Design, Synthesis, and Bioactivity Assessment of Modified Vemurafenib Analog
Abstract
1. Introduction
2. Results
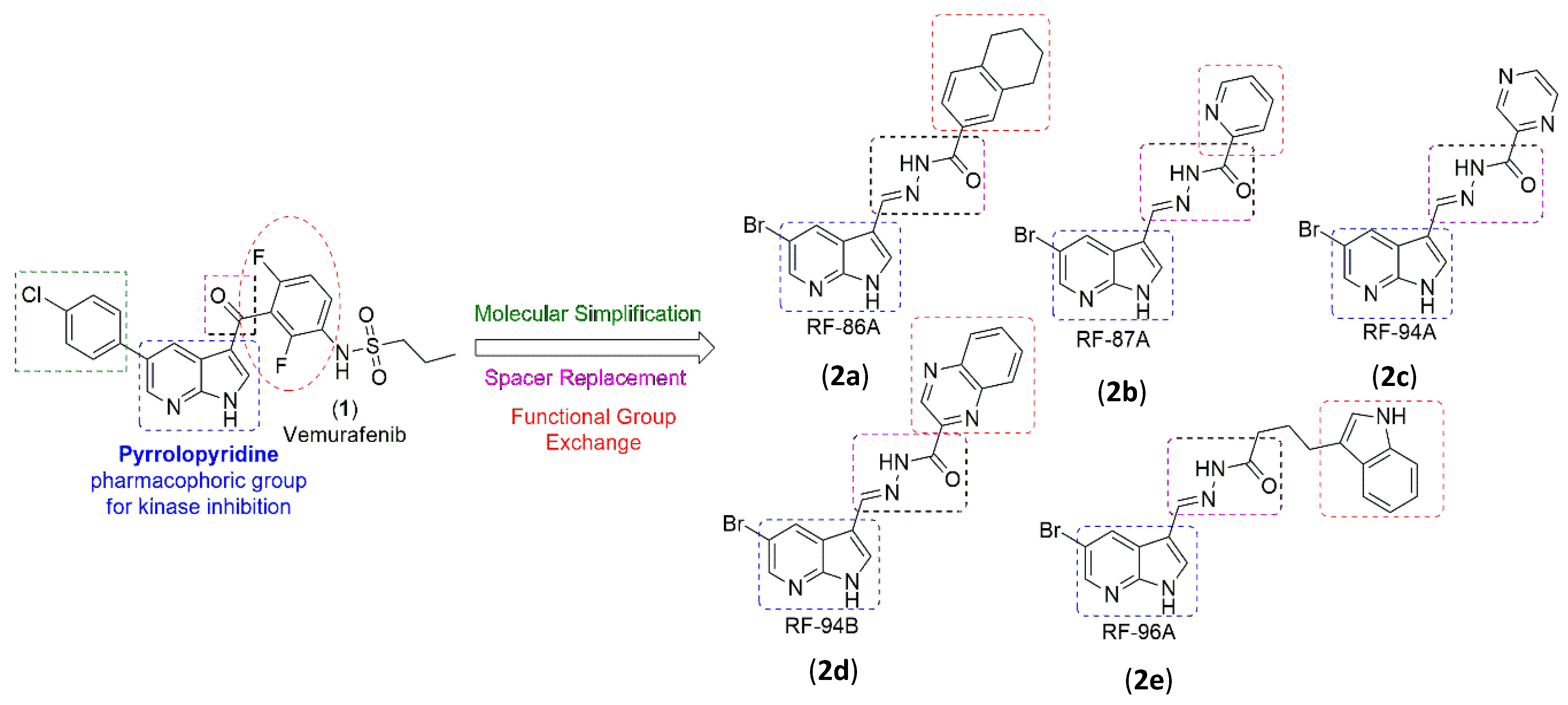
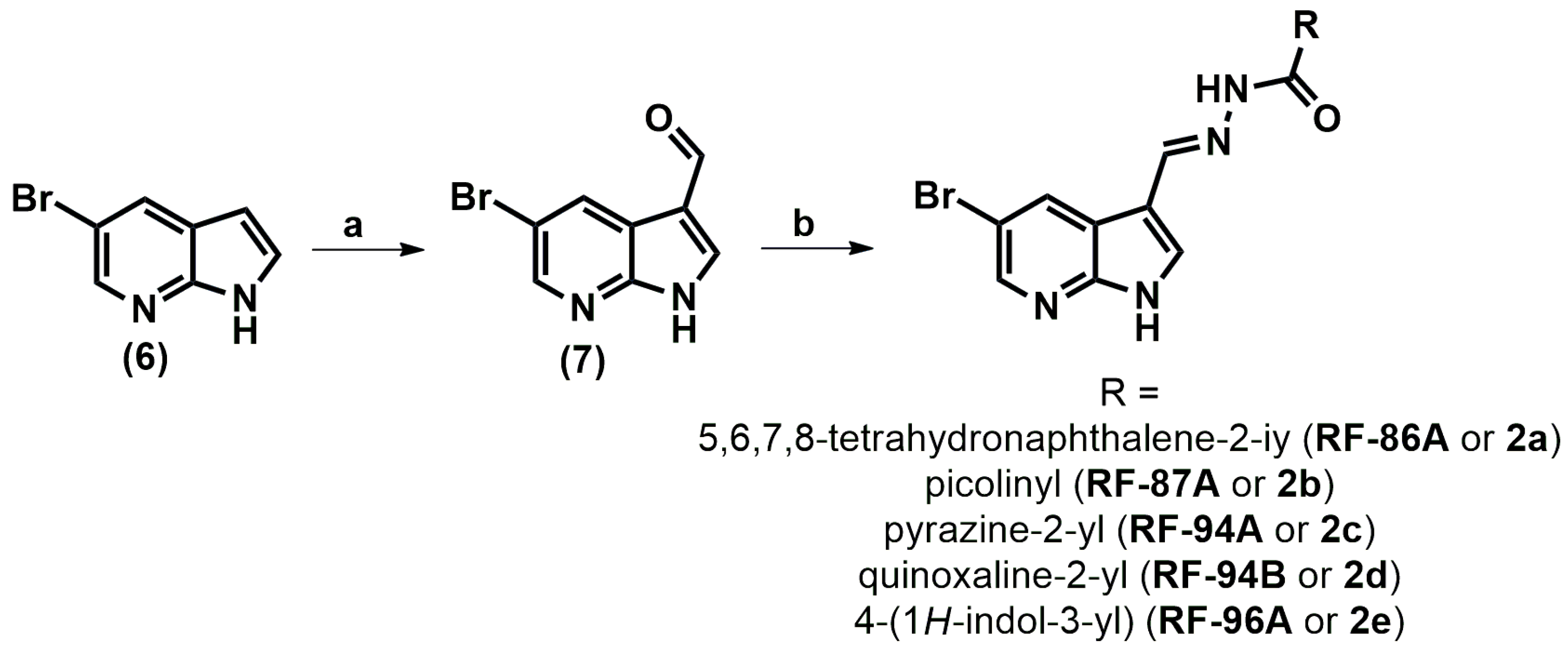
2.1. Analogs Synthesis
2.2. Effects on Cell Viability
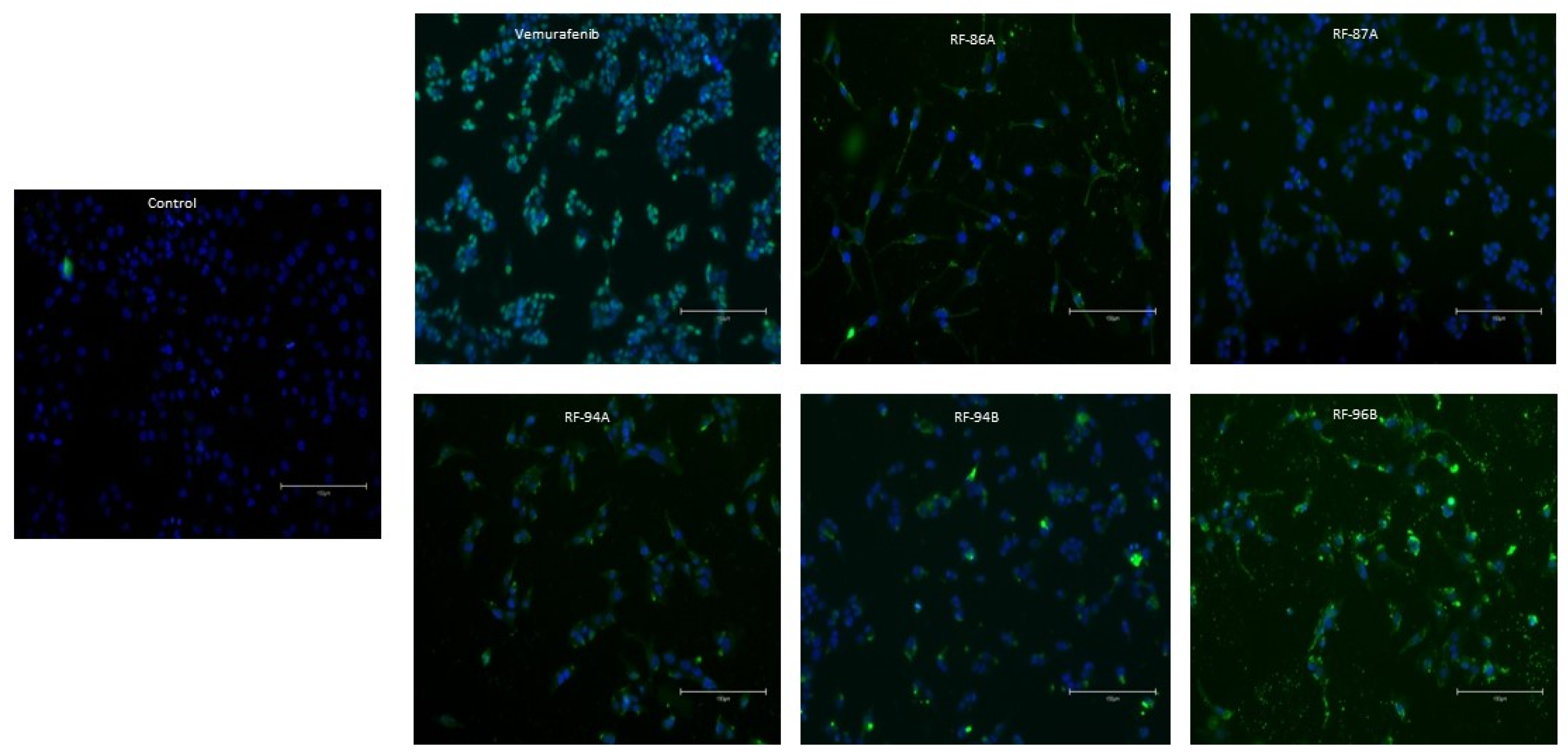
2.3. Vemurafenib and Analogs Caused DNA Fragmentation in A375 Cells
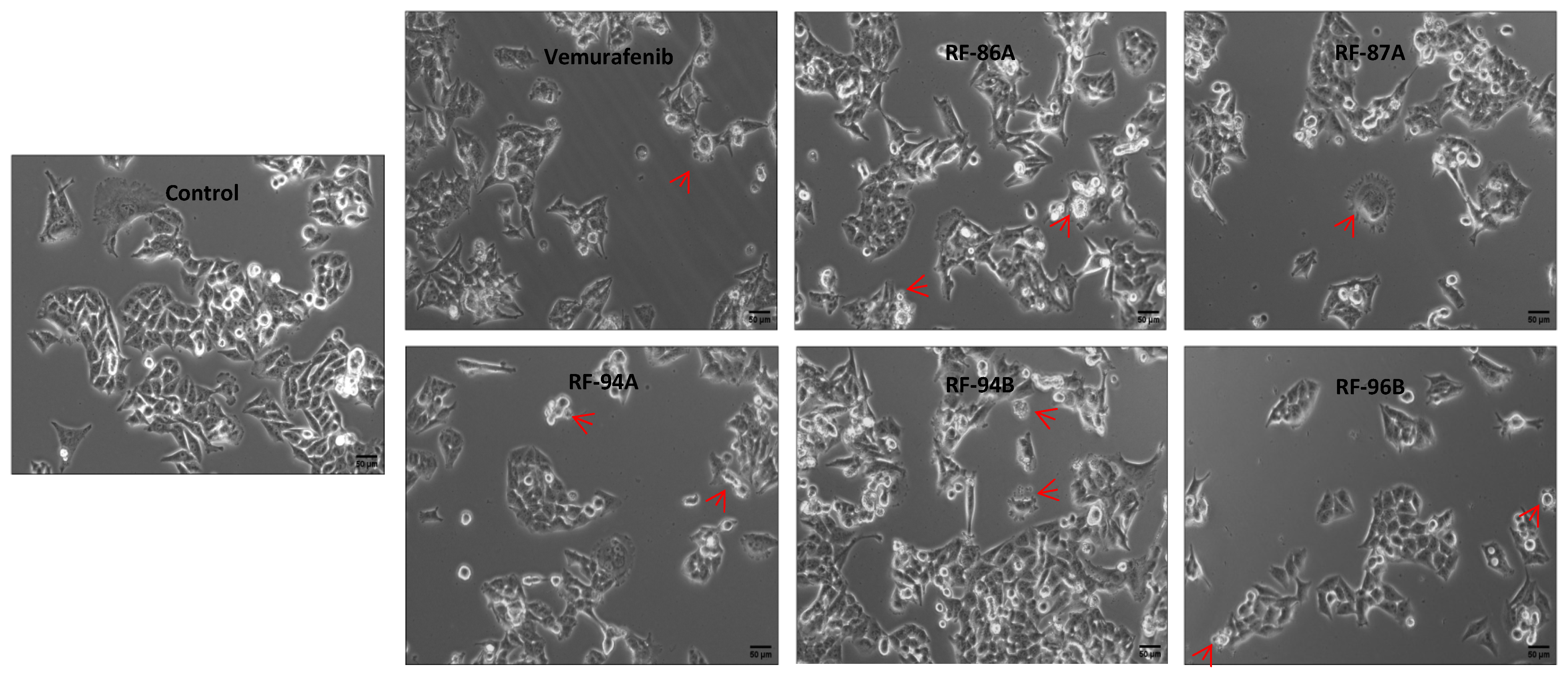
2.4. Cell Apoptosis Detected by Cell Morphology Analysis
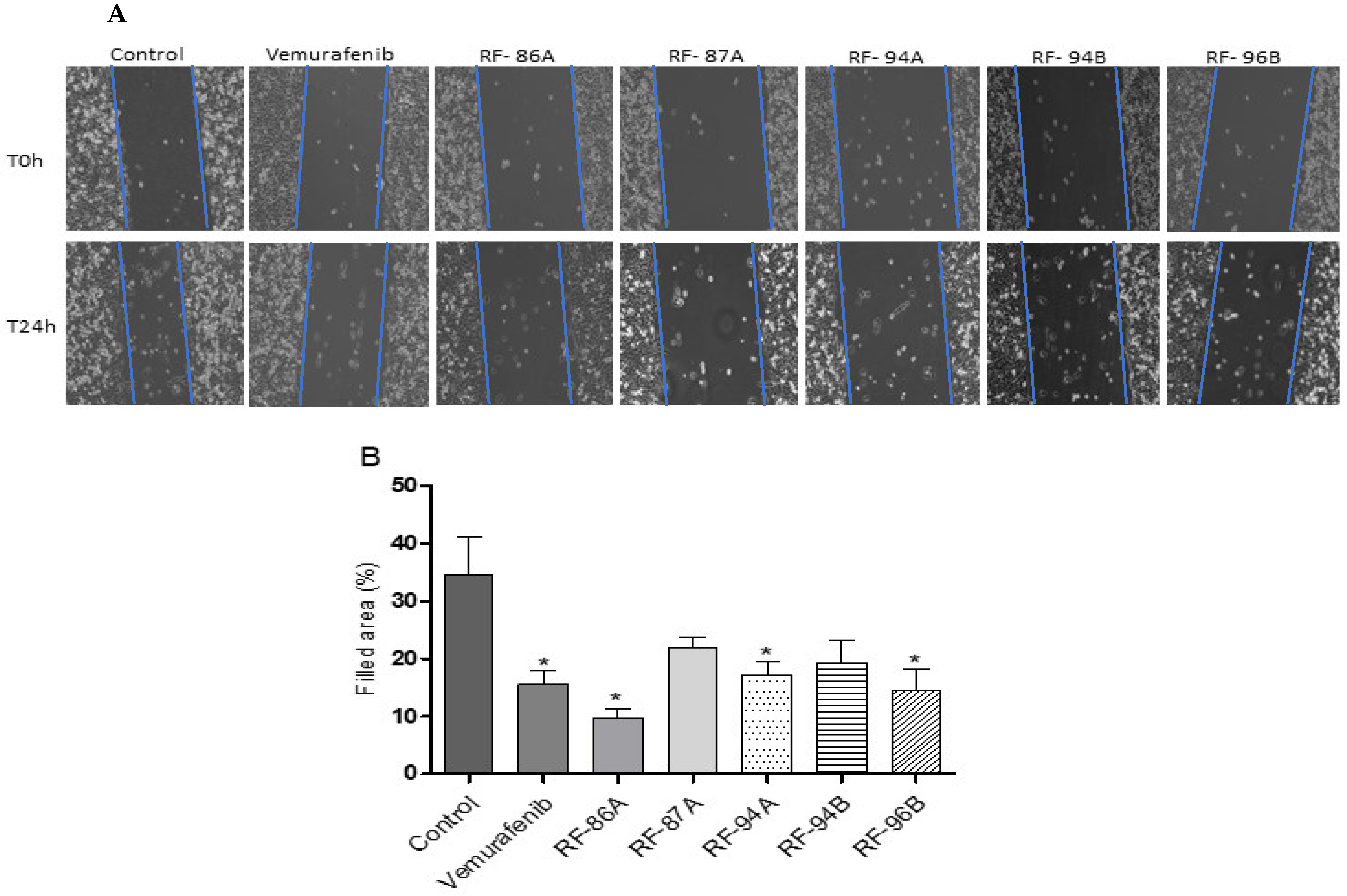
2.5. Cell Migration Quantified by the Cell-Based Scratch Assay
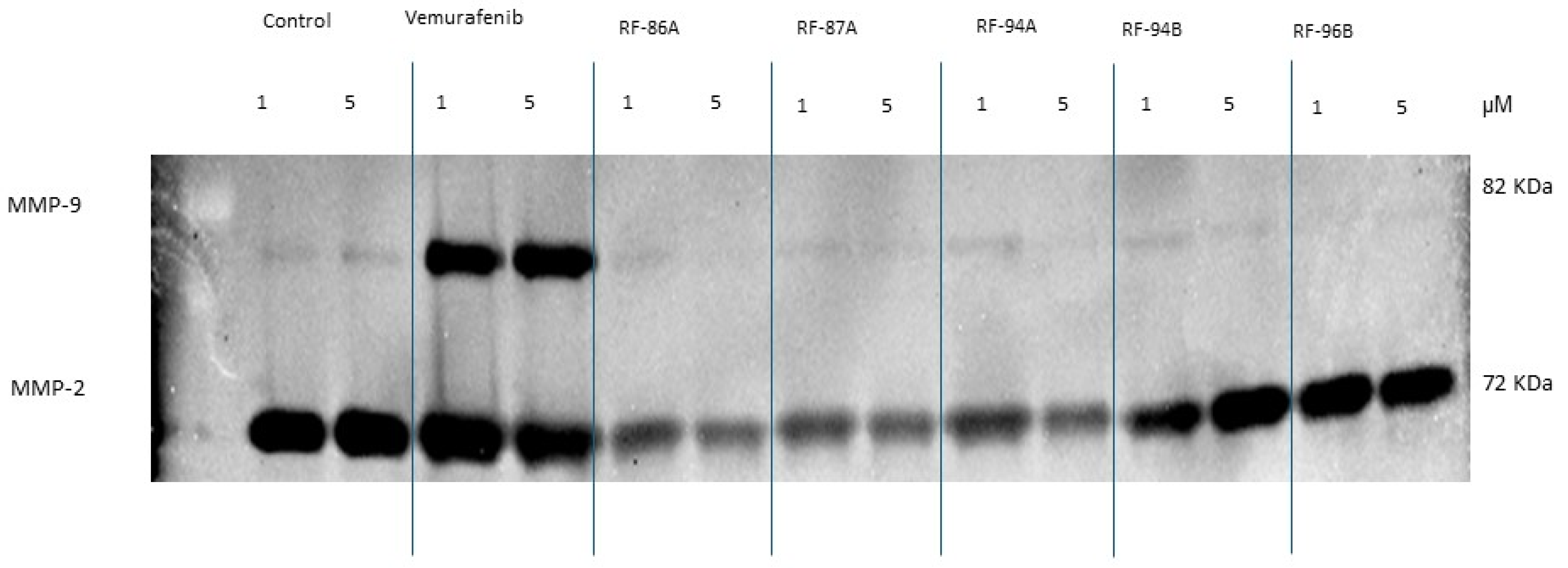
2.6. Effects of Vemurafenib and Analogs on A375 Cell Secretion of MMP-2 and MMP-9
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. General Mechanism for Obtaining N-Acylhydrazones (2a–e)
4.2.1. (E)-N-((5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)methylene)-5,6,7,8-tetrahydronaphthalene-2-carbohydrazide (2a or RF-86B)
4.2.2. (E)-N-((5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)methylene)picolinohydrazide (2b or RF-87A)
4.2.3. (E)-N-((5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)methylene)pyrazine-2-carbohydrazide (2c or RF-94A)
4.2.4. (E)-N-((5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)methylene)quinoxaline-2-carbohydrazide (2d or RF-94B)
4.2.5. (EZ)-N-((1H-Pyrrolo[2,3-b]pyridin-3-yl)methylene)-4-(1H-indol-3-yl)butanehydrazide (2e or RF-96B)
4.3. Chemicals
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. DNA Fragmentation Analyses
4.7. Cell Morphology Analysis
4.8. Cell Scratch Assay
4.9. Gelatin Zymography
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, C.J.; Yen, G.C. The in vitro and in vivo experimental evidences disclose the chemopreventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin. Exp. Metastasis 2010, 27, 361–369. [Google Scholar] [CrossRef]
- Fecher, L.A.; Amaravadi, R.K.; Flaherty, K.T. The MAPK pathway in melanoma. Curr. Opin. Oncol. 2008, 20, 183–189. [Google Scholar] [CrossRef]
- Sala, E.; Mologni, L.; Truffa, S.; Gaetano, C.; Bollag, G.E.; Gambacorti-Passerini, C. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol. Cancer Res. 2008, 6, 751–759. [Google Scholar] [CrossRef]
- Di Nunno, V.; Gatto, L.; Tosoni, A.; Bartolini, S.; Franceschi, E. Implications of BRAF V600E mutation in gliomas: Molecular considerations, prognostic value and treatment evolution. Front. Oncol. 2023, 12, 1067252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, G.; Sheng, C. Structural simplification: An efficient strategy in lead optimization. Eur. J. Med. Chem. 2019, 178, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Mérour, J.Y.; Buron, F.; Plé, K.; Bonnet, P.; Routier, S. The azaindole framework in the design of kinase inhibitors. Molecules 2014, 19, 19935–19979. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Sawa, M. 7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors. Chem. Pharm. Bull. 2018, 66, 29–36. [Google Scholar] [CrossRef]
- Duarte, C.D.; Barreiro, E.J.; Fraga, C.A.M. Privileged structures: A useful concept for the rational design of new lead drug candidates. Mini-Reviews Med. Chem. 2007, 7, 1108–1119. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Pinheiro, P.S.M.; Lima, L.M.; Fraga, C.A.M.; Barreiro, E.J. N-Acylhydrazones as drugs. Bioorganic Med. Chem. Lett. 2018, 28, 2797–2806. [Google Scholar] [CrossRef]
- Cordeiro, N.M.; Freitas, R.H.C.N.; Fraga, C.A.M.; Fernandes, P.D. Discovery of novel orally active tetrahydro-naphthyl-N-acylhydrazones with in vivo anti-TNF-α effect and remarkable anti-inflammatory properties. PLoS ONE 2016, 11, e0154606. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Léda, P.H.O.; de Miranda, A.L.P.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Freitas, R.H.C.N.; Cordeiro, N.M.; Carvalho, P.R.; Alves, M.A.; Guedes, I.A.; Valerio, T.S.; Dardenne, L.E.; Lima, L.M.; Barreiro, E.J.; Fernandes, P.D.; et al. Discovery of naphthyl-N-acylhydrazone p38α MAPK inhibitors with in vivo anti-inflammatory and anti-TNF-α activity. Chem. Biol. Drug Des. 2018, 91, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, R.H.; Jain, M.R.; Goel, A.; Patel, D.N.; Prajapati, V.M.; Gupta, A.A.; Jadav, P.A.; Patel, P.R. Design, synthesis, and biological evaluation of substituted-N-(thieno[2,3-b]pyridin-3-yl)-guanidines, N-(1H-pyrrolo[2,3-b]pyridin-3-yl)-guanidines, and N-(1H-indol-3-yl)-guanidines. Bioorganic Med. Chem. 2007, 15, 3248–3265. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.; Javid, N.; Zia-Ur-Rehman, M.; Zaheer, M.; Huma, R.; Roohi, A.; Athar, M.M. Synthesis of novel N-Acylhydrazones and their C-N/N-N bond conformational characterization by NMR spectroscopy. Molecules 2021, 26, 4908. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, N.D.M.; Freitas, R.H.C.N.; Fraga, C.A.M.; Fernandes, P.D. New 2-amino-pyridinyl-N-acylhydrazones: Synthesis and identification of their mechanism of anti-inflammatory action. Biomed. Pharmacother. 2020, 123, 109739. [Google Scholar] [CrossRef]
- Cardoso, L.N.F.; Nogueira, T.C.M.; Kaiser, C.R.; Wardell, J.L.; Wardell, S.M.S.V.; de Souza, M.V.N. Synthesis and anti-tubercular activity of Thienyl and Furanyl derivatives. Mediterr. J. Chem. 2016, 5, 356–366. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Bai, J.; Xue, Y.; Peng, Q. MMP1 and MMP9 are potential prognostic biomarkers and targets for uveal melanoma. BMC Cancer 2021, 21, 1068. [Google Scholar] [CrossRef]
- Salemi, R.; Falzone, L.; Madonna, G.; Polesel, J.; Cinà, D.; Mallardo, D.; Ascierto, P.A.; Libra, M.; Candido, S. MMP-9 as a candidate marker of response to BRAF inhibitors in melanoma patients with BRAFV600E mutation detected in circulating-free DNA. Front. Pharmacol. 2018, 9, 856. [Google Scholar] [CrossRef]
- Marusak, C.; Bayles, I.; Ma, J.; Gooyit, M.; Gao, M.; Chang, M.; Bedogni, B. The thiirane-based selective MT1-MMP/MMP2 inhibitor ND-322 reduces melanoma tumor growth and delays metastatic dissemination. Pharmacol. Res. 2016, 113, 515–520. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kawai, T.; Sato, H.; Aida, S.; Kasamatsu, H.; Aurues, T.; Ikeda, T. Expression of Matrix Metalloproteinase-2 (MMP-2) and of Membrane-Type-1-Matrix Metalloproteinase (MT1-MMP) in Transitional Cell Carcinoma of the Upper Urinary Tract. Hum. Pathol. 2000, 31, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.E.; Suling, W.J.; Reynolds, R.C. Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J. Med. Chem. 2002, 45, 5604–5606. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.A.; Guerra, F.S.; Sagrillo, F.S.; Pinheiro, P.d.S.M.; Alves, M.A.; Thota, S.; Chaves, L.S.; Sant’Anna, C.M.R.; Fernandes, P.D.; Fraga, C.A.M. Design, Synthesis, and Pharmacological Evaluation of First-in-Class Multitarget N-Acylhydrazone Derivatives as Selective HDAC6/8 and PI3Kα Inhibitors. ChemMedChem 2020, 15, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Saczewski, J.; Paluchowska, A.; Klenc, J.; Raux, E.; Barnes, S.; Sullivan, S.; Duszynska, B.; Bojarski, A.J.; Strekowskia, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino) pyrimidines and Quinazoline Analogs as Serotonin 5-HT2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. [Google Scholar] [CrossRef]
- Freitas, R.H.C.N.; Barbosa, J.M.; Bernardino, P.; Sueth-Santiago, V.; Wardell, S.M.; Wardell, J.L.; Decoté-Ricardo, D.; Melo, T.G.; da Silva, E.F.; Salomão, K.; et al. Synthesis and trypanocidal activity of novel pyridinyl-1,3,4-thiadiazole derivatives. Biomed. Pharmacother. 2020, 127, 110162. [Google Scholar] [CrossRef]
- Filho, J.; Castro, M. Synthesis, structural characterization, and antimicrobial activity of novel ferrocene-N-acyl hydrazones designed by means of molecular simplification strategy Celebrating the 100th anniversary of the birth of Professor Paulo Freire. J. Organometallic Chem. 2022, 979, 1–15. [Google Scholar] [CrossRef]
- Guerra, F.S.; Sampaio, L.d.S.; Konig, S.; Bonamino, M.; Rossi, M.I.D.; Costa, M.L.; Fernandes, P.; Mermelstein, C. Membrane cholesterol depletion reduces breast tumor cell migration by a mechanism that involves non-canonical Wnt signaling and IL-10 secretion. Transl. Med. Commun. 2016, 1, 3. [Google Scholar] [CrossRef]
- Guerra, F.S.; De Oliveira, R.G.; Fraga, C.A.M.; Mermelstein, C.D.S.; Fernandes, P.D. ROCK inhibition with Fasudil induces beta-catenin nuclear translocation and inhibits cell migration of MDA-MB 231 human breast cancer cells. Sci. Rep. 2017, 7, 13723. [Google Scholar] [CrossRef]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 2012, 878, 121–135. [Google Scholar] [CrossRef]

| A375 | HEK293T | ||||
|---|---|---|---|---|---|
| Compound | IC50 (µM) | 95% CI | IC50 (µM) | 95% CI | Selectivity Index |
| Vemurafenib | 7.68 | 5.334 to 11.15 | >1000 | - | >1000 |
| RF-86A (2a) | 6.99 | 4.864 to 10.15 | 114.4 | 43.47 to ∞ | 16.37 |
| RF-87A (2b) | 47.22 | 36.72 to 64.08 | 142.3 | 38.60 to ∞ | 3.01 |
| RF-94A (2c) | 53.01 | 39.29 to 77.77 | 25.76 | 22.62 to 29.61 | 0.49 |
| RF-94B (2d) | 46.33 | 35.81 to 63.40 | 25.52 | 15.79 to 48.59 | 0.55 |
| RF-96B (2e) | 21.32 | 15.54 to 30.84 | 14.32 | 9.572 to 22.76 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.S.; Freitas, R.H.C.N.d.; Moldovan, F.; Rocha, D.R.d.; Carvalho, R.S.; Fernandes, P.D. Design, Synthesis, and Bioactivity Assessment of Modified Vemurafenib Analog. Pharmaceuticals 2025, 18, 1161. https://doi.org/10.3390/ph18081161
Guerra FS, Freitas RHCNd, Moldovan F, Rocha DRd, Carvalho RS, Fernandes PD. Design, Synthesis, and Bioactivity Assessment of Modified Vemurafenib Analog. Pharmaceuticals. 2025; 18(8):1161. https://doi.org/10.3390/ph18081161
Chicago/Turabian StyleGuerra, Fabiana Sélos, Rosana Helena Coimbra Nogueira de Freitas, Florina Moldovan, David Rodrigues da Rocha, Renato Sampaio Carvalho, and Patricia Dias Fernandes. 2025. "Design, Synthesis, and Bioactivity Assessment of Modified Vemurafenib Analog" Pharmaceuticals 18, no. 8: 1161. https://doi.org/10.3390/ph18081161
APA StyleGuerra, F. S., Freitas, R. H. C. N. d., Moldovan, F., Rocha, D. R. d., Carvalho, R. S., & Fernandes, P. D. (2025). Design, Synthesis, and Bioactivity Assessment of Modified Vemurafenib Analog. Pharmaceuticals, 18(8), 1161. https://doi.org/10.3390/ph18081161








