HIV Infection and Antiretroviral Therapy Impair Liver Function in People Living with HIV: Systematic Review and Meta-Analysis
Abstract
1. Introduction
- Aim and Objectives
- Objectives
2. Materials and Methods
2.1. Information Sources, Search Strategy
2.2. Eligibility Criteria and Selection Process
2.3. Data Items and Extraction
2.4. Quality Assessment and Risk of Bias
2.5. Synthesis Method and Statistical Analysis
3. Results
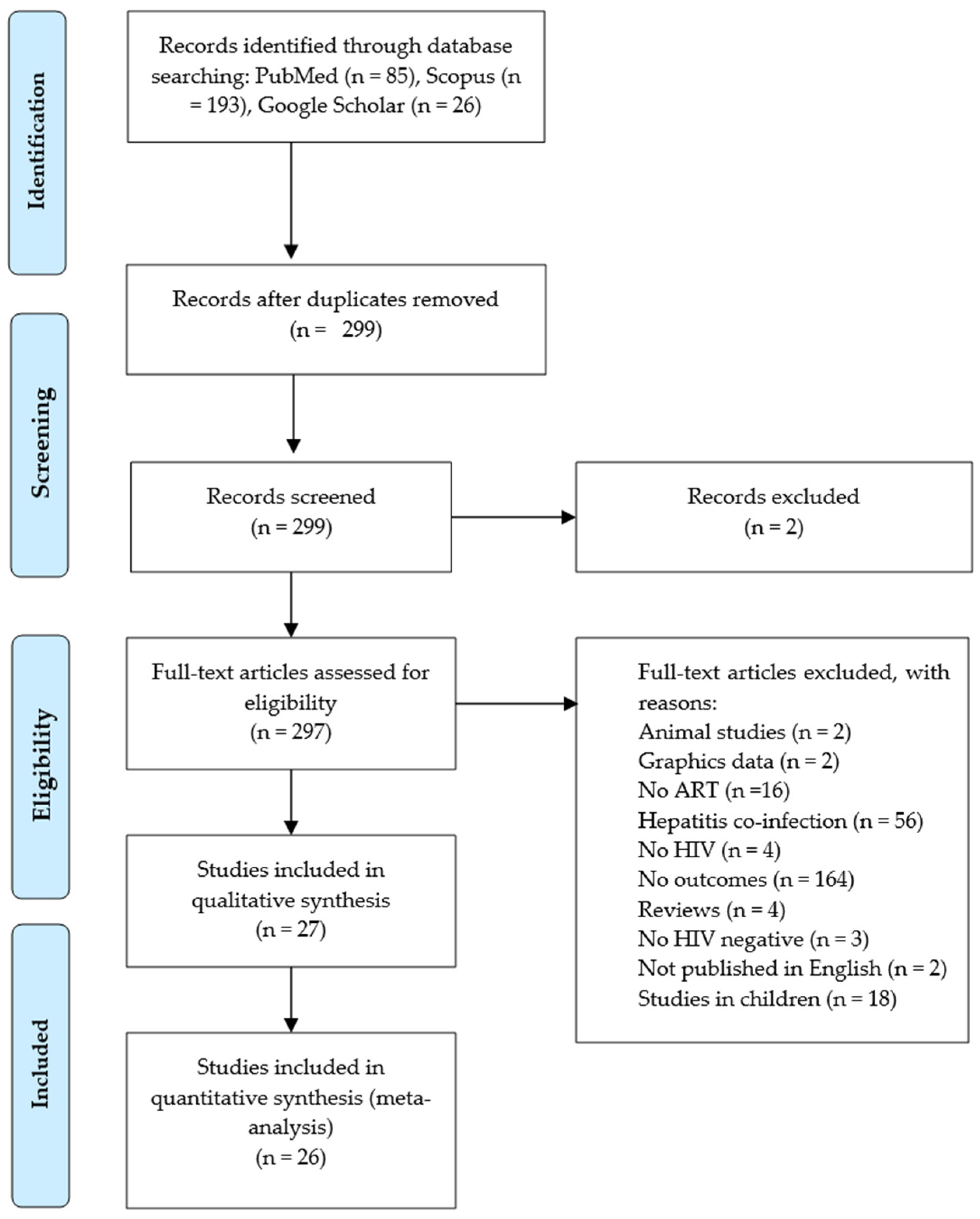
3.1. Literature Search and Study Selection
3.2. The Demographic Features of the Studies
3.3. Quality Assessment and Risk of Bias
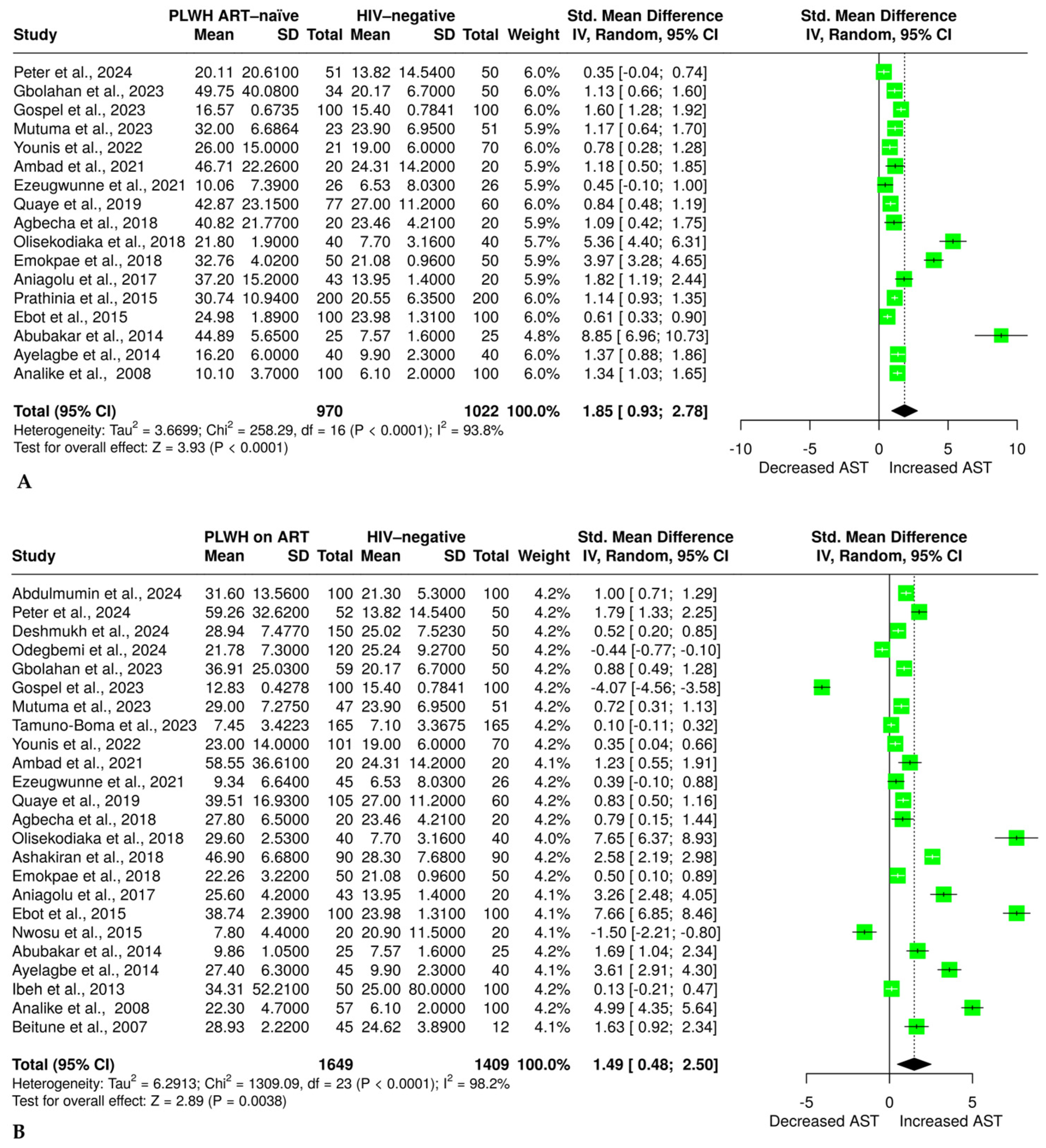
3.4. The Effect of HIV and ART on Aspartate Aminotransferase
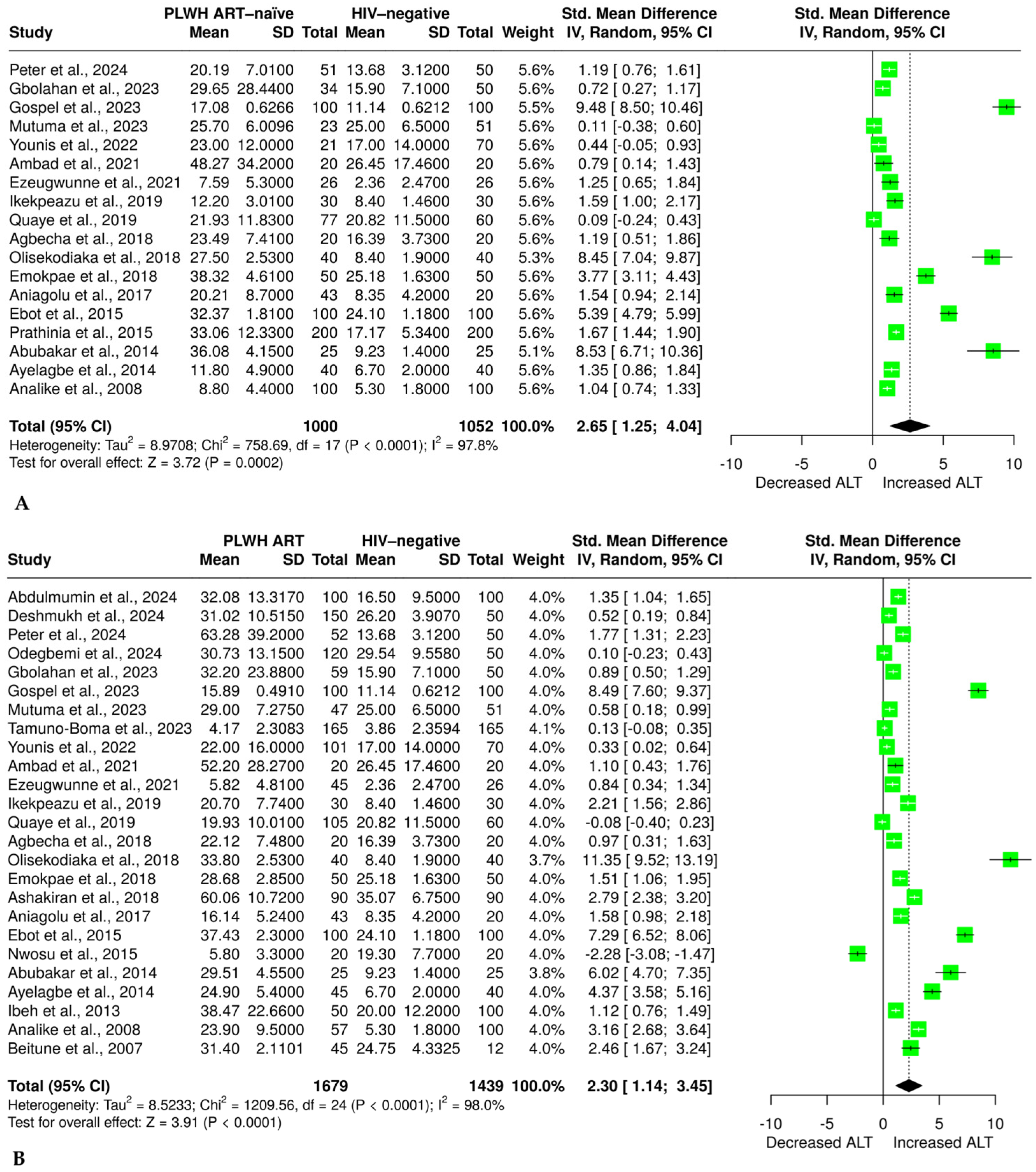
3.5. Effect of HIV Infection and ART on ALT
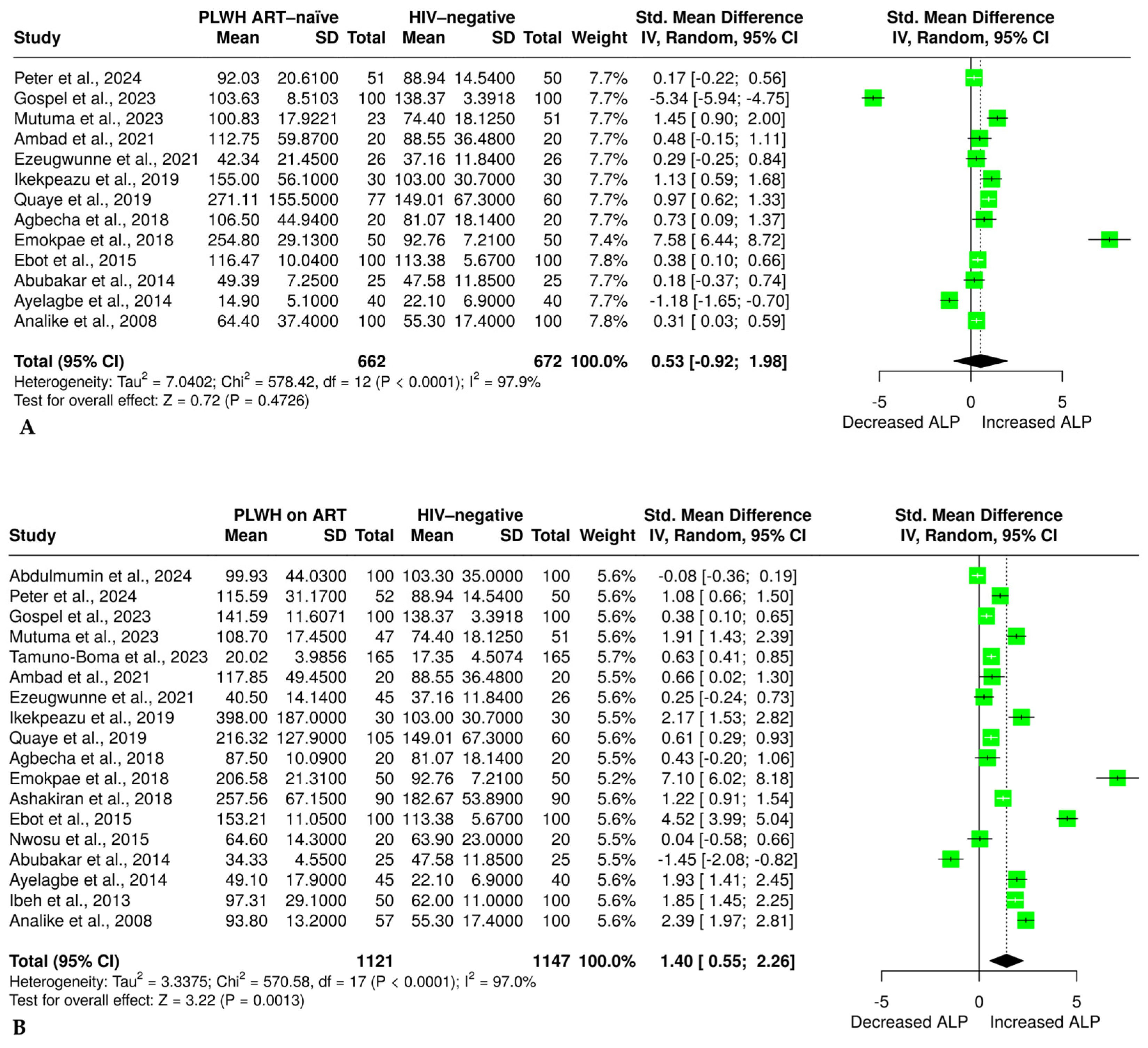
3.6. The Effect of HIV Infection and ART on Alkaline Phosphatase
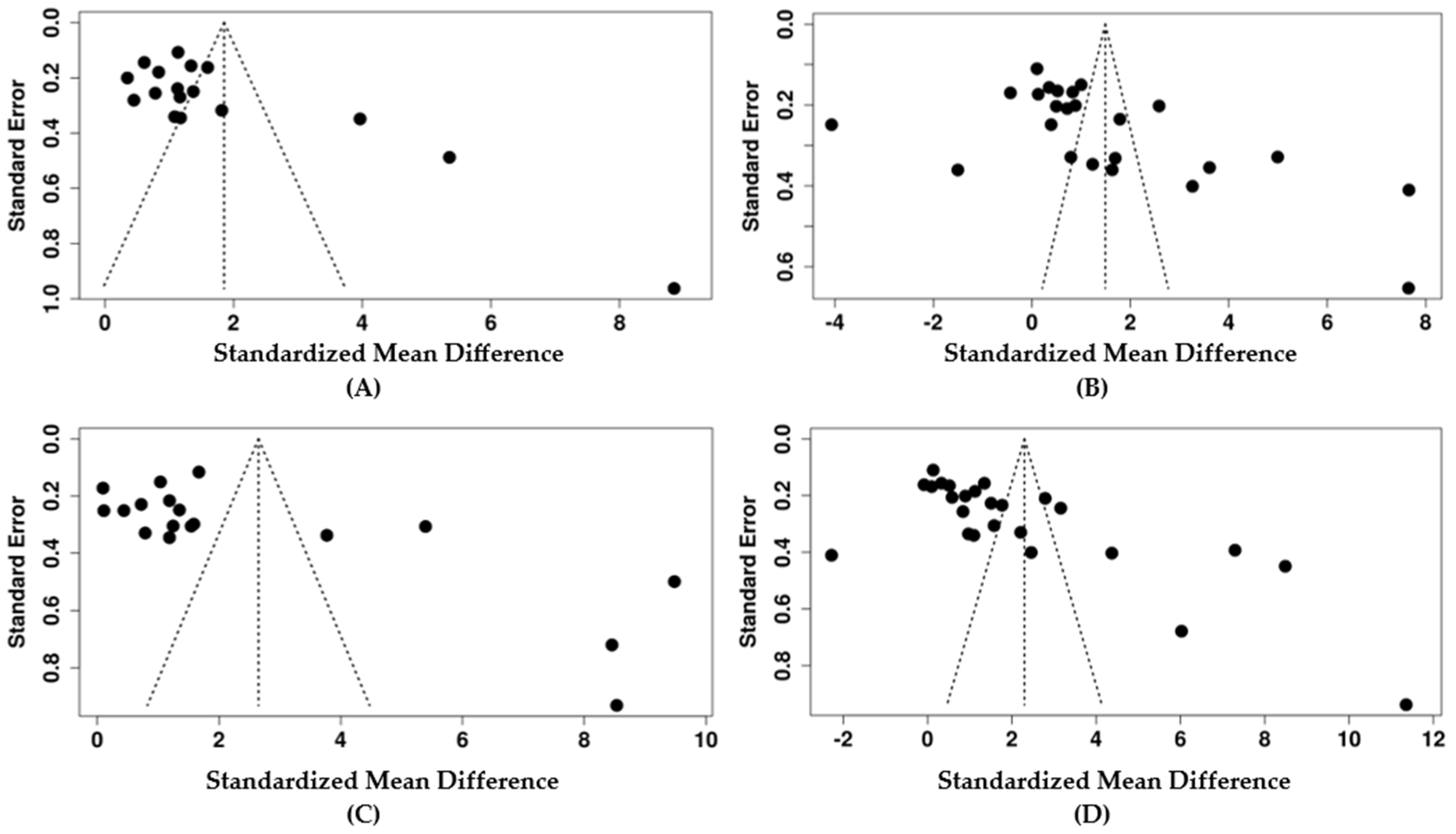
3.7. Publication Bias and Sensitivity Analysis
3.8. Subgroup Analysis
4. Discussion
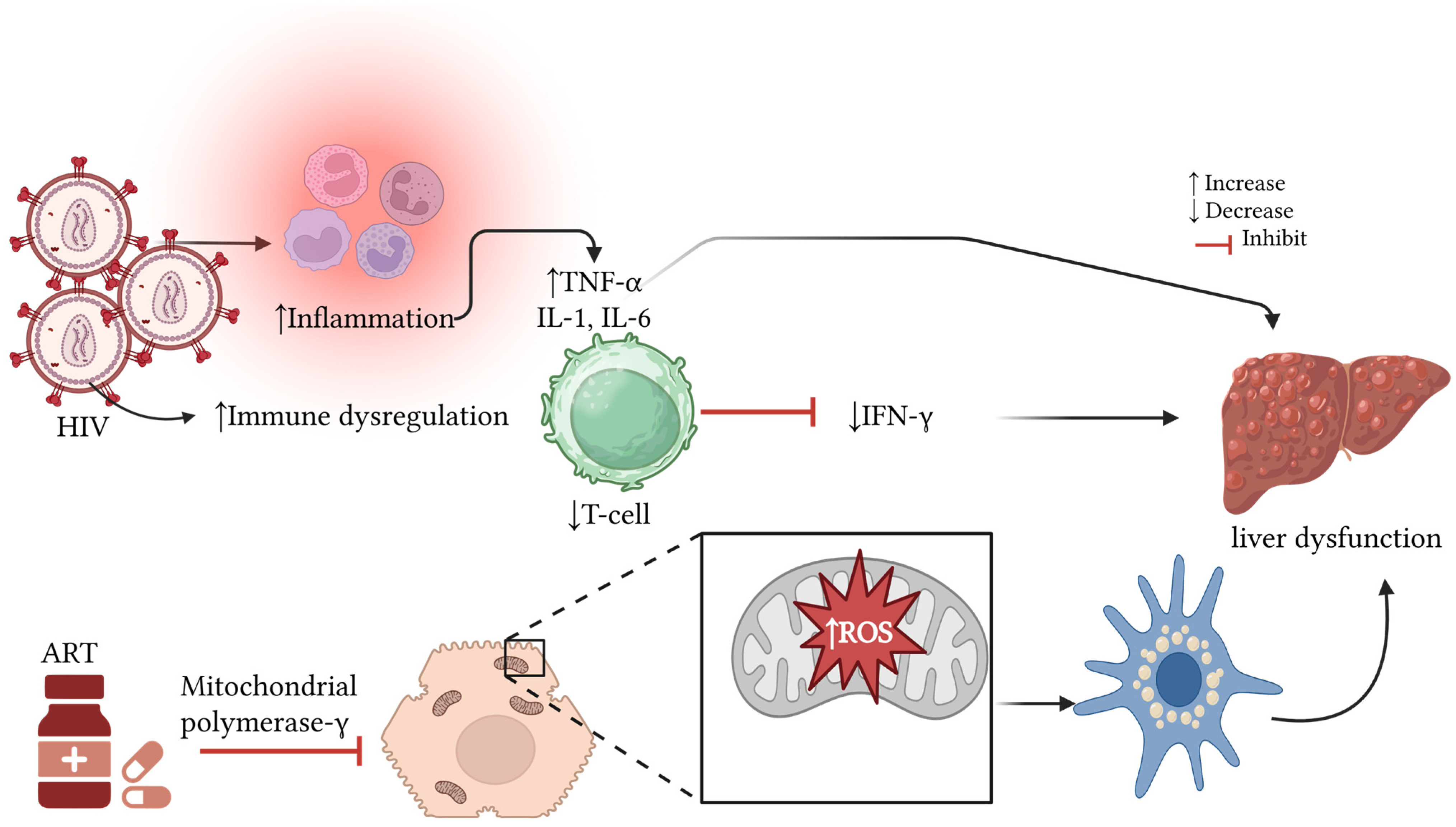
Strengths and Limitations
5. Conclusions
Recommendation and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | acquired immunodeficiency virus |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| ART | antiretroviral therapy |
| ARV | antiretroviral |
| AST | aspartate aminotransferase |
| CD4 | cluster of differentiation 4 |
| CI | confidence interval |
| DTG | dolutegravir |
| HAART | highly active antiretroviral therapy |
| HBV | hepatitis B Virus |
| HCV | hepatitis C Virus |
| HIV | human immunodeficiency virus |
| MeSH | Medical Subject Headings |
| NNRTI | non-nucleoside reverse transcriptase inhibitors |
| NOS | Newcastle–Ottawa Scale |
| NRTI | nucleoside reverse transcriptase inhibitor |
| PICO | population, intervention, comparator, outcome |
| PLWH | people living with HIV |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SD | standard deviation |
| SEM | standard error of mean |
| SMD | standardized mean difference |
References
- Vijayan, K.V.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Word Health Organisation. HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 24 November 2024).
- Al-Jabri, A.A. How Does HIV-1 Infect a Susceptible Human Cell? Current Thinking. SQU J. Sci. Res. Med. Sci. 2003, 5, 31–44. [Google Scholar] [CrossRef]
- Gebremedhin, T.; Aynalem, M.; Adem, M.; Geremew, D.; Aleka, Y.; Kiflie, A. Dolutegravir Based Therapy Showed CD4+ T Cell Count Recovery and Viral Load Suppression among ART Naïve People Living with HIV AIDS: A Pilot Evaluation. Sci. Rep. 2024, 14, 3297. [Google Scholar] [CrossRef]
- Shiferaw, M.B.; Tulu, K.T.; Zegeye, A.M.; Wubante, A.A. Liver Enzymes Abnormalities among Highly Active Antiretroviral Therapy Experienced and HAART Naïve HIV-1 Infected Patients at Debre Tabor Hospital, North West Ethiopia: A Comparative Cross-Sectional Study. AIDS Res. Treat. 2016, 2016, 1985452. [Google Scholar] [CrossRef]
- Moses, J.S.; Pau, A.K.; Kuriakose, S.; Grandits, G.; Reilly, C.; Sherman, B.T.; Chang, W.; Dai, L.; Khan, M.A.; Highbarger, H.; et al. HIV-1 Suppression and Rare Dolutegravir Resistance in Antiretroviral-Experienced People with HIV in Liberia. Commun. Med. 2025, 5, 164. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N.; Kumar, S.; Sashindran, V.K.; Puri, P. A Cross-Sectional Study of Liver Function Tests in HIV-Infected Persons in Western India. Med. J. Armed Forces India 2017, 73, 23–28. [Google Scholar] [CrossRef]
- Rwegerera, G.M.; Rimbi, M.; Mudhina, V.; Simone, M.T.; Sefo, M.; Segona, B. Dolutegravir Induced Sub-Acute Hepatic Failure in HIV Positive Treatment Naïve Man in Botswana. Case Rep. Intern. Med. 2019, 6, 5–8. [Google Scholar] [CrossRef]
- Mengistu, E.F.; Malik, D.T.; Molla, M.D.; Adugna, A.; Jemal, M. Liver Function Tests, CD4+ Counts, and Viral Load among People Living with HIV on Dolutegravir Compared to Efavirenz-Based CART; a Comparative Cross-Sectional Study. Heliyon 2024, 10, e33054. [Google Scholar] [CrossRef]
- Suffrin, J.C.D.; Allan-Blitz, L.-T.; Taylor, E.; Ruderman, T.; Boti, M.; Moyo, J.; Phiri, F.M.; Ndalama, E.; Connolly, E. Presumed Severe Hepatocellular Toxicity after Initiation on a Dolutegravir-Based HIV Treatment Regimen in Rural Malawi: A Case Report. Ann. Clin. Case Rep. Hepatol. 2022, 7, 2098. [Google Scholar] [CrossRef]
- Thornton, A.C.; Jose, S.; Bhagani, S.; Chadwick, D.; Dunn, D.; Gilson, R.; Main, J.; Nelson, M.; Rodger, A.; Taylor, C.; et al. Hepatitis B, Hepatitis C, and Mortality among HIV-Positive Individuals. AIDS 2017, 31, 2525–2532. [Google Scholar] [CrossRef]
- Shalanyuy, L.; Feh-Alanyuy, F.; Moses, S.; Mengnjo, T.; Chongsi, W.; Hervis, T. Effects of Antiretroviral Therapy on Liver Based Enzymes, AST, ALT, ALP and Total Bilirubin in HIV Patients Attending the Bamenda Regional Hospital. Pharm. Sci. Anal. Res. J. 2025, 7, 180117. [Google Scholar]
- Mataranyika, P.A.; Kibuule, D.; Kalemeera, F.; Kaura, H.; Godman, B.; Rennie, T.W. Liver Enzyme Elevations in a Cohort of HIV/AIDS Patients on First-Line Antiretroviral Therapy in Namibia: Findings and Implications. Alex. J. Med. 2018, 54, 49–56. [Google Scholar] [CrossRef]
- Abongwa, L.E.; Nyamache, A.K.; Charles, F.; Torimiro, J.; Emmanuel, N.; Domkam, I.; Eyongetah, M.; Jude, B.; Mua, F.H.; Bella, S.; et al. Risk Factors of Severe Hepatotoxicity among HIV-1 Infected Individuals Initiated on Highly Active Antiretroviral Therapy in the Northwest Region of Cameroon. BMC Gastroenterol. 2022, 22, 286. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The Interaction of Hepatic Lipid and Glucose Metabolism in Liver Diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Tang, L.W.T.; Varma, M.V.S. Hepatic Impairment and the Differential Effects on Drug Clearance Mechanisms: Analysis of Pharmacokinetic Changes in Disease State. Clin. Pharmacol. Ther. 2025. [Google Scholar] [CrossRef]
- Yap, C.Y.; Aw, T.C. Liver Function Tests (LFTs). Proc. Singap. Healthc. 2010, 19, 80–82. [Google Scholar] [CrossRef]
- Parsons, G. Understanding Liver Function Tests: Part 1. Prescriber 2023, 34, 19–23. [Google Scholar] [CrossRef]
- Sterling, R.K.; Chiu, S.; Snider, K.; Nixon, D. The Prevalence and Risk Factors for Abnormal Liver Enzymes in HIV-Positive Patients without Hepatitis B or C Coinfections. Dig. Dis. Sci. 2008, 53, 1375–1382. [Google Scholar] [CrossRef]
- Yeni, P. Update on HAART in HIV. J. Hepatol. 2006, 44, S100–S103. [Google Scholar] [CrossRef]
- Shafer, R.W.; Vuitton, D.A. Highly Active Antiretroviral Therapy (HAART) for the Treatment of Infection with Human Immunodeficiency Virus Type 1. Biomed. Pharmacother. 1999, 53, 73–86. [Google Scholar] [CrossRef]
- Benedicto, A.M.; Fuster-Martínez, I.; Tosca, J.; Esplugues, J.V.; Blas-García, A.; Apostolova, N. Nnrti and Liver Damage: Evidence of Their Association and the Mechanisms Involved. Cells 2021, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zeng, J.; Wu, J.; Qiao, L.; Chen, Q.; Chen, D.; Zhang, Y. Nucleoside Reverse Transcriptase Inhibitors Induced Hepatocellular Mitochondrial DNA Lesions and Compensatory Enhancement of Mitochondrial Function and DNA Repair. Int. J. Antimicrob. Agents 2018, 51, 385–392. [Google Scholar] [CrossRef]
- Paemanee, A.; Sornjai, W.; Kittisenachai, S.; Sirinonthanawech, N.; Roytrakul, S.; Wongtrakul, J.; Smith, D.R. Nevirapine Induced Mitochondrial Dysfunction in HepG2 Cells. Sci. Rep. 2017, 7, 9194. [Google Scholar] [CrossRef]
- Gao, S.; Gui, X.; Deng, L.; Zhang, Y.; Liang, K.; Yang, R.; Yan, Y.; Rong, Y. Antiretroviral Therapy Hepatotoxicity: Prevalence, Risk Factors, and Clinical Characteristics in a Cohort of Han Chinese. Hepatol. Res. 2010, 40, 287–294. [Google Scholar] [CrossRef]
- Van Welzen, B.J.; Mudrikova, T.; Arends, J.E.; Hoepelman, A. No Increased Risk of Hepatotoxicity in Long-Term Use of Nonnucleoside Reverse Transcriptase Inhibitors in HIV-Infected Patients. HIV Med. 2012, 13, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.M.; Chen, P.C.; Hsieh, H.C.; Calkins, M.J. Mitochondrial Defects Arise from Nucleoside/Nucleotide Reverse Transcriptase Inhibitors in Neurons: Potential Contribution to HIV-Associated Neurocognitive Disorders. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 406–413. [Google Scholar] [CrossRef]
- Saitoh, A.; Fenton, T.; Alvero, C.; Fletcher, C.V.; Spector, S.A. Impact of Nucleoside Reverse Transcriptase Inhibitors on Mitochondria in Human Immunodeficiency Virus Type 1-Infected Children Receiving Highly Active Antiretroviral Therapy. Antimicrob. Agents Chemother. 2007, 51, 4236–4242. [Google Scholar] [CrossRef]
- Isoda, A.; Mihara, M.; Matsumoto, M.; Sawamura, M. Severe Lactic Acidosis during Tenofovir Disoproxil Fumarate and Cobicistat Combination for HIV Patient. BMJ Case Rep. 2023, 16, e255751. [Google Scholar] [CrossRef]
- Muñoz-Muela, E.; Mejías-Trueba, M.; Serna-Gallego, A.; Saborido-Alconchel, A.; Fernández-Pérez, S.; Herrero, M.; Sotomayor, C.; Gutiérrez-Valencia, A.; Trujillo-Rodríguez, M.; López-Cortés, L.F. Mitochondrial Disorders After 12 Months of Human Immunodeficiency Virus Type 1 Preexposure Prophylaxis Based on Tenofovir Disoproxil Fumarate Plus Emtricitabine in Healthy Adults. J. Infect. Dis. 2025, jiaf156. [Google Scholar] [CrossRef]
- Mata-Marín, J.A.; Gaytán-Martínez, J.; Grados-Chavarría, B.H.; Fuentes-Allen, J.L.; Arroyo-Anduiza, C.I.; Alfaro-Mejía, A. Correlation between HIV Viral Load and Aminotransferases as Liver Damage Markers in HIV Infected Naive Patients: A Concordance Cross-Sectional Study. Virol. J. 2009, 6, 181. [Google Scholar] [CrossRef]
- Abubakar, M.; Abduljalil, M.; Nasiru, Y. Changes in Liver Function Enzymes of HIV/AIDS Patients Treated with Antiretroviral Drugs (ARVS) in Specialist Hospital. Niger. J. Basic Appl. Sci. 2014, 22, 85–89. [Google Scholar] [CrossRef]
- Moya-Salazar, J.; Barrial-Vega, M.; Arrieta-Calderón, R.; Contreras-Pulache, H. Changes in Liver Function Test Levels in HIV Patients Undergoing Highly Active Antiretroviral Therapy (HAART). Longitudinal Study in Lima, Peru. Rev. Fac. Med. 2022, 70, e86775. [Google Scholar] [CrossRef]
- Ezeugwunne, J.; Ogbodo, E.; Ezeuduji, O.; Iwuji, J.; Okwara, N.; Obi-Ezeani, C.; Amah, A.; Odumodu, I.; Izuchukwu, E. Assessment of Alpha-Fetoprotein, Albumin, CD4+ and Some Liver Enzymes in HIV Infected Adult on ART in Nauth Nnewi, South Eastern Nigeria. Adv. Biores. 2021, 12, 199–205. [Google Scholar]
- Ambad, R.S.; Shinde, R.V.; Jha, R.K.; Bhatt, N.; Jha, R.K. Study on Activity of Liver Enzymes in HIV Affected Women. Ann. Rom. Soc. Cell Biol. 2021, 25, 7093–7098. [Google Scholar]
- Ebot, W.; Achidi, E.; Kamga, H.-L.; Njunda, A.; Apinjoh, T. Liver Function Tests of HIV/AIDS Patients at the Nylon District Hospital, Douala, Cameroon. Int. J. Res. Med. Sci. 2015, 3, 2549–2552. [Google Scholar] [CrossRef]
- Abdulmumin, Y.; Haruna, I.U.; Danjaji, H.I.; Muhammad, M.; Mikail, T.A.; Rabiu, Z.; Lawan, U. Effect of Highly Active Antiretroviral Drugs Therapy (HAART) on Serum Hepatic and Renal Function Indices on HIV Patients in Kano Metropolitan. Sahel J. Life Sci. FUDMA 2024, 2, 134–141. [Google Scholar] [CrossRef]
- Tamuno-Boma, O.; Azuonwu, O.; Opusunju Boma, H.; Tee Popnen, G.; Gabriel-Brisibe, C.U.; Ihua, N.; Akuru Udiomine, B.; Akram, M. Assessment on Liver Function Biomarkers in HIV Positive Pregnant and Non-Pregnant Women on Antiretroviral Therapy in Rivers State, Nigeria. J. HIV Clin. Sci. Res. 2023, 10, 001–005. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 February 2025).
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.Com: An Online Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Hassani, H.; Ghodsi, M.; Howell, G. A Note on Standard Deviation and Standard Error. Teach. Math. Its Appl. 2010, 29, 108–112. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing Heterogeneity in Meta-Analysis: Q Statistic or I2 Index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- El Beitune, P.; Duarte, G.; Campbell, O.; Quintana, S.M.; Rodrigues, L.C. Effects of Antiretroviral Agents During Pregnancy on Liver Enzymes and Amylase in HIV-Exposed, Uninfected Newborn Infants. Braz. J. Infect. Dis. 2007, 11, 314–317. [Google Scholar] [CrossRef]
- Analike, R.; Nnamah, N.; Dioka, C.; Meludu, S.; Osuji, C.; Asomugha, A. Evaluation of Liver Function Tests of HIV Positive Patients on Antiretroviral Therapy in Nnewi, Nigeria. J. Biomed. Investig. 2008, 4, 42–48. [Google Scholar] [CrossRef]
- Ibeh, B.O.; Omodamiro, O.D.; Ibeh, U.; Habu, J.B. Biochemical and Haematological Changes in HIV Subjects Receiving Winniecure Antiretroviral Drug in Nigeria. J. Biomed. Sci. 2013, 20, 73. [Google Scholar] [CrossRef]
- Ayelagbe, O.G.; Akerele, O.P.; Onuegbu, A.J.; Oparinde, D.P. Drug Hepatotoxicity in HIV Patients on Highly Active Antiretroviral Therapy [HAART] in Southwest Nigeria. IOSR J. Dent. Med. Sci. 2014, 13, 67–70. [Google Scholar] [CrossRef]
- Prathinia, M.B.; Reshma, S.; Madan Gopal, R.; Sushith; Pravira, K.; Nair, S. Significance of Liver Enzymes as a Baseline Investigation in Recently Diagnosed HIV Positive Patients. Int. J. Biomed. Adv. Res. 2015, 6, 768–770. [Google Scholar]
- Aniagolu, M.; Ugwuene, F.O.; Ikegwuonu, I. The Effects of Highly Active Antiretroviral Therapy on the Activities of Some Liver Enzymes and the Concentrations of Protein and Albumin in HIV Positive Patients in Nsukka South East Nigeria. Int. J. Health Sci. Res. 2017, 7, 67–71. [Google Scholar]
- Agbecha, A.; Ikyernum, J. Impact of HIV-Infection on Serum Liver Enzymes: A Comparative Study among Anti-Retroviral Therapy (ART) Naïve Patients, ART Follow-Up Patients, and HIV Sero-Negative Controls. Int. J. Healthc. Med. Sci. 2018, 4, 196–200. [Google Scholar] [CrossRef]
- Ashakiran, N.; ARSatyanarayana, V.; Ravikanth, M.; SGirish Kumar, P. Abnormalities of Liver Enzymes in HIV Positive Patients on Antiretroviral Therapy. Int. J. Clin. Biochem. Res. 2019, 6, 61–63. [Google Scholar] [CrossRef]
- Olisekodiaka, M.J.; Onuegbu, A.; Igbeneghu, C.; Garuba, W.O.; Amah, U.; Okwara, J.E. Measurement of CD4+ Cells and Liver Functions in HIV Patients on Antiretroviral Therapy. Ann. Int. Med. Dent. Res. 2018, 4, PT01–PT05. [Google Scholar]
- Emokpae, M.A.; Akhimien, J.O. Abnormal Biomarkers of Liver Function in Human Immunodeficiency Virus Type 1 Infected Subjects without Hepatitis B or C Co-Infection and Their Association with Disease Severity. J. Med. Discov. 2018, 3, jmd17058. [Google Scholar] [CrossRef]
- Quaye, O.; Kuleape, J.A.; Bonney, E.Y.; Puplampu, P.; Tagoe, E.A. Imbalance of Antioxidant Enzymes Activities and Trace Elements Levels in Ghanaian HIV-Infected Patients. PLoS ONE 2019, 14, e0220181. [Google Scholar] [CrossRef]
- Ikekpeazu, J.E.; Ibegbu, M.D.; Onyekwelu, K.C.; Uche, O.S. Liver Enzyme Activities in HIV Seropositive Pregnant Women on Highly Active Antiretroviral Therapy (HAART). Int. J. HIV AIDS Res. 2019, 2, 7–10. [Google Scholar]
- Younis, M.Y.G.; El-Sherif, M.; Alhaddad, A.B. Lipid Abnormalities among Libyan HIV-Infected Patients Receiving Antiretroviral (ARV) Drugs and ARV Naïve Patients. J. Adv. Med. Med. Res 2022, 34, 470–481. [Google Scholar] [CrossRef]
- Mutuma, B.; Omedo, R.; Wafula, P.; Demba, N.; Zablon, J.; Shaviya, N.; Were, T. Hepatic Function and Its Association with Clinical Outcomes in Non-Adherent HIV-1 Adults. Afro-Egypt. J. Infect. Endem. Dis. 2023, 13, 146–156. [Google Scholar] [CrossRef]
- Gospel, A.; Chimezie, D.N.; Chimerenka, J.I.; Tochukwu, N. Effects of Anti-Retroviral Therapy on Some Liver Parameters of HIV Sero-Positive Individuals in Rivers State, Nigeria. Int. J. Adv. Acad. Res. 2023, 9, 73–85. [Google Scholar]
- Gbolahan, I.A.; Victoria, M.; Ugbomoiko, D.O.; Gambo, E.D.; Ibrahim, M.A. Estimation of Serum Minerals, Total Protein and Liver Enzymes in HIV Patients Receiving Haart in Federal Medical Centre, Keffi, Nasarawa State, Nigeria. Asian J. Res. Biochem. 2023, 13, 1–11. [Google Scholar] [CrossRef]
- Peter, A.S.; Matthias, G.S.; Ezekiel, C.; Benedo, O.H. Evaluation of Some Liver Enzymes in HIV/AIDS Patients on Antiretroviral Therapy in University of Abuja Teaching Hospital, Nigeria. Int. J. Hum. Health Sci. 2024, 8, 126–131. [Google Scholar] [CrossRef]
- Deshmukh, H.; Patil, V.; Joshi, N.; Nagar, V. Relevance of Hepatic Enzymes in People Living with HIV on Antiretroviral Therapy. Int. J. Pharm. Sci. Rev. Res. 2024, 84, 100–104. [Google Scholar] [CrossRef]
- Odegbemi, O.B.; Olaniyan, M.F.; Muhibi, M.A. Hepatic Toxicity Assessment in HIV’s Interaction with Reverse Transcriptase and Integrase Strand Transfer Inhibitors at a Military Hospital, Southsouth Nigeria. Egypt. Liver J. 2024, 14, 77. [Google Scholar] [CrossRef]
- Nwosu, D.C.; Okolie, N.J.C.; Ajero, C.M.U.; Ojiegbe, G.C.; Oze, G.O.; Ifeanyi, E.; Nnatunanya, I.; Amajuoyi, O.; Ochei, K.C.; Okpara, K.E. Biochemical Alteration in Adults HIV Patients on Antiretroviral Therapy. World J. Pharm. Pharm. Sci. 2015, 4, 153–160. [Google Scholar]
- Dusingize, J.C.; Hoover, D.R.; Shi, Q.; Mutimura, E.; Rudakemwa, E.; Ndacyayisenga, V.; Gakindi, L.; Mulvihill, M.; Sinayobye, J.D.A.; Musabeyezu, E.; et al. Association of Abnormal Liver Function Parameters with HIV Serostatus and CD4 Count in Antiretroviral-Naive Rwandan Women. AIDS Res. Hum. Retroviruses 2015, 31, 723–730. [Google Scholar] [CrossRef]
- Tesfa, E.; Siefu, D.; Belayneh, Y.; Mekonnen, Z. Liver Enzyme Elevation in Patients Taking HAART Compared with Treatment Naïve Controls at Debre Berhan Referral Hospital: A Comparative Cross-Sectional Study, Northeast Ethiopia. BMC Res. Notes 2019, 12, 714. [Google Scholar] [CrossRef]
- Abarca, J.C.; Huerta, L.; Fierro, N.A. Antiretroviral Therapies for Human Immunodeficiency Virus and Liver Disease: Challenges and Opportunities. Ann. Hepatol. 2020, 19, 121–122. [Google Scholar] [CrossRef]
- Osakunor, D.N.M.; Obirikorang, C.; Fianu, V.; Asare, I.; Dakorah, M. Hepatic Enzyme Alterations in HIV Patients on Antiretroviral Therapy: A Case-Control Study in a Hospital Setting in Ghana. PLoS ONE 2015, 10, e0134449. [Google Scholar] [CrossRef]
- Anyanwu, C.F.; JohnBull, T.O.; Usman, I.M.; Aigbogun, E.O.; Ochai, J.; Qasem, A.H.; Alkhayyat, S.S.; Alexiou, A.; Batiha, G.E.S. Substance Use, Highly Active Antiretroviral Therapy, and Liver Enzymes: Evidence From a Cross-Sectional Study of HIV-Infected Adult Patients Without Comorbidities on HAART in the University of Port Harcourt Teaching Hospital. Front. Reprod. Health 2021, 3, 664080. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, D.; Cai, Y.; Zhu, L.; Xiao, Q.; Peng, W.; Chen, B. A Multi-Omic Analysis Reveals That Gamabufotalin Exerts Anti-Hepatocellular Carcinoma Effects by Regulating Amino Acid Metabolism through Targeting STAMBPL1. Phytomedicine 2024, 135, 156094. [Google Scholar] [CrossRef]
- Schank, M.; Zhao, J.; Moorman, J.P.; Yao, Z.Q. The Impact of HIV- and ART-Induced Mitochondrial Dysfunction in Cellular Senescence and Aging. Cells 2021, 10, 174. [Google Scholar] [CrossRef]
- Xuan, W.; Song, D.; Yan, Y.; Yang, M.; Sun, Y. A Potential Role for Mitochondrial DNA in the Activation of Oxidative Stress and Inflammation in Liver Disease. Oxid. Med. Cell. Longev. 2020, 2020, 5835910. [Google Scholar] [CrossRef]
- Sereti, I. Immune Reconstruction Inflammatory Syndrome in HIV Infection: Beyond What Meets the Eye. Top. Antivir. Med. 2020, 27, 106–111. [Google Scholar] [PubMed]
- Jong, E.; Conradie, F.; Berhanu, R.; Black, A.; John, M.A.; Meintjes, G.; Menezes, C. Guideline: Consensus Statement: Management of Drug-Induced Liver Injury in HIV-Positive Patients Treated for TB. South. Afr. J. HIV Med. 2013, 14, 113–119. [Google Scholar] [CrossRef]
- Kaspar, M.B.; Sterling, R.K. Mechanisms of Liver Disease in Patients Infected with HIV. BMJ Open Gastroenterol. 2017, 4, e000166. [Google Scholar] [CrossRef]
- Sherman, K.E.; Thomas, D.L. HIV and Liver Disease: A Comprehensive Update. Top. Antivir. Med. 2022, 40, 547–558. [Google Scholar]
- Ganesan, M.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Liver as a Target of Human Immunodeficiency Virus Infection. World J. Gastroenterol. 2018, 24, 4728–4737. [Google Scholar] [CrossRef]
- Pillaye, J.N.; Marakalala, M.J.; Khumalo, N.; Spearman, W.; Ndlovu, H. Mechanistic Insights into Antiretroviral Drug-Induced Liver Injury. Pharmacol. Res. Perspect. 2020, 8, e00598. [Google Scholar] [CrossRef]
- Gökengin, D.; Yamazhan, T. Hepatic Adverse Events during Highly Active Antiretroviral Therapy Containing Nevirapine: A Case Report. Ann. Clin. Microbiol. Antimicrob. 2002, 1, 1. [Google Scholar] [CrossRef]
- Strauss, K.L.E.; Phoswa, W.N.; Mokgalaboni, K. The Impact of Antiretroviral Therapy on Liver Function Among Pregnant Women Living with HIV in Co-Existence with and Without Pre-Eclampsia. Viruses 2025, 17, 28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss, K.-L.E.; Phoswa, W.N.; Hanser, S.; Mokgalaboni, K. HIV Infection and Antiretroviral Therapy Impair Liver Function in People Living with HIV: Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 955. https://doi.org/10.3390/ph18070955
Strauss K-LE, Phoswa WN, Hanser S, Mokgalaboni K. HIV Infection and Antiretroviral Therapy Impair Liver Function in People Living with HIV: Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(7):955. https://doi.org/10.3390/ph18070955
Chicago/Turabian StyleStrauss, Kay-Lee E., Wendy N. Phoswa, Sidney Hanser, and Kabelo Mokgalaboni. 2025. "HIV Infection and Antiretroviral Therapy Impair Liver Function in People Living with HIV: Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 7: 955. https://doi.org/10.3390/ph18070955
APA StyleStrauss, K.-L. E., Phoswa, W. N., Hanser, S., & Mokgalaboni, K. (2025). HIV Infection and Antiretroviral Therapy Impair Liver Function in People Living with HIV: Systematic Review and Meta-Analysis. Pharmaceuticals, 18(7), 955. https://doi.org/10.3390/ph18070955





