Impact of Gliflozins on Right Heart Remodeling in Italian Patients with Type 2 Diabetes and Heart Failure: Results from the GLISCAR Real-World Study
Abstract
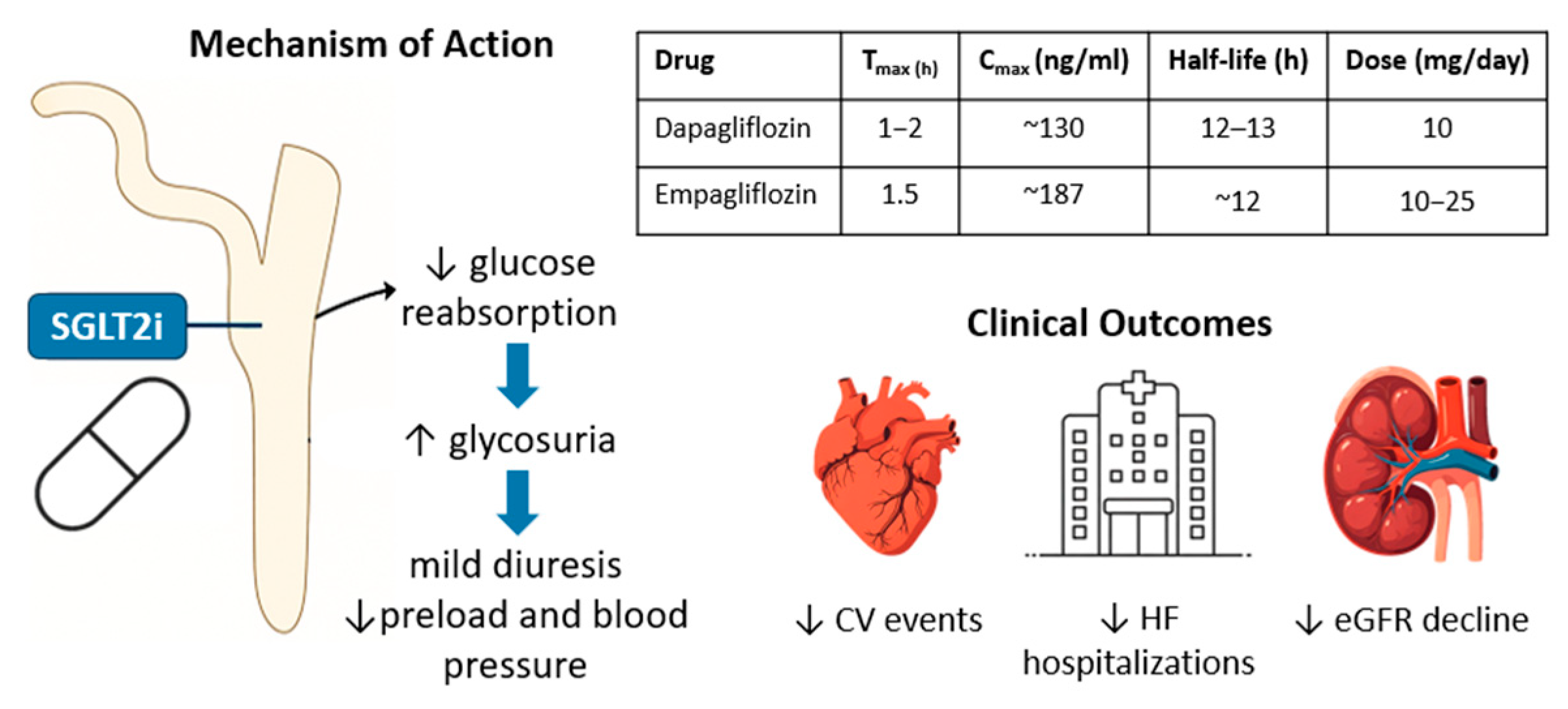
1. Introduction
2. Result
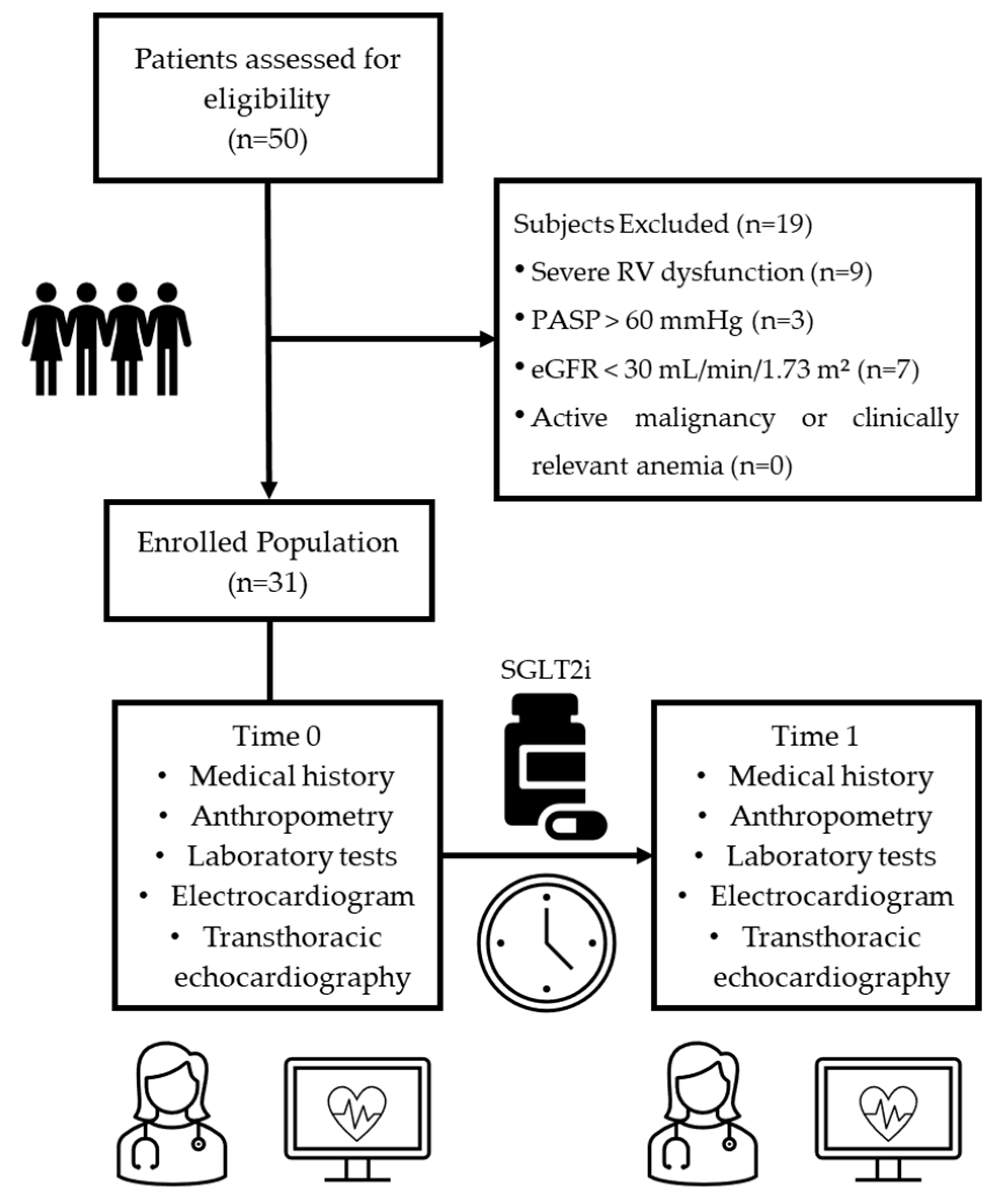
2.1. General and Clinical Characteristics of the Study Population at Baseline
2.2. Changes in Clinical Characteristics and Blood Sample Findings
2.3. Assessment of Echocardiography in the Study Population: Time 0’ and 1’ (12 Months Follow-Up)
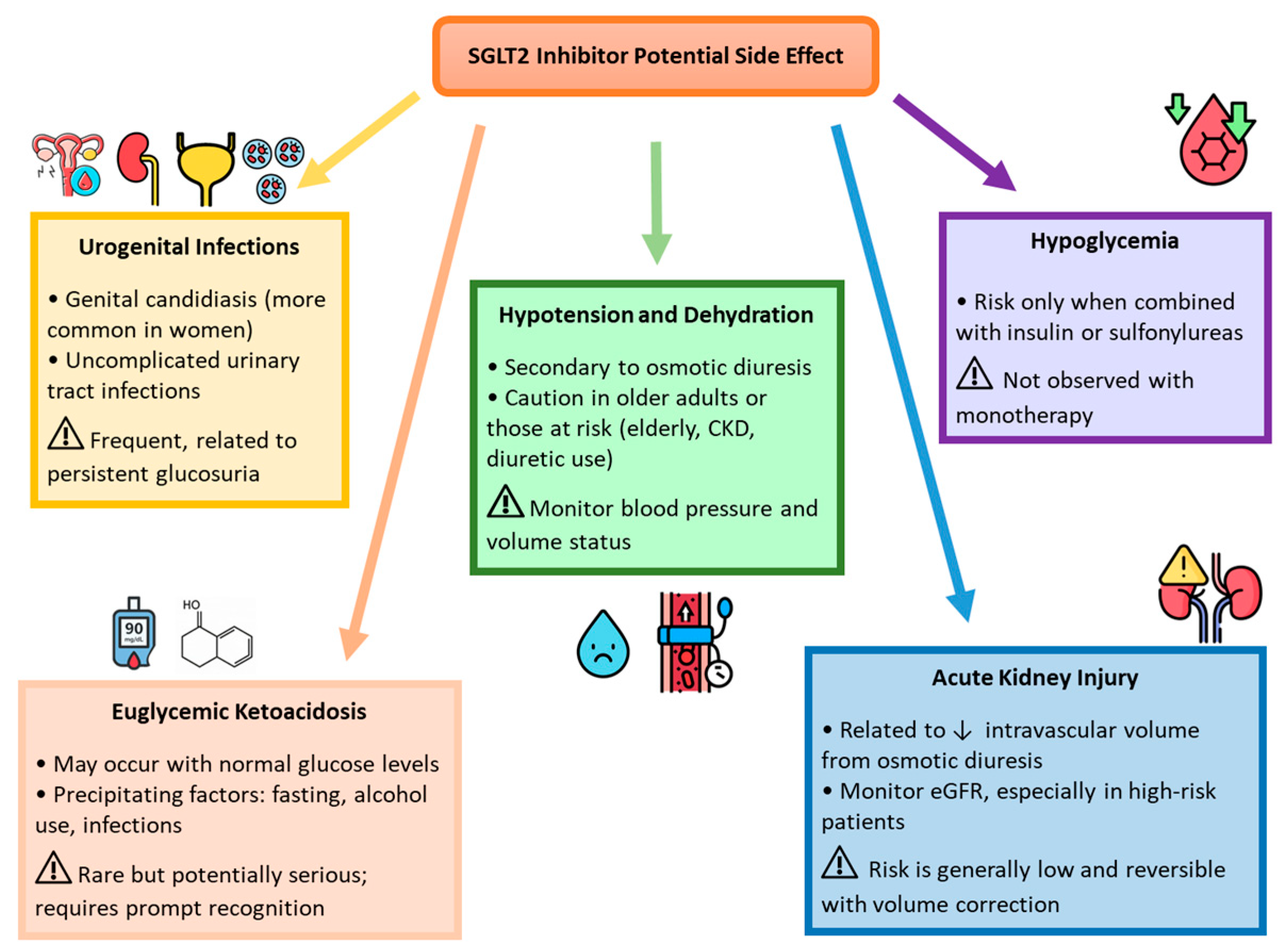
3. Discussion
3.1. Perspective for Clinical Practice
3.2. Limitations
4. Materials and Methods
4.1. Study Population
4.2. Clinical Assessment
4.3. Laboratory Assessment
4.4. Echocardiography
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palazzuoli, A.; Iacoviello, M. Diabetes Leading to Heart Failure and Heart Failure Leading to Diabetes: Epidemiological and Clinical Evidence. Heart Fail. Rev. 2023, 28, 585–596. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Goncalves, E. Heart Failure in the Patient with Diabetes: Epidemiology, Aetiology, Prognosis, Therapy and the Effect of Glucose-Lowering Medications. Diabetes Obes. Metab. 2019, 21, 1277–1290. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Pantanetti, P.; Cangelosi, G.; Alberti, S.; Di Marco, S.; Michetti, G.; Cerasoli, G.; Di Giacinti, M.; Coacci, S.; Francucci, N.; Petrelli, F.; et al. Changes in Body Weight and Composition, Metabolic Parameters, and Quality of Life in Patients with Type 2 Diabetes Treated with Subcutaneous Semaglutide in Real-World Clinical Practice. Front. Endocrinol. 2024, 15, 1394506. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Jiménez, B.; Rodríguez de Vera Gómez, P.; Belmonte Lomas, S.; Mesa Díaz, Á.M.; Caballero Mateos, I.; Galán, I.; Morales Portillo, C.; Martínez-Brocca, M.A. Transforming Body Composition with Semaglutide in Adults with Obesity and Type 2 Diabetes Mellitus. Front. Endocrinol. 2024, 15, 1386542. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J. Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation. Front. Endocrinol. 2021, 12, 645617. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes: Developed by the Task Force on the Management of Cardiovascular Disease in Patients with Diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Elrakaybi, A.; Laubner, K.; Zhou, Q.; Hug, M.J.; Seufert, J. Cardiovascular Protection by SGLT2 Inhibitors—Do Anti-Inflammatory Mechanisms Play a Role? Mol. Metab. 2022, 64, 101549. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Fudim, M.; Salah, H.M.; Heerspink, H.J.L.; Voors, A.A.; Ponikowski, P. Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure: Potential Decongestive Mechanisms and Current Clinical Studies. Eur. J. Heart Fail. 2023, 25, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Çamcı, S.; Yılmaz, E. Effects of Sodium-Glucose Co-Transporter-2 Inhibition on Pulmonary Arterial Stiffness and Right Ventricular Function in Heart Failure with Reduced Ejection Fraction. Medicina 2022, 58, 1128. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’Antonio, A.; Ambrosio, G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: Superiority of Longitudinal Strain over Tricuspid Annular Plane Systolic Excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef]
- Cinar, T.; Saylik, F.; Cicek, V.; Pay, L.; Khachatryan, A.; Alejandro, J.; Erdem, A.; Hayiroglu, M.I. Effects of SGLT2 Inhibitors on Right Ventricular Function in Heart Failure Patients: Updated Meta-Analysis of the Current Literature. Kardiol. Pol. 2024, 82, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R. Right Heart Phenotype in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007840. [Google Scholar] [CrossRef]
- Vijiiac, A.; Onciul, S.; Guzu, C.; Scarlatescu, A.; Petre, I.; Zamfir, D.; Onut, R.; Deaconu, S.; Dorobantu, M. Forgotten No More—The Role of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: An Echocardiographic Perspective. Diagnostics 2021, 11, 548. [Google Scholar] [CrossRef]
- Riccardi, M.; Pagnesi, M.; Corso, R.; Sammartino, A.M.; Tomasoni, D.; Inciardi, R.M.; Lombardi, C.M.; Adamo, M.; Nodari, S.; Metra, M. Prognostic Role of TAPSE to PASP Ratio in Outpatients with Left Ventricular Systolic Dysfunction. ESC Heart Fail. 2024, 12, 912–922. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Jiang, J.; Luo, Z.; Tan, X.; Ren, R.; Tanimura, T.L.; Wang, M.; Zhang, C. Right Ventricular–Pulmonary Arterial Coupling and Outcome in Heart Failure with Preserved Ejection Fraction. Clin. Cardiol. 2024, 47, e24308. [Google Scholar] [CrossRef]
- Antit, S.; Mrabet, A.; Fathi, M.; Fekih, R.; Boussabeh, E.; Zakhama, L. Tricuspid Annular Plane Systolic Excursion/Pulmonary Arterial Systolic Pressure Ratio as a Predictor of Outcome in Acute Heart Failure. Tunis. Medicale 2025, 103, 104–111. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, M.; Zhang, L.; He, Q.; Gao, L.; Ji, M.; Lin, Y.; Li, Y. Prognostic Implication of Right Ventricular–Pulmonary Artery Coupling in Valvular Heart Disease. Front. Cardiovasc. Med. 2025, 11, 1504063. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Galiero, R.; Loffredo, G.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Salvatore, T.; Ruggiero, R.; Di Palo, M.R.; et al. Impact of Gliflozins on Cardiac Remodeling in Patients with Type 2 Diabetes and Reduced Ejection Fraction Heart Failure: A Pilot Prospective Study. GLISCAR Study. Diabetes Res. Clin. Pract. 2023, 200, 110686. [Google Scholar] [CrossRef] [PubMed]
- Gamaza-Chulián, S.; Díaz-Retamino, E.; González-Testón, F.; Gaitero, J.C.; Castillo, M.J.; Alfaro, R.; Rodríguez, E.; González-Caballero, E.; Martín-Santana, A. Effect of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Left Ventricular Remodelling and Longitudinal Strain: A Prospective Observational Study. BMC Cardiovasc. Disord. 2021, 21, 456. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Tse, G.; Korantzopoulos, P.; Letsas, K.P.; Zhang, Q.; Li, G.; Lip, G.Y.H.; Liu, T. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Cardiac Remodelling: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2022, 28, 1961–1973. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lamet, L.S.P.; Martinez, F.; et al. SGLT-2 Inhibitors in Patients with Heart Failure: A Comprehensive Meta-Analysis of Five Randomised Controlled Trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Abbate, M.; Fusco, F.; Scognamiglio, G.; Merola, A.; Palma, M.; Grimaldi, N.; Barracano, R.; Borrelli, N.; Ppaccioli, G.; Sorice, D.; et al. Dapagliflozin in Adults with a Systemic Right Ventricle: Initial Results from the DAPA-SERVE Study. Eur. Heart J. 2023, 44, ehad655.1914. [Google Scholar] [CrossRef]
- Amsallem, M.; Mercier, O.; Kobayashi, Y.; Moneghetti, K.; Haddad, F. Forgotten No More: A Focused Update on the Right Ventricle in Cardiovascular Disease. JACC Heart Fail. 2018, 6, 891–903. [Google Scholar] [CrossRef]
- Chemla, D.; Berthelot, E.; Assayag, P.; Attal, P.; Hervé, P. Physiopathologie Hémodynamique du Ventricule Droit [Pathophysiology of Right Ventricular Hemodynamics]. Rev. Mal. Respir. 2018, 35, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, J.S.; Nielsen-Kudsk, A.H.; Schwab, J.; Ringgaard, S.; Nielsen-Kudsk, J.E.; de Man, F.S.; Andersen, A.; Andersen, S. Effects of Empagliflozin on Right Ventricular Adaptation to Pressure Overload. Front. Cardiovasc. Med. 2023, 10, 1302265. [Google Scholar] [CrossRef]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Seno, H.; Yamada, H.; Nishio, S.; Torii, Y.; Hirata, Y.; Saijo, Y.; Ise, T.; Yamaguchi, K.; Fukuda, D.; et al. Right Ventricular Function and Beneficial Effects of Cardiac Rehabilitation in Patients with Systolic Chronic Heart Failure. Can. J. Cardiol. 2018, 34, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, A.; Rauwolf, T.; Groscheck, T.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Steendijk, P.; Braun-Dullaeus, C.R. Pressure-Volume Loop Validation of TAPSE/PASP for Right Ventricular Arterial Coupling in Heart Failure with Pulmonary Hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rako, Z.A.; Kremer, N.; Yogeswaran, A.; Richter, M.J.; Tello, K. Adaptive versus Maladaptive Right Ventricular Remodelling. ESC Heart Fail. 2023, 10, 762–775. [Google Scholar] [CrossRef]
- Saucedo-Orozco, H.; Voorrips, S.N.; Yurista, S.R.; de Boer, R.A.; Westenbrink, B.D. SGLT2 Inhibitors and Ketone Metabolism in Heart Failure. J. Lipid Atheroscler. 2022, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Russo, V.; Malvezzi Caracciolo D’Aquino, M.; Caturano, A.; Scognamiglio, G.; Pezzullo, E.; Fabiani, D.; Del Giudice, C.; Carbone, A.; Bottino, R.; Caso, V.; et al. Improvement of Global Longitudinal Strain and Myocardial Work in Type 2 Diabetes Patients on Sodium-Glucose Cotransporter 2 Inhibitors Therapy. J. Cardiovasc. Pharmacol. 2023, 82, 196–200. [Google Scholar] [CrossRef]
- Nevola, R.; Alfano, M.; Pafundi, P.C.; Brin, C.; Gragnano, F.; Calabrò, P.; Adinolfi, L.E.; Rinaldi, L.; Sasso, F.C.; Caturano, A. Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in the Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev. Cardiovasc. Med. 2022, 23, 106. [Google Scholar] [CrossRef]
- Rivera, F.B.; Tang, V.A.S.; De Luna, D.V.; Lerma, E.V.; Vijayaraghavan, K.; Kazory, A.; Shah, N.S.; Volgman, A.S. Sex Differences in Cardiovascular Outcomes of SGLT-2 Inhibitors in Heart Failure Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Am. Heart J. Plus 2023, 26, 100261. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Gender Difference in Cardiovascular Outcomes with SGLT-2 Inhibitors and GLP-1 Receptor Agonist in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Diabetes Metab. Syndr. 2020, 14, 181–187. [Google Scholar] [CrossRef]
- Wittnich, C.; Tan, L.; Wallen, J.; Belanger, M. Sex Differences in Myocardial Metabolism and Cardiac Function: An Emerging Concept. Pflugers Arch. 2013, 465, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Filippatos, G.; Siddiqi, T.J.; Ferreira, J.P.; Brueckmann, M.; Bocchi, E.; Böhm, M.; Chopra, V.K.; Giannetti, N.; Iwata, T.; et al. Effects of Empagliflozin in Women and Men with Heart Failure and Preserved Ejection Fraction. Circulation 2022, 146, 1046–1055. [Google Scholar] [CrossRef]
- Obokata, M.; Olson, T.P.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Borlaug, B.A. Haemodynamics, Dyspnoea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2018, 39, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Sorimachi, H.; Omar, M.; Popovic, D.; Alogna, A.; Jensen, M.D.; Borlaug, B.A. Dapagliflozin and Right Ventricular–Pulmonary Vascular Interaction in Heart Failure with Preserved Ejection Fraction: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 843–851. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Angermann, C.E.; Collins, S.P.; Teerlink, J.R.; Ponikowski, P.; Biegus, J.; Comin-Colet, J.; Ferreira, J.P.; Mentz, J.R.; Nassif, M.E.; et al. Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results from the EMPULSE Trial. Circulation 2022, 146, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Oriecuia, C.; Tomasoni, D.; Sala, I.; Bonfioli, G.B.; Adamo, M.; Gussago, C.; Lombardi, C.M.; Pagnesi, M.; Savarese, G.; Metra, M.; et al. Sodium Glucose Co-Transporter 2 Inhibitors and Quality of Life in Patients with Heart Failure: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 147–157. [Google Scholar] [CrossRef]
- Iatridi, F.; Carrero, J.J.; Cornec-Le Gall, E.; Kanbay, M.; Luyckx, V.; Shroff, R.; Ferro, C.J. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105 (Suppl. 4), S117–S314. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (n = 31) |
|---|---|
| Age, yrs, mean (SD) | 63.10 (15.71) |
| Sex, n (%) | |
| Male | 24 (76.7) |
| Female | 7 (23.3) |
| Height, mean (SD) | 170.55 (9.30) |
| NYHA Class, n (%) | 12 (19.0) |
| I | - |
| II | 17 (54.9) |
| III | 13 (41.9) |
| IV | 1 (3.2) |
| Arterial Hypertension, n (%) | 28 (93.3) |
| Duration of Heart Failure, yrs, median [IQR] | 9.5 (8.2–11.5) |
| Ischemic etiology, n (%) | 22 (73.3) |
| Dilated cardiomyopathy, n (%) | 9 (26.7) |
| Atrial fibrillation, n (%) | 9 (30.0) |
| Smoking, n (%) | 9 (30.0) |
| Retinopathy, n (%) | 5 (16.7) |
| Neuropathy, n (%) | 4 (13.3) |
| CKD, n (%) | 9 (30.0) |
| Beta-blockers, n (%) | 31 (100) |
| ACE-i, n (%) | 7 (22) |
| Sartans, n (%) | 10 (32) |
| Nepryl-i, n (%) | 21 (68) |
| Anti-Mineralcorticoids, n (%) | 24 (77) |
| Diuretics, n (%) | 26 (84) |
| Ivabradine, n (%) | 3 (10) |
| Ranolazine, n (%) | 2 (6) |
| Ca2+-Antagonists, n (%) | 6 (20) |
| Digoxin, n (%) | 3 (10) |
| Nitrates, n (%) | 1 (3) |
| Metformin, n (%) | 9 (30) |
| Insulin, n (%) | 6 (19) |
| Acetylsalicylic acid, n (%) | 22 (70) |
| P2Y12-i, n (%) | 22 (70) |
| Oral anticoagulant, n (%) | 5 (16.1) |
| Ezetimibe, n (%) | 18 (58) |
| Statins, n (%) | 25 (80) |
| PCSK9-i, n (%) | 6 (19.3) |
| Progression Time | |||
|---|---|---|---|
| Variable | Time 0 | Time 1 (=12 Months) | p |
| Weight, median [IQR] | 89.00 [72.50–97.00] | 86.50 [67.75–92.75] | <0.001 |
| BMI, median [IQR] | 29.39 [25.69–32.71] | 29.00 [24.25–33.00] | <0.001 |
| SBP, mean (SD) | 111.40 (15.98) | 119 (10.20) | 0.277 |
| DBP, mean (SD) | 64.17 (10.51) | 70.66 (8.71) | 0.178 |
| Hb, mean (SD) | 12.81 (2.29) | 13.57 (1.92) | 0.298 |
| HcT, mean (SD) | 38.20 (4.60) | 40.88 (7.78) | 0.833 |
| HbA1c, mean (SD) | 8.85 (2.20) | 6.72 (0.83) | <0.001 |
| Creatinine, mean (SD) | 1.01 (0.31) | 1.16 (0.41) | 0.248 |
| eGFR, median [IQR] | 63.00 [52.50–85.00] | 63.5 [46.5–76.5] | 0.206 |
| Total cholesterol, median [IQR] | 128.00 [103.25–174.75] | 130.00 [111.00–162.00] | 0.978 |
| LDL cholesterol, median [IQR] | 66.00 [40.00–91.00] | 62.00 [43.0–81.5] | 0.597 |
| HDL cholesterol, median [IQR] | 38.50 [31.50–54.00] | 40.00 [31.25–56.50] | 0.211 |
| Triglyceride, median [IQR] | 112.00 [87.50–147.50] | 111.00 [81.00–126.50] | 0.229 |
| NT Pro-BNP, median [IQR] | 1159.00 [892.50–2758.50] | 538.00 [328.25–2458.00] | 0.027 |
| Progression Time | |||
|---|---|---|---|
| Variable | Time 0 | Time 1 (=12 Months) | p |
| LVEDD (mm), mean (SD) | 61.29 (7.93) | 58.34 (7.03) | 0.014 |
| LVESD (mm), mean (SD) | 49.61 (10.11) | 45.03 (9.01) | 0.003 |
| IVSD (mm), median [IQR] | 11.00 [9.50–12.00] | 11.00 [10.00–12.00] | 0.006 |
| LVEDV (ml), mean (SD) | 183.52 (59.00) | 171 (57.37) | 0.009 |
| LVESV (ml), mean (SD) | 123.45 (46.96) | 106.10 (44.02) | 0.007 |
| LVEF (%), mean (SD) | 33.06 (5.36) | 38.71 (7.21) | 0.075 |
| GLS (%), median [IQR] | −8.40 [−10.30–−7.80] | −9.70 [−8.05–−12.05] | 0.009 |
| LAD (mm), median [IQR] | 46.00 [42.50–50.00] | 43.00 [41.00–49.00] | 0.026 |
| RAA (cm2), mean (SD) | 17.12 (3.61) | 16.73 (3.37) | 0.100 |
| RAEI (ml), mean (SD) | 78.22 (51.69) | 92.00 (48.40) | 0.003 |
| TRAEF (%), mean (SD) | 40 (15.16) | 45.05 (13.00) | 0.012 |
| RVD1 (mm), mean (SD) | 39.55 (5.43) | 40.00 (5.39) | 0.069 |
| RVD2 (mm), mean (SD) | 30.87 (5.77) | 32.21 (5.90) | 0.363 |
| RVD3 (mm), mean (SD) | 75.84 (10.86) | 75.37 (8.83) | 0.001 |
| TAPSE (mm), mean (SD) | 18.00 (4.23) | 19.40 (4.13) | 0.035 |
| FAC (%), mean (SD) | 39.20 (7.17) | 40.03 (7.13) | <0.001 |
| PASP (mmHg), mean (SD) | 35.23 (14.61) | 30.89 (7.77) | <0.001 |
| TAPSE/PAPs ratio, mean (SD) | 0.58 (0.24) | 0.69 (0.23) | 0.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetrano, E.; Galiero, R.; Simeon, V.; Palmiero, G.; Cesaro, A.; Caturano, A.; Rinaldi, L.; Salvatore, T.; Ruggiero, R.; Di Palo, M.R.; et al. Impact of Gliflozins on Right Heart Remodeling in Italian Patients with Type 2 Diabetes and Heart Failure: Results from the GLISCAR Real-World Study. Pharmaceuticals 2025, 18, 1200. https://doi.org/10.3390/ph18081200
Vetrano E, Galiero R, Simeon V, Palmiero G, Cesaro A, Caturano A, Rinaldi L, Salvatore T, Ruggiero R, Di Palo MR, et al. Impact of Gliflozins on Right Heart Remodeling in Italian Patients with Type 2 Diabetes and Heart Failure: Results from the GLISCAR Real-World Study. Pharmaceuticals. 2025; 18(8):1200. https://doi.org/10.3390/ph18081200
Chicago/Turabian StyleVetrano, Erica, Raffaele Galiero, Vittorio Simeon, Giuseppe Palmiero, Arturo Cesaro, Alfredo Caturano, Luca Rinaldi, Teresa Salvatore, Roberto Ruggiero, Maria Rosaria Di Palo, and et al. 2025. "Impact of Gliflozins on Right Heart Remodeling in Italian Patients with Type 2 Diabetes and Heart Failure: Results from the GLISCAR Real-World Study" Pharmaceuticals 18, no. 8: 1200. https://doi.org/10.3390/ph18081200
APA StyleVetrano, E., Galiero, R., Simeon, V., Palmiero, G., Cesaro, A., Caturano, A., Rinaldi, L., Salvatore, T., Ruggiero, R., Di Palo, M. R., Sardu, C., Marfella, R., Calabrò, P., & Sasso, F. C. (2025). Impact of Gliflozins on Right Heart Remodeling in Italian Patients with Type 2 Diabetes and Heart Failure: Results from the GLISCAR Real-World Study. Pharmaceuticals, 18(8), 1200. https://doi.org/10.3390/ph18081200











