The AURKA-Selective Inhibitor Alisertib Attenuates Doxorubicin-Induced Hepatotoxicity in Mice via Modulation of IL-17A/NF-κB and STAT3 Signaling Pathways
Abstract
1. Introduction
2. Results
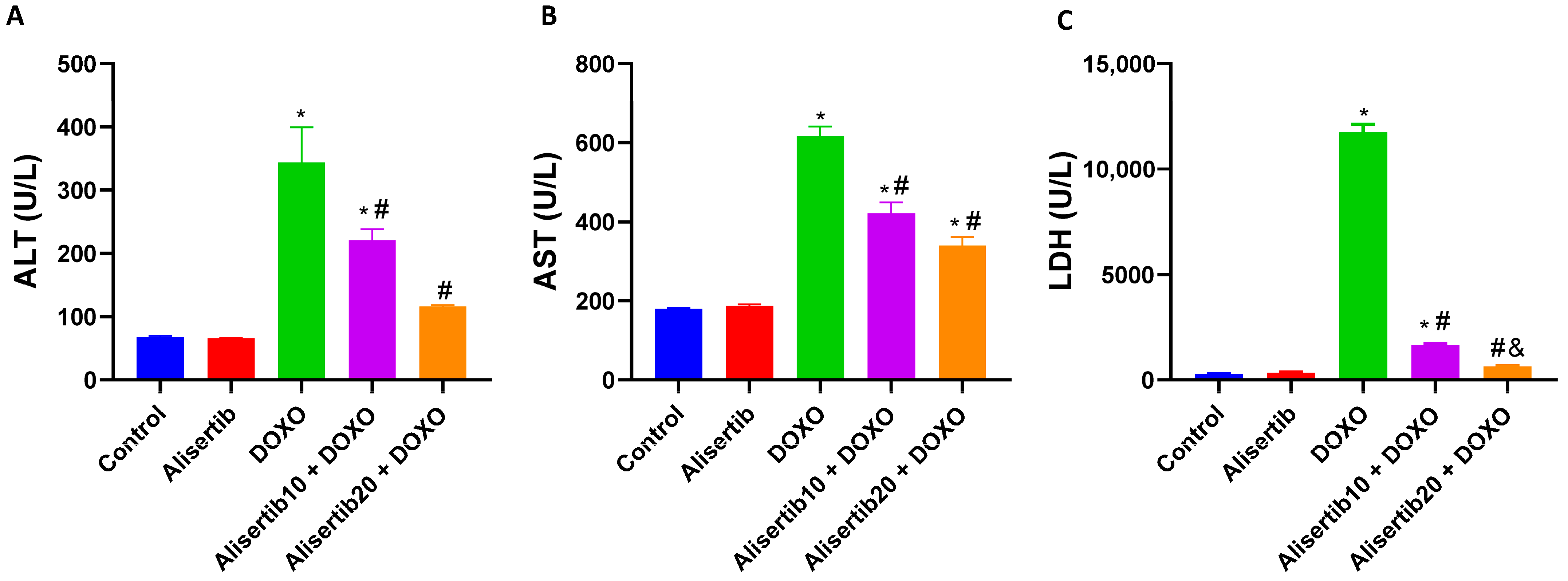
2.1. Effect of Alisertib on DOXO-Induced Hepatocellular Injury
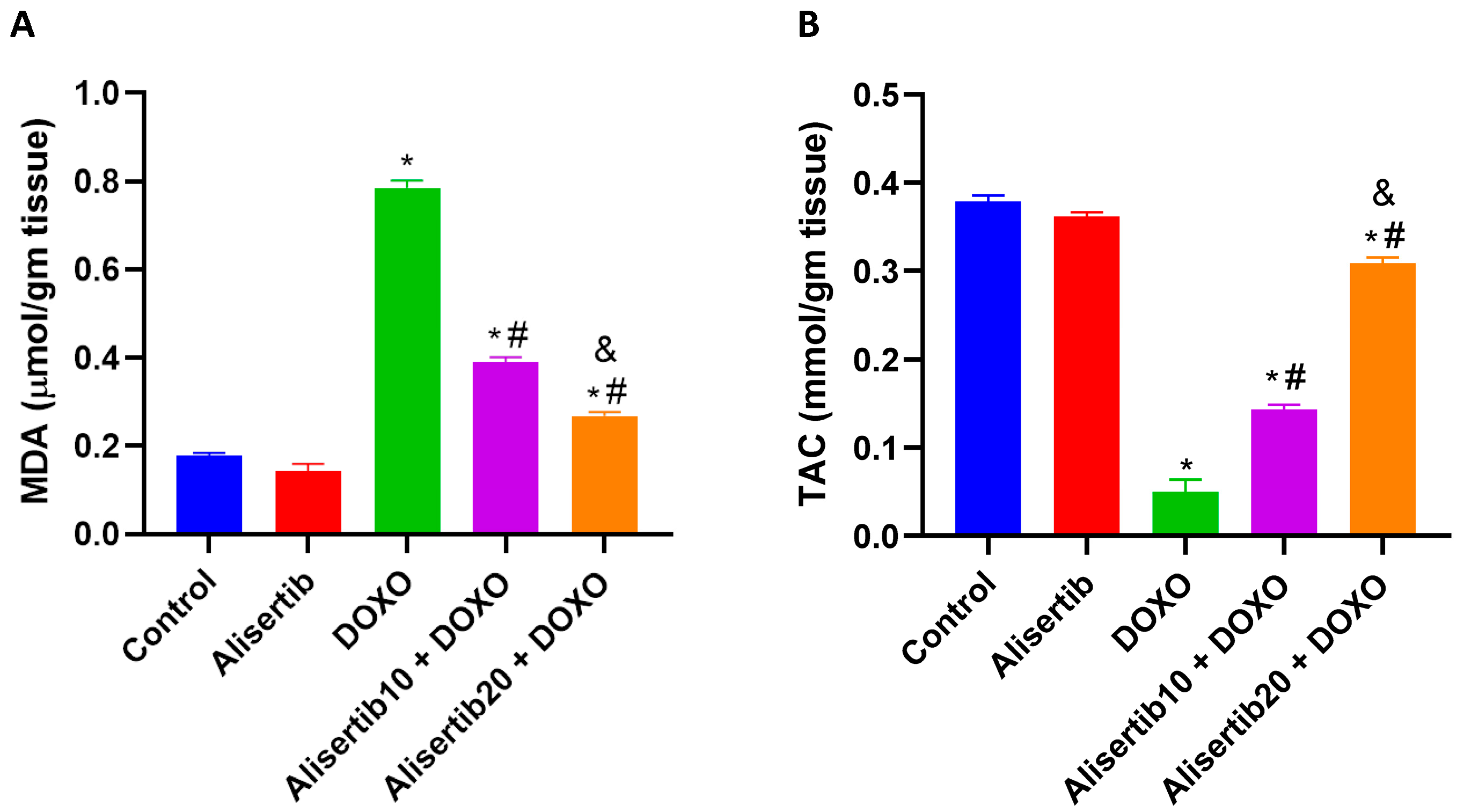
2.2. Effect of Alisertib on DOXO-Induced Hepatic Oxidative Stress
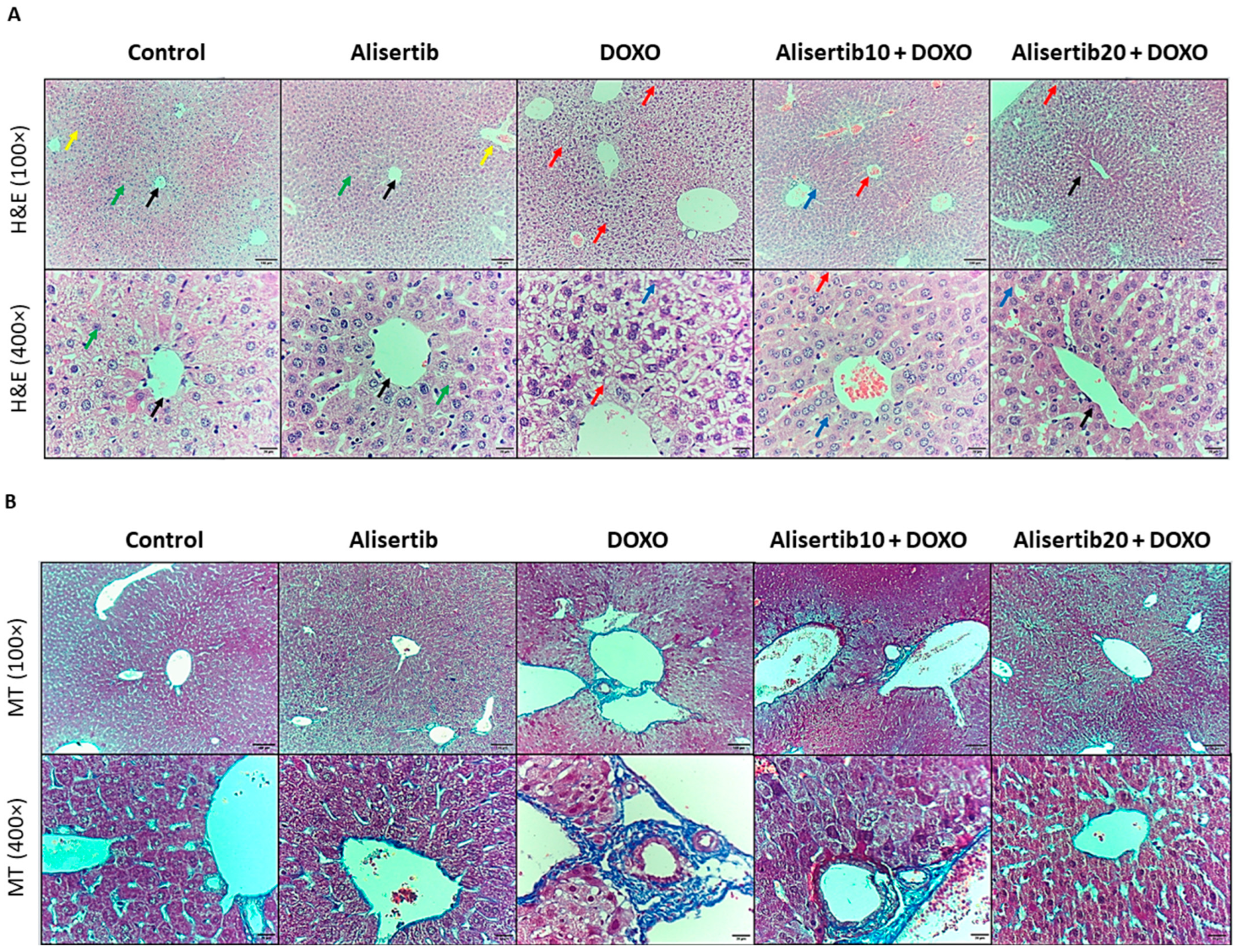
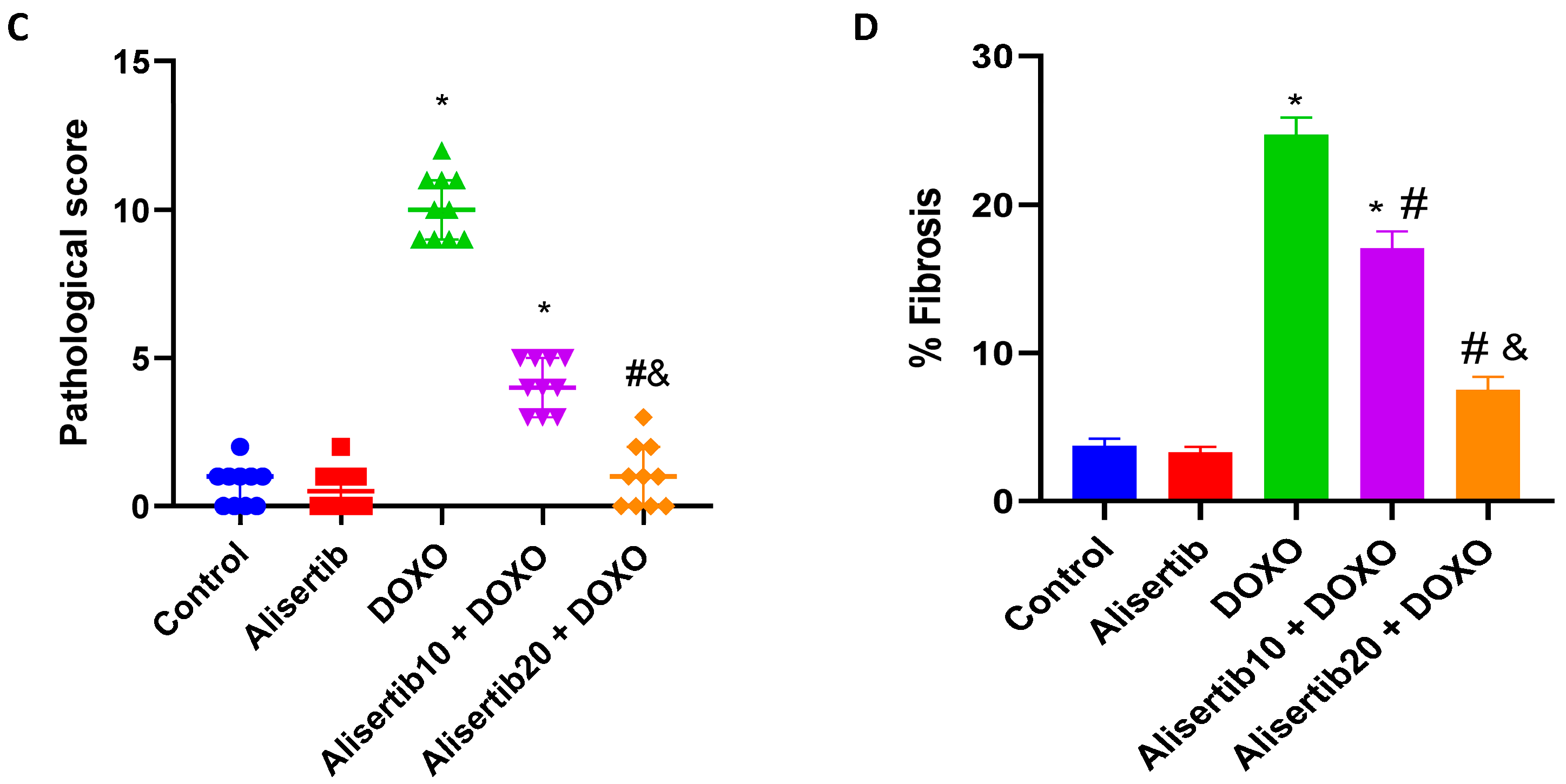
2.3. Effect of Alisertib on DOXO-Induced Hepatic Histopathological Abnormalities
2.4. Effect of Alisertib on DOXO-Induced Liver Activation of NF-κB
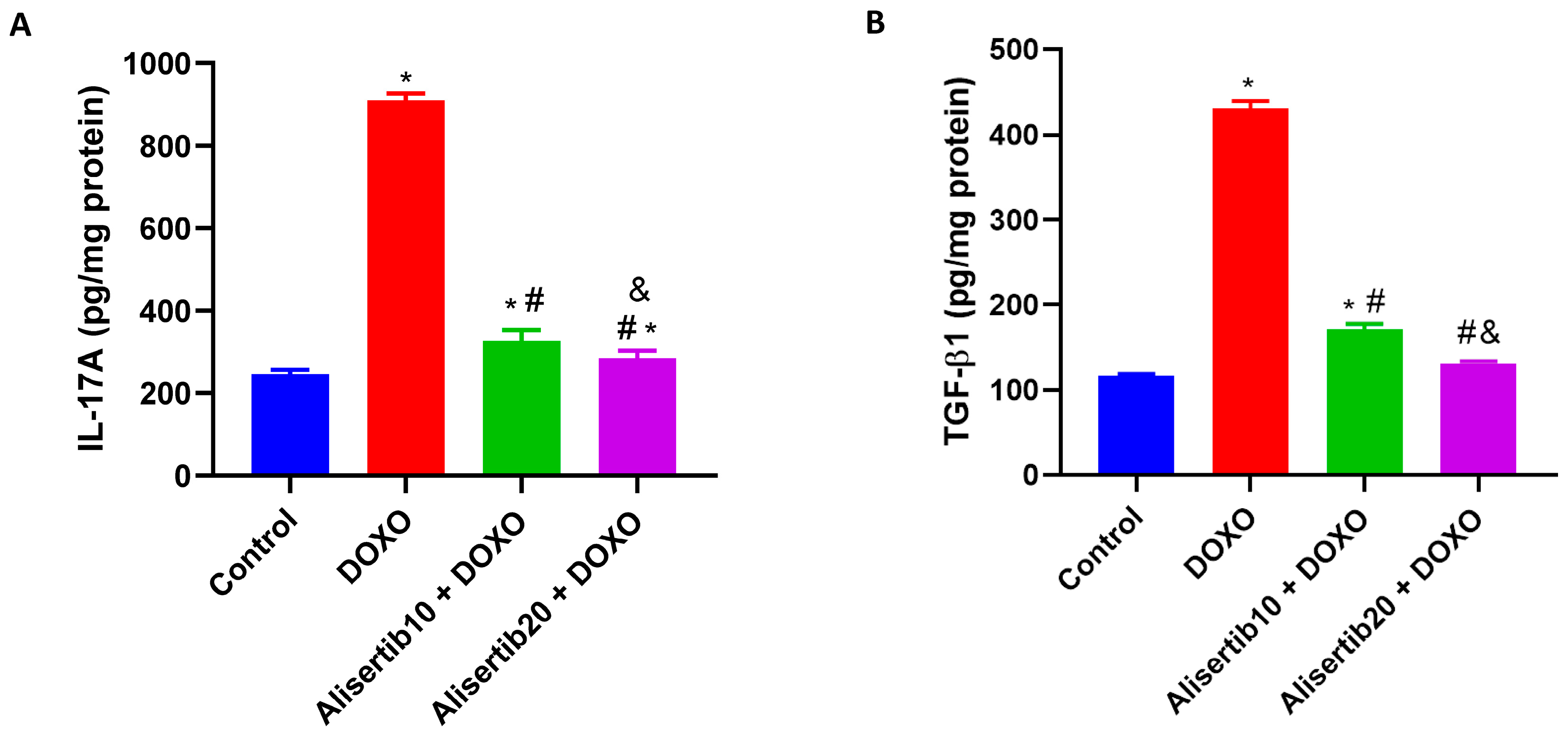
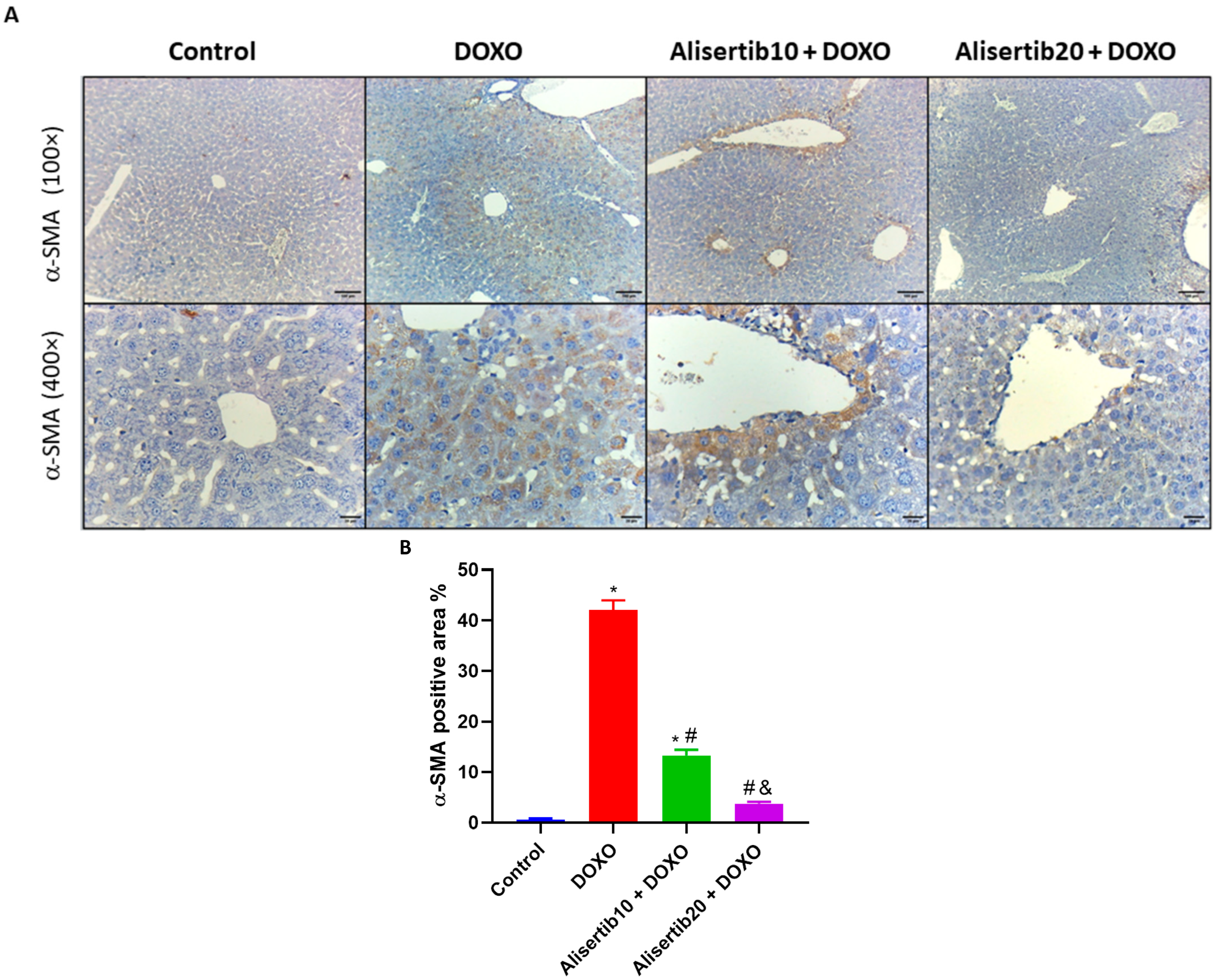
2.5. Effect of Alisertib on DOXO-Induced Elevation of IL-17A, TGF-β1, and α-SMA Hepatic Expression
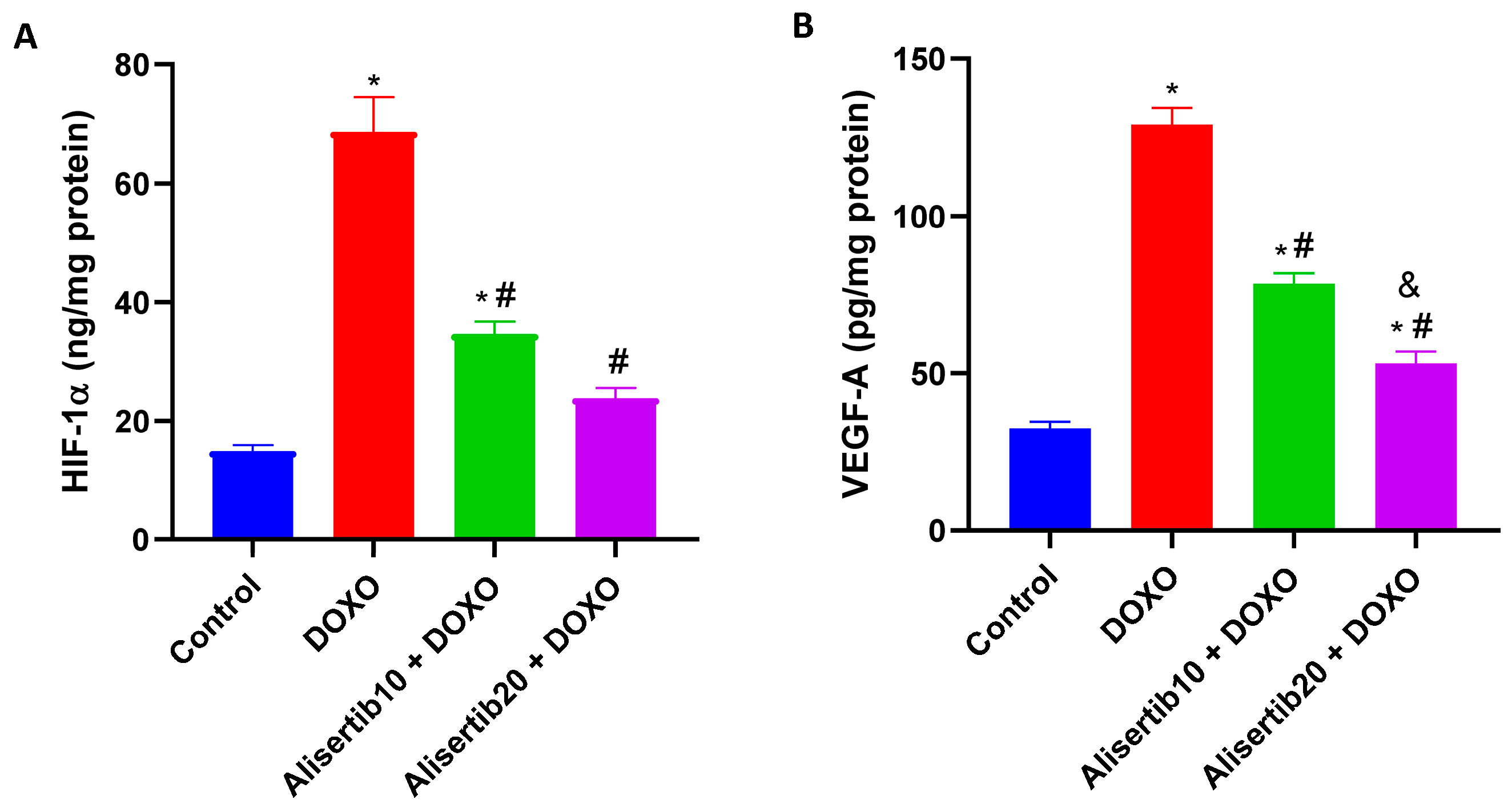
2.6. Effect of Alisertib on DOXO-Induced Elevation of HIF-1α and VEGF-A Hepatic Expression
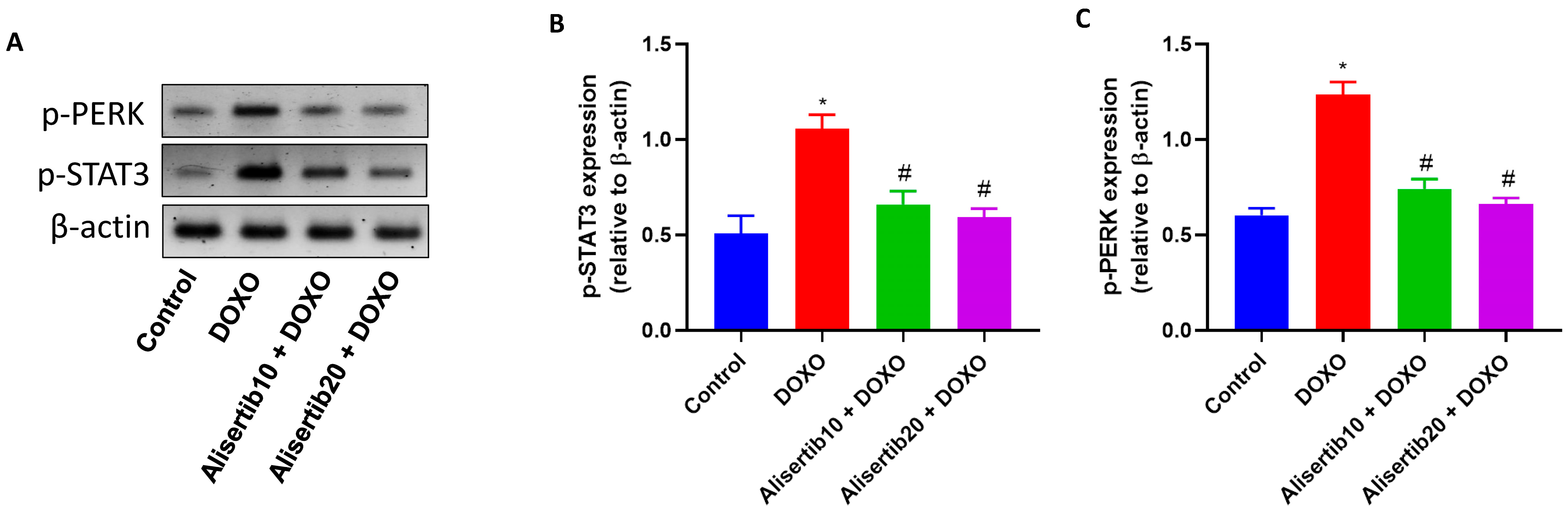
2.7. Effect of Alisertib on DOXO-Induced Hepatic Activation of STAT3 and PERK
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Sample Collection and Preparation
4.5. Assessment of Liver Function
4.6. Assessment of Hepatic Redox Status
4.7. Histopathological Assessment of the Liver
4.8. Immunohistochemistry
4.9. ELISA Measurements
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.; Ismail, M.F.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.G.; Ouhtit, A. Histopathological Effects of Cisplatin, Doxorubicin, and 5-Fluorouracil (5-FU) on the Liver of Male Albino Rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Arasteh, O.; Elyasi, S. Preventive and Therapeutic Use of Herbal Compounds Against Doxorubicin-Induced Hepatotoxicity: A Comprehensive Review. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1595–1617. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Alzahrani, A.A.; Bawazir, N.M.; Khawtani, R.O.; Arab, H.H. Targeting the Proinflammatory Cytokines, Oxidative Stress, Apoptosis and TGF-β1/STAT-3 Signaling by Irbesartan to Ameliorate Doxorubicin-Induced Hepatotoxicity. J. Infect. Chemother. 2018, 24, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Aljobaily, N.; Viereckl, M.J.; Hydock, D.S.; Aljobaily, H.; Wu, T.Y.; Busekrus, R.; Jones, B.; Alberson, J.; Han, Y. Creatine Alleviates Doxorubicin-Induced Liver Damage by Inhibiting Liver Fibrosis, Inflammation, Oxidative Stress, and Cellular Senescence. Nutrients 2020, 13, 41. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity via Suppressing Oxidative Stress and Modulating iNOS, NF-κB, and TNF-α in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166. [Google Scholar] [CrossRef]
- Liu, Z.; Suo, C.; Jiang, Y.; Zhao, R.; Zhang, T.; Jin, L.; Chen, X. Phenome-Wide Association Analysis Reveals Novel Links Between Genetically Determined Levels of Liver Enzymes and Disease Phenotypes. Phenomics 2022, 2, 295–311. [Google Scholar] [CrossRef]
- Peng, L.; Chen, H.G.; Zhou, X. Lipidomic Investigation of the Protective Effects of Polygonum perfoliatum Against Chemical Liver Injury in Mice. J. Integr. Med. 2023, 21, 289–301. [Google Scholar] [CrossRef]
- Carmena, M.; Ruchaud, S.; Earnshaw, W.C. Making the Auroras Glow: Regulation of Aurora A and B Kinase Function by Interacting Proteins. Curr. Opin. Cell Biol. 2009, 21, 796–805. [Google Scholar] [CrossRef]
- Diallo, A.; Prigent, C. Les Sérine/Thréonine Kinases Contrôlant la Progression du Cycle Cellulaire Comme Cibles Thérapeutiques. Bull. Cancer 2011, 98, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, J.; Chen, D.; Huang, J.; Feng, B.; Han, S.; Chen, Y.; Song, H.; De, W.; Zhu, Z.; et al. Aurora-A Promotes Chemoresistance in Hepatocellular Carcinoma by Targeting NF-κB/MicroRNA-21/PTEN Signaling Pathway. Oncotarget 2014, 5, 12916–12935. [Google Scholar] [CrossRef] [PubMed]
- Katsha, A.; Arras, J.; Soutto, M.; Belkhiri, A.; El-Rifai, W. AURKA Regulates JAK2–STAT3 Activity in Human Gastric and Esophageal Cancers. Mol. Oncol. 2014, 8, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Pitts, T.M.; Bradshaw-Pierce, E.L.; Bagby, S.M.; Hyatt, S.L.; Selby, H.M.; Spreafico, A.; Tentler, J.J.; McPhillips, K.; Klauck, P.J.; Capasso, A.; et al. Antitumor Activity of the Aurora A Selective Kinase Inhibitor, Alisertib, Against Preclinical Models of Colorectal Cancer. Oncotarget 2016, 7, 50290–50301. [Google Scholar] [CrossRef]
- Ganapathi, R.N.; Norris, E.J.; Sutker, A.P.; Klotz, K.E.; Ganapathi, M.K. Targeting Aurora A Kinase (AAK) in Platinum-Resistant High Grade Serous Ovarian Cancer. Front. Oncol. 2020, 10, 1354. [Google Scholar] [CrossRef]
- Biswas, S.; Mahapatra, E.; Ghosh, A.; Das, S.; Roy, M.; Mukherjee, S. Curcumin Rescues Doxorubicin Responsiveness via Regulating Aurora a Signaling Network in Breast Cancer Cells. Asian. Pac. J. Cancer Prev. 2021, 22, 957–970. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L. Silencing Aurora-Kinase-A (AURKA) Reinforced the Sensitivity of Diffuse Large B-Cell Lymphoma Cells to Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) via Suppressing β-Catenin and RAS-Extracellular Signal-Regulated Protein Kinase (ERK1/2) Pathway. Bioengineered 2021, 12, 8296–8308. [Google Scholar] [CrossRef]
- Saleh, D.O.; Mahmoud, S.S.; Hassan, A.; Sanad, E.F. Doxorubicin-induced hepatic toxicity in rats: Mechanistic protective role of Omega-3 fatty acids through Nrf2/HO-1 activation and PI3K/Akt/GSK-3β axis modulation. Saudi J. Biol. Sci. 2022, 29, 103308. [Google Scholar] [CrossRef]
- Dewanjee, S.; Joardar, S.; Bhattacharjee, N.; Dua, T.K.; Das, S.; Kalita, J.; Manna, P. Edible leaf extract of Ipomoea aquatica Forssk attenuates doxorubicin-induced liver injury via inhibiting oxidative impairment, MAPK activation, and intrinsic pathway of apoptosis. Food Chem. Toxicol. 2017, 105, 322–336. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; AlSwayyed, M.; Alzarea, S.I.; Al-Alallah, I.A.; Alghamdi, A.; Hakami, H.M.; Alyousef, M.K.; et al. Diosmin Alleviates Doxorubicin-Induced Liver Injury via Modulation of Oxidative Stress-Mediated Hepatic Inflammation and Apoptosis via NfkB and MAPK Pathway: A Preclinical Study. Antioxidants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Ng, K.T.; Liu, J.; Liu, H.; Zhang, W.; Xiao, F.; Li, X.; Lo, C.M.; Lu, L.; et al. IL-17a exacerbates hepatic ischemia-reperfusion injury in fatty liver by promoting neutrophil infiltration and mitochondria-driven apoptosis. J. Leukoc. Biol. 2020, 108, 1603–1613. [Google Scholar] [CrossRef]
- Elshal, M.; Hazem, S.H. Escin suppresses immune cell infiltration and selectively modulates Nrf2/HO-1, TNF-α/JNK, and IL-22/STAT3 signaling pathways in concanavalin A-induced autoimmune hepatitis in mice. Inflammopharmacology 2022, 30, 2317–2329. [Google Scholar] [CrossRef]
- Narayanan, A.; Amaya, M.; Voss, K.; Chung, M.; Benedict, A.; Sampey, G.; Kehn-Hall, K.; Luchini, A.; Liotta, L.; Bailey, C.; et al. Reactive oxygen species activate NFκB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology 2014, 449, 270–286. [Google Scholar] [CrossRef]
- Madani, B.; Burzangi, A.; Alkreathy, H.; Karim, S.; Shaik, R.A.; Khan, L. Thymoquinone Prevents Doxorubicin-induced Hepatic Injury by Mitigating the Impairment of Mitochondrial Respiration and Electron Transport. Int. J. Pharm. Res. Allied Sci. 2022, 11, 89–97. [Google Scholar] [CrossRef]
- Tan, S.; Lu, X.; Chen, W.; Pan, B.; Kong, G.; Wei, L. Analysis and experimental validation of IL-17 pathway and key genes as central roles associated with inflammation in hepatic ischemia-reperfusion injury. Sci. Rep. 2024, 14, 6423. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.R.; Kannerup, A.S.; Grønbæk, H.; Andersen, K.J.; Funch-Jensen, P.; Frystyk, J.; Flyvbjerg, A.; Mortensen, F.V. Effects of ischemic pre- and postconditioning on HIF-1α, VEGF, and TGF-β expression after warm ischemia and reperfusion in the rat liver. Comp. Hepatol. 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hu, M.; Chen, Z.; Zeng, L. The roles and mechanisms of hypoxia in liver fibrosis. J. Transl. Med. 2021, 19, 186. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, H.; Zhang, Z.; Li, Z.; Chen, X.; Zhao, Y.; Ju, R.; He, J.; Pan, H.; Liu, X.; et al. Ongoing involvers and promising therapeutic targets of hepatic fibrosis: The hepatic immune microenvironment. Front. Immunol. 2023, 14, 1131588. [Google Scholar] [CrossRef]
- Xu, M.; Pang, Q.; Xu, S.; Ye, C.; Lei, R.; Shen, Y.; Xu, J. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 2017, 9, 3188–3197. [Google Scholar] [CrossRef]
- Elshal, M.; Abu-Elsaad, N.; El-Karef, A.; Ibrahim, T.M. The multi-kinase inhibitor pazopanib targets hepatic stellate cell activation and apoptosis alleviating progression of liver fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 1293–1304. [Google Scholar] [CrossRef]
- Luo, R.; Yi, Z.; Wu, W.; Meng, W. The mRNA levels of PPARα, HIF-1α, and VEGF in the liver tissues of rats with alcoholic liver disease. Am. J. Transl. Res. 2021, 13, 11932–11937. [Google Scholar] [PubMed] [PubMed Central]
- Jiang, M.; Bai, M.; Xu, S.; Wang, T.; Lei, J.; Xu, M.; Huang, S.; Jia, Z.; Zhang, A. Blocking AURKA with MK-5108 attenuates renal fibrosis in chronic kidney disease. Biochim. Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166227. [Google Scholar] [CrossRef]
- Wen, Q.J.; Yang, Q.; Goldenson, B.; Malinge, S.; Lasho, T.; Schneider, R.K.; Breyfogle, L.J.; Schultz, R.; Gilles, L.; Koppikar, P.; et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat. Med. 2015, 21, 1473–1480. [Google Scholar] [CrossRef]
- Yin, T.; Zhao, Z.B.; Guo, J.; Wang, T.; Yang, J.B.; Wang, C.; Long, J.; Ma, S.; Huang, Q.; Zhang, K.; et al. Aurora A Inhibition Eliminates Myeloid Cell-Mediated Immunosuppression and Enhances the Efficacy of Anti-PD-L1 Therapy in Breast Cancer. Cancer Res. 2019, 79, 3431–3444. [Google Scholar] [CrossRef] [PubMed]
- Zarco, N.; Dovas, A.; de Araujo Farias, V.; Nagaiah, N.K.H.; Haddock, A.; Sims, P.A.; Hambardzumyan, D.; Meyer, C.T.; Canoll, P.; Rosenfeld, S.S.; et al. Resistance to spindle inhibitors in glioblastoma depends on STAT3 and therapy induced senescence. iScience 2024, 27, 111311. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Paul, P.; Kim, K.W.; Lee, S.; Qiao, J.; Chung, D.H. Targeting Aurora kinase-A downregulates cell proliferation and angiogenesis in neuroblastoma. J. Pediatr. Surg. 2014, 49, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Whately, K.M.; Voronkova, M.A.; Maskey, A.; Gandhi, J.; Loskutov, J.; Choi, H.; Yanardag, S.; Chen, D.; Wen, S.; Margaryan, N.V.; et al. Nuclear Aurora-A kinase-induced hypoxia signaling drives early dissemination and metastasis in breast cancer: Implications for detection of metastatic tumors. Oncogene 2021, 40, 5651–5664. [Google Scholar] [CrossRef]
- Shreya, S.; Grosset, C.F.; Jain, B.P. Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef]
- Kropski, J.A.; Blackwell, T.S. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Investig. 2018, 128, 64–73. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Kang, N.; Malhi, H.; Shah, V.H.; Maiers, J.L. Transforming growth factor β (TGFβ) cross-talk with the unfolded protein response is critical for hepatic stellate cell activation. J. Biol. Chem. 2019, 294, 3137–3151. [Google Scholar] [CrossRef]
- Darmadi, D.; Ruslie, R.H.; Pakpahan, C. Vascular Endothelial Growth Factor (VEGF) in Liver Disease. In Tumor Angiogenesis and Modulators; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, Y.H.; Li, J.C.; Li, Q.; Ye, D.; Zhang, X.X.; Li, J.T. Vascular endothelial growth factor protein and gene delivery by novel nanomaterials for promoting liver regeneration after partial hepatectomy. World J. Gastroenterol. 2023, 29, 3748–3757. [Google Scholar] [CrossRef]
- Briassouli, P.; Chan, F.; Savage, K.; Reis-Filho, J.S.; Linardopoulos, S. Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res. 2007, 67, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Sasai, K.; Treekitkarnmongkol, W.; Kai, K.; Katayama, H.; Sen, S. Functional Significance of Aurora Kinases-p53 Protein Family Interactions in Cancer. Front. Oncol. 2016, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.G.; Ecsedy, J.A.; Chakravarty, A.; Silverman, L.; Zhang, M.; Hoar, K.M.; Stroud, S.G.; Chen, W.; Shinde, V.; Huck, J.J.; et al. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin. Cancer Res. 2011, 17, 7614–7624. [Google Scholar] [CrossRef]
- Stockwell, S.R.; Scott, D.E.; Fischer, G.; Guarino, E.; Rooney, T.P.C.; Feng, T.S.; Moschetti, T.; Srinivasan, R.; Alza, E.; Asteian, A.; et al. Selective Aurora A-TPX2 Interaction Inhibitors Have In Vivo Efficacy as Targeted Antimitotic Agents. J. Med. Chem. 2024, 67, 15521–15536. [Google Scholar] [CrossRef]
- Palani, S.; Patel, M.; Huck, J.; Zhang, M.; Balani, S.K.; Yang, J.; Chen, S.; Mettetal, J.; Manfredi, M.; Shyu, W.C.; et al. Preclinical pharmacokinetic/pharmacodynamic/efficacy relationships for alisertib, an investigational small-molecule inhibitor of Aurora A kinase. Cancer Chemother. Pharmacol. 2013, 72, 1255–1264. [Google Scholar] [CrossRef]
- Alherz, F.A.; Negm, W.A.; El-Masry, T.A.; Elmorshedy, K.E.; El-Kadem, A.H. The potential beneficial role of Ginkgetin in doxorubicin-induced hepatotoxicity: Elucidating the underlying claim. Biomed. Pharmacother. 2023, 165, 115010. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Suzuki, S.; Toledo-Pereyra, L.H.; Rodriguez, F.J.; Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993, 55, 1265–1272. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqussair, F.; Elshal, M.; Makled, M.N.; Abu-Elsaad, N.M. The AURKA-Selective Inhibitor Alisertib Attenuates Doxorubicin-Induced Hepatotoxicity in Mice via Modulation of IL-17A/NF-κB and STAT3 Signaling Pathways. Pharmaceuticals 2025, 18, 1201. https://doi.org/10.3390/ph18081201
Alqussair F, Elshal M, Makled MN, Abu-Elsaad NM. The AURKA-Selective Inhibitor Alisertib Attenuates Doxorubicin-Induced Hepatotoxicity in Mice via Modulation of IL-17A/NF-κB and STAT3 Signaling Pathways. Pharmaceuticals. 2025; 18(8):1201. https://doi.org/10.3390/ph18081201
Chicago/Turabian StyleAlqussair, Faisal, Mahmoud Elshal, Mirhan N. Makled, and Nashwa M. Abu-Elsaad. 2025. "The AURKA-Selective Inhibitor Alisertib Attenuates Doxorubicin-Induced Hepatotoxicity in Mice via Modulation of IL-17A/NF-κB and STAT3 Signaling Pathways" Pharmaceuticals 18, no. 8: 1201. https://doi.org/10.3390/ph18081201
APA StyleAlqussair, F., Elshal, M., Makled, M. N., & Abu-Elsaad, N. M. (2025). The AURKA-Selective Inhibitor Alisertib Attenuates Doxorubicin-Induced Hepatotoxicity in Mice via Modulation of IL-17A/NF-κB and STAT3 Signaling Pathways. Pharmaceuticals, 18(8), 1201. https://doi.org/10.3390/ph18081201





