Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri
Abstract
1. Introduction
2. Results and Discussion
2.1. Repurposing Rational of Conformationally-Restricted Cyclopentane-Based Miltefosine Analogs
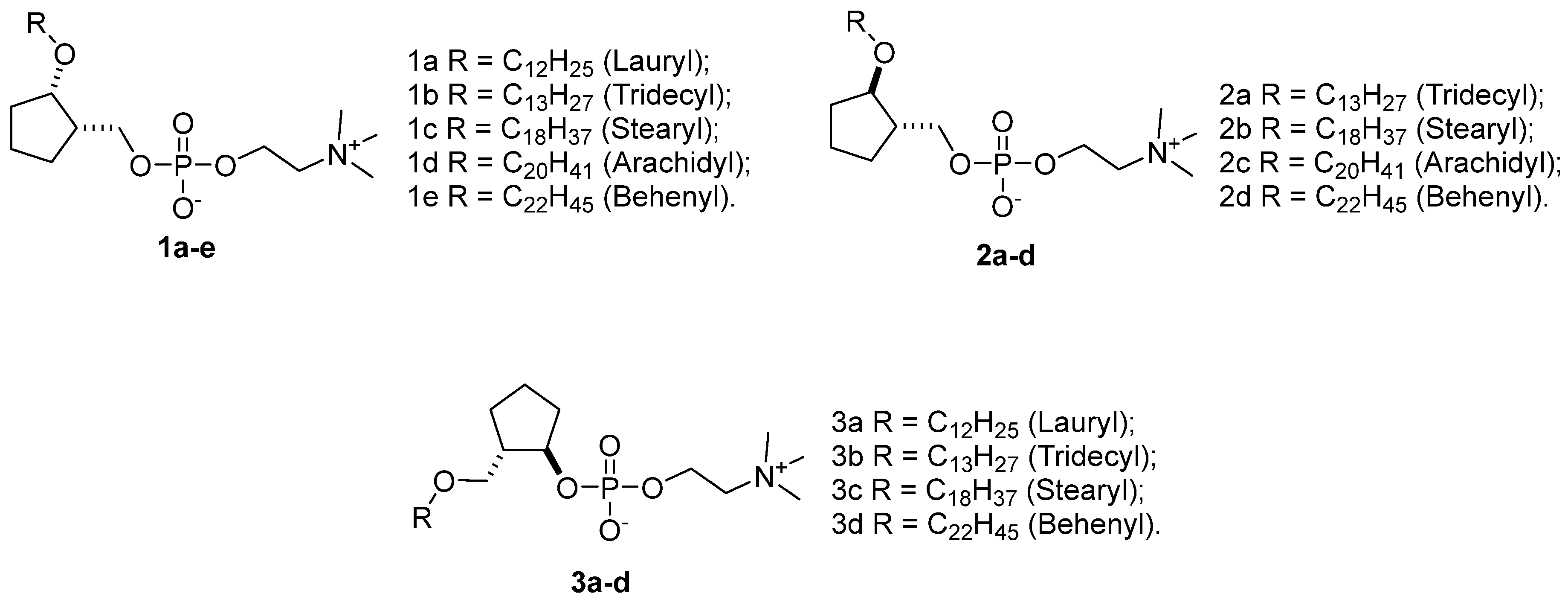
2.2. Chemistry
2.3. Biological Evaluations
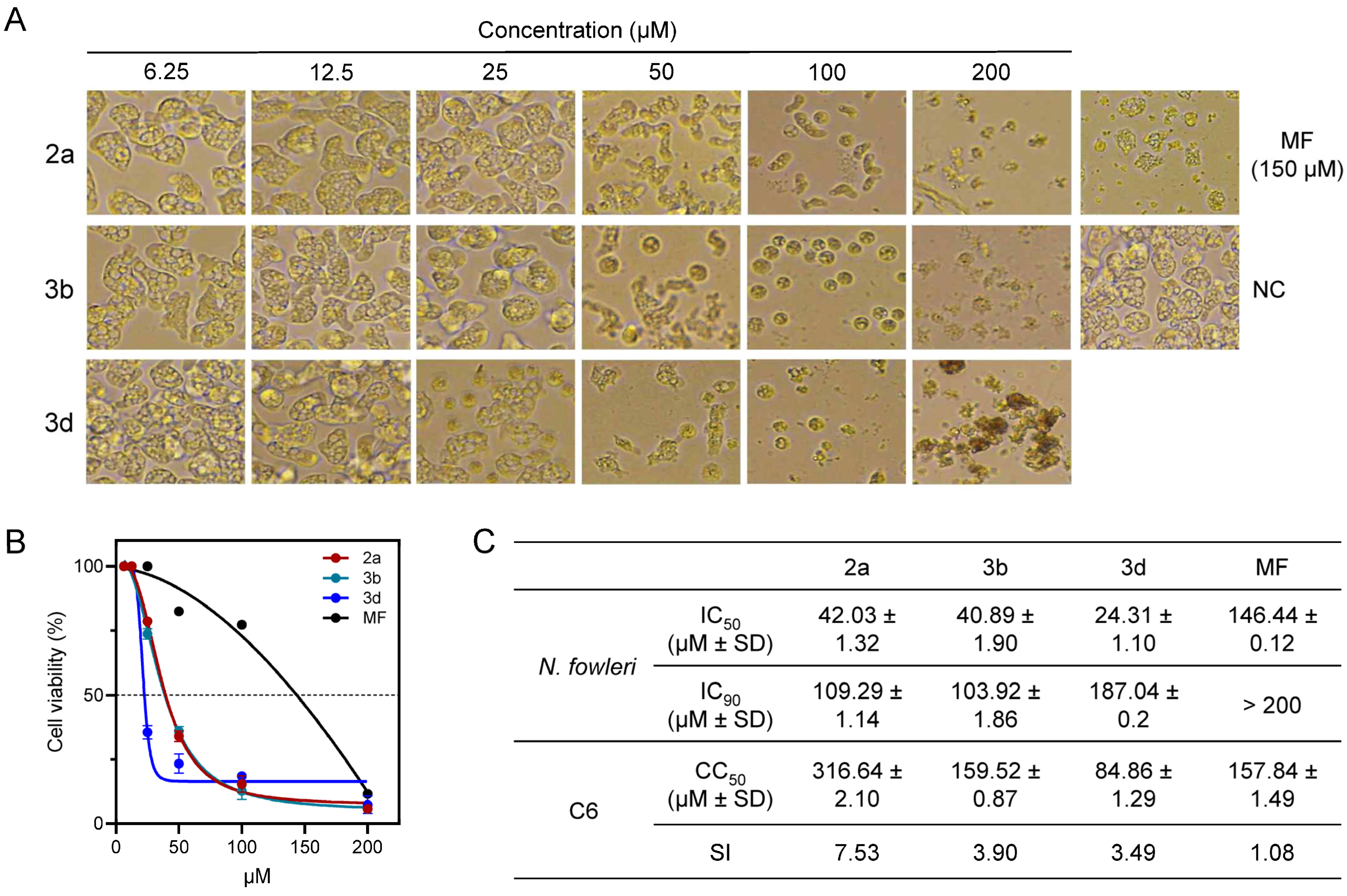
2.3.1. In Vitro Evaluation of Anti-Amoebic Activity and Selectivity Against N. fowleri
2.3.2. Compounds 2a, 3b and 3d Induce Morphological Changes in N. fowleri
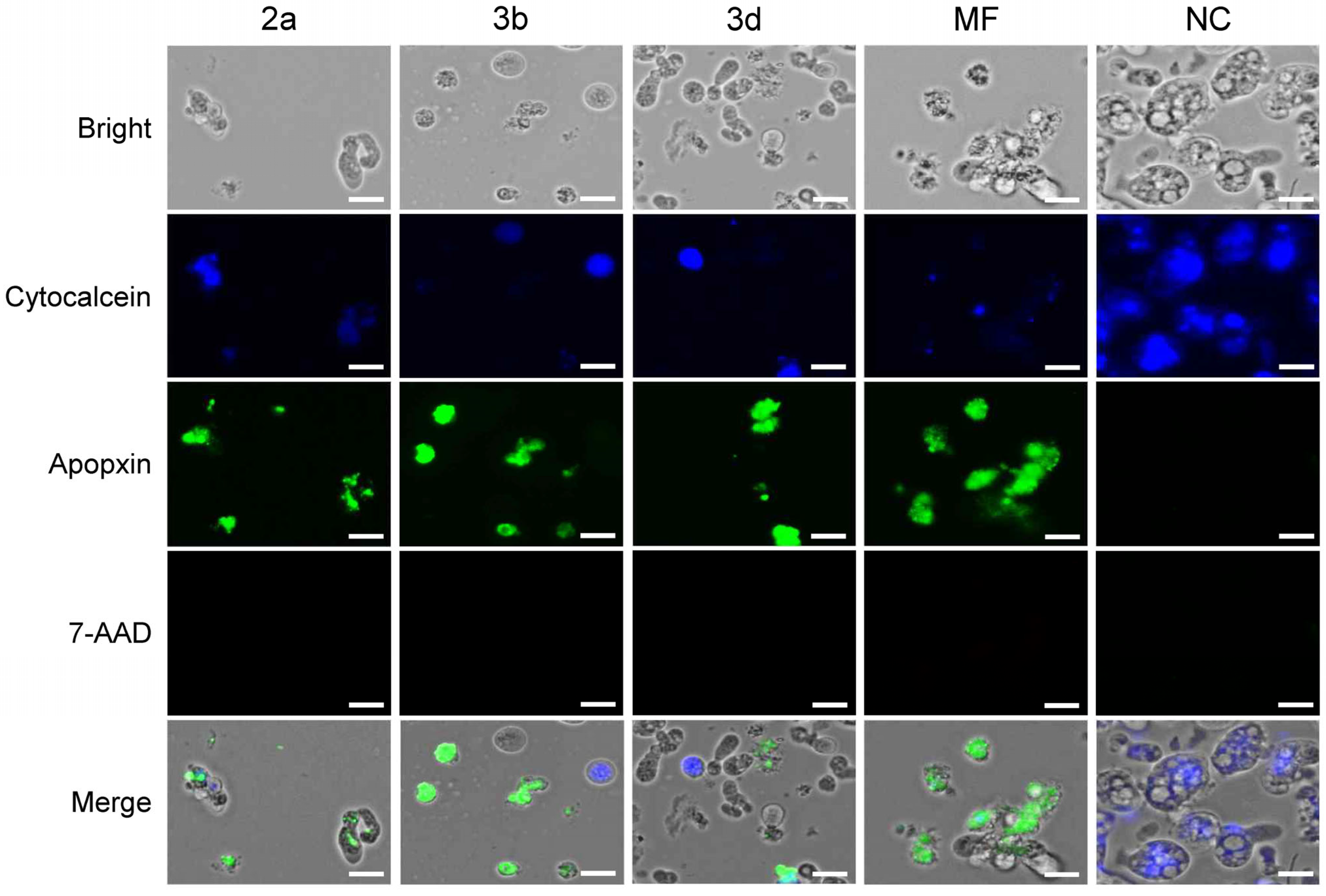
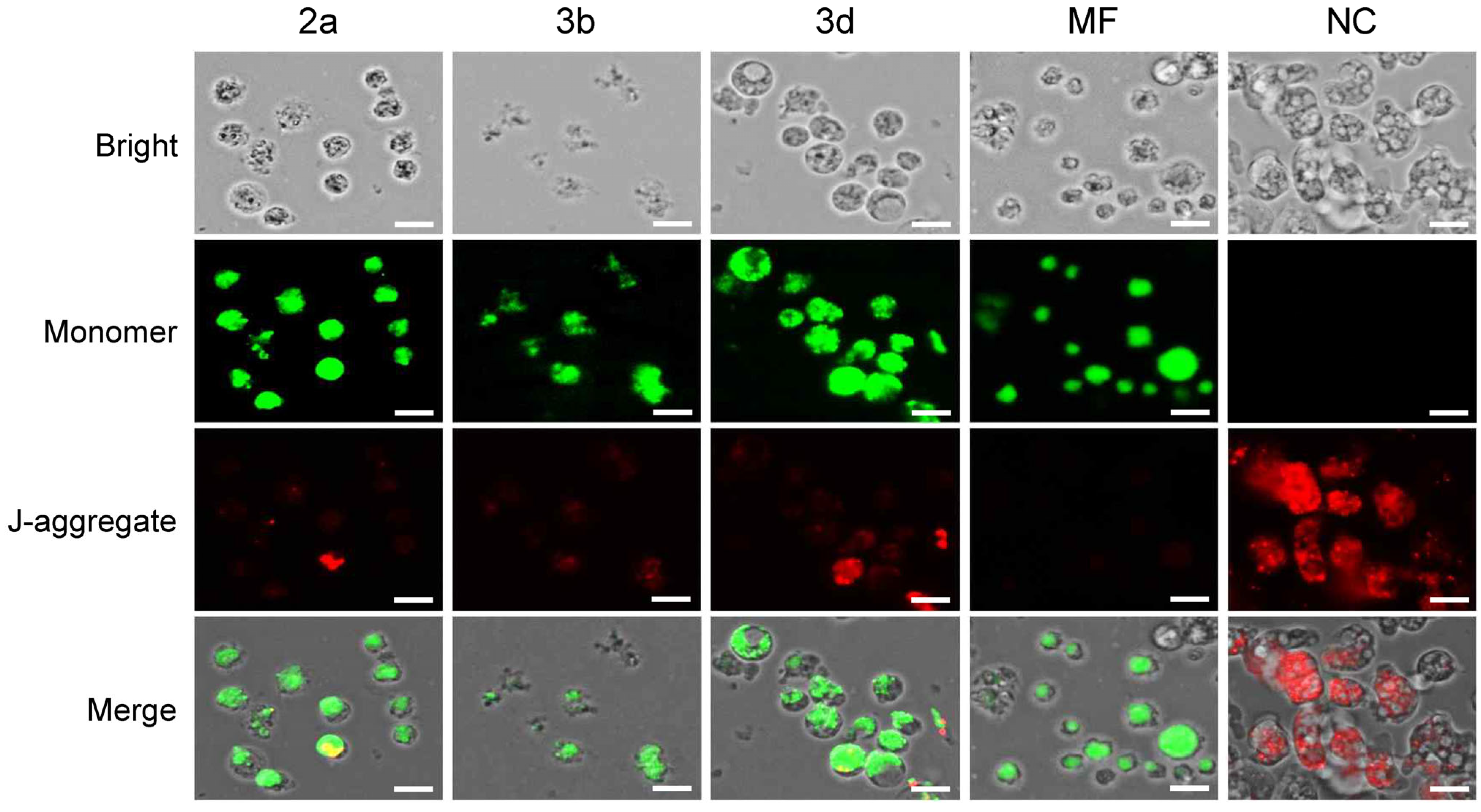
2.3.3. Compounds 2a, 3b and 3d Induce Apoptotic-like but Not Necrotic Death of N. fowleri
2.3.4. Compounds 2a, 3b and 3d Induce DNA Fragmentation in N. fowleri
2.3.5. Compounds 2a, 3b and 3d Result in Mitochondrial Dysfunction in N. fowleri
3. Materials and Methods
3.1. Cells and Cultures
3.2. Anti-Amoebic Activity Assays
3.3. Cytotoxicity Assays for C6 Cells
3.4. Apoptosis/Necrosis Assay
3.5. TUNEL Assay
3.6. Mitochondrial Membrane Potential Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Ali, I.K.M.; Cope, J.R.; Khan, N.A. Biology and pathogenesis of Naegleria fowleri. Acta Trop. 2016, 164, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.E.; Chávez-Munguía, B.; Omaña-Molina, M.; Lorenzo-Morales, J. Naegleria fowleri. Trends Parasitol. 2019, 35, 848–849. [Google Scholar] [CrossRef]
- Ata, I.; Riaz, N.; Ata, F.; Farooq, U.; Mallhi, T.H. Waking up to the Naegleria threat: Urgent measures needed to protect public health in Pakistan. Expert Rev. Anti-Infect. Ther. 2024, 22, 129–130. [Google Scholar] [CrossRef]
- Cooper, A.M.; Aouthmany, S.; Shah, K.; Rega, P.P. Killer amoebas: Primary amoebic meningoencephalitis in a changing climate. JAAPA 2019, 32, 30–35. [Google Scholar] [CrossRef]
- De Jonckheere, J.F. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect. Genet. Evol. 2011, 11, 1520–1528. [Google Scholar] [CrossRef]
- Gharpure, R.; Bliton, J.; Goodman, A.; Ali, I.K.M.; Yoder, J.; Cope, J.R. Epidemiology and Clinical Characteristics of Primary Amebic Meningoencephalitis Caused by Naegleria fowleri: A Global Review. Clin. Infect. Dis. 2021, 73, e19–e27. [Google Scholar] [CrossRef]
- Alanazi, A.; Younas, S.; Ejaz, H.; Alruwaili, M.; Alruwaili, Y.; Mazhari, B.B.Z.; Atif, M.; Junaid, K. Advancing the understanding of Naegleria fowleri: Global epidemiology, phylogenetic analysis, and strategies to combat a deadly pathogen. J. Infect. Public Health 2025, 18, 102690. [Google Scholar] [CrossRef]
- Gharpure, R.; Gleason, M.; Salah, Z.; Blackstock, A.J.; Hess-Homeier, D.; Yoder, J.S.; Ali, I.K.M.; Collier, S.A.; Cope, J.R. Geographic Range of Recreational Water-Associated Primary Amebic Meningoencephalitis, United States, 1978–2018. Emerg. Infect. Dis. 2021, 27, 271–274. [Google Scholar] [CrossRef]
- Cope, J.R.; Ali, I.K. Primary Amebic Meningoencephalitis: What Have We Learned in the Last 5 Years? Curr. Infect. Dis. Rep. 2016, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Exploring therapeutic approaches against Naegleria fowleri infections through the COVID box. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100545. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Guzmán-Téllez, P.; Flores-Huerta, N.; Silva-Olivares, A.; Serrano-Luna, J.; Shibayama, M. Chapter 158—Naegleria. In Molecular Medical Microbiology, 3rd ed.; Tang, Y.-W., Hindiyeh, M.Y., Liu, D., Sails, A., Spearman, P., Zhang, J.-R., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 3121–3133. [Google Scholar]
- Capewell, L.G.; Harris, A.M.; Yoder, J.S.; Cope, J.R.; Eddy, B.A.; Roy, S.L.; Visvesvara, G.S.; Fox, L.M.; Beach, M.J. Diagnosis, Clinical Course, and Treatment of Primary Amoebic Meningoencephalitis in the United States, 1937–2013. J. Pediatr. Infect. Dis. Soc. 2015, 4, e68–e75. [Google Scholar] [CrossRef]
- Bellini, N.K.; Santos, T.M.; da Silva, M.T.A.; Thiemann, O.H. The therapeutic strategies against Naegleria fowleri. Exp. Parasitol. 2018, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jahangeer, M.; Mahmood, Z.; Munir, N.; Waraich, U.-E.-A.; Tahir, I.M.; Akram, M.; Ali Shah, S.M.; Zulfqar, A.; Zainab, R. Naegleria fowleri: Sources of infection, pathophysiology, diagnosis, and management; a review. Clin. Exp. Pharmacol. Physiol. 2020, 47, 199–212. [Google Scholar] [CrossRef]
- Pedro de Sena, M.P.; Daniel, A.R.; Rodolfo do Couto, M.; Sreekanth, T.; Carlos, A.M.F. The Use of Conformational Restriction in Medicinal Chemistry. Curr. Top. Med. Chem. 2019, 19, 1712–1733. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Hu, X.; Xu, H.; Yang, B.; Zhan, L.; Cai, Y.; Gu, Y.; Chen, X.; Zheng, Y.; et al. Conformational restriction enables discovering a series of chroman derivatives as potent and selective NaV1.8 inhibitors with improved pharmacokinetic properties. Eur. J. Med. Chem. 2025, 293, 117697. [Google Scholar] [CrossRef]
- Qin, Y.; Poulsen, C.; Narayanan, D.; Chan, C.B.; Chen, X.; Montes, B.R.; Tran, K.T.; Mukminova, E.; Lin, C.; Gajhede, M.; et al. Structure-Guided Conformational Restriction Leading to High-Affinity, Selective, and Cell-Active Tetrahydroisoquinoline-Based Noncovalent Keap1-Nrf2 Inhibitors. J. Med. Chem. 2024, 67, 18828–18864. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Allison, M. NCATS launches drug repurposing program. Nat. Biotechnol. 2012, 30, 571–572. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Phan, T.-N.; Choi, Y.; Moon, S.; No, J.H.; Lee, Y.S. Design, Rational Repurposing, Synthesis, In Vitro Evaluation, Homology Modeling and In Silico Study of Sulfuretin Analogs as Potential Antileishmanial Hit Compounds. Pharmaceuticals 2022, 15, 1058. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Yu, B.; Ouyang, L. Drug repurposing: An effective strategy to accelerate contemporary drug discovery. Drug Discov. Today 2022, 27, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.E.; Phan, T.-N.; Moon, S.; Lee, C.H.; Kim, Y.J.; Cho, S.B.; El-Sayed, S.M.; Choi, Y.; No, J.H.; Lee, Y.S. Design, synthesis, and repurposing of O6-aminoalkyl-sulfuretin analogs towards discovery of potential lead compounds as antileishmanial agents. Eur. J. Med. Chem. 2023, 251, 115256. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Wang, C.Y.; Lee, C.J.; Jeon, H.R.; Choi, Y.; Moon, S.; Lee, C.H.; Kim, Y.J.; Cho, S.B.; Mahmoud, K.; et al. Repurposing Synthetic Congeners of a Natural Product Aurone Unveils a Lead Antitumor Agent Inhibiting Folded P-Loop Conformation of MET Receptor Tyrosine Kinase. Pharmaceuticals 2023, 16, 1597. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Bayoumi, W.A.; El-Sayed, S.M.; Phan, T.-N.; Oh, T.; Ham, G.; Mahmoud, K.; No, J.H.; Lee, Y.S. Design, Synthesis, and Repurposing of Rosmarinic Acid-β-Amino-α-Ketoamide Hybrids as Antileishmanial Agents. Pharmaceuticals 2023, 16, 1594. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Bayoumi, W.A.; El-Sayed, S.M.; Phan, T.-N.; Kim, Y.J.; Lee, C.H.; Cho, S.B.; Oh, T.; Ham, G.; Mahmoud, K.; et al. Rational repurposing, synthesis, in vitro and in silico studies of chromone-peptidyl hybrids as potential agents against Leishmania donovani. J. Enzym. Inhib. Med. Chem. 2023, 38, 2229071. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Mahmoud, K.; Phan, T.-N.; Shaldam, M.A.; Lee, C.H.; Kim, Y.J.; Cho, S.B.; Bayoumi, W.A.; El-Sayed, S.M.; Choi, Y.; et al. Bestatin analogs-4-quinolinone hybrids as antileishmanial hits: Design, repurposing rational, synthesis, in vitro and in silico studies. Eur. J. Med. Chem. 2023, 250, 115211. [Google Scholar] [CrossRef]
- Scherman, D.; Fetro, C. Drug repositioning for rare diseases: Knowledge-based success stories. Therapies 2020, 75, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Roessler, H.I.; Knoers, N.V.A.M.; van Haelst, M.M.; van Haaften, G. Drug Repurposing for Rare Diseases. Trends Pharmacol. Sci. 2021, 42, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, C.H.; Seynaeve, C.; Bontenbal, M.; Planting, A.S.; Sindermann, H.; Verweij, J. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anti-Cancer Drugs 2000, 11, 825–828. [Google Scholar] [CrossRef]
- Cope, J.R. Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 666. [Google Scholar]
- Alli, A.; Ortiz, J.F.; Morillo Cox, Á.; Armas, M.; Orellana, V.A. Miltefosine: A Miracle Drug for Meningoencephalitis Caused by Free-Living Amoebas. Cureus 2021, 13, e13698. [Google Scholar] [CrossRef]
- Martínez, D.Y.; Seas, C.; Bravo, F.; Legua, P.; Ramos, C.; Cabello, A.M.; Gotuzzo, E. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin. Infect. Dis. 2010, 51, e7–e11. [Google Scholar] [CrossRef]
- Marschner, N.; Kötting, J.; Eibl, H.; Unger, C. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother. Pharmacol. 1992, 31, 18–22. [Google Scholar] [CrossRef]
- Astman, N.; Arbel, C.; Katz, O.; Barzilai, A.; Solomon, M.; Schwartz, E. Tolerability and Safety of Miltefosine for the Treatment of Cutaneous Leishmaniasis. Trop. Med. Infect. Dis. 2024, 9, 218. [Google Scholar] [CrossRef]
- Pandey, K.; Ravidas, V.; Siddiqui, N.A.; Sinha, S.K.; Verma, R.B.; Singh, T.P.; Dhariwal, A.C.; Das Gupta, R.K.; Das, P. Pharmacovigilance of Miltefosine in Treatment of Visceral Leishmaniasis in Endemic Areas of Bihar, India. Am. J. Trop. Med. Hyg. 2016, 95, 1100–1105. [Google Scholar] [CrossRef]
- Pal, B.; Daniel, A.T.; Sweta, K.; Krishna, M.; Rishikesh, K.; Krishna, P.; Ali, S.N.; Sameer, D.; Chaudhary, V. Ophthalmic adverse effects of miltefosine in the treatment of leishmaniasis: A systematic review. Cutan. Ocul. Toxicol. 2024, 43, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Matoba, A.; Weikert, M.P.; Kim, S. Corneal Manifestations of Miltefosine Toxicity in Acanthamoeba Keratitis. Ophthalmology 2021, 128, 1273. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, F.; Sharifi, I.; Khosravi, A.; Molaakbari, E.; Tavakkoli, H.; Salarkia, E.; Bahraminejad, S.; Bamorovat, M.; Dabiri, S.; Salari, Z.; et al. Comparison of cytotoxicity of Miltefosine and its niosomal form on chick embryo model. Sci. Rep. 2024, 14, 2482. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Seyedi, F.; Mohamadi, N.; Sharifi, I.; Pardakhty, A.; Khosravi, A.; Kamali, A. Cytotoxicity Effects of Miltefosine and Niosomal form on Human Umbilical Vein Endothelial Cells: Colorimetric Assay, Apoptosis, and Gene Expression Profiling. Lett. Drug Des. Discov. 2023, 20, 1936–1946. [Google Scholar] [CrossRef]
- Spadari, C.d.C.; Borba-Santos, L.P.; Rozental, S.; Ishida, K. Miltefosine repositioning: A review of potential alternative antifungal therapy. J. Med. Mycol. 2023, 33, 101436. [Google Scholar] [CrossRef]
- de Bastiani, F.W.M.d.S.; Spadari, C.d.C.; de Matos, J.K.R.; Salata, G.C.; Lopes, L.B.; Ishida, K. Nanocarriers Provide Sustained Antifungal Activity for Amphotericin B and Miltefosine in the Topical Treatment of Murine Vaginal Candidiasis. Front. Microbiol. 2020, 10, 2976. [Google Scholar] [CrossRef]
- Kolter, T. Conformational Restriction of Sphingolipids. In Highlights in Bioorganic Chemistry: Methods and Applications; Schmuck, C., Wennemers, H., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2004; pp. 48–62. [Google Scholar] [CrossRef]
- Fang, Z.; Song, Y.; Zhan, P.; Zhan, Q.; Liu, X. Conformational Restriction: An Effective Tactic in ‘Follow-On’-Based Drug Discovery. Future Med. Chem. 2014, 6, 885–901. [Google Scholar] [CrossRef]
- Borsari, C.; Rageot, D.; Dall’Asen, A.; Bohnacker, T.; Melone, A.; Sele, A.M.; Jackson, E.; Langlois, J.-B.; Beaufils, F.; Hebeisen, P.; et al. A Conformational Restriction Strategy for the Identification of a Highly Selective Pyrimido-pyrrolo-oxazine mTOR Inhibitor. J. Med. Chem. 2019, 62, 8609–8630. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Alam, M.M.; Phan, T.N.; Baek, K.H.; Lee, H.; Cho, S.B.; Lee, C.H.; Kim, Y.J.; No, J.H.; Lee, Y.S. Repurposing of conformationally-restricted cyclopentane-based AKT-inhibitors leads to discovery of potential and more selective antileishmanial agents than miltefosine. Bioorg. Chem. 2023, 141, 106890. [Google Scholar] [CrossRef]
- Cope, J.R.; Conrad, D.A.; Cohen, N.; Cotilla, M.; DaSilva, A.; Jackson, J.; Visvesvara, G.S. Use of the Novel Therapeutic Agent Miltefosine for the Treatment of Primary Amebic Meningoencephalitis: Report of 1 Fatal and 1 Surviving Case. Clin. Infect. Dis. 2015, 62, 774–776. [Google Scholar] [CrossRef]
- Ni Nyoman, A.D.; Lüder, C.G.K. Apoptosis-like cell death pathways in the unicellular parasite Toxoplasma gondii following treatment with apoptosis inducers and chemotherapeutic agents: A proof-of-concept study. Apoptosis 2013, 18, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, J. Programmed Cell Death in Protozoa; Landes Bioscience: Austin, TX, USA, 2008. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Flucofuron as a Promising Therapeutic Agent against Brain-Eating Amoeba. ACS Infect. Dis. 2024, 10, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.G.; Kang, J.-M.; Võ, T.C.; Na, B.-K. Kaempferol induces programmed cell death in Naegleria fowleri. Phytomedicine 2023, 119, 154994. [Google Scholar] [CrossRef] [PubMed]
- Arberas-Jiménez, I.; Rodríguez-Expósito, R.L.; San Nicolás-Hernández, D.; Chao-Pellicer, J.; Sifaoui, I.; Díaz-Marrero, A.R.; Fernández, J.J.; Piñero, J.E.; Lorenzo-Morales, J. Marine Meroterpenoids Isolated from Gongolaria abies-marina Induce Programmed Cell Death in Naegleria fowleri. Pharmaceuticals 2023, 16, 1010. [Google Scholar] [CrossRef]
- Arberas-Jiménez, I.; Rizo-Liendo, A.; Nocchi, N.; Sifaoui, I.; Chao-Pellicer, J.; Souto, M.L.; Suárez-Gómez, B.; Díaz-Marrero, A.R.; Fernández, J.J.; Piñero, J.E.; et al. Sesquiterpene lactones as potential therapeutic agents against Naegleria fowleri. Biomed. Pharmacother. 2022, 147, 112694. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Rizo-Liendo, A.; Sifaoui, I.; Arberas-Jiménez, I.; Reyes-Batlle, M.; Piñero, J.E.; Lorenzo-Morales, J. Fluvastatin and atorvastatin induce programmed cell death in the brain eating amoeba Naegleria fowleri. Biomed. Pharmacother. 2020, 130, 110583. [Google Scholar] [CrossRef]
- Zeouk, I.; Sifaoui, I.; Rizo-Liendo, A.; Arberas-Jiménez, I.; Reyes-Batlle, M.; L. Bazzocchi, I.; Bekhti, K.; E. Piñero, J.; Jiménez, I.A.; Lorenzo-Morales, J. Exploring the Anti-Infective Value of Inuloxin A Isolated from Inula viscosa against the Brain-Eating Amoeba (Naegleria fowleri) by Activation of Programmed Cell Death. ACS Chem. Neurosci. 2021, 12, 195–202. [Google Scholar] [CrossRef]


| Compound | Alkyl Chain | IC50 of Inhibition of N. fowleri | Relative Potency to Miltefosine a | CC50 of C6 Glial Cells | Selectivity Index b |
|---|---|---|---|---|---|
| 1a | Lauryl (C12H25) | 161.98 ± 1.02 | 0.90 | >400 | >2.47 |
| 1b | Tridecyl (C13H27) | 39.14 ± 0.60 | 3.74 | 105.37 ± 4.76 | 2.69 |
| 1c | Stearyl (C18H37) | 72.73 ± 2.32 | 2.01 | 158.4 ± 0.71 | 2.18 |
| 1d | Arachidyl (C20H41) | 43.64 ± 1.02 | 3.4 | 69.49 ± 0.16 | 1.59 |
| 1e | Behenyl (C22H45) | 165.82 ± 068 | 0.88 | 113.65 ± 3.20 | 0.69 |
| 2a | Tridecyl (C13H27) | 42.03 ± 1.32 | 3.49 | 316.64 ± 2.10 | 7.53 |
| 2b | Stearyl (C18H37) | 23.72 ± 0.50 | 6.18 | 69.71 ± 0.04 | 2.94 |
| 2c | Arachidyl (C20H41) | 19.63 ± 0.44 | 7.46 | 42.96 ± 8.37 | 2.19 |
| 2d | Behenyl (C22H45) | 55.65 ± 3.32 | 2.63 | 71.03 ± 0.61 | 1.28 |
| 3a | Lauryl (C12H25) | 279.45 ± 0.94 | 0.52 | >400 | >1.43 |
| 3b | Tridecyl (C13H27) | 40.89 ± 1.90 | 3.58 | 159.52 ± 0.87 | 3.90 |
| 3c | Stearyl (C18H37) | 76.33 ± 1.56 | 1.92 | 58.48 ± 2.08 | 0.77 |
| 3d | Behenyl (C22H45) | 24.31 ± 1.10 | 6.03 | 84.86 ± 1.29 | 3.49 |
| Miltefosine | 146.53 ± 0.12 | 1.00 | 158.89 ± 1.49 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.H.E.; Lê, H.G.; Võ, T.C.; Kim, M.; No, J.H.; Aboutaleb, M.H.; Sim, J.; Na, B.-K.; Lee, Y.S. Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri. Pharmaceuticals 2025, 18, 984. https://doi.org/10.3390/ph18070984
Hassan AHE, Lê HG, Võ TC, Kim M, No JH, Aboutaleb MH, Sim J, Na B-K, Lee YS. Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri. Pharmaceuticals. 2025; 18(7):984. https://doi.org/10.3390/ph18070984
Chicago/Turabian StyleHassan, Ahmed H. E., Hương Giang Lê, Tuấn Cường Võ, Minji Kim, Joo Hwan No, Mohamed H. Aboutaleb, Jaehoon Sim, Byoung-Kuk Na, and Yong Sup Lee. 2025. "Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri" Pharmaceuticals 18, no. 7: 984. https://doi.org/10.3390/ph18070984
APA StyleHassan, A. H. E., Lê, H. G., Võ, T. C., Kim, M., No, J. H., Aboutaleb, M. H., Sim, J., Na, B.-K., & Lee, Y. S. (2025). Discovery of Cyclopentane-Based Phospholipids as Miltefosine Analogs with Superior Potency and Enhanced Selectivity Against Naegleria fowleri. Pharmaceuticals, 18(7), 984. https://doi.org/10.3390/ph18070984










