Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials
Abstract
1. Introduction
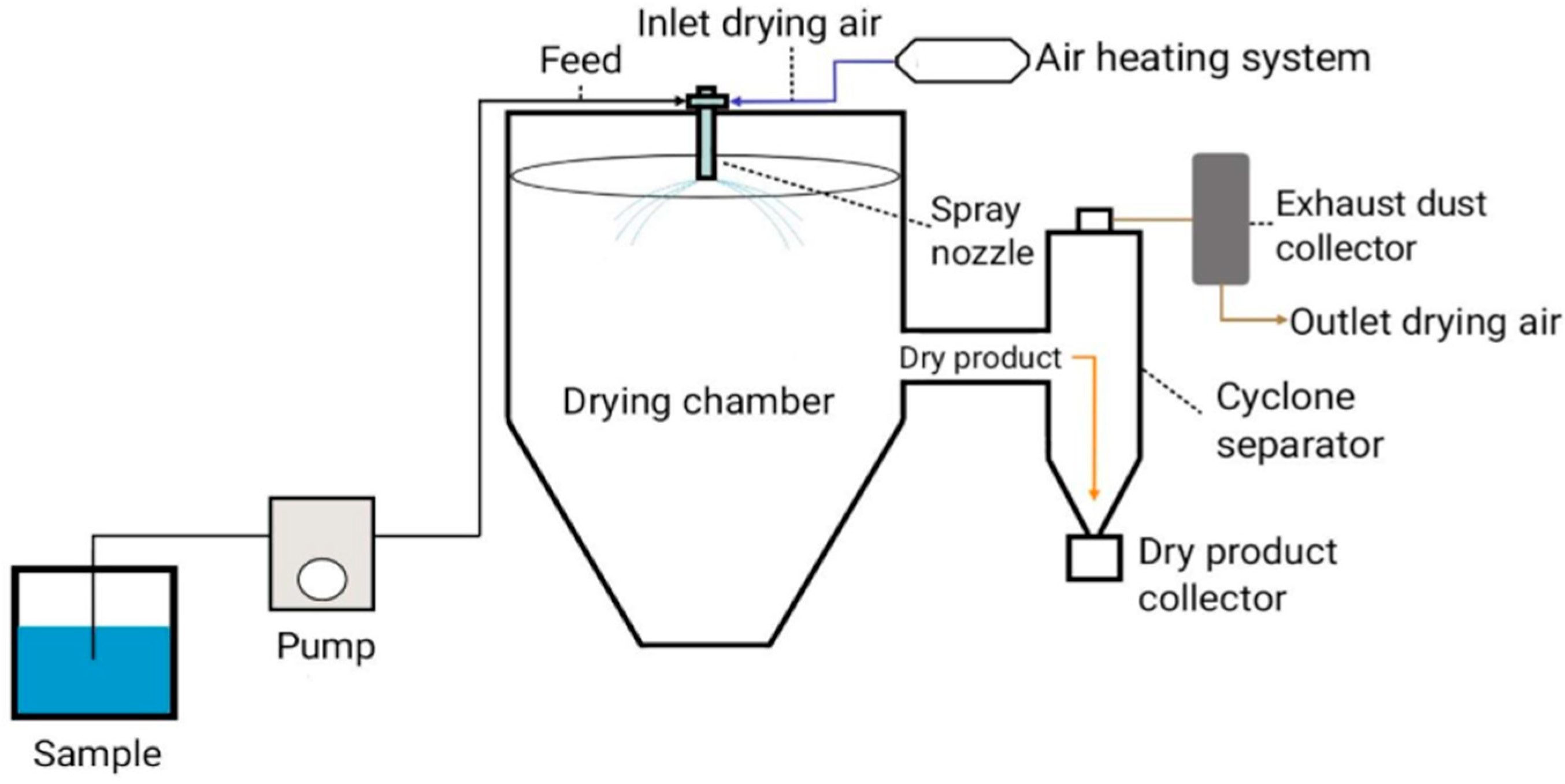
2. Mechanism of Microencapsulation by Spray-Drying
2.1. Liquid Feedstock Preparation
2.2. Atomization
2.3. Drying
2.4. Particle Collection
3. Wall Materials Used for Microencapsulation by Spray-Drying
3.1. Polysaccharides
3.2. Proteins
3.3. Innovative Wall Materials for Spray-Dying Microencapsulation
3.4. Fibers
3.4.1. Wood Hemicelluloses
3.4.2. Orange Waste Fiber
3.4.3. Yeast
3.4.4. Canola Protein Isolate
3.4.5. Pea Protein Isolate
4. Microencapsulated Substances
4.1. Phytochemicals
| No. | Ingredient(s) | Wall Materials, Ratio | Processing Parameters | Encapsulation Efficiency, % | Benefits of Encapsulated Ingredients (Increase Solubility, Antioxidant Activity, etc.) | References |
|---|---|---|---|---|---|---|
| 1. | Red cabbage (Brassica oleracea) anthocyanin-rich extract | Maltodextrin and Arabic gum, with citric acid. Different ratios were used; the best ratio was the one with the highest maltodextrin content: 25:25 (+1% of citric acid) | 8 mL/min Tinlet = 130 ± 2 °C | The microparticles yield was higher than 40%. | The moisture content in microparticles varied between 6.25 and 16.42%, and the water activity ranged from 0.48 to 0.64, which is a positive value for future food application (not confirmed in other articles). The microparticles presented a uniform appearance and a homogenous size distribution, with spherical shapes and smooth surfaces. | [49] |
| 2. | Barberry (Berberis vulgaris) extract, rich in anthocyanins | Combination of gum Arabic and maltodextrin (3:1 most effective), maltodextrin and gelatin (3:1), and only maltodextrin. The ratio between the extract solid content and the wall material was 1:4 | Tinlet = 150 °C Toutlet = 100 °C | Efficiency: About 93% for Arabic gum with maltodextrin and 91% for others, according to the presented graph. Encapsulation efficiency of maltodextrin: Gelatin was significantly lower than maltodextrin/gum Arabic (p < 0.05). | Colorant has higher efficiency and the longest anthocyanin stability under all conditions evaluated. Formulating a jelly using the encapsulated anthocyanin color at the level of 7% was achieved with acceptable sensory attributes and physicochemical evaluations. | [50] |
| 3. | Extracts of Hibiscus sabdariffa calyces with natural red–purple pigment and antioxidant properties | Different concentrations of mesquite gum (100:1–100:5 v/w) | Tinlet = 180 ± 2 °C Toutlet = 100 ± 2.3 °C | The highest yield was 74.9 ± 4.3% when there was 5% mesquite gum. | Powders solubilize easily in water or in aqueous ethanol. Good storage, physicochemical, and antioxidant properties (kept almost constant in different conditions at room T for 1 year). | [51] |
| 4. | Renealmia alpinia (Rottb.) Maas fruit pericarp extract. Potential purple colorant | Maltodextrin, gum Arabic, and a 1:1 mixture of both | Tinlet = 150 ± 2 °C Toutlet = 98 ± 2 °C | Maltodextrin/Arabic gum yield (21.58%), only Arabic gum (19.47%), and Maltodextrin only (18.59%). Maltodextrin/gum Arabic coating significantly (p < 0.05) increased the yield when compared with gum Arabic and maltodextrin only microencapsulates. | The mixture with MDX:GA showed lower humidity content and the highest yield of powders. However, GA was better as an encapsulating material for the conservation of anthocyanins and phenolic compounds; MDX showed superior coating capacity in encapsulates stored at 4 °C. It improved and preserved storage and antioxidant properties. | [52] |
| 5. | Pomegranate juice powder | Maltodextrin (25, 35, and 45% w/w) | 0.5–1.5 kg/h Tinlet 123–143 °C Toutlet 48–76 °C | The production yield of pomegranate juice powder was between 17 and 25%, with higher maltodextrin rates and temperatures leading to higher yields. | Affected density, anthocyanin level, and antioxidant properties; produced larger particles; and improved stability. | [53] |
| 6. | Antioxidant-rich blueberry waste extracts | Sodium alginate (3% w/w) or inulin (3% w/w) | 12 mL/min Tinlet = 150 ± 1 °C Toutlet = 80 ± 2 °C | The yields of “BWM-Alginate” and “BWM-Inulin” powders were 64∼72 and 60~68%, respectively. | The product generated from BWM had desirable color, water activity, and reconstitution properties in water or milk and can be used as a food colorant and supplement. Compared with inulin, alginate gave a greater powder yield, greater Bifidobacterium-boosting effects, better protection of antioxidants during spray-drying, and prolonged storage at 20 or 38 °C. | [54] |
| 7. | Cabernet Sauvignon and Bordeaux grape pomace extracts | Brewery waste yeast Saccharomyces cerevisiae (5% (w/w) of dry yeast) | Tinlet = 130 °C Toutlet = 80 °C | Yeasts were proven to be a great wall material for the encapsulation of bioactive compounds by spray-drying. It was possible to obtain powders with characteristics that enhance the shelf-life of the product (1 year). Bordeaux grape pomace extracts are better as a colorant. | [55] | |
| 8. | Elderberry (Sambucus nigra) extract | Maltodextrin–β-glucan (0.5, 1, 2, and 3%) and control/maltodextrin and Arabic gum (92.5:7.5) | The flow of the pump was 25%. Tinlet = 140 °C | 3% BG—77.97% ± 2.350; control sample—80.45% ± 1.3855. The highest encapsulation efficiency was achieved with the powder containing the lowest ratio of maltodextrin/β-glucan, reaching around 93.9% ± 2.717 compared to other maltodextrin/β-glucan ratios tested (p < 0.05). | The 0.5% β-glucan ratio is recommended for more efficient microencapsulation due to the encapsulation efficiency, storage loss, ascorbic acid, and anthocyanin total content characteristics. However, while the higher content of maltodextrin or Arabic gum is undesirable, the higher β-glucan content as its replacement is favorable due to its health-beneficial effect on the human body. | [56] |
| 9. | Blue maize (Zea mays) polyphenols | Maltodextrin (120 g/L total solids) or maltodextrin/pectin (120 g/L total solids, 84/16 w/w) | Tinlet = 150 ± 1 °C Toutlet = 80 ± 1 °C | 80.61 ± 1.32 for maltodextrin and 65.76 ± 0.19 for the maltodextrin–pectin combination. The maltodextrin particles had a higher yield, as compared to maltodextrin–pectin particles (p < 0.05). | The combined matrix showed better protection during storage, with a significantly higher half-life and antioxidant activity. The release of phenolics after in vitro digestion was nearly complete from both matrices; the combined matrix favored intestinal release but less absorption. | [57] |
| 10. | Anthocyanins from chokeberry (Aronia melanocarpa) | 92.5% maltodextrin and 7.5% guar gum, gum Arabic, pectin, β-glucan, or inulin | The flow of the pump was 25%. Tinlet = 140 °C | Efficiency varied from 78.61% for Maltodextrin + Arabic gum to 92.98% for Maltodextrin + guar gum. GG showed the highest efficiency of encapsulation. The lowest efficiency was obtained with microcapsules coated by maltodextrin and gum Arabic compared to the other groups (p < 0.05). | The particles of the GG powder were the smallest, preferably distributed, and of uniform size and shape. These features directly influenced the highest solubility of this preparation among all powders. Guar gum powder also had the best protecting properties. | [58] |
4.2. Oils
| No. | Ingredient(s) | Wall Materials, Ratio | Processing Parameters | Encapsulation Efficiency, % | Benefits of Encapsulated Ingredients (Increase Solubility, Antioxidant Activity, etc.) | References |
|---|---|---|---|---|---|---|
| 1. | Citronella (Cymbopogon winterianus) essential oil | Gum Arabic, maltodextrin (40%), and whey protein concentrate (60%) | Tinlet = 120 °C Toutlet = 65–70 °C | 53–100% | Enhanced thermal stability, improved oxidative stability, and enabled controlled release | [63] |
| 2. | Persian lime (Citrus latifolia) essential oil | Maltodextrin (20–35%) | 120–300 mL/h Tinlet 120–180 °C | 56–87% The encapsulation efficiency exhibited an increasing trend with the elevation of the maltodextrin concentration from 20% to 30% (p < 0.05). | Improved antimicrobial and thermal stability and the release of active substances | [64] |
| 3. | Cardamom (Elletaria cardamomum) essential oil | Skim milk (10, 20, and 30%); modified starch (10, 20, and 30%) | 10 mL/min Tinlet = 180 ± 10 °C Toutlet = 90 ± 10 °C | 79–95% | Modified the size of particles and improved the release of active substances | [65] |
| 4. | Cinnamon essential oil | Maltodextrin (15.75%) and whey protein isolate (7%) | Tinlet = 180 °C Toutlet = 90 °C | 84–89% The encapsulation efficiency increased with decreasing whey protein isolate/maltodextrin ratios (p < 0.05). | Demonstrated strong protection with the optimized formulation during storage | [66] |
| 5. | Pomelo (Citrus grandis (L.) Osbeck) essential oil | Maltodextrin (20–35%) | 120 mL/h Tinlet = 140 °C | 89.44% The effect of maltodextrin concentrations on encapsulation efficiency was statistically significant (p < 0.05). | Improved thermal stability and higher amount of components | [67] |
| 6. | Corn mint (Mentha arvensis L.) essential oil | Maltodextrin (20–30%) | 4–10 mL/min Tinlet 130–150 °C | 68.6–98.9% Encapsulation efficiency increased with increasing maltodextrin concentrations (p < 0.05). | Higher amount of components | [68] |
| 7. | Lavender essential oil | Gum acacia, sodium caseinate, gelatin, chitosan, β-cyclodextrin, and polyvinyl alcohol (1:1) | Tinlet = 180 °C Toutlet = 80 ± 2 °C | 20–65% The encapsulation efficiency of lavender oil significantly improved when gelatin was used as a carrier material during spray-drying with gum acacia and sodium caseinate, increasing from 28.6% to 65.9% (p < 0.05). | Combinations of different wall materials would change the retention and release of essential oil and modify the surface morphology of microcapsules | [69] |
| 8. | Ginger (Zingiber officinale) essential oil | Gum Arabic (20%) and inulin (20%) | 0.8 L/h Tinlet = 160 °C Toutlet = 68 °C | 9.46–35.69% | Improving the retention of bioactive compounds | [70] |
| 9. | Basil (Ocimum basilicum L.) essential oil | Sodium alginate with sodium caseinate and maltodextrin (1:2 and 1:1) | Tinlet = 140 °C Toutlet = 70 °C | 43–78% The encapsulation efficiency increased with increasing concentrations of sodium alginate in the mixture with maltodextrin (p < 0.05). | Increases solubility and release and improves encapsulation efficiency and morphological characteristics | [71] |
| 10. | Clove (Syzigium aromaticum) essential oil | Casein (4.65%) | Tinlet 110–120 °C Toutlet 55–65 °C | 97.78% | Improves encapsulation efficiency and antibacterial activity | [72] |
4.3. Vitamins
4.3.1. Water-Soluble Vitamins
4.3.2. Fat-Soluble Vitamins
| No. | Ingredient(s) | Wall Materials | Processing Parameters | Encapsulation Efficiency, % | Benefits of Encapsulated Ingredients (Increase Solubility, Antioxidant Activity, etc.) | References |
|---|---|---|---|---|---|---|
| 1. | Vitamin D3 | Maltodextrin (25%), modified starch (3%), and whey protein (2%) | Tinlet = 170 °C Toutlet = ±80 °C | Maltodextrin/modified starch/whey protein yield 96.4% | Improved stability and bioavailability. An increase in maltodextrin concentration in the feed solution led to the formation of smoother and more spherical spray-dried powder particles. | [78] |
| 2. | Vitamin E | Whey protein (1:3) | 4 mL/min Tinlet = 100 °C Toutlet = 80 °C | 89.6 ± 2.5% | Improved stability and bioavailability. | [16] |
| 3. | Vitamin A | Gum Arabic, maltodextrin, and starch (15%) | 4 mL/min Tinlet = 150 °C Toutlet = 80 °C | 88–98% | Improved stability and bioavailability, extending the release time of vitamin A. | [79] |
| 4. | Vitamin B1 | Chitosan and ferulic acid (1:1) | 10 mL/min Tinlet = 140 °C Toutlet = 77 °C | 91 ± 2.31% | Vitamin B1-loaded microspheres showed potential anti-inflammatory activity via the inhibition of carrageenan-induced paw edema in albino rats. | [73] |
| 5. | Vitamin B6 | Chitosan and ferulic acid (1:1) | 10 mL/min Tinlet = 140 °C Toutlet = 77 °C | 83 ± 3.17% | Vitamin B6-loaded microspheres showed potential anti-inflammatory activity via the inhibition of carrageenan-induced paw edema in albino rats. | [73] |
| 6. | Folic acid | Maltodextrin (40%) | 1.5 L/h Tinlet = 194.2 °C Toutlet = 87.7 °C | 90.9 ± 1.8% | Improved stability and bioavailability. | [80] |
| 7. | Vitamin B12 | Zein (20%) | 4 mL/min Tinlet = 90 °C Toutlet ± 50 °C | 82.3% | Improved stability, bioavailability, and release profiles of vitamin B12. | [81] |
| 8. | Vitamin C | Sodium alginate (3.5%) | Tinlet = 110 °C Toutlet = 65 °C | 93.48% | Alginate-based microparticles acted as a protective barrier, effectively preventing vitamin C from degrading or being lost during the 30-day storage period. After 30 days of storage, there was no statistically significant difference in encapsulation efficiency (p < 0.05). | [82] |
4.4. Controlled Release Systems
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Research and Markets. Available online: https://www.researchandmarkets.com/reports/3972885/bioactive-ingredients-market-size-share-and?srsltid=AfmBOopzI5qtUqaY-wydakkcE14MZEuCdQUziA--FSL5A6ZKzYKVV6ty (accessed on 12 June 2025).
- Grand View Research. Available online: https://www.grandviewresearch.com/horizon/outlook/bioactive-ingredients-market-size/global (accessed on 12 June 2025).
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of Anthocyanins—Critical Review of Techniques and Wall Materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, X.; Shen, J.; Li Zongze Tan, S.; Liu, W.; Cheng, Z. Choosing the Appropriate Wall Materials for Spray-Drying Microencapsulation of Natural Bioactive Ingredients: Taking Phenolic Compounds as Examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry, 2nd ed.; Pignatello, R., Musumeci, T., Eds.; Intechopen: London, UK, 2018; Volume 2, pp. 9–36. [Google Scholar]
- Szczap, J.; Jacobs, I. Atomization and spray drying processes. In Microencapsulation in the Food Industry: A Practical Implementation Guide, 2nd ed.; Sobel, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 6, pp. 59–71. [Google Scholar]
- Estrada-Cervantes, L.B.; Dublan-Garcia, O.; Rojas-Rivas, E.; Perea-Flores, M.J.; Velazquez, G.; Hernandez-Jabalera, A.; Guadarrama-Lezama, A.Y. Analysis of glycolysis, proteolysis and bioactive compounds during in vitro gastrointestinal digestion of blackberry juice microcapsules. Food Meas. 2025, 19, 3162–3178. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Kumar, L.R.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104 Pt B, 1986–1995. [Google Scholar] [CrossRef]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-Based Bionanocomposite Films Prepared by Emulsion Technique for Food Preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef]
- Afzal, A.; Afzaal, M.; Saeed, F.; Shah, Y.A.; Raza, M.A.; Khan, M.H.; Asghar, A.; Akram, N.; Ateeq, H.; Teferi Asres, D. Milk protein based encapsulation of probiotics and other food material: Comprehensive review. Int. J. Food Prop. 2018, 27, 245–262. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Anandharamakrishnan, C. Enhancement of oral bioavailability of vitamin E by spray-freeze drying of whey protein microcapsules. Food Bioprod. Process. 2016, 100, 469–476. [Google Scholar] [CrossRef]
- Ho, T.M.; Lehtonen, M.; Räikkönen, H.; Kilpeläinen, P.O.; Mikkonen, K.S. Wood hemicelluloses as effective wall materials for spray-dried microcapsulation of polyunsaturated fatty acid-rich oils. Food Res. Int. 2023, 164, 112333. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Wood Hemicelluloses as Innovative Wall Materials for Spray-Dried Microencapsulation of Berry Juice: Part 1—Effect of Homogenization Techniques on their Feed Solution Properties. Food Bioprocess Technol. 2023, 16, 909–929. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M. Encapsulation of pomegranate peel extract with a new carrier material from orange juice by-products. J. Food Eng. 2019, 253, 1–13. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Saturno, R.P.; Marson, G.V.; Hubinger, M.D. Spent brewer’s yeast proteins and cell debris as innovative emulsifiers and carrier materials for edible oil microencapsulation. Food Res. Int. 2021, 140, 109853. [Google Scholar] [CrossRef]
- Sultana, A.; Miyamoto, A.; Lan Hy, Q.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Microencapsulation of flavors by spray drying using Saccharomyces cerevisiae. J. Food Eng. 2017, 199, 36–41. [Google Scholar] [CrossRef]
- Aktaş, H.; Custodio-Mendoza, J.A.; Moczkowska, M.; Szpicer, A. The role of canola, black caraway, and wheat bran protein isolates in anthocyanin microencapsulation via double emulsions. Ind. Crops Prod. 2024, 222, 119644. [Google Scholar] [CrossRef]
- Cháirez-Jiménez, C.; Castro-López, C.; Serna-Saldívar, S.; Chuck-Hernández, C. Partial characterization of canola (Brassica napus L.) protein isolates as affected by extraction and purification methods. Heliyon 2023, 9, e21938. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Sanz, T.; Beltrán, S. Microencapsulation of rice bran oil using pea protein and maltodextrin mixtures as wall material. Heliyon 2020, 6, e03615. [Google Scholar] [CrossRef] [PubMed]
- Niño-Vásquez, I.A.; Muñiz-Márquez, D.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C. Co-microencapsulation: A promising multi-approach technique for enhancement of functional properties. Bioengineered 2022, 13, 5168–5189. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef]
- Busto, M.D.; González-Temiño, Y.; Albillos, S.M.; Ramos-Gómez, S.; Pilar-Izquierdo, M.C.; Palacios, D.; Ortega, N. Microencapsulation of a Commercial Food-Grade Protease by Spray Drying in Cross-Linked Chitosan Particles. Foods 2022, 11, 2077. [Google Scholar] [CrossRef]
- Vijeth, S.B.; Heggannavar, G.; Kariduraganavar, M.Y. Encapsulating Wall Materials for Micro/Nanocapsules. In Microencapsulation—Processes, Technologies and Industrial Applications, 1st ed.; Salaün, F., Ed.; IntechOpen: London, UK, 2018; Volume 1, pp. 3–22. [Google Scholar]
- Negro, V.; Ruggeri, B.; Fino, D.; Tonini, D. Life cycle assessment of orange peel waste management. Resour. Conserv. Recycl. 2017, 127, 148–158. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Lionetto, F.; Nisi, R.; Stoppa, M.; Licciulli, A. Sustainable Production of Stiff and Crystalline Bacterial Cellulose from Orange Peel Extract. Sustainability 2022, 14, 2247. [Google Scholar] [CrossRef]
- Bigi, F.; Maurizzi, E.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Waste Orange Peels as a Source of Cellulose Nanocrystals and Their Use for the Development of Nanocomposite Films. Foods 2023, 12, 960. [Google Scholar] [CrossRef]
- Kobayashi, Y.; El-Wali, M.; Guðmundsson, H.; Guðmundsdóttir, E.E.; Friðjónsson, Ó.H.; Karlsson, E.N.; Roitto, M.; Tuomisto, H.L. Life-cycle assessment of yeast-based single-cell protein production with oat processing side-stream. Sci. Total Environ. 2023, 873, 162318. [Google Scholar] [CrossRef]
- Fernandes, C.; Gurgel, L.V.A.; Baêta, B.E.L. Comparative life cycle assessment of early-stage technological layouts for brewers’ spent grain upcycling: A sustainable approach for adding value to waste. J. Water Process Eng. 2024, 66, 105904. [Google Scholar] [CrossRef]
- Halahlah, A.; Räikkönen, H.; Piironen, V.; Valoppi, F.; Mikkonen, K.S.; Ho, T.M. Wood hemicelluloses as sustainable wall materials to protect bioactive compounds during spray drying of bilberries. Powder Technol. 2023, 415, 118148. [Google Scholar] [CrossRef]
- Utama, G.L.; Oktaviani, L.; Balia, R.L.; Rialita, T. Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers 2023, 15, 3481. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, X.; He, R.; Yuan, J.; Aluko, R.E. Effect of high pressure treatment on rapeseed protein microparticle properties and gastrointestinal release behavior of the encapsulated peptides. Food Res. Int. 2015, 77, 549–555. [Google Scholar] [CrossRef]
- Chen, F.P.; Liu, L.L.; Tang, C.H. Spray-drying microencapsulation of curcumin nanocomplexes with soy protein isolate: Encapsulation, water dispersion, bioaccessibility and bioactivities of curcumin. Food Hydrocoll. 2020, 105, 105821. [Google Scholar] [CrossRef]
- Linke, A.; Hinrichs, J.; Kohlus, R. Impact of the oil droplet size on the oxidative stability of microencapsulated oil. J. Microencapsul. 2020, 37, 170–181. [Google Scholar] [CrossRef]
- Tambade, P.B.; Sharma, M.; Singh, A.K.; Surendranath, B. Flaxseed Oil Microcapsules Prepared Using Soy Protein Isolate and Modified Starch: Process Optimization, Characterization and In Vitro Release Behaviour. Agric. Res. 2020, 9, 652–662. [Google Scholar] [CrossRef]
- Le Priol, L.; Dagmey, A.; Morandat, S.; Saleh, K.; El Kirat, K.; Nesterenko, A. Comparative study of plant protein extracts as wall materials for the improvement of the oxidative stability of sunflower oil by microencapsulation. Food Hydrocoll. 2019, 95, 105–115. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Zanoni, F.; Primiterra, M.; Angeli, N.; Zoccatelli, G. Microencapsulation by spray-drying of polyphenols extracted from red chicory and red cabbage: Effects on stability and color properties. Food Chem. 2020, 307, 125535. [Google Scholar] [CrossRef]
- Cegledi, E.; Garofulić, I.E.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. [Google Scholar] [CrossRef] [PubMed]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Antioxidant activities of spray-dried carotenoids using maltodextrin-Arabic gum as wall materials. Bull. Natl. Res. Cent. 2021, 45, 58. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. A Comparative Study of Encapsulation of β-Carotene via Spray-Drying and Freeze-Drying Techniques Using Pullulan and Whey Protein Isolate as Wall Material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Córdoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef]
- Machado, M.H.; da Rosa Almeida, A.; Maciel, M.V.D.O.B.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Salazar-González, C.; Cid-Ortega, S.; Guerrero-Beltrán, J.A. Antioxidant characteristics of extracts of Hibiscus sabdariffa calyces encapsulated with mesquite gum. J. Food Sci. Technol. 2017, 54, 1747–1756. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, O.; Ruiz-Espinosa, H.; Luna-Guevara, J.J.; Ochoa-Velasco, C.E.; Luna Vital, D.; Luna-Guevara, M.L. A potential natural coloring agent with antioxidant properties: Microencapsulates of Renealmia alpinia (Rottb.) Maas fruit pericarp. NFS J. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalenoei, M.G.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Waterhouse, G.I.; Sun-Waterhouse, D.; Su, G.; Zhao, H.; Zhao, M. Spray-Drying of Antioxidant-Rich Blueberry Waste Extracts; Interplay Between Waste Pretreatments and Spray-Drying Process. Food Bioprocess Technol. 2017, 10, 1074–1092. [Google Scholar] [CrossRef]
- Rubio, F.V.T.; Haminiuk, I.W.C.; Marterlli-Tosi, M.; da Silva, M.P.; Makinmori, G.Y.F.; Favaro-Trindade, C.S. Utilization of grape pomaces and brewery waste Saccharomyces cerevisiae for the production of bio-based microencapsulated pigments. Food Res. Int. 2020, 136, 109470. [Google Scholar] [CrossRef]
- Sobieralska, M.; Kurek, M.A. Beta-Glucan as Wall Material in Encapsulation of Elderberry (Sambucus nigra) Extract. Plant Foods Hum. Nutr. 2019, 74, 334–341. [Google Scholar] [CrossRef]
- Ruiz Canizales, J.; Heredia, J.B.; Domínguez Avila, J.A.; Madera Santana, T.J.; Villegas Ochoa, M.A.; Robles Sánchez, R.M.; González Aguilar, G.A. Microencapsulation of blue maize (Zea mays L.) polyphenols in two matrices: Their stability during storage and in vitro digestion release. Food Meas. 2019, 13, 892–900. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Qiao, Z.; Wang, X.; Tang, H.; Yang, C.; Huang, F. Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity. Foods 2022, 11, 2993. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.; Guadix, E.M. Development of Fish Oil-Loaded Microcapsules Containing Whey Protein Hydrolysate as Film-Forming Material for Fortification of Low-Fat Mayonnaise. Foods 2020, 9, 545. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Durmus, M.; Özogul, Y.; Ozyurt, G.; Ucar, Y.; Kosker, A.R.; Yazgan, H.; Ibrahim, S.A.; Özogul, F. Effects of citrus essential oils on the oxidative stability of microencapsulated fish oil by spray-drying. Front. Nutr. 2023, 9, 978130. [Google Scholar] [CrossRef]
- Duarte, P.F.; Wlodarkievicz, M.E.; Nascimento, L.H.; Puton, B.M.S.; Fischer, B.; Fernandes, I.A.; Colet, R.; Paroul, N.; Cansian, R.L.; Junges, A. Microencapsulation of Citronella Essential Oil (Cymbopogon winterianus) with Different Wall Materials Using Spray Drying. Lett. Appl. NanoBioSci. 2023, 12, 71. [Google Scholar] [CrossRef]
- Van, C.K.; Nguyen, P.T.N.; Nguyen, T.T.T.; Bach, L.G. Microencapsulation of Citrus latifolia peel essential oil by spray-drying using maltodextrin: Characterization, antimicrobial activities, and release profile. LWT 2024, 197, 115825. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Kadkhodaee, R.; Mortazavi, S.A. Effect of Drying Process and Wall Material on the Properties of Encapsulated Cardamom Oil. Food Biophys. 2011, 6, 68–76. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Chen, F.; Wan, R.; Yu, C.W.; Li, J.; McClements, D.J.; Deng, Z. Microencapsulation of an essential oil (cinnamon oil) by spray drying: Effects of wall materials and storage conditions on microcapsule properties. J. Food Process. Preserv. 2020, 44, e14805. [Google Scholar] [CrossRef]
- Nguyen, T.N.P.; Van, C.K.; Nguyen, T.T.T.; Van Tran, T.; Hoang, Q.B.; Bach, L.G. Influence of spray drying parameters on the physicochemical characteristics of microencapsulated pomelo (Citrus grandis (L.) Osbeck) essential oil. Food Sci. Biotechnol. 2020, 31, 1679–1689. [Google Scholar] [CrossRef]
- Truong, C.B.H.; Nguyen, T.K.H.; Tran, T.T.T.; Nguyen, T.N.L.; Mai, H.C. Microencapsulation of corn mint (Mentha arvensis L.) essential oil using spray-drying technology. Food Res. 2020, 6, 154–160. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Xiong, X.; Qian, M.C.; Ji, H. Preparation and release mechanism of lavender oil microcapsules with different combinations of coating materials. Flavor Fragr. J. 2019, 35, 157–166. [Google Scholar] [CrossRef]
- de Souza, H.J.B.; Dessimoni, A.L.d.A.; Ferreira, M.L.A.; Botrel, D.A.; Borges, S.V.; Viana, L.C.; Fernandes, R.V.d.B. Microparticles obtained by spray-drying technique containing ginger essential oil with the addition of cellulose nanofibrils extracted from the ginger vegetable fiber. Dry. Technol. 2020, 39, 1912–1926. [Google Scholar] [CrossRef]
- Veena Paul, A.; Rai, D.C.; Pandhi, S.; Seth, A. Effect of coating materials for microencapsulation of basil oil using spray drying. Med. Plants 2020, 12, 251–257. [Google Scholar] [CrossRef]
- Sahlan, M.; Lestari, S.F.; Indrawati, T.; Pratami, D.K.; Wijarnako, A.; Hermansyah, H.; Lischer, K.; Rabbani, A.N. Microencapsulation of clove oil using spray dry with casein encapsulator and activity test towards Streptococcus mutan. AIP Conf. Proc. 2019, 2193, 030006. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Anandan, R.; Navitha, M.; Asha, K.K.; Kumar, K.A.; Mathew, S.; Ravishankar, C.N. Development of thiamine and pyridoxine loaded ferulic acid-grafted chitosan microspheres for dietary supplementation. J. Food Sci. Technol. 2016, 53, 551–560. [Google Scholar] [CrossRef]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Lazăr, R.; Blaga, A.C.; Rocha, F.A. Preliminary evaluation and studies on the preparation, characterization and in vitro release studies of different biopolymer microparticles for controlled release of folic acid. Powder Technol. 2020, 369, 279–288. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Edtrvinho, B.N.; Rocha, F. Improvement of vitamin E microencapsulation and release using different biopolymers as encapsulating agents. Food Bioprod. Process. 2021, 130, 23–33. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Design and characterization of controlled-release vitamin A microparticles prepared by a spray-drying process. Powder Technol. 2017, 305, 411–417. [Google Scholar] [CrossRef]
- Bashir, I.; Wani, S.M.; Bhat, A.A.; Khan, A.A.; Hussain, S.Z.; Ganai, S.A.; Anjum, N. Effect of freeze drying and spray drying on physical properties, morphology and in vitro release kinetics of vitamin D3 nanoparticles. Powder Technol. 2024, 432, 119164. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch and maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Cortés, R.M.; Hernández, G.; Estrada, M.E.M. Optimization of the spray drying process for obtaining cape gooseberry powder: An innovative and promising functional food. Vitae 2017, 24, 59–67. [Google Scholar] [CrossRef]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A new approach to the production of zein microstructures with vitamin B12, by electrospinning and spray drying techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Marcela, F.; Lucía, C.; Esther, F.; Elena, M. Microencapsulation of L-Ascorbic Acid by Spray Drying Using Sodium Alginate as Wall Material. J. Encapsulation Adsorpt. Sci. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Gonciarz, W.; Brzeziński, M.; Orłowska, W.; Wawrzyniak, P.; Lewandowski, A.; Narayanan, V.H.B.; Chmiela, M. Spray-dried pH-sensitive chitosan microparticles loaded with Mycobacterium bovis BCG intended for supporting treatment of Helicobacter pylori infection. Sci. Rep. 2024, 14, 4747. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Lopes, M.V.; Rossi-Bergmann, B.; Ré, M.I.; Santos, A.M. Synthesis and characterization of poly(N-vinylcaprolactam)-based spray-dried microparticles exhibiting temperature and pH-sensitive properties for controlled release of ketoprofen. Drug Dev. Ind. Pharm. 2017, 43, 1519–1529. [Google Scholar] [CrossRef]
- Tallian, C.; Tegl, G.; Quadlbauer, L.; Vielnascher, R.; Weinberger, S.; Cremers, R.; Pellis, A.; Salari, J.W.O.; Guebitz, G.M. Lysozyme-Responsive Spray-Dried Chitosan Particles for Early Detection of Wound Infection. ACS Appl. Bio Mater. 2019, 2, 1331–1339. [Google Scholar] [CrossRef]

| Wall Material | Type | Water Solubility | Emulsifying Capacity | Film-Forming Ability | Encapsulation Efficiency | Cost | References |
|---|---|---|---|---|---|---|---|
| Maltodextrin | Polysaccharide | High | Low | Good | Moderate–High | Low | [11] |
| Gum Arabic | Polysaccharide | Very High | High | Excellent | High | Medium–High | [12] |
| Chitosan | Polysaccharide | Low–Moderate | Moderate | Good | High | Medium | [13,14] |
| Whey Protein Isolate | Protein | High | High | Good | High | Medium | [15,16] |
| Sodium Caseinate | Protein | High | High | Good | High | Medium | [15] |
| Wood Hemicelluloses | Polysaccharide | Moderate–High | Good | Moderate | High | Low | [17,18] |
| Orange Waste Fiber | Fiber | Moderate | Low | Moderate | High | Very Low | [19] |
| Yeast Cell Wall | β-glucan composite | Low–Moderate | Moderate–High | High | High | Very Low | [20,21] |
| Canola Protein Isolate | Protein | Moderate | Good | Good | High | Low | [22,23] |
| Pea Protein Isolate | Protein | Moderate | Moderate | Good | Moderate–High | Low | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudžiuvelytė, L.; Petrauskaitė, E.; Stabrauskienė, J.; Bernatonienė, J. Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals 2025, 18, 963. https://doi.org/10.3390/ph18070963
Pudžiuvelytė L, Petrauskaitė E, Stabrauskienė J, Bernatonienė J. Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals. 2025; 18(7):963. https://doi.org/10.3390/ph18070963
Chicago/Turabian StylePudžiuvelytė, Lauryna, Eglė Petrauskaitė, Jolita Stabrauskienė, and Jurga Bernatonienė. 2025. "Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials" Pharmaceuticals 18, no. 7: 963. https://doi.org/10.3390/ph18070963
APA StylePudžiuvelytė, L., Petrauskaitė, E., Stabrauskienė, J., & Bernatonienė, J. (2025). Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals, 18(7), 963. https://doi.org/10.3390/ph18070963









