Eugenia uniflora L.: Analysis of Chemical Profile and Cytotoxic Action on Tumor (HeLa) and Non-Tumor Cells (NIH/3T3)
Abstract
1. Introduction
2. Results
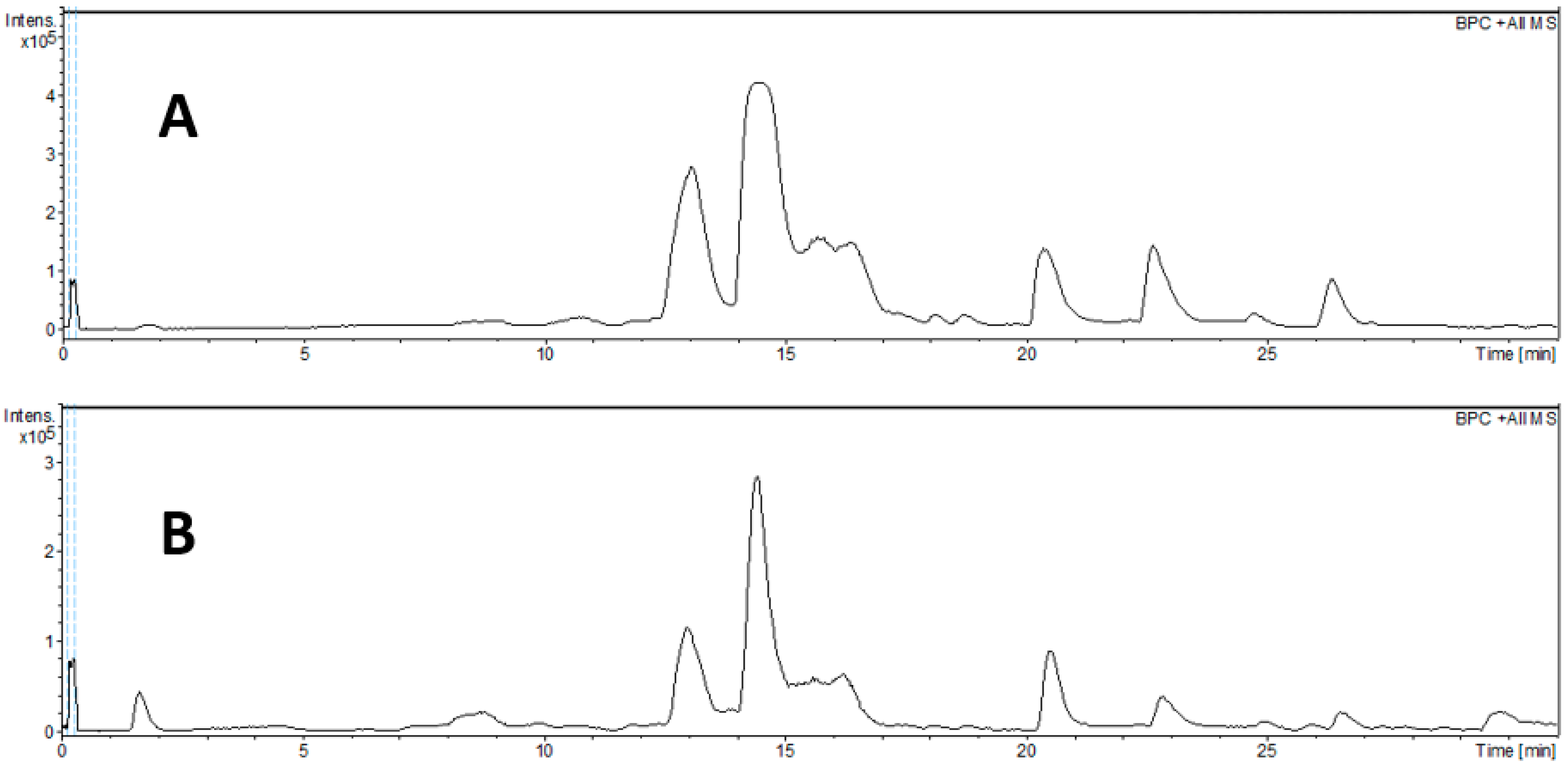
2.1. Chemical Profile
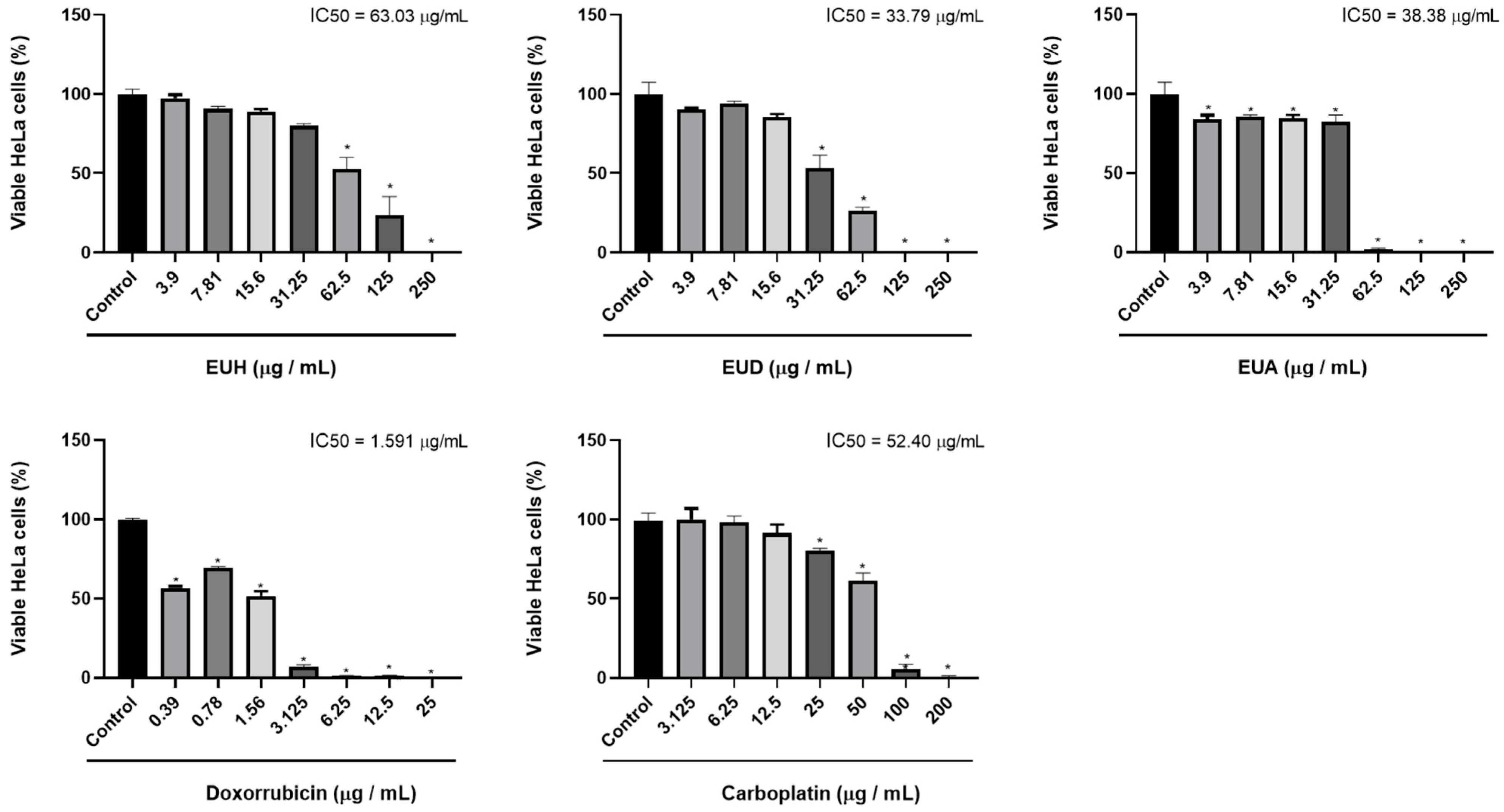
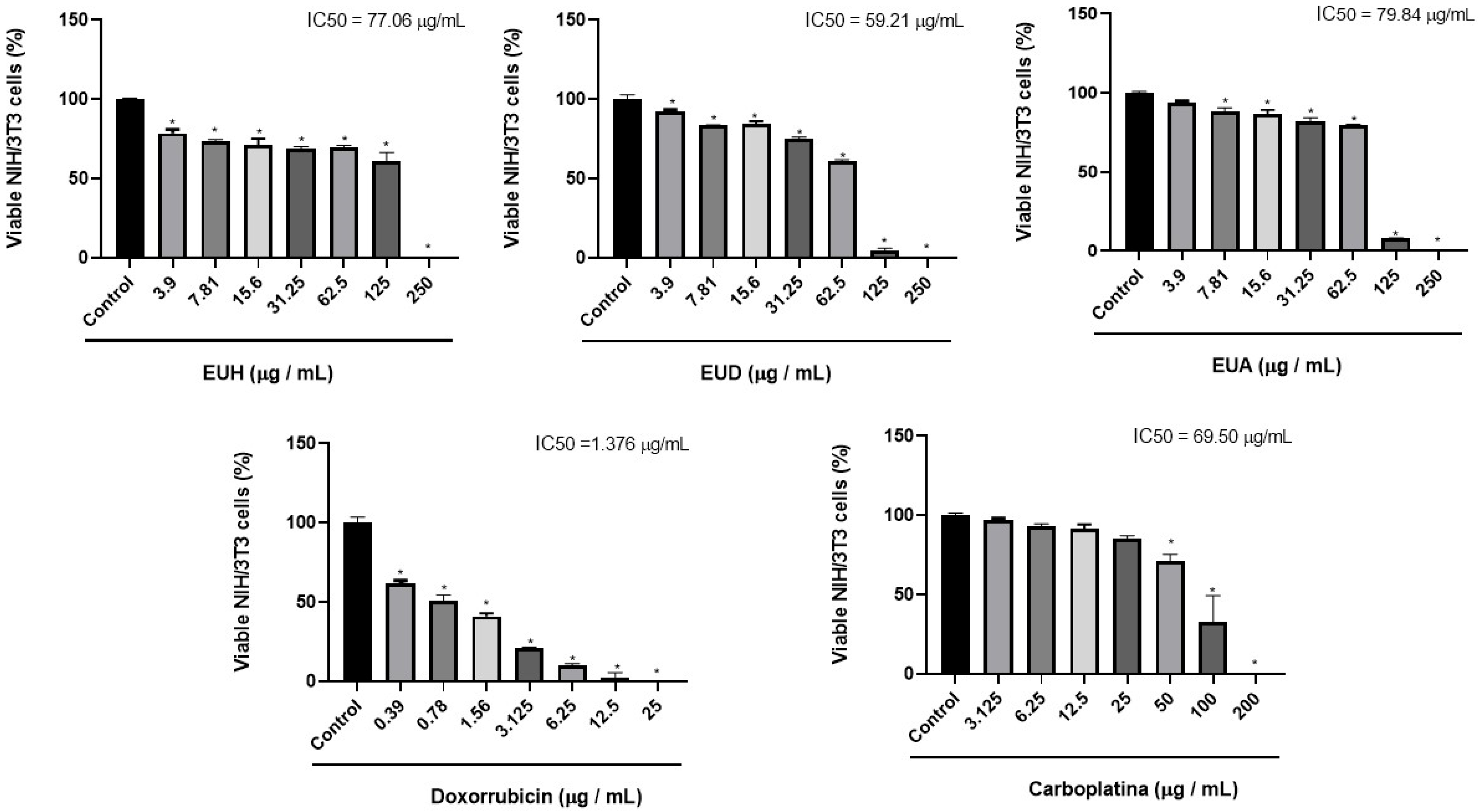
2.2. Cell Viability
2.3. Selectivity Index (SI)
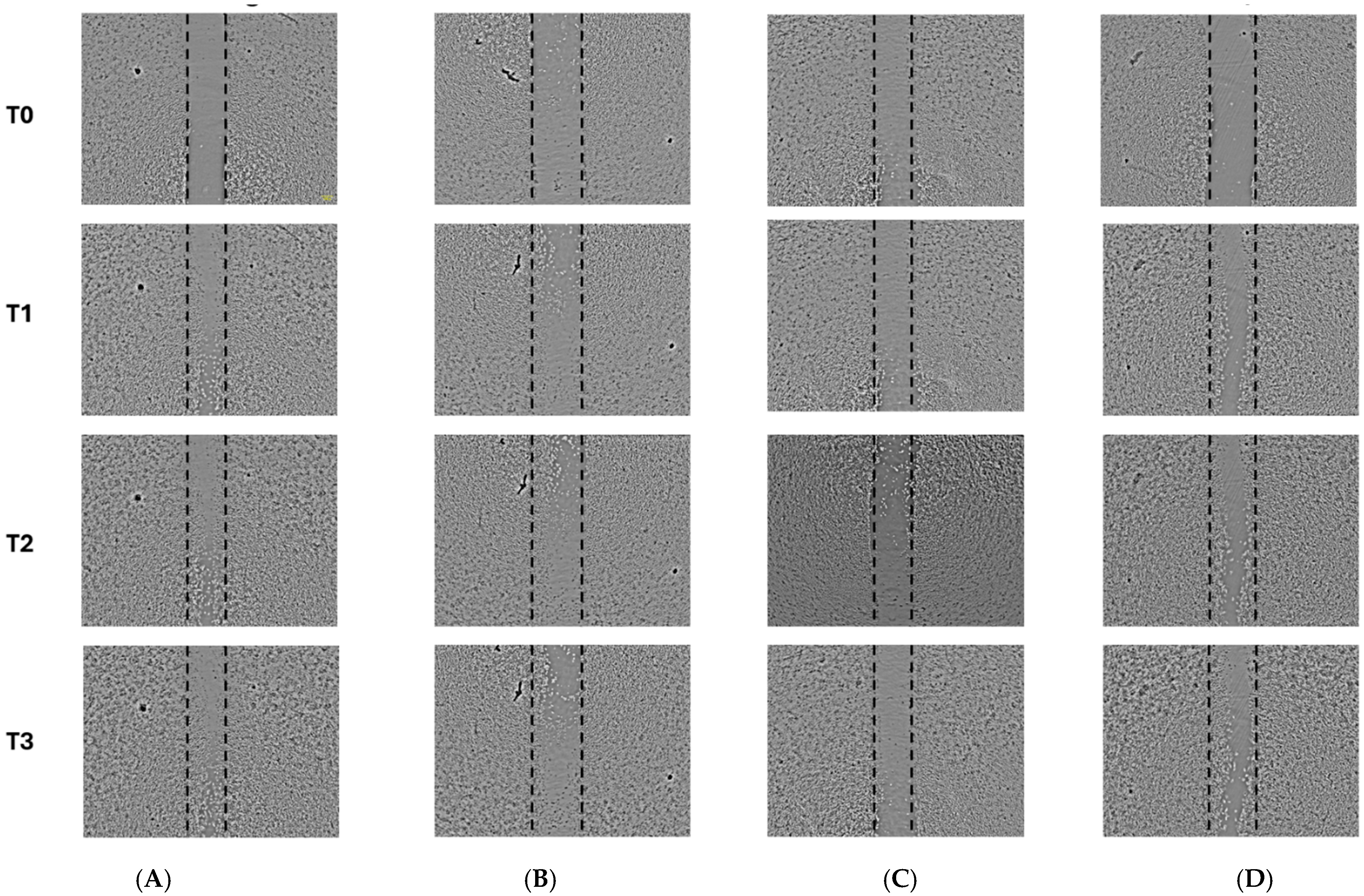
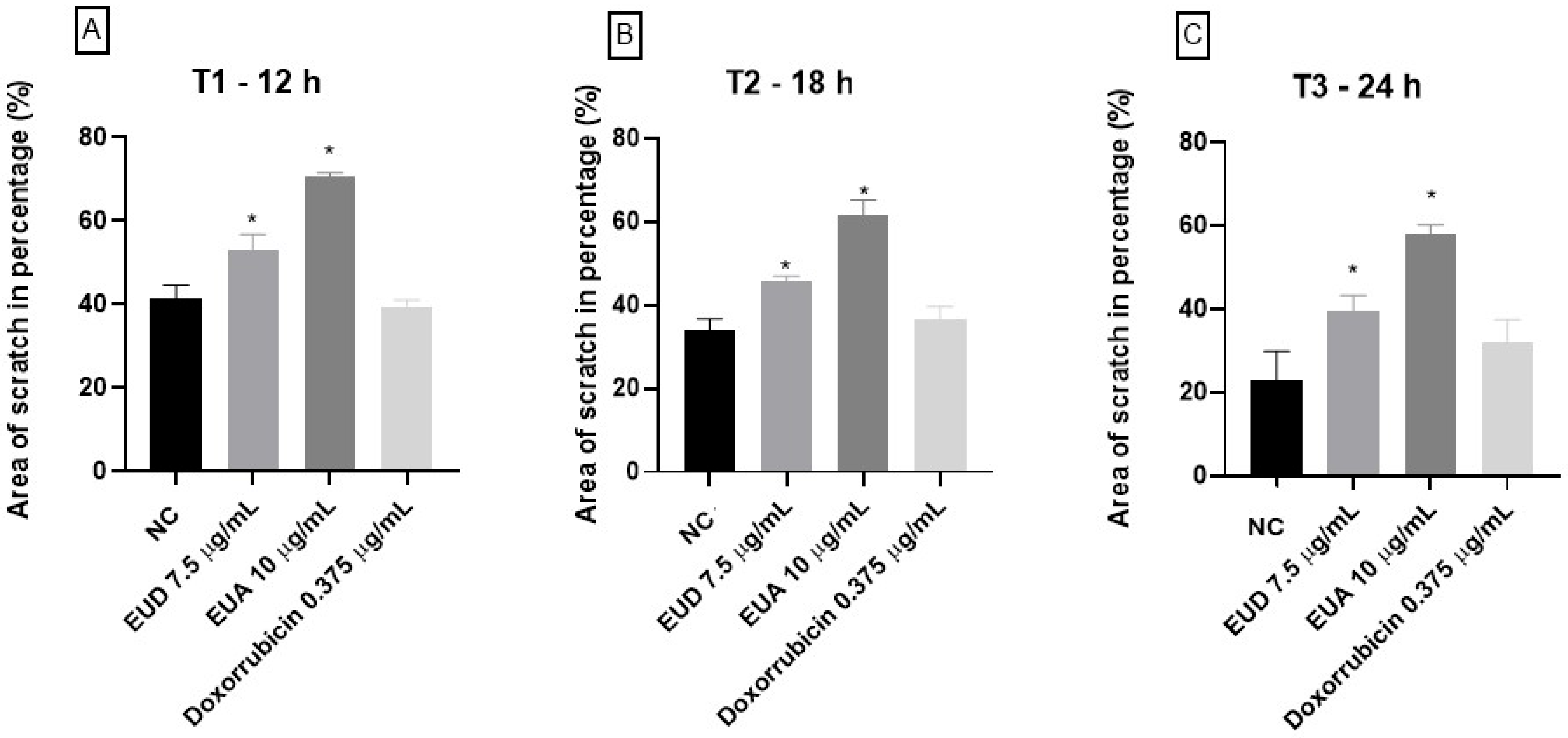
2.4. Cell Migration Assay—Scratch Assay
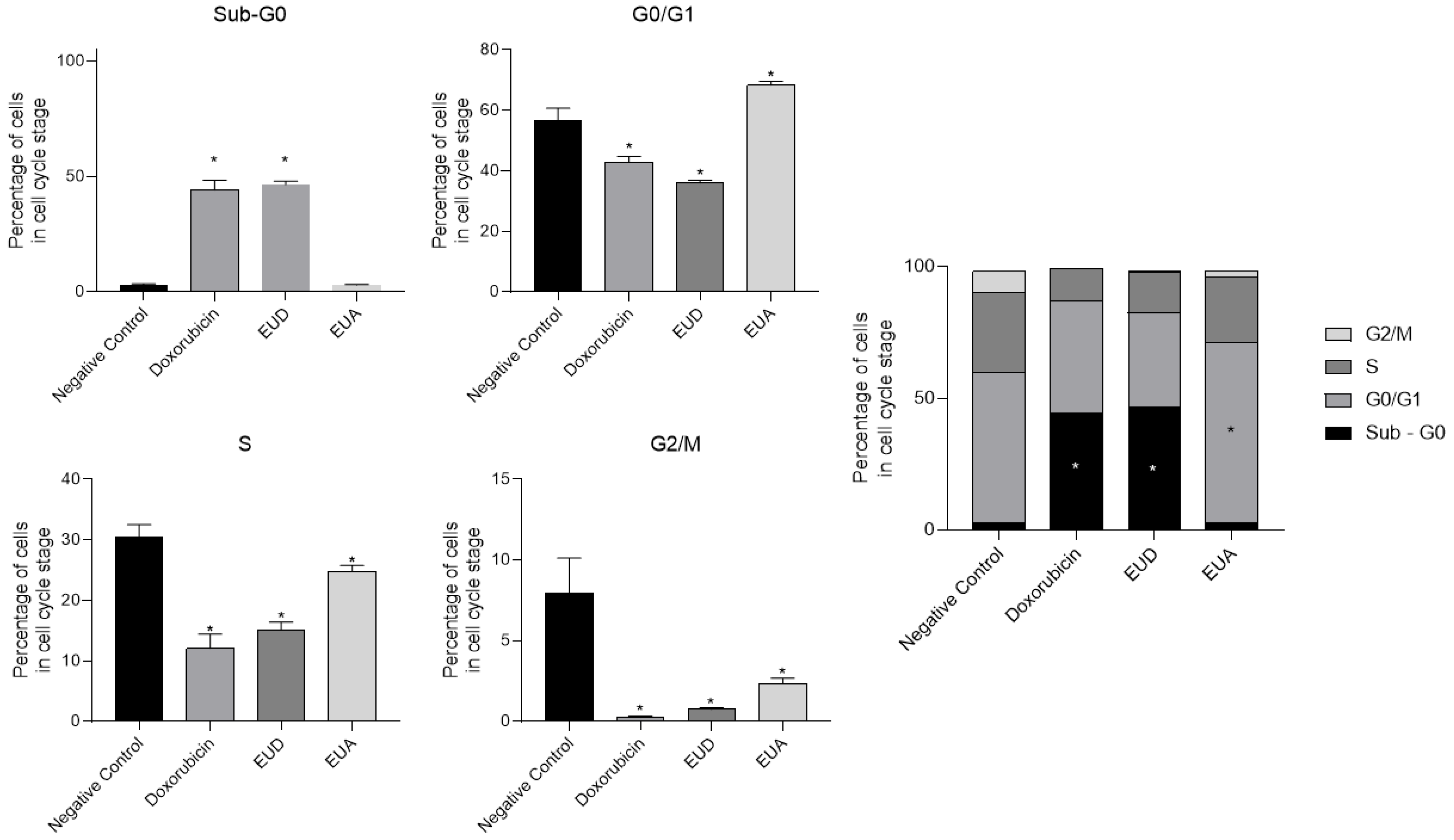
2.5. Cell Cycle Arrest Analysis Using Flow Cytometry
3. Discussion
4. Materials and Methods
4.1. Material Plant and Preparation of Extracts
4.2. Determination of Total Soluble Flavonoid Content
4.3. Determination of Total Soluble Phenolic Content
4.4. Chemical Profile Analysis of the Samples by LC-MS/MS
4.5. Cell Viability Assay
4.6. Selectivity Index (SI) Calculation
4.7. Cell Migration Assay
4.8. Cell Cycle Analysis by Flow Cytometry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.T.M.; Charret, T.S.; Wermelinger, G.F.; Nogueira, T.S.R.; Robbs, B.K.; Castiglione, R.C.; Simões, R.L.; Machado, R.L.D.; Vieira, I.J.C.; Abreu, L.S.; et al. Evaluation of the Antiproliferative Potential of yellow Jaboticaba (Myrciaria glazioviana) extracts against Human Cervical Cancer (HeLa cells line) and the Analysis of their Chemical Composition by HPLC-HRESIMS. Chem. Biodivers. 2024, 21, e202301467. [Google Scholar] [CrossRef]
- Drețcanu, G.; Iuhas, C.I.; Diaconeasa, Z. The involvement of natural polyphenols in the chemoprevention of cervical cancer. Int. J. Mol. Sci. 2021, 22, 8812. [Google Scholar] [CrossRef]
- HPV. Instituto Nacional de Câncer—INCA. 2022. Available online: https://www.gov.br/inca/pt-br/acesso-a-informacao/perguntas-frequentes/hpv (accessed on 6 May 2024).
- International Agency for Research on Cancer (IARC). 2022. Available online: https://www.iarc.who.int/cancer-type/cervical-cancer/ (accessed on 27 April 2025).
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Paci, A.; Veal, G.; Bardin, C.; Widmer, N.; Beijnen, J.; Astier, A.; Chatelut, E. Review of therapeutic drug monitoring of anticancer drugs part 1—Cytotoxics. Eur. J. Cancer 2014, 50, 2010–2019. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Núñez, J.G.; Pinheiro, J.S.; Silveira, G.F.; Beckenkamp, A.; Buffon, A.; Bruno, A. N Antineoplastic potential of the aqueous crude extract of Eugenia uniflora L. In human cervical cancer. Braz. J. Pharm. Sci. 2018, 54, e17267. [Google Scholar] [CrossRef]
- de Souza, P.S.O.; dos Santos, M.T.; Monteiro, R.G.; Espindola, M.T.A.; de Souza, H.J.S.; Monteiro, A.L.B.; Camara, C.d.A.; Silva, T.M.S. Tannins and flavonoids from the flowers of Eugenia uniflora (myrtaceae). Quim. Nova 2022, 45, 1083–1091. [Google Scholar]
- Rattmann, Y.D.; de Souza, L.M.; Malquevicz-Paiva, S.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.; Iacomini, M. Analysis of flavonoids from Eugenia uniflora leaves and its protective effect against murine sepsis. Evid.-Based Complement. Altern. Med. 2012, 2012, 623940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Dominguez-López, I.; Pérez, M.; Lamuela-Raventós, R.M. Total (poly)phenol analysis by the Folin-Ciocalteu assay as an anti-inflammatory biomarker in biological samples. Crit. Rev. Food Sci. Nutr. 2023, 64, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, S.R.S.; Scherruth, M.S.F.; Calixto, P.S.; Carraro, M.M.; Silveira, A.C.; Lazzaratto, M. Método de Folin Ciocalteau adaptado para quantificar polifenóis em extratos de erva-mate. Movimenta 2020, 13, 419–426. [Google Scholar]
- Alves, T.P.; Pereira, M.T.M.; Charret, T.S.; Thurler-Júnior, J.C.; Wermelinger, G.F.; Baptista, A.R.; Robbs, B.K.; Sawaya, A.C.H.F.; Pascoal, V.D.B.; Cristina Pascoal, A.C.R.F. Evaluation of the Antiproliferative Potential of Eugenia pyriformis Leaves in Cervical Cancer Cells. Chem. Biodivers. 2022, 19, e202200114. [Google Scholar] [CrossRef] [PubMed]
- Tambara, A.L.; Moraes, L.L.S.; Forno, A.H.; Boldori, J.R.; Soares, A.T.G.; Rodrigues, C.F.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Chen, M.; Cao, J.Q.; Ang, S.; Zeng, T.N.; Li, N.P.; Yang, T.J.; Liu, J.S.; Wu, Y.; Ye, W.C.; Wang, L. Eugenunilones A–H: Rearranged sesquiterpenoids from Eugenia uniflora. Org. Chem. Front. 2022, 9, 667–675. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.Q.; Guo, Y.; Chen, J.X.; Jin, Q.; Chen, M.H.; Chen, B.Y.; Tu, Z.C.; Ye, W.C.; Wang, L. Eugenilones A-N: Sesquiterpenoids from the fruits of Eugenia uniflora. Phytochemistry 2023, 211, 113699. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. An Overview of the Cell Cycle. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26869/ (accessed on 6 May 2024).
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Niu, Y.; Xu, J.; Sun, T. Cyclin-Dependent Kinases 4/6 Inhibitors in Breast Cancer: Current Status, Resistance, and Combination Strategies. J. Cancer 2019, 10, 5504–5517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Monteiro, J.R.B.; Ardisson, J.S.; Athaydes, B.R.; Gonçalves, R.C.R.; Rodrigues, R.P.; Kuster, R.M.; Kitagawa, R.R. Anti-Helicobacter pylori and Anti-inflammatory Properties of Eugenia uniflora L. Braz. Arch. Biol. Technol. 2019, 62, e19180285. [Google Scholar] [CrossRef]
- Correia, V.T.V.; Silva, P.R.; Ribeiro, C.M.S.; Ramos, A.L.C.C.; Mazzinghy, A.C.D.C.; Silva, V.D.M.; Júnior, A.H.O.; Nunes, B.V.; Vieira, A.L.S.; Ribeiro, L.V.; et al. An Integrative Review on the Main Flavonoids Found in Some Species of the Myrtaceae Family: Phytochemical Characterization. Health Benefits and Development of Products. Plants 2022, 11, 2796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismiyati, N.; Putri, D.D.P.; Kusumastuti, S.A.; Febriansyah, R. Antiproliferative Effect of Ethanolic Extract Eugenia uniflora Lam. Leaves on T47D Cells. Indones. J. Cancer Chemoprev. 2012, 3, 370–375. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Ma, L.; Chen, R.; Yuan, Q.; Zheng, Y.; Li, C.; Peng, G. Phenolic Compounds from Polygonum chinense Induce Growth Inhibition and Apoptosis of Cervical Cancer SiHa Cells. BioMed Res. Int. 2020, 2020, 8868508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, C.H.; Zhao, J.F.; Zhang, X.G.; Ding, C.H.; Hao, H.H.; Ji, Y.H.; Li, L.P.; Guo, Z.T.; Liu, W.S. Discovery of ellagic acid as a competitive inhibitor of Src homology phosphotyrosyl phosphatase 2 (SHP2) for cancer treatment: In vitro and in silico study. Int. J. Biol. Macromol. 2023, 254, 127845. [Google Scholar] [CrossRef] [PubMed]
- Oriola, A.O.; Miya, G.M.; Singh, M.; Oyedeji, A.O. Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities. Sci. Pharm. 2023, 91, 42. [Google Scholar] [CrossRef]
- Ahmad, S.; Sayeed, S.; Bano, N.; Sheikh, K.; Raza, K. In-silico analysis reveals Quinic acid as a multitargeted inhibitor against Cervical Cancer. J. Biomol. Struct. Dyn. 2022, 41, 9770–9786. [Google Scholar] [CrossRef]
- Modzelewska, A.; Sur, S.; Kumar, S.K.; Khan, S.R. Sesquiterpenes: Natural products that decrease cancer growth. Anti-Cancer Agents Med. Chem. 2005, 5, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [CrossRef] [PubMed]
| Samples | Total Soluble Phenolic (μg GAE/mg) | Total Flavonoid (μg E Quercetin/mg) |
|---|---|---|
| EUD | 10.71 ± 4.29 | 23.82 ± 1.31 |
| EUA | 45.94 ± 8.25 | 14.22 ± 3.44 |
| Peak No. | tR (min) | m/z [M + H]+ | m/z [M + Na]+ | m/z [M − H]− | Molecular Formula | Error (ppm) | MS/MS | Tentative Assignment | Ext | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.7 | - | - | 191.0560 | C7H12O6 | −5.0 | 127 | Quinic acid | A | [20] |
| 1.7 | - | - | 331.0671 | C13H16O10 | −3.4 | 221; 169; 125 | Galloyl hexoside | A | [20,21] | |
| 2 | 1.7 | - | - | 361.0768 | C14H18O11 | −0.7 | 191; 169; 125 | Galloylquinic acid | A | [20] |
| 3 | 2.3 | - | - | 169.0139 | C7H6O5 | −4.4 | 125 | Gallic acid | A | [20,21] |
| 4 | 8.3 | - | - | 463.0877 | C21H20O12 | −1.3 | 317; 316; 271 | Myricetin-O-rhamnoside | A | [20] |
| 5 | 10.3 | - | - | 300.9981 | C14H6O8 | −0.7 | 284; 257; 229; 201; 173 | Ellagic acid | A | [21] |
| 6 | 10.8 | 247.1318 | - | - | C15H18O3 | 4.3 | 145; 119; 105; 93 | Eugenunilone D or Eugenilone F | D and A | [22,23] |
| 7 | 10.8 | 265.1424 | - | - | C15H20O4 | 3.9 | 187; 164; 131; 125; 107; 91 | Eugenilone L I | D and A | [22,23] |
| 8 | 11.6 | - | 291.1580 | - | C17H22O4 | 4.4 | 257; 147; 119; 105; 91 | NI | D and A | [22,23] |
| 9 | 11.6 | - | 305.1371 | - | C17H20O5 | 4.1 | 185; 151; 114; 105; | NI | D and A | [22,23] |
| 10 | 12.3 | 245.1178 | - | - | C15H16O3 | −2.4 | 201; 156; 129; 105; 91 | NI | D and A | [22,23] |
| 11 | 12.3 | 263.1283 | - | - | C15H18O4 | −2.0 | 193; 156; 147; 105; 91 | Eugenilone B | D and A | [22,23] |
| 12 | 13.0 | 247.1339 | - | - | C15H18O3 | −4.2 | 145; 131; 119; 105; 93 | Eugenunilone D or Eugenilone F | D and A | [22,23] |
| 13 | 13.0 | 265.1445 | - | - | C15H20O4 | −4.0 | 229; 187; 164; 131; 125; 107; 91 | Eugenilone L II | D and A | [22,23] |
| 14 | 14.1 | 249.1497 | - | - | C15H20O3 | −4.7 | 185; 143; 131; 119; 105; 91 | Eugenunilone C or Eugenilone A or E | D and A | [22,23] |
| 15 | 14.1 | 265.1428 | - | - | C15H20O4 | 2.4 | 229; 179; 131; 119; 105; 91 | Eugenilone L III | D and A | [22,23] |
| 16 | 15.6 | 249.1499 | - | - | C15H20O3 | −5.5 | 185; 143; 131; 119; 105; 91 | Eugenunilone C or Eugenilone A or E | D and A | [22,23] |
| 17 | 15.6 | 231.1370 | - | - | C15H18O2 | 4.1 | 155; 128; 119; 105; 91 | Eugenunilone F or H | D and A | [22,23] |
| 18 | 17.0 | 289.1420 | - | - | C17H20O4 | 5.0 | 213; 179; 128; 119; 105; 91 | NI | D and A | [22,23] |
| 19 | 18.2 | 245.1174 | - | - | C15H16O4 | −0.7 | 171; 156; 119; 105; 91 | NI | D and A | [22,23] |
| 20 | 18.2 | 307.1550 | - | - | C17H22O5 | −3.3 | 183; 143; 117; 105; 91 | Eugenilone M or N | D and A | [22,23] |
| 21 | 18.7 | 249.1493 | - | - | C15H20O3 | −3.1 | 185; 143; 131; 128; 119; 105; 91 | Eugenunilone C or Eugenilone A or E | D and A | [22,23] |
| 22 | 20.4 | 247.1336 | - | - | C15H18O3 | −3.0 | 147; 131; 119; 105; 91 | Eugenunilone D or Eugenilone F | D and A | [22,23] |
| 23 | 20.4 | 307.1551 | - | - | C17H22O5 | −3.6 | 187; 159; 131; 105; 91 | Eugenilone M or N | D and A | [22,23] |
| 24 | 22.6 | 231.1392 | - | - | C15H18O2 | −5.4 | 209; 155; 142; 128; 119; 105; 91 | Eugenunilone F or H | D and A | [22,23] |
| 25 | 22.6 | 291.1599 | - | - | C17H22O4 | −2.8 | 213; 157; 142; 128; 119; 105; 91 | NI | D and A | [22,23] |
| 26 | 24.7 | 291.1603 | - | - | C17H22O4 | −4.2 | 213; 145; 105; 91 | NI | D and A | [22,23] |
| 27 | 26.4 | 289.1450 | - | - | C17H20O4 | −5.4 | 229; 183; 155; 109; 91 | NI | D and A | [22,23] |
| 28 | 26.4 | 329.1401 | - | - | C19H20O5 | −5.3 | 229; 183; 155; 149; 109; | NI | D and A | [22,23] |
| Samples | IC50 Tumor Cells HeLa (μg/mL) | IC50 Non-Tumor cells NIH/3T3 (μg/mL) | Selectivity Index (SI) |
|---|---|---|---|
| EUH | 63.03 | >100 | >6.3 |
| EUD | 33.75 | >100 | >2.96 |
| EUA | 38.38 | >100 | >2.60 |
| Doxorubicin | 1.591 | 2.321 | 1.5 |
| Carboplatin | 52.40 | 38.32 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescinelli, L.M.R.; Longue, M.F.; de Oliveira, G.G.F.V.; Thurler-Júnior, J.C.; Charret, T.S.; Nogueira, T.S.R.; Pereira, M.T.M.; Vieira, I.J.C.; Abreu, L.S.; Pascoal, V.D.B.; et al. Eugenia uniflora L.: Analysis of Chemical Profile and Cytotoxic Action on Tumor (HeLa) and Non-Tumor Cells (NIH/3T3). Pharmaceuticals 2025, 18, 1199. https://doi.org/10.3390/ph18081199
Pescinelli LMR, Longue MF, de Oliveira GGFV, Thurler-Júnior JC, Charret TS, Nogueira TSR, Pereira MTM, Vieira IJC, Abreu LS, Pascoal VDB, et al. Eugenia uniflora L.: Analysis of Chemical Profile and Cytotoxic Action on Tumor (HeLa) and Non-Tumor Cells (NIH/3T3). Pharmaceuticals. 2025; 18(8):1199. https://doi.org/10.3390/ph18081199
Chicago/Turabian StylePescinelli, Letícia M. R., Milena França Longue, Giovana G. F. V. de Oliveira, Júlio C. Thurler-Júnior, Thiago S. Charret, Thalya S. R. Nogueira, Mariana T. M. Pereira, Ivo J. C. Vieira, Lucas S. Abreu, Vinicius D. B. Pascoal, and et al. 2025. "Eugenia uniflora L.: Analysis of Chemical Profile and Cytotoxic Action on Tumor (HeLa) and Non-Tumor Cells (NIH/3T3)" Pharmaceuticals 18, no. 8: 1199. https://doi.org/10.3390/ph18081199
APA StylePescinelli, L. M. R., Longue, M. F., de Oliveira, G. G. F. V., Thurler-Júnior, J. C., Charret, T. S., Nogueira, T. S. R., Pereira, M. T. M., Vieira, I. J. C., Abreu, L. S., Pascoal, V. D. B., & Pascoal, A. C. R. F. (2025). Eugenia uniflora L.: Analysis of Chemical Profile and Cytotoxic Action on Tumor (HeLa) and Non-Tumor Cells (NIH/3T3). Pharmaceuticals, 18(8), 1199. https://doi.org/10.3390/ph18081199











