Antimalarial Drug Repurposing of Epirubicin and Pelitinib in Combination with Artemether and Lumefantrine
Abstract
1. Introduction
2. Results
- i.
- Synergism (FIC50 < 1) is where one drug potentiates the activity of another drug, leading to a greater combined effect of the two drugs than when used alone.
- ii.
- Additivity (FIC50 = 1) is where the combined effect of the two drugs is the same as the effect of the individual drugs when used alone.
- iii.
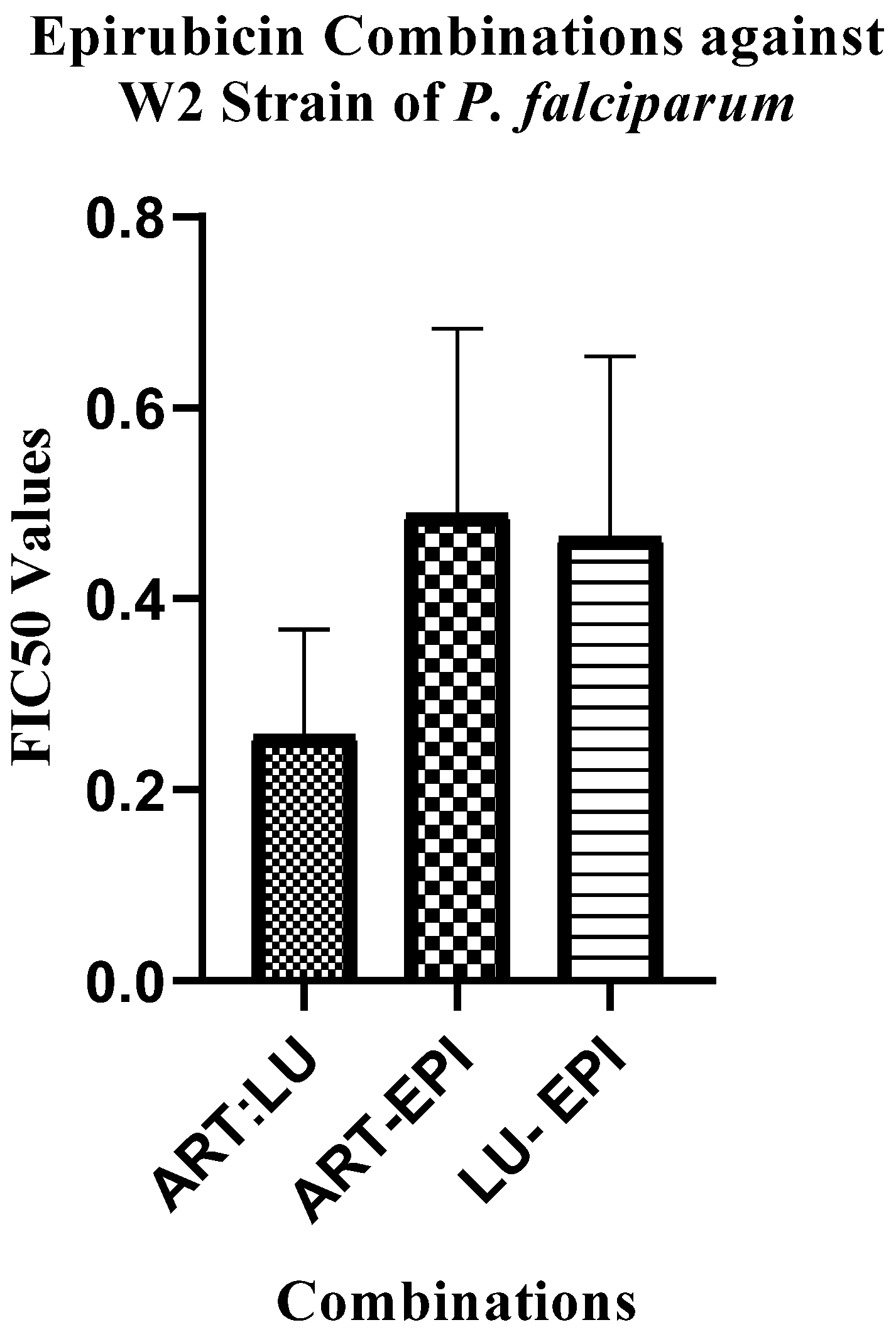
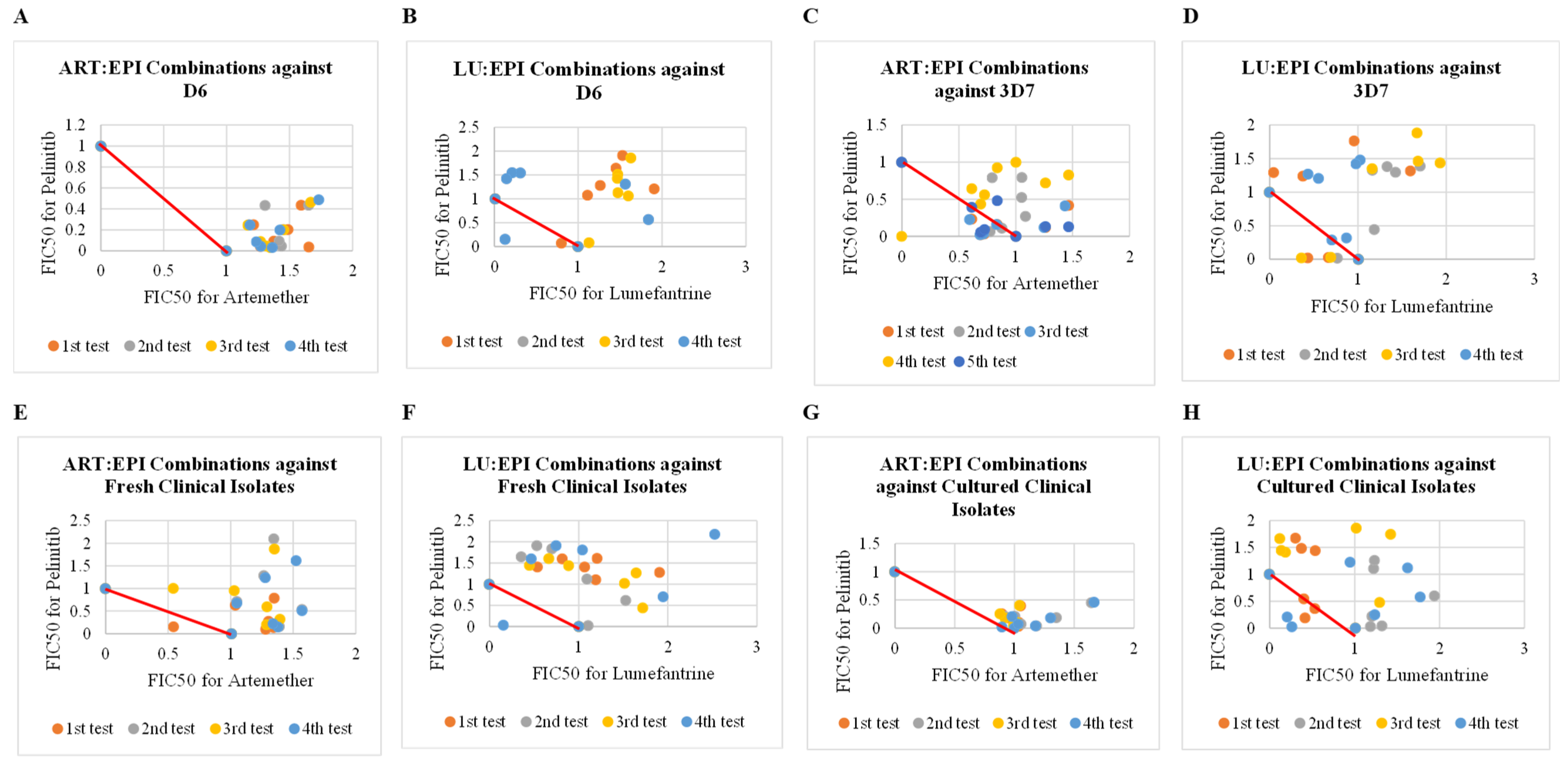
2.1. Antiplasmodial Activities of Artemether and Lumefantrine Each Combined with Epirubicin
2.2. Antiplasmodial Activities of Artemether and Lumefantrine Each Combined with Pelitinib
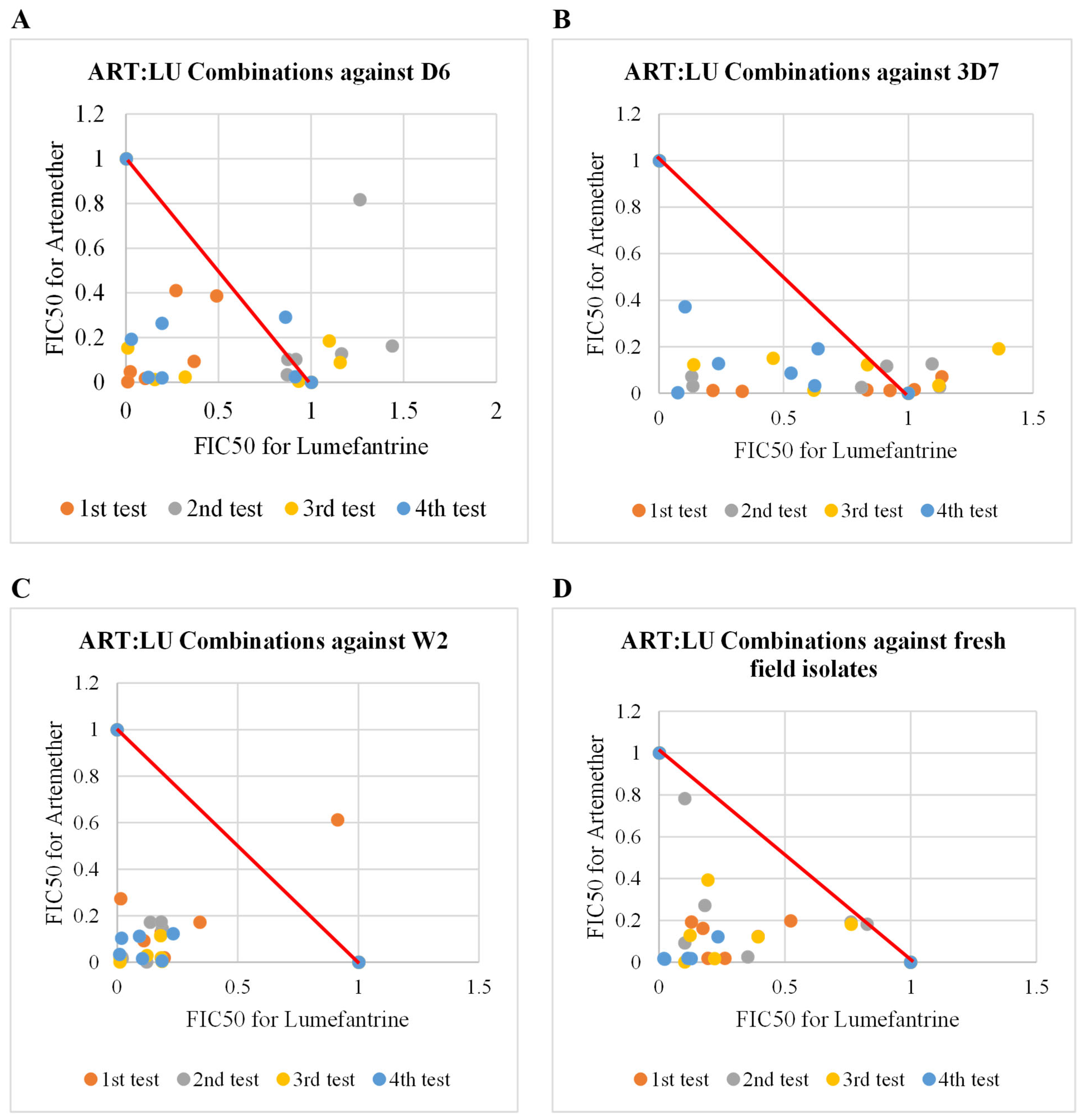
2.3. Antiplasmodial Activities of Artemether in Combination with Lumefantrine
2.4. Antiplasmodial Activities of Reference Antimalarial Drugs
3. Discussion
3.1. Combinations of Epirubicin with Artemether and Lumefantrine
3.2. Combinations of Pelitinib with Artemether and Lumefantrine
3.3. Antiplasmodial Activities of Epirubicin and Pelitinib When Tested Alone
3.4. Safety of Epirubicin
3.5. Strengths and Limitations
3.5.1. Strengths
3.5.2. Limitations
4. Materials and Methods
4.1. Test Drugs and Reference Antimalarial Drugs
4.2. Plasmodium falciparum Parasite Strains
4.3. Drug Combinations
4.4. Antiplasmodial Activities
4.4.1. Ex Vivo Antiplasmodial Assays
4.4.2. In Vitro Antiplasmodial Assays
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Chen, J.; Ma, X.; Tang, J.; Xu, S.; Gu, Y.; Tang, F.; Cao, Y.; Wang, W.; Zhou, H.; Zhang, J.; et al. Disparate selection of mutations in the dihydrofolate reductase gene (dhfr) of Plasmodium ovale curtisi and P. o. wallikeri in Africa. PLoS Negl. Trop. Dis. 2022, 16, e0010977. [Google Scholar] [CrossRef]
- Song, X.; Wei, W.; Cheng, W.; Zhu, H.; Wang, W.; Dong, H.; Li, J. Cerebral malaria induced by Plasmodium falciparum: Clinical features, pathogenesis, diagnosis, and treatment. Front. Cell. Infect. Microbiol. 2022, 12, 939532. [Google Scholar] [CrossRef]
- World Health Organization: World Malaria Report. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 18 May 2025).
- Ouji, M.; Augereau, J.M.; Paloque, L.; Benoit-Vical, F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 2018, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: WHO Guidelines for Malaria. 2023. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria (accessed on 24 July 2024).
- Ochora, D.O.; Kakudidi, E.K.; Namukobe, J.; Ipulet, P.; Wakoli, D.M.; Okore, W.; Mwakio, E.W.; Yeda, R.A.; Cheruiyot, A.C.; Juma, D.W.; et al. Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata. Plants 2022, 11, 47. [Google Scholar] [CrossRef]
- World Health Organization: Global Malaria Programme. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy. 2019. Available online: https://www.who.int/docs/default-source/documents/publications/gmp/who-cds-gmp-2019-17-eng.pdf?ua=1 (accessed on 24 July 2024).
- Conrad, M.D.; Rosenthal, P.J. Antimalarial drug resistance in Africa: The calm before the storm? Lancet Infect. Dis. 2019, 19, e338–e351. [Google Scholar] [CrossRef] [PubMed]
- Ounjaijean, S.; Somsak, V. Synergistic antimalarial treatment of Plasmodium berghei infection in mice with dihydroartemisinin and Gymnema inodorum leaf extract. BMC Complement. Med. Ther. 2023, 23, 20. [Google Scholar] [CrossRef]
- Savi, M.K. An Overview of Malaria Transmission Mechanisms, Control, and Modeling. Med. Sci. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Sinnis, P.; Fidock, D.A. The RTS,S vaccine—A chance to regain the upper hand against malaria? Cell 2022, 185, 750–754. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Good, M.F. Malaria Vaccines: Progress to Date. BioDrugs 2023, 37, 737–756. [Google Scholar] [CrossRef]
- Batista, R.; De Jesus, J.A.; De Oliveira, A.B. Plant-derived antimalarial agents: New leads and efficient phytomedicines. part II. non-alkaloidal natural products. Molecules 2009, 14, 3037–3072. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Plants as antimalarial agents in Sub-Saharan Africa. Acta Trop. 2015, 152, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Ochora, D.O.; Murithi, C.; Masai, R.J.; Abdi, F.; Cheruyiot, A.; Katuura, E.; Asiimwe, S.; Nabatanzi, A.; Anywar, G.; Oryem-origa, H.; et al. Ex. vivo and in vitro antiplasmodial activity and toxicity of Caesalpinia decapetala (Roth) Alston (Fabaceae). J. Ethnopharmacol. 2024, 318 Pt B, 117007. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evid.-Based Complement. Altern. Med. 2020, 12, 8749083. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Chetia, D. Plant Flavonoids as Potential Source of Future Antimalarial leads. Syst. Rev. Pharmacol. 2017, 8, 13–18. [Google Scholar] [CrossRef]
- Ochora, D.O.; Kakudidi, E.; Namukobe, J.; Heydenreich, M.; Coghi, P.; Yang, L.J.; Mwakio, E.W.; Andagalu, B.; Roth, A.; Akala, H.M.; et al. A new benzophenone, and the antiplasmodial activities of the constituents of Securidaca longipedunculata fresen (Polygalaceae). Nat. Prod. Res. 2021, 36, 2758–2766. [Google Scholar] [CrossRef]
- Riscoe, M.; Kelly, J.X.; Winter, R. Xanthones as Antimalarial Agents: Discovery, Mode of Action, and Optimization. Curr. Med. Chem. 2005, 12, 2539–2549. [Google Scholar] [CrossRef]
- Erhirhie, E.O.; Ikegbune, C.; Okeke, A.I.; Onwuzuligbo, C.C.; Madubuogwu, N.U.; Chukwudulue, U.M.; Okonkwo, O.B. Antimalarial herbal drugs: A review of their interactions with conventional antimalarial drugs. Clin. Phytosci. 2021, 7, 7. [Google Scholar] [CrossRef]
- Hinkson, I.V.; Madej, B.; Stahlberg, E.A. Accelerating Therapeutics for Opportunities in Medicine: A Paradigm Shift in Drug Discovery. Front. Pharmacol. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Choudhuri, S. Drug Design for Malaria with Artificial Intelligence (AI). In Plasmodium Species and Drug Resistance, 1st ed.; Tyagi, R.K., Ed.; IntechOpen: London, UK, 2021; pp. 181–196. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Harrison, R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016, 15, 817–818. [Google Scholar] [CrossRef]
- Abd-Rahman, A.N.; Zaloumis, S.; McCarthy, J.S.; Simpson, J.A.; Commons, R.J. Scoping Review of Antimalarial Drug Candidates in Phase I and II Drug Development. Antimicrob. Agents Chemother. 2022, 66, e01659-21. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, M. Antimalarial drugs—What is in use and what is in the pipeline. Arch. Pharm. 2008, 341, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, B.T.; Mehlotra, R.K. Expanding the antimalarial drug arsenal-now, but how? Pharmaceuticals 2011, 4, 681–712. [Google Scholar] [CrossRef]
- Choudhuri, S.; Yendluri, M.; Poddar, S.; Li, A.; Mallick, K.; Mallik, S.; Ghosh, B. Recent Advancements in Computational Drug Design Algorithms through Machine Learning and Optimization. Kinases Phosphatases 2023, 1, 117–140. [Google Scholar] [CrossRef]
- Ikerionwu, C.; Ugwuishiwu, C.; Okpala, I.; James, I.; Okoronkwo, M.; Nnadi, C.; Orji, U.; Ebem, D.; Ike, A. Application of machine and deep learning algorithms in optical microscopic detection of Plasmodium: A malaria diagnostic tool for the future. Photodiagn. Photodyn. Ther. 2022, 40, 103198. [Google Scholar] [CrossRef]
- Alsanousi, W.A.; Ahmed, N.Y.; Hamid, E.M.; Elbashir, M.K.; Musa, M.M.; Wang, J.; Khan, N. A novel deep learning-assisted hybrid network for Plasmodium falciparum parasite mitochondrial proteins classification. PLoS ONE 2022, 17, e0275195. [Google Scholar] [CrossRef]
- MacLeod, A.K.; Coquelin, K.S.; Huertas, L.; Simeons, F.C.; Riley, J.; Casado, P.; Guijarro, L.; Casanueva, R.; Frame, L.; Pinto, E.G.; et al. Acceleration of infectious disease drug discovery and development using a humanized model of drug metabolism. Proc. Natl. Acad. Sci. USA 2024, 121, e2315069121. [Google Scholar] [CrossRef]
- Weisman, J.L.; Liou, A.P.; Shelat, A.A.; Cohen, F.E.; Guy, R.K.; DeRisi, J.L. Searching for new antimalarial therapeutics amongst known drugs. Chem. Biol. Drug Des. 2006, 67, 409–416. [Google Scholar] [CrossRef]
- Haldar, K.; Bhattacharjee, S.; Innocent, S. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2019, 16, 156–170. [Google Scholar] [CrossRef]
- World Health Organization: WHO Guidelines for Malaria. 2021. Available online: https://files.magicapp.org/guideline/67ecb5f5-80be-4515-899f-c003434a611b/published_guideline_5438-2_0.pdf (accessed on 24 July 2024).
- Hanscheid, T.; Del Portal Luyten, C.R.; Hermans, S.M.; Grobusch, M.P. Repurposing of anti-malarial drugs for the treatment of tuberculosis: Realistic strategy or fanciful dead end? Malar. J. 2024, 23, 132–139. [Google Scholar] [CrossRef]
- Mogire, R.M.; Akala, H.M.; Macharia, R.W.; Juma, D.W.; Cheruiyot, A.C.; Andagalu, B.; Brown, M.L.; El-Shemy, H.A.; Nyanjom, S.G. Target-similarity search using Plasmodium falciparum proteome identifies approved drugs with anti-malarial activity and their possible targets. PLoS ONE 2017, 12, e0186364. [Google Scholar] [CrossRef] [PubMed]
- Ochora, D.O.; Mogire, R.M.; Masai, R.J.; Yeda, R.A.; Mwakio, E.W.; Amwoma, J.G.; Wakoli, D.M.; Yenesew, A.; Akala, H.M. Ex. vivo and In vitro antiplasmodial activities of approved drugs predicted to have antimalarial activities using chemogenomics and drug repositioning approach. Heliyon 2023, 9, e18863. [Google Scholar] [CrossRef]
- Akala, H.M.; Waters, C.N.; Yenesew, A.; Wanjala, C.; Akenga, T.A. In vitro antiplasmodial and cyclin-dependent protein kinase (pfmrk) inhibitory activities of sele c ted flavonoids in combination with chloroquine (CQ) and artemisinin. Res. Pharm. Biotechnol. 2010, 2, 40–50. [Google Scholar]
- Talele, T.T.; Khedkar, S.A.; Rigby, A.C. Successful Applications of Computer Aided Drug Discovery: Moving Drugs from Concept to the Clinic. Curr. Top. Med. Chem. 2010, 10, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Shivahare, R.; Shaham, S.H.; Joshi, P.; Sharma, A.; Tripathi, R. Repurposing of existing therapeutics to combat drug-resistant malaria. Biomed. Pharmacother. 2021, 136, 111275. [Google Scholar] [CrossRef]
- Martin, J.H.; Bowden, N.A. Drug Repurposing-Overcoming the translational hurdles to clinical use. Pharmacol. Res. Perspect. 2019, 7, e00548. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial drug discovery: Progress and approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Rodrigues, J.; Cassiano, G.C.; Tavella, T.A.; Tomaz, K.P.; Baia-da-Silva, D.C.; Souza, M.F.; Lima, M.N.; Mottin, M.; Almeida, L.D.; et al. Computational Chemogenomics Drug Repositioning Strategy Enables the Discovery of Epirubicin as a New Repurposed Hit for Plasmodium falciparum and P. vivax. Antimicrob. Agents Chemother. 2020, 64, e02041-19. [Google Scholar] [CrossRef]
- Conrad, M.D.; Asua, V.; Garg, S.; Giesbrecht, D.; Niaré, K.; Smith, S.; Namuganga, J.F.; Katairo, T.; Legac, J.; Crudale, R.M.; et al. Evolution of Partial Resistance to Artemisinins in Malaria Parasites in Uganda. N. Engl. J. Med. 2023, 389, 722–732. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Akinbo, J.; Ojurongbe, O. The effect of lopinavir/ritonavir on the antimalarial activity of artemether or artemether/lumefantrine in a mouse model of Plasmodium berghei. J. Chemother. 2015, 27, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Ogundeyi, K.J.; Ajayi, A.M.; Oduyomi, O.J.; Adeyemo, S.A.; Ologe, M.O.; Ademowo, O.G. Vitamin C co-administration with artemether-lumefantrine abrogates chronic stress exacerbated Plasmodium berghei-induced sickness behaviour, inflammatory and oxidative stress responses in mice. J. Neuroimmunol. 2025, 399, 578518. [Google Scholar] [CrossRef] [PubMed]
- DrugBank 6.0, DB00445, DB05524. Available online: https://go.drugbank.com/drugs/DB00445 (accessed on 2 March 2025).
- Owumi, S.E.; Adebisi, G.E.; Odunola, O.A. Epirubicin toxicity in rat’s ovary and uterus: A protective role of 3-Indolepropionic acid supplementation. Chem.-Biol. Interact. 2023, 374, 110414. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.; Capone, V.; Cocchia, R.; Cademartiri, F.; Riccardi, F.; Arcopinto, M.; Alshahid, M.; Anwar, K.; Carafa, M.; Carbone, A.; et al. Cardiovascular Side Effects of Anthracyclines and HER2 Inhibitors among Patients with Breast Cancer: A Multidisciplinary Stepwise Approach for Prevention, Early Detection, and Treatment. J. Clin. Med. 2023, 12, 2121. [Google Scholar] [CrossRef]
- Sui, D.; Tang, X.; Ding, J.; Wang, Y.; Qin, Y.; Zhang, N.; Liu, X.; Deng, Y.; Song, Y. Sequential administration of sialic acid-modified liposomes as carriers for epirubicin and zoledronate elicit stronger antitumor effects with reduced toxicity. Int. J. Pharm. 2021, 602, 120552. [Google Scholar] [CrossRef] [PubMed]
- Chaa, S.; Boufadi, M.Y.; Keddari, S.; Benchaib, A.H. Protective effect of propolis from Tigzirt on epirubicin-induced cardiotoxicity and nephrotoxicity. J. Pharm. Pharmacogn. Res. 2021, 9, 549–562. [Google Scholar] [CrossRef]
- Lourens, C.; Watkins, W.M.; Barnes, K.I.; Sibley, C.H.; Guerin, P.J.; White, N.J.; Lindegardh, N. Implementation of a reference standard and proficiency testing programme by the World Wide Antimalarial Resistance Network (WWARN). Malar. J. 2010, 9, 375–378. [Google Scholar] [CrossRef]
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef]
- Yenesew, A.; Akala, H.M.; Twinomuhwezi, H.; Chepkirui, C.; Irungu, B.N.; Eyase, F.L.; Kamatenesi-Mugisha, M.; Kiremire, B.T.; Johnson, J.D.; Waters, N.C. The antiplasmodial and radical scavenging activities of flavonoids of Erythrina burttii. Acta Trop. 2012, 123, 123–127. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Wakoli, D.M.; Ondigo, B.N.; Ochora, D.O.; Amwoma, J.G.; Okore, W.; Mwakio, E.W.; Chemwor, G.; Juma, J.; Okoth, R.; Okudo, C.; et al. Impact of parasite genomic dynamics on the sensitivity of Plasmodium falciparum isolates to piperaquine and other antimalarial drugs. BMC Med. 2022, 20, 448. [Google Scholar] [CrossRef]
- Akala, H.M.; Eyase, F.L.; Cheruiyot, A.C.; Omondi, A.A.; Ogutu, B.R.; Waters, N.C.; Johnson, J.D.; Polhemus, M.E.; Schnabel, D.C.; Walsh, D.S. Antimalarial drug sensitivity profile of western Kenya Plasmodium falciparum field isolates determined by a SYBR Green I in vitro assay and molecular analysis. Am. J. Trop. Med. Hyg. 2011, 85, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ongeri, O.D. Pharmacological Properties of Ginger Combinations. In Ginger—Cultivation and Use, 1st ed.; Prashant, K., Rabia, S.A., Eds.; IntechOpen: London, UK, 2023; pp. 64–89. [Google Scholar] [CrossRef]

| Drug Combinations ART/LU: EPI | Mean of Sum FIC50 ± STDEV | |||||||
|---|---|---|---|---|---|---|---|---|
| P. falciparum Strains (In Vitro) | Clinical Field Isolates | |||||||
| D6 | 3D7 | W2 | KOM (Ex Vivo) | |||||
| ART-EPI | LU-EPI | ART-EPI | LU-EPI | ART-EPI | LU-EPI | ART-EPI | LU-EPI | |
| 4:1 | 0.63 ± 0.05 | 0.60 ± 0.23 | 0.65 ± 0.04 | 0.28 ± 0.09 | 0.82 ± 0.24 | 0.85 ± 0.73 | 1.04 ± 0.51 | 0.53 ± 0.35 |
| 3:1 | 0.46 ± 0.09 | 0.44 ± 0.18 | 0.43 ± 0.33 | 0.40 ± 0.31 | 0.59 ± 0.31 | 0.54 ± 0.33 | 1.14 ± 0.30 | 0.71 ± 0.57 |
| 1:1 | 0.82 ± 0.44 | 0.76 ± 0.57 | 0.53 ± 0.35 | 0.058 ± 0.05 | 0.52 ± 0.30 | 0.31 ± 0.21 | 0.67 ± 0.52 | 0.19 ± 0.07 |
| 1:2 | 0.68 ± 0.23 | 0.64 ± 0.44 | 0.48 ± 0.27 | 0.10 ± 0.05 | 0.24 ± 0.22 | 0.43 ± 0.34 | 0.26 ± 0.17 | 0.20 ± 0.13 |
| 1:3 | 0.21 ± 0.12 | 0.43 ± 0.21 | 0.59 ± 0.42 | 0.09 ± 0.05 | 0.48 ± 0.36 | 0.33 ± 0.25 | 0.44 ± 0.32 | 0.18 ± 0.12 |
| 1:4 | 0.27 ± 0.19 | 0.39 ± 0.24 | 0.51 ± 0.34 | 0.13 ± 0.14 | 0.28 ± 0.13 | 0.33 ± 0.20 | 0.39 ± 0.27 | 0.28 ± 0.16 |
| Mean * | 0.51 ± 0.19 | 0.54 ± 0.31 | 0.53 ± 0.36 | 0.18 ± 0.11 | 0.49 ± 0.29 | 0.46 ± 0.34 | 0.65 ± 0.42 | 0.35 ± 0.28 |
| Drug Combinations ART/LU: PEL | Mean of Sum FIC50 ± STDEV | |||||||
|---|---|---|---|---|---|---|---|---|
| P. falciparum Strains (In Vitro) | Clinical Field Isolates | |||||||
| D6 | 3D7 | KOM (Ex Vivo) | MGT (In Vitro) | |||||
| ART-PEL | LU-PEL | ART-PEL | LU-PEL | ART-PEL | LU-PEL | ART-PEL | LU-PEL | |
| 4:1 | 1.48 ± 0.13 | 1.95 ± 0.92 | 0.75 ± 0.06 | 0.8 ± 0.12 | 1.48 ± 0.06 | 1.57 ± 1.00 | 1.03 ± 0.07 | 1.11 ± 1.08 |
| 3:1 | 1.43 ± 0.18 | 0.34 ± 0.12 | 0.83 ± 0.11 | 0.91 ± 0.52 | 1.58 ± 0.08 | 2.72 ± 0.38 | 1.14 ± 0.08 | 1.62 ± 0.66 |
| 1:1 | 1.41 ± 0.07 | 2.69 ± 0.31 | 1.36 ± 0.01 | 2.33 ± 0.43 | 1.91 ± 0.21 | 2.93 ± 1.02 | 1.12 ± 0.01 | 1.99 ± 0.58 |
| 1:2 | 1.65 ± 0.03 | 2.13 ± 0.33 | 1.19 ± 0.27 | 2.94 ± 0.43 | 1.78 ± 0.12 | 2.55 ± 0.19 | 1.31 ± 0.2 | 2.28 ± 0.44 |
| 1:3 | 1.43 ± 0.02 | 2.31 ± 0.59 | 1.86 ± 0.01 | 2.24 ± 0.73 | 2.6 ± 0.39 | 2.44 ± 0.14 | 1.17 ± 0.03 | 1.13 ± 0.61 |
| 1:4 | 2.12 ± 0.07 | 2.29 ± 0.79 | 1.08 ± 0.35 | 2.62 ± 0.61 | 2.2 ± 1.13 | 1.97 ± 0.07 | 1.78 ± 0.33 | 2.12 ± 0.23 |
| Mean * | 1.58 ± 0.08 | 1.95 ± 0.51 | 1.18 ± 0.14 | 1.97 ± 0.47 | 1.93 ± 0.33 | 2.36 ± 0.47 | 1.26 ± 0.12 | 1.71 ± 0.06 |
| Drug Combinations ART:LU | Mean of Sum FIC50 ± STDEV | |||
|---|---|---|---|---|
| P. falciparum Strains (In Vitro) | Clinical Field Isolates | |||
| D6 | 3D7 | W2 | KOM (Ex Vivo) | |
| ART:LU | ART:LU | ART:LU | ART:LU | |
| 4:1 | 0.94 ± 0.44 | 1.07 ± 0.30 | 0.26 ± 0.06 | 0.66 ± 0.32 |
| 3:1 | 1.01 ± 0.23 | 0.92 ± 0.34 | 0.25 ± 0.08 | 0.55 ± 0.29 |
| 1:1 | 1.06 ± 0.67 | 0.91 ± 0.26 | 0.18 ± 0.07 | 0.26 ± 0.15 |
| 1:2 | 0.62 ± 0.50 | 0.83 ± 0.23 | 0.43 ± 0.33 | 0.37 ± 0.30 |
| 1:3 | 0.37 ± 0.25 | 0.31 ± 0.21 | 0.34 ± 0.11 | 0.27 ± 0.27 |
| 1:4 | 0.34 ± 0.29 | 0.32 ± 0.18 | 0.08 ± 0.06 | 0.32 ± 0.17 |
| Mean * | 0.72 ± 0.43 | 0.72 ± 0.25 | 0.26 ± 0.17 | 0.40 ± 0.25 |
| Drugs | Mean IC50 ± STDEV (µM) | |||||
|---|---|---|---|---|---|---|
| P. falciparum Strains | Field Isolates (Ex Vivo) | |||||
| W2 | DD2 | D6 | 3D7 | F32 ART | KOM | |
| Epirubicin | 0.08 ± 0.01 | 0.12 ± 0.04 | 0.17 ± 0.01 | 0.15 ± 0.02 | 0.18 ± 0.03 | 0.08 ± 0.01 |
| Pelitinib | 1.21 ± 0.09 | 1.36 ± 0.29 | 2.62 ± 0.39 | 1.48 ± 0.31 | 1.41 ± 0.07 | 0.27 ± 0.01 |
| Artemether | 0.0046 ± 0.0016 | 0.011 ± 0.006 | 0.0032 ± 0.001 | 0.0052 ± 0.002 | 0.004 ± 0.001 | 0.008 ± 0.003 |
| Lumefantrine | 0.061 ± 0.042 | 0.026 ± 0.0053 | 0.049 ± 0.003 | 0.072 ± 0.005 | 0.21 ± 0.06 | 0.046 ± 0.007 |
| Mefloquine | 0.0043 ± 0.0012 | 0.066 ± 0.001 | 0.0091 ± 0.003 | 0.0042 ± 0.002 | 0.038 ± 0.003 | 0.0058 ± 0.002 |
| Chloroquine | 0.12 ± 0.02 | 0.263 ± 0.04 | 1.37 ± 0.04 | 0.095 ± 0.03 | 0.015 ± 0.005 | 0.18 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochora, D.O.; Mogire, R.M.; Murithi, B.M.; Abdi, F.; Ondari, E.N.; Masai, R.J.; Mwakio, E.; Cheruyiot, A.; Yenesew, A.; Akala, H.M. Antimalarial Drug Repurposing of Epirubicin and Pelitinib in Combination with Artemether and Lumefantrine. Pharmaceuticals 2025, 18, 956. https://doi.org/10.3390/ph18070956
Ochora DO, Mogire RM, Murithi BM, Abdi F, Ondari EN, Masai RJ, Mwakio E, Cheruyiot A, Yenesew A, Akala HM. Antimalarial Drug Repurposing of Epirubicin and Pelitinib in Combination with Artemether and Lumefantrine. Pharmaceuticals. 2025; 18(7):956. https://doi.org/10.3390/ph18070956
Chicago/Turabian StyleOchora, Douglas O., Reagan M. Mogire, Bernard M. Murithi, Farid Abdi, Erick N. Ondari, Rael J. Masai, Edwin Mwakio, Agnes Cheruyiot, Abiy Yenesew, and Hoseah M. Akala. 2025. "Antimalarial Drug Repurposing of Epirubicin and Pelitinib in Combination with Artemether and Lumefantrine" Pharmaceuticals 18, no. 7: 956. https://doi.org/10.3390/ph18070956
APA StyleOchora, D. O., Mogire, R. M., Murithi, B. M., Abdi, F., Ondari, E. N., Masai, R. J., Mwakio, E., Cheruyiot, A., Yenesew, A., & Akala, H. M. (2025). Antimalarial Drug Repurposing of Epirubicin and Pelitinib in Combination with Artemether and Lumefantrine. Pharmaceuticals, 18(7), 956. https://doi.org/10.3390/ph18070956









