Abstract
Background: Taraxacum officinale (T. officinale), commonly known as dandelion, is a plant with recognized therapeutic properties in both traditional and modern medicine. Historically, it has been used to treat various conditions, particularly liver disorders, owing to its antioxidant and anti-inflammatory activities. This narrative review focuses on its biological activity, with an emphasis on hepatoprotective effects. Methods: We performed a compilation and analysis of published studies on the effects of T. officinale in animal models and its potential application in liver diseases. Results: Preclinical studies have reported that extracts of this plant protect against liver damage induced by toxic agents such as alcohol, carbon tetrachloride, and paracetamol. Among the most relevant and predominant bioactive compounds of T. officinale is taraxasterol, which modulates inflammatory and oxidative stress pathways, helping to prevent liver damage. Conclusions: While preclinical studies are promising, further clinical trials are essential to confirm the safety and efficacy of T. officinale in the treatment of liver diseases. Determining the optimal dosing, evaluating its potential as an adjuvant in pharmacological treatments, as well as evaluating possible interactions with conventional drugs, is necessary for the potential use of T. officinale as an adjuvant agent in the treatment of liver diseases.
1. Introduction
Medicinal plants have been used since ancient times. Different civilizations have gained knowledge about the medicinal properties of a large number of plants found in their environment. For centuries, plant species used as sources of food and medicine have shown therapeutic properties, produced by substances or active principles found in their constituents, with protective effects against diseases [1]. Historically, communities worldwide have depended on herbal preparations to treat various health disorders. Nowadays, the World Health Organization (WHO) has promoted the use of traditional medicinal plants for the management and prevention of several diseases [2]. In 1997, the WHO indicated that a medicinal plant is “any plant in which one or more of its organs contain substances that can be used for therapeutic purposes or as precursors in the synthesis of other drugs”. The WHO description discriminates between plants/herbs whose compounds and beneficial properties have been scientifically well studied, from those that, despite being considered medicinal, have not yet been fully assessed. Among them, Taraxacum officinale Weber ex F.H.Wigg [3] has recently received scientific attention due to its high nutritional value and its beneficial effects on human health [4]. Taraxacum officinale (T. officinale) is commonly known as “dandelion”, a plant of the genus Taraxacum from the Asteraceae family. T. officinale is a non-toxic perennial herbaceous plant frequently considered a weed [5] that can be exploited due to its beneficial properties [6]. The common name “dandelion” probably comes from the French “dent-de-lion”, which refers to the “toothed” leaves of the plant [7]. As Stearn defined in 1973, the coin “officinale” literally means “be appropriate to an office”, indicating the storehouse of a monastery, where “medicines and other necessary items were lay-up”. Therefore, this spice designation is coined for plants that have recognized medicinal, gastronomic, or other uses [8].
2. Botanical, Ethnopharmacological, and Experimental Overview of Taraxacum officinale
2.1. Distribution, Habitat, and Botanical Description
It is thought that T. officinale emerged in Eurasia, from where it was involuntarily disseminated by population movement, spreading it almost all over the world [9]. T. officinale can tolerate a varied range of environmental conditions, growing in optimal conditions in well-drained soils, enriched in calcium and high humus content [10]. It grows in grasslands, roadsides, parks, orchards, and vacant lots, adapting to light and growing strongly in spaces with direct sunlight, although it can also grow in indirect or diffuse light. Additionally, it tolerates drought seasons and frost [11].
T. officinale is a perennial herbaceous plant, about 10–50 cm high. It has a tapering, brown, and poorly branched root, which releases a white and bitter latex (Figure 1A). The plant has a radical rosette of simple, lobed leaves with irregular teeth, arranged in a rosette, light to dark green in color and bitter in flavor, about 40 cm tall, and 0.7 to 15 cm wide (Figure 1B). The basal rosette produces hollow, cylindrical stems, topped with large, solitary yellow flowers (on average, 5 to 10 flowers) that close at dusk and when rain is approaching (Figure 1C). The fruits/seeds are tapered achenes of grayish-brown color and a white umbrella-shaped papilla (Figure 1D) [12].
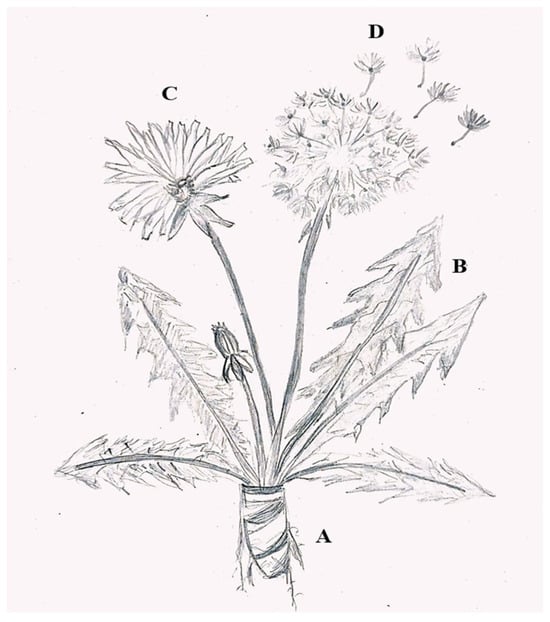
Figure 1.
The morphology of Taraxacum officinale. (A) Roots; (B) leaves; (C) flower; (D) seed with fruit. (achene).
2.2. History and Traditional Uses of T. officinale
T. officinale has been used since ancient times for the treatment of several health disorders. Theophrastus (371 BC-287 BC), a Greek naturalist, was one of the first to describe this plant. He recommended it to be used as a stimulant, especially against freckles and other liver-related skin conditions [13]. In the past, it was often consumed fermented into wine. Celts and Anglo-Saxon tribes used it to prevent scurvy, as a diuretic, and a laxative. Traditional Chinese medicine applied T. officinale roots for the treatment of swelling. Also, T. officinale juice was recommended for the protection of the liver and against dropsy (fluid retention in one or more areas of the body), as well as an antidote against scorpion stings [14]. In the 10th and 11th centuries, Arab physicians used it to treat ailments of the liver and spleen [15]. In 1543, Leonhard Fuchs, a botanist, defined its medicinal use in gout, diarrhea, blisters, liver conditions, among others. In native North American medicine, they used T. officinale root-based concoctions and cooking to treat kidney disease, stomachache pains, and heartburn [16]. It has also been used in folk medicine as a “blood purifier and laxative”, for skin conditions such as eczema, and for the treatment of arthritic and rheumatic conditions [7]. In Mexico, a whole herb decoction is traditionally used to control diabetes mellitus. In the case of traditional Chinese medicine, its main use is in combination with other herbs to treat hepatitis, improve the immune response to infections of the upper respiratory tract, such as bronchitis or pneumonia, and at a folk level, it is also used in compresses due to its anti-mastopathic activity. All of the above indicate that Chinese culture considers this a non-toxic plant [17]. In the 19th century, studies began to find scientific explanations related to the mechanism of action of T. officinale relative to its multiple beneficial actions: antioxidant, cholagogue, anti-inflammatory, analgesic, anticoagulant, choleretic, angiogenic, and anticancer, among others [18]. Currently, all its parts (roots, foliage, and flowers) are commercially available in different pharmacological and supplemental preparations that are suggested to treat, for example, some liver, gallbladder, and kidney disorders. Since the 1950s, biochemical composition and bioactive compounds identification of T. officinale have been described. A common use is as an antioxidant, due to its free radical scavenging activity, as well as the anticancer effect of the root [19]. This might lead to T. officinale and its bioactive derivatives being established as interesting therapeutic tools.
Therefore, the aim of this narrative review is to critically synthesize the current preclinical evidence regarding the hepatoprotective effects of T. officinale, focusing on its bioactive compounds, mechanisms of action, and potential applications in liver disease. Unlike previous reviews that broadly cover the general medicinal uses of dandelion, this work specifically highlights its role in liver protection and metabolic liver disorders, emphasizing recent findings published in the last two decades.
2.3. Chemical Composition of T. officinale
The characterization of T. officinale composition has been a subject of interest, mainly to find bioactive components associated with its pharmacological properties [20]. T. officinale is rich in vitamins, inulin, and is a rich source of phytochemicals, including terpenes, phenolic acids, and flavonoids, alongside being a good source of amino acids and minerals, particularly potassium [14]. In addition, its bitter substances are known to stimulate gut function, while phenolic derivatives have anti-inflammatory and antioxidant properties, making the nutritional composition of T. officinale of high interest when used as food and fodder [21,22]. Research on T. officinale bioactive compounds, particularly phenols, terpenes, and flavonoids, accounts for almost half of all research supporting the importance of this plant in providing health and medical benefits, reducing the high cost, and rethinking the technological/chemical approaches to disease treatments currently used in medical care [18]. For this reason, it is necessary to know the organic compounds and some examples of their respective biological activity, which are presented in Table 1.

Table 1.
Main organic compounds identified in T. officinale, their anatomical origin, and associated biological activities reported in preclinical models.
2.4. In Vitro and In Vivo Evidence of T. officinale Biological Activities
To date, most research on T. officinale has reported the biological activity of plant extracts. A literature search on the use of T. officinale in various models of diseases and disorders is summarized in Table 2, which highlights the versatility of the different plant extracts and fractions. These fractions confer metabolic benefits (diabetes, obesity, dyslipidemia), protect against bone pathologies and bacterial infections associated with counteracting oxidative processes, and inhibit tumor growth in experimental models. Notably, consistent antioxidant and anti-inflammatory properties emerge from most of these studies, suggesting that many of the reported effects are mediated through modulation of oxidative stress and inflammatory pathways. Table 3 summarizes the evidence attributing hepatoprotective properties to T. officinale in various pathological contexts. Over the past two decades, this species has gained prominence in biomedical research for its antioxidant, anti-inflammatory, and lipid metabolism-regulating properties. In experimental hepatology, it is currently being assessed both as a prophylactic agent promoting liver protection and regeneration and as a therapeutic agent, intervening in established lesions and even in neoplastic processes.

Table 2.
Biological effects of T. officinale in various disease models.

Table 3.
Hepatoprotective activity of T. officinale in liver disease.
It is important to note that outcomes vary depending on the part of the plant used, extraction method, and experimental model; consequently, clinical extrapolation must be approached with caution. Nevertheless, the body of work compiled in these tables supports T. officinale as an important source of bioactive compounds with broad therapeutic applications and underscores the need for further preclinical and clinical studies, aimed at standardizing extracts, elucidating molecular mechanisms, and defining safe and effective dosing regimens.
2.5. T. officinale and Liver Pathologies
Chronic liver disorders (CLDs) are among the main health complications worldwide, becoming an important public health problem due to the high morbidity rate [80]. CLDs are related to malnourishment, metabolic syndrome, viral infections, alcohol, and drug abuse, among others [81]. Pharmacological treatments for the disorders mentioned above are often difficult to achieve and may have limited efficacy [82]. Consequently, complementary and alternative medicines for the treatment of liver disorders have become of special interest in recent years, as potential natural agents to diminish or avoid the risk of harm when these compounds are used clinically [83].
As mentioned above, T. officinale has been used for many years as the main component of various herbal preparations for hepatoprotection. The monographs of the European Scientific Cooperative on Phytotherapy (ESCOP) certify the action of the root as a restorer of liver and biliary function, and its indication for dyspepsia and loss of appetite was scientifically proven (Taraxaci Radix (Dandelion Root)) [84]. The Expert Commission of the German Ministry of Health (German Commission E) approved the traditional use of leaves and roots with aerial parts to activate urinary elimination, as an adjuvant in mild urinary complaints, relief of digestive disorders, as well as for loss of appetite [9]. The European Medicines Agency (EMA) supports the traditional use of T. officinale, particularly leaves and root extracts, to improve the elimination of body fluids. The EMA also approved the traditional consumption of aerial parts for the relief of mild gastrointestinal disorders, such as satiety, flatulence, and slow digestion. It is also indicated as a stimulant of liver function [59]. Scarce data are available on preclinical and clinical studies with this plant extract. As the concept of reverse pharmacology is rapidly growing from the discovery of ‘leads’ from plants, it is worthwhile conducting scientific studies on T. officinale to support its traditional use.
2.6. In Vivo Liver Studies of T. officinale Effects on Acute and Chronic Liver Disease
The hepatoprotective role of T. officinale has been investigated, particularly as an alternative for the treatment of hepatic diseases, gaining special momentum in the last decade. In the context of acute liver disease, Colle et al. assessed the protective effect of T. officinale leaf extracts (0.1 and 0.5 mg/mL) on acetaminophen (APAP)-induced hepatotoxicity [40]. These authors showed that the foliar extract reduced the levels of reactive oxygen species (ROS), decreased serum transaminases, and caused hepatic histopathological alterations. These beneficial effects were attributable to the high content of phenolic compounds. Domitrović et al. [85] showed that a T. officinale extract was able to modulate the regenerative hepatic capabilities through inactivation of stellate cells, decreasing liver fibrosis. Interestingly, these authors found that the extract restored the liver antioxidant capacity by normalizing the levels of Cu/Zn SOD. A similar antioxidant effect was observed in a model of sodium dichromate-induced hepatotoxicity and genotoxicity, whereas T. officinale reduced the DNA fragmentation in hepatocytes and re-established the antioxidant enzymes [64].
Regarding liver fibrosis, several studies evaluated the hepatoprotective effect of T. officinale using the carbon tetrachloride (CCL4)-induced liver injury model. As mentioned previously, Domitrovic’s group [85] and Huseini et al. found that hepatic microvesicular steatosis and liver damage were ameliorated at T. officinale doses higher than the average for humans (over 750 mg/kg/day) [86]. These results were corroborated by Al-Malki et al. [87] and Favari et al. [69] using aqueous leaf extracts and the aerial part of the plant, respectively. Also, ethanol and n-hexane extracts of T. officinale leaves were evaluated, showing that not only the aqueous extract could be hepatoprotective [88]. This work showed that a methanol extract significantly restored the levels of critical liver enzymes. In addition, bilirubin, lipid profile, and antioxidant enzymes were improved, thus protecting against oxidative stress. More recently, Hamza et al. [89] found that T. officinale root preparation (500 mg/kg, oral administration) presented anti-inflammatory activity through the inactivation of NF-κB pathways, negatively modulating the levels of IL-1β, TNF-α, transforming growth factor-β1 (TGF-β1), and COX-2.
One relevant consideration in CLDs is cirrhosis decompensation, generating acute-on-chronic liver failure (ACLF), which is associated with more than a 20% mortality rate one month after occurrence [90]. Similar results are observed in animal models [24].
2.7. Impact on Metabolic-Related Liver Diseases
Among liver diseases, metabolic dysfunction-associated fatty liver disease (MAFLD) has gained primary importance in recent years worldwide [91]. MAFLD is often linked to overweight/obesity and associated comorbidities such as insulin resistance and diabetes [92]. It can evolve from simple steatosis to steatohepatitis, or to major complications such as fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [93]. At present, there are no pharmacological treatments approved for the prevention or management of MAFLD [94], although there are several clinical trials ongoing [95]. Given the complexity of the underlying mechanisms, monotherapies are unlikely to provide effective solutions [96], and there is an evident need for new therapeutic alternatives [94]. Several natural compounds have been shown to exert beneficial effects against metabolic liver disease [83], and T. officinale may become one of them [97].
A leaf extract from T. officinale was evaluated in a liver steatosis model [67]. This report indicated that increased insulin and fasting glucose levels were normalized, followed by a dramatic decrease in hepatic lipid accumulation, as well as body and liver weight, with a suppression of triglycerides (TG) and total cholesterol (TC), among others. The hepatoprotective results observed for T. officinale provide evidence for the use of leaf extract as a therapy in the treatment of fatty liver and obesity-related disorders.
Since diabetes is a metabolic disease associated with liver function [95], it is interesting to evaluate the action of T. officinale in this pathology. T. officinale leaf extract activated the antioxidant response and decreased ROS, lipid peroxidation, and nitrite levels in streptozotocin (STZ)-induced diabetic rats. The authors concluded that a T. officinale leaf extract could improve the antioxidant status of the liver, suggesting its usefulness in improving diabetes-induced liver injury [68]. Another T. officinale root aqueous extract (400 mg/kg) was reported to improve glycemic control in diabetic mice [27]. The polysaccharides present in the aqueous extract might have been responsible for these effects due to their ability to inhibit α-glucosidase and α-amylase, which would indicate that this herb can be developed as functional foods or drugs (bioactive compounds) for the prevention and treatment of diabetes due to their effective hypoglycemic effects, promising natural alternatives for the treatment of diabetes [33,98].
2.8. T. officinale Bioactive Compounds on Liver Pathologies
T. officinale is rich in saccharides, peptides, flavonoids, terpenes, and several other phytochemicals [97]. Its polysaccharides are important functional substances [99,100], with immune regulatory and antioxidant activities. Two of these polysaccharides (TOP1 and 2) obtained from T. officinale exhibited hepatoprotective effects in a model of liver fibrosis. Specifically, these compounds decreased AST (aspartate aminotransferase) and ALT (alanine aminotransferase), reversed reduced glutathione (GSH) depletion and nuclear factor kappa B (NF-κB) activity, and decreased expression of its regulatory inflammatory mediators iNOS, COX-2, TNF-α, IL-1β. These data suggest that TOPs may be a potential preventive or therapeutic compound against CCL4-induced liver damage [77].
Another relevant active component isolated from T. officinale is taraxasterol (TA), a pentacyclic-triterpenoid [101]. TA exhibits pharmacological properties: antioxidant, anti-inflammatory, and anti-tumor effects, contributing to its protective potential against a diversity of maladies. In neurons, it has been described that the antioxidant effect of TA is achieved through the nuclear factor erythroid-related factor 2 (Nrf2) signaling pathway [101]. The protective role of TA on acute liver disease has also been studied in APAP models, finding that TA reduces liver damage associated with a decrease in ALT, AST, LDH, and ROS, normalizing the antioxidant response (catalase (CAT) and GSH). These hepatoprotective effects were mediated by activation of the Nrf2/HO-1 pathway [61]. Comparable results were also observed when acute liver injury was generated by concavalin A (Con A). TA reduced the hepatic damage generated by Con A through anti-inflammatory pathways, in particular a reduction in the activity of toll-like receptors 2 and 4 (TLR2 and TLR4) and NF-κB. Interestingly, TA prevented apoptosis by decreasing the pro-apoptotic protein Bcl-2-associated X protein (Bax) and increasing the anti-apoptotic protein leukemia protein 2 (Bcl-2), which reduced apoptosis in liver cells, demonstrating to be a potent hepatoprotective molecule [102]. TA also exerts a hepatoprotective role on ethanol-induced liver disease, mainly mediated by upregulating antioxidant defense mediated by i) antioxidant improvement due to activation of Nrf2: ROS reduction with decreasing of nitric oxide (NO), and increasing CAT, superoxide dismutase (SOD) and GSH activity; ii) anti-inflammatory actions: inhibition of the degradation of α inhibitor kappa B (Iκ-Bα), and the expression level of NF-κB, along with the reduction in the inflammatory pathway TLR4/MyD88/NF-κB, both of them associated with a depletion of pro-inflammatory TNF-α, IL-6, and IL-1β cytokines [31,103]. These findings indicate that TA may offer protective effects against ethanol-induced liver injury in mice by regulating inflammation and oxidative stress. To clarify the mechanism of action of TA, He et al. performed an RNA sequencing analysis of liver tissues subjected to fibrosis and treated with TA. Their analysis revealed that TA treatment was associated with the expression of 2675 genes related to the extracellular matrix (ECM). Specifically, TA blocked the gene expression of hypoxia-inducible factor 1 subunit alpha (HIF-1α), Smad-dependent transforming growth factor-beta (TGF)-signaling pathway (TGF-β/SMAD), and Wnt-signaling pathways, thus inhibiting the activation pathways of Ito cells, decreasing excessive ECM production [104].
Finally, the impact of TA on liver damage associated with mycotoxin exposition (aflatoxin B1) has also been investigated. It has been reported anti-inflammatory and antioxidant effects of TA are associated with inhibition of hepatocyte apoptosis by regulation of caspase-3, Bax, and Bcl-2 [63]. These authors found that TA enhanced hepatocyte autophagy by modulating the PI3K/AKT/mTOR pathway. This suggests that the mechanism of action of TA is complex and related to the activation of survival pathways. Therefore, TA could be one of the most relevant hepatoprotective agents present in T. officinale, but future studies regarding their safety and use as adjuvant therapies are necessary.
2.9. T. officinale in Liver Cancer
One of the most severe complications of liver fibrosis is hepatic cancer. Over the past decade, the incidence of liver cancer has increased by 25%, with HCC being the most prevalent form [105]. Therapeutic strategies for the treatment of HCC have experienced important advances, including surgical resection, liver transplantation, and other surgical interventions [106]. On the other hand, drug therapies against HCC, including immunotherapies, have a high rate of recurrence and resistance [107]. Alternative therapies, including herb extracts, could be of interest in this case. For this purpose, investigations have tried to elucidate the effect of TA on liver cancer. Bao et al. [62] developed an in vivo model of HCC, generated by subcutaneous injections of SK-Hep1 (liver adenocarcinoma) cells in mice and then treated orally with TA. The TA treatment inhibited tumor growth by up-regulating Histidine Triad Nucleotide-Binding Protein 1 (Hint1) and Bax expression, decreasing CyclinD1 and Bcl-2 protein expression in tumors. In the same study, TA induced cell cycle arrest in the G0/G1 phase in Hep2 cells and promoted apoptosis. Another interesting study was developed by Ren et al. [78], where they established a hepatocarcinoma model in tumor-bearing mice (mouse HCC cell line H22). In this model, the TA treatment inhibited tumor cell proliferation by depletion of Ki-67, and enhanced antitumor immunity, associated with a significant increase in CD4+ T cells in the spleen, but without effect on CD8+ T cells. As has been extensively described, hepatocarcinoma is considered an immunogenic tumor; therefore, the anti-inflammatory and antioxidant actions of T. officinale could be tested in the future to be used as an alternative therapeutic strategy.
Despite promising results in vitro and in preclinical studies, these hepatoprotective properties of T. officinale require further validation in clinical studies in humans. Both cellular and animal models help us to understand the mechanisms of action of bioactive compounds. For example, the anti-inflammatory and antioxidant properties of TA or polysaccharides are present in the plant. However, there are major differences between animal and human metabolism, which poses a challenge to clinical studies. Nowadays, there are no established clinical protocols that support the consumption of T. officinale as a management for liver diseases in humans, highlighting the need for rigorous clinical trials that assess safety, efficacy, and optimal dosage in human populations. This will help us to determine whether T. officinale can be a possible complementary or adjuvant treatment for conventional therapies, especially in chronic or acute liver pathologies where current treatments are limited.
2.10. Safety Considerations, Contraindications, and Interactions of Taraxacum officinale
Although T. officinale possesses multiple therapeutic properties, its use is not without risks. Cases of hypersensitivity have been reported in individuals allergic to plants of the Asteraceae family, which may trigger mild to moderate cutaneous or respiratory allergic reactions [108].
Due to its well-known diuretic activity, extracts of T. officinale may interact with antihypertensive drugs, other diuretics, and lithium salts, potentially increasing the risk of dehydration or electrolyte imbalances [109].
Furthermore, some of its bioactive compounds exhibit antiplatelet activity; therefore, its use should be approached with caution in patients taking anticoagulant medications, as it could increase the risk of bleeding [75,110].
Its use is not recommended without medical supervision in individuals with severe hepatic or renal conditions, nor in pregnant or breastfeeding women, due to the lack of conclusive clinical studies supporting its safety in these populations [111,112].
Various in vitro studies have demonstrated that T. officinale extracts exhibit low toxicity in normal cells, supporting its traditional use and suggesting a favorable safety profile when used at conventional concentrations. According to Schütz et al., no significant adverse effects have been reported in experimental studies [7]. However, at high concentrations, some extracts have shown cytotoxic activity against tumor cell lines, which may indicate potential antitumor effects. These findings highlight both the therapeutic potential of T. officinale and the need for further research, particularly in animal models and clinical trials, to confirm its safety and efficacy [7].
2.11. Limitations and Future Perspectives
Although the beneficial effects of T. officinale extracts and isolated compounds have been widely documented over the past two decades, no approved drug or therapy based on this knowledge has yet been developed. This absence may be explained by several factors, including the high variability in the chemical composition of plant extracts, which is influenced by species differences, extraction methods, and cultivation conditions. Moreover, the lack of robust and large-scale clinical trials limits the validation of their efficacy and safety in humans [7]. Other significant obstacles include the insufficient standardization of formulations, the complexity of regulatory frameworks for herbal medicines, and the need for comprehensive toxicity and pharmacokinetic studies [113]. Therefore, advancing toward the development of T. officinale-based therapies requires the implementation of rigorous protocols to ensure product quality, safety, and efficacy.
Future perspectives should focus on the design of well-controlled clinical trials to validate the therapeutic effects observed in preclinical models. Additionally, efforts should be directed toward the isolation and characterization of specific bioactive compounds with pharmacological potential. Furthermore, it is essential to deepen our understanding of the molecular mechanisms of action and optimize formulations to enhance their bioavailability and therapeutic effectiveness.
In conclusion, integrating these strategies may facilitate the translation of scientific knowledge into concrete clinical applications, offering new therapeutic alternatives based on T. officinale.
3. Literature Search Strategy and Selection Criteria
This narrative review was conducted to compile and critically analyze the scientific evidence regarding the biological and hepatoprotective properties of Taraxacum officinale. A literature search was carried out using three major scientific databases: PubMed, Scopus, and Web of Science, covering the period from 1973 to April 2024.
The search strategy included combinations of the following keywords and Boolean operators:
“Taraxacum officinale” OR “dandelion” AND “liver” OR “hepatoprotection” OR “hepatotoxicity” OR “liver fibrosis” OR “steatosis” OR “antioxidant” OR “anti-inflammatory”. Inclusion criteria: peer-reviewed original articles, reviews, and ethnopharmacological reports; studies conducted in vitro, in vivo, and clinical trials; articles written in English or Spanish; studies focused on the effects of T. officinale or its isolated bioactive compounds on liver-related diseases or hepatocellular protection. Exclusion criteria: studies with insufficient methodological detail; articles not related to liver or hepatic function; non-scientific reports, news articles, or commercial reviews.
From the bibliographical review, approximately 200 articles were initially identified across selected databases. After applying inclusion and exclusion criteria and evaluating the relevance and quality of each study, around 100 articles were selected and included in this review. Data were extracted manually and organized into thematic sections addressing the plant’s phytochemistry, pharmacological mechanisms, and evidence for hepatoprotective activity.
4. Conclusions
T. officinale has several bioactive compounds that have caused considerable interest in the scientific community. Compounds that, when studied in vitro or preclinical studies, have been revealed to have anti-inflammatory, antioxidant, diuretic, and hepatoprotective properties. As a result, it has started to be evaluated as a treatment for diseases, mainly obesity, diabetes, osteoporosis, and cancer. In recent years, since the diagnosis of liver disease has increased dramatically, especially fatty liver and metabolic diseases, the research focused on the potential use of T. officinale in liver diseases has increased considerably. Most of this research has been developed in animal models or cell lines. In this way, scientists have been able to identify a variety of mechanisms of action, mainly antioxidant and anti-inflammatory responses. However, further research is still required to fully understand how T. officinale or its components interact molecularly with hepatic metabolic pathways and how these can influence metabolic homeostasis. To date, research has identified T. officinale as a potential therapeutic; however, detailed research is needed to establish its efficacy and safety in clinical trials. Optimal doses could be determined and interactions evaluated with other medications commonly used in patients with liver disease to improve their therapeutic effect.
Author Contributions
All authors contributed significantly to the work as follows: conceptualization, F.H.V. and J.Z.-H.; design, F.H.V., M.Q.S.M. and J.Z.-H.; data acquisition, F.H.V., M.Q.S.M., N.M.-C., F.B.-N. and J.Z.-H.; data interpretation and analysis, F.H.V., M.Q.S.M., N.M.-C., D.R.G. and J.Z.-H.; manuscript draft, F.H.V., M.Q.S.M., N.M.-C., D.R.G. and J.Z.-H.; critical revision of the manuscript, F.H.V., D.R.G. and J.Z.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by the National Agency for Research and Development (ANID). National Doctorate Grant N° 21212100 and N° 21220031, and the project INGE210025, University of Talca.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this review. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no relevant interests to disclose.
Abbreviations
| TA | Taraxasterol |
| T. officinale | Taraxacum officinale |
| WHO | World Health Organization |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NF-κB/NF-kB | Nuclear factor kappa B/Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PTBP1 | Polypyrimidine tract-binding protein 1 |
| SIRT1 | Sirtuin-1 NAD-dependent deacetylase |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor alpha |
| TLR2 | Toll-like receptor 2 |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| ICR Mice | Outbred mice derived from Swiss albino mice |
| INS-1 Cells | Rat insulinoma-1 cells |
| IR-HepG2 Cells | Human insulin-resistant HepG2 cells |
| V79-4 Cell Line | Subline of Chinese hamster lung fibroblast V79 cells |
| MAC-T | Primary bovine mammary alveolar cells |
| HepG2 Cell Line | Liver biopsy from a 15-year-old Caucasian male |
| HeLa Cell Line | Cervical cancer cells from a female human |
| Raw264 Cell Line | Tumor in a male mouse induced with Abelson murine leukemia virus (A-MuLV) |
| HepG2/2E1 Cells | Human liver cancer cells expressing cytochrome P450 2E1 (CYP2E1) |
| HepG 2.2.15 Cells | Human hepatoblastoma-derived cells |
| Huh7 Cell Line | Hepatoma-derived cells from a human male |
| HCC H22 Cells | Mouse hepatocellular carcinoma cells |
| Hep3B Cell Line | Liver cancer cells from an 8-year-old Black male |
| CLD | Chronic liver disorders |
| ESCOP | European Scientific Cooperative on Phytotherapy |
| German Commission E | Expert Commission of the German Ministry of Health |
| EMA | European Medicines Agency |
| APAP | Acetaminophen |
| CCL4 | Carbon tetrachloride |
| ACLF | Acute-on-chronic liver failure |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| HCC | Hepatocellular carcinoma |
| TG | Triglycerides |
| TC | Total cholesterol |
| STZ | Streptozotocin |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| GSH | Reduced glutathione |
| CAT | Catalase |
| Con A | Concanavalin A |
| TLR4 | Toll-like receptor 4 |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| NO | Nitric oxide |
| SOD | Superoxide dismutase |
| IκBα | Inhibitor alpha of kappa B |
| ECM | Extracellular matrix |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| TGF-β/SMAD | Transforming growth factor-beta signaling pathway |
| Hint1 | Histidine triad nucleotide-binding protein 1 |
| Mouse HCC H22 | Mouse hepatocellular carcinoma cell line H22 |
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aremu, O.O.; Oyedeji, A.O.; Oyedeji, O.O.; Nkeh-Chungag, B.N.; Sewani Rusike, C.R. In Vitro and In Vivo Antioxidant Properties of Taraxacum officinale in Nω-Nitro-l-Arginine Methyl Ester (L-NAME)-Induced Hypertensive Rats. Antioxidants 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- GBIF Secretariat. GBIF Backbone Taxonomy 2022. Available online: https://doi.org/10.15468/39omei (accessed on 3 October 2023).
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Jinchun, Z.; Jie, C. The effects of Taraxacum officinale extracts (TOE) supplementation on physical fatigue in mice. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 128–133. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Stearn, W.T. Botanical Latin: History, Grammar Syntax, Terminology and Vocabulary, 3rd ed.; David & Charles: Trowbridge, UK, 1973; 472p. [Google Scholar]
- Siedentopp, U. Nutrición: El diente de león. Rev. Int. Acupunt. 2007, 1, 44–46. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols Content, Antioxidant Activity, and Cytotoxicity Assessment of Taraxacum officinale Extracts Prepared through the Micelle-Mediated Extraction Method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef]
- Rasool, S.; Sharma, B. Taraxacum officinale: A high value less known medicinal plant. Ann. Plant Sci. 2014, 3, 908–915. [Google Scholar]
- Stewart-Wade, S.M.; Neumann, S.; Collins, L.L.; Boland, G.J. The biology of Canadian weeds. 117. Taraxacum officinale G. H. Weber ex Wiggers. Can. J. Plant Sci. 2002, 82, 825–853. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Awortwe, C.; Sackeyfio, A.C.; Osei-Safo, D.; Bugyei, K.A.; Asiedu-Gyekye, I.J. Dual effect of Taraxacum officinale leaves: Anticholinergic and inhibitory effect on inflammatory cells in ovalbumin-sensitized guinea-pigs. Afr. J. Pharm. Pharmacol. 2011, 5, 2613–2619. [Google Scholar] [CrossRef]
- Sweeney, B.; Vora, M.; Ulbricht, C.; Basch, E. Evidence-Based Systematic Review of Dandelion (Taraxacum officinale) by Natural Standard Research Collaboration. J. Herb. Pharmacother. 2009, 5, 79–93. [Google Scholar] [CrossRef]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological Effects of Dandelion (Taraxacum officinale) in Type 2 Diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, J.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Escudero, N.L.; De Arellano, M.L.; Fernández, S.; Albarracín, G.; Mucciarelli, S. Taraxacum officinale as a food source. Plant Foods Hum. Nutr. 2003, 58, 1–10. [Google Scholar] [CrossRef]
- Vondrášková, B.; Čermák, B.; Martínková, L.; Brouček, J. Examination of the nutritional quality of forbs from mountainous pastures in the southwestern Bohemia region. Ekol. Bratisl. 2012, 31, 231–237. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Concepts in functional foods: The case of inulin and oligofructose. J. Nutr. 1999, 129, 1398S–1401S. [Google Scholar] [CrossRef] [PubMed]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and Identification of Compounds from Bioactive Extracts of Taraxacum officinale Weber ex F. H. Wigg. (Dandelion) as a Potential Source of Antibacterial Agents. Evid.-Based Complement. Altern. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Juee, L.Y.M.; Naqishbandi, A.M. In vivo and in vitro antidiabetic potential of Taraxacum officinale root extracts. Curr. Issues Pharm. Med. Sci. 2020, 33, 168–175. [Google Scholar] [CrossRef]
- Jeon, D.; Kim, S.J.; Kim, H.S. Anti-inflammatory evaluation of the methanolic extract of Taraxacum officinale in LPS-stimulated human umbilical vein endothelial cells. BMC Complement. Altern. Med. 2017, 17, 508. [Google Scholar] [CrossRef]
- Jędrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef]
- Petlevski, R.; Hadžija, M.; Slijepčević, M.; Juretić, D.; Petrik, J. Glutathione S-transferases and malondialdehyde in the liver of NOD mice on short-term treatment with plant mixture extract P-9801091. Phytother. Res. 2003, 17, 311–314. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.; Sang, R.; Li, J.; Ge, B.; Zhang, X. Protective Effects of Taraxasterol against Ethanol-Induced Liver Injury by Regulating CYP2E1/Nrf2/HO-1 and NF-κB Signaling Pathways in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 8284107. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, Q.M.; Khaliq, T. Effects of Euphorbia prostrata and Fumaria parviflora in normoglycaemic and alloxan-treated hyperglycaemic rabbits. Planta Med. 1984, 50, 138–142. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.Y.; Park, E.M.; Choi, M.S.; Lee, M.K.; Jeon, S.M.; Jang, M.K.; Kim, M.J.; Park, Y.B. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin. Chim. Acta 2002, 317, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Waheed, A.; Qureshi, R.A.; Burdi, D.K.; Verspohl, E.J.; Khan, N.; Hasan, M. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother. Res. 2004, 18, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Z.; Ma, L.; Wang, X.; Li, Z.; Li, J. Taraxacum officinale Extracts Alleviate Anhedonia and Depression by Inhibiting UPRmt and Mitophagy in the Hippocampi of T2DM Rats. Pharmacogn. Mag. 2024, 20, 794–801. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Yim, J.H.; Cho, C.W.; Rhee, Y.K.; Lim, S.I.; Kim, Y.C. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef]
- Alfaifi, A.M.; Tashkandi, M.A.; Yousef, J.M. Roles for Taraxacum officinale and Vitamins (C, K) on Bone Formation and Resorption Heparin-Induced in Rats. Arch. Pharm. Pract. 2023, 14, 82–91. [Google Scholar] [CrossRef]
- Heo, J.; Kim, M.; Kim, J.H.; Shin, H.; Lim, S.E.; Jung, H.S.; Sohn, Y.; Ku, J. Effect of Taraxaci Herba on Bone Loss in an OVX-Induced Model through the Regulation of Osteoclast Differentiation. Nutrients 2022, 14, 4354. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Gubert, P.; da Luz, S.C.A.; Athayde, M.L.; Teixeira Rocha, J.B.; Soares, F.A.A. Antioxidant properties of Taraxacum officinale leaf extract are involved in the protective effect against hepatoxicity induced by acetaminophen in mice. J. Med. Food 2012, 15, 549–556. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef]
- Salem, M.O.A.; Salem, T.A.; Yürüten Özdemir, K.; Sönmez, A.Y.; Bilen, S.; Güney, K. Antioxidant enzyme activities and immune responses in rainbow trout (Onchorhynchus mykiss) juveniles fed diets supplemented with dandelion (Taraxacum officinalis) and lichen (Usnea barbata) extracts. Fish Physiol. Biochem. 2021, 47, 1053–1062. [Google Scholar] [CrossRef]
- Yoo, K.; Hwang, I.K.; Moon, B. Comparative flavonoids contents of selected herbs and associations of their radical scavenging activity with antiproliferative actions in V79-4 cells. J. Food Sci. 2009, 74, C419–C425. [Google Scholar] [CrossRef] [PubMed]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Genovese, S.; Epifano, F.; Tirillini, B.; Ferrante, C.; Leporini, L. Antiproliferative, protective and antioxidant effects of artichoke, dandelion, turmeric and rosemary extracts and their formulation. Int. J. Immunopathol. Pharmacol. 2010, 23, 601–610. [Google Scholar] [CrossRef] [PubMed]
- KaKamal, F.Z.; Lefter, R.; Mihai, C.T.; Farah, H.; Ciobica, A.; Ali, A.; Radu, L.; Mavroudis, L.; Ech-Chahad, A. Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil. Molecules 2022, 27, 6477. [Google Scholar] [CrossRef]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef]
- Yoon, H.S.; Park, C.M. Alleviated Oxidative Damage by Taraxacum officinale through the Induction of Nrf2-MAPK/PI3K Mediated HO-1 Activation in Murine Macrophages RAW 264.7 Cell Line. Biomolecules 2019, 9, 288. [Google Scholar] [CrossRef]
- Koh, Y.J.; Cha, D.S.; Ko, J.S.; Park, H.J.; Choi, H.D. Anti-inflammatory effect of Taraxacum officinale leaves on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Med. Food 2010, 13, 870–878. [Google Scholar] [CrossRef]
- Dong, L.; Dongzhi, Z.; Jin, Y.; Kim, Y.C.; Lee, D.S.; Huang, S.; Panichayupakaranant, P.; Li, B. Taraxacum officinale Wigg. Attenuates Inflammatory Responses in Murine Microglia through the Nrf2/HO-1 and NF-κB Signaling Pathways. Am. J. Chin. Med. 2020, 48, 445–462. [Google Scholar] [CrossRef]
- Hu, G.; Wang, J.; Hong, D.; Zhang, T.; Duan, H.; Mu, X.; Yang, Z. Effects of aqueous extracts of Taraxacum Officinale on expression of tumor necrosis factor-alpha and intracellular adhesion molecule 1 in LPS-stimulated RMMVECs. BMC Complement. Altern. Med. 2017, 17, 38. [Google Scholar] [CrossRef]
- Chen, W.; Fan, H.; Liang, R.; Zhang, R.; Zhang, J.; Zhu, J. Taraxacum officinale extract ameliorates dextran sodium sulphate-induced colitis by regulating fatty acid degradation and microbial dysbiosis. J. Cell. Mol. Med. 2019, 23, 8161–8172. [Google Scholar] [CrossRef]
- Abdel-Magied, N.; Abdel Fattah, S.M.; Elkady, A.A. Differential effect of Taraxacum officinale L. (dandelion) root extract on hepatic and testicular tissues of rats exposed to ionizing radiation. Mol. Biol. Rep. 2019, 46, 4893–4907. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, E. Hepatoprotective properties of Dandelion: Recent update. J. Appl. Pharm. Sci. 2016, 6, 202–205. [Google Scholar] [CrossRef]
- Racz Kotilla, E.; Racz, G.; Solomon, A. The action of Taraxacum officinale extracts on the body weight and diuresis of laboratory animals. Planta Med. 1974, 26, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lal, B. Ethnobotanical notes on some medicinal and aromatic plants of Himachal Pradesh. Indian J. Tradit. Knowl. 2005, 4, 424–428. [Google Scholar]
- Brondz, I. Analytical Methods in the Quality Control of Scientific Publications Part III: Publishers’ Ethics and Editors’ Complicity. Int. J. Anal. Mass Spectrom. Cromatogr. 2014, 2, 77–102. [Google Scholar] [CrossRef]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef]
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.-T.; Lee, J.; Cho, H.Y.; Jun, W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef]
- Yang, Y.; Ying, G.; Wu, S.; Wu, F.; Chen, Z. In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol. Infect. Agent. Cancer 2020, 15, 44. [Google Scholar] [CrossRef]
- Lin, W.; Gu, B.; Gu, Y.; Zhao, R.; Huang, Y.; Fan, R.; Rong, W.; Liu, Z. Taraxasterol protects against acetaminophen-induced hepatotoxicity by reducing liver inflammatory response and ameliorating oxidative stress in mice. Int. Immunopharmacol. 2024, 138, 112580. [Google Scholar] [CrossRef]
- Bao, T.; Ke, Y.; Wang, Y.; Wang, W.; Li, Y.; Wang, Y.; Kui, X.; Zhou, Q.; Zhou, H.; Zhang, C.; et al. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J. Mol. Med. 2018, 96, 661–672. [Google Scholar] [CrossRef]
- Sang, R.; Ge, B.; Li, H.; Zhou, H.; Yan, K.; Wang, W.; Cui, Q.; Zhang, X. Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy. Ecotoxicol. Environ. Saf. 2023, 251, 114546. [Google Scholar] [CrossRef] [PubMed]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ. Toxicol. 2016, 31, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Wang, J.; Li, J.; Ding, J.; He, X. Protective Effect of Dandelion Leaf Water Extracts on APAP-Induced Liver Injury in Rats and Its Mechanism. Cell Mol. Biol. 2022, 68, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.S.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Dandelion leaf extract protects against liver injury induced by methionine- and choline-deficient diet in mice. J. Med. Food 2013, 16, 26–33. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.-T.; Park, J.H.; Kim, H.-J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- Nzekwe, S.; Morakinyo, A.; Oguntibeju, O.; Ayeleso, A. Effect of Taraxacum officinale Leaf Extract on Liver Antioxidant Status in Streptozotocin-Induced Diabetic Male Wistar Rats. Afr. J. Biomed. Res. 2020, 23, 421–428. [Google Scholar]
- Favari, L.; Arce-Díaz, C.; Ortíz-Martínez, J.; Pablo-Pérez, S.; Soto, C.; Meléndez-Camargo, M.E. Efectos hepatoprotector y antioxidante de Taraxacum officinale en el daño hepático agudo inducido por el tetracloruro de carbono en la rata. Rev. Mex. Cienc. Farm. 2013, 44, 53–61. [Google Scholar]
- Esatbeyoglu, T.; Obermair, B.; Dorn, T.; Siems, K.; Rimbach, G.; Birringer, M. Sesquiterpene Lactone Composition and Cellular Nrf2 Induction of Taraxacum officinale Leaves and Roots and Taraxinic Acid β-d-Glucopyranosyl Ester. J. Med. Food 2017, 20, 71–78. [Google Scholar] [CrossRef]
- Yi, R.K.; Song JLe Lim, Y.I.; Kim, Y.K.; Park, K.Y. Preventive Effect of the Korean Traditional Health Drink (Taemyeongcheong) on Acetaminophen-Induced Hepatic Damage in ICR Mice. Prev. Nutr. Food Sci. 2015, 20, 52–59. [Google Scholar] [CrossRef]
- Zheng, Y.; Lei, L.; Liang, S.; Ai, J.; Deng, X.; Li, Y.; Zhang, T.P.; Pu, S.B.; Ren, Y.S. Protective Effect of Fresh/Dry Dandelion Extracts on APAP-Overdose-Induced Acute Liver Injury. Chin. J. Integr. Med. 2022, 28, 683–692. [Google Scholar] [CrossRef]
- Ren, Y.S.; Zheng, Y.; Duan, H.; Lei, L.; Deng, X.; Liu, X.Q.; Mei, Z.N.; Deng, X.K. Dandelion polyphenols protect against acetaminophen-induced hepatotoxicity in mice via activation of the Nrf-2/HO-1 pathway and inhibition of the JNK signaling pathway. Chin. J. Nat. Med. 2020, 18, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Macía, M.J.; García, E.; Vidaurre, P.J. An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol. 2005, 97, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a Source of Biologically Active Compounds Supporting the Therapy of Co-Existing Diseases in Metabolic Syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Park, C.M.; Youn, H.J.; Chang, H.K.; Song, Y.S. TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem. Toxicol. 2010, 48, 1255–1261. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.; Qin, Y.; Shang, J.; Wang, Y.; Wei, P.; Guo, J.; Jia, H.; Zhao, T. Taraxasterol prompted the anti-tumor effect in mice burden hepatocellular carcinoma by regulating T lymphocytes. Cell Death Discov. 2022, 8, 264. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Abusaliya, A.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Vetrivel, P.; Heo, J.D.; Kim, J.; Won, C.K.; et al. Apigetrin Promotes TNFα-Induced Apoptosis, Necroptosis, G2/M Phase Cell Cycle Arrest, and ROS Generation through Inhibition of NF-κB Pathway in Hep3B Liver Cancer Cells. Cells 2022, 11, 2734. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.W.; Kim, Y.S.; Kang, S.S.; Kim, J.A.; Lee, S.H.; Lee, S.M. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol. Pharm. Bull. 2007, 30, 1898–1904. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Wang, N.; Tsao, S.W.; Feng, Y. Current Status of Herbal Medicines in Chronic Liver Disease Therapy: The Biological Effects, Molecular Targets and Future Prospects. Int. J. Mol. Sci. 2015, 16, 28705–28745. [Google Scholar] [CrossRef] [PubMed]
- Taraxaci Radix (Dandelion Root)—ESCOP n.d. Available online: https://www.escop.com/downloads/taraxaci-radix-dandelion-root-escop-2024/ (accessed on 13 October 2024).
- Domitrović, R.; Jakovac, H.; Romić, Ž.; Rahelić, D.; Tadić, Ž. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J. Ethnopharmacol. 2010, 130, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Huseini, F.H.; Mahmoudabady, Z.A. The Effects of Taraxacum officinale L. and Berberis vulgaris L. Root Extracts on Carbon Tetrachloride Induced Liver Toxicity in Rats. J. Med. Plants 2010, 9, 45–52. [Google Scholar]
- Al-Malki, A.L.; Abo-Golayel, M.K.; Abo-Elnaga, G.; Al-Beshri, H. Hepatoprotective effect of dandelion (Taraxacum officinale) against induced chronic liver cirrhosis. J. Med. Plants Res. 2013, 7, 1494–1505. [Google Scholar] [CrossRef]
- Ahmad, D.; Gulfraz, M.; Ahmad, M.S.; Nazir, H.; Gul, H.; Asif, S. Protective action of Taraxacum officinale on CCl4 induced hepatotoxicity in rats. Afr. J. Pharm. Pharmacol. 2014, 8, 775–780. [Google Scholar] [CrossRef]
- Ahmed Hamza, A.; Gamel Mohamed, M.; Mohamed Lashin, F.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 81, 43. [Google Scholar] [CrossRef]
- Moreau, R.; Tonon, M.; Krag, A.; Angeli, P.; Berenguer, M.; Berzigotti, A.; Fernandez, J.; Francoz, C.; Gustot, T.; Jalan, R.; et al. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J. Hepatol. 2023, 79, 461–491. [Google Scholar] [CrossRef]
- Ohno, R.; Kaneko, H.; Suzuki, Y.; Okada, A.; Matsuoka, S.; Ueno, K.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; et al. Association of Metabolic Dysfunction-Associated Fatty Liver Disease With Risk of HF and AF. JACC Asia 2023, 3, 908–921. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, F.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A multisystem disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 1–23. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Bhopale, K.K.; Srinivasan, M.P. Therapeutics for Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Livers 2023, 3, 597–617. [Google Scholar] [CrossRef]
- Sangro, P.; de la Torre Aláez, M.; Sangro, B.; D’Avola, D. Metabolic dysfunction–associated fatty liver disease (MAFLD): An update of the recent advances in pharmacological treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Valenzuela, R.; Zúñiga-Hernández, J.; Del Campo, A. Relevant Aspects of Combined Protocols for Prevention of N(M)AFLD and Other Non-Communicable Diseases. Mol. Nutr. Food Res. 2024, 68, 2400062. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, M.V.; Negreanu-Pirjol, T.; Olariu, L.; Negreanu-Pirjol, B.S.; Lepadatu, A.C.; Anghel, L.; Rosoiu, N. Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review. Int. J. Mol. Sci. 2025, 26, 450. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Chai, Y.; Guo, Y.; Tianzhi, Y.; Bao, Y. Hypoglycemic effect of Taraxacum officinale root extract and its synergism with Radix Astragali extract. Food Sci. Nutr. 2021, 9, 2075–2085. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Chen, S.; Chen, S.; Huang, Z.; Zhou, C.; Zou, C.; Liu, Q.; Ye, H.; Lin, H.; et al. Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2017, 66, 198–206. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- He, Y.; Jiang, K.; Zhao, X. Taraxasterol protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activation of Nrf2 signalling pathway. Artif. Cells Nanomed. Biotechnol. 2020, 48, 252–258. [Google Scholar] [CrossRef]
- Sang, R.; Yu, Y.; Ge, B.; Xu, L.; Wang, Z.; Zhang, X. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3929–3937. [Google Scholar] [CrossRef]
- Li, Z.; Lian, Y.; Wei, R.; Jin, L.; Cao, H.; Zhao, T.; Ma, X.; Zhong, M.; Gao, Y.; Zhang, K. Effects of taraxasterol against ethanol and high-fat diet-induced liver injury by regulating TLR4/MyD88/NF-κB and Nrf2/HO-1 signaling pathways. Life Sci. 2020, 262, 118546. [Google Scholar] [CrossRef]
- He, H.; Xu, B.; Ge, P.; Gao, Y.; Wei, M.; Li, T.; Zhang, R.; Li, B.; Cao, H.; Zhang, K. The effects of taraxasterol on liver fibrosis revealed by RNA sequencing. Int. Immunopharmacol. 2023, 114. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Garza, L.E.; González-Huezo, M.S.; Moctezuma-Velázquez, C.; Ladrón de Guevara-Cetina, L.; Vilatobá, M.; García-Juárez, I.; Alvarado-Reyes, R.; Álvarez-Treviño, G.A.; Allende-Pérez, S.; Bornstein-Quevedo, L.; et al. II Consenso Mexicano de Carcinoma Hepatocelular. Parte I: Epidemiología y diagnóstico. Rev. Gastroenterol. Mex. 2022, 87, 216–234. [Google Scholar] [CrossRef]
- Aguirre-Maldonado, I.; Herrera-López, E.E.; López-Zenteno, F.; Ramírez-Nava, J.C.; López-Hernández, N.A.; Arellanes-Robledo, J.; Yauner-Pozo, L.; Román-García, R.; Montero, H.; Aguilera-Alexander, A.; et al. Intriguing hepatoprotective effects of sucrose on hepatocellular carcinoma pathogenesis. Sci. Rep. 2024, 14, 23689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.; Jiang, L.; Chen, Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark. Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Denisow-Pietrzyk, M.; Pietrzyk, Ł.; Denisow, B. Asteraceae species as potential environmental factors of allergy. Environ. Sci. Pollut. Res. 2019, 26, 6290–6300. [Google Scholar] [CrossRef]
- Clare, B.A.; Conroy, R.S.; Spelman, K. The diuretic effect in human subjects of an extract of Taraxacum officinale folium over a single day. J. Altern. Complement. Med. 2009, 15, 929–934. [Google Scholar] [CrossRef]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- Petrović, S. Biljni i tradicionalni biljni lekovi, monografije EU i lista EU. Arh. Farm. 2019, 69, 221–269. [Google Scholar] [CrossRef]
- Pawlików, M.; Aebisher, D.; Bartusik-Aebisher, D. Dandelion. In The Biochemical Guide to Toxins; Nova Science Pub Inc: Hauppauge, NY, USA, 2024; pp. 217–220. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014, 4, 66193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).