In Vitro Evaluation of Olive Leaf (Olea europaea L.) Extract as a Functional Food Component in Combination with Chemotherapeutics in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Results of Minerals and Vitamins Analysis of OWE
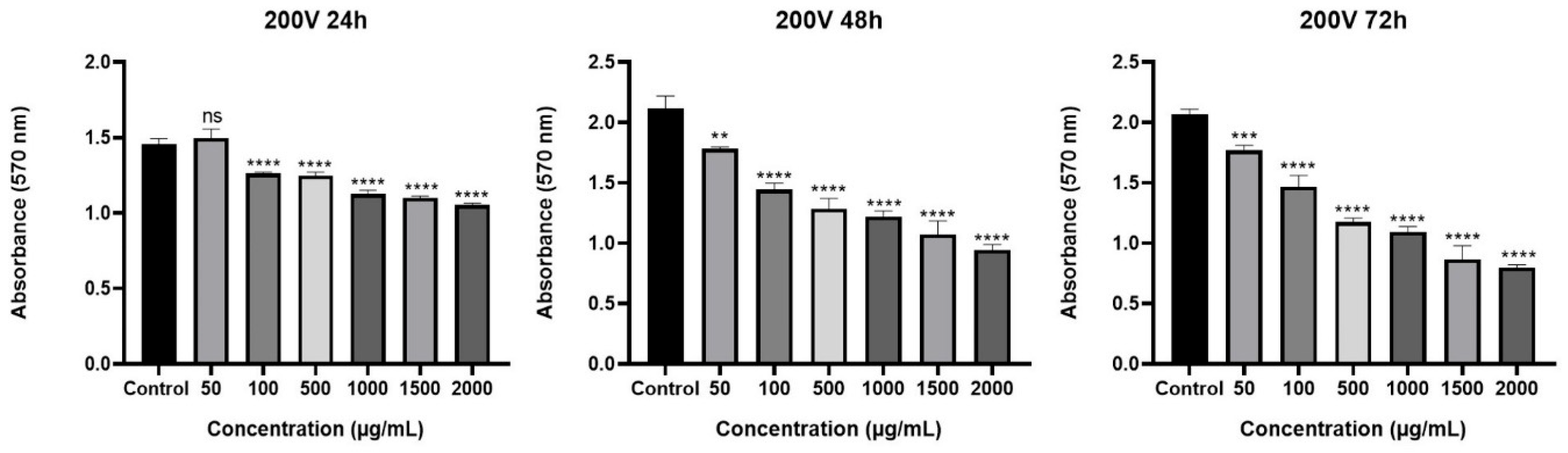
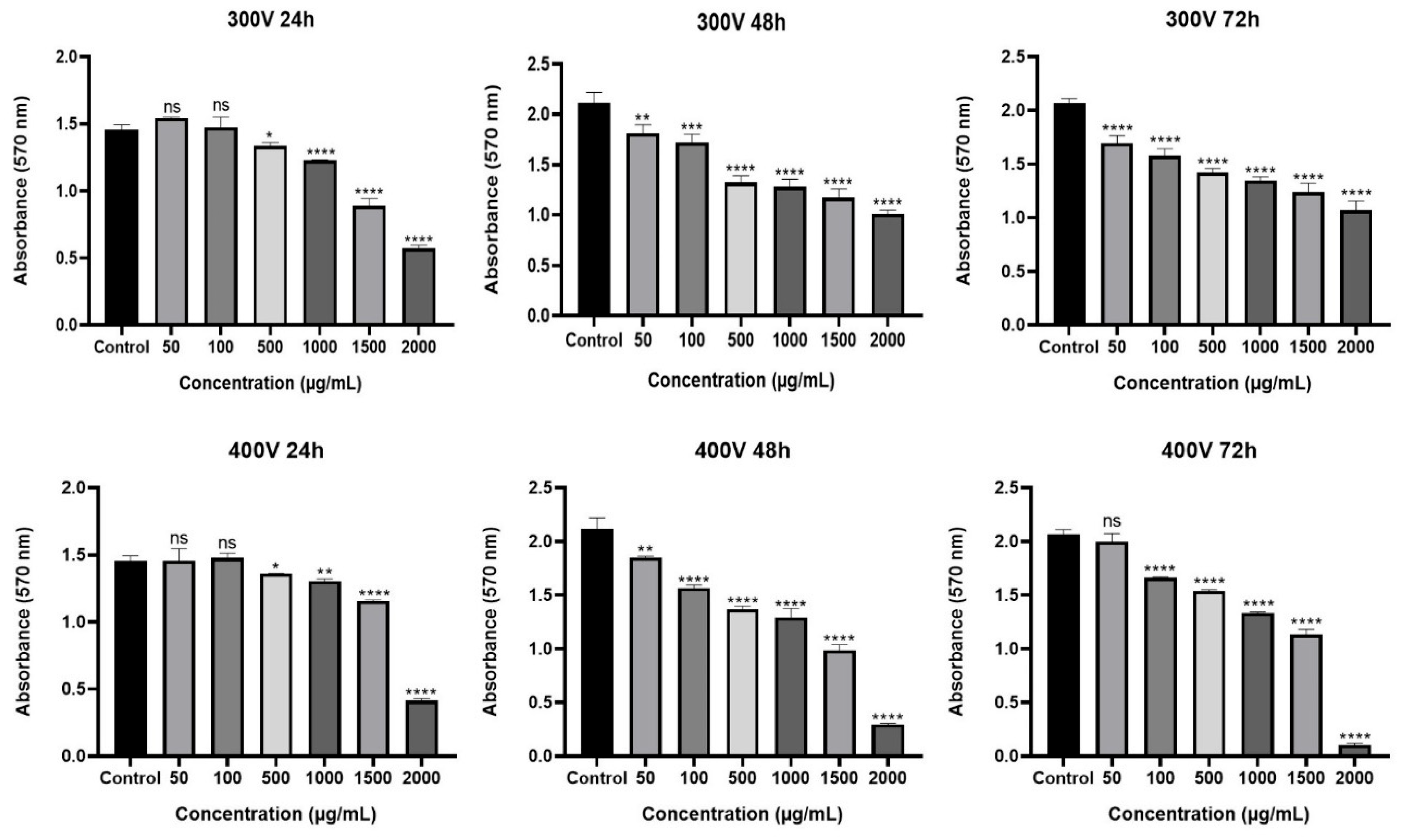
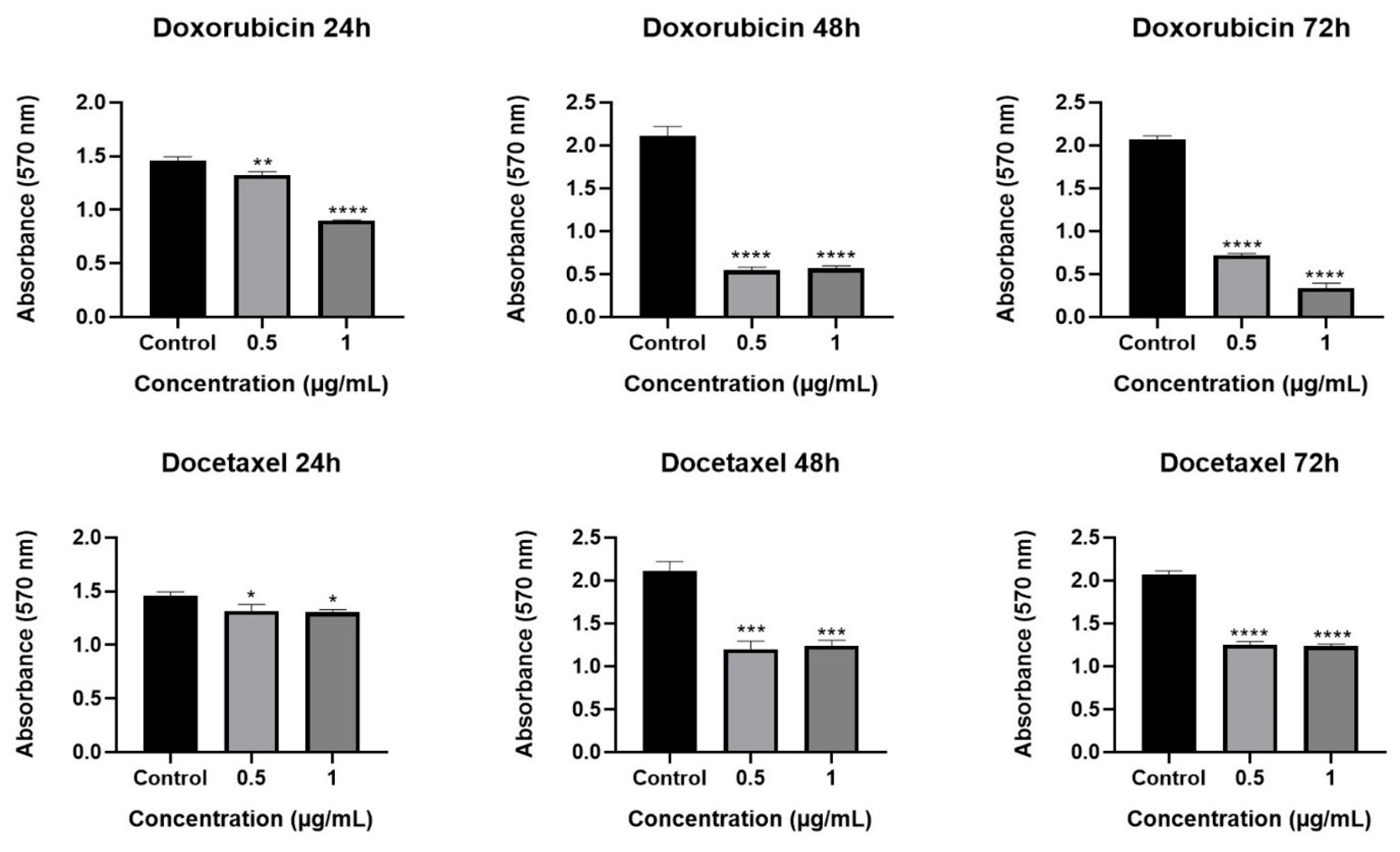
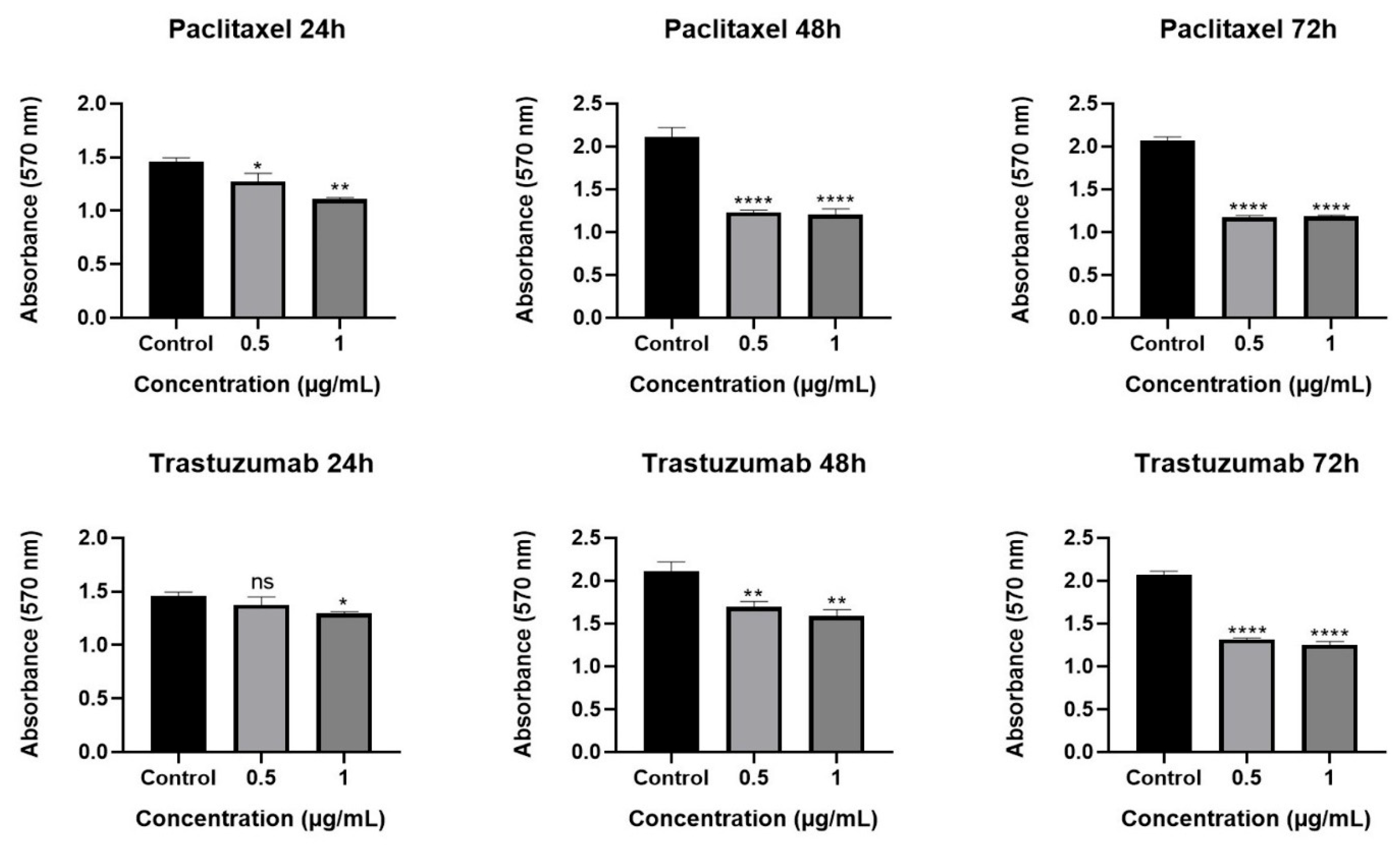
2.2. Cytotoxic Effect of Olea europaea L. Extract and Drugs
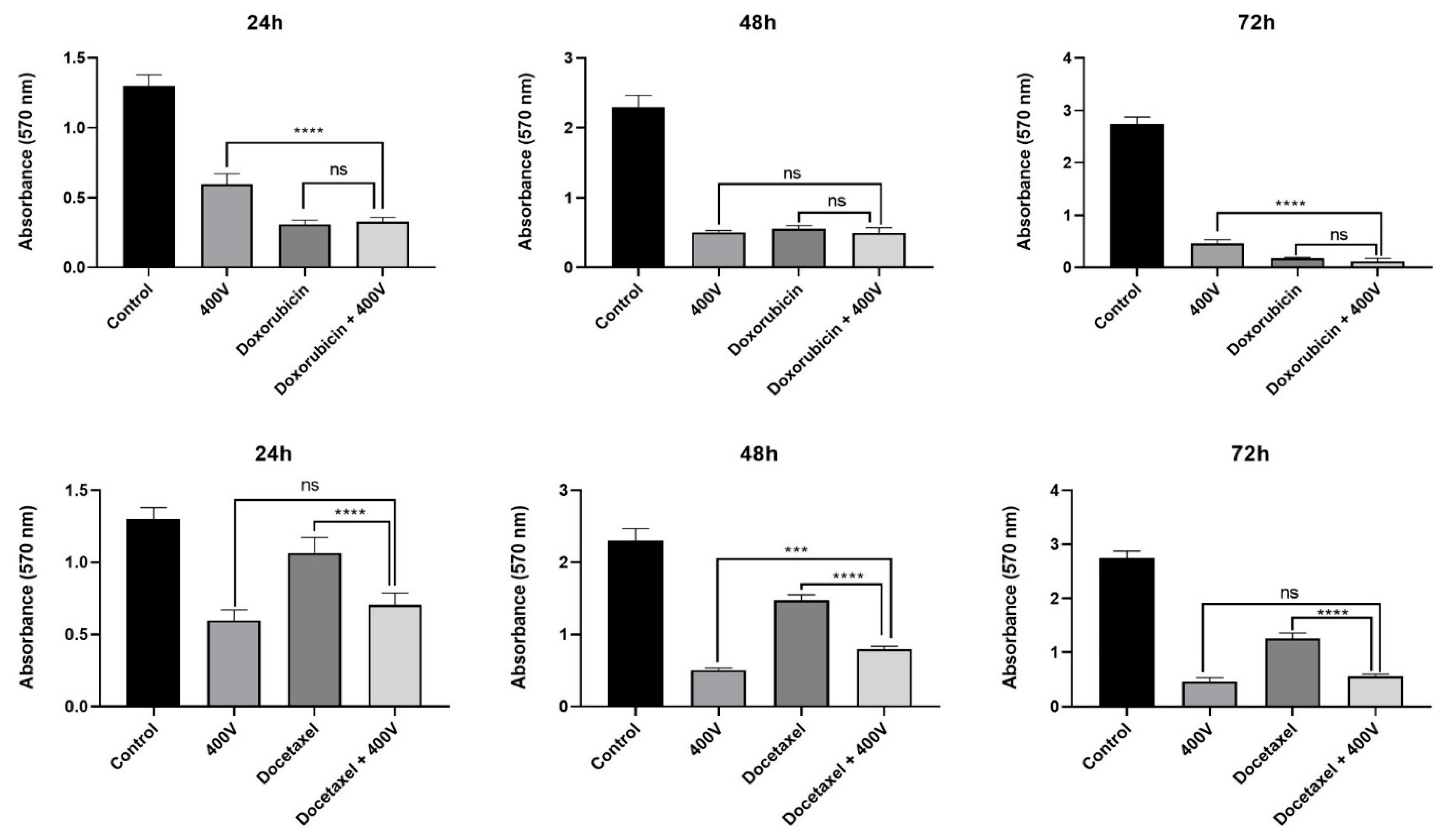
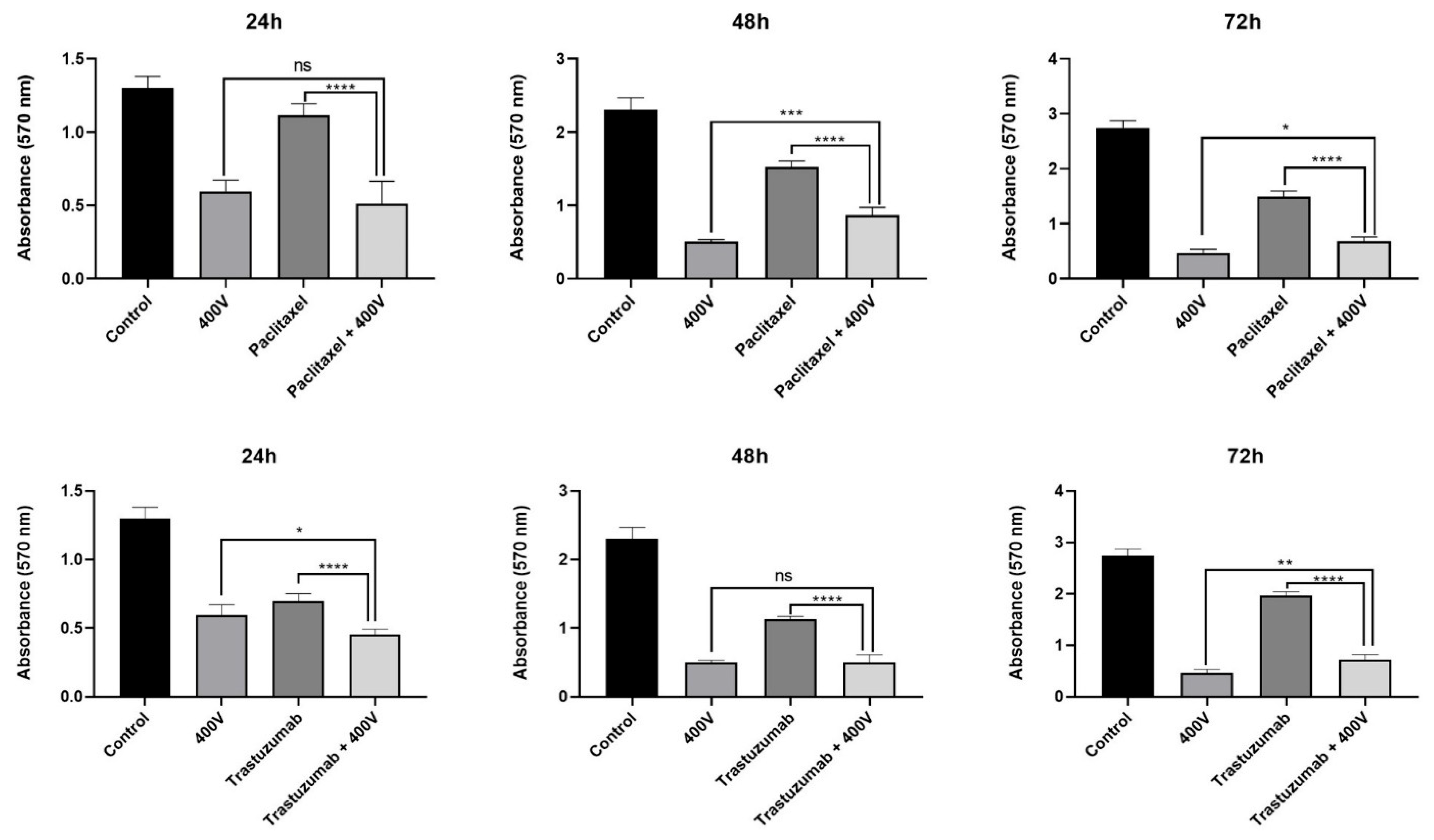
2.3. Effect of Olea europaea L. Extract on Drug Efficacy
3. Discussion
4. Material and Methods
4.1. Extraction of Olea europaea L. Leaf
4.2. Mineral and Vitamin Analysis of OWE
4.3. Cell Culture
4.4. MTT Assay
4.5. Synergistic Effects of Olea europaea L. Extracts
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ito, T.; Urushima, H.; Sakaue, M.; Yukawa, S.; Honda, H.; Hirai, K.; Igura, T.; Hayashi, N.; Maeda, K.; Kitagawa, T.; et al. Reduction of adverse effects by a mushroom product, active hexose correlated compound (AHCC) in patients with advanced cancer during chemotherapy: The significance of the levels of HHV-6 DNA in saliva as a surrogate biomarker during chemotherapy. Nutr. Cancer 2014, 66, 377–382. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Global Cancer: Facts and Figures, 3rd ed.; American Cancer Society: New York, NY, USA, 2015. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef]
- Mohini, C.; Romi, S.; Srija, S.; Flora, S.J.S. A review of FDA approved drugs and their formulations for the treatment of breast cancer. Front. Pharmacol. 2023, 14, 1184472. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Fenton, C. Docetaxel. Drugs 2005, 65, 2513–2531. [Google Scholar] [CrossRef]
- Milross, C.G.; Mason, K.A.; Hunter, N.R.; Chung, W.-K.; Peters, L.J.; Milas, L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J. Natl. Cancer Inst. 1996, 88, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C. Antineoplastic agents from plants. Ann. Rev. Pharmacol. Toxicol. 1977, 17, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H.; Davies, K.J.A. Redox cycling of anthracyclins by cardiac mitochondria: Formation of superoxide anion, hydrogen peroxide and hydroxyl radical. J. Biol. Chem. 1986, 261, 3068–3074. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclins: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharm. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leylandjones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Urvashi, L.; Vishal, K.; Palak, A.; Charan, S.; Arti, S. An update on breast cancer chemotherapy-associated toxicity and their management approaches. Health Sci. Rev. 2023, 9, 100119. [Google Scholar] [CrossRef]
- Reddy, K.P.; Fayanju, O.M. ASO Author Reflections: The Financial Impact of Delayed and Forgone Care Among Patients with Breast Cancer. Ann. Surg. Oncol. 2025, 32, 3335–3336. [Google Scholar] [CrossRef]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am. Health Drug Benefits 2016, 9, 23–32. [Google Scholar]
- Yang, L.J.; Han, T.; Liu, R.N.; Shi, S.M.; Luan, S.Y.; Meng, S.N. Plant-derived natural compounds: A new frontier in inducing immunogenic cell death for cancer treatment. Biomed. Pharmacother. 2024, 177, 117099. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, D.J.; Shi, J.X.; Huang, M.Y.; Yu, J.M.; Chen, X.J.; Wei, X.; Zou, L.; Lu, J.J. Immunogenic cell death inducers for cancer therapy: An emerging focus on natural products. Phytomedicine 2024, 132, 155828. [Google Scholar] [CrossRef]
- Isleem, R.M.; Alzaharna, M.M.; Sharif, F.A. Synergistic anticancer effect of combining metformin with olive (Olea europaea L.) leaf crude extract on the human breast cancer cell line MCF-7. J. Med. Plants Stud. 2020, 8, 30–37. [Google Scholar]
- Cheng, Y.; Li, J.; Feng, X.; Wu, Y.; Wu, X.; Lau, B.W.M.; Shamay, S.M.N.; Simon, M.Y.L.; Sai-Wang, S.; George, P.H.L.; et al. Taohong Siwu decoction enhances the chemotherapeutic efficacy of doxorubicin by promoting tumor vascular normalization. Phytomedicine 2024, 134, 155995. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D. Intervention studies on Mediterranean diet and cardiovascular risk. Mol. Nutr. Food Res. 2007, 51, 1209–1214. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Rossi, M.; Negri, E.; La, V.C. Selected aspects of Mediterranean diet and cancer risk. Nutr. Cancer. 2009, 61, 756–766. [Google Scholar] [CrossRef]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) leaf extract induces apoptosis and monocyte/macrophage differentiation in human chronic myelogenous leukemia K562 cells: Insight into the underlying mechanism. Oxid. Med. Cell. Longev. 2014, 2014, 927619. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant Food Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef]
- Mijatovic, S.A.; Timotijevic, G.S.; Miljkovic, D.M.; Radovic, J.M.; Maksimovic—Ivanic, D.D.; Dekanski, D.P.; Stosic-Grujicic, S.D. Multiple antimelanoma potential of dry olive leaf extract. Int. J. Cancer 2011, 128, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Kumral, A.; Giriş, M.; Soluk-Tekkeşin, M.; Olgaç, V.; Doğru-Abbasoğlu, S.; Türkoğlu, Ü.; Uysal, M. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology 2015, 22, 117–123. [Google Scholar] [CrossRef]
- Babich, H.; Visioli, F. In vitro cytotoxicity to human cells in culture of some phenolics from olive oil. Farmaco 2003, 58, 403–407. [Google Scholar] [CrossRef]
- Hamdi, K.H.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Owen, R.W.; Giocosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.B. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. BioMed Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef]
- Goey, A.K.; Meijerman, I.; Rosing, H.; Burgers, J.A.; Mergui-Roelvink, M.; Keessen, M.; Marchetti, S.; Beijnen, J.H.; Schellens, J.H. The effect of echinacea purpurea on the pharmacokinetics of docetaxel. Br. J. Clin. Pharmacol. 2013, 76, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ganji-Harsini, S.; Khazaei, M.; Rashidi, Z.; Ghanbari, A. Thymoquinone could increase the efficacy of tamoxifen induced apoptosis in human breast cancer cells: An in vitro study. Cell J. 2016, 18, 245–254. [Google Scholar] [CrossRef]
- Quispe-Soto, E.T.; Calaf, G.M. Effect of curcumin and paclitaxel on breast carcinogenesis. Int. J. Oncol. 2016, 49, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Konczak, I.; Rheman, A.; Robard, K. Chemistry and bioactivity of olive biophenols in some antioxidant and antiproliferative in vitro bioassays. Chem. Res. Toxicol. 2009, 22, 227–234. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.; Yamada, P.; Isoda, H. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired auto resistance to trastuzumab (Herceptin™) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80–98. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to support the anti-cancer effect of olive leaf extract and future directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Morandi, F.; Bensa, V.; Calarco, E.; Pastorino, F.; Perri, P.; Corrias, M.V.; Ponzoni, M.; Brignole, C. The Olive Leaves Extract Has Anti-Tumor Effects against Neuroblastoma through Inhibition of Cell Proliferation and Induction of Apoptosis. Nutrients 2021, 13, 2178. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical properties and antiproliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Klebanoff, C.A.; Restifo, N.P. Paths to stemness: Building the ultimate antitumour T cell. Nat. Rev. Cancer. 2012, 12, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Araki, K.; Li, S.; Han, J.H.; Ye, L.; Tan, W.G.; Konieczny, B.T.; Bruinsma, M.W.; Martinez, J.; Pearce, E.L.; et al. Autophagy is essential for effector CD8+ T cell survival and memory formation. Nat. Immunol. 2014, 15, 1152–1161. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Ling, M.T.; Luk, S.U.; Al-Ejeh, F.; Khanna, K.K. Tocotrienol as a potential anticancer agent. Carcinogenesis 2012, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Su, S.; Zhao, Y.; Long, J. Intrinsic relationship between viscosity, viscosity index, and molecular structure of isoalkanes. J. Mol. Model. 2023, 29, 101. [Google Scholar] [CrossRef]
- Azman, N.A.N.; Ab Rahman, S.S.; Anuar, N.F.; Wan Taib, W.R.; Mohd Rawi, R.I. In Vitro Cytotoxic Effect of Dichloromethane Extract of Prismatomeris glabra in Human Breast Cancer Cells. Asian J. Med. Biomed. 2020, 4, 59–66. [Google Scholar] [CrossRef]
| Experiments | Unit | Experimental Method | Results with ± SD | ||
|---|---|---|---|---|---|
| 200 V | 300 V | 400 V | |||
| Minerals | |||||
| Potassium (K) | mg/100 g | ICP-OES | 1536 ± 56 | 1507 ± 9 | 1744 ± 33 |
| Phosphorus (P) | mg/100 g | ICP-OES | 188 ± 5 | 202 ± 2 | 218 ± 7 |
| Calcium (Ca) | mg/100 g | ICP-OES | 284 ± 3 | 296 ± 4 | 301 ± 12 |
| Ferritin (Fe) | mg/100 g | ICP-OES | 5.50 ± 0.50 | 5.28 ± 0.21 | 3.74 ± 0.08 |
| Magnesium (Mg) | mg/100 g | ICP-OES | 120 ± 4 | 118 ± 1 | 118 ± 5 |
| Manganese (Mn) | mg/100 g | ICP-OES | 1.29 ± 0.03 | 1.30 ± 0.02 | 1.30 ± 0.01 |
| Zinc (Zn) | mg/100 g | ICP-OES | 16.3 ± 2.8 | 20.5 ± 2.0 | 19.8 ± 1.2 |
| Sodium (Na) | mg/100 g | ICP-OES | 85.4 ± 6.3 | 86.7 ± 2.4 | 45.8 ± 2.4 |
| Selenium (Se) | µg/100 g | ICP-MS | 6.78 ± 0.08 | 6.67 ± 0.09 | 6.87 ± 0.19 |
| Vitamins | |||||
| A | mg/100 g | HPLC | <0.01 | <0.01 | <0.01 |
| B5 | mg/100 g | HPLC | < 0.3 | <0.3 | <0.3 |
| C | mg/100 g | HPLC | 16.2 ± 0.5 | 17.8 ± 0.1 | 13.9 ± 0.4 |
| D2 | mg/100 g | HPLC | <0.04 | <0.04 | <0.04 |
| E | mg/100 g | HPLC | 0.20 ± 0.01 | 0.08 ± 0.01 | 0.36 ± 0.01 |
| K1 | mg/100 g | HPLC-FLD | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.010 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büker, E.; Kiran, F.; Taliboglu, S.; Casoni, D.; Ipekel, A. In Vitro Evaluation of Olive Leaf (Olea europaea L.) Extract as a Functional Food Component in Combination with Chemotherapeutics in MCF-7 Breast Cancer Cells. Pharmaceuticals 2025, 18, 965. https://doi.org/10.3390/ph18070965
Büker E, Kiran F, Taliboglu S, Casoni D, Ipekel A. In Vitro Evaluation of Olive Leaf (Olea europaea L.) Extract as a Functional Food Component in Combination with Chemotherapeutics in MCF-7 Breast Cancer Cells. Pharmaceuticals. 2025; 18(7):965. https://doi.org/10.3390/ph18070965
Chicago/Turabian StyleBüker, Eda, Fadime Kiran, Seval Taliboglu, Dorina Casoni, and Ayşe Ipekel. 2025. "In Vitro Evaluation of Olive Leaf (Olea europaea L.) Extract as a Functional Food Component in Combination with Chemotherapeutics in MCF-7 Breast Cancer Cells" Pharmaceuticals 18, no. 7: 965. https://doi.org/10.3390/ph18070965
APA StyleBüker, E., Kiran, F., Taliboglu, S., Casoni, D., & Ipekel, A. (2025). In Vitro Evaluation of Olive Leaf (Olea europaea L.) Extract as a Functional Food Component in Combination with Chemotherapeutics in MCF-7 Breast Cancer Cells. Pharmaceuticals, 18(7), 965. https://doi.org/10.3390/ph18070965







