Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications
Abstract
1. Introduction
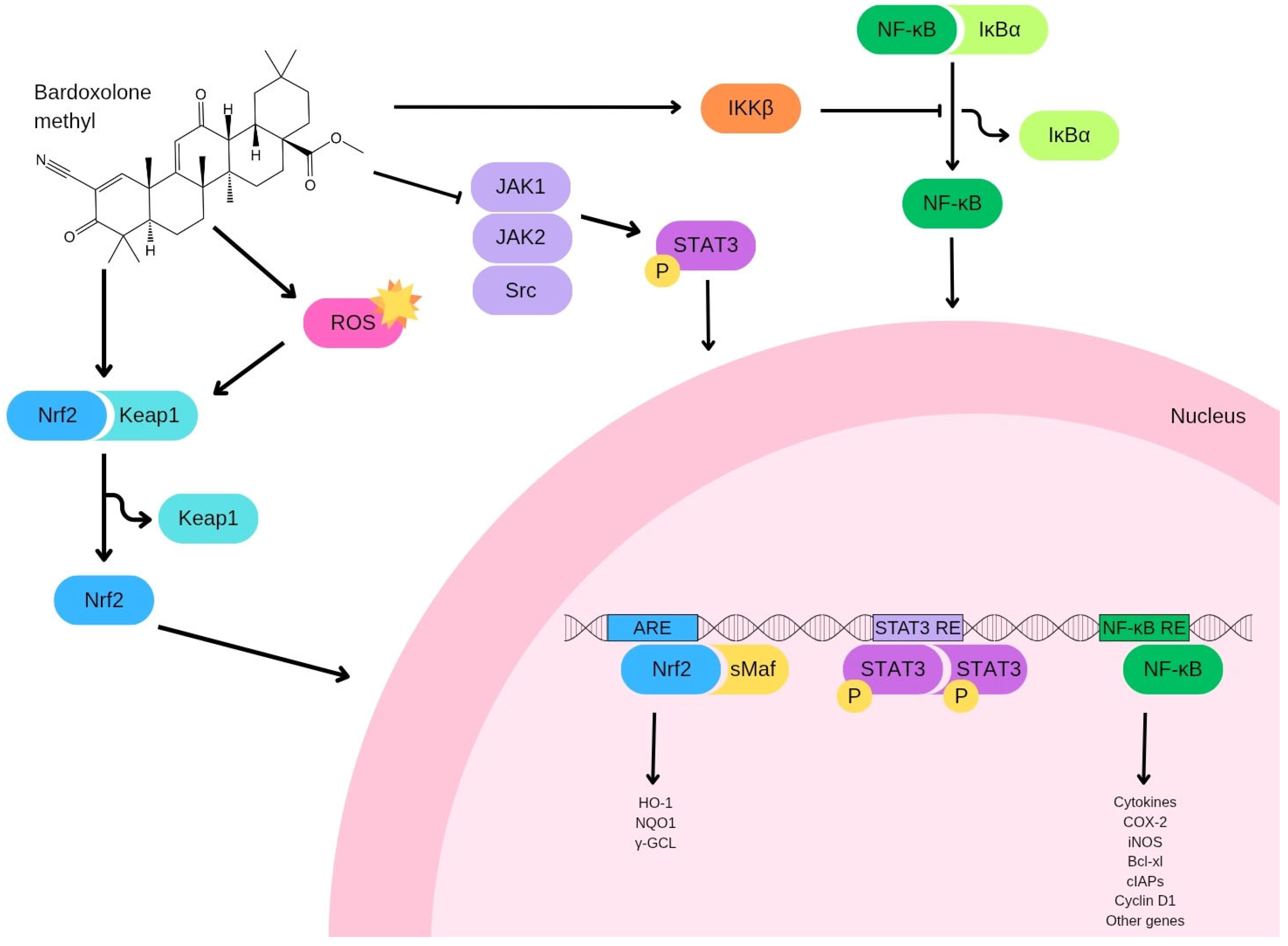
2. Bardoxolone Methyl: Pharmacokinetic Properties and Biological Mechanisms
3. Targets of Bardoxolone Methyl
4. Bardoxolone Methyl and Cancer
4.1. Lung Cancer
4.2. Breast Cancer
4.3. Prostate Cancer
4.4. Pancreatic Cancer
4.5. Colorectal Cancer
4.6. Ovarian Cancer
4.7. Glioblastoma
4.8. Osteosarcoma
4.9. Oral Squamous Cell Carcinoma
4.10. Other Malignancies
5. Limitations, Adverse Effects, and Safety Concerns
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARE | antioxidant response element |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma extra large |
| bZip | basic leucine zipper |
| CDDO | 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid |
| CETSA | cellular thermal shift assays |
| CHOP | CCAAT/enhancer binding protein homologous protein |
| CKD | chronic kidney disease |
| COX-2 | inducible cyclooxygenase 2 |
| CSC | cancer stem cell |
| Cul3 | Cullin3 |
| DARTS | drug affinity responsive target stability |
| DLTs | dose-limiting toxicities |
| DMF | dose-modifying factor |
| DVL2 | Disheveled 2 |
| eGFR | estimated glomerular filtration rate |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| FZD7 | Frizzled-7 |
| GCLC | glutamate-cysteine ligase catalytic |
| GCLM | glutamate-cysteine ligase modifier |
| GPx | glutathione peroxidase |
| GSK3 | glycogen synthase kinase 3 |
| GST | glutathione S-transferase |
| HO-1 | heme oxygenase-1 |
| IKK | IκB kinase |
| IL-10 | interleukin-10 |
| IL-6 | interleukin-6 |
| iNOS | nitric oxide synthase |
| JAK | Janus Kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LC–MS/MS | liquid chromatography–tandem mass spectrometry |
| LRP6 | lipoprotein receptor-related protein 6 |
| Maf | musculoaponeurotic fibrosarcoma |
| MAPK | mitogen-activated protein kinase |
| MDSCs | myeloid-derived suppressor cells |
| MMP-9 | matrix metalloproteinase-9 |
| MMTV | mouse mammary tumor virus |
| MTD | maximum tolerated dose |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| NAC | N-acetylcysteine |
| NQO1 | NADPH dehydrogenase quinone 1 |
| Nrf2 | erythroid 2-related factor 2 |
| p-4E-BP1 | phosphorylated 4E-BP1 |
| PBMCs | peripheral blood mononuclear cells |
| p-eIF-4E | phosphorylated eIF-4E |
| pErbB2 | phosphorylated ErbB2 |
| p-Foxo3a | Foxo3a |
| PI3K | phosphoinositide 3-kinase |
| p-S6K1 | phosphorylated S6 kinase 1 |
| PyMT | polyoma-middle T |
| Rbx1 | Ring-box 1 |
| ROS | reactive oxygen species |
| SDD | spray-dried dispersion |
| SOD-1 | superoxide dismutase-1 |
| STATs | Signal Transducers and Activators of Transcription |
| TERT | telomerase reverse transcriptase |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Tossetta, G. Role of Natural Compounds as Anti-Cancer Agents. Anti-Cancer Agents Med. Chem. 2025, 25, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Bizzoca, M.E.; Leuci, S.; Mignogna, M.D.; Muzio, E.L.; Caponio, V.C.A.; Muzio, L.L. Natural compounds may contribute in preventing SARS-CoV-2 infection: A narrative review. Food Sci. Hum. Wellness 2022, 11, 1134–1142. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Busilacchi, E.M.; Marzioni, D.; Mazzucchelli, R. Dose-dependent effects of curcumin on 22Rv1 prostate cancer cell line. Mol. Biol. Rep. 2025, 52, 339. [Google Scholar] [CrossRef] [PubMed]
- Bizzoca, M.E.; Caponio, V.C.A.; Lo Muzio, L.; Claudio, P.P.; Cortese, A. Methods for Overcoming Chemoresistance in Head and Neck Squamous Cell Carcinoma: Keeping the Focus on Cancer Stem Cells, a Systematic Review. Cancers 2024, 16, 3004. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qi, X. Biosynthesis of triterpenoids in plants: Pathways, regulation, and biological functions. Curr. Opin. Plant Biol. 2025, 85, 102701. [Google Scholar] [CrossRef]
- Fu, L.; Gribble, G.W. Efficient and scalable synthesis of bardoxolone methyl (cddo-methyl ester). Org. Lett. 2013, 15, 1622–1625. [Google Scholar] [CrossRef]
- Wimmerova, M.; Bildziukevich, U.; Wimmer, Z. Selected Plant Triterpenoids and Their Derivatives as Antiviral Agents. Molecules 2023, 28, 7718. [Google Scholar] [CrossRef]
- Li, S.; Kuo, H.D.; Yin, R.; Wu, R.; Liu, X.; Wang, L.; Hudlikar, R.; Peter, R.M.; Kong, A.N. Epigenetics/epigenomics of triterpenoids in cancer prevention and in health. Biochem. Pharmacol. 2020, 175, 113890. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Tsao, T.; Estrov, Z.; Lee, R.M.; Wang, R.Y.; Jackson, C.E.; McQueen, T.; Monaco, G.; Munsell, M.; Belmont, J.; et al. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004, 64, 7927–7935. [Google Scholar] [CrossRef] [PubMed]
- Suh, N.; Wang, Y.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Hickey, W.F.; Maue, R.A.; Place, A.E.; Porter, D.M.; Spinella, M.J.; et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999, 59, 336–341. [Google Scholar]
- Baer-Dubowska, W.; Narozna, M.; Krajka-Kuzniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Suh, N.; Honda, T.; Finlay, H.J.; Barchowsky, A.; Williams, C.; Benoit, N.E.; Xie, Q.W.; Nathan, C.; Gribble, G.W.; Sporn, M.B. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998, 58, 717–723. [Google Scholar]
- Konopleva, M.; Tsao, T.; Ruvolo, P.; Stiouf, I.; Estrov, Z.; Leysath, C.E.; Zhao, S.; Harris, D.; Chang, S.; Jackson, C.E.; et al. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood 2002, 99, 326–335. [Google Scholar] [CrossRef]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef]
- Teuscher, N.S.; Kelley, R.J.; Dumas, E.O.; Klein, C.E.; Awni, W.M.; Meyer, C.J. A food effect study and dose proportionality study to assess the pharmacokinetics and safety of bardoxolone methyl in healthy volunteers. Clin. Pharmacol. Drug Dev. 2014, 3, 314–320. [Google Scholar] [CrossRef]
- Ling, T.; Boyd, L.; Rivas, F. Triterpenoids as Reactive Oxygen Species Modulators of Cell Fate. Chem. Res. Toxicol. 2022, 35, 569–584. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. C. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and its Analogues in Oral Squamous Cell Carcinoma: State-of-the-art and Therapeutic Potential. Anti-Cancer. Agents Med. Chem. 2024, 25, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Cecati, M.; Vignini, A. The Multifaceted Role of the Polyphenol Curcumin: A Focus on Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2024, 21, e15733998313402. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Fantone, S.; Marzioni, D.; Tossetta, G. NRF2/KEAP1 signaling inhibitors in gynecologic cancers. Expert Rev. Anti-Cancer Ther. 2024, 24, 1191–1194. [Google Scholar] [CrossRef]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of NRF2/KEAP1 Signaling by Phytotherapeutics in Periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2022, 50, 873–881. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, J.; Chen, X.; Yang, K.; Wang, K. Oxidative Stress and Redox Signaling in Gastric Cancer: From Mechanisms to Therapeutic Implications. Antioxidants 2025, 14, 258. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular Modulators of the NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Front. Biosci. (Landmark Ed.) 2023, 28, 143. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mateuszuk, L.; Wojnar-Lason, K.; Kaczara, P.; Tworzydlo, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef] [PubMed]
- Annesi, L.; Tossetta, G.; Borghi, C.; Piani, F. The Role of Xanthine Oxidase in Pregnancy Complications: A Systematic Review. Antioxidants 2024, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhou, X.; Qiu, J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. Pharmacother. 2020, 131, 110676. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/beta-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.I.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep. 2019, 28, 746–758.e4. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxid. Med. Cell Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Calkins, M.J.; Jakel, R.J.; Johnson, D.A.; Chan, K.; Kan, Y.W.; Johnson, J.A. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 244–249. [Google Scholar] [CrossRef]
- Lee, J.M.; Shih, A.Y.; Murphy, T.H.; Johnson, J.A. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J. Biol. Chem. 2003, 278, 37948–37956. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Cecati, M.; Sartini, D.; Campagna, R.; Biagini, A.; Ciavattini, A.; Emanuelli, M.; Giannubilo, S.R. Molecular analysis of endometrial inflammation in preterm birth. Cell. Mol. Biol. 2017, 63, 51–57. [Google Scholar] [CrossRef]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Goswami, S.K.; Ranjan, P.; Dutta, R.K.; Verma, S.K. Management of inflammation in cardiovascular diseases. Pharmacol. Res. 2021, 173, 105912. [Google Scholar] [CrossRef]
- Licini, C.; Tossetta, G.; Avellini, C.; Ciarmela, P.; Lorenzi, T.; Toti, P.; Gesuita, R.; Voltolini, C.; Petraglia, F.; Castellucci, M.; et al. Analysis of cell-cell junctions in human amnion and chorionic plate affected by chorioamnionitis. Histol. Histopathol. 2016, 31, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Di Renzo, G.C.; Meyyazhagan, A.; Tersigni, C.; Scambia, G.; Di Simone, N.; Marzioni, D. HtrA1 in Gestational Diabetes Mellitus: A Possible Biomarker? Diagnostics 2022, 12, 2705. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Ahmad, R.; Raina, D.; Meyer, C.; Kharbanda, S.; Kufe, D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J. Biol. Chem. 2006, 281, 35764–35769. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; An, J.; Zhang, G.; Chen, Y.; Fu, L.; Tao, L.; Liang, G.; Shen, X. Cyclovirobuxine D alleviates aldosterone-induced myocardial hypertrophy by protecting mitochondrial function depending on the mutual regulation of Nrf2-SIRT3. Biomed. Pharmacother. 2023, 167, 115618. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Cao, J.; Wu, C.; Tang, L.; Bian, W.; Chen, Y.; Yu, L.; Wu, Y.; Li, S.; et al. Targeting epigenetic and post-translational modifications of NRF2: Key regulatory factors in disease treatment. Cell Death Discov. 2025, 11, 189. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, L.; Wang, X.; Ji, Y.; Tu, Y. Nrf2 in human cancers: Biological significance and therapeutic potential. Am. J. Cancer Res. 2024, 14, 3935–3961. [Google Scholar] [CrossRef]
- Peng, X.; Feng, J.; Yang, H.; Xia, P.; Pu, F. Nrf2: A key regulator in chemoradiotherapy resistance of osteosarcoma. Genes. Dis. 2025, 12, 101335. [Google Scholar] [CrossRef]
- Luchkova, A.; Mata, A.; Cadenas, S. Nrf2 as a regulator of energy metabolism and mitochondrial function. FEBS Lett. 2024, 598, 2092–2105. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal transducer and activator of transcription-3, inflammation, and cancer: How intimate is the relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Raina, D.; Meyer, C.; Kufe, D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)→signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008, 68, 2920–2926. [Google Scholar] [CrossRef]
- Liby, K.; Royce, D.B.; Williams, C.R.; Risingsong, R.; Yore, M.M.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Sporn, T.A.; Sporn, M.B. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007, 67, 2414–2419. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Giorgini, S.; Morichetti, D.; Goteri, G.; Sartini, D.; Serritelli, E.N.; Emanuelli, M. Paraoxonase-2 is upregulated in triple negative breast cancer and contributes to tumor progression and chemoresistance. Hum. Cell 2023, 36, 1108–1119. [Google Scholar] [CrossRef]
- Liby, K.; Risingsong, R.; Royce, D.B.; Williams, C.R.; Yore, M.M.; Honda, T.; Gribble, G.W.; Lamph, W.W.; Vannini, N.; Sogno, I.; et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl Ester and the rexinoid LG100268. Clin. Cancer Res. 2008, 14, 4556–4563. [Google Scholar] [CrossRef]
- Liby, K.T.; Royce, D.B.; Risingsong, R.; Williams, C.R.; Maitra, A.; Hruban, R.H.; Sporn, M.B. Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev. Res. 2010, 3, 1427–1434. [Google Scholar] [CrossRef]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Gilardini Montani, M.S.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Cirone, M. NRF2 and STAT3: Friends or foes in carcinogenesis? Discov. Oncol. 2023, 14, 37. [Google Scholar] [CrossRef]
- Tran, K.; Risingsong, R.; Royce, D.; Williams, C.R.; Sporn, M.B.; Liby, K. The synthetic triterpenoid CDDO-methyl ester delays estrogen receptor-negative mammary carcinogenesis in polyoma middle T mice. Cancer Prev. Res. 2012, 5, 726–734. [Google Scholar] [CrossRef]
- Kim, E.H.; Deng, C.; Sporn, M.B.; Royce, D.B.; Risingsong, R.; Williams, C.R.; Liby, K.T. CDDO-methyl ester delays breast cancer development in BRCA1-mutated mice. Cancer Prev. Res. 2012, 5, 89–97. [Google Scholar] [CrossRef]
- Ling, X.; Konopleva, M.; Zeng, Z.; Ruvolo, V.; Stephens, L.C.; Schober, W.; McQueen, T.; Dietrich, M.; Madden, T.L.; Andreeff, M. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007, 67, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.A.; Kim, I.Y.; Lee, A.R.; Yoon, M.J.; Cho, H.; Lee, J.S.; Choi, K.S. Ca2+ influx-mediated dilation of the endoplasmic reticulum and c-FLIPL downregulation trigger CDDO-Me-induced apoptosis in breast cancer cells. Oncotarget 2015, 6, 21173–21192. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Mo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. J. Tissue Eng. Regen. Med. 2020, 14, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Chitoran, E.; Rotaru, V.; Stefan, D.C.; Gullo, G.; Simion, L. Blocking Tumoral Angiogenesis VEGF/VEGFR Pathway: Bevacizumab-20 Years of Therapeutic Success and Controversy. Cancers 2025, 17, 1126. [Google Scholar] [CrossRef]
- Fantone, S.; Tossetta, G.; Di Simone, N.; Tersigni, C.; Scambia, G.; Marcheggiani, F.; Giannubilo, S.R.; Marzioni, D. CD93 a potential player in cytotrophoblast and endothelial cell migration. Cell Tissue Res. 2022, 387, 123–130. [Google Scholar] [CrossRef]
- Ball, M.S.; Bhandari, R.; Torres, G.M.; Martyanov, V.; ElTanbouly, M.A.; Archambault, K.; Whitfield, M.L.; Liby, K.T.; Pioli, P.A. CDDO-Me Alters the Tumor Microenvironment in Estrogen Receptor Negative Breast Cancer. Sci. Rep. 2020, 10, 6560. [Google Scholar] [CrossRef]
- El-Ashmawy, M.; Delgado, O.; Cardentey, A.; Wright, W.E.; Shay, J.W. CDDO-Me protects normal lung and breast epithelial cells but not cancer cells from radiation. PLoS ONE 2014, 9, e115600. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Yu, S.; Xiong, Y.; Fan, J.; Lyu, Y.; Su, Z.; Song, J.; Liu, S.; Sun, Q.; et al. CDDO-Me Elicits Anti-Breast Cancer Activity by Targeting LRP6 and FZD7 Receptor Complex. J. Pharmacol. Exp. Ther. 2020, 373, 149–159. [Google Scholar] [CrossRef]
- Pozzi, V.; Salvolini, E.; Lucarini, G.; Salvucci, A.; Campagna, R.; Rubini, C.; Sartini, D.; Emanuelli, M. Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life 2020, 72, 1415–1425. [Google Scholar] [CrossRef]
- Refaat, A.; Pararasa, C.; Arif, M.; Brown, J.E.; Carmichael, A.; Ali, S.S.; Sakurai, H.; Griffiths, H.R. Bardoxolone-methyl inhibits migration and metabolism in MCF7 cells. Free Radic. Res. 2017, 51, 211–221. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Spinelli, G.; Sartini, D.; Milanese, G.; Galosi, A.B.; Emanuelli, M. The Utility of Nicotinamide N-Methyltransferase as a Potential Biomarker to Predict the Oncological Outcomes for Urological Cancers: An Update. Biomolecules 2021, 11, 1214. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Goteri, G.; Senzacqua, M.; Marcheggiani, F.; Tiano, L.; Marzioni, D.; Mazzucchelli, R. Ciliary Neurotrophic Factor Modulates Multiple Downstream Signaling Pathways in Prostate Cancer Inhibiting Cell Invasiveness. Cancers 2022, 14, 5917. [Google Scholar] [CrossRef] [PubMed]
- Wasinger, G.; Oszwald, A.; Shariat, S.F.; Comperat, E. Histological patterns, subtypes and aspects of prostate cancer: Different aspects, different outcomes. Curr. Opin. Urol. 2022, 32, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Montironi, R.; Marzioni, D.; Mazzucchelli, R. AT-rich interactive domain 1A (ARID1A) cannot be considered a morphological marker for prostate cancer progression: A pilot study. Acta Histochem. 2022, 124, 151847. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Liu, Y.; Jiang, D.; Divine, G.W.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. Carcinogenesis 2011, 32, 757–764. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Liu, Y.; Arbab, A.S.; Divine, G.W.; Dulchavsky, S.A.; Gautam, S.C. Prevention of Prostate Cancer with Oleanane Synthetic Triterpenoid CDDO-Me in the TRAMP Mouse Model of Prostate Cancer. Cancers 2011, 3, 3353–3369. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Deeb, D.; Arbab, A.S.; Gautam, S.C. Telomerase reverse transcriptase (TERT) is a therapeutic target of oleanane triterpenoid CDDO-Me in prostate cancer. Molecules 2012, 17, 14795–14809. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Dulchavsky, S.A.; Gautam, S.C. CDDO-me induces apoptosis and inhibits Akt, mTOR and NF-kappaB signaling proteins in prostate cancer cells. Anticancer. Res. 2007, 27, 3035–3044. [Google Scholar]
- Deeb, D.; Gao, X.; Jiang, H.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro-survival Akt and mTOR. Prostate 2009, 69, 851–860. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Janic, B.; Arbab, A.S.; Rojanasakul, Y.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 2010, 79, 350–360. [Google Scholar] [CrossRef]
- Khurana, N.; Chandra, P.K.; Kim, H.; Abdel-Mageed, A.B.; Mondal, D.; Sikka, S.C. Bardoxolone-Methyl (CDDO-Me) Suppresses Androgen Receptor and Its Splice-Variant AR-V7 and Enhances Efficacy of Enzalutamide in Prostate Cancer Cells. Antioxidants 2020, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Arbab, A.S.; Barton, K.; Dulchavsky, S.A.; Gautam, S.C. CDDO-Me: A Novel Synthetic Triterpenoid for the Treatment of Pancreatic Cancer. Cancers 2010, 2, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.B.; Gautam, S.C. Inhibition of cell proliferation and induction of apoptosis by CDDO-Me in pancreatic cancer cells is ROS-dependent. J. Exp. Ther. Oncol. 2012, 10, 51–64. [Google Scholar]
- Deeb, D.; Gao, X.; Liu, Y.; Kim, S.H.; Pindolia, K.R.; Arbab, A.S.; Gautam, S.C. Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity. Biochem. Biophys. Res. Commun. 2012, 422, 561–567. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Liu, Y.; Varma, N.R.; Arbab, A.S.; Gautam, S.C. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules 2013, 18, 3250–3265. [Google Scholar] [CrossRef]
- Jutooru, I.; Chadalapaka, G.; Abdelrahim, M.; Basha, M.R.; Samudio, I.; Konopleva, M.; Andreeff, M.; Safe, S. Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: Role of microRNA-27a. Mol. Pharmacol. 2010, 78, 226–236. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Liu, Y.; Liu, P.; Zhang, Y.; Shaw, J.; Gautam, S.C. CDDO-Me inhibits tumor growth and prevents recurrence of pancreatic ductal adenocarcinoma. Int. J. Oncol. 2015, 47, 2100–2106. [Google Scholar] [CrossRef]
- Altobelli, E.; Latella, G.; Morroni, M.; Licini, C.; Tossetta, G.; Mazzucchelli, R.; Profeta, V.F.; Coletti, G.; Leocata, P.; Castellucci, M.; et al. Low HtrA1 expression in patients with long-standing ulcerative colitis and colorectal cancer. Oncol. Rep. 2017, 38, 418–426. [Google Scholar] [CrossRef]
- Pozzi, V.; Campagna, R.; Sartini, D.; Emanuelli, M. Nicotinamide N-Methyltransferase as Promising Tool for Management of Gastrointestinal Neoplasms. Biomolecules 2022, 12, 1173. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Hao, J.; Liu, Y.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Synthetic triterpenoids inhibit growth, induce apoptosis and suppress pro-survival Akt, mTOR and NF-kappaB signaling proteins in colorectal cancer cells. Anticancer. Res. 2010, 30, 785–792. [Google Scholar]
- Gao, X.; Deeb, D.; Liu, P.; Liu, Y.; Arbab-Ali, S.; Dulchavsky, S.A.; Gautam, S.C. Role of reactive oxygen species (ROS) in CDDO-Me-mediated growth inhibition and apoptosis in colorectal cancer cells. J. Exp. Ther. Oncol. 2011, 9, 119–127. [Google Scholar] [PubMed]
- Tossetta, G.; Inversetti, A. Ovarian Cancer: Advances in Pathophysiology and Therapies. Int. J. Mol. Sci. 2023, 24, 8930. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Y.; Deeb, D.; Arbab, A.S.; Guo, A.M.; Dulchavsky, S.A.; Gautam, S.C. Synthetic oleanane triterpenoid, CDDO-Me, induces apoptosis in ovarian cancer cells by inhibiting prosurvival AKT/NF-kappaB/mTOR signaling. Anticancer Res. 2011, 31, 3673–3681. [Google Scholar] [PubMed]
- Gao, X.; Liu, Y.; Deeb, D.; Liu, P.; Liu, A.; Arbab, A.S.; Gautam, S.C. ROS mediate proapoptotic and antisurvival activity of oleanane triterpenoid CDDO-Me in ovarian cancer cells. Anticancer. Res. 2013, 33, 215–221. [Google Scholar]
- Duan, Z.; Ames, R.Y.; Ryan, M.; Hornicek, F.J.; Mankin, H.; Seiden, M.V. CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 681–689. [Google Scholar] [CrossRef]
- Qin, D.; Wang, W.; Lei, H.; Luo, H.; Cai, H.; Tang, C.; Wu, Y.; Wang, Y.; Jin, J.; Xiao, W.; et al. CDDO-Me reveals USP7 as a novel target in ovarian cancer cells. Oncotarget 2016, 7, 77096–77109. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.; Dulchavsky, S.A.; Gautam, S.C. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J. Neurooncol. 2007, 84, 147–157. [Google Scholar] [CrossRef]
- Ryu, K.; Susa, M.; Choy, E.; Yang, C.; Hornicek, F.J.; Mankin, H.J.; Duan, Z. Oleanane triterpenoid CDDO-Me induces apoptosis in multidrug resistant osteosarcoma cells through inhibition of Stat3 pathway. BMC Cancer 2010, 10, 187. [Google Scholar] [CrossRef]
- Serritelli, E.N.; Sartini, D.; Campagna, R.; Pozzi, V.; Martin, N.I.; van Haren, M.J.; Salvolini, E.; Cecati, M.; Rubini, C.; Emanuelli, M. Targeting nicotinamide N-methyltransferase decreased aggressiveness of osteosarcoma cells. Eur. J. Clin. Investig. 2024, 54, e14185. [Google Scholar] [CrossRef]
- Zou, W.; Liu, X.; Yue, P.; Zhou, Z.; Sporn, M.B.; Lotan, R.; Khuri, F.R.; Sun, S.Y. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res. 2004, 64, 7570–7578. [Google Scholar] [CrossRef]
- Zou, W.; Yue, P.; Khuri, F.R.; Sun, S.Y. Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res. 2008, 68, 7484–7492. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, S.; Liu, X.; Yue, P.; Sporn, M.B.; Khuri, F.R.; Sun, S.Y. c-FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl-2-cyano-3, 12-dioxooleana-1, 9-dien-28-oate (CDDO-Me) in human lung cancer cells. Cancer Biol. Ther. 2007, 6, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.; Campagna, R.; Notarstefano, V.; Pozzi, V.; Orilisi, G.; Pompei, V.; Togni, L.; Mascitti, M.; Sartini, D.; Giorgini, E.; et al. Deepening Cisplatin sensitivity on Oral Squamous cell Carcinoma cell lines after PON2 knockdown: A FTIRM investigation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 330, 125726. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pozzi, V.; Salvucci, A.; Togni, L.; Mascitti, M.; Sartini, D.; Salvolini, E.; Santarelli, A.; Lo Muzio, L.; Emanuelli, M. Paraoxonase-2 expression in oral squamous cell carcinoma. Hum. Cell 2023, 36, 1211–1213. [Google Scholar] [CrossRef]
- Campagna, R.; Belloni, A.; Pozzi, V.; Salvucci, A.; Notarstefano, V.; Togni, L.; Mascitti, M.; Sartini, D.; Giorgini, E.; Salvolini, E.; et al. Role Played by Paraoxonase-2 Enzyme in Cell Viability, Proliferation and Sensitivity to Chemotherapy of Oral Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2023, 24, 338. [Google Scholar] [CrossRef]
- Sartini, D.; Campagna, R.; Lucarini, G.; Pompei, V.; Salvolini, E.; Mattioli-Belmonte, M.; Molinelli, E.; Brisigotti, V.; Campanati, A.; Bacchetti, T.; et al. Differential immunohistochemical expression of paraoxonase-2 in actinic keratosis and squamous cell carcinoma. Hum. Cell 2021, 34, 1929–1931. [Google Scholar] [CrossRef]
- Hermann, C.; Lang, S.; Popp, T.; Hafner, S.; Steinritz, D.; Rump, A.; Port, M.; Eder, S. Bardoxolone-Methyl (CDDO-Me) Impairs Tumor Growth and Induces Radiosensitization of Oral Squamous Cell Carcinoma Cells. Front. Pharmacol. 2020, 11, 607580. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, Y.X.; Zhao, R.; Pan, S.T.; Zhe, H.; He, Z.X.; Duan, W.; Zhang, X.; Yang, T.; Qiu, J.X.; et al. Bardoxolone methyl induces apoptosis and autophagy and inhibits epithelial-to-mesenchymal transition and stemness in esophageal squamous cancer cells. Drug Des. Dev. Ther. 2015, 9, 993–1026. [Google Scholar] [CrossRef]
- Nagaraj, S.; Youn, J.I.; Weber, H.; Iclozan, C.; Lu, L.; Cotter, M.J.; Meyer, C.; Becerra, C.R.; Fishman, M.; Antonia, S.; et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin. Cancer Res. 2010, 16, 1812–1823. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010, 59, 1593–1600. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
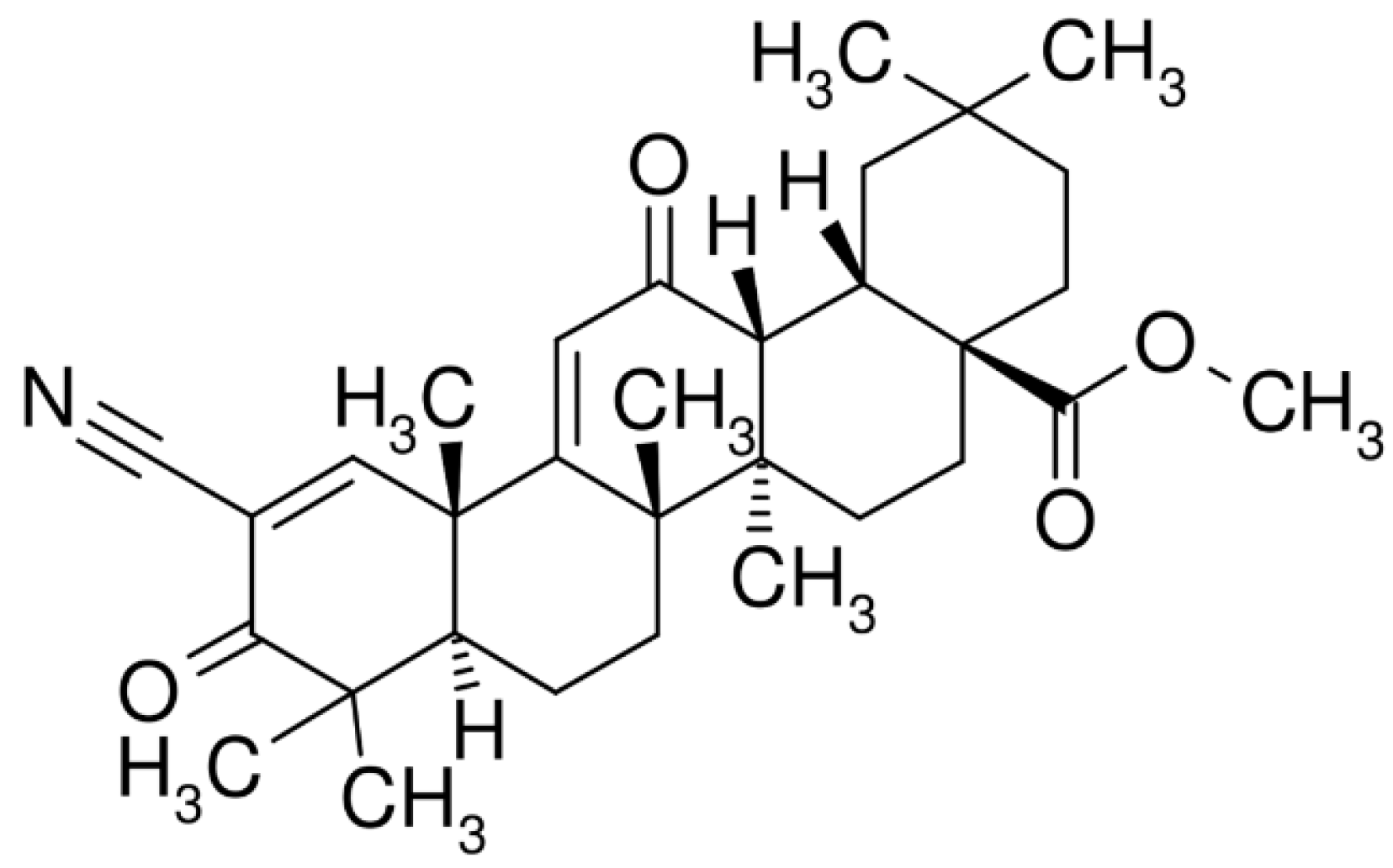

| Cancer Type | In Vivo/In Vitro | Anticancer Effects | Reference |
|---|---|---|---|
| Lung adenocarcinoma | In vivo (A/J mice + vinyl carbamate) | ↓ 92% average tumor burden; ↓ number/size of tumors; ↓ high-grade tumors (1 vs. 36% in control) | [64] |
| Breast cancer (ER-) | In vivo (MMTV-neu mice) | Delayed the onset of ER- tumors | [66] |
| Pancreatic cancer (KPC mouse model) | In vivo | ↑ survival of mice by 3 to 4 weeks | [67] |
| Breast cancer (ER-) | In vivo | Delayed the initiation of tumor formation; ↑ overall survival by 5.2 weeks; ↓ tumor-associated macrophages into the mammary tissue; ↓ CXCL12 and CCL2 in the primary tumor cells and MMP9; ↓ proliferation, ↓ cyclin D1 expression; ↓ EGFR and STAT3 phosphorylation | [69] |
| Breast cancer (BRCA1-/p53+/−) | In vivo | Delayed tumor development; ↓ phosphorylation of ErbB2; ↓ cell proliferation; induced a G0/G1 cell cycle arrest | [70] |
| Chemoresistant breast cancer | In vitro/in vivo | In vitro: ↓ STAT3, Src, Akt, c-Myc levels; arrest cell cycle in G2-M phase; ↓ invasive growth of 4T1 cells no induction of apoptosis. In vivo: ↓ tumor progression | [71] |
| Breast cancer | In vitro | Induced extensive vacuolization originating from the endoplasmic reticulum triggers apoptotic death; ↑ calcium concentrations; ↑ ROS; ↓ c-FLIPL | [72] |
| Breast cancer | In vivo (PyMT mice) | ↓ IL-10 and VEGF; ↑ TNF; ↓ CD206 and CD115 markers; ↓ CD4+ T; ↑ CD8+ T cells | [76] |
| Breast and lung cancer | In vitro (γ-irradiated cells) | ↓ radiation-induced cytotoxicity; ↑ Nrf2; ↓ DNA damage | [77] |
| Breast cancer and mammary tumors | In vitro/in vivo | In vitro: ↑ lysosome-dependent ubiquitination and proteolytic degradation of LRP6 and FZD7; ↓ cytoplasmic β-catenin activation; ↓ Wnt target genes and CSC markers In vivo: impaired tumor progression; ↓ Wnt/β-catenin signaling inhibitor | [78] |
| Breast cancer | In vitro | Time-dependent decrease in cell migration; ↓ mitochondrial respiratory function; ↑ phosphorylation of AKT, DNA damage; ↓ cell proliferation. Changes in p65 phosphorylation and SOD2 expression, implying a role for non-canonical NF-κB signaling in bardoxolone methyl downstream effects | [80] |
| Prostate adenocarcinoma | In vivo (murine model) | ↓ progression of preneoplastic lesions ↓ the metastatic process; ↓ TERT production and phosphorylation | [87] |
| Prostate cancer (LNCaP, PC-3 and DU145) | In vitro | ↑ apoptosis in LNCaP and PC-3 cell lines; ↑ caspases-3, -8, and -9; ↓ anti-apoptotic proteins Bcl-2, Bcl-xL and XIAP; ↓ NF-κB signaling pathway | [88] |
| Prostate cancer | In vitro | ↓ cell growth; ↑ apoptosis; ↓ p-Akt, mTOR, and NF-κB. | [89] |
| Prostate cancer | In vitro | ↓ androgen receptors expression, cell viability, migration, and colony formation | [91] |
| Pancreatic cancer | In vitro | ↓ growth of both K-Ras mutated (MiaPaca2, Panc1, and Capan2) and wild-type K-Ras (BxPC3) pancreatic cancer cells; ↑ apoptosis; triggered the loss of mitochondrial membrane potential and the release of cytochrome C; ↓ p-Akt, NF-κB, mTOR, p-Foxo3a, p-S6K1, p-eIF-4E, and p-4E-BP1 | [92] |
| Pancreatic cancer | In vitro | ↓ cell proliferation and ↑ apoptosis in MiaPaCa-2 and Panc-1 pancreatic cancer cells; ↓ p-Akt, p-mTOR, and NF-κB; ↑ Erk1/2 | [95] |
| Pancreatic cancer | In vitro | ↓ hTERT gene expression; ↓ hTERT telomerase activity; and modulation of several proteins involved in regulating hTERT expression and activity | [94] |
| Pancreatic cancer | In vitro | ↑ hydrogen peroxide and superoxide anions; ↓ telomerase activity | [95] |
| Pancreatic cancer | In vivo (orthotopic pancreatic cancer model) | ↓ pancreatic tumors size; ↓ expression of Sp1, Sp3, and Sp4 proteins; ↓ VEGF, cyclin D1, and surviving | [96] |
| Pancreatic ductal adenocarcinoma | In vitro/in vivo (heterotopic (subcutaneous) and orthotopic (pancreatic tail) xenograft models) | In vitro: ↓ inhibition of cell proliferation; ↑ apoptosis; ↓ p-Akt, NF-κB, and p-mTOR. In vivo: downregulation of key survival signaling mediators, including p-Akt, NF-κB, and p-mTOR In vivo: ↓tumor volume, expression of p-Akt and p-mTOR within the tumor microenvironment; ↑ survival | [97] |
| Colorectal cancer | In vitro | ↓ cell growth and viability; ↑ apoptosis; cleavage of PARP-1; ↑ caspases-3, -8, and -9, and mitochondrial depolarization; ↓ Akt, NF-κB, and mTOR, NF-κB, Bcl-2, Bcl-xL, Bad, and survivin | [100] |
| Colorectal cancer | In vitro | ↑ ROS generation | [101] |
| Ovarian cancer | In vitro | ↓ growth of ovarian cancer cells; ↑ apoptosis; ↓ p-AKT, NF-κB, and p-mTOR | [103] |
| Ovarian cancer | In vitro | ↑ ROS generation | [104] |
| Ovarian and breast cancer | In vitro | ↓ IL-6 secretion in paclitaxel-resistant ovarian cancer cells; ↓ nuclear translocation of Stat3 induced by IL-6 or oncostatin; ↓ phosphorylation levels of Stat3, Jak2, and Src; ↓ Bcl-X(L), survivin, and Mcl-1, ↑ cleavage of PARP and the release of its fragments; ↑ cytotoxic effects of chemotherapeutics paclitaxel | [105] |
| Ovarian cancer | In vitro | ↓ USP7; ↓ MDM2, MDMX, and UHRF1 | [106] |
| Glioblastoma and neuroblastoma | In vitro | ↓ cell proliferation; ↑ caspases-3, -8, and -9; mitochondrial depolarization; release of cytochrome c from mitochondria; ↓ p-AKT, NF-κB, and Notch1 | [107] |
| Osteosarcoma | In vitro | ↓ STAT3 pathway, ↓ p-STAT3, Bcl-XL, Survivin, MCL-1 expression; apoptosis induction; ↑ doxorubicin cytotoxicity | [108] |
| Lung Cancer | In vitro | Activation of DR5 via the JNK-CHOP pathway, caspase-8 activation, ER stress induction, c-FLIP degradation, enhanced TRAIL-induced apoptosis | [110,111,112] |
| Colon Carcinoma (MC38), Lung (LLC), Thymoma (EL-4) | In vivo (mouse models) | Reversal of MDSC-mediated immunosuppression, reduction in ROS, enhanced immune response, tumor growth inhibition | [119,120] |
| Pancreatic adenocarcinoma | In vivo (clinical samples) | Enhanced immune response in combination with gemcitabine, without affecting MDSC counts, well tolerated in patients | [119] |
| Mantle Cell Lymphoma, Anaplastic Thyroid Carcinoma | In vivo (Phase I trial) | One complete response (mantle cell lymphoma), one partial (thyroid carcinoma), reduced NF-κB and cyclin D1, increased NQO1 mRNA (Nrf2 activation), elevated eGFR | [18] |
| Oral Squamous Cell Carcinoma (OSCC) | In vitro | Cytotoxic and radiosensitizing effects, redox disruption, selective toxicity to cancer vs. normal cells, suppressed proliferation and clonogenic survival | [117] |
| Esophageal Squamous Cell Carcinoma (ESCC) | In vitro | G2/M arrest (↑ p21, p53), apoptosis (↑ Bax, ↓ Bcl-2/Bcl-xL), caspase-9 and PARP cleavage, autophagy via PI3K/mTOR inhibition, Nrf2 activation, EMT and stemness suppression | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavoni, V.; Di Crescenzo, T.; Membrino, V.; Alia, S.; Fantone, S.; Salvolini, E.; Vignini, A. Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications. Pharmaceuticals 2025, 18, 966. https://doi.org/10.3390/ph18070966
Schiavoni V, Di Crescenzo T, Membrino V, Alia S, Fantone S, Salvolini E, Vignini A. Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications. Pharmaceuticals. 2025; 18(7):966. https://doi.org/10.3390/ph18070966
Chicago/Turabian StyleSchiavoni, Valentina, Tiziana Di Crescenzo, Valentina Membrino, Sonila Alia, Sonia Fantone, Eleonora Salvolini, and Arianna Vignini. 2025. "Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications" Pharmaceuticals 18, no. 7: 966. https://doi.org/10.3390/ph18070966
APA StyleSchiavoni, V., Di Crescenzo, T., Membrino, V., Alia, S., Fantone, S., Salvolini, E., & Vignini, A. (2025). Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications. Pharmaceuticals, 18(7), 966. https://doi.org/10.3390/ph18070966








