Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential
Abstract
1. Introduction
2. Flavonoids
3. Chrysin
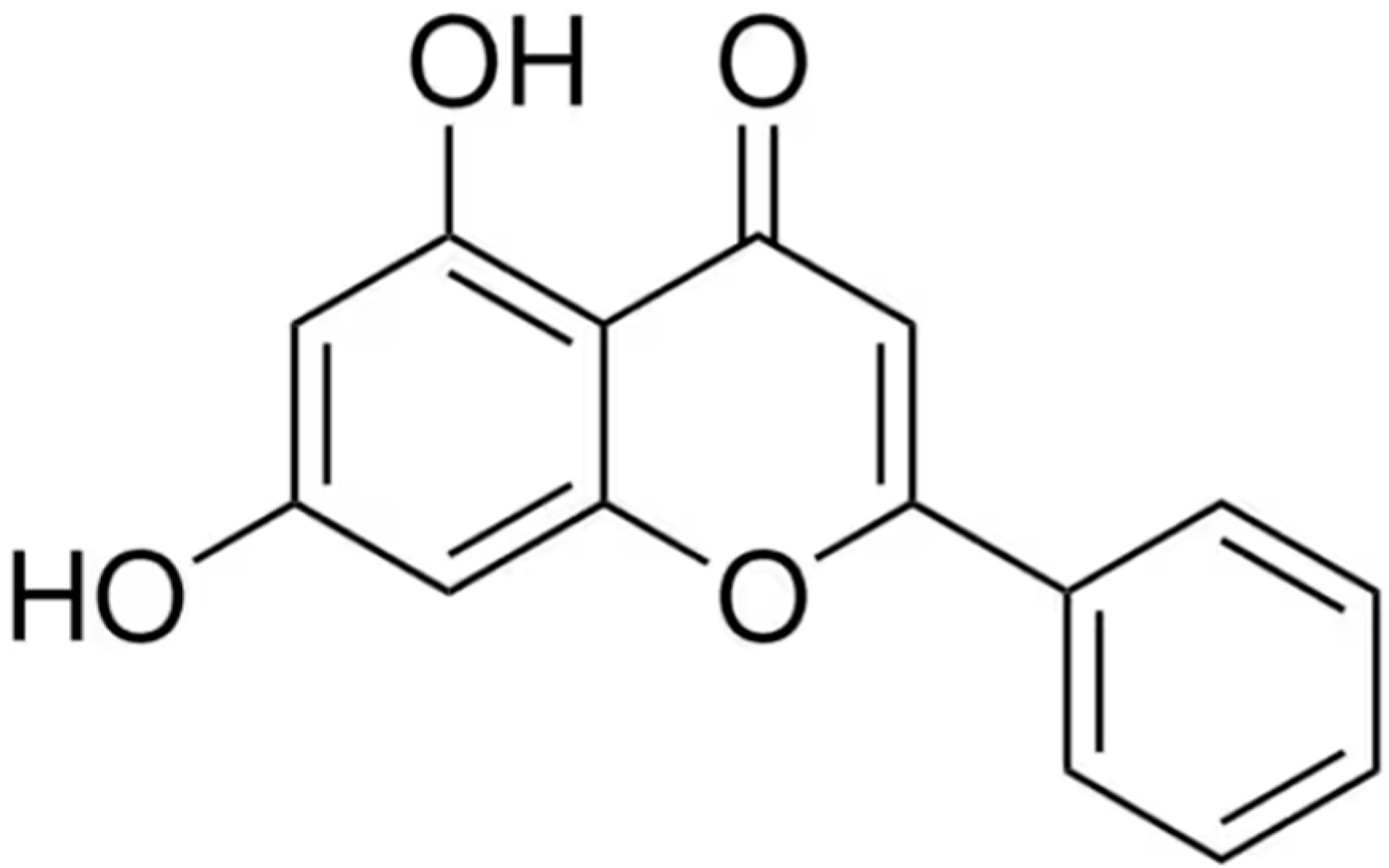
3.1. Chemical Structure
| Characteristic | Chrysin | Quercetin | Apigenin | Catechins (e.g., EGCG from Green Tea) | Genistein |
|---|---|---|---|---|---|
| Main sources | Propolis, honey, passionflower (Passiflora) [20,21] | Onion, apples, broccoli, capers, grapes [20,21] | Chamomile, celery, parsley, oranges [20,21]. | Green tea, black tea, cocoa, berries [20,21]. | Soy and its products (tofu, soy milk) [20,21]. |
| In vitro efficacy | High anti-inflammatory, antioxidant, and anticancer activity. Aromatase inhibitor [20,21]. | Very high antioxidant and anti-inflammatory activity. Antiviral and anticancer effects [20,21]. | Strong anxiolytic, calming, anti-inflammatory, and anticancer effects [20,21]. | Exceptionally strong antioxidant and anticancer effects. Supports metabolism and cardiovascular health [20,21]. | Estrogen-like activity (phytoestrogen). Strong anticancer properties (breast and prostate cancer) [20,21]. |
| Bioavailability | Very low. It is rapidly metabolized in the intestines and liver (first-pass effect), which drastically limits its concentration in the bloodstream. Delivery systems (e.g., nanoparticles) are required [22]. | Low to moderate. Better than chrysin, but it also undergoes extensive metabolism. Its absorption improves in the presence of fats and vitamin C [23,24]. | Low. Similar metabolism issues as with other flavonoids, but it shows the ability to cross the blood-brain barrier [25,26]. | Moderate. Bioavailability is variable and relatively low, but sufficient to produce biological effects with regular tea consumption [27,28]. | Moderate to high. One of the most well-absorbed flavonoids, especially in Asian populations that regularly consume soy [29,30]. |
| Key therapeutic benefits | Potential anticancer effects (mainly observed in laboratory studies). | Support for the cardiovascular system (blood pressure reduction). | Natural calming and sleep aid (acts on GABA receptors). | Strong cancer prevention. | Relief of menopause symptoms. |
| Neuroprotective and anxiolytic properties. | Reduction of inflammation and allergy symptoms. | Cancer prevention. | Heart and brain protection (neuroprotective and cardioprotective effects). | Osteoporosis prevention. | |
| Support in bodybuilding as an aromatase inhibitor (effect not clinically confirmed) [21]. | Immune support (antiviral activity) [20,21,22,23]. | Skin health (used in cosmetics) [25,31]. | Support for weight management and type 2 diabetes treatment [27,32]. | Significant role in prevention and treatment of hormone-dependent cancers [20,21,29]. | |
| Limitations and considerations | The main limitation is extremely low bioavailability, which undermines its therapeutic effectiveness in vivo without advanced delivery systems [22]. | It may interact with certain medications (e.g., blood thinners) and chemotherapy [23,24]. | It may enhance the effects of sedative medications [25,26]. | High doses may be toxic to the liver. It may also inhibit iron absorption [33]. | Due to its phytoestrogenic effects, its supplementation is controversial in patients with hormone-dependent cancers [21,29,30]. |
3.2. Chemical Properties Derived from the Structure
- Hydroxylation: The introduction of additional hydroxyl groups, especially into the B ring, leads to the formation of other well-known flavonoids, such as apigenin (–OH group at the 4′ position) or luteolin (–OH groups at the 3′ and 4′ positions). This process can mimic natural biosynthetic pathways found in plants [42].
- Glycosylation: The attachment of sugar molecules to hydroxyl groups improves the compound’s water solubility and affects its bioavailability [44].
3.3. Chrysin as a Precursor for the Synthesis of Prodrugs and Carriers
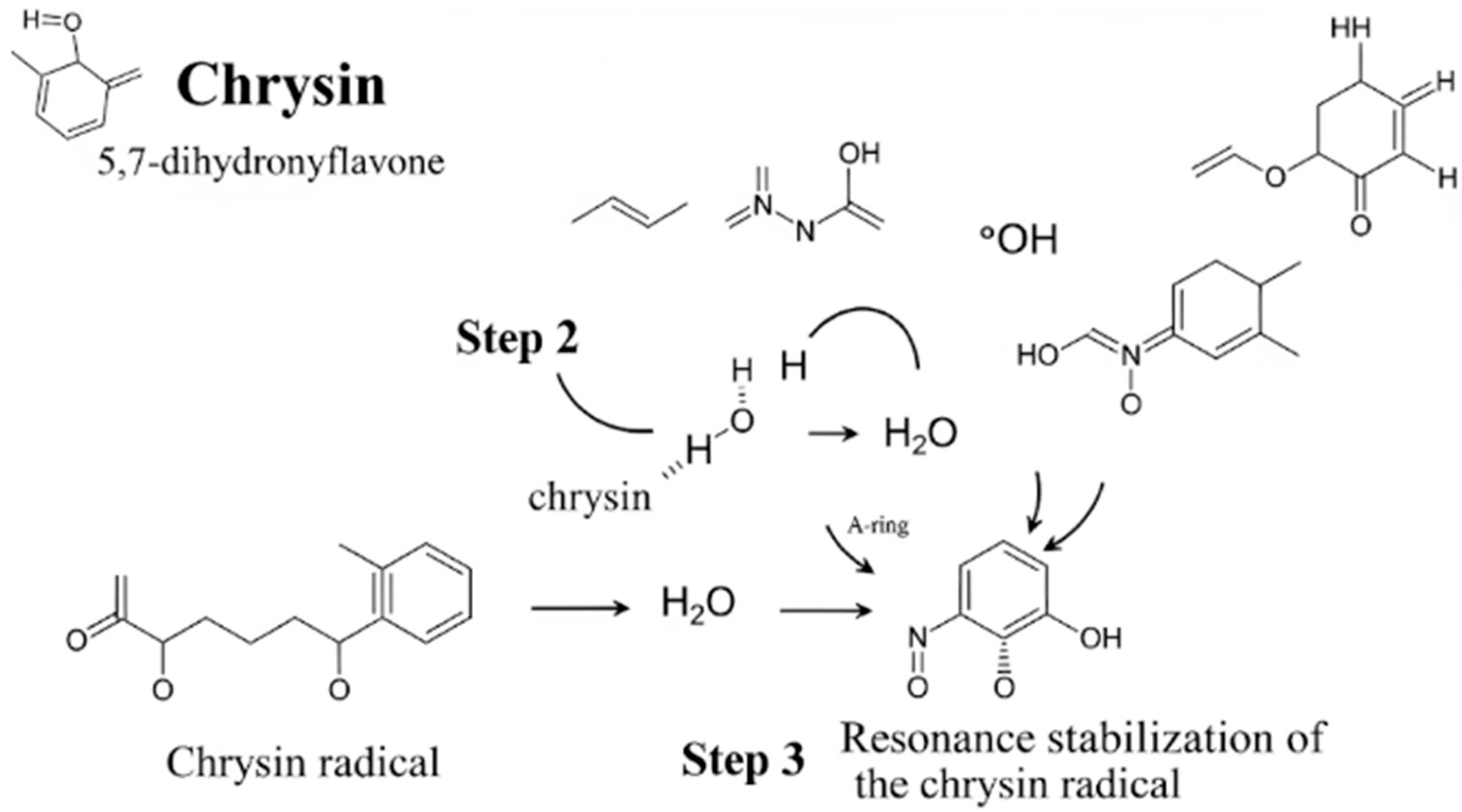
4. Mechanisms of Action of Chrysin
Anticancer Activity in Various Types of Cancer
5. Methods of Phytochemical Transport into the Cell
6. The Most Important Limitations in the Use of Chrysin
6.1. Extremely Low Bioavailability and Poor Absorption
6.2. Lack of Solid Evidence from Clinical Trials in Humans
6.3. Questionable Efficacy as an Aromatase Inhibitor in Humans
6.4. Potential Drug Interactions
6.5. Lack of Regulation and Standardization of Supplements
6.5.1. Anti-Inflammatory and Antioxidant Activity
6.5.2. Neuroprotective Potential
6.5.3. Summary and Contextual Relevance
7. Conclusions
Perspectives and Future Research Directions
- Development of novel drug delivery systems: Research on formulations (e.g., nanoparticles, liposomes, cyclodextrin complexes) to increase the bioavailability and solubility of chrysin.
- Synthesis and biological evaluation of new derivatives: Design and synthesis of modified chrysin analogs with increased potency, selectivity towards biological targets (e.g., cancer cells), and improved pharmacokinetic properties.
- Studies on synergistic mechanisms: Evaluating the efficacy of chrysin in combination therapies with conventional chemotherapeutics to potentially reduce drug doses and limit their toxicity.
- Advanced preclinical and clinical trials: Conducting detailed studies in animal models and, in the longer term, well-designed clinical trials to confirm its efficacy and safety in humans.
- Exploration of new therapeutic targets: Investigating the effect of chrysin on other, less-studied signaling pathways and pathological processes, such as autophagy, cellular senescence, or metabolic disorders.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, E.L.; Sales Maia, B.H.L.N.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. Intech 2017, 13, 78–94. [Google Scholar]
- Majewska, M.; Czeczot, H. Flawonoidy w profilaktyce i terapii. Terapia I Leki 2009, 65, 369–377. [Google Scholar]
- Koes, R.E.; Quattrocchio, F. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Simmonds, M.S. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.; Stevenson, P.C. Effects of Isoflavonoids from Cicer on Larvae of Heliocoverpa armigera. J. Chem. Ecol. 2001, 27, 965–977. [Google Scholar] [CrossRef]
- Tsuboi, T.; Lu, R.; Yonezawa, T.; Watanbe, A.; Woo, J.-T.; Abe-Dohmae, S.; Yokoyama, S. Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis 2020, 297, 32–37. [Google Scholar] [CrossRef]
- Feng, X.; Haohan, Q.; Shi, Q.; Zhang, Y.; Zhou, F.; Haochen, W.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef]
- Siddiqui, A.; Akhtar, J.; Uddin, M.S.S.; Khan, M.I.; Khalid, M.; Ahmad, M. A Naturally Occurring Flavone (Chrysin): Chemistry, Occurrence, Pharmacokinetic, Toxicity, Molecular Targets and Medicinal Properties. J. Biol. Act. Prod. Nat. 2018, 8, 208–227. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Baernatoniene, J.; Samarghadian, S. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Samarghandian, A.; Farkhondeh, T.; Azimi-Nezhad, M. Protective effects of chrysin against drugs and toxic agents. Dose Response 2017, 15, 1559325817711782. [Google Scholar] [CrossRef]
- Piska, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Edible mushroom Pleurotus ostreatus (Oyster mushroom)—Its dietary significance and biological activity. Acta Sci. Pol. Horotrum Cultus 2017, 16, 151–161. [Google Scholar]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2018, 38, 107316. [Google Scholar] [CrossRef]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing nutritional component chrysin as a therapeutic agent: Bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, R.; Liao, X.; Aa, J.; Wang, G. UDP-glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: A systematic review of UGT isoforms for precision medicine. Pharm. Res. 2017, 121, 169–183. [Google Scholar] [CrossRef]
- Quan, E.; Wang, H.; Dong, D.; Zhang, X.; Wu, B. Characterization of chrysin glucuronidation in UGT1A1-overexpressing HeLa cells: Elucidating the transporters responsible for efflux of glucuronide. Drug Metab. Dispos. 2015, 43, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Otake, Y.; Galijatovic, A.; Ritter, J.K.; Walle, U.K. Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in the human hepatoma cell line Hep G2. Drug Metab. Dispos. 2000, 28, 1077–1082. [Google Scholar] [CrossRef]
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica 1999, 29, 1241–1256. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243–255. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Michala, A.S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, B.E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, and Bioactivities. J. Food Sci. 2019, 84, 2767–2780. [Google Scholar]
- Lambert, J.D.; Yang, C.S. Cancer chemopreventive activity and bioavailability of tea polyphenols. J. Nutr. 2003, 133, 3248–3254. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Park, J. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int. J. Mol. Med. 2016, 38, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Hase, T.; Tokimitsu, I. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 1001–1008. [Google Scholar] [CrossRef]
- Ho, C.K.; Choi, S.W.; Fung, M.S.; Benzie, I.F.F. Tea polyphenols: Absorption, bioavailability and potential toxicity. CAB Rev. 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Chandra, P.; Pathak, R.; Sachan, N. Chrysin: Chemistry, Occurrence, Pharmacokinetics, Toxicity, Molecular Targets, and Medicinal Properties of a Naturally Occurring Flavone. Curr. Bioact. Compd. 2024, 21. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.P.; He, J.; Liu, D.; Zhang, Q.-Z.; Li, K.; Zheng, X.; Tang, G.-T.; Guo, Y.; Liu, Y. The Relationship between Pharmacological Properties and Structure-Activity of Chrysin Derivatives. Mini Rev. Med. Chem. 2019, 19, 555–568. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; He, J.; Zheng, X.; Wu, H. Synthetic derivatives of chrysin and their biological activities. Med. Chem. Res. 2014, 23, 555–563. [Google Scholar] [CrossRef]
- Raina, R.; Bhatt, R.; Hussain, A. Chrysin targets aberrant molecular signatures and pathways in carcinogenesis (Review). World Acad. Sci. J. 2024, 6, 45. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, C.; Hu, M.; Wang, X.; Li, S.; An, Z.; Yang, X.; Xie, Y. Synthesis and biological evaluation of the novel chrysin prodrug for non-alcoholic fatty liver disease treatment. Front. Pharmacol. 2024, 15, 1336232. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Mruczek, P.; Niedoba, M.; Dewalska, A.; Stocerz, K.; Kadela-Tomanek, M. Anticancer Activity of Ether Derivatives of Chrysin. Molecules 2025, 30, 960. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Kopustinskiene, D.M.; Simal-Gandara, J.; Bernatoniene, J.; Samarghandian, S. An updated review on the versatile role of chrysin in neurological diseases: Chemistry, pharmacology, and drug delivery approaches. Biomed Pharmacother. 2021, 141, 111906. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Xu, C.; Li, X.; Chen, K. Hydroxylation decoration patterns of flavonoids in horticultural crops: Chemistry, bioactivity, and biosynthesis. Hortic. Res. 2022, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Goracy, A.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Pereira, R.M.; Campos, H.M.; Ferreira, P.Y.; Uchenna, N.; Silva, Y.S.; Okoh, V.I.; Pruccoli, L.; Arruda, E.L.; Liao, L.M.; Mota, P.A. Glycosylation of chrysin with β-d-glucose tetraacetate (LQFM280) enhances its in vitro and in vivo neuroprotective effects against the toxicity induced by 3-nitropropionic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 4095–4109. [Google Scholar] [CrossRef]
- Zhang, S.; Sadhasivam, D.R.; Soundrajan, S.; Shanmugavel, P.; Raji, A.; Xu, M. In vitro and in vivo investigation of chrysin chelated copper complex as biocompatible materials for bone tissue engineering applications. 3 Biotech 2023, 13, 45. [Google Scholar] [CrossRef]
- Goto, H.; Yanagimachi, M.; Goto, S.; Takeuchi, M.; Kato, H.; Yokosuka, T.; Ryosuke, K.; Shumpei, Y. Methylated chrysin reduced cell proliferation, but antagonized cytotoxicity of other anticancer drugs in acute lymphoblastic leukemia. Anticancer Drugs 2012, 23, 417–425. [Google Scholar] [CrossRef]
- Boaru, D.L.; Fraile-Martinez, O.; Leon-Oliva, D.; Garcia-Montero, C.; Castro-Maertinez, P.; Miranda-Gonzalez, A.; Saez, M.A.; Munon-Zamarron, L.; Castillo-Ruiz, E.; Barrena-Blazquez, S.; et al. Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Sci. 2024, 20, 5608–5672. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Long, J.; Li, R.; Liu, Y.; Yang, Z.; Zheng, X. Fluorine-Containing Chrysin Derivatives: Synthesis and Biological Activity. Nat. Prod. Commun. 2019, 14, 1934578X19878921. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Wang, W.-X.; Wang, Z.-Q.; Chen, L.-J.; Zhang, J.-Y.; Liu, X.-C.; Wu, S.; Zhang, Y. Synthesis and antitumor activity evaluation of chrysin derivatives. Eur. J. Med. Chem. 2014, 75, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, J.; Szyk, P.; Czarczynska-Goslinska, B.; Goslinski, T. Flavonoids, Chalcones, and Their Fluorinated Derivatives—Recent Advances in Synthesis and Potential Medical Applications. Molecules 2025, 30, 2395. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef]
- Oriquat, G.; Masoud, I.M.; Kamel, M.A.; Aboudeya, H.M.; Bakir, M.B.; Shaker, S.A. The Anti-Obesity and Anti-Steatotic Effects of Chrysin in a Rat Model of Obesity Mediated through Modulating the Hepatic AMPK/mTOR/lipogenesis Pathways. Molecules 2023, 28, 1734. [Google Scholar] [CrossRef]
- Tarahomi, M.; Firouzi Amandi, A.; Eslami, M.; Yazdani, Y.; Salek Farrokhi, A.; Ghorbani, F.; Taherian, M.; Yousefi, B. Niosomes nanoparticles as a novel approach in drug delivery enhances anticancer properties of chrysin in human ovarian carcinoma cells (SKOV3): An in vitro study. Med. Oncol. 2023, 40, 87. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Keglevich, P.; Ábrányi-Balogh, P.; Szigetvári, Á.; Dékány, M.; Szántay, C., Jr.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Novel Chrysin and 7-Aminochrysin Derivatives. Molecules 2020, 25, 888. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Mavroidi, B.; Varna, D.; Zahariou, G.; Litsardakis, G.; Pelecanou, M.; Hatzidimitriou, A.G. Structurally Characterized Cobalt and Nickel Complexes of Flavonoid Chrysin as Potential Radical Scavenging Compounds. Inorganics 2025, 13, 230. [Google Scholar] [CrossRef]
- Avila-Roman, J.; Quevedo-Tinoco, L.; Oliveros-Ortiz, A.; Garcia-Gil, S.; Rodriguez-Garcia, G.; Motilva, V.; Gomez-Hurtado, M.; Talero, E. Synthesis and Bioevaluation of New Stable Derivatives of Chrysin-8-C-Glucoside That Modulate the Antioxidant Keap1/Nrf2/HO-1 Pathway in Human Macrophages. Pharmaceuticals 2024, 17, 1388. [Google Scholar] [CrossRef]
- Wadibhasme, P.G.; Ghaisas, M.M.; Thakurdesai, P.A. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm. Biol. 2011, 49, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jiang, M.; Zhang, Y.; Liu, X.; Du, Q.; Feng, G. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, Y.H.; Lee, S.; Lee, S.R.; Choi, Y.A.; Jin, M.; Choi, J.H.; Park, J.H.; Park, P.H.; Choi, H.; et al. Chrysin attenuates atopic dermatitis by suppressing inflammation of keratinocytes. Food Chem. Toxicol. 2017, 110, 142–150. [Google Scholar] [CrossRef]
- Bae, Y.; Lee, S.; Kim, S.H. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharmacol. 2011, 254, 56–64. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Koh, D.; Lim, Y.; Shin, S.Y. Chrysin Inhibits NF-κB-Dependent CCL5 Transcription by Targeting IκB Kinase in the Atopic Dermatitis-Like Inflammatory Microenvironment. Int. J. Mol. Sci. 2020, 21, 7348. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van Oers, R.F.; Bacabac, R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J. Biomech. 2015, 48, 855–865. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Bońka, A.; Kaczmarczyk-Sedlak, I. Wpływ chryzyny na parametry histomorfometryczne kości owariektomizowanych szczurów. Herbalism 2017, 1, 41–54. [Google Scholar] [CrossRef]
- Oroslić, N.; Nemrava, J.; Jelec, Z.; Kukolij, M.; Odeh, D.; Jakopović, B.; Jembrek, M.J.; Bagatin, T.; Fures, R.; Bagatin, D. Antioxidative and Anti-Inflammatory Activities of Chrysin and Naringenin in a Drug-Induced Bone Loss Model in Rats. Int. J. Mol. Sci. 2022, 23, 2872. [Google Scholar]
- Zeng, W.; Yan, Y.; Zhang, F.; Zhang, C.; Liang, W. Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell 2013, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Xingyue, L.; Shuang, L.; Qiang, W.; Jinjuan, F.; Yongjian, Y. Chrysin Ameliorates Sepsis-Induced Cardiac Dysfunction Through Upregulating Nrf2/Heme Oxygenase 1 Pathway. J. Cardiovasc. Pharmacol. 2021, 77, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Tang, Y.; Qian, G.; Lv, H.; Song, X.; Liu, Y. Chrysin improves endothelial inflammation via the NFAT pathway in Kawasaki disease. Mol. Biol. Rep. 2025, 52, 428. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Sasikumar, S.; Mohamed Puhari, S.S.; Ramprasath, T.; Baskaran, N.; Vasudevan, V.; Selvam, G.S. Chrysin reduces hypercholesterolemia-mediated atherosclerosis through modulating oxidative stress, microflora, and apoptosis in experimental rats. J. Food Biochem. 2023, 46, e14349. [Google Scholar] [CrossRef] [PubMed]
- Anandhi, R.; Thomas, P.; Geraldine, P. Evaluation of the anti-atherogenic potential of chrysin in Wistar rats. Mol. Cell. Biochem. 2013, 385, 103–113. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.-W.; Park, W.-K.; Kong, J.-Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.-K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Fabbro, L.; Bortolotto, V.C.; Ferreira, L.M.; Sari, M.H.M.; Furian, A.F. Chrysin’s anti-inflammatory action in the central nervous system: A scoping review and an evidence-gap mapping of its mechanisms. Eur. J. Pharmacol. 2025, 997, 177602. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsenes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghadian, S.; Bafandeh, F. The Cardiovascular Protective Effects of Chrysin: A Narrative Review on Experimental Researches. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 17–27. [Google Scholar] [CrossRef]
- Li, X.-W.; Guo, B.; Shen, Y.-Y.; Yang, J.-R. Effect of chrysin on expression of NOX4 and NF-κB in right ventricle of monocrotaline-induced pulmonary arterial hypertension of rats. Yao Xue Xue Bao 2015, 50, 1128–1134. [Google Scholar]
- Verma, S.; Anderson, T.J. Fundamentals of Endothelial Function for the Clinical Cardiologist. Circulation 2002, 105, 546–549. [Google Scholar] [CrossRef]
- Veerappan, R.; Malarvili, T. Chrysin pretreatment improves angiotensin system, c-GMP concentration in L-NAME induced hypertensive rats. Indian J. Clin. Biochem. 2019, 34, 288–295. [Google Scholar] [CrossRef]
- Villar, I.C.; Vera, R.; Galisteo, M.; O’Valle, F.; Romero, M.; Zarzuelo, A.; Duarte, J. Endothelial Nitric Oxide Production Stimulated by the Bioflavonoid Chrysin in Rat Isolated Aorta. Planta Med. 2005, 71, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Afzal, O.; Kazmi, I.; Al-Abbasi, F.A.; Altamimi, A.S.A.; Yang, Z. Neuroprotective role of chrysin-loaded poly(lactic-co-glycolic acid) nanoparticle against kindling-induced epilepsy through Nrf2/ARE/HO-1 pathway. J. Biochem. Mol. Toxicol. 2020, 35, e22634. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Landa, J.F.; Guillén-Ruiz, G.; Hernández-López, F.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav. Brain Res. 2021, 397, 112952. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Faheem, M.A.; Akhtar, T.; Naseem, N.; Aftab, U.; Zafar, M.S.; Hussain, S.; Shahzad, M.; Gobe, G.C. Chrysin Is Immunomodulatory and Anti-Inflammatory against Complete Freund’s Adjuvant-Induced Arthritis in a Pre-Clinical Rodent Model. Pharmaceutics 2023, 15, 1225. [Google Scholar] [CrossRef]
- Rashno, M.; Ghaderi, S.; Nesari, A.; Khorsandi, L.; Farbood, Y.; Sarkaki, A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: Insights into underlying mechanisms. Psychopharmacology 2020, 237, 1607–1619. [Google Scholar] [CrossRef]
- Prajit, R.; Saenno, R.; Suwannakot, K.; Kaewngam, S.; Anosri, T.; Sritawan, N.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; et al. Chrysin mitigates neuronal apoptosis and impaired hippocampal neurogenesis in male rats subjected to D-galactose-induced brain aging. Biogerontology 2024, 25, 1275–1284. [Google Scholar] [CrossRef]
- Mantawy, E.M.; Esmat, A.; El-Bakly, W.M.; ElDin, R.A.S.; El-Demerdash, E. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci. Rep. 2017, 7, 4795. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Sevanan, M. Chrysin exerts anti-oxidant properties and restores motor function in MPTP induced mouse model of Parkinson disease. Int. J. Res. Pharm. Sci. 2020, 11, 4388–4394. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Upadhyay, R.; Sevanan, M. Chrysin for Neurotrophic and Neurotransmitter Balance in Parkinson’s Disease. In Neuroprotection: Methods and Protocols; Ray, S.K., Ed.; Springer: New York, NY, USA, 2024; Volume 2761, pp. 477–490. [Google Scholar]
- Meda, L.; Cassatella, M.; Szendrei, G.; Otvos, L., Jr.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 1995, 374, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Zhisan, D. Flavonoids from Carya cathayensis Sarg. leaves inhibit carotid artery lesion formation induced by low blood flow. Biomed. Pharmacother. 2017, 94, 88–92. [Google Scholar] [CrossRef]
- Jurcau, A.; Andronie-Cioara, F.L.; Nistor-Cseppento, D.C.; Pascalau, N.; Rus, M.; Vasca, E.; Jurcau, M.C. The Involvement of Neuroinflammation in the Onset and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 14582. [Google Scholar] [CrossRef] [PubMed]
- Gresa-Arribas, N.; Serratosa, J.; Saura, J.; Sola, C. Inhibition of CCAAT/enhancer binding proteindelta expression by chrysin in microglial cells results in anti-inflammatory and neuroprotective effects. J. Neurochem. 2010, 115, 526–536. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.-F.; Hernández-López, F.; Cueto-Escobedo, J.; Herrera-Huerta, E.V.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Romero-Avendaño, E. Chrysin (5,7-dihydroxyflavone) exerts anxiolytic-like effects through GABA(A) receptors in a surgical menopause model in rats. Biomed. Pharmacother. 2019, 109, 2387–2395. [Google Scholar] [CrossRef]
- Wolfman, C.; Viola, H.; Paladini, A.; Dajas, F.; Medina, J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol. Biochem. Behav. 1994, 47, 1–4. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, X.; Wang, Y.; Niu, L.; Niu, C. Recent advances in nutrition for the treatment of depressive disorder. Curr. Pharm. Des. 2018, 24, 2583–2590. [Google Scholar] [CrossRef]
- Goyal, A.; Singh, G.; Verma, A. A Comprehensive Review on Therapeutic Potential of Chrysin in Brain Related Disorders. CNS Neurol. Disord. Drug. Targets 2023, 22, 789–800. [Google Scholar] [CrossRef]
- Długosz, E.; Misiak, J.; Grabowska, K.; Jasicka-Misiak, I. Neuroprotective properties of the polyphenol agent of honey and bee pollen. Farm. Pol. 2024, 80, 241–253. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Micozzi, M.S.; Dog, T.L. Women’s Health in Complementary and Integrative Medicine: A Clinical Guide; Churchill Livingstone: London, UK, 2004. [Google Scholar]
- Park, W.; Park, M.Y.; Song, G.; Lim, W. 5,7-Dimethoxyflavone induces apoptotic cell death in human endometriosis cell lines via activation of endoplasmic reticulum stress pathway. Phytother. Res. 2020, 34, 2275–2286. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell. Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.F.; Sohn, J.; Chen, S.N.; Yao, P.; Bolton, J.L.; Burdette, J.E. Biological characterization of non-steroidal progestins from botanicals used for women’s health. Steroids 2012, 77, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef] [PubMed]
- Rudzitis-Auth, J.; Korbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef]
- Goleij, P.; Khandan, M.; Tabari, M.A.K.; Sanaye, P.M.; Alijanzadeh, D.; Soltani, A.; Hosseini, Z.; Larsen, D.S.; Khan, H.; Kumar, A.P.; et al. Unlocking the potential: How flavonoids affect angiogenesis, oxidative stress, inflammation, proliferation, invasion, and alter receptor interactions in endometriosis. Food Sci. Nutr. 2024, 13, e4607. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, K.; Jiang, L.; Liu, B.; Jian, X. Effect of dietary patterns and nutritional supplementation in the management of endometriosis: A review. Front. Nutr. 2025, 12, 1539665. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuang, L.; Liu, Q.; Yu, X.; Min, Q.; Chen, M.; Chen, Q. Rosiglitazone affects the progression of surgically induced endometriosis in a rat model. Mol. Med. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Park, H.; Jin, U.H.; Orr, A.A.; Echegaray, S.P.; Davidson, L.A.; Allred, C.D.; Chapkin, R.S.; Jayaraman, A.; Lee, K.; Tamamis, P.; et al. Isoflavones as Ah receptor agonists in colon-derived cell lines: Structure–activity relationships. Chem. Res. Toxicol. 2019, 32, 2353–2364. [Google Scholar] [CrossRef]
- Shrestha, R.; Mohankumar, K.; Martin, G.; Hailemariam, A.; Lee, S.-O.; Jin, U.H.; Burghardt, R.; Safe, S. Flavonoids kaempferol and quercetin are nuclear receptor 4A1 (NR4A1, Nur77) ligands and inhibit rhabdomyosarcoma cell and tumor growth. J. Exp. Clin. Cancer Res. 2021, 40, 392. [Google Scholar] [CrossRef]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-cancer activity of chrysin in cancer therapy: A systematic review. Indian J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Ma, J.; Liu, P.; Pan, L. Network pharmacology unveils the intricate molecular landscape of chrysin in breast cancer therapeutics. Discov. Oncol. 2025, 16, 228. [Google Scholar] [CrossRef]
- Hasan, S.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. OncoTargets Ther. 2020, 13, 3277–3287. [Google Scholar] [CrossRef]
- Rahmani-Moghadam, E.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-spectrum preclinical antitumor activity of chrysin: Current trends and future perspectives. Biomolecules 2020, 10, 1374. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, P.; Zhang, G.; Li, W.; Lin, H.; Hu, X. Inhibition of toll-like receptor 4 activation by apigenin and chrysin via competition for sites and conformational changes. Int. J. Biol. Macromol. 2023, 252, 126415. [Google Scholar] [CrossRef]
- Pawar, J.S.; Mustafa, S.; Ghosh, I. Chrysin and capsaicin induces premature senescence and apoptosis via mitochondrial dysfunction and p53 elevation in cervical cancer cells. Saudi J. Biol. Sci. 2022, 29, 3838–3847. [Google Scholar] [CrossRef]
- Raina, R.; Afroze, N.; Kedhari Sundaram, M.; Haque, S.; Bajbouj, K.; Hamad, M.; Hussain, A. Chrysin inhibits propagation of HeLa cells by attenuating cell survival and inducing apoptotic pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2206–2220. [Google Scholar] [PubMed]
- Xu, S.; Murtagh, S.; Han, Y.; Wan, F.; Toriola, A.T. Breast cancer incidence among US women aged 20 to 49 years by race, stage, and hormone receptor status. JAMA Netw. Open. 2024, 7, e2353331. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Shao, Z.; Zhong, X.; Ding, X.; Wu, L.; Chen, J.; He, P.; Cheng, Y.; Zhu, K.; et al. Pyrotinib and chrysin synergistically potentiate autophagy in HER2-positive breast cancer. Signal Transduct. Target. Ther. 2023, 8, 463. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoo, E.-S.; Han, S.-H.; Jung, G.-H.; Han, E.-J.; Choi, E.-Y.; Jeon, S.-J.; Jung, S.-H.; Kim, B.; Cho, S.-D.; et al. Chrysin induces apoptosis and autophagy in human melanoma cells via the mTOR/S6K pathway. Biomedicines 2022, 10, 1467. [Google Scholar] [CrossRef]
- Puls Medycyny. Rak Jelita Grubego—Nowe Terapie. Puls Medycyny, 3 Lipca 2025. Available online: https://pulsmedycyny.pl/medycyna/onkologia/rak-jelita-grubego-nowe-terapie/ (accessed on 13 July 2025).
- Lin, Y.-M.; Chen, C.-I.; Hsiang, Y.-P.; Hsu, Y.-C.; Cheng, K.-C.; Chien, P.-H.; Pan, H.-L.; Lu, C.-C.; Chen, Y.-J. Chrysin attenuates cell viability of human colorectal cancer cells through autophagy induction unlike 5-fluorouracil/oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tong, Y.; Ying, J.; Lei, Z.; Wan, L.; Zhu, X.; Ye, F.; Mao, P.; Wu, X.; Pan, R.; et al. Chrysin induces growth arrest, apoptosis, ER stress, and inhibits STAT3 activation via ROS generation in bladder cancer cells. Oncol. Lett. 2018, 15, 9117–9125. [Google Scholar] [PubMed]
- Restani, R.B.; Morgado, P.I.; Ribeiro, M.P.; Correia, I.J.; Aguiar-Ricardo, A.; Bonifácio, V.D.B. Biocompatible polyurea dendrimers with pH-dependent fluorescence. Angew. Chem. 2012, 124, 5252–5255. [Google Scholar] [CrossRef]
- Cruz, A.; Mota, P.; Ramos, C.; Pires, R.F.; Mendes, C.; Silva, J.P.; Nunes, S.C.; Bonifácio, V.D.B.; Serpa, J. Polyurea dendrimer folate-targeted nanodelivery of l-buthionine sulfoximine as a tool to tackle ovarian cancer chemoresistance. Antioxidants 2020, 9, 133. [Google Scholar] [CrossRef]
- Davaran, S.; Fazeli, H.; Ghamkhari, A.; Rahimi, F.; Molavi, O.; Anzabi, M.; Salehi, R. Synthesis and characterization of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of methotrexate and chrysin in combination cancer chemotherapy. J. Biomater. Sci. Polym. 2018, 29, 1265–1286. [Google Scholar] [CrossRef]
- Srikun, D.; Albers, A.E.; Chang, C.J. A dendrimer-based platform for simultaneous dual fluorescence imaging of hydrogen peroxide and pH gradients produced in living cells. Chem. Sci. 2011, 2, 1156–1165. [Google Scholar] [CrossRef]
- Santos, I.; Ramos, C.; Mendes, C.; Sequeira, C.O.; Tomé, C.S.; Fernandes, D.G.; Mota, P.; Pires, R.F.; Urso, D.; Hipólito, A.; et al. Targeting glutathione and cystathionine β-synthase in ovarian cancer treatment by selenium-chrysin polyurea dendrimer nanoformulation. Nutrients 2019, 11, 2523. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Darabi, M.; Afraidooni, L.; Zarghami, N.; Daraee, H.; Eskandari, L.; Mellatyar, H.; Akbarzadeh, A. Comparison, synthesis and evaluation of anticancer drug-loaded polymeric nanoparticles on breast cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Aiyelabegan, H.T.; Negahdari, B.; Rajeian, B.; Zarghami, N. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed. Pharmacother. 2016, 84, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J. Natl. Cancer Inst. 1994, 86, 299–304. [Google Scholar] [CrossRef]
- Tavakoli, F.; Jahanban-Esfahlan, R.; Seidi, K.; Jabbari, M.; Behzadi, R.; Soltanahmadid, Y.P.; Zarghami, N. Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–86. [Google Scholar] [CrossRef]
- Dabiri, S.; Jafari, S.; Molavi, O. Advances in nanocarrier-mediated delivery of chrysin: Enhancing solubility, bioavailability, and anticancer efficacy. Bioimpacts 2024, 15, 30269. [Google Scholar] [CrossRef]
- Sood, A.; Mehrotra, A.; Sharma, U.; Aggarwal, D.; Singh, T.; Shahwan, M.; Jairoun, A.A.; Rani, I.; Ramniwas, S.; Tuli, H.S.; et al. Advancements and recent explorations of anti-cancer activity of chrysin: From molecular targets to therapeutic perspective. Explor. Target Antitumor. Ther. 2024, 5, 477–494. [Google Scholar] [CrossRef]
- Ting, P.; Srinuanchai, W.; Suttisansanee, U.; Tuntipopipat, S.; Charoenkiatkul, S.; Praengam, K.; Chantong, B.; Temviriyanukul, P.; Nuchuchua, O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods 2021, 10, 1912. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Lan, H.; Bi, B.; Peng, X.; Li, D.; Wang, H.; Zhy, K.; Shao, F.; Yin, M. Enhancing breast cancer treatment: Mesoporous dopamine nanoparticles in synergy with chrysin for photothermal therapy. Front. Oncol. 2024, 14, 1427858. [Google Scholar] [CrossRef]
- Abdelmawgood, I.A.; Badr, A.M.; Abdelkader, A.E.; Mahana, N.A.; Mohamed, A.S.; Abdelfattah, H.H. Chrysin-loaded poly (lactic-co-glycolic acid) nanoparticles alleviate sepsis-induced splenic injury by regulating myeloid-derived suppressor cells. Immunol. Res. 2025, 73, 80. [Google Scholar] [CrossRef]
- Garg, A. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles—Storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar]
- Singh, M.; Pathak, K. Solid lipid nanoparticles: A promising drug delivery system. Pharm. Nanotechnol. 2017, 5, 85–98. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Walle, U.K.; Wilson, F.A. The fate of the flavonoid chrysin in human Caco-2 cells: Conjugation and efflux. Drug Metab. Dispos. 2001, 29, 1294–1299. [Google Scholar]
- Garg, A.; Chaturvedi, S. A Comprehensive Review on Chrysin: Emphasis on Molecular Targets, Pharmacological Actions and Bio-pharmaceutical Aspects. Curr. Drug Targets 2022, 23, 420–436. [Google Scholar] [CrossRef]
- Adangale, S.C.; Wairkar, S. Potential therapeutic activities and novel delivery systems of chrysin-a nature’s boon. Food Biosci. 2022, 45, 101316. [Google Scholar] [CrossRef]
- Dean, W. Chrysin: Is It An Effective Aromatase Inhibitor? Vitam. Res. News 2000, 14, 4–5. [Google Scholar]
- Saarinen, N.; Joshi, S.C.; Ahotupa, M.; Li, X.; Ammälä, J.; Mäkelä, S. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J. Steroid Biochem. Mol. Biol. 2004, 92, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Bakos, É.; Kuffa, K.; Bencsik, T.; Zsidó, B.Z.; Hetényi, C.; Telbisz, Á.; Özvegy-Laczka, C.; et al. Effects of chrysin and its major conjugated metabolites chrysin-7-sulfate and chrysin-7-glucuronide on cytochrome P450 enzymes and on OATP, P-gp, BCRP, and MRP2 transporters. Drug Metab. Dispos. 2020, 48, 1064–1073. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Gong, N.; Xu, W.; Guo, Y.; Du, G.; Lu, Y. Development of a new certified reference material of high-purity chrysin for the quality control of traditional Chinese medicine. Accred. Qual. Assur. 2016, 21, 287–293. [Google Scholar] [CrossRef]
- Kang, J.K.; Chung, Y.C.; Hyun, C.G. Anti-Inflammatory Effects of 6-Methylcoumarin in LPS-Stimulated RAW 264.7 Macrophages via Regulation of MAPK and NF-κB Signaling Pathways. Molecules 2021, 26, 5351. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, C.H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Lee, S.; Choi, Y.H.; Seo, Y.T.; Kim, G.Y. Hydrangenol inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-mediated HO-1 pathway. Int. Immunopharmacol. 2016, 35, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Tang, D.; Xu, K.; Jiang, Z.-F. Curcumin-mediated neuroprotection against amyloid-β-induced mitochondrial dysfunction involves suppression of oxidative stress and apoptosis. J. Alzheimer’s Dis. 2012, 32, 981–996. [Google Scholar] [CrossRef] [PubMed]
| Biological Activity | Main Mechanism of Action | Example Effects |
|---|---|---|
| Anticancer | Induction of apoptosis, inhibition of proliferation and angiogenesis, cell cycle arrest [112,115]. | Inhibition of breast, prostate, lung, and colon cancer cell growth [112,116,122,126]. |
| Antioxidant | Neutralization of free radicals (ROS/RNS) by donating a hydrogen atom from –OH groups [73,74,75]. | Protection of cells against oxidative stress [73,74,75]. |
| Anti-inflammatory | Inhibition of NF-κB and MAPK signaling pathways, reduction of pro-inflammatory cytokine production (e.g., TNF-α, IL-6) [70,71,72]. | Reduction of inflammation in various disease models [70,71,72]. |
| Neuroprotective | Protection of neurons from apoptosis, inhibition of neuroinflammation, antioxidant action in the CNS [91,92,94]. | Potential application in neurodegenerative diseases (Alzheimer’s, Parkinson’s) [91,92,94]. |
| Antiviral | Inhibition of viral replication, e.g., by inhibiting key viral enzymes [2,9]. | Activity against influenza, HIV, Herpes Simplex viruses [2,9]. |
| Anxiolytic | Modulation of GABA-A receptors (action similar to benzodiazepines) [95,96]. | Calming and anti-anxiety effect without typical side effects [95,96]. |
| Drug Delivery System | Efficacy | Limitations |
|---|---|---|
| Micelles | Improvement of chrysin solubility thanks to the hydrophobic core. Increased stability and bioavailability. Possibility of surface modification for targeted delivery [139,140,141]. | Low drug encapsulation efficiency. Possibility of premature drug release due to micelle instability under certain biological conditions [139,140,141]. |
| Dendrimers | Precise, controlled structure and size. High surface functionality enabling attachment of targeting molecules. Enhanced solubility and bioavailability of chrysin [142]. | Potential toxicity, especially with higher-generation dendrimers and positive charges. Complex and costly synthesis process [142]. |
| Polymeric nanoparticles | High encapsulation efficiency. Possibility of controlled, prolonged drug release. Protection of chrysin from degradation. Improvement of therapeutic efficacy in vivo [143,144]. | Possibility of drug leakage during storage. Complexity of the manufacturing process. Potential issues with biodegradability and toxicity of certain polymers [143,144]. |
| Solid Lipid Nanoparticles (SLNs) | High biocompatibility and low toxicity due to the use of physiological lipids. Feasibility of large-scale production. Protection of the drug from chemical degradation. Improved bioavailability following oral administration [145]. | Lower encapsulation efficiency compared to polymeric nanoparticles. Tendency for drug expulsion during storage due to the crystalline structure of lipids [145]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkiewicz, M.; Moździerz, A.; Rzepecka-Stojko, A.; Stojko, J. Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential. Pharmaceuticals 2025, 18, 1162. https://doi.org/10.3390/ph18081162
Kurkiewicz M, Moździerz A, Rzepecka-Stojko A, Stojko J. Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential. Pharmaceuticals. 2025; 18(8):1162. https://doi.org/10.3390/ph18081162
Chicago/Turabian StyleKurkiewicz, Magdalena, Aleksandra Moździerz, Anna Rzepecka-Stojko, and Jerzy Stojko. 2025. "Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential" Pharmaceuticals 18, no. 8: 1162. https://doi.org/10.3390/ph18081162
APA StyleKurkiewicz, M., Moździerz, A., Rzepecka-Stojko, A., & Stojko, J. (2025). Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential. Pharmaceuticals, 18(8), 1162. https://doi.org/10.3390/ph18081162








