5-Yl)Pyrazine-2-Carboxamide: A Novel, Selective, and Competitive Indole-Based Lead Inhibitor for Human Monoamine Oxidase B. J. Enzym. Inhib. Med. Chem. 2020, 35, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, Z.V.; Kabanova, M.V.; Filimonov, S.I.; Abramov, I.G.; Petzer, A.; Petzer, J.P.; Firgang, S.I.; Suponitsky, K.Y. Inhibition of Monoamine Oxidase by Indole-5,6-Dicarbonitrile Derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, Z.V.; Kabanova, M.V.; Filimonov, S.I.; Abramov, I.G.; Petzer, A.; Engelbrecht, I.; Petzer, J.P.; Abramov, I.G.; Suponitsky, K.Y.; Veselovsky, A.V. An Investigation of the Monoamine Oxidase Inhibition Properties of Pyrrolo [3,4-f]Indole-5,7-Dione and Indole-5,6-Dicarbonitrile Derivatives. Drug Dev. Res. 2018, 79, 81–93. [Google Scholar] [CrossRef]
- Chirkova, Z.V.; Kabanova, M.V.; Filimonov, S.I.; Abramov, I.G.; Petzer, A.; Hitge, R.; Petzer, J.P.; Suponitsky, K.Y. Optimization of Pyrrolo[3,4-f]Indole-5,7-Dione and Indole-5,6-Dicarbonitrile Derivatives as Inhibitors of Monoamine Oxidase. Drug Dev. Res. 2019, 80, 1–11. [Google Scholar] [CrossRef]
- Nam, M.; Park, M.; Park, H.; Kim, Y.; Yoon, S.; Sawant, V.S.; Choi, J.W.; Park, J.; Park, K.D.; Min, S.; et al. Indole-Substituted Benzothiazoles and Benzoxazoles as Selective and Reversible MAO- B Inhibitors for Treatment of Parkinson’ s Disease. ACS Chem. Neurosci. 2017, 8, 1430–1628. [Google Scholar] [CrossRef]
- Jismy, B.; El Qami, A.; Pišlar, A.; Frlan, R.; Kos, J.; Gobec, S.; Knez, D.; Abarbri, M. Pyrimido[1,2-b]Indazole Derivatives: Selective Inhibitors of Human Monoamine Oxidase B with Neuroprotective Activity. Eur. J. Med. Chem. 2021, 209, 112911. [Google Scholar] [CrossRef]
- Can, N.Ö.; Osmaniye, D.; Levent, S.; Sağlik, B.N.; Inci, B.; Lgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of New Hydrazone Derivatives for MAO Enzymes Inhibitory Activity. Molecules 2017, 22, 1381. [Google Scholar] [CrossRef]
- Evranos-Aksöz, B.; Yabanoglu-Çiftçi, S.; Uçar, G.; Yelekçi, K.; Ertan, R. Synthesis of Some Novel Hydrazone and 2-Pyrazoline Derivatives: Monoamine Oxidase Inhibitory Activities and Docking Studies. Bioorg. Med. Chem. Lett. 2014, 24, 3278–3284. [Google Scholar] [CrossRef]
- Turan-zitouni, G.; Hussein, W.; Saglık, B.N.; Tabbi, A.; Korkut, B. Design, Synthesis and Biological Evaluation of Novel N-Pyridyl-Hydrazone Derivatives as Potential Monoamine Oxidase (MAO) Inhibitors. Molecules 2018, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Salgin-Gökşen, U.; Gökhan-Kelekçi, N.; Yabanoglu-Çiftci, S.; Yelekçi, K.; Uçar, G. Synthesis, Molecular Modeling, and In Vitro Screening of Monoamine Oxidase Inhibitory Activities of Some Novel Hydrazone Derivatives. J. Neural Transm. 2013, 120, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Befani, O.; Turini, P.; Alcaro, S.; Ortuso, F.; et al. Selective Inhibitory Activity against MAO and Molecular Modeling Studies of 2-Thiazolylhydrazone Derivatives. J. Med. Chem. 2007, 50, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Distinto, S.; Yáñez, M.; Alcaro, S.; Cardia, M.C.; Gaspari, M.; Sanna, M.L.; Meleddu, R.; Ortuso, F.; Kirchmair, J.; Markt, P.; et al. Synthesis and Biological Assessment of Novel 2-Thiazolylhydrazones and Computational Analysis of Their Recognition by Monoamine Oxidase B. Eur. J. Med. Chem. 2012, 48, 284–295. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Ayyannan, S.R. Design, Synthesis, and Evaluation of 2-Amino-6-Nitrobenzothiazole-Derived Hydrazones as MAO Inhibitors: Role of the Methylene Spacer Group. ChemMedChem 2016, 11, 1551–1567. [Google Scholar] [CrossRef]
- Can, Ö.D.; Osmaniye, D.; Özkay, Ü.D.; Sağlık, B.N.; Levent, S.; Ilgın, S.; Baysal, M.; Özkay, Y.; Kaplancıklı, Z.A. MAO Enzymes Inhibitory Activity of New Benzimidazole Derivatives Including Hydrazone and Propargyl Side Chains. Eur. J. Med. Chem. 2017, 131, 92–106. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Petzer, A.; Guglielmi, P.; D’Ascenzio, M.; Chimenti, P.; Bagetta, D.; Alcaro, S.; Zengin, G.; Petzer, J.P.; et al. 4-(3-Nitrophenyl)Thiazol-2-Ylhydrazone Derivatives as Antioxidants and Selective HMAO-B Inhibitors: Synthesis, Biological Activity and Computational Analysis. J. Enzym. Inhib. Med. Chem. 2019, 34, 597–612. [Google Scholar] [CrossRef]
- Chimenti, P.; Petzer, A.; Carradori, S.; Ascenzio, M.D.; Silvestri, R.; Alcaro, S.; Ortuso, F.; Petzer, J.P.; Secci, D. Exploring 4-Substituted-2-Thiazolylhydrazones from 2-, 3-, and 4-Acetylpyridine as Selective and Reversible HMAO-B Inhibitors. Eur. J. Med. Chem. 2013, 66, 221–227. [Google Scholar] [CrossRef]
- Carradori, S.; D’Ascenzio, M.; De Monte, C.; Secci, D.; Yáñez, M. Synthesis and Selective Human Monoamine Oxidase B Inhibition of Heterocyclic Hybrids Based on Hydrazine and Thiazole Scaffolds. Arch. Pharm. 2013, 346, 17–22. [Google Scholar] [CrossRef]
- Kumar, S.; Oh, J.M.; Prabhakaran, P.; Awasti, A.; Kim, H.; Mathew, B. Isatin-Tethered Halogen-Containing Acylhydrazone Derivatives as Monoamine Oxidase Inhibitor with Neuroprotective Effect. Sci. Rep. 2024, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Mateev, E.; Valkova, I.; Kuteva, H.; Tzankova, D.; Stefanova, D.; Yordanov, Y.; Lybomirova, K.; Zlatkov, A.; Tzankova, V.; et al. Neurotoxicity, Neuroprotection, In Vitro MAOA/MAOB Inhibitory Activity Assessment, Molecular Docking, and Permeability Assay Studies of Newly Synthesized Hydrazones Containing a Pyrrole Ring. Molecules 2024, 29, 4338. [Google Scholar] [CrossRef] [PubMed]
- Mattev, E. Design, Synthesis, Biological Evaluation and Molecular Docking of Pyrrole-based Compounds as Antioxidant and MAO-B Inhibitory Agents. Farmacia 2022, 70, 344–354. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Mateev, E.; Angelov, B.; Tzankova, V.; Georgieva, M. In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson’s Disease. Molecules 2022, 27, 8485. [Google Scholar] [CrossRef]
- Mateev, E.; Karatchobanov, V.; Dedja, M.; Diamantakos, K.; Mateeva, A.; Muhammed, M.T.; Irfan, A.; Kondeva-Burdina, M.; Valkova, I.; Georgieva, M.; et al. Novel Pyrrole Derivatives as Multi-Target Agents for the Treatment of Alzheimer’s Disease: Microwave-Assisted Synthesis, In Silico Studies and Biological Evaluation. Pharmaceuticals 2024, 17, 1171. [Google Scholar] [CrossRef]
- Georgieva, M.; Sharkov, M.; Mateev, E.; Tzankova, D.; Popov, G.; Manov, V.; Zlatkov, A.; Simeonova, R.; Kondeva-Burdina, M. Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease. Molecules 2025, 30, 3154. [Google Scholar] [CrossRef]
- Strydom, B.; Bergh, J.J.; Petzer, J.P. Inhibition of Monoamine Oxidase by Phthalide Analogues. Bioorg. Med. Chem. Lett. 2013, 23, 1269–1273. [Google Scholar] [CrossRef]
- Engelbrecht, I.; Petzer, J.P.; Petzer, A. The Synthesis and Evaluation of Sesamol and Benzodioxane Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2015, 25, 1896–1900. [Google Scholar] [CrossRef]
- Van Der Walt, M.M.; Terre’Blanche, G.; Petzer, A.; Petzer, J.P. Novel Sulfanylphthalimide Analogues as Highly Potent Inhibitors of Monoamine Oxidase B. Bioorg. Med. Chem. Lett. 2012, 22, 6632–6635. [Google Scholar] [CrossRef]
- Delogu, G.L.; Kumar, A.; Gatto, G.; Bustelo, F.; Saavedra, L.M.; Rodríguez-Franco, M.I.; Laguna, R.; Viña, D. Synthesis and in Vitro Study of Nitro- and Methoxy-2-Phenylbenzofurans as Human Monoamine Oxidase Inhibitors. Bioorg Chem 2021, 107, 104616. [Google Scholar] [CrossRef]
- Matos, M.J.; Vilar, S.; García-Morales, V.; Tatonetti, N.P.; Uriarte, E.; Santana, L.; Viña, D. Insight into the Functional and Structural Properties of 3-Arylcoumarin as an Interesting Scaffold in Monoamine Oxidase B Inhibition. ChemMedChem 2014, 9, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.L.; Pintus, F.; Mayán, L.; Matos, M.J.; Vilar, S.; Munín, J.; Fontenla, J.A.; Hripcsak, G.; Borges, F.; Viña, D. MAO Inhibitory Activity of Bromo-2-Phenylbenzofurans: Synthesis, in Vitro Study, and Docking Calculations. Medchemcomm 2017, 8, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, E.; Barboza, B.; Delgado-Esteban, M.; Escala, N.; Jiménez-Blasco, D.; Lopez-Pérez, J.L.; Cillero de la Fuente, L.; Quezada, E.; Munín, J.; Viña, D.; et al. Potent, Selective and Reversible HMAO-B Inhibition by Benzalphthalides: Synthesis, Enzymatic and Cellular Evaluations and Virtual Docking and Predictive Studies. Bioorg. Chem. 2024, 146, 107255. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, A.; Petzer, J.; Petzer, A.; Legoabe, L. 3-Coumaranone Derivatives as Inhibitors of Monoamine Oxidase. Drug Des. Devel. Ther. 2015, 9, 5479–5489. [Google Scholar] [CrossRef]
- Mostert, S.; Petzer, A.; Petzer, J.P. Indanones as High-Potency Reversible Inhibitors of Monoamine Oxidase. ChemMedChem 2015, 10, 862–873. [Google Scholar] [CrossRef]
- Nel, M.S.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. 2-Heteroarylidene-1-Indanone Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Chem. 2016, 69, 20–28. [Google Scholar] [CrossRef]
- Li, S.; Lv, X.; Cheng, K.; Tian, Y.; Huang, X.; Kong, H.; Duan, Y.; Han, J.; Liao, C.; Xie, Z. Discovery of Novel 2,3-Dihydro-1H-Inden-1-Amine Derivatives as Selective Monoamine Oxidase B Inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1090–1093. [Google Scholar] [CrossRef]
- Mathew, B.; Haridas, A.; Uçar, G.; Baysal, I.; Joy, M.; Mathew, G.E.; Lakshmanan, B.; Jayaprakash, V. Synthesis, Biochemistry, and Computational Studies of Brominated Thienyl Chalcones: A New Class of Reversible. ChemMedChem 2016, 11, 1109–1187. [Google Scholar] [CrossRef]
- Mellado, M.; Salas, C.O.; Uriarte, E.; Viña, D.; Jara-Gutiérrez, C.; Matos, M.J.; Cuellar, M. Design, Synthesis and Docking Calculations of Prenylated Chalcones as Selective Monoamine Oxidase B Inhibitors with Antioxidant Activity. ChemistrySelect 2019, 4, 7659–7783. [Google Scholar] [CrossRef]
- Hitge, R.; Smit, S.; Petzer, A.; Petzer, J.P. Evaluation of Nitrocatechol Chalcone and Pyrazoline Derivatives as Inhibitors of Catechol-O-Methyltransferase and Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2020, 30, 127188. [Google Scholar] [CrossRef] [PubMed]
- Kamecki, F.; Knez, D.; Carvalho, D.; Marcucci, C.; Rademacher, M.; Higgs, J.; Zakelj, S.; Marcos, A.; Pinto, F.d.T.; Abin-Carriquiry, J.A.; et al. Multitarget 2′-Hydroxychalcones as Potential Drugs for the Treatment of Neurodegenerative Disorders and Their Comorbidities. Neuropharmacol. J. 2021, 201, 108837. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Mathew, G.E.; Uçar, G.; Baysal, I.; Suresh, J.; Vilapurathu, J.K.; Prakasan, A.; Suresh, J.K.; Thomas, A. Development of Fluorinated Methoxylated Chalcones as Selective Monoamine Oxidase-B Inhibitors: Synthesis, Biochemistry and Molecular Docking Studies. Bioorg. Chem. 2015, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Uçar, G.; Yabanog¢lu-Çiftçi, S.; Baysal, I.; Suresh, J.; Mathew, G.E.; Vilapurathu, J.K.; Nadeena, A.M.; Nabeela, P.; Lakshmi, V.; et al. Development of Fluorinated Thienylchalcones as Monoamine Oxidase-B Inhibitors: Design, Synthesis, Biological Evaluation and Molecular Dock-Ing Studies. Lett. Org. Chem. 2015, 12, 605–613. [Google Scholar] [CrossRef]
- Mathew, B.; Mathew, G.E.; Uçar, G.; Baysal, I.; Suresh, J.; Mathew, S.; Haridas, A.; Jayaprakash, V. Potent and Selective Monoamine Oxidase-B Inhibitory Activity: Fluoro- vs. Trifluoromethyl-4-Hydroxylated Chalcones Derivatives. Chem. Biodivers. 2016, 13, 969–1110. [Google Scholar] [CrossRef]
- Mathew, B.; Uçar, G.; Mathew, G.E.; Mathew, S.; Purapurath, P.K.; Moolayil, F.; Mohan, S.; Gupta, S.V. Monoamine Oxidase Inhibitory Activity: Methyl- versus Chlorochalcone Derivatives. ChemMedChem 2016, 11, 2649–2655. [Google Scholar] [CrossRef]
- Iacovino, L.G.; Pinzi, L.; Facchetti, G.; Bortolini, B.; Christodoulou, M.S.; Binda, C.; Rastelli, G.; Rimoldi, I.; Passarella, D.; Di Paolo, M.L.; et al. Promising Non-Cytotoxic Monosubstituted Chalcones to Target Monoamine Oxidase-B. ACS Med. Chem. Lett. 2021, 12, 1151–1158. [Google Scholar] [CrossRef]
- Facchetti, G.; Marchese, S.; Coccè, V.; Doneda, L.; Alessandri, G.; Paino, F.; Pessina, A.; Pinzi, L.; Rastelli, G.; Binda, C.; et al. Design, Synthesis, and Biological Evaluation of Chalcone Derivatives as Selective Monoamine Oxidase-B Inhibitors with Potential Neuroprotective Effects. Eur. J. Med. Chem. 2025, 298, 117990. [Google Scholar] [CrossRef]
- Desideri, N.; Fioravanti, R.; Proietti Monaco, L.; Biava, M.; Yáñez, M.; Ortuso, F.; Alcaro, S. 1,5-Diphenylpenta-2,4-Dien-1-Ones as Potent and Selective Monoamine Oxidase-B Inhibitors. Eur. J. Med. Chem. 2013, 59, 91–100. [Google Scholar] [CrossRef]
- Minders, C.; Petzer, J.P.; Petzer, A.; Lourens, A.C.U. Monoamine Oxidase Inhibitory Activities of Heterocyclic Chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. [Google Scholar] [CrossRef]
- Rehuman, N.A.; Oh, J.M.; Abdelgawad, M.A.; Beshr, E.A.M.; Abourehab, M.A.S.; Gambacorta, N.; Nicolotti, O.; Jat, R.K.; Kim, H.; Mathew, B. Development of Halogenated-Chalcones Bearing with Dimethoxy Phenyl Head as Monoamine Oxidase-B Inhibitors. Pharmaceuticals 2022, 15, 1152. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Oh, J.M.; Parambi, D.G.T.; Kumar, S.; Musa, A.; Ghoneim, M.M.; Nayl, A.A.; El-Ghorab, A.H.; Ahmad, I.; Patel, H.; et al. Development of Bromo- and Fluoro-Based α, β-Unsaturated Ketones as Highly Potent MAO-B Inhibitors for the Treatment of Parkinson’s Disease. J. Mol. Struct. 2022, 1266, 133545. [Google Scholar] [CrossRef]
- Guglielmi, P.; Secci, D.; Petzer, A.; Bagetta, D.; Rotondi, G.; Ferrante, C.; Recinella, L.; Leone, S.; Alcaro, S.; Zengin, G.; et al. Benzo[b]Tiophen-3-Ol Derivatives as Effective Inhibitors of Human Monoamine Oxidase: Design, Synthesis, and Biological Activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 1511–1525. [Google Scholar] [CrossRef]
- Nel, M.S.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. 2-Benzylidene-1-Indanone Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2016, 26, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Łażewska, D.; Olejarz-maciej, A.; Reiner, D.; Kaleta, M.; Latacz, G.; Zygmunt, M.; Doroz-Płonka, A.; Karcz, T.; Frank, A.; Stark, H.; et al. Dual Target Ligands with 4-Tert-Butylphenoxy Scaffold as Histamine H3 Receptor Antagonists and Monoamine Oxidase B Inhibitors. Int. J. Mol. Sci. 2020, 21, 3411. [Google Scholar] [CrossRef] [PubMed]
- Huleatt, P.B.; Khoo, M.L.; Chua, Y.Y.; Tan, T.W.; Liew, R.S.; Balogh, B.; Deme, R.; Gölöncsér, F.; Magyar, K.; Sheela, D.P.; et al. Novel Arylalkenylpropargylamines as Neuroprotective, Potent, and Selective Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s Disease. J. Med. Chem. 2015, 58, 1400–1419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, M.; Dwivedi, A.R.; Kumar, V. Synthesis, Biological Evaluation and Molecular Modeling Studies of Propargyl-Containing 2,4,6-Trisubstituted Pyrimidine Derivatives as Potential Anti-Parkinson Agents. ChemMedChem 2018, 13, 705–712. [Google Scholar] [CrossRef]
- Meiring, L.; Petzer, J.P.; Legoabe, L.J.; Petzer, A. The Evaluation of N-Propargylamine-2-Aminotetralin as an Inhibitor of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2022, 67, 128746. [Google Scholar] [CrossRef]
- Van Der Walt, M.M.; Terre’Blanche, G.; Lourens, A.C.U.; Petzer, A.; Petzer, J.P. Sulfanylphthalonitrile Analogues as Selective and Potent Inhibitors of Monoamine Oxidase B. Bioorg. Med. Chem. Lett. 2012, 22, 7367–7370. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Ozalp, L.; Danıs, O.; Odabas, Z. Synthesis and Human Monoamine Oxidase Inhibitory Activity of Novel C2-, C3- and C4-Substituted Phthalonitriles. Bioorg. Med. Chem. Lett. 2022, 74, 128917. [Google Scholar] [CrossRef]
- Strydom, B.; Bergh, J.J.; Petzer, J.P. The Inhibition of Monoamine Oxidase by 8-(2-Phenoxyethoxy)Caffeine Analogues. Arzneimittelforschung-Drug Res. 2012, 62, 513–518. [Google Scholar] [CrossRef]
- Petzer, A.; Grobler, P.; Bergh, J.J.; Petzer, J.P. Inhibition of Monoamine Oxidase by Selected Phenylalkylcaffeine Analogues. J. Pharm. Pharmacol. 2014, 66, 677–687. [Google Scholar] [CrossRef]
- Booysen, H.P.; Moraal, C.; Terre’Blanche, G.; Petzer, A.; Bergh, J.J.; Petzer, J.P. Thio- and Aminocaffeine Analogues as Inhibitors of Human Monoamine Oxidase. Bioorg. Med. Chem. 2011, 19, 7507–7518. [Google Scholar] [CrossRef]
- Song, B.; Xiao, T.; Qi, X.; Li, L.N.; Qin, K.; Nian, S.; Hu, G.X.; Yu, Y.; Liang, G.; Ye, F. Design and Synthesis of 8-Substituted Benzamido-Phenylxanthine Derivatives as MAO-B Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 1739–1742. [Google Scholar] [CrossRef]
- Chavarria, D.; Cagide, F.; Pinto, M.; Gomes, L.R.; Low, J.N.; Borges, F. Development of Piperic Acid-Based Monoamine Oxidase Inhibitors: Synthesis, Structural Characterization and Biological Evaluation. J. Mol. Struct. 2019, 1182, 298–307. [Google Scholar] [CrossRef]
- Chavarria, D.; Fernandes, C.; Silva, V.; Silva, C.; Gil-Martins, E.; Soares, P.; Silva, T.; Silva, R.; Remião, F.; Oliveira, P.J.; et al. Design of Novel Monoamine Oxidase-B Inhibitors Based on Piperine Scaffold: Structure-Activity-Toxicity, Drug-Likeness and Efflux Transport Studies. Eur. J. Med. Chem. 2020, 185, 111770. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Oh, J.M.; Baty, R.S.; Batiha, G.E.S.; Parambi, D.G.T.; Gambacorta, N.; Nicolotti, O.; Kim, H. Piperazine-Substituted Chalcones: A New Class of MAO-B, AChE, and BACE-1 Inhibitors for the Treatment of Neurological Disorders. Environ. Sci. Pollut. Res. 2021, 28, 38855–38866. [Google Scholar] [CrossRef]
- Kumar, B.; Sheetal; Mantha, A.K.; Kumar, V. Synthesis, Biological Evaluation and Molecular Modeling Studies of Phenyl-/Benzhydrylpiperazine Derivatives as Potential MAO Inhibitors. Bioorg. Chem. 2018, 77, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, F.; Viña, D.; Uriarte, E.; Laguna, R.; Matos, M.J. 7-Amidocoumarins as Multitarget Agents Against Neurodegenerative Diseases: Substitution Pattern Modulation. ChemMedChem 2021, 16, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Rodríguez-Enríquez, F.; Borges, F.; Santana, L.; Uriarte, E.; Estrada, M.; Rodríguez-Franco, M.I.; Laguna, R.; Viña, D. 3-Amidocoumarins as Potential Multifunctional Agents against Neurodegenerative Diseases. ChemMedChem 2015, 10, 2071–2079. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, S.; Ferino, G.; Cadoni, E.; Matos, M.J.; Quezada, E.; Uriarte, E.; Santana, L.; Vilar, S.; Tatonetti, N.P.; Yáñez, M.; et al. Benzopyran-2-Ones as Attractive Scaffold for MAO Inhibitors: Synthesis, Biological Evaluation and Docking Studies. In Proceedings of the 15th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-15), Online, 1–30 November 2011. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zeng, R.F.; Liu, Y.; Xie, S.S.; Lan, J.S.; Ding, Y.; Yang, Y.T.; Yang, J.; Zhang, T. Design and Synthesis of Novel 3,4-Dihydrocoumarins as Potent and Selective Monoamine Oxidase-B Inhibitors with the Neuroprotection against Parkinson’s Disease. Bioorg. Chem. 2021, 109, 104685. [Google Scholar] [CrossRef]
- Guglielmi, P.; Coluccia, M.; Marconi, G.D.; Ortuso, F.; Procopio, F.; Carradori, S.; Pizzicannella, J.; Arrighi, F.; Troiani, A.; Salvitti, C.; et al. Design, Synthesis, and Biological Activity of 2-Aroylbenzofuran-3-Ols and 2-Aroylbenzofuran Derivatives: A New Route Towards HMAO-B Inhibition. Eur. J. Med. Chem. 2025, 297, 117983. [Google Scholar] [CrossRef]
- Matos, M.J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and Study of a Series of 3-Arylcoumarins as Potent and Selective Monoamine Oxidase B Inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; Nicolotti, O.; Grossi, G.; Di Braccio, M.; Soto-Otero, R.; Mendez-Alvarez, E.; Stefanachi, A.; Gadaleta, D.; Carotti, A. Fine Molecular Tuning at Position 4 of 2H-Chromen-2-One Derivatives in the Search of Potent and Selective Monoamine Oxidase B Inhibitors. Eur. J. Med. Chem. 2013, 70, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Barletta, M.; Soto-Otero, R.; Nicolotti, O.; Mendez-Alvarez, E.; Catto, M.; Introcaso, A.; Stefanachi, A.; Cellamare, S.; Altomare, C.; et al. Discovery, Biological Evaluation, and Structure-Activity and -Selectivity Relationships of 6′-Substituted (E)-2-(Benzofuran-3(2H)-Ylidene)-N-Methylacetamides, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors. J. Med. Chem. 2013, 56, 2651–2664. [Google Scholar] [CrossRef]
- Meletli, F.; Gündüz, C.; Alparslan, M.M.; Attar, A.; Demir, S.; İskit, E.; Danış, Ö. Design, Synthesis and Biological Evaluation of Novel Benzocoumarin Derivatives as Potent Inhibitors of MAO-B Activity. Bioorg. Med. Chem. Lett. 2024, 113, 129984. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Wang, Y.; Bao, X.Q.; Yang, B.B.; Gao, F.; Wang, L.; Zhang, D.; Li, L. Discovery of Coumarin Mannich Base Derivatives as Multifunctional Agents Against Monoamine Oxidase B and Neuroinflammation for the Treatment of Parkinson’s Disease. Eur. J. Med. Chem. 2019, 173, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Viña, D.; Janeiro, P.; Borges, F.; Santana, L.; Uriarte, E. New Halogenated 3-Phenylcoumarins as Potent and Selective MAO-B Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 5157–5160. [Google Scholar] [CrossRef]
- Viña, D.; Matos, M.J.; Ferino, G.; Cadoni, E.; Laguna, R.; Borges, F.; Uriarte, E.; Santana, L. 8-Substituted 3-Arylcoumarins as Potent and Selective MAO-B Inhibitors: Synthesis, Pharmacological Evaluation, and Docking Studies. ChemMedChem 2012, 7, 464–470. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, F.; Viña, D.; Uriarte, E.; Fontenla, J.A.; Matos, M.J. Discovery and Optimization of 3-Thiophenylcoumarins as Novel Agents Against Parkinson’s Disease: Synthesis, In Vitro and In Vivo Studies. Bioorg. Chem. 2020, 101, 103986. [Google Scholar] [CrossRef]
- Siju, E.N.; Vishnupriya, C.P.; Balan, S.; Hariraj, N.; Rahul, K.; Vivek, D.; Lakshmi, A.A.G.; Minil, M.; Vatakeel, B. Design, Synthesis and Evaluation of Substituted Coumarin Derivatives as New Scaffold on Parkinson’s Disease. World J. Pharm. Res. 2020, 9, 1702–1717. [Google Scholar] [CrossRef]
- Mpitimpiti, A.N.; Petzer, J.P.; Petzer, A.; Jordaan, J.H.L.; Lourens, A.C.U. Synthesis and Evaluation of Chromone Derivatives as Inhibitors of Monoamine Oxidase. Mol. Divers. 2019, 23, 897–913. [Google Scholar] [CrossRef]
- Reis, J.; Cagide, F.; Chavarria, D.; Silva, T.; Fernandes, C.; Gaspar, A.; Uriarte, E.; Remião, F.; Alcaro, S.; Ortuso, F.; et al. Discovery of New Chemical Entities for Old Targets: Insights on the Lead Optimization of Chromone-Based Monoamine Oxidase B (MAO-B) Inhibitors. J. Med. Chem. 2016, 59, 5879–5893. [Google Scholar] [CrossRef]
- Gaspar, A.; Reis, J.; Fonseca, A.; Milhazes, N.; Viña, D.; Uriarte, E.; Borges, F. Chromone 3-Phenylcarboxamides as Potent and Selective MAO-B Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Silva, T.; Yáñez, M.; Vina, D.; Orallo, F.; Ortuso, F.; Uriarte, E.; Alcaro, S.; Borges, F. Chromone, a Privileged Scaffold for the Development of Monoamine Oxidase Inhibitors. J. Med. Chem. 2011, 54, 5165–5173. [Google Scholar] [CrossRef] [PubMed]
- Cagide, F.; Silva, T.; Reis, J.; Gaspar, A.; Borges, F.; Gomes, L.R.; Low, J.N. Discovery of Two New Classes of Potent Monoamine Oxidase-B Inhibitors by Tricky Chemistry. Chem. Commun. 2015, 51, 2832–2835. [Google Scholar] [CrossRef]
- Cloete, S.J.; N’Da, C.I.; Legoabe, L.J.; Petzer, A.; Petzer, J.P. The Evaluation of 1-Tetralone and 4-Chromanone Derivatives as Inhibitors of Monoamine Oxidase. Mol. Divers. 2021, 25, 491–507. [Google Scholar] [CrossRef]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. Inhibition of Monoamine Oxidase by Selected C6-Substituted Chromone Derivatives. Eur. J. Med. Chem. 2012, 49, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Alcaro, S.; Gaspar, A.; Ortuso, F.; Milhazes, N.; Orallo, F.; Uriarte, E.; Yáñez, M.; Borges, F. Chromone-2- and -3-Carboxylic Acids Inhibit Differently Monoamine Oxidases A and B. Bioorg. Med. Chem. Lett. 2010, 20, 2709–2712. [Google Scholar] [CrossRef]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. Selected Chromone Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2012, 22, 5480–5484. [Google Scholar] [CrossRef]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. α-Tetralone Derivatives as Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. Lett. 2014, 24, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. The Synthesis and Evaluation of C7-Substituted α-Tetralone Derivatives as Inhibitors of Monoamine Oxidase. Chem. Biol. Drug Des. 2015, 86, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Meiring, L.; Petzer, J.P.; Petzer, A. Inhibition of Monoamine Oxidase by 3,4-Dihydro-2(1H)-Quinolinone Derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 5498–5502. [Google Scholar] [CrossRef]
- Meiring, L.; Petzer, J.P.; Petzer, A. C6- and C7-Substituted 3,4-Dihydro-2(1H)-Quinolinones as Inhibitors of Monoamine Oxidase. Drug Res. 2017, 67, 170–178. [Google Scholar] [CrossRef]
- Hitge, R.; Petzer, A.; Petzer, J.P. Isatoic Anhydrides as Novel Inhibitors of Monoamine Oxidase. Bioorg. Med. Chem. 2022, 73, 117030. [Google Scholar] [CrossRef]
- Guo, H.; Lu, Z.; Chen, S.; Yu, Y. Synthesis and Biological Evaluation of 1,2,3,4-Tetrahydroisoquinolines Derivatives as Monoamine Oxidase Inhibitors for Treatment of Alzheimer’s and Parkinson’s Diseases. Asian J. Chem. 2015, 27, 3651–3654. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Luckay, R.C.; Olalekan, S.O.; Badeji, A.A.; Matinise, N.; Tshikhudo, F. Investigating the Inhibitory Potential of Halogenated Quinoline Derivatives against MAO-A and MAO-B: Synthesis, Crystal Structure, Density Functional Theory, and Molecular Dynamics Simulations. ACS Omega 2025, 10, 26500–26519. [Google Scholar] [CrossRef]
- Khattab, S.N.; Haiba, N.S.; Asal, A.M.; Bekhit, A.A.; Amer, A.; Abdel-Rahman, H.M.; El-Faham, A. Synthesis and Evaluation of Quinazoline Amino Acid Derivatives as Mono Amine Oxidase (MAO) Inhibitors. Bioorg. Med. Chem. 2015, 23, 3574–3585. [Google Scholar] [CrossRef]
- Qhobosheane, M.A.; Legoabe, L.J.; Petzer, A.; Petzer, J.P. The Monoamine Oxidase Inhibition Properties of C6-Mono- and N3/C6-Disubstituted Derivatives of 4(3H)-Quinazolinone. Bioorg. Chem. 2019, 85, 60–65. [Google Scholar] [CrossRef]
- Qhobosheane, M.A.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. Synthesis and Evaluation of 2-Substituted 4(3H)-Quinazolinone Thioether Derivatives as Monoamine Oxidase Inhibitors. Bioorg. Med. Chem. 2018, 26, 5531–5537. [Google Scholar] [CrossRef]
- Di Paolo, M.L.; Salerno, S.; Nordio, G.; Piazzola, F.; Sarno, S.; Sarno, G.; Natale, B.; Poggetti, V.; Borreca, A.; Baglini, E.; et al. 2-(Phenylamino)-7,8-Dihydroquinazolin-5(6H)-One, a Promising Scaffold for MAO-B Inhibitors with Potential GSK3β Targeting. Eur. J. Med. Chem. 2025, 291, 117580. [Google Scholar] [CrossRef]
- Marais, L.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. The Monoamine Oxidase Inhibition Properties of C6- and N1-Substituted 3-Methyl-3,4-Dihydroquinazolin-2(1H)-One Derivatives. Mol. Divers. 2020, 24, 391–406. [Google Scholar] [CrossRef]
- Yeon, S.K.; Choi, J.W.; Park, J.H.; Lee, Y.R.; Kim, H.J.; Shin, S.J.; Jang, B.K.; Kim, S.; Bahn, Y.S.; Han, G.; et al. Synthesis and Evaluation of Biaryl Derivatives for Structural Characterization of Selective Monoamine Oxidase B Inhibitors Toward Parkinson’s Disease Therapy. Bioorg. Med. Chem. 2018, 26, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Yang, X.; Cai, P.; Liu, Q.; Wang, K.D.G.; Kong, L.; Wang, X. Neuroprotective Effects of Benzyloxy Substituted Small Molecule Monoamine Oxidase B Inhibitors in Parkinson’s Disease. Bioorg. Med. Chem. 2016, 24, 5929–5940. [Google Scholar] [CrossRef]
- Mostert, S.; Petzer, A.; Petzer, J.P. Inhibition of Monoamine Oxidase by Benzoxathiolone Analogues. Bioorg. Med. Chem. Lett. 2016, 26, 1200–1204. [Google Scholar] [CrossRef]
- Van der Walt, M.M.; Terre’Blanche, G.; Petzer, J.P.; Petzer, A. Benzyloxynitrostyrene Analogues—A Novel Class of Selective and Highly Potent Inhibitors of Monoamine Oxidase B. Eur. J. Med. Chem. 2017, 125, 1193–1199. [Google Scholar] [CrossRef]
- Kaya, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of Some Novel 2-Substituted Benzothiazole Derivatives Containing Benzylamine Moiety as Monoamine Oxidase Inhibitory Agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 1654–1661. [Google Scholar] [CrossRef]
- Sağlık, B.N.; Osmaniye, D.; Acar Çevik, U.; Levent, S.; Kaya Çavuşoğlu, B.; Atlı Eklioğlu, Ö.; Özkay, Y.; Koparal, A.S.; Kaplancıklı, Z.A. Synthesis, in Vitro Enzyme Activity and Molecular Docking Studies of New Benzylamine-Sulfonamide Derivatives as Selective MAO-B Inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1422–1432. [Google Scholar] [CrossRef]
- Legoabe, L.J.; Petzer, A.; Petzer, J.P. 2-Acetylphenol Analogs as Potent Reversible Monoamine Oxidase Inhibitors. Drug Des. Devel. Ther. 2015, 9, 3635–3644. [Google Scholar] [CrossRef]
- Reis, J.; Oliveira, C.; Milhazes, N.; Vina, D.; Borges, F. Exploring Nitrostyrene as a Scaffold for a New Class a of Monoamine Oxidase Inhibitors. Lett. Drug Des. Discov. 2012, 9, 958–961. [Google Scholar] [CrossRef]
- Mostert, S.; Petzer, A.; Petzer, J.P. The Evaluation of 1,4-Benzoquinones as Inhibitors of Human Monoamine Oxidase. Eur. J. Med. Chem. 2017, 135, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Zaib, S.; Bakht, S.M.; Ibrar, A.; Khan, I.; Batool, S.; Saeed, A.; Iqbal, J. Symmetrical Aryl Linked Bis-Iminothiazolidinones as New Chemical Entities for the Inhibition of Monoamine Oxidases: Synthesis, in Vitro Biological Evaluation and Molecular Modelling Analysis. Bioorg. Chem. 2017, 70, 17–26. [Google Scholar] [CrossRef]
- Sawant, V.S.; Park, H.; Baek, S.Y.; Lee, J.; Choi, J.W.; Park, K.D.; Choi, K.I.; Seong, J.; Lee, S.; Choo, H. Benzoxazoles as Selective Monoamine Oxidase B (MAO-B) Inhibitors. Bull. Korean Chem. Soc. 2019, 40, 457–460. [Google Scholar] [CrossRef]
- Al-Saad, O.M.; Gabr, M.; Darwish, S.S.; Rullo, M.; Pisani, L.; Miniero, D.V.; Liuzzi, G.M.; Kany, A.M.; Hirsch, A.K.H.; Abadi, A.H.; et al. Novel 6-Hydroxybenzothiazol-2-Carboxamides as Potent and Selective Monoamine Oxidase B Inhibitors Endowed with Neuroprotective Activity. Eur. J. Med. Chem. 2024, 269, 116266. [Google Scholar] [CrossRef]
- Shetnev, A.; Shlenev, R.; Efimova, J.; Ivanovskii, S.; Tarasov, A.; Petzer, A.; Petzer, J.P. 1,3,4-Oxadiazol-2-Ylbenzenesulfonamides as Privileged Structures for the Inhibition of Monoamine Oxidase B. Bioorg. Med. Chem. Lett. 2019, 29, 126677. [Google Scholar] [CrossRef]
- Distinto, S.; Meleddu, R.; Yanez, M.; Cirilli, R.; Bianco, G.; Sanna, M.L.; Arridu, A.; Cossu, P.; Cottiglia, F.; Faggi, C.; et al. Drug Design, Synthesis, in Vitro and in Silico Evaluation of Selective Monoaminoxidase B Inhibitors Based on 3-Acetyl-2-Dichlorophenyl-5-Aryl-2,3-Dihydro-1,3,4-Oxadiazole Chemical Scaffold. Eur. J. Med. Chem. 2016, 108, 542–552. [Google Scholar] [CrossRef]
- Presnukhina, S.I.; Kotlyarova, V.D.; Shetnev, A.A.; Baykov, S.V.; Turmanov, R.; Appazov, N.; Zhapparbergenov, R.; Zhussupova, L.; Togyzbayeva, N.; Cloete, S.J.; et al. Synthesis of 1,2,4-Oxadiazin-5(6H)-One Derivatives and Their Biological Investigation as Monoamine Oxidase Inhibitors. Molecules 2024, 29, 5550. [Google Scholar] [CrossRef]
- Shetnev, A.; Osipyan, A.; Baykov, S.; Sapegin, A.; Chirkova, Z.; Korsakov, M.; Petzer, A.; Engelbrecht, I.; Petzer, J.P. Novel Monoamine Oxidase Inhibitors Based on the Privileged 2-Imidazoline Molecular Framework. Bioorg. Med. Chem. Lett. 2019, 29, 40–46. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P. Novel Isoxazole Derivatives as Potential Antiparkinson Agents: Synthesis, Evaluation of Monoamine Oxidase Inhibitory Activity and Docking Studies. Med. Chem. Res. 2019, 28, 1488–1501. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P. Synthesis, Monoamine Oxidase Inhibitory Activity and Computational Study of Novel Isoxazole Derivatives as Potential Antiparkinson Agents. Comput. Biol. Chem. 2019, 79, 63–72. [Google Scholar] [CrossRef]
- Panova, V.A.; Filimonov, S.I.; Chirkova, Z.V.; Kabanova, M.V.; Shetnev, A.A.; Korsakov, M.K.; Petzer, A.; Petzer, J.P.; Suponitsky, K.Y. Investigation of Pyrazolo[1,5-a]Quinoxalin-4-Ones as Novel Monoamine Oxidase Inhibitors. Bioorg. Chem. 2021, 108, 104563. [Google Scholar] [CrossRef]
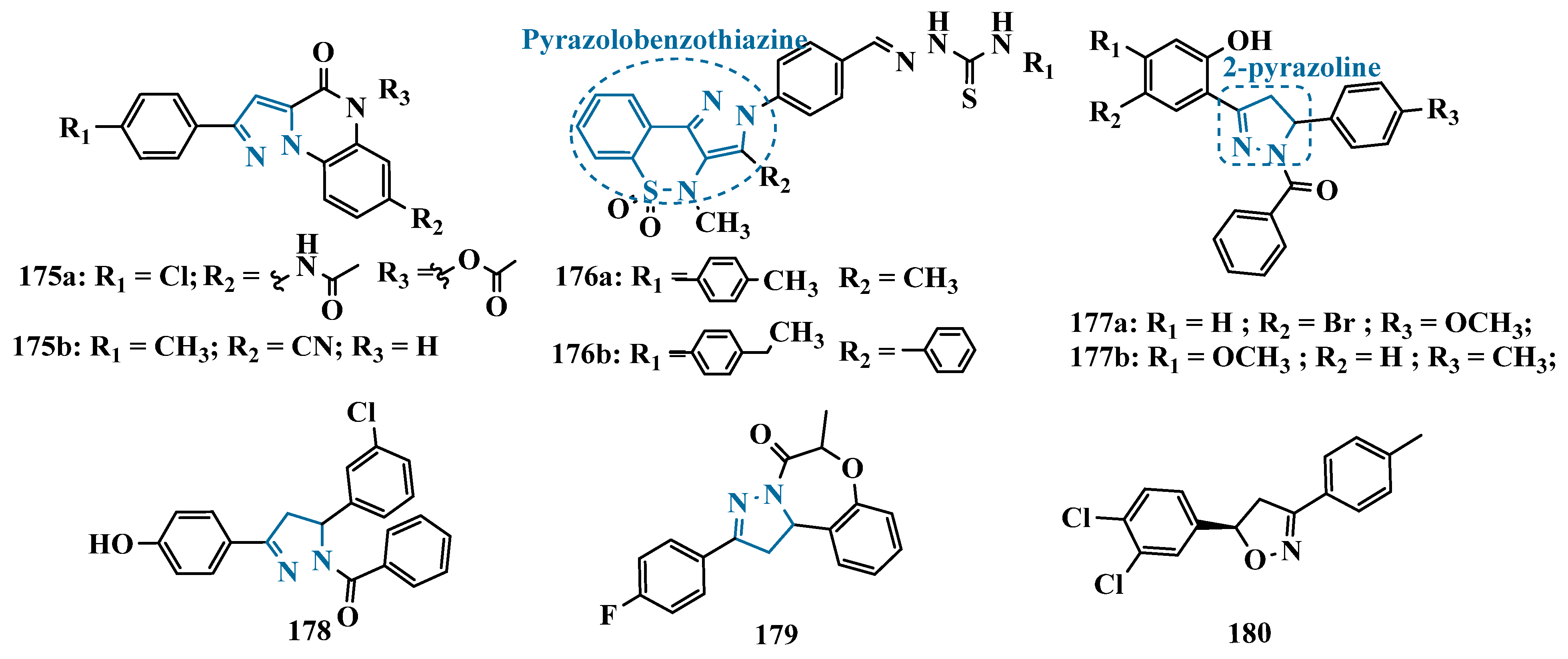
- Abid, S.M.A.; Aslam, S.; Zaib, S.; Bakht, S.M.; Ahmad, M.; Athar, M.M.; Gardiner, J.M.; Iqbal, J. Pyrazolobenzothiazine-Based Carbothioamides as New Structural Leads for the Inhibition of Monoamine Oxidases: Design, Synthesis, in Vitro Bioevaluation and Molecular Docking Studies. MedChemComm 2017, 8, 452–464. [Google Scholar] [CrossRef]
- Evranos-Aksoz, B.; Ucar, G.; Yelekci, K. Design, Synthesis and HMAO Inhibitory Screening of Novel 2-Pyrazoline Analogues. Comb. Chem. High. Throughput Screen. 2017, 20, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.N.; Zhang, L.H.; Ge, R.; Feng, X.E.; Li, Q.S. Triphenylpyrazoline Ketone Chlorophenols as Potential Candidate Compounds against Parkinson’s Disease: Design, Synthesis, and Biological Evaluation. Med. Chem. Res. 2022, 31, 1517–1544. [Google Scholar] [CrossRef]
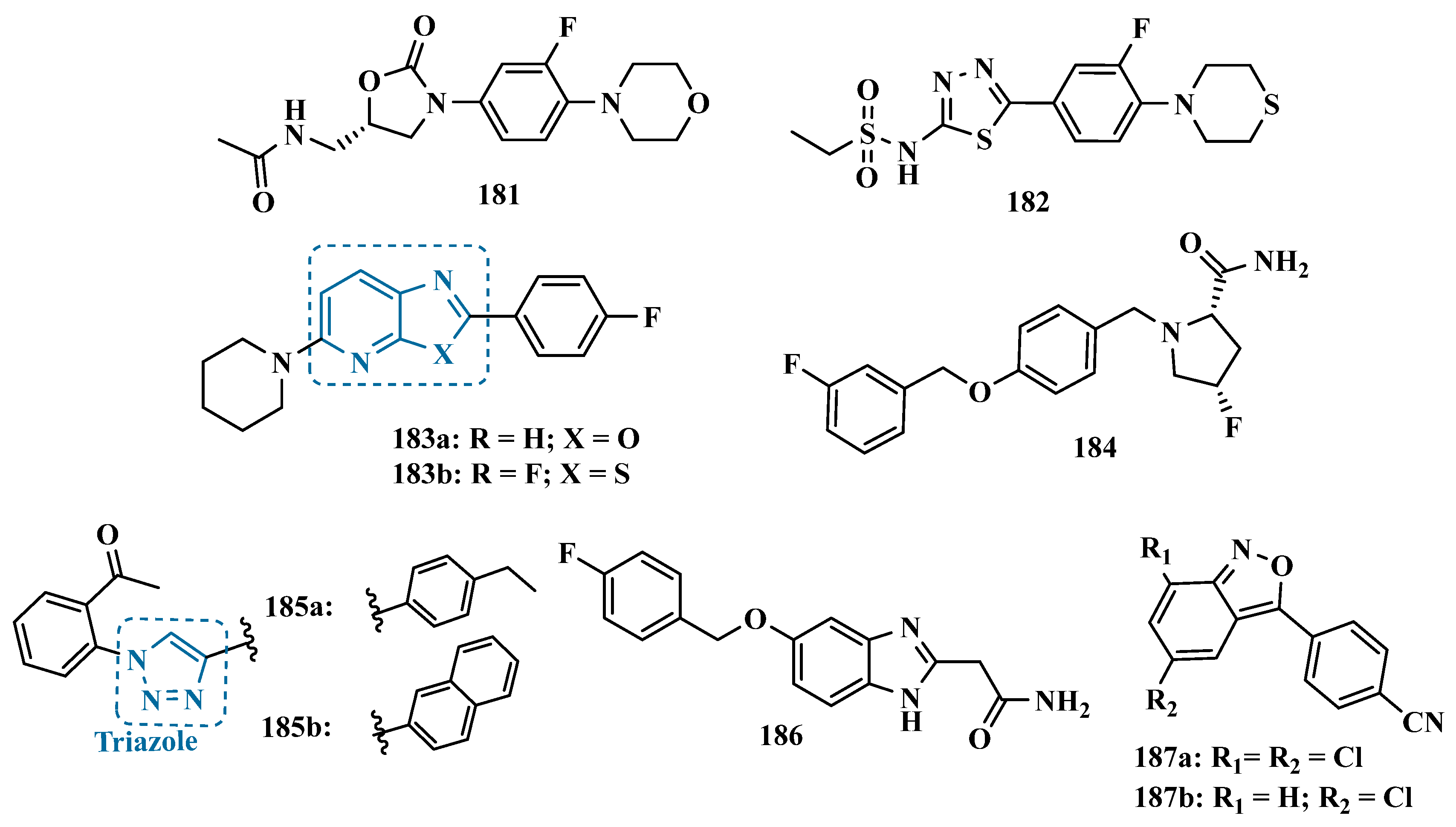
- Chen, R.; Xiao, J.; Ni, Y.; Xu, H.F.; Zheng, M.; Tong, X.; Zhang, T.T.; Liao, C.; Tang, W.J. Novel Tricyclic Pyrazolo[1,5-d][1,4]Benzoxazepin-5(6H)-One: Design, Synthesis, Model and Use as HMAO-B Inhibitors. Bioorg. Med. Chem. 2016, 24, 1741–1748. [Google Scholar] [CrossRef]
- Cardia, M.C.; Sanna, M.L.; Meleddu, R.; Distinto, S.; Yañez, M.; Viña, D.; Lamela, M.; Maccioni, E. A Novel Series of 3,4-Disubstituted Dihydropyrazoles: Synthesis and Evaluation for MAO Enzyme Inhibition. J. Heterocycl. Chem. 2013, 50, E87–E92. [Google Scholar] [CrossRef]
- Carradori, S.; Silvestri, R. New Frontiers in Selective Human MAO-B Inhibitors. J. Med. Chem. 2015, 58, 6717–6732. [Google Scholar] [CrossRef]
- Maccioni, E.; Alcaro, S.; Orallo, F.; Cardia, M.C.; Distinto, S.; Costa, G.; Yanez, M.; Sanna, M.L.; Vigo, S.; Meleddu, R. Synthesis of New 3-Aryl-4,5-Dihydropyrazole-1-Carbothioamide Derivatives. An Investigation on Their Ability to Inhibit Monoamine Oxidase. Eur. J. Med. Chem. 2010, 45, 4490–4498. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bolasco, A.; Bizzarri, B.; D’Ascenzio, M.; Maccioni, E. Discovery and Optimization of Pyrazoline Derivatives as Promising Monoamine Oxidase Inhibitors. Curr. Top. Med. Chem. 2012, 12, 2240–2257. [Google Scholar] [CrossRef]
- Cerioni, G.; Maccioni, E.; Cardia, M.C.; Vigo, S.; Mocci, F. Characterization of 2,5-Diaryl-1,3,4-Oxadiazolines by Multinuclear Magnetic Resonance and Density Functional Theory Calculations. Investigation on a Case of Very Remote Hammett Correlation. Magn. Reson. Chem. 2009, 47, 727–733. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Carradori, S.; Befani, O.; Turini, P.; Alcaro, S.; Ortuso, F. Synthesis, Molecular Modeling Studies, and Selective Inhibitory Activity against Monoamine Oxidase of N,N′-Bis[2-Oxo-2H-Benzopyran]-3-Carboxamides. Bioorg. Med. Chem. Lett. 2006, 16, 4135–4140. [Google Scholar] [CrossRef]
- MacCioni, E.; Alcaro, S.; Cirilli, R.; Vigo, S.; Cardia, M.C.; Sanna, M.L.; Meleddu, R.; Yanez, M.; Costa, G.; Casu, L.; et al. 3-Acetyl-2,5-Diaryl-2,3-Dihydro-1,3,4-Oxadiazoles: A New Scaffold for the Selective Inhibition of Monoamine Oxidase B. J. Med. Chem. 2011, 54, 6394–6398. [Google Scholar] [CrossRef]
- Meleddu, R.; Distinto, S.; Cirilli, R.; Alcaro, S.; Yanez, M.; Sanna, M.L.; Corona, A.; Melis, C.; Bianco, G.; Matyus, P.; et al. Through Scaffold Modification to 3,5-Diaryl-4,5-Dihydroisoxazoles: New Potent and Selective Inhibitors of Monoamine Oxidase B. J. Enzym. Inhib. Med. Chem. 2017, 32, 264–270. [Google Scholar] [CrossRef]
- Dhangar, M.S.; Ahmad, I.; Oh, J.M.; Patil, B.R.; Chinnam, S.; Sriram, D.; Kumari, J.; Mathew, B.; Sayyed, R.A.; Chaudhari, S.B.; et al. Optimizing Linezolid: Transforming It into a Selective MAO-B Inhibitor via a Toxicity-to-Activity Optimization Approach. ACS Med. Chem. Lett. 2025, 16, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Kim, J.; Kim, T.; Jo, S.; Yeom, M.; Moon, B.; Choo, I.H.; Lee, J.; Lim, E.J.; Park, K.D.; et al. Oxazolopyridines and Thiazolopyridines as Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s Disease. Bioorg. Med. Chem. 2013, 21, 5480–5487. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, C.; Chen, K.; Wang, T.; Deng, K.; Jin, C.; Hao, G. Enhancing Monoamine Oxidase B Inhibitory Activity via Chiral Fluorination: Structure-Activity Relationship, Biological Evaluation, and Molecular Docking Study. Eur. J. Med. Chem. 2022, 228, 114025. [Google Scholar] [CrossRef]
- Costa, G.P.; Dias, Í.F.C.; Fronza, M.G.; Besckow, E.M.; Fetter, J.; Nascimento, J.E.R.; Jacob, R.G.; Savegnago, L.; Bortolatto, C.F.; Brüning, C.A.; et al. Synthesis of 2′-(1,2,3-Triazoyl)-Acetophenones: Molecular Docking and Inhibition of: In Vitro Monoamine Oxidase Activity. New J. Chem. 2021, 45, 714–724. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, M.; He, J.; Song, X.; Guo, J.; Gao, B.; Zhang, J.; Zhang, C.; Xie, Y. Discovery of Novel Benzimidazole Derivatives as Selective and Reversible Monoamine Oxidase B Inhibitors for Parkinson’s Disease Treatment. Eur. J. Med. Chem. 2024, 274, 116566. [Google Scholar] [CrossRef]
- Shetnev, A.; Kotov, A.; Kunichkina, A.; Proskurina, I.; Baykov, S.; Korsakov, M.; Petzer, A.; Petzer, J.P. Monoamine Oxidase Inhibition Properties of 2,1-Benzisoxazole Derivatives. Mol. Divers 2024, 28, 1009–1021. [Google Scholar] [CrossRef]
- Clark, D.; Tuor, U.I.; Thompson, R.; Institoris, A.; Kulynych, A.; Zhang, X.; Kinniburgh, D.W.; Bari, F.; Busija, D.W.; Barber, P.A. Protection against Recurrent Stroke with Resveratrol: Endothelial Protection. PLoS ONE 2012, 7, e47792. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol Attenuates Oxidative Damage through Activating Mitophagy in an in Vitro Model of Alzheimer’s Disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Koryciński, K.; Chomentowski, A.; Kiełczewska, A.; Odrzygóźdź, C.; Modzelewska, B. Resveratrol and Alzheimer’s Disease. From Molecular Pathophysiology to Clinical Trials. Exp. Gerontol. 2018, 113, 36–47. [Google Scholar] [CrossRef]
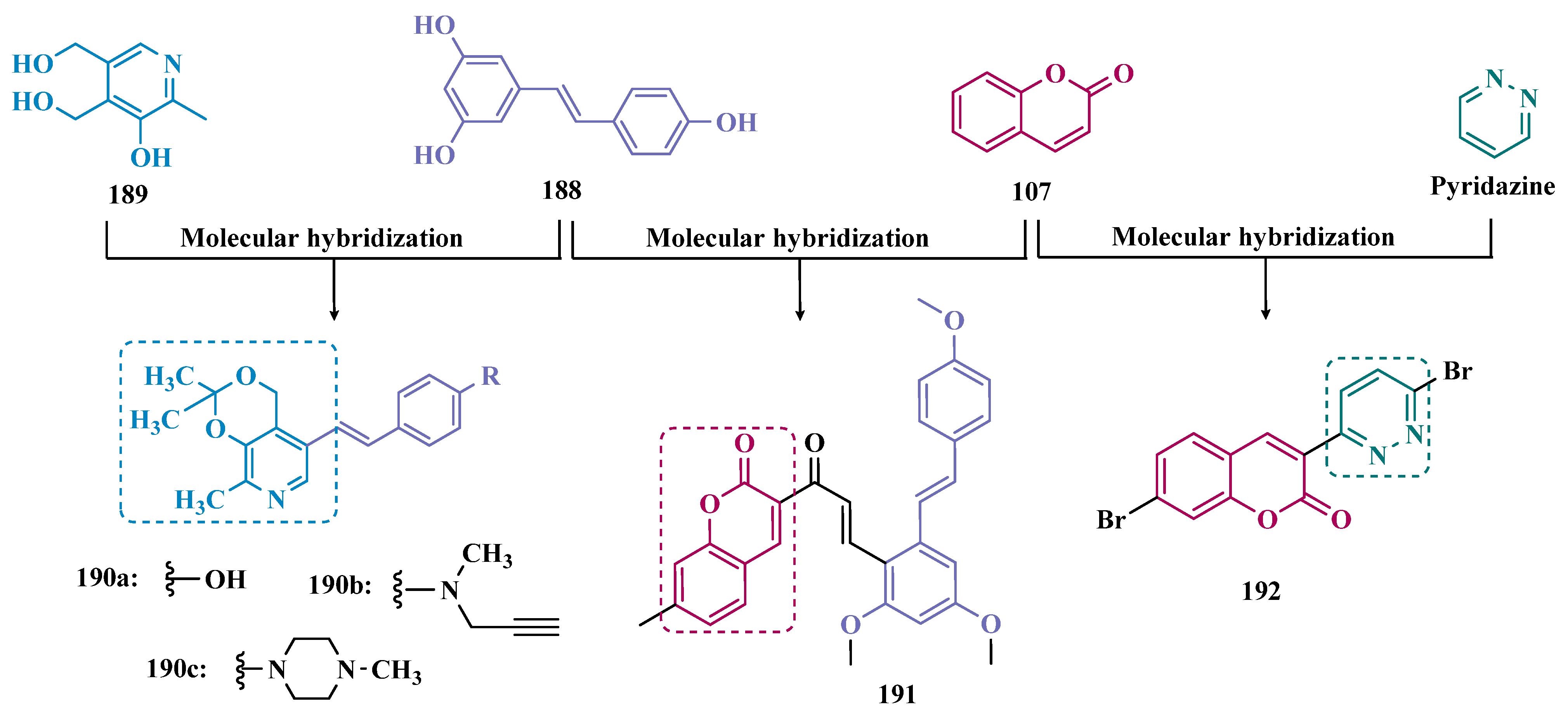
- Li, W.; Yang, X.; Song, Q.; Cao, Z.; Shi, Y.; Deng, Y.; Zhang, L. Pyridoxine-Resveratrol Hybrids as Novel Inhibitors of MAO-B with Antioxidant and Neuroprotective Activities for the Treatment of Parkinson’s Disease. Bioorg. Chem. 2020, 97, 103707. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, F.; Costas-Lago, M.C.; Besada, P.; Alonso-Pena, M.; Torres-Terán, I.; Viña, D.; Fontenla, J.Á.; Sturlese, M.; Moro, S.; Quezada, E.; et al. Novel Coumarin-Pyridazine Hybrids as Selective MAO-B Inhibitors for the Parkinson’s Disease Therapy. Bioorg. Chem. 2020, 104, 104203. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.F.; Cheng, H.J.; Ren, J.; Li, H.L.; Guo, L.L.; Zhang, X.X.; Liao, C. Novel 2H-Chromen-2-One Derivatives of Resveratrol: Design, Synthesis, Modeling and Use as Human Monoamine Oxidase Inhibitors. Eur. J. Med. Chem. 2015, 103, 185–190. [Google Scholar] [CrossRef]
- Matos, M.J.; Herrera Ibatá, D.M.; Uriarte, E.; Viña, D. Coumarin-Rasagiline Hybrids as Potent and Selective HMAO-B Inhibitors, Antioxidants, and Neuroprotective Agents. ChemMedChem 2020, 15, 532–538. [Google Scholar] [CrossRef]
- Moya-Alvarado, G.; Yañez, O.; Morales, N.; González-González, A.; Areche, C.; Núñez, M.T.; Fierro, A.; García-Beltrán, O. Coumarin-Chalcone Hybrids as Inhibitors of MAO-B: Biological Activity and in Silico Studies. Molecules 2021, 26, 2430. [Google Scholar] [CrossRef]
- Kavully, F.S.; Oh, J.M.; Dev, S.; Kaipakasseri, S.; Palakkathondi, A.; Vengamthodi, A.; Abdul Azeez, R.F.; Tondo, A.R.; Nicolotti, O.; Kim, H.; et al. Design of Enamides as New Selective Monoamine Oxidase-B Inhibitors. J. Pharm. Pharmacol. 2020, 72, 916–926. [Google Scholar] [CrossRef]
- Osmaniye, D.; Kurban, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Novel Thiosemicarbazone Derivatives: In Vitro and in Silico Evaluation as Potential Mao-b Inhibitors. Molecules 2021, 26, 6640. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Hu, Y.; Wang, W.; Gao, X. Discovery of Novel Procaine-Imidazole Derivative as Inhibitor of Monoamine Oxidase-B for Potential Benefit in Parkinson’s Disease. ChemistrySelect 2020, 5, 10928–10932. [Google Scholar] [CrossRef]
- El-Halaby, L.O.; Al-Sanea, M.M.; Elgazar, A.A.; Tawfik, S.S.; Hamdi, A.; Ewes, W.A. New Phenylpiperazine-Thiazolidine-2,4-Dione Hybrids Targeting MAO Inhibition: Synthesis, Biological Evaluation, Kinetic Study and in Silico Insights. Bioorg. Med. Chem. 2025, 121, 118123. [Google Scholar] [CrossRef]
- Oh, J.M.; Zenni, Y.N.; Özdemir, Z.; Kumar, S.; Kılıç, S.; Akdağ, M.; Özçelik, A.B.; Kim, H.; Mathew, B. Inhibition of Monoamine Oxidases by Pyridazinobenzylpiperidine Derivatives. Molecules 2024, 29, 3097. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. In Silico Design, Synthesis of Hybrid Combinations: Quercetin Based MAO Inhibitors with Antioxidant Potential. Curr. Top. Med. Chem. 2019, 19, 156–170. [Google Scholar] [CrossRef]
- Cheong, S.L.; Federico, S.; Spalluto, G.; Klotz, K.-N.; Pastorin, G. The Current Status of Pharmacotherapy for the Treatment of Parkinson’s Disease: Transition from Single-Target to Multitarget Therapy. Drug Discov. Today 2019, 24, 1769–1783. [Google Scholar] [CrossRef] [PubMed]
- Katsoulaki, E.-E.; Dimopoulos, D.; Hadjipavlou-Litina, D. Multitarget Compounds Designed for Alzheimer, Parkinson, and Huntington Neurodegeneration Diseases. Pharmaceuticals 2025, 18, 831. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, K.; Hagenow, S.; Affini, A.; Reiner, D.; Stark, H. Rasagiline Derivatives Combined with Histamine H3 Receptor Properties. Bioorg. Med. Chem. Lett. 2019, 29, 126612. [Google Scholar] [CrossRef] [PubMed]
- Łażewska, D.; Olejarz-Maciej, A.; Kaleta, M.; Bajda, M.; Siwek, A.; Karcz, T.; Doroz-Płonka, A.; Cichoń, U.; Kuder, K.; Kieć-Kononowicz, K. 4-Tert-Pentylphenoxyalkyl Derivatives—Histamine H3 Receptor Ligands and Monoamine Oxidase B Inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3596–3600. [Google Scholar] [CrossRef]
- Łażewska, D.; Ligneau, X.; Schwartz, J.-C.; Schunack, W.; Stark, H.; Kieć-Kononowicz, K. Ether Derivatives of 3-Piperidinopropan-1-Ol as Non-Imidazole Histamine H3 Receptor Antagonists. Bioorg. Med. Chem. 2006, 14, 3522–3529. [Google Scholar] [CrossRef]
- Eissa, N.; Khan, N.; Ojha, S.K.; Lazewska, D.; Kiec-Kononowicz, K.; Sadek, B. The Histamine H3 Receptor Antagonist DL77 Ameliorates MK801-Induced Memory Deficits in Rats. Front. Neurosci. 2018, 12, 42. [Google Scholar] [CrossRef]
- Sadek, B.; Saad, A.; Subramanian, D.; Shafiullah, M.; Łażewska, D.; Kieć-Kononowiczc, K. Anticonvulsant and Procognitive Properties of the Non-Imidazole Histamine H3 Receptor Antagonist DL77 in Male Adult Rats. Neuropharmacology 2016, 106, 46–55. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Rai, G.K.; Ayyannan, S.R. Exploration of a Library of 3,4-(Methylenedioxy)Aniline-Derived Semicarbazones as Dual Inhibitors of Monoamine Oxidase and Acetylcholinesterase: Design, Synthesis, and Evaluation. ChemMedChem 2016, 11, 1145–1160. [Google Scholar] [CrossRef]
- Carradori, S.; Ortuso, F.; Petzer, A.; Bagetta, D.; De Monte, C.; Secci, D.; De Vita, D.; Guglielmi, P.; Zengin, G.; Aktumsek, A.; et al. Design, Synthesis and Biochemical Evaluation of Novel Multi-Target Inhibitors as Potential Anti-Parkinson Agents. Eur. J. Med. Chem. 2018, 143, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, M.S.; Pavankumar, V.; Kumar, S.; Raja, A.S. Experimental and Computational Evaluation of Piperonylic Acid Derived Hydrazones Bearing Isatin Moieties as Dual Inhibitors of Cholinesterases and Monoamine Oxidases. ChemMedChem 2019, 14, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Rullo, M.; Maramai, S.; de Candia, M.; Pisani, L.; Catto, M.; Mugnaini, C.; Brizzi, A.; Cappelli, A.; Olivucci, M.; et al. Design, Synthesis and Biological Evaluation of Light-Driven on–off Multitarget AChE and MAO-B Inhibitors. RSC Med. Chem. 2022, 13, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Poggialini, F.; Governa, P.; Vagaggini, C.; Maramai, S.; Lamponi, S.; Mugnaini, C.; Brizzi, A.; Purgatorio, R.; de Candia, M.; Catto, M.; et al. Light-Mediated Activation/Deactivation Control and in Vitro ADME-Tox Profiling of a Donepezil-like Dual AChE/MAO-B Inhibitor. Eur. J. Pharm. Sci. 2025, 209, 107066. [Google Scholar] [CrossRef]
- Mathew, G.E.; Oh, J.M.; Mohan, K.; Tengli, A.; Mathew, B.; Kim, H. Development of Methylthiosemicarbazones as New Reversible Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease. J. Biomol. Struct. Dyn. 2021, 39, 4786–4794. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Weinreb, O.; Amit, T.; Mandel, S.; Bar-Am, O.; Youdim, M.B.H. Novel Molecular Targets of the Neuroprotective/Neurorescue Multimodal Iron Chelating Drug M30 in the Mouse Brain. Neuroscience 2011, 189, 345–358. [Google Scholar] [CrossRef]
- Avramovich-Tirosh, Y.; Amit, T.; Bar-Am, O.; Zheng, H.; Fridkin, M.; Youdim, M.B.H. Therapeutic Targets and Potential of the Novel Brain-Permeable Multifunctional Iron Chelator–Monoamine Oxidase Inhibitor Drug, M-30, for the Treatment of Alzheimer’s Disease 1. J. Neurochem. 2007, 100, 490–502. [Google Scholar] [CrossRef]
- Youdim, M.B.H. M30, a Brain Permeable Multi Target Neurorestorative Drug in Post Nigrostriatal Dopamine Neuron Lesion of Parkinsonism Animal Models. Park. Relat. Disord. 2012, 18, S151–S154. [Google Scholar] [CrossRef]
- Gal, S.; Zheng, H.; Fridkin, M.; Youdim, M.B.H. Restoration of Nigrostriatal Dopamine Neurons in Post-MPTP Treatment by the Novel Multifunctional Brain-Permeable Iron Chelator-Monoamine Oxidase Inhibitor Drug, M30. Neurotox. Res. 2010, 17, 15–27. [Google Scholar] [CrossRef]
- Zheng, H.; Weiner, L.M.; Bar-Am, O.; Epsztejn, S.; Cabantchik, Z.I.; Warshawsky, A.; Youdim, M.B.H.; Fridkin, M. Design, Synthesis, and Evaluation of Novel Bifunctional Iron-Chelators as Potential Agents for Neuroprotection in Alzheimer’s, Parkinson’s, and Other Neurodegenerative Diseases. Bioorg. Med. Chem. 2005, 13, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gal, S.; Weiner, L.M.; Bar-Am, O.; Warshawsky, A.; Fridkin, M.; Youdim, M.B.H. Novel Multifunctional Neuroprotective Iron Chelator-monoamine Oxidase Inhibitor Drugs for Neurodegenerative Diseases: In Vitro Studies on Antioxidant Activity, Prevention of Lipid Peroxide Formation and Monoamine Oxidase Inhibition. J. Neurochem. 2005, 95, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Youdim, M.B.H.; Fridkin, M. Site-Activated Chelators Targeting Acetylcholinesterase and Monoamine Oxidase for Alzheimer’s Therapy. ACS Chem. Biol. 2010, 5, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Košak, U.; Knez, D.; Pišlar, A.; Horvat, S.; Žakelj, S.; Igert, A.; Dias, J.; Nachon, F.; Brazzolotto, X.; Gobec, S. N-Propargylpyrrolidine-Based Butyrylcholinesterase and Monoamine Oxidase Inhibitors. Chem. Biol. Interact. 2025, 420, 111681. [Google Scholar] [CrossRef]
- Van Der Walt, M.M.; Terre’Blanche, G.; Petzer, A.; Petzer, J.P. The Adenosine Receptor Affinities and Monoamine Oxidase B Inhibitory Properties of Sulfanylphthalimide Analogues. Bioorg. Chem. 2015, 59, 117–123. [Google Scholar] [CrossRef]
- Kuder, K.J.; Załuski, M.; Schabikowski, J.; Latacz, G.; Olejarz-Maciej, A.; Jaśko, P.; Doroz-Płonka, A.; Brockmann, A.; Müller, C.E.; Kieć-Kononowicz, K. Novel, Dual Target-Directed Annelated Xanthine Derivatives Acting on Adenosine Receptors and Monoamine Oxidase B. ChemMedChem 2020, 15, 772–786. [Google Scholar] [CrossRef]
- Brunschweiger, A.; Koch, P.; Schlenk, M.; Pineda, F.; Küppers, P.; Hinz, S.; Köse, M.; Ullrich, S.; Hockemeyer, J.; Wiese, M.; et al. 8-Benzyltetrahydropyrazino[2,1-f]Purinediones: Water-Soluble Tricyclic Xanthine Derivatives as Multitarget Drugs for Neurodegenerative Diseases. ChemMedChem 2014, 9, 1704–1724. [Google Scholar] [CrossRef]
- Koch, P.; Akkari, R.; Brunschweiger, A.; Borrmann, T.; Schlenk, M.; Küppers, P.; Köse, M.; Radjainia, H.; Hockemeyer, J.; Drabczyńska, A.; et al. 1,3-Dialkyl-Substituted Tetrahydropyrimido[1,2-f]Purine-2,4-Diones as Multiple Target Drugs for the Potential Treatment of Neurodegenerative Diseases. Bioorg. Med. Chem. 2013, 21, 7435–7452. [Google Scholar] [CrossRef]
- Załuski, M.; Schabikowski, J.; Schlenk, M.; Olejarz-Maciej, A.; Kubas, B.; Karcz, T.; Kuder, K.; Latacz, G.; Zygmunt, M.; Synak, D.; et al. Novel Multi-Target Directed Ligands Based on Annelated Xanthine Scaffold with Aromatic Substituents Acting on Adenosine Receptor and Monoamine Oxidase B. Synthesis, In Vitro and In Silico Studies. Bioorg. Med. Chem. 2019, 27, 1195–1210. [Google Scholar] [CrossRef]
- Drabczyńska, A.; Schumacher, B.; Müller, C.E.; Karolak-Wojciechowska, J.; Michalak, B.; Pȩkala, E.; Kieć-Kononowicz, K. Impact of the Aryl Substituent Kind and Distance from Pyrimido[2,1-f]Purindiones on the Adenosine Receptor Selectivity and Antagonistic Properties. Eur. J. Med. Chem. 2003, 38, 397–402. [Google Scholar] [CrossRef]
- Drabczyńska, A.; Müller, C.E.; Lacher, S.K.; Schumacher, B.; Karolak-Wojciechowska, J.; Nasal, A.; Kawczak, P.; Yuzlenko, O.; Pekala, E.; Kieć-Kononowicz, K. Synthesis and Biological Activity of Tricyclic Aryloimidazo-, Pyrimido-, and Diazepinopurinediones. Bioorg. Med. Chem. 2006, 14, 7258–7281. [Google Scholar] [CrossRef]
- Drabczyńska, A.; Müller, C.E.; Schiedel, A.; Schumacher, B.; Karolak-Wojciechowska, J.; Fruziński, A.; Zobnina, W.; Yuzlenko, O.; Kieć-Kononowicz, K. Phenylethyl-Substituted Pyrimido[2,1-f]Purinediones and Related Compounds: Structure-Activity Relationships as Adenosine A1 and A2A Receptor Ligands. Bioorg. Med. Chem. 2007, 15, 6956–6974. [Google Scholar] [CrossRef]
- Drabczyńska, A.; Yuzlenko, O.; Köse, M.; Paskaleva, M.; Schiedel, A.C.; Karolak-Wojciechowska, J.; Handzlik, J.; Karcz, T.; Kuder, K.; Müller, C.E.; et al. Synthesis and Biological Activity of Tricyclic Cycloalkylimidazo-, Pyrimido- and Diazepinopurinediones. Eur. J. Med. Chem. 2011, 46, 3590–3607. [Google Scholar] [CrossRef]
- Koch, P.; Brunschweiger, A.; Namasivayam, V.; Ullrich, S.; Maruca, A.; Lazzaretto, B.; Küppers, P.; Hinz, S.; Hockemeyer, J.; Wiese, M.; et al. Probing Substituents in the 1- and 3-Position: Tetrahydropyrazino-Annelated Water-Soluble Xanthine Derivatives as Multi-Target Drugs with Potent Adenosine Receptor Antagonistic Activity. Front. Chem. 2018, 6, 206. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Xu, Y.; Wu, K.; Chen, S.; Hu, M.; Wang, L.; Ye, Y.; Ye, F. Synthesis and Evaluation of Phenylxanthine Derivatives as Potential Dual A2AR Antagonists/MAO-B Inhibitors for Parkinson’s Disease. Molecules 2017, 22, 1010. [Google Scholar] [CrossRef] [PubMed]
- Rivara, S.; Piersanti, G.; Bartoccini, F.; Diamantini, G.; Pala, D.; Riccioni, T.; Stasi, M.A.; Cabri, W.; Borsini, F.; Mor, M.; et al. Synthesis of (E)-8-(3-Chlorostyryl)Caffeine Analogues Leading to 9-Deazaxanthine Derivatives as Dual A2A Antagonists/MAO-B Inhibitors. J. Med. Chem. 2013, 56, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Stößel, A.; Schlenk, M.; Hinz, S.; Küppers, P.; Heer, J.; Gütschow, M.; Müller, C.E. Dual Targeting of Adenosine A2A Receptors and Monoamine Oxidase B by 4H-3,1-Benzothiazin-4-Ones. J. Med. Chem. 2013, 56, 4580–4596. [Google Scholar] [CrossRef]
- Engelbrecht, I.; Petzer, J.P.; Petzer, A. Nitrocatechol Derivatives of Chalcone as Inhibitors of Monoamine Oxidase and Catechol-O-Methyltransferase. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wu, Y.; Lan, Y.; Xie, H.; Sun, H.; Liu, W.; Feng, F.; Jiang, X. Identification and Optimization of Nitrophenolic Analogues as Dopamine Metabolic Enzyme Inhibitors for the Treatment of Parkinson’s Disease. Bioorg. Chem. 2024, 148, 107488. [Google Scholar] [CrossRef]
- de Beer, A.D.; Legoabe, L.J.; Petzer, A.; Petzer, J.P. The Inhibition of Catechol O-Methyltransferase and Monoamine Oxidase by Tetralone and Indanone Derivatives Substituted with the Nitrocatechol Moiety. Bioorg. Chem. 2021, 114, 105130. [Google Scholar] [CrossRef]
- Chavarria, D.; Benfeito, S.; Soares, P.; Lima, C.; Garrido, J.; Serrão, P.; Soares-da-Silva, P.; Remião, F.; Oliveira, P.J.; Borges, F. Boosting Caffeic Acid Performance as Antioxidant and Monoamine Oxidase B/Catechol-O-Methyltransferase Inhibitor. Eur. J. Med. Chem. 2022, 243, 114740. [Google Scholar] [CrossRef]
- Tavari, M.; Malan, S.F.; Joubert, J. Design, Synthesis, Biological Evaluation and Docking Studies of Sulfonyl Isatin Derivatives as Monoamine Oxidase and Caspase-3 Inhibitors. Medchemcomm 2016, 7, 1628–1639. [Google Scholar] [CrossRef]
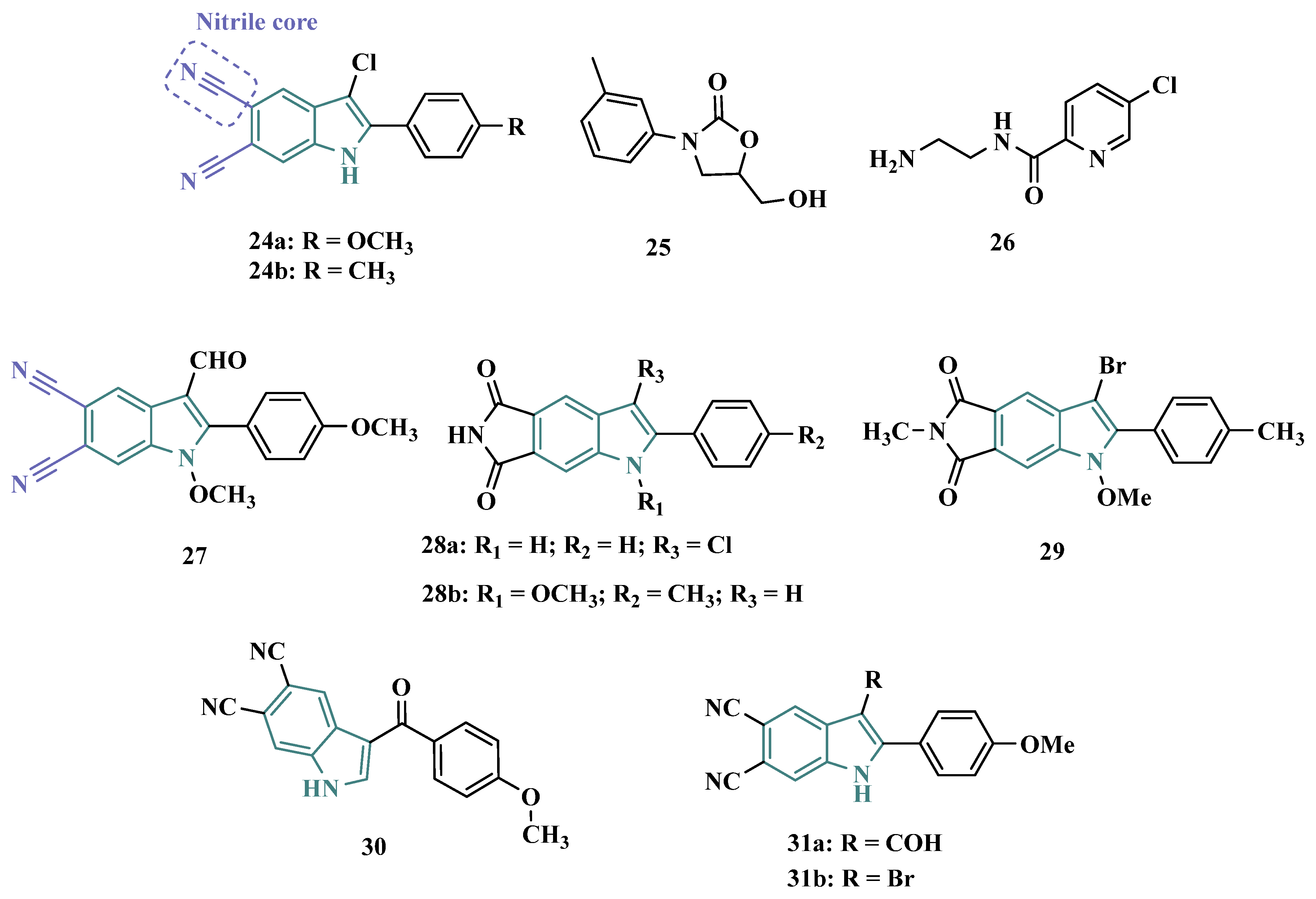
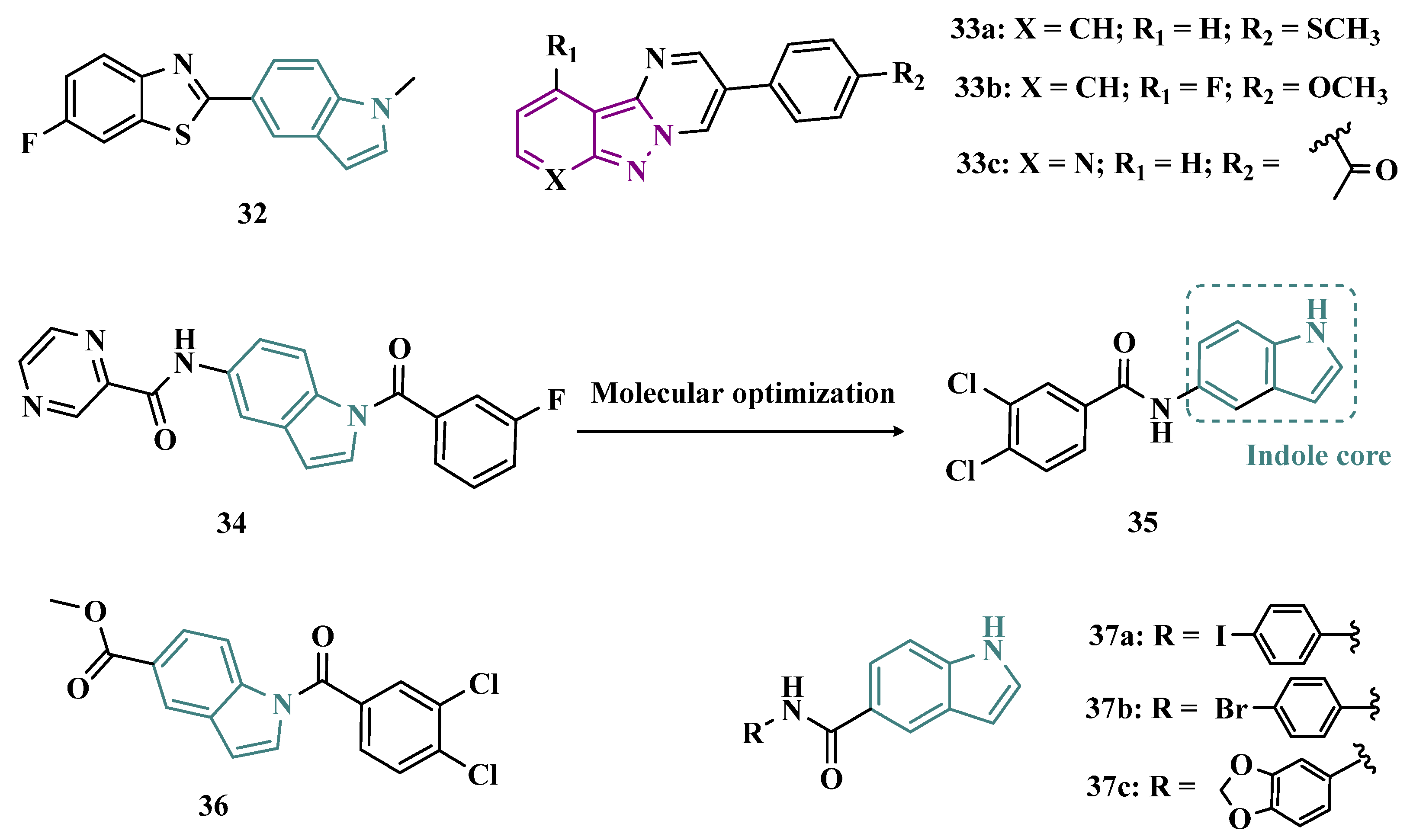
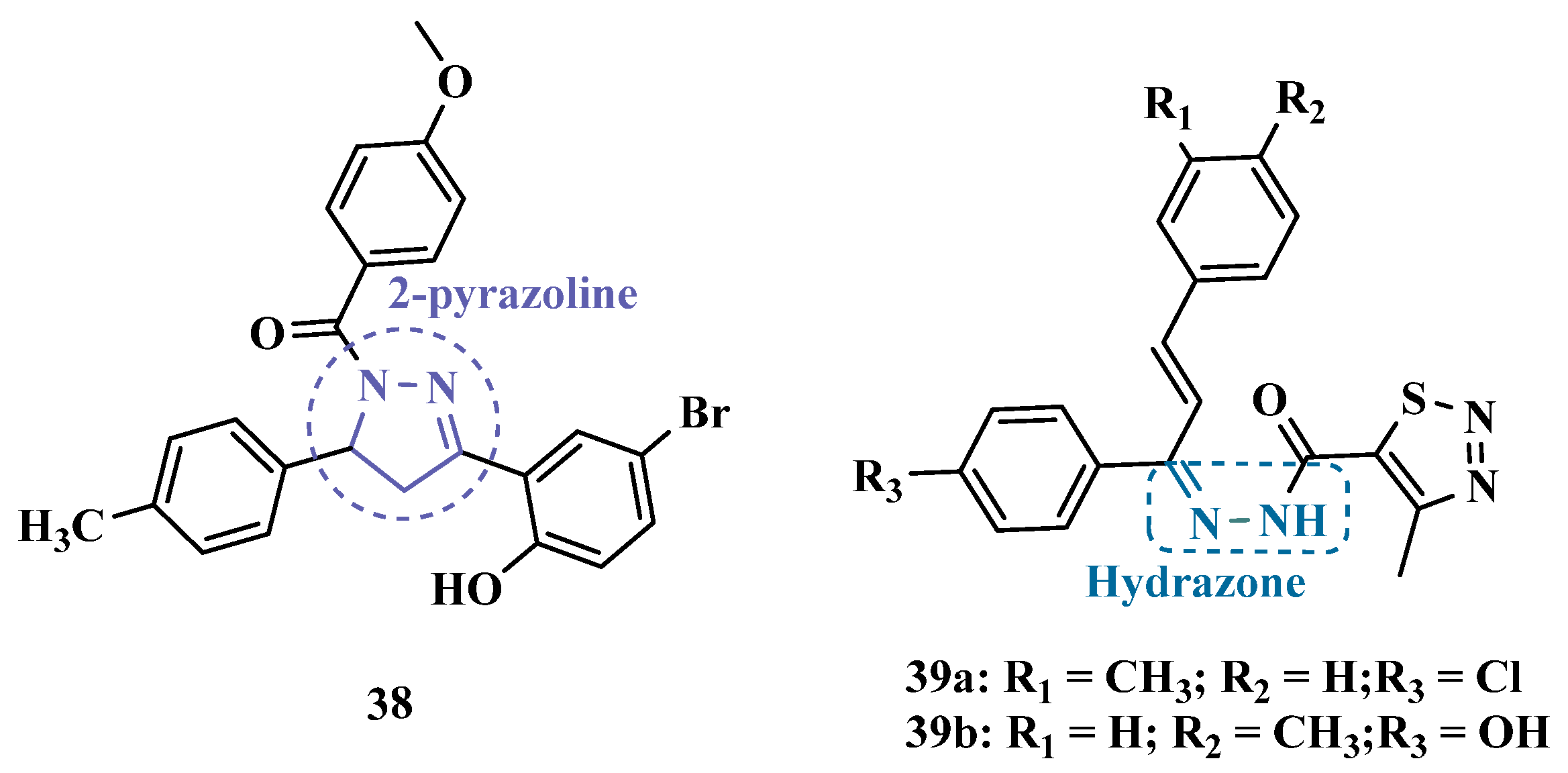
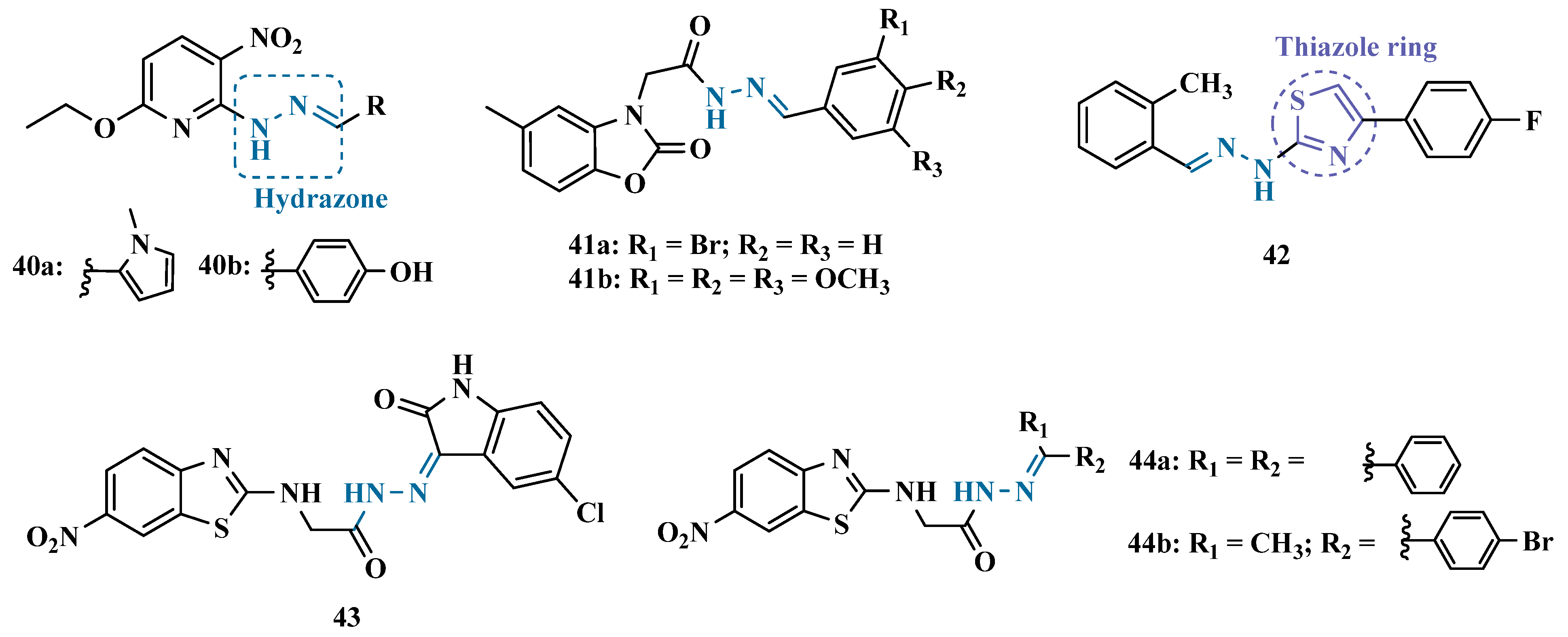

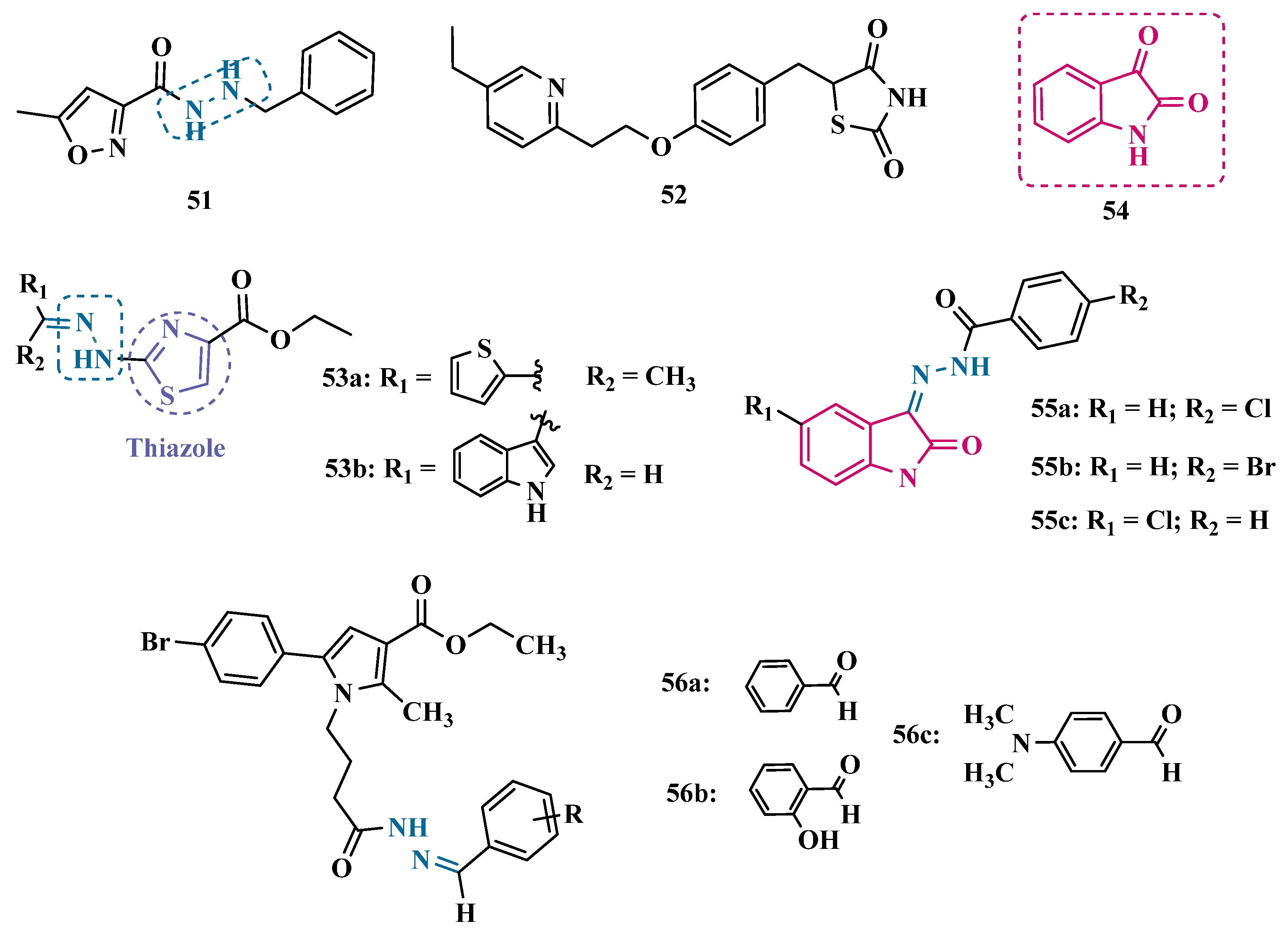
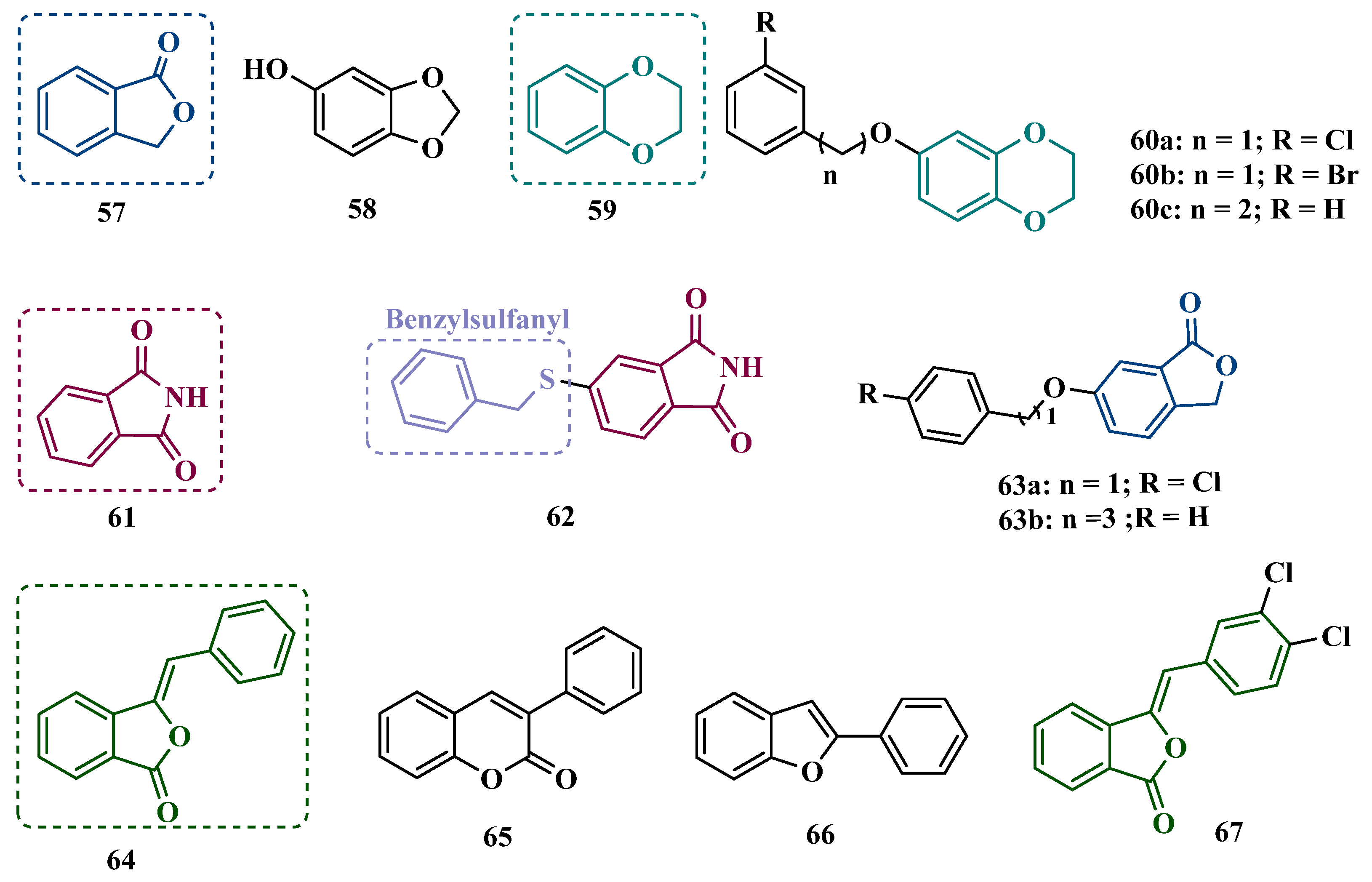
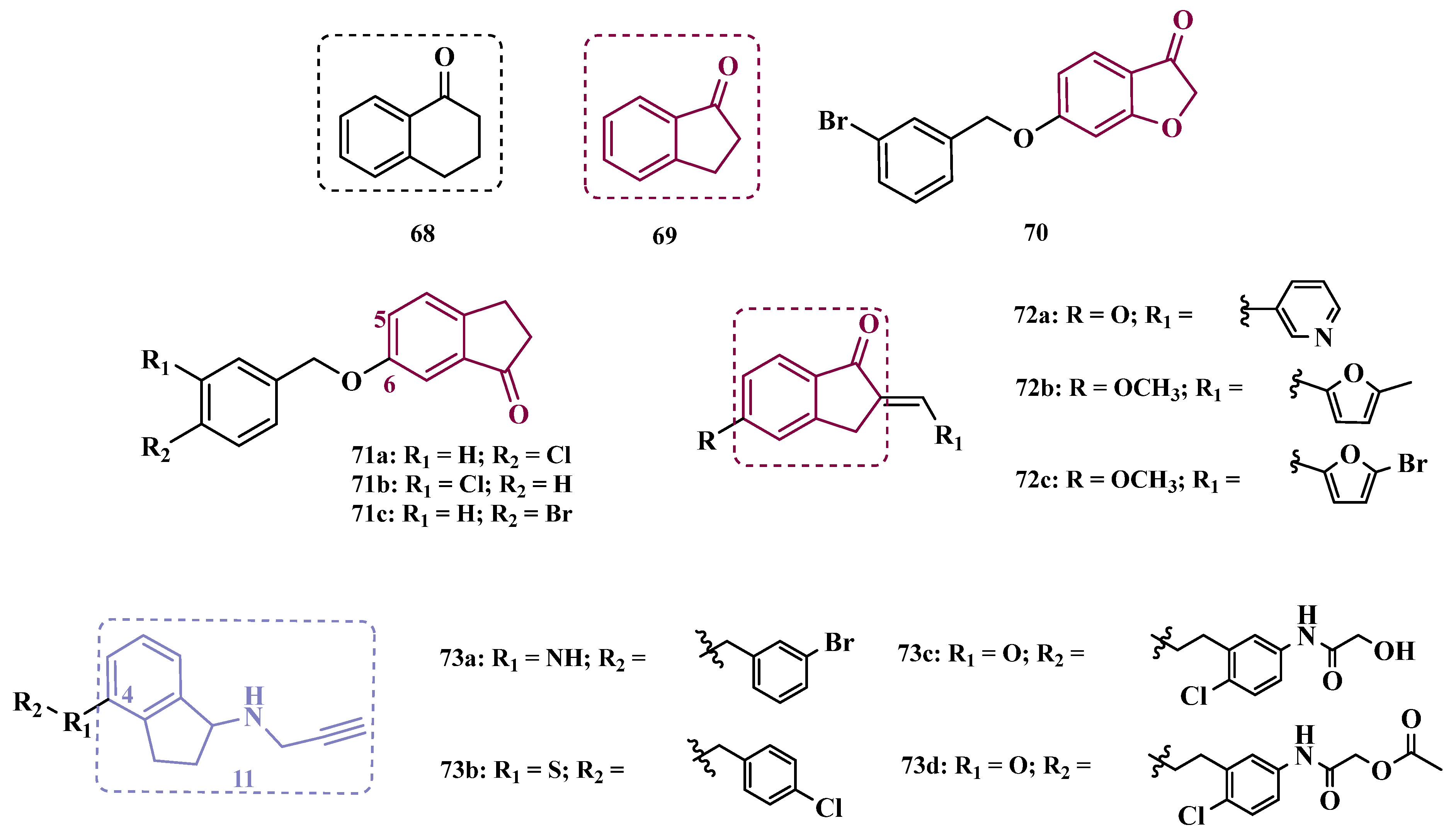
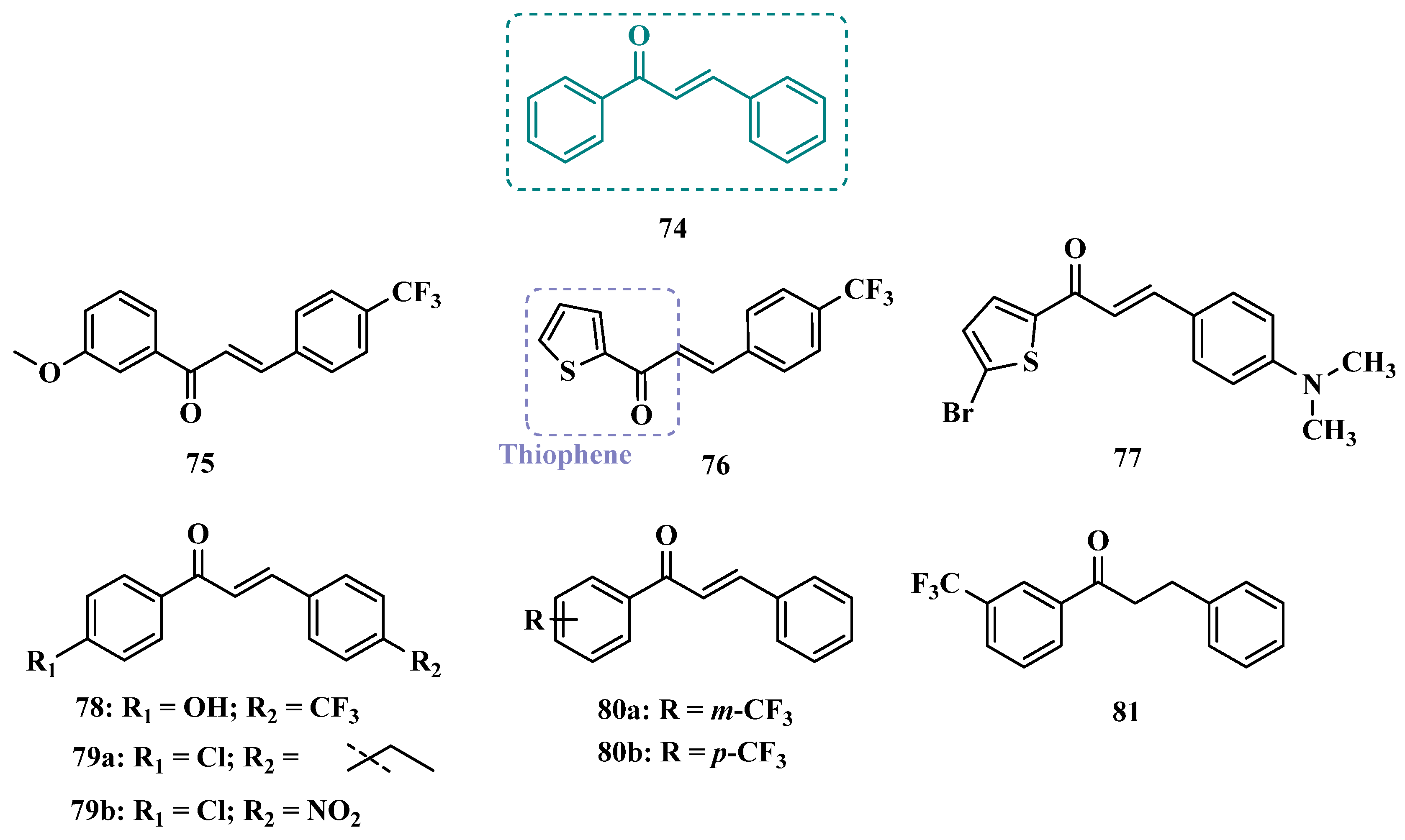
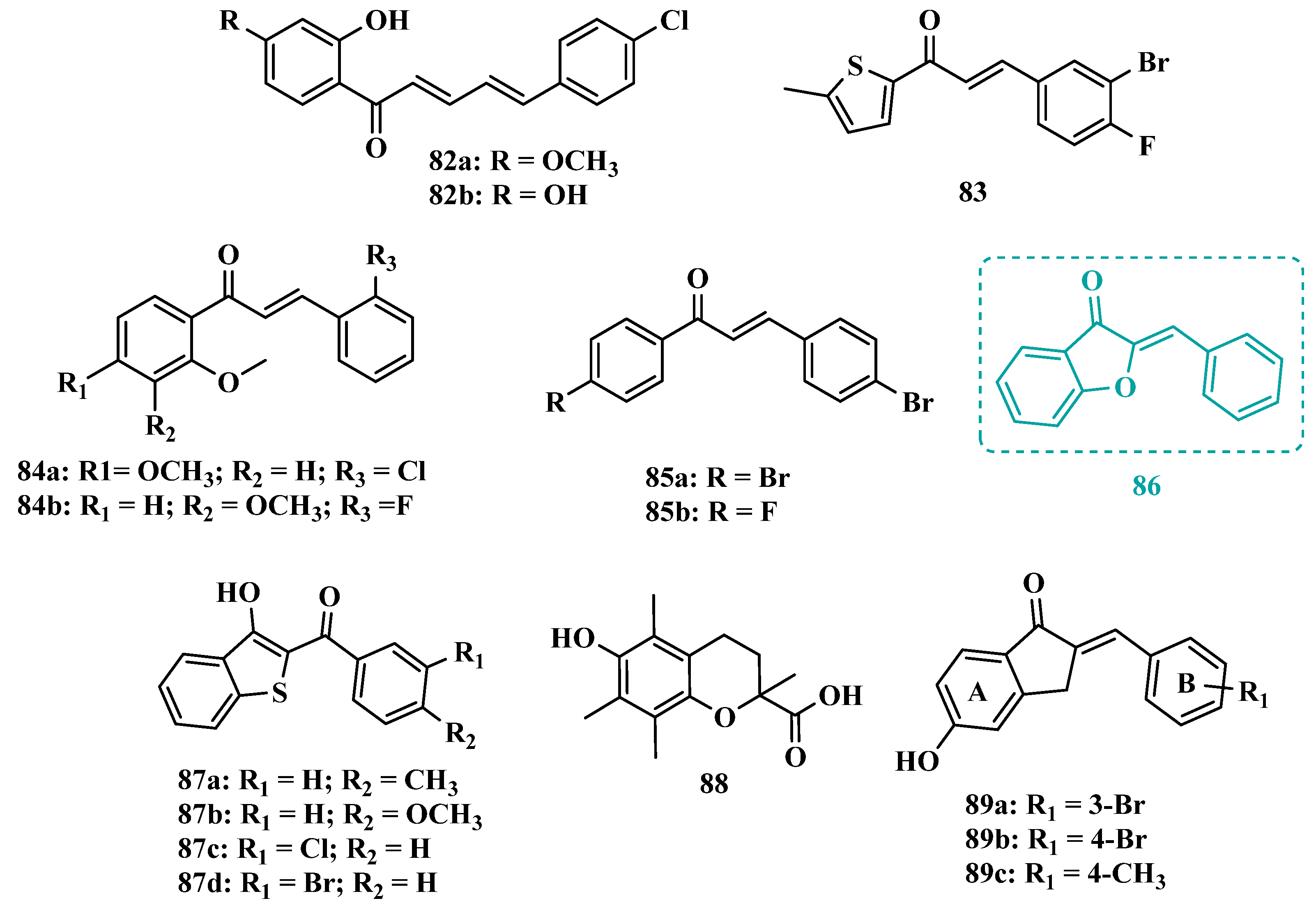
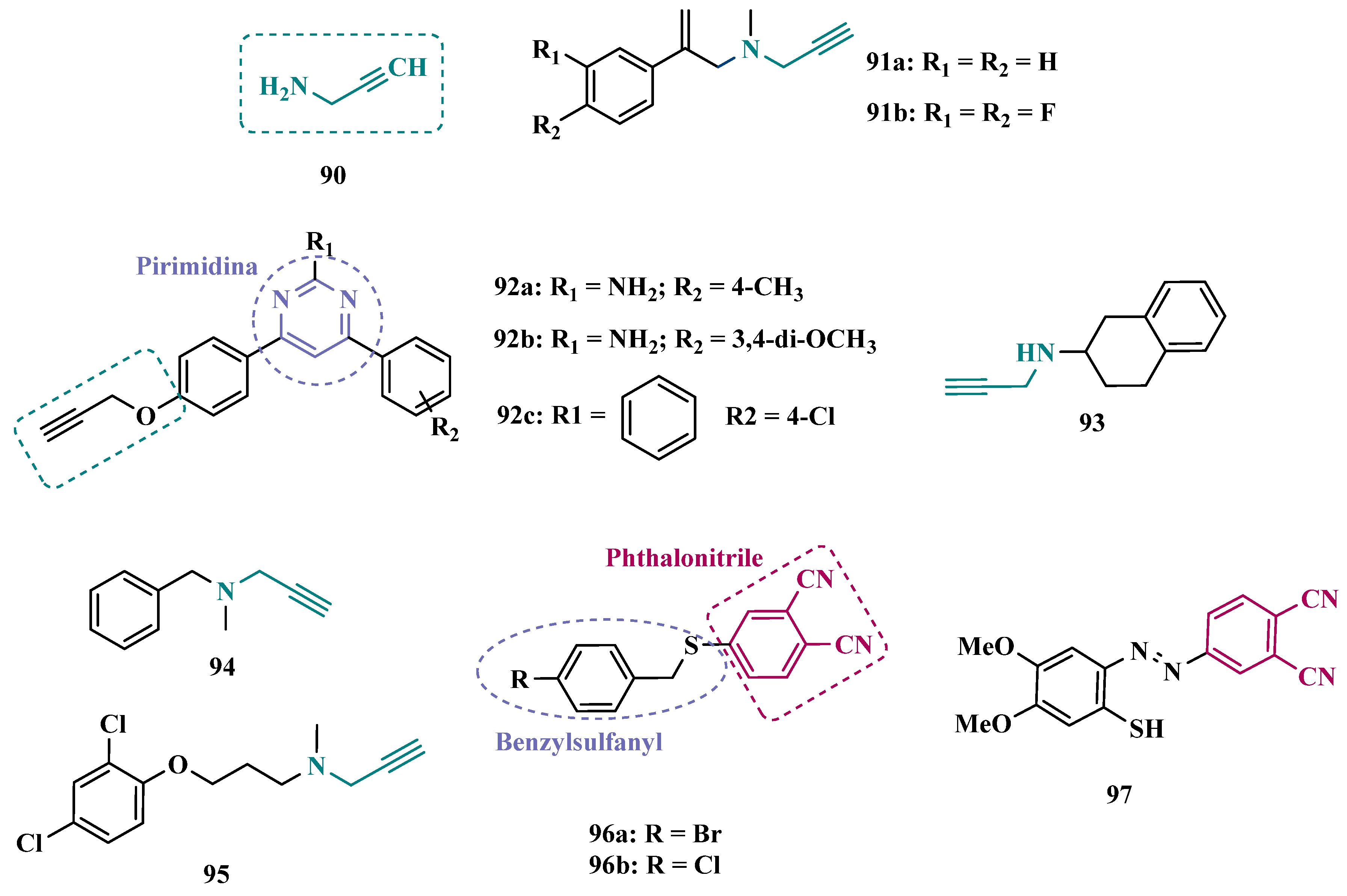

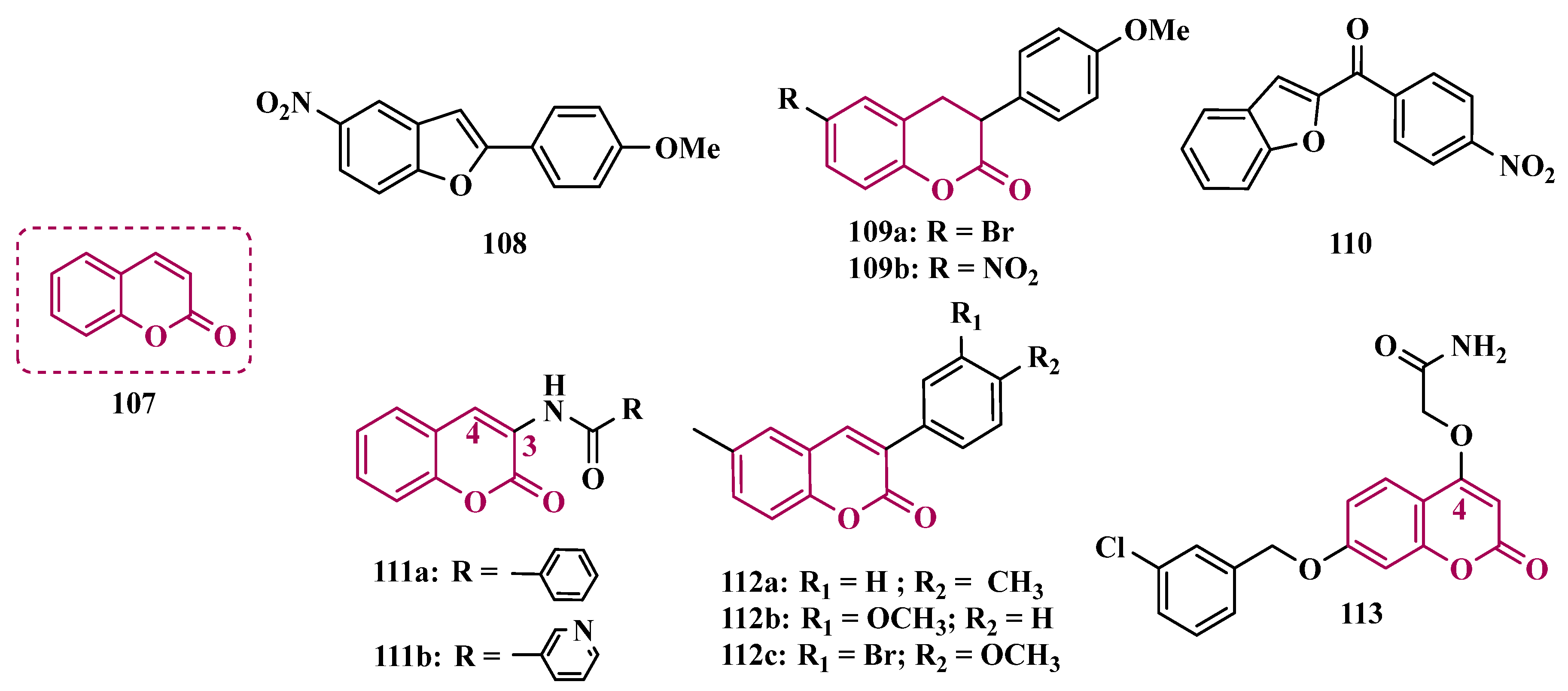
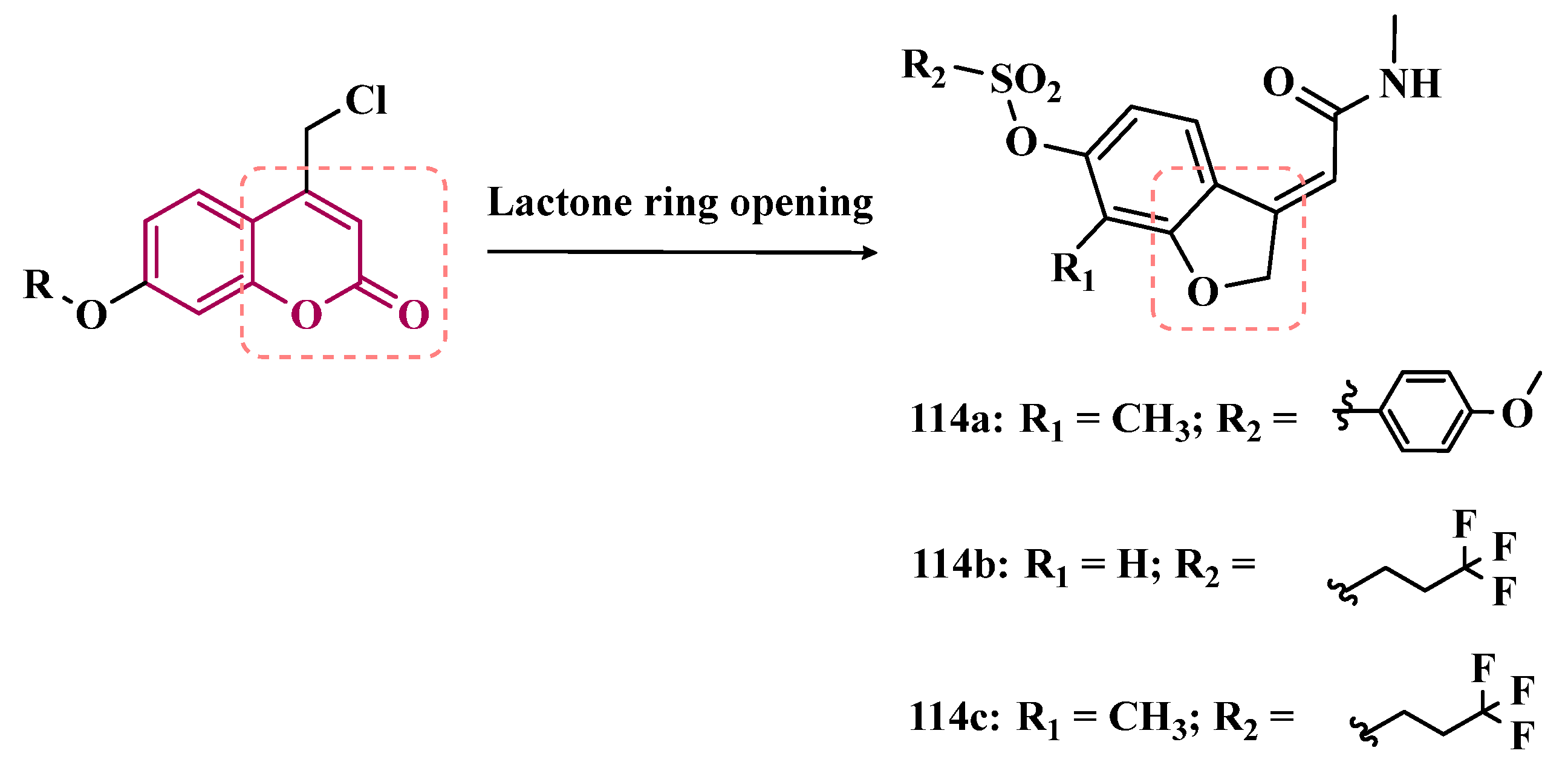
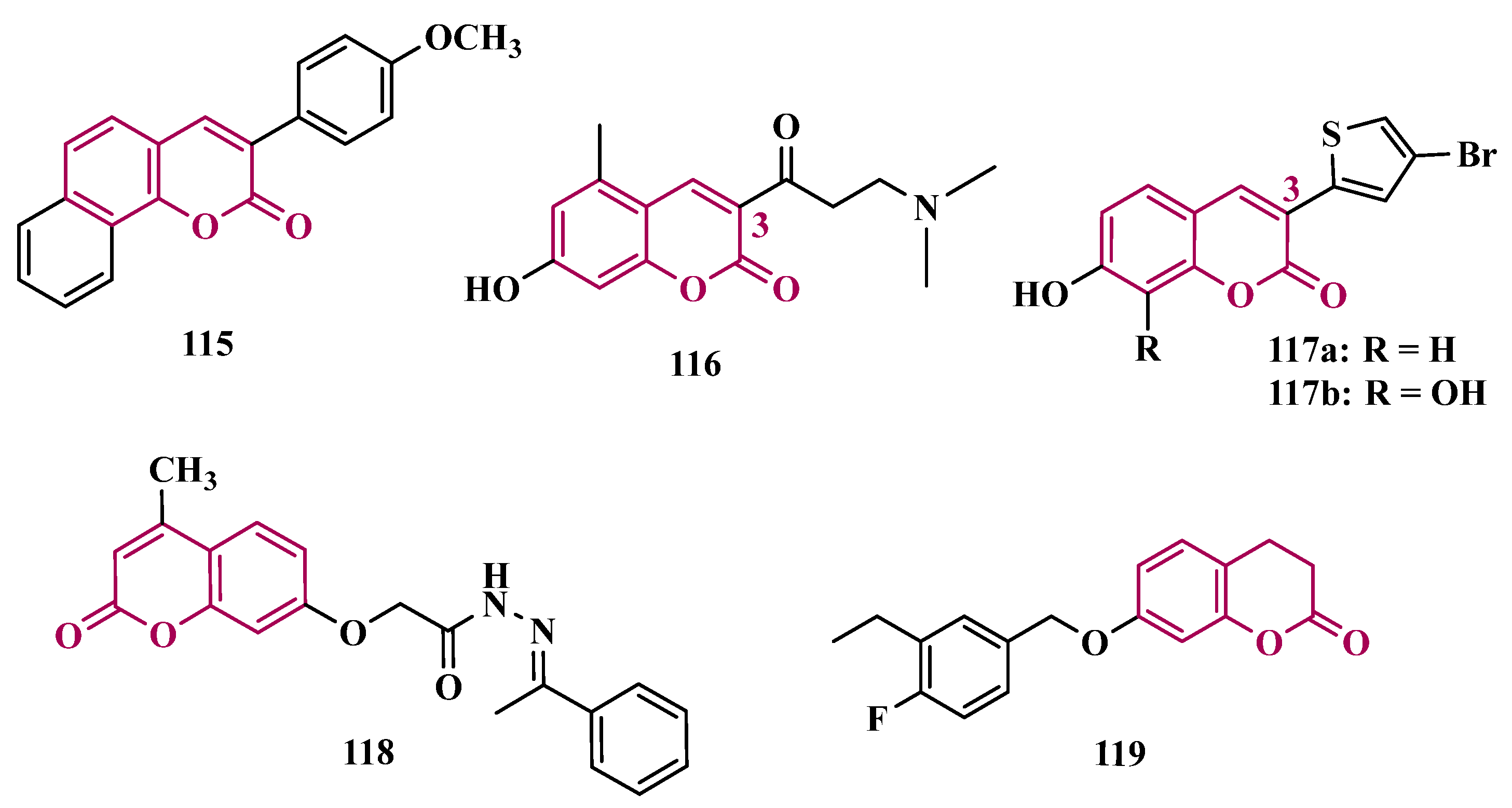
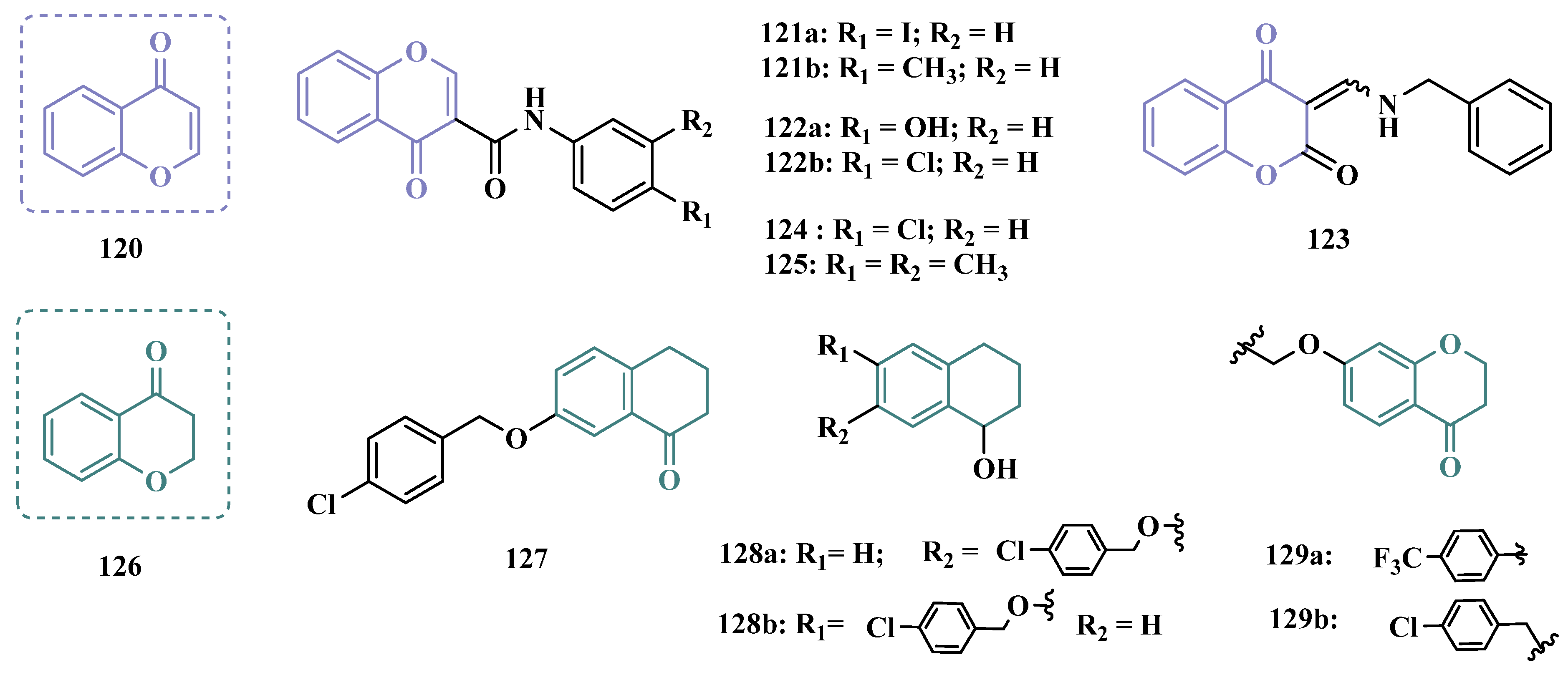
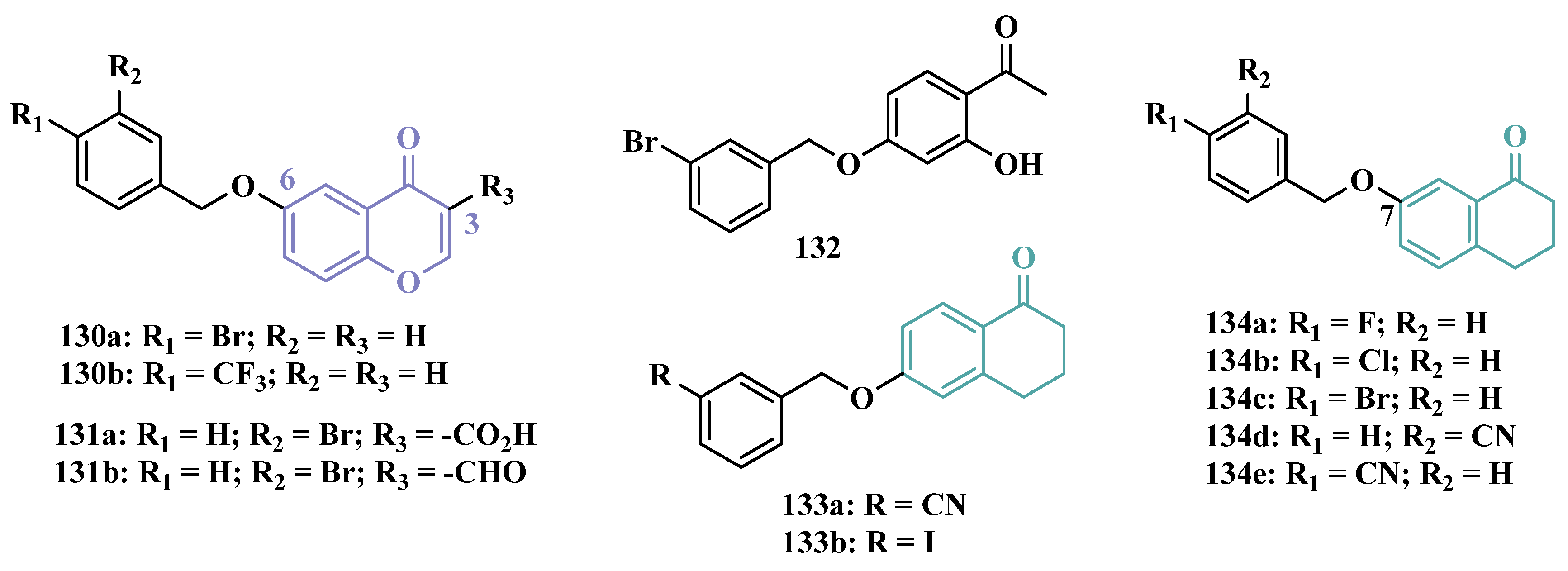
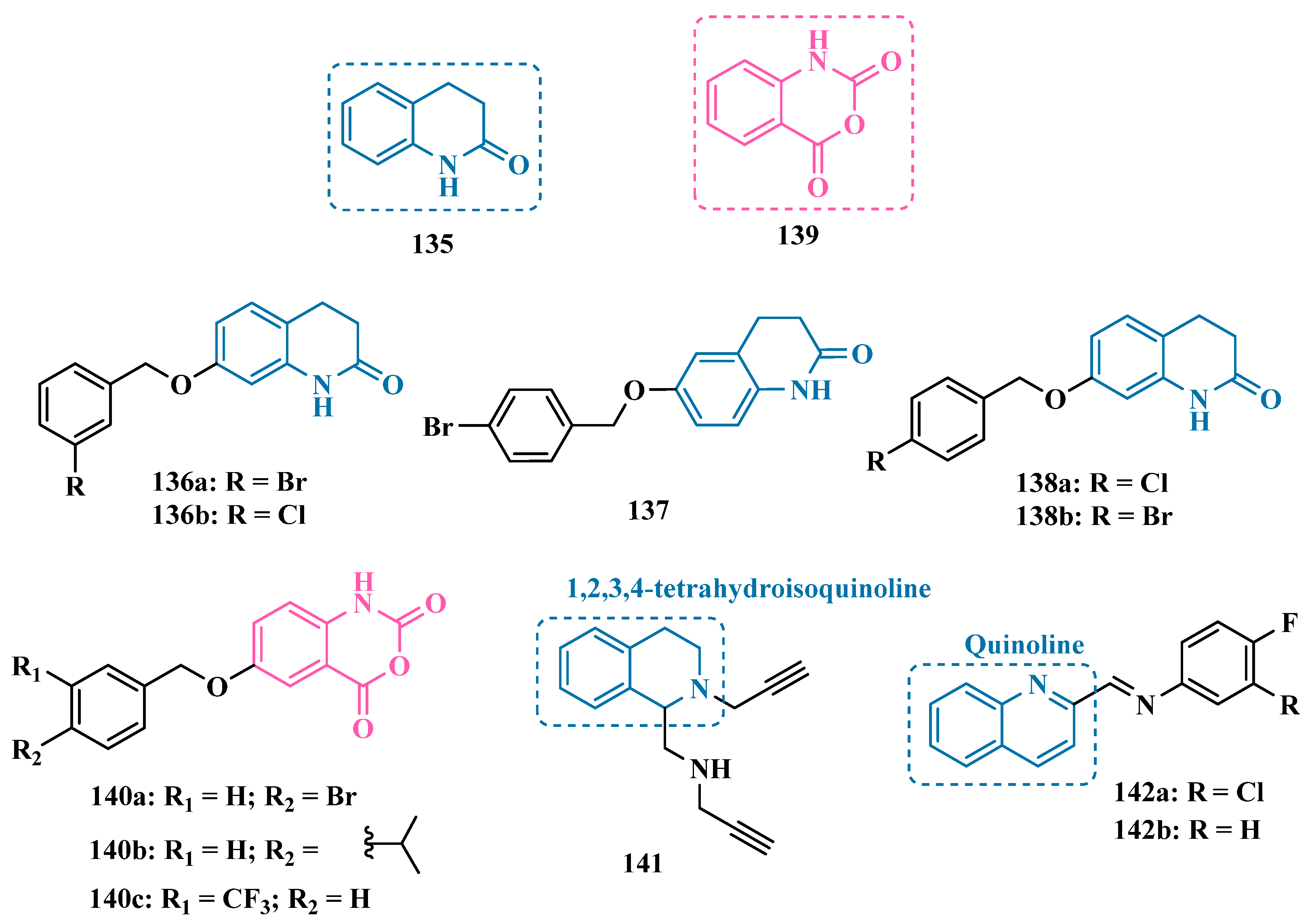
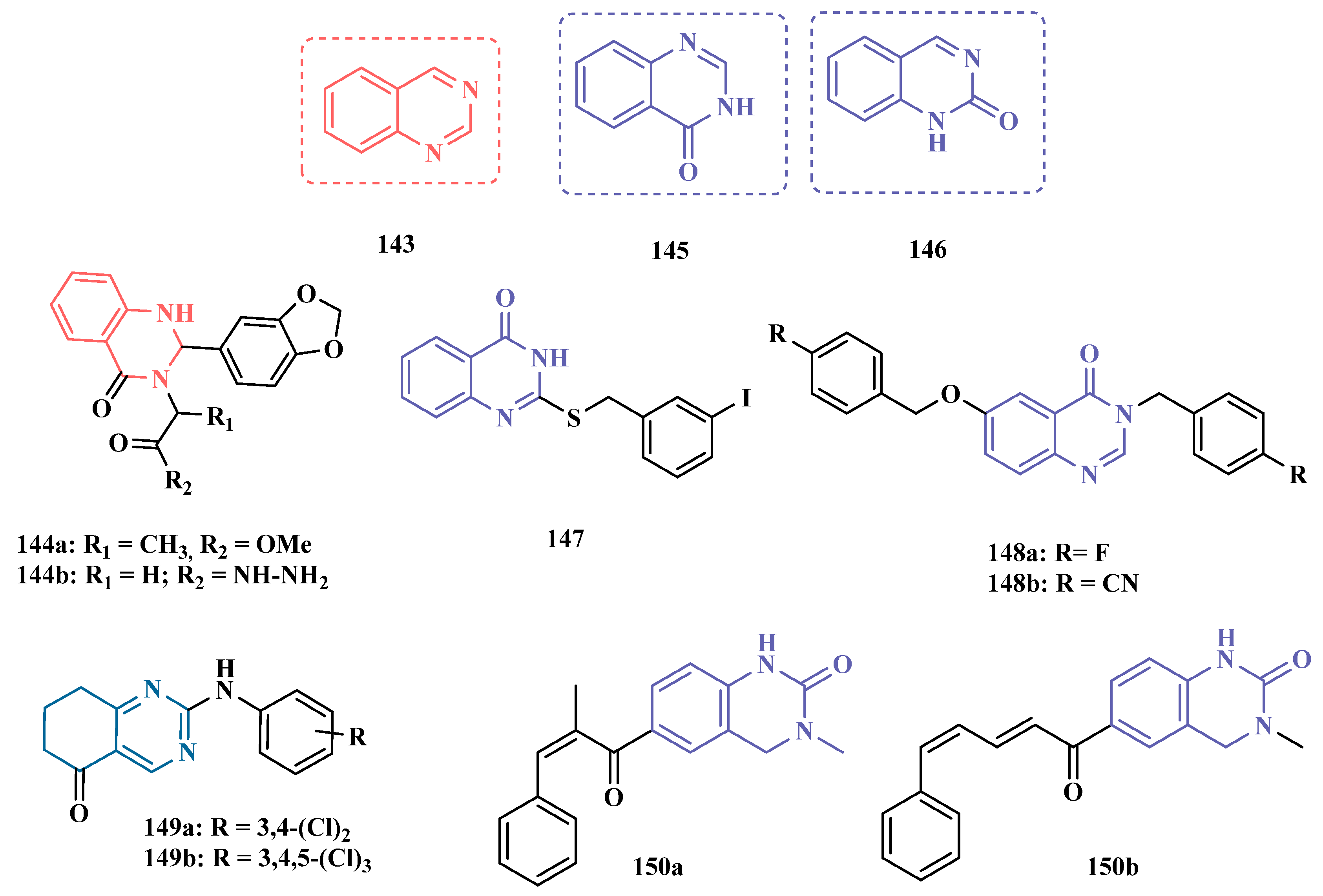
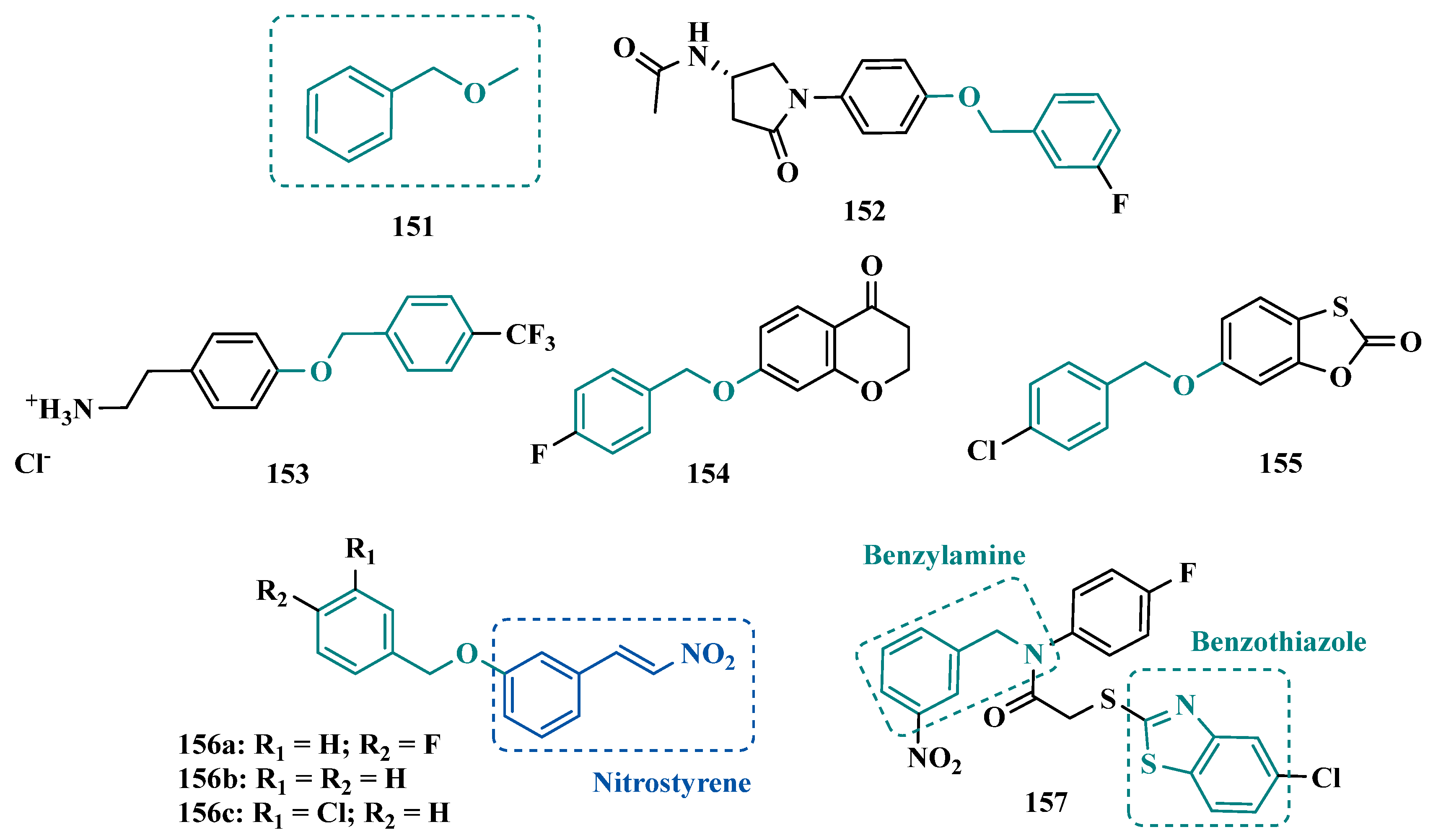


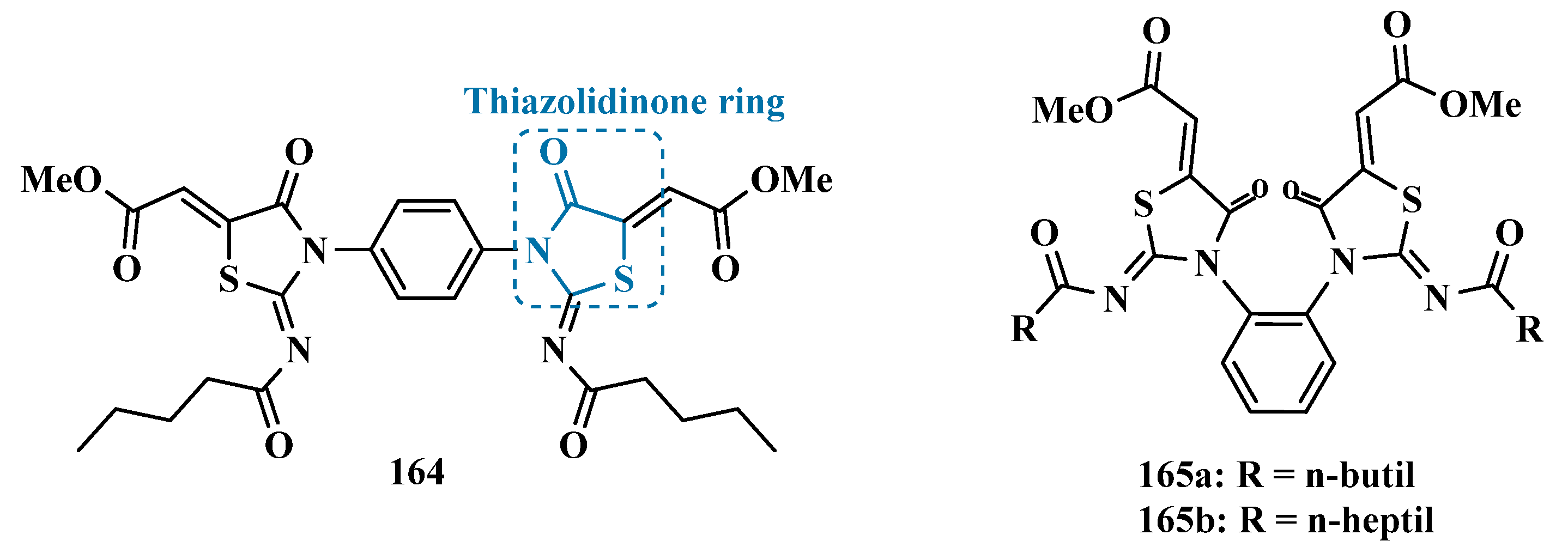
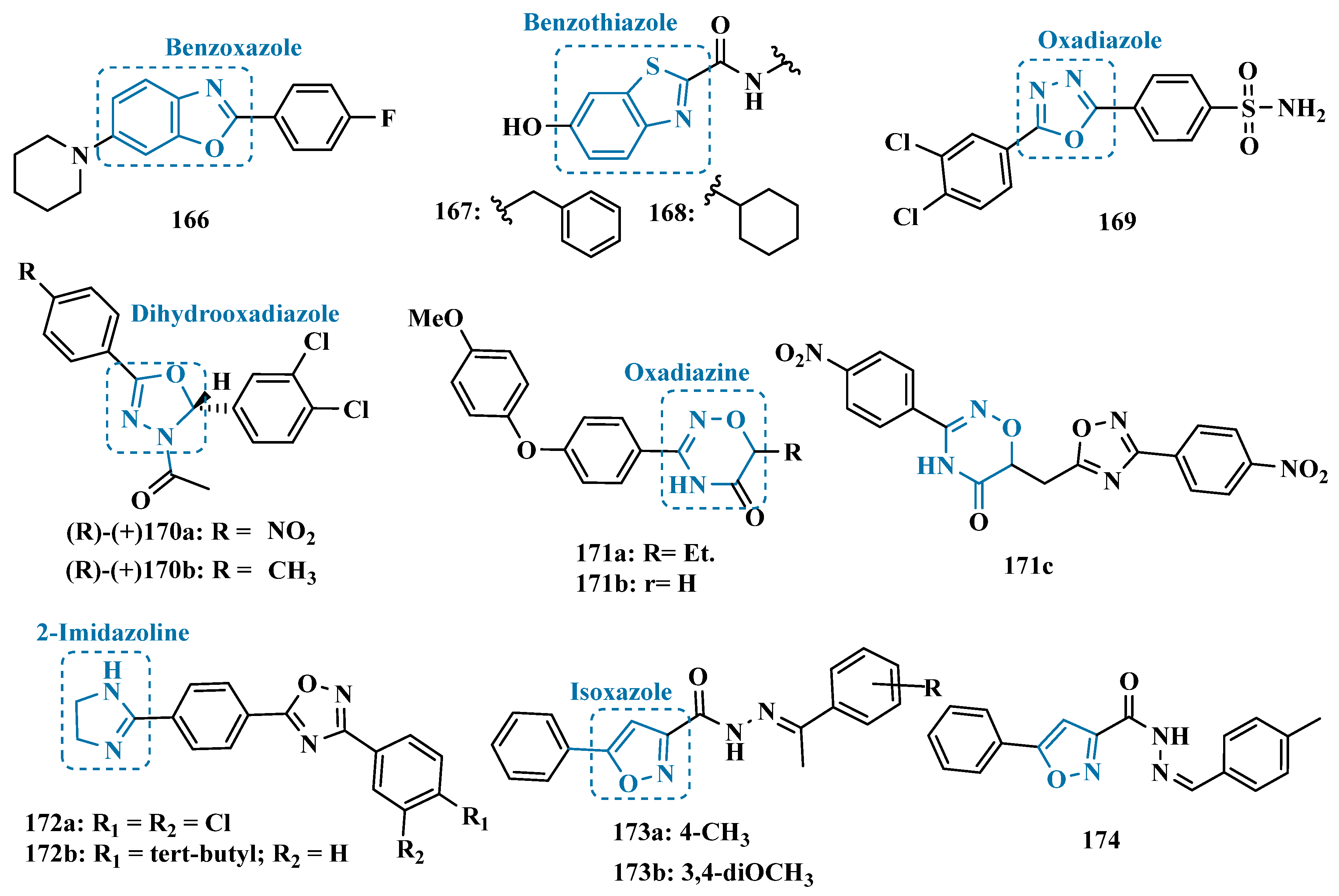
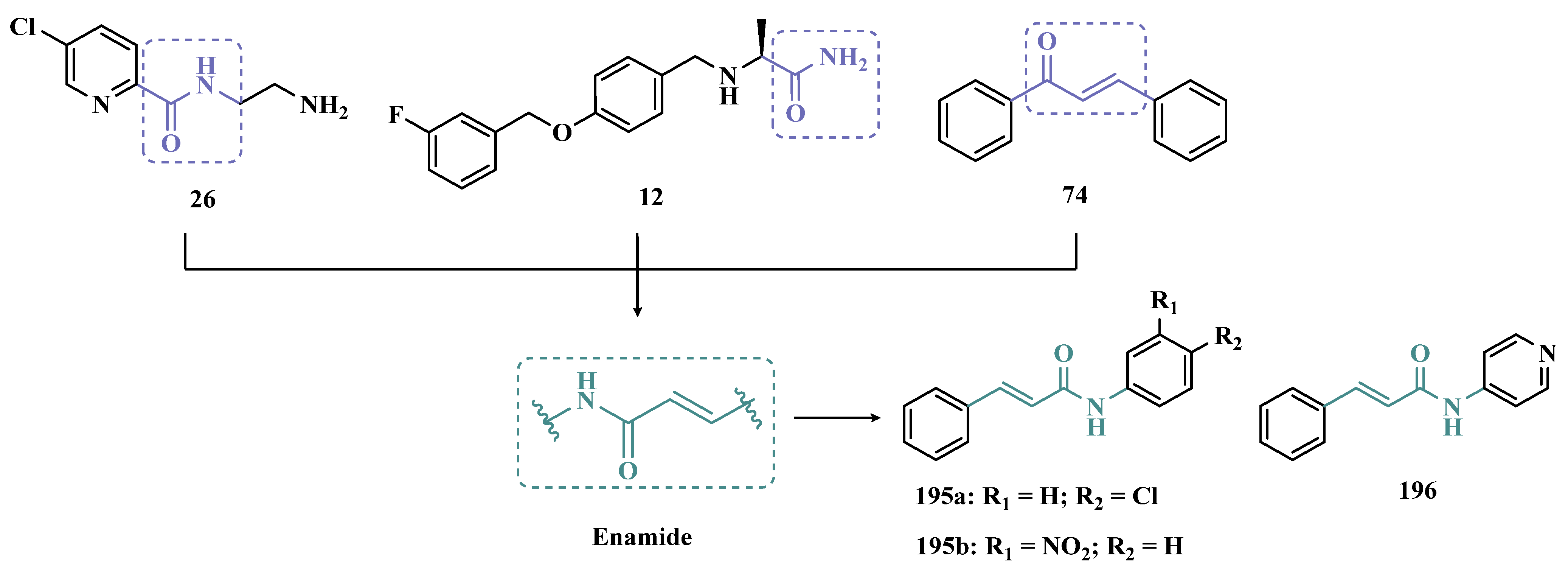
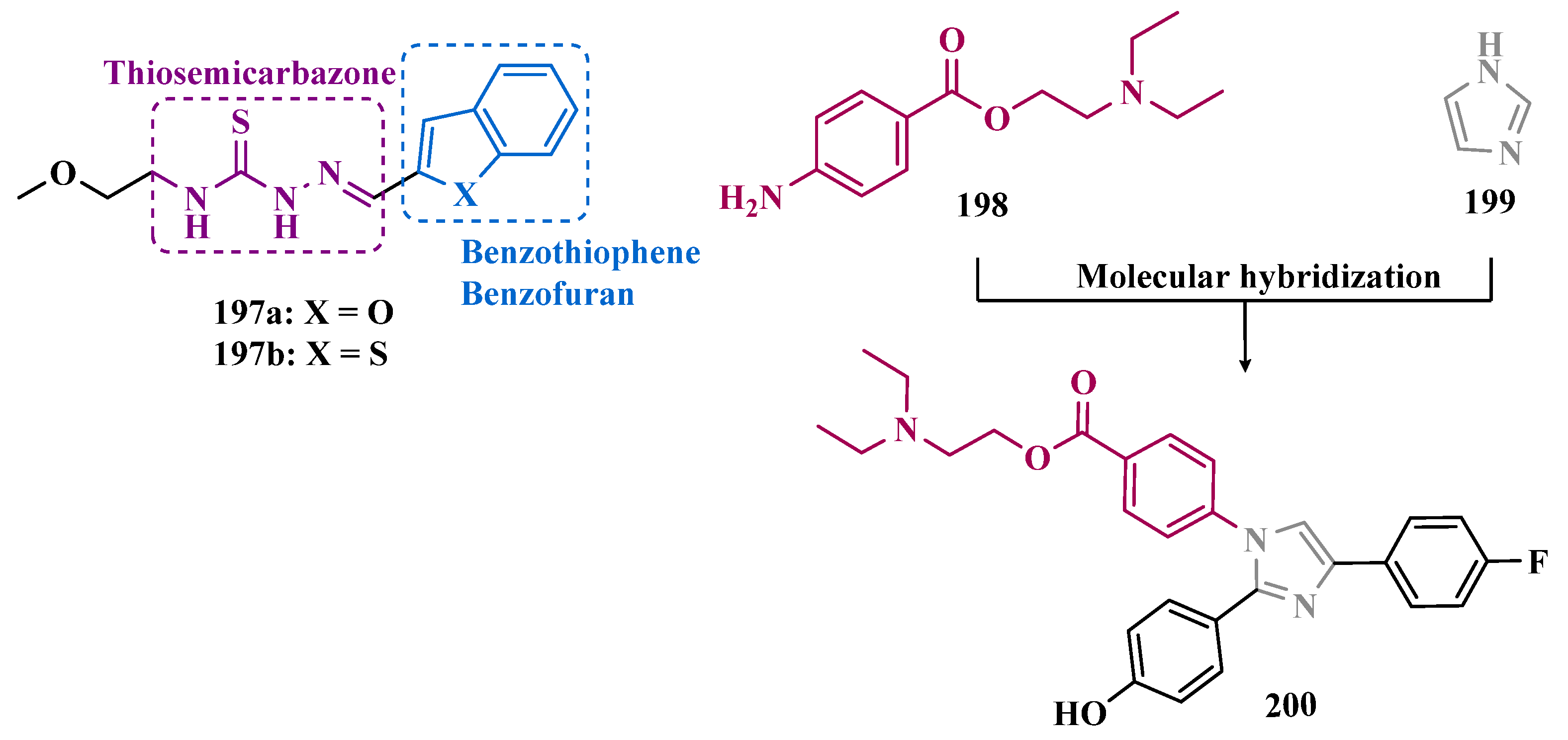
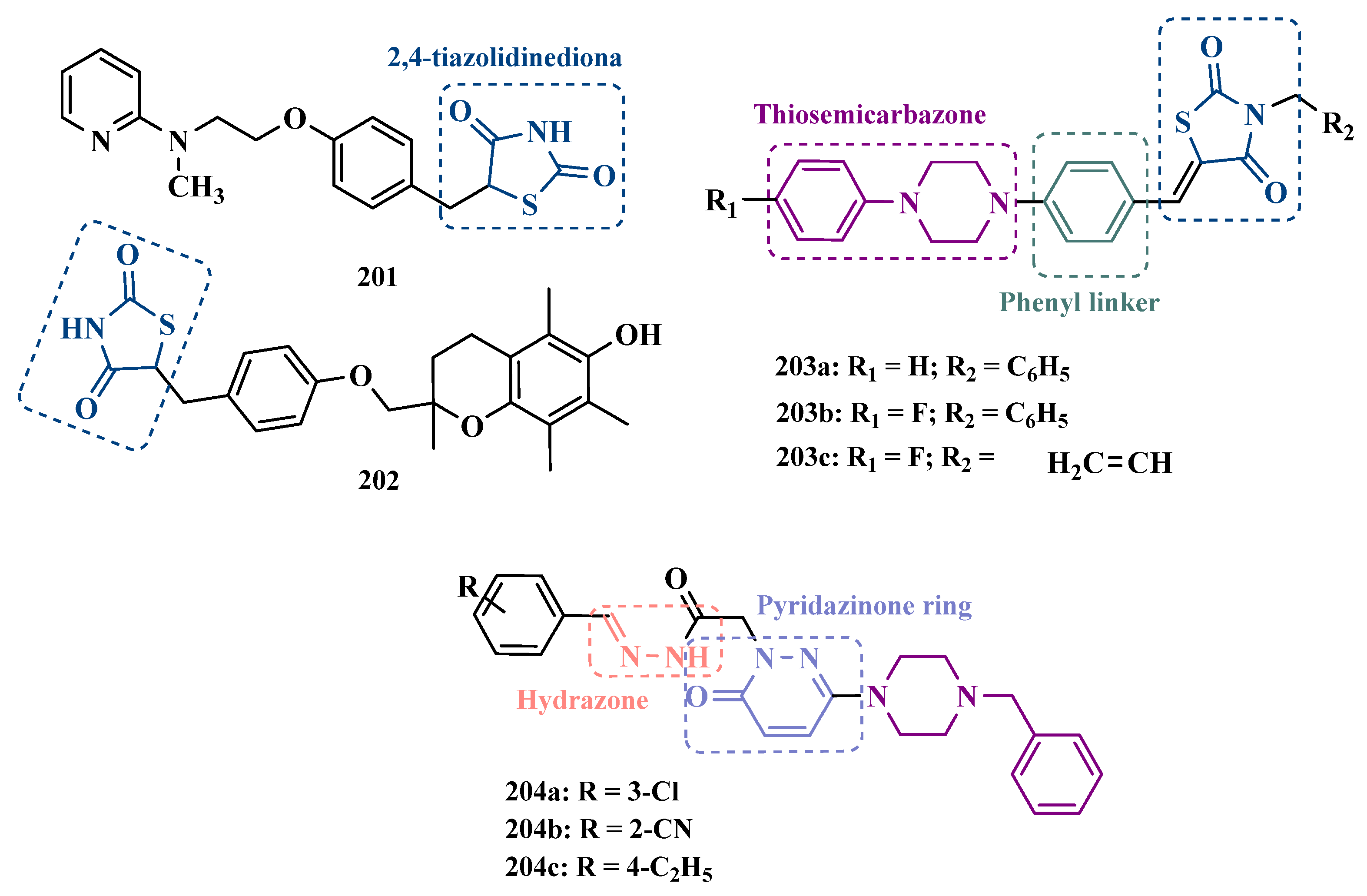
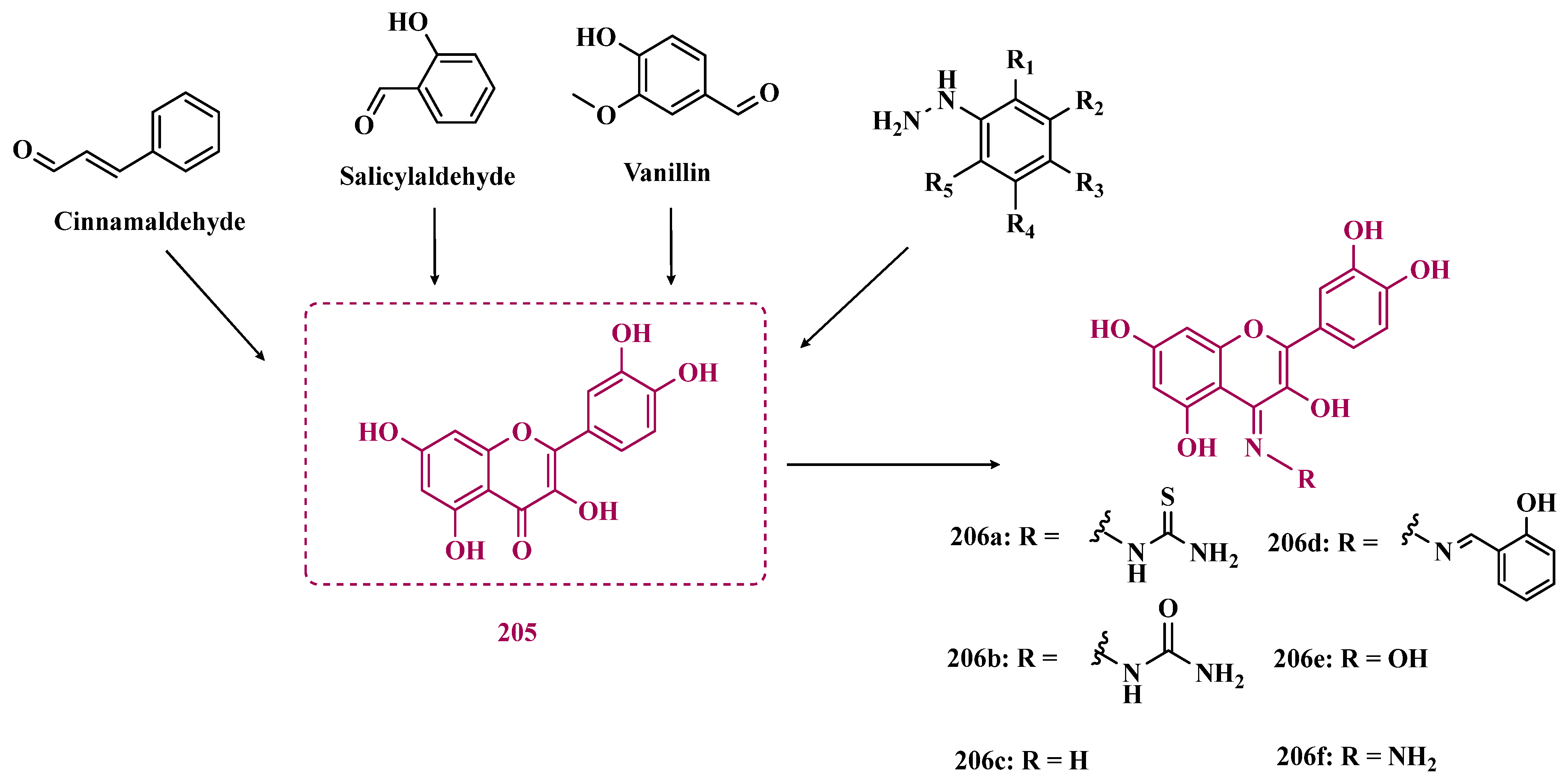



| Inhibitor | IC50 MAO-A (nM) | IC50 MAO-B (nM) | SI | Inhibitory Profile | LogP | TPSA | BBB Penetration | Toxicity |
|---|---|---|---|---|---|---|---|---|
| 16a | >10,000 | 0.586 ± 0.087 | 17,064 | Rev/Comp | 3.60 | 57.8 | - | - |
| 16b | >10,000 | 0.386 ± 0.052 | 25,906 | - | 2.67 | 57.8 | - | - |
| 16c | >10,000 | 1.59 ± 0.16 | 6289 | - | 3.72 | 46.9 | - | - |
| 18 | >10,000 | 0.612 ± 0.065 | 16,339 | - | 4.38 | 41 | - | - |
| 19a | >10,000 | 0.662 ± 0.059 | 15,105 | - | - | 46.92 | Yes | - |
| 44a | 87,900 ± 4780 | 4.4 ± 0.2 | 19,977 | - | - | - | - | - |
| 48b | 6470 ± 1250 | 2.5 ± 0.15 | 2588 | - | - | - | - | - |
| 70 | 3920 ± 827 | 4.0 ± 1 | 980 | Rev/Comp | - | - | - | - |
| 73c | 25,2200 ± 20,400 | 270 ± 20 | 934 | - | - | - | - | - |
| 73d | 436,500 ± 40,300 | 480 ± 4 | 909 | - | - | - | - | - |
| 82b | 15,370 | 11.35 | 1354 | Rev | - | - | - | - |
| 85a | 5820 ± 720 | 6.2 ± 0.9 | 938.7 | Rev/Comp | - | - | - | - |
| 96a | 218,000 | 25 | 8720 | Rev | - | - | - | - |
| 105 | - | 47.4 | >211 | Rev/Comp | - | 68.55 | - | Low |
| 112a | NA | 0.31 ± 0.02 | >333,333 | - | - | - | - | - |
| 112b | NA | 0.80 ± 0.05 | >125,000 | - | - | - | - | - |
| 112c | NA | 0.74 ± 0.02 | >135,870 | - | - | - | - | - |
| 119 | 99,999 ± 0.53 | 0.37 ± 40 | >270,270 | Rev/Comp | - | 35.53 | Yes | Low |
| 125 | NA | 0.67 ± 0.13 | >149,254 | Rev/Comp | 3.69 | 59.31 | Yes | Low |
| 138a | 28,900 ± 4220 | 1.4 ± 0.3 | 20,643 | - | - | - | - | - |
| 138b | >100,000 | 2.5 ± 0.7 | >40,000 | - | - | - | - | - |
| 151 | >100,000 | 9 ± 1 | 110,000 | Comp | - | - | - | - |
| 152 | >100,000 | 12.34 ± 1.62 | >8104 | - | 3.66 | 35.53 | Yes | Low |
| 158 | - | 3.9 ± 0.7 | >25,641 | - | - | - | - | - |
| 159a | 50,700 ± 4450 | 2.9 ± 0.3 | 17,482 | - | - | - | - | - |
| 159b | 17,700 ± 2940 | 1.3 ± 0.3 | 13,615 | - | - | - | - | - |
| 159c | 38,200 ± 3130 | 4 ± 1 | 9550 | - | - | - | - | - |
| 169 | 46,200 ± 11,200 | 2.7 ± 0.64 | 17,111 | Rev | - | - | - | - |
| 180 | NA | 11.97 ± 0.37 | >8354 | - | - | - | - | - |
| 192 | >100,000 | 60 ± 4 | 1666.67 | Rev | - | - | Yes | - |
| 195a | >40,000 | 110 ± 24 | >363 | Rev/Comp | - | - | - | - |
| 200 | 15,220 ± 3400 | 32 ± 2 | 475 | - | - | - | - | Low |
| 213b | 2660 ± 51 | 5.3 ± 0.8 | 501 | Rev/Comp | - | - | Yes | - |
| 213c | 29,100 ± 2520 | 7.2 ± 1.8 | 4041 | - | - | - | Yes | - |
| 240 | >10,000 | 34.9 ± 2.5 | 286 | Rev/Comp | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.V.; Massuda, L.E.; Gontijo, V.S.; Viegas, C., Jr. Monoamine Oxidase Inhibitors in Drug Discovery Against Parkinson’s Disease: An Update. Pharmaceuticals 2025, 18, 1526. https://doi.org/10.3390/ph18101526
Ribeiro LV, Massuda LE, Gontijo VS, Viegas C Jr. Monoamine Oxidase Inhibitors in Drug Discovery Against Parkinson’s Disease: An Update. Pharmaceuticals. 2025; 18(10):1526. https://doi.org/10.3390/ph18101526
Chicago/Turabian StyleRibeiro, Luana Vergueiro, Larissa Emika Massuda, Vanessa Silva Gontijo, and Claudio Viegas, Jr. 2025. "Monoamine Oxidase Inhibitors in Drug Discovery Against Parkinson’s Disease: An Update" Pharmaceuticals 18, no. 10: 1526. https://doi.org/10.3390/ph18101526
APA StyleRibeiro, L. V., Massuda, L. E., Gontijo, V. S., & Viegas, C., Jr. (2025). Monoamine Oxidase Inhibitors in Drug Discovery Against Parkinson’s Disease: An Update. Pharmaceuticals, 18(10), 1526. https://doi.org/10.3390/ph18101526







