Repurposing Analysis of Nitroxoline (8-Hydroxy-5-nitroquinoline) as an Antichagasic Compound
Abstract
1. Introduction
2. Results
2.1. Biological Activity Results
2.2. Mechanisms of Cell Death
2.2.1. Chromatin Condensation Analysis
2.2.2. Mitochondrial Membrane Potential Analysis
2.2.3. ATP Levels Analysis
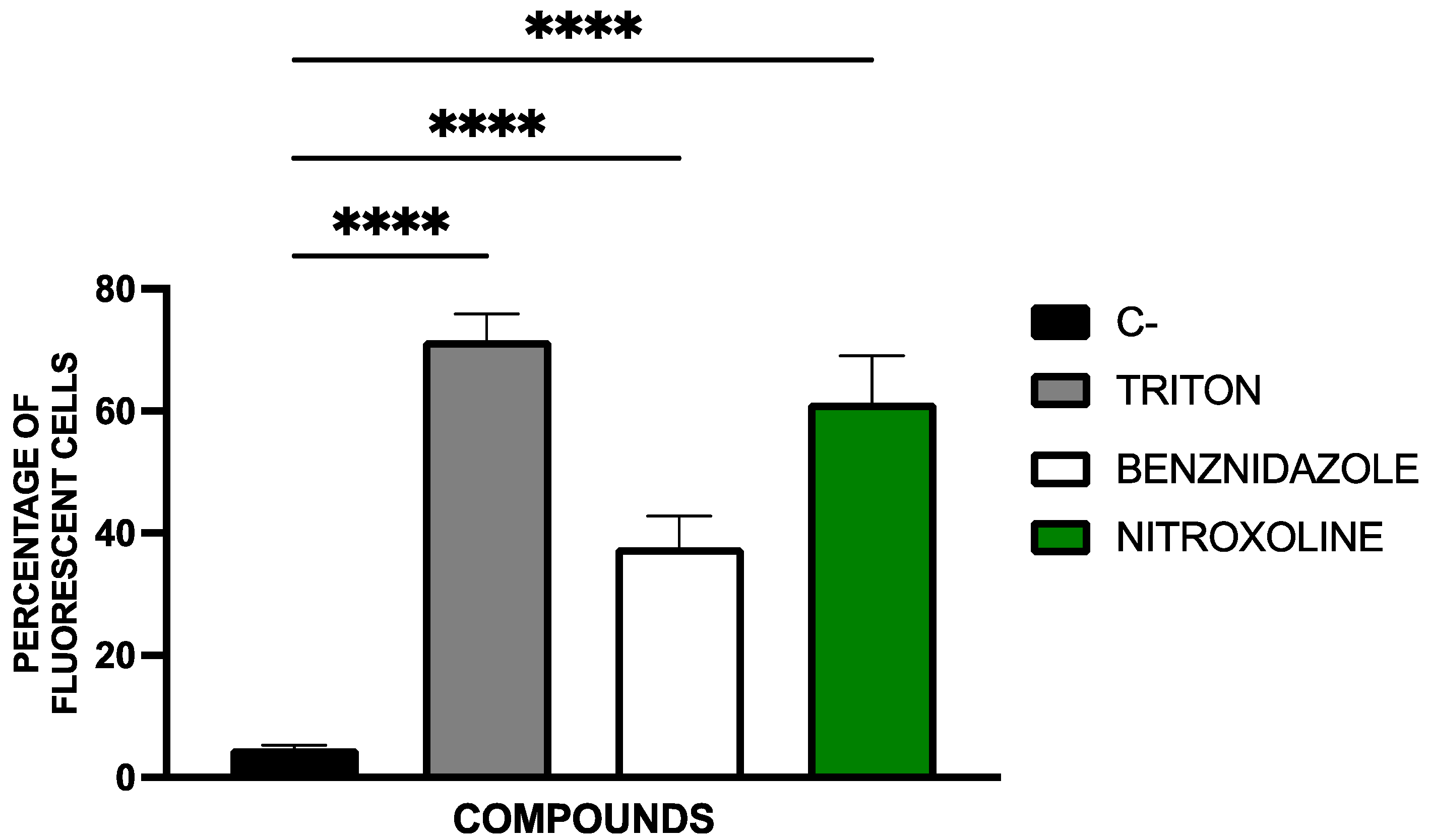
2.2.4. Plasma Membrane Permeability Analysis
2.2.5. Accumulation of Reactive Oxygen Species Analysis
3. Discussion
4. Materials and Methods
4.1. Compounds
4.2. Cultures
4.3. Activity Against Epimastigote Stage
4.4. Activity Against Amastigote Stage
4.5. Mechanisms of Cell Death
4.5.1. Chromatin Condensation Analysis
4.5.2. Mitochondrial Membrane Potential Analysis
4.5.3. ATP Levels Analysis
4.5.4. Plasmatic Membrane Permeability Analysis
4.5.5. Accumulation of Reactive Oxygen Species Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| LIT | Liver Infusion Tryptose |
| FBS | Foetal Bovine Serum |
| DMEM | Dulbecco’s Modified Eagle Medium |
| IC50 | Inhibitory concentration 50 |
| SDS | Sodium dodecyl sulfate |
| PI | Propidium iodide |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| IC90 | Inhibitory concentration 90 |
| ROS | Reactive oxygen species |
References
- Filigheddu, M.T.; Górgolas, M.; Ramos, J.M. Orally-Transmitted Chagas Disease. Med. Clin. 2017, 148, 125–131. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.S.; Vermeij, D.; Ramos, A.N.J.; Luquetti, A.O. Chagas Disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kluyber, D.; Desbiez, A.L.J.; Attias, N.; Massocato, G.F.; Gennari, S.M.; Soares, H.S.; Bagagli, E.; Bosco, S.M.G.; Garcés, H.G.; Ferreira, J.d.S.; et al. Zoonotic Parasites Infecting Free-Living Armadillos from Brazil. Transbound. Emerg. Dis. 2021, 68, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.A.; Furtado, C.; Lisboa, C.V.; de Oliveira, F.; Santos, F.M.; D’Andrea, P.S.; Roque, A.L.R.; Xavier, S.C.d.C.; Jansen, A.M. Trypanosomatid Richness Among Rats, Opossums, and Dogs in the Caatinga Biome, Northeast Brazil, a Former Endemic Area of Chagas Disease. Front. Cell. Infect. Microbiol. 2022, 12, 851903. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Acevedo, C.Y.; Parada-García, A.S.; Olivera, M.J.; Torres-Torres, F.; Zuleta-Dueñas, L.P.; Hernández, C.; Ramírez, J.D. Clinical and Epidemiological Characterization of Acute Chagas Disease in Casanare, Eastern Colombia, 2012–2020. Front. Med. 2021, 8, 681635. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Bringaud, F.; Nolan, D.P.; Figueiredo, L.M. Metabolic Reprogramming during the Trypanosoma brucei Life Cycle. F1000Research 2017, 6, 683. [Google Scholar] [CrossRef] [PubMed]
- Zíková, A. Mitochondrial Adaptations throughout the Trypanosoma brucei Life Cycle. J. Eukaryot. Microbiol. 2022, 69, e12911. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Prata, A. Clinical and Epidemiological Aspects of Chagas Disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rincón, G.J.; Gallo-Bernal, S.; Jiménez, P.A.; Cruz-Saavedra, L.; Ramírez, J.D.; Rodríguez, M.J.; Medina-Mur, R.; Díaz-Nassif, G.; Valderrama-Achury, M.D.; Medina, H.M. Molecular and Clinical Aspects of Chronic Manifestations in Chagas Disease: A State-of-the-Art Review. Pathogens 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. What Is Chagas Disease? JAMA 2024, 332, 2158. [Google Scholar] [CrossRef] [PubMed]
- Schmunis, G.A.; Yadon, Z.E. Chagas Disease: A Latin American Health Problem Becoming a World Health Problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.; Marcus, R.; Standley, C.J. The Importance of Screening for Chagas Disease Against the Backdrop of Changing Epidemiology in the USA. Curr. Trop. Med. Rep. 2022, 9, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.J.; Chiodini, P.L. Diagnosis and Clinical Management of Chagas Disease: An Increasing Challenge in Non-Endemic Areas. Res. Rep. Trop. Med. 2022, 13, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal Treatment of Chagas Disease. Enfermedades Infecc. Microbiol. Clin. Engl. Ed. 2021, 39, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, W.; Łaszkiewicz, J.; Tomczak, W.; Nowak, Ł.; Chorbińska, J.; Sójka, A.; Małkiewicz, B.; Szydełko, T. Nitroxoline: Treatment and Prevention of Urinary Tract Infections from the Urologist’s Perspective. Cent. Eur. J. Urol. 2024, 77, 339–343. [Google Scholar] [CrossRef]
- Fuchs, F.; Becerra-Aparicio, F.; Xanthopoulou, K.; Seifert, H.; Higgins, P.G. In Vitro Activity of Nitroxoline against Carbapenem-Resistant Acinetobacter baumannii Isolated from the Urinary Tract. J. Antimicrob. Chemother. 2022, 77, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Hof, H.; Juretschke, C. Nitroxoline: An Option for the Treatment of Urinary Tract Infection with Multi-Resistant Uropathogenic Bacteria. Infection 2019, 47, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Huang, L.; Li, X.; Watanabe, M.; Li, C.; Xu, A.; Liu, C.; Li, Q.; Araki, M.; Wada, K.; et al. The Novel Combination of Nitroxoline and PD-1 Blockade, Exerts a Potent Antitumor Effect in a Mouse Model of Prostate Cancer. Int. J. Biol. Sci. 2019, 15, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Hsu, L.-C.; Leu, W.-J.; Chen, C.-S.; Guh, J.-H. Repurposing of Nitroxoline as a Potential Anticancer Agent Against Human Prostate Cancer—A Crucial Role on AMPK/mTOR Signaling Pathway and the Interplay with Chk2 Activation. Oncotarget 2015, 6, 39806–39820. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Jiang, M.; Xue, D.; Wang, H.; Lu, Z.; Ding, L.; Xie, H.; Wang, R.; Luo, W.; Xu, L.; et al. Nitroxoline Suppresses Metastasis in Bladder Cancer via EGR1/circNDRG1/miR-520h/smad7/EMT Signaling Pathway. Int. J. Biol. Sci. 2022, 18, 5207–5220. [Google Scholar] [CrossRef] [PubMed]
- Lazovic, J.; Guo, L.; Nakashima, J.; Mirsadraei, L.; Yong, W.; Kim, H.J.; Ellingson, B.; Wu, H.; Pope, W.B. Nitroxoline Induces Apoptosis and Slows Glioma Growth In Vivo. Neuro Oncol. 2015, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Zöller, N.; Tietgen, M.; Steinhorst, K.; Bechtel, M.; Rothenburger, T.; Kandler, J.D.; Schneider, J.; Corman, V.M.; Ciesek, S.; et al. Repurposing of the Antibiotic Nitroxoline for the Treatment of mpox. J. Med. Virol. 2023, 95, e28652. [Google Scholar] [CrossRef] [PubMed]
- Milan Bonotto, R.; Mitrović, A.; Sosič, I.; Martínez-Orellana, P.; Dattola, F.; Gobec, S.; Kos, J.; Marcello, A. Cathepsin Inhibitors Nitroxoline and Its Derivatives Inhibit SARS-CoV-2 Infection. Antivir. Res. 2023, 216, 105655. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez de Garayo, M.; Liu, W.; Rondeau, N.C.; Damoci, C.B.; Miranda, J.J.L. Rationally Repurposed Nitroxoline Inhibits Preclinical Models of Epstein-Barr Virus-Associated Lymphoproliferation. J. Antibiot. 2021, 74, 763–766. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information Nitroxoline|C9H6N2O3|CID 19910—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nitroxoline (accessed on 26 February 2025).
- Fuchs, F.; Hamprecht, A. Susceptibility of Carbapenemase-Producing Enterobacterales (CPE) to Nitroxoline. J. Antimicrob. Chemother. 2019, 74, 2934–2937. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.M.; Wolke, M.; Rybniker, J.; Plum, G.; Fuchs, F. Activity of the Old Antimicrobial Nitroxoline against Mycobacterium abscessus Complex Isolates. J. Glob. Antimicrob. Resist. 2023, 33, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Hof, H.; Hofmann, S.; Kurzai, O.; Meis, J.F.; Hamprecht, A. Antifungal Activity of Nitroxoline against Candida auris Isolates. Clin. Microbiol. Infect. 2021, 27, 1697.e7–1697.e10. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Expósito, R.L.; Sifaoui, I.; Reyes-Batlle, M.; Fuchs, F.; Scheid, P.L.; Piñero, J.E.; Sutak, R.; Lorenzo-Morales, J. Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants 2023, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, W.; Jing, W.; Feng, M.; Zhou, Q.; Cheng, X. Nitroxoline Evidence Amoebicidal Activity Against Acanthamoeba castellanii through DNA Damage and the Stress Response Pathways. Int. J. Parasitol. Drugs Drug Resist. 2025, 27, 100578. [Google Scholar] [CrossRef] [PubMed]
- Tongkrajang, N.; Kobpornchai, P.; Dubey, P.; Chaisri, U.; Kulkeaw, K. Modelling Amoebic Brain Infection Caused by Balamuthia mandrillaris Using a Human Cerebral Organoid. PLoS Negl. Trop. Dis. 2024, 18, e0012274. [Google Scholar] [CrossRef] [PubMed]
- Spottiswoode, N.; Pet, D.; Kim, A.; Gruenberg, K.; Shah, M.; Ramachandran, A.; Laurie, M.T.; Zia, M.; Fouassier, C.; Boutros, C.L.; et al. Successful Treatment of Balamuthia mandrillaris Granulomatous Amebic Encephalitis with Nitroxoline. Emerg. Infect. Dis. 2023, 29, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Fuchs, F.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J.; Scheid, P. Repurposing of Nitroxoline as an Alternative Primary Amoebic Meningoencephalitis Treatment. Antibiotics 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.; Medeiros, A.; Comini, M.; Suescun, L.; Rodríguez Arce, E.; Martins, M.; Pinheiro, T.; Marques, F.; Gambino, D. Pt-Fe Ferrocenyl Compounds with Hydroxyquinoline Ligands Show Selective Cytotoxicity on Highly Proliferative Cells. J. Inorg. Biochem. 2019, 199, 110779. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.A.; Huttner, A.; Koch, B.C.P.; Mouton, J.W.; Muller, A.E. Review of the Pharmacokinetic Properties of Nitrofurantoin and Nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Niggemann, H.; Stein, G.; Stein, G. Review of the Literature and Individual Patients’ Data Meta-Analysis on Efficacy and Tolerance of Nitroxoline in the Treatment of Uncomplicated Urinary Tract Infections. BMC Infect. Dis. 2014, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Sorel, R.H.; Snelleman, C.; Hulshoff, A. High-Performance Liquid Chromatographic Analysis of Nitroxoline in Plasma and Urine. J. Chromatogr. 1981, 222, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jiangsu Yahong Meditech Co., Ltd. Base Addition Salts of Nitroxoline and Uses Thereof. U.S. Patent 2016/0031819 A1, 12 September 2017. [Google Scholar]
- Repac Antić, D.; Parčina, M.; Gobin, I.; Petković Didović, M. Chelation in Antibacterial Drugs: From Nitroxoline to Cefiderocol and Beyond. Antibiotics 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cherdtrakulkiat, R.; Lawung, R.; Nabu, S.; Tantimavanich, S.; Sinthupoom, N.; Prachayasittikul, S.; Prachayasittikul, V. Nitroxoline: A Potent Antimicrobial Agent against Multidrug Resistant Enterobacteriaceae. EXCLI J. 2019, 18, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Machado, I.; Fontana, C.; Risi, G.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. New Heteroleptic Oxidovanadium(V) Complexes: Synthesis, Characterization and Biological Evaluation as Potential Agents Against Trypanosoma cruzi. J. Biol. Inorg. Chem. 2018, 23, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Kresken, M.; Wohlfarth, E.; Bahrs, C.; Grabein, B.; Strohmaier, W.L.; Naber, K.G. Therapy of cystitis with nitroxoline-NitroxWin: Prospective, multicenter, non-interventional study and microbiological resistance surveillance. Urologie 2023, 62, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.I.; Wang, S.; Yang, D.; Pan, K.; Li, L.; Yuan, S. Preclinical Pharmacodynamic Evaluation of Antibiotic Nitroxoline for Anticancer Drug Repurposing. Oncol. Lett. 2016, 11, 3265–3272. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S. Cell Death Pathways in Pathogenic Trypanosomatids: Lessons of (over)Kill. Cell Death Dis. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Debrabant, A.; Lee, N.; Bertholet, S.; Duncan, R.; Nakhasi, H.L. Programmed Cell Death in Trypanosomatids and Other Unicellular Organisms. Int. J. Parasitol. 2003, 33, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ouaissi, A. Apoptosis-like Death in Trypanosomatids: Search for Putative Pathways and Genes Involved. Kinetoplastid Biol. Dis. 2003, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Akarid, K.; Grodet, A.; Petit, P.X.; Estaquier, J.; Ameisen, J.C. On the Evolution of Programmed Cell Death: Apoptosis of the Unicellular Eukaryote Leishmania Major Involves Cysteine Proteinase Activation and Mitochondrion Permeabilization. Cell Death Differ. 2002, 9, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Debrabant, A.; Nakhasi, H. Programmed Cell Death in Trypanosomatids: Is It an Altruistic Mechanism for Survival of the Fittest? Kinetoplastid Biol. Dis. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Prasad, S.; Webb, L.J.; Aggarwal, B.B. Cancer Drug Discovery by Repurposing: Teaching New Tricks to Old Dogs. Trends Pharmacol. Sci. 2013, 34, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Liu, J.O. Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Mrhar, A.; Kopitar, Z.; Kozjek, F.; Presl, V.; Karba, R. Clinical Pharmacokinetics of Nitroxoline. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 476–481. [Google Scholar] [PubMed]
- Hu, D.; Xu, H.; Xiao, B.; Li, D.; Zhou, Z.; Liu, X.; Tang, J.; Shen, Y. Albumin-Stabilized Metal-Organic Nanoparticles for Effective Delivery of Metal Complex Anticancer Drugs. ACS Appl. Mater. Interfaces 2018, 10, 34974–34982. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Fard, M.M.; Mirian, M.; Hassanzadeh, F. Targeted Nanoparticles for Co-Delivery of 5-FU and Nitroxoline, a Cathepsin B Inhibitor, in HepG2 Cells of Hepatocellular Carcinoma. Anticancer Agents Med. Chem. 2020, 20, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.M.; Wolke, M.; Rybniker, J.; Plum, G.; Fuchs, F. In Vitro Activity of Repurposed Nitroxoline Against Clinically Isolated Mycobacteria Including Multidrug-Resistant Mycobacterium tuberculosis. Front. Pharmacol. 2022, 13, 906097. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.M.; Martins, L.C.; Marques, G.V.; Silva, C.A.; Faria, G.; Caldas, S.; Dos Santos, J.S.; Leclercq, S.Y.; Maltarollo, V.G.; Ferreira, R.S.; et al. Synthesis of Quinoline Derivatives as Potential Cysteine Protease Inhibitors. Future Med. Chem. 2020, 12, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, D.; Cueto-Díaz, E.J.; Medina-Gil, T.; Chevillard, R.; Bernal-Fraile, T.; López-Sastre, R.; Aldfer, M.M.; Ungogo, M.A.; Elati, H.A.A.; Arai, N.; et al. Imidazoline- and Benzamidine-Based Trypanosome Alternative Oxidase Inhibitors: Synthesis and Structure-Activity Relationship Studies. ACS Med. Chem. Lett. 2022, 13, 312–318. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.E.; Joussef, A.C.; Pacheco, L.K.; da Silva, D.G.; Steindel, M.; Rebelo, R.A.; Schmidt, B. Synthesis and In Vitro Evaluation of Leishmanicidal and Trypanocidal Activities of N-Quinolin-8-yl-arylsulfonamides. Bioorg. Med. Chem. 2007, 15, 7553–7560. [Google Scholar] [CrossRef] [PubMed]
- Cartuche, L.; Sifaoui, I.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Lorenzo-Morales, J.; Piñero, J.E.; Díaz-Marrero, A.R.; Fernández, J.J. Antikinetoplastid Activity of Indolocarbazoles from Streptomyces sanyensis. Biomolecules 2020, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Salazar-Villatoro, L.; Omaña-Molina, M.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. Acrylonitrile Derivatives: In Vitro Activity and Mechanism of Cell Death Induction against Trypanosoma cruzi and Leishmania amazonensis. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100531. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Marrero, A.R.; López-Arencibia, A.; Bethencout-Estrella, C.J.; Cen-Pacheco, F.; Sifaoui, I.; Hernández Creus, A.; Duque-Ramírez, M.C.; Souto, M.L.; Hernández Daranas, A.; Lorenzo-Morales, J.; et al. Antiprotozoal Activities of Marine Polyether Triterpenoids. Bioorg. Chem. 2019, 92, 103276. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.J.; Martínez, M.L.; López-Arencibia, A.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Jiménez, I.A.; Lorenzo-Morales, J.; Piñero, J.E.; Bazzocchi, I.L. In Vitro Susceptibility of Kinetoplastids to Celastroloids from Maytenus chiapensis. Antimicrob. Agents Chemother. 2021, 65, e02236-20. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Nocchi, N.; López-Arencibia, A.; San Nicolás-Hernández, D.; Souto, M.L.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Fernández, J.J.; Lorenzo-Morales, J.; Piñero, J.E. Antikinetoplastid Activity of Sesquiterpenes Isolated from the Zoanthid Palythoa aff. clavata. Pharmaceuticals 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Sifaoui, I.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. Acrylonitrile Derivatives against Trypanosoma cruzi: In Vitro Activity and Programmed Cell Death Study. Pharmaceuticals 2021, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- San Nicolás-Hernández, D.; Bethencourt-Estrella, C.J.; López-Arencibia, A.; Hernández-Álvarez, E.; Sifaoui, I.; Bazzocchi, I.L.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Withaferin A-Silyl Ether Analogs as Potential Anti-Kinetoplastid Agents Targeting the Programmed Cell Death. Biomed. Pharmacother. 2023, 157, 114012. [Google Scholar] [CrossRef] [PubMed]








| Activity Against Epimastigote Forms (IC50 µM) | |||
| Nitroxoline | 3.00 ± 0.44 | Benznidazole | 6.92 ± 0.77 |
| Activity Against Amastigote Forms (IC50 µM) | |||
| Nitroxoline | 1.24 ± 0.23 | Benznidazole | 2.67 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bethencourt-Estrella, C.J.; López-Arencibia, A.; Calero-Docina, I.M.; Fuchs, F.; Scheid, P.; Lorenzo-Morales, J.; Piñero, J.E. Repurposing Analysis of Nitroxoline (8-Hydroxy-5-nitroquinoline) as an Antichagasic Compound. Pharmaceuticals 2025, 18, 1106. https://doi.org/10.3390/ph18081106
Bethencourt-Estrella CJ, López-Arencibia A, Calero-Docina IM, Fuchs F, Scheid P, Lorenzo-Morales J, Piñero JE. Repurposing Analysis of Nitroxoline (8-Hydroxy-5-nitroquinoline) as an Antichagasic Compound. Pharmaceuticals. 2025; 18(8):1106. https://doi.org/10.3390/ph18081106
Chicago/Turabian StyleBethencourt-Estrella, Carlos J., Atteneri López-Arencibia, Isabel M. Calero-Docina, Frieder Fuchs, Patrick Scheid, Jacob Lorenzo-Morales, and José E. Piñero. 2025. "Repurposing Analysis of Nitroxoline (8-Hydroxy-5-nitroquinoline) as an Antichagasic Compound" Pharmaceuticals 18, no. 8: 1106. https://doi.org/10.3390/ph18081106
APA StyleBethencourt-Estrella, C. J., López-Arencibia, A., Calero-Docina, I. M., Fuchs, F., Scheid, P., Lorenzo-Morales, J., & Piñero, J. E. (2025). Repurposing Analysis of Nitroxoline (8-Hydroxy-5-nitroquinoline) as an Antichagasic Compound. Pharmaceuticals, 18(8), 1106. https://doi.org/10.3390/ph18081106











