Therapeutic Potential of Glucose Oxidase-Loaded Biogenic Mesoporous Silica Nanoparticles in Ovarian Cancer
Abstract
1. Introduction
2. Results and Discussion
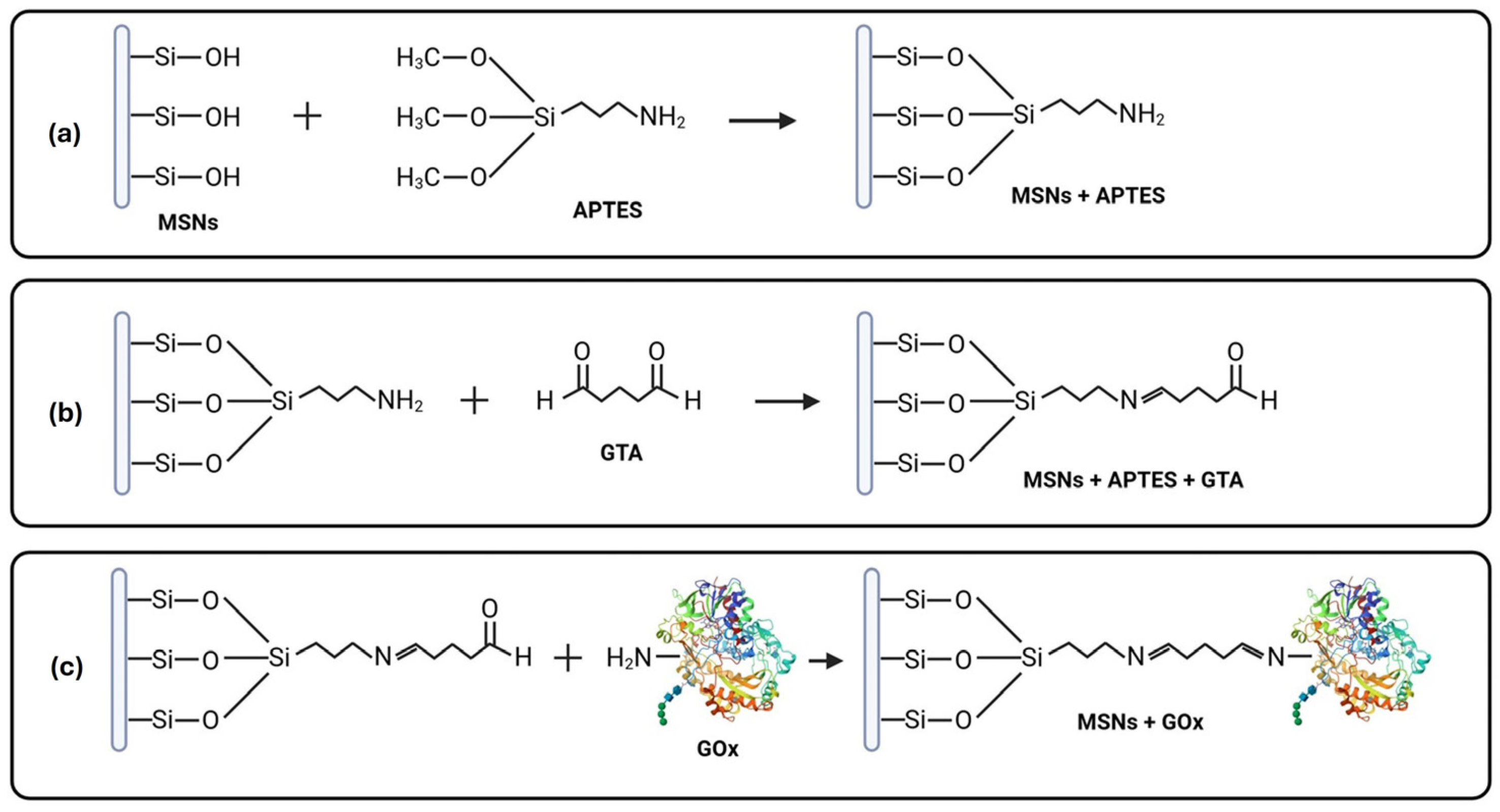
2.1. Extraction of Biogenic MSNs and Immobilization of GOx
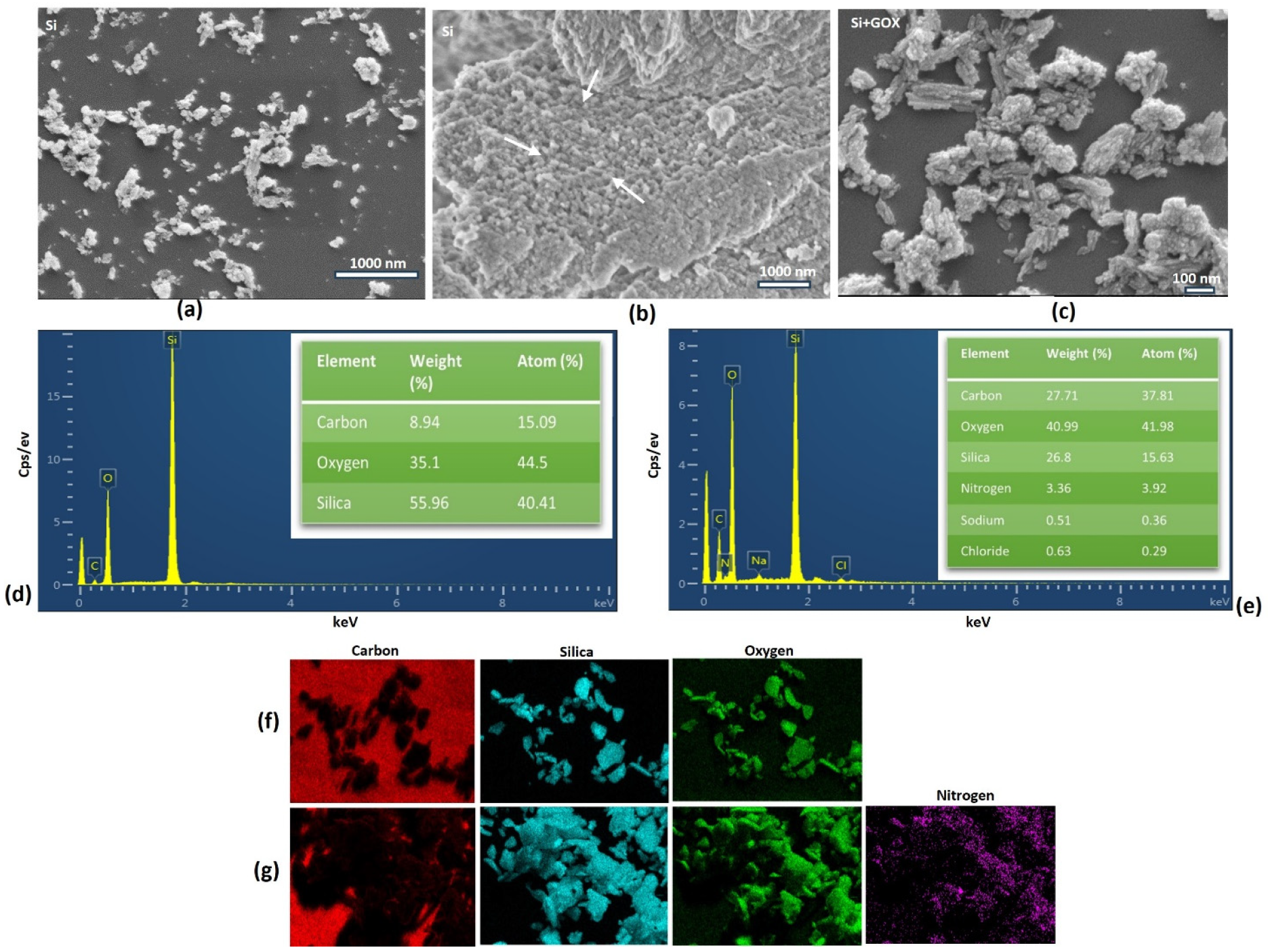
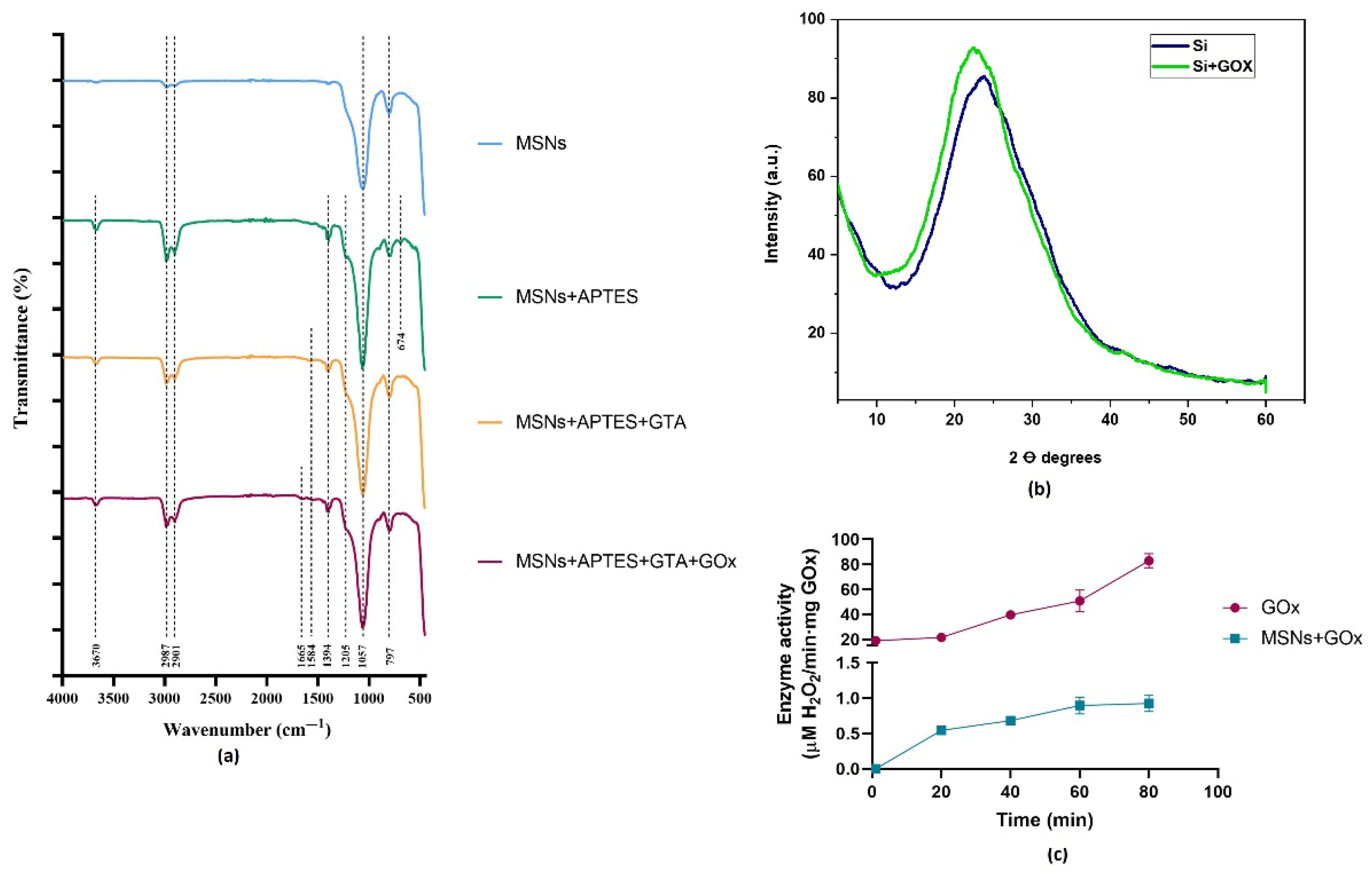
2.2. Characterization of the MSNs Pre- and Post-Immobilization
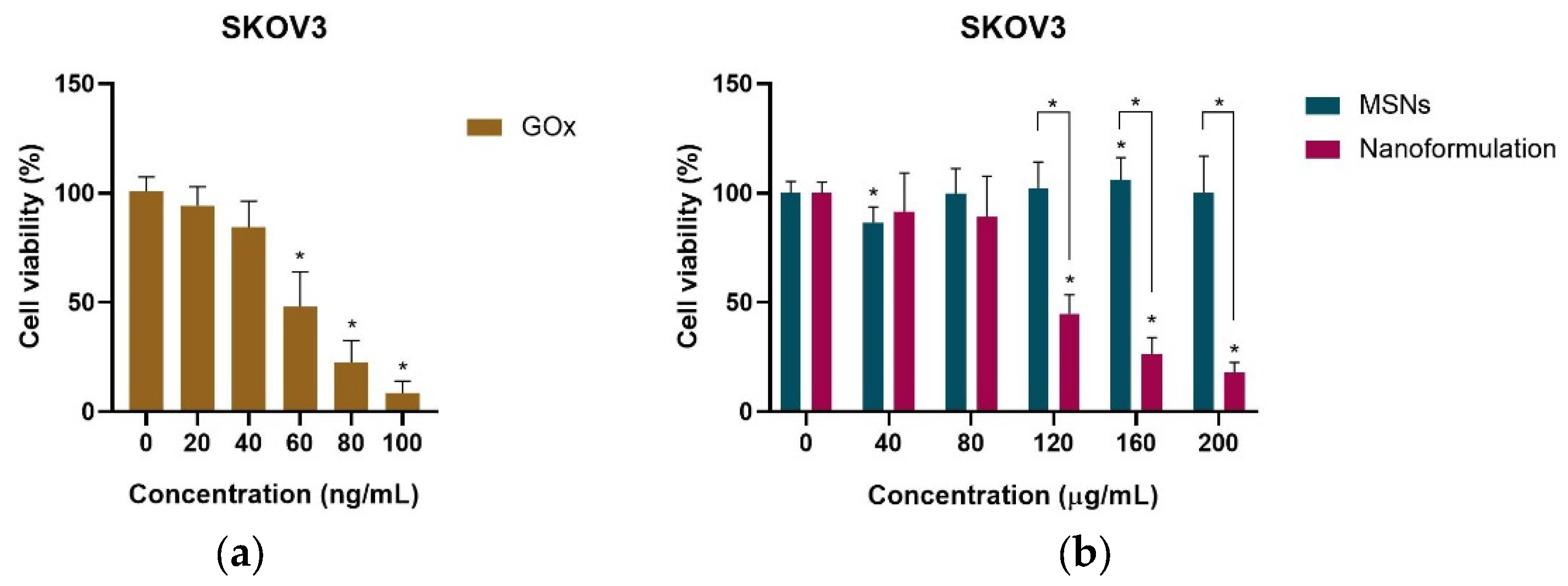
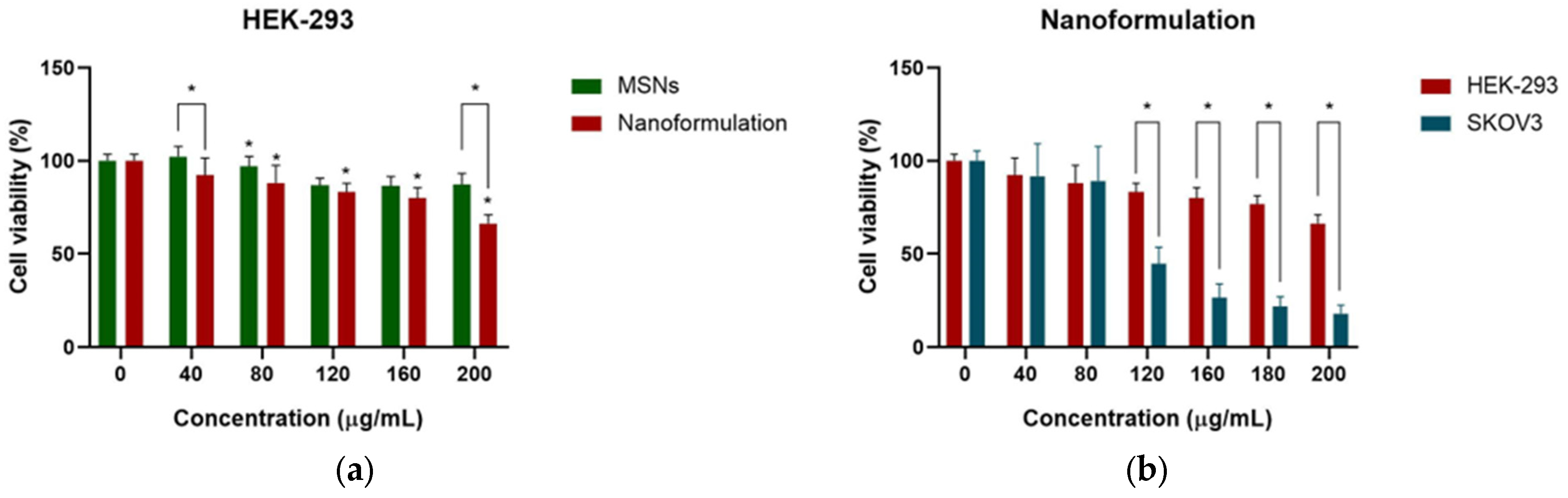
2.3. Cell Viability Assays of SKOV3 and HEK-293 Cells
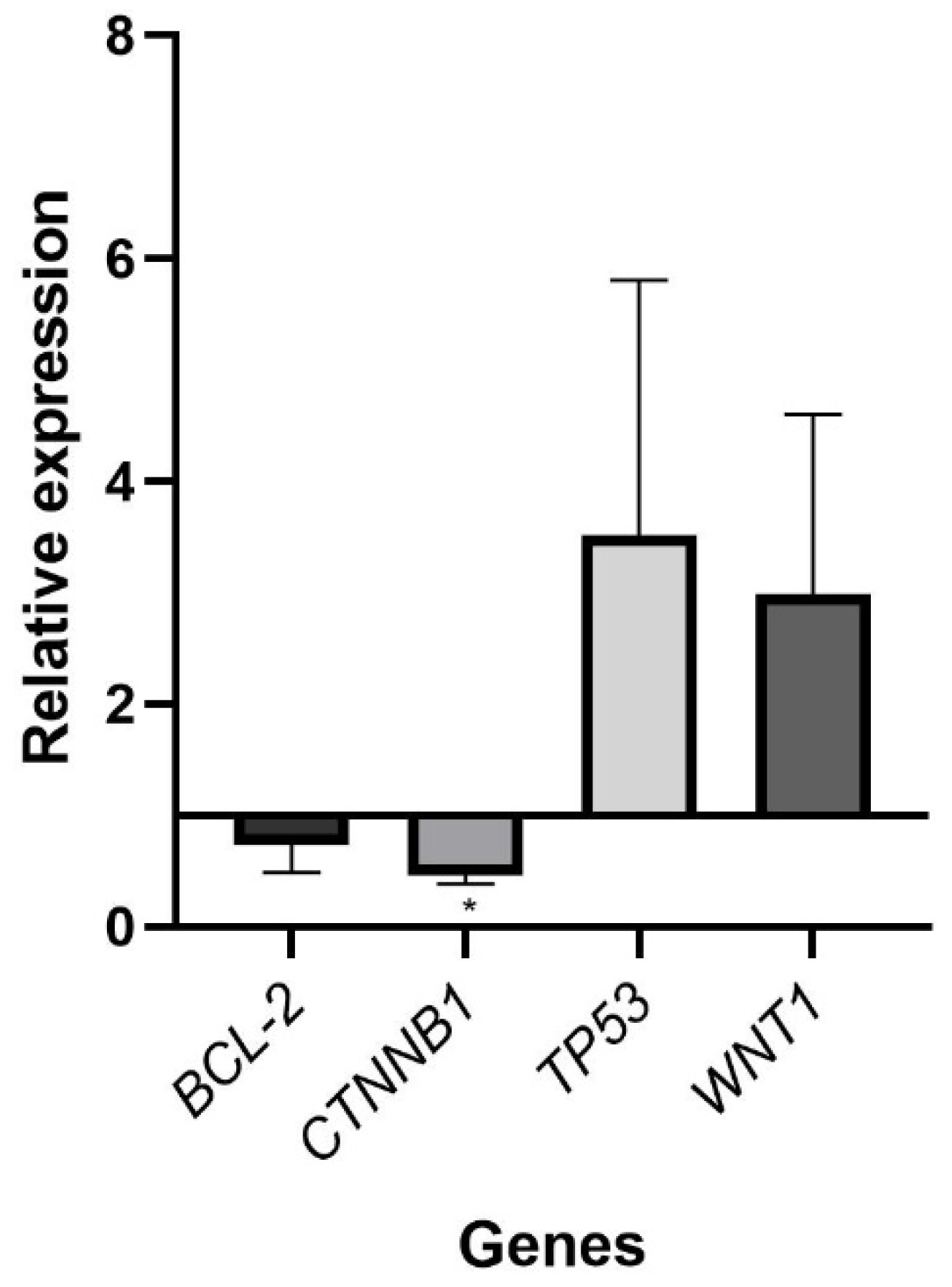
2.4. Differential Gene Expression Analysis by qPCR
2.5. Future Perspectives
3. Materials and Methods
3.1. Materials
3.2. Biogenic MSNs Extraction
3.3. Glucose Oxidase Immobilization
3.4. Determination of Drug Encapsulation Efficiency
3.5. Activity Assay
3.6. Biogenic MSNs Characterization Pre- and Post-Immobilization
3.7. Cell Culture
3.8. Cell Viability Assay
3.9. RNA Extraction, cDNA Synthesis, and Expression Analysis by RT-qPCR
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OC | Ovarian cancer |
| GOx | Glucose oxidase |
| MSNs | Mesoporous silica nanoparticles |
| DDS | Drug delivery systems |
| APTES | 3-aminopropiltrietoxysilane |
| GTA | Glutaraldehyde |
| CTNNB1 | Β-catenin |
| EOC | Epithelial ovarian cancer |
| H2O2 | Hydrogen peroxide |
| FAD | Flavin adenine dinucleotide |
| ROS | Reactive oxygen species |
| NP | Nanoparticles |
| HNO3 | Nitric acid |
| H2SO4 | Sulfuric acid |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| RPMI | Roswell Park Memorial Institute 1640 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| SKOV3 | Human ovarian adenocarcinoma |
| HEK-293 | Human embryonic kidney |
| MTT | 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide |
| BSA | Bovine serum albumin |
| DEE | Drug encapsulation efficiency |
| FTIR | Fourier transform infrared spectroscopy |
| DLS | Dynamic light scattering |
| XRD | X-ray diffractometer |
| SEM | Scanning electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| BET | Brunauer–Emmett–Teller |
| BJH | Barret–Joyner–Halenda |
| FBS | Fetal bovine serum |
| SAM | Self-assembled monolayer |
| MSS | Mesoporous silica spheres |
| HGSOC | High-grade serous ovarian cancer |
| PEG | Polyethylene glycol |
| HER2 | Human epidermal growth factor 2 |
| FA | Folic acid |
| CD44 | Cluster of differentiation 44 |
| VEGF | Vascular endothelial growth factor |
| DOX | Doxorubicin |
| PLGA | Poly(d,l-lactide-co-glycolide) |
| FAK | Focal adhesion kinase |
| CCM | Cancer cell membrane |
| HMON | Hollow mesoporous organosilica nanoparticle |
| TPZ | Tirapazamine |
References
- Győrffy, B. Discovery and Ranking of the Most Robust Prognostic Biomarkers in Serous Ovarian Cancer. Geroscience 2023, 45, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Al-Ani, O.; Al-Ani, F. Epidemiology and Risk Factors for Ovarian Cancer. Menopause Rev. 2023, 22, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Mullangi, S.; Vadakekut, E.S.; Lekkala, M.R. Epithelial Ovarian Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567760/ (accessed on 20 August 2024).
- Schorge, J.O.; Modesitt, S.C.; Coleman, R.L.; Cohn, D.E.; Kauff, N.D.; Duska, L.R.; Herzog, T.J. SGO White Paper on Ovarian Cancer: Etiology, Screening and Surveillance. Gynecol. Oncol. 2010, 119, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barriga, J.J. Mortality Trends and Potential Years of Life Lost Due to Ovarian Cancer in Mexico, 2000–2014. Gac. Med. Mex. 2018, 154, 367–375. [Google Scholar] [CrossRef]
- Lopez, A.; Reyna, D.E.; Gitego, N.; Kopp, F.; Zhou, H.; Miranda-Roman, M.A.; Nordstrøm, L.U.; Narayanagari, S.R.; Chi, P.; Vilar, E.; et al. Co-Targeting of BAX and BCL-XL Proteins Broadly Overcomes Resistance to Apoptosis in Cancer. Nat. Commun. 2022, 13, 1199. [Google Scholar] [CrossRef] [PubMed]
- Spitz, A.Z.; Gavathiotis, E. Physiological and Pharmacological Modulation of BAX. Trends Pharmacol. Sci. 2022, 43, 206–220. [Google Scholar] [CrossRef]
- Yoon, O.; Roh, J. Downregulation of KLF4 and the Bcl-2/Bax Ratio in Advanced Epithelial Ovarian Cancer. Oncol. Lett. 2012, 4, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Hu, C.; Li, H.; Jiang, M. Wnt Signaling and Tumors. Mol. Clin. Oncol. 2024, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garza, L.E.; Dominguez-Vigil, I.G.; Garza-Martinez, J.; Valdez-Aparicio, E.A.; Barrera-Barrera, S.A.; Barrera-Saldana, H.A. Personalized Medicine in Ovarian Cancer: A Perspective From Mexico. World J. Oncol. 2021, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose Oxidase: Natural Occurrence, Function, Properties and Industrial Applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Vitolo, M. Overview on Glucose Oxidase. World J. Pharm. Res. 2021, 10, 130–155. [Google Scholar]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Mano, N. Engineering Glucose Oxidase for Bioelectrochemical Applications. Bioelectrochemistry 2019, 128, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Wang, Y.; Li, X.; Wan, M.; Zhang, G.; Leng, F.; Zhang, H. Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. Int. J. Mol. Sci. 2022, 23, 9841. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, J.; Wang, Y.; Li, W. Glucose Oxidase and Metal Catalysts Combined Tumor Synergistic Therapy: Mechanism, Advance and Nanodelivery System. J. Nanobiotechnol. 2023, 21, 400. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Y.; Cheng, H.; Meng, Y.; Shi, J.; Chen, Y.; Wu, Y. Enhanced Cancer Starvation Therapy Based on Glucose Oxidase/3-Methyladenine-Loaded Dendritic Mesoporous Organosilicon Nanoparticles. Biomolecules 2021, 11, 1363. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Hu, Y.R.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Multimodal Synergistic Cancer Therapy. Adv. Mater. 2019, 31, 1808325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Jia, Z.; Da, M.; Zhao, J.; Yang, R.; Chen, D. Recent Advances in Glucose Oxidase-Based Nanocarriers for Tumor Targeting Therapy. Heliyon 2023, 9, 2405–8440. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Chen, Q.; Li, C.; Li, Z.; Lin, J. Recent Advances in Glucose-Oxidase-Based Nanocomposites for Tumor Therapy. Small 2019, 15, 1903895. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.R.M.M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215. [Google Scholar] [CrossRef] [PubMed]
- Uriostegui-Pena, A.G.; Torres-Copado, A.; Ochoa-Sanchez, A.; Luna-Bárcenas, G.; Sahare, P.; Paul, S. Nanoformulated Phytochemicals in Skin Anti-Aging Research: An Updated Mini Review. 3 Biotech 2025, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Sahare, P.; Ruiz-Manriquez, L.M.; Anguiano, B.; Banerjee, A.; Pathak, S.; Duttaroy, A.K.; Luna-Bárcenas, G.; Paul, S. Recent Advances in Nanomedicine for the Diagnosis and Therapy of Thyroid Disorders. 3 Biotech 2025, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 558493. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 486705. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and Its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef] [PubMed]
- Djayanti, K.; Maharjan, P.; Cho, K.H.; Jeong, S.; Kim, M.S.; Shin, M.C.; Min, K.A. Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations. Int. J. Mol. Sci. 2023, 24, 6349. [Google Scholar] [CrossRef] [PubMed]
- Sola-Rabada, A.; Sahare, P.; Hickman, G.J.; Vasquez, M.; Canham, L.T.; Perry, C.C.; Agarwal, V. Biogenic porous silica and silicon sourced from Mexican Giant Horsetail (Equisetum myriochaetum) and their application as supports for enzyme immobilization. Colloids Surf. B Biointerfaces 2018, 166, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Živojević, K.; Mladenović, M.; Djisalov, M.; Mundzic, M.; Ruiz-Hernandez, E.; Gadjanski, I.; Knežević, N.Ž. Advanced mesoporous silica nanocarriers in cancer theranostics and gene editing applications. J. Control. Release 2021, 337, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, Y.; Ke, W.; Chen, W.; Wang, W.; Ge, Z. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett. 2017, 17, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Anraku, Y.; Kataoka, K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew. Chem. Int. Ed. 2020, 59, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Pang, H.; Ma, T.; Du, F.; Li, L.; Huang, J.; Ma, L.; Qiu, L. Ultrasound Targeted Microbubble Destruction Combined with Fe-MOF Based Bio-/Enzyme-Mimics Nanoparticles for Treating of Cancer. J. Nanobiotechnol. 2021, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Ye, R.; Wang, Y.; Guo, M.; Zhu, M.; Yin, F.; Wang, Y.; Lai, X.; Wang, Y.; Qi, Z.; et al. Targeted PH-Responsive Biomimetic Nanoparticle-Mediated Starvation-Enhanced Chemodynamic Therapy Combined with Chemotherapy for Ovarian Cancer Treatment. Int. J. Pharm. 2024, 661, 124426. [Google Scholar] [CrossRef] [PubMed]
- Alsamarat, R.; Sunoqrot, S. A Glucose Oxidase-Curcumin Composite Nanoreactor for Multimodal Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2024, 7, 4611–4621. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lu, Z.; Lv, Q.; Jiang, Y.; Zhang, L.; Wang, Z. Tumor Metabolism Destruction via Metformin-Based Glycolysis Inhibition and Glucose Oxidase-Mediated Glucose Deprivation for Enhanced Cancer Therapy. Acta Biomater. 2022, 145, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Youden, B.; Yang, Y.; Chen, Y.; Carrier, A.; Cui, S.; Oakes, K.; Servos, M.; Jiang, R.; Zhang, X. Synergistic Multimodal Cancer Therapy Using Glucose Oxidase@CuS Nanocomposites. ACS Appl. Mater. Interfaces 2021, 13, 41464–41472. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Sanchez, A.; Sahare, P.; Pathak, S.; Banerjee, A.; Estevez, M.; Duttaroy, A.K.; Luna-Bárcenas, G.; Paul, S. Evaluation of the Synergistic Effects of Curcumin-Resveratrol Co-Loaded Biogenic Silica on Colorectal Cancer Cells. Front. Pharmacol. 2024, 15, 1341773. [Google Scholar] [CrossRef] [PubMed]
- Currie, H.A.; Perry, C.C. Silica in Plants: Biological, Biochemical and Chemical Studies. Ann. Bot. 2007, 100, 1383. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V.V.; Hickman, G.J.; Sola-Rabada, A.; Perry, C.C. Distributions of Silica and Biopolymer Structural Components in the Spore Elater of Equisetum arvense, an Ancient Silicifying Plant. Front. Plant Sci. 2019, 10, 442724. [Google Scholar] [CrossRef] [PubMed]
- Jenie, S.N.A.; Plush, S.E.; Voelcker, N.H. Recent Advances on Luminescent Enhancement-Based Porous Silicon Biosensors. Pharm. Res. 2016, 33, 2314–2336. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Promptmas, C.; Thanapitak, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Chaowicharat, E.; Jeamsaksiri, W. Optimization of 3-Aminopropyltriethoxysilane Functionalization on Silicon Nitride Surface for Biomolecule Immobilization. Talanta 2020, 207, 120305. [Google Scholar] [CrossRef] [PubMed]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and Characterization of Biomolecule Immobilization on Silicon Substrates Using (3-Aminopropyl)Triethoxysilane (APTES) and Glutaraldehyde Linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, Challenges and Opportunities of Enzyme Immobilization on Porous Silicon for Biosensing Applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- To, T.D.; Nguyen, A.T.; Phan, K.N.T.; Truong, A.T.T.; Doan, T.C.D.; Dang, C.M. Modification of Silicon Nitride Surfaces with GOPES and APTES for Antibody Immobilization: Computational and Experimental Studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045006. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)Triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Huang, A.; Zhu, H.; Ding, J.; Zhang, W.; Chen, Y. Immobilization of Lipase on Silica Nanoparticles by Adsorption Followed by Glutaraldehyde Cross-Linking. Bioprocess. Biosyst. Eng. 2023, 46, 25–38. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization; Springer: Cham, Switzerland, 2018; pp. 113–145. [Google Scholar] [CrossRef]
- Idris, A.M.; El-Zahhar, A.A. Indicative Properties Measurements by SEM, SEM-EDX and XRD for Initial Homogeneity Tests of New Certified Reference Materials. Microchem. J. 2019, 146, 429–433. [Google Scholar] [CrossRef]
- Velez, J.; Arce, R.; Alburquenque, D.; Gautier, J.L.; Zuñiga, C.; Herrera, F. Simple steps for synthesis of silicon oxide mesoporous materials used as template. J. Chil. Chem. Soc. 2013, 58, 1998–2000. [Google Scholar] [CrossRef][Green Version]
- Chang, S.S.; Clair, B.; Ruelle, J.; Beauchêne, J.; Di Renzo, F.; Quignard, F.; Zhao, G.J.; Yamamoto, H.; Gril, J. Mesoporosity as a New Parameter for Understanding Tension Stress Generation in Trees. J. Exp. Bot. 2009, 60, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yanfei, Z.; Jiao, W. Mesoporous Carbon Materials: Synthesis Methods, Properties, and Advanced Applications. Front. Mater. 2025, 12, 1548671. [Google Scholar] [CrossRef]
- Zhou, G.; Fung, K.K.; Wong, L.W.; Chen, Y.; Renneberg, R.; Yang, S. Immobilization of glucose oxidase on rod-like and vesicle-like mesoporous silica for enhancing current responses of glucose biosensors. Talanta 2011, 84, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Kleitz, F.; Linden, M.; Yu, C.; Popat, A. Silica Nanoparticles: A Review of Their Safety and Current Strategies to Overcome Biological Barriers. Adv. Drug Deliv. Rev. 2023, 203, 115115. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lin, W.; Lai, H.; Tong, J.; Lei, J.; Yuan, M.; Zhang, Y.; Cui, C. Understanding the Relationship between Particle Size and Ultrasonic Treatment during the Synthesis of Metal Nanoparticles. Ultrason. Sonochem 2021, 73, 105497. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W.S.W. Ultrasonication an Intensifying Tool for Preparation of Stable Nanofluids and Study the Time Influence on Distinct Properties of Graphene Nanofluids—A Systematic Overview. Ultrason. Sonochem 2021, 73, 105479. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi Firouzabadi, B.; Gigliobianco, M.R.; Joseph, J.M.; Censi, R.; Di Martino, P. Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties. Pharmaceutics 2022, 14, 2708. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of Amorphous Silica and Sulfonic Acid Functionalized Silica Used as Reinforced Phase for Polymer Electrolyte Membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef]
- Bayu, A.; Nandiyanto, D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Shokri, B.; Firouzjah, M.; Hosseini, S.I. FTIR Analysis of Silicon Dioxide Thin Film Deposited by Metal Organic-Based PECVD. In Proceedings of the 19th International Symposium on Plasma Chemistry Society, Bochum, Germany, 6–31 July 2009; Volume 2631, pp. 1–4. [Google Scholar]
- Li, K.M.; Jiang, J.G.; Tian, S.C.; Chen, X.J.; Yan, F. Influence of Silica Types on Synthesis and Performance of Amine-Silica Hybrid Materials Used for CO2 Capture. J. Phys. Chem. 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R.S. Synthesis of SiO2 Nanoparticles by Sol-Gel Method and Their Optical and Structural Properties. Rom. J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Ramasubramaniam, S.; Govindarajan, C.; Nasreen, K.; Sudha, P.N. Removal of Cadmium (II) Ions from Aqueous Solution Using Chitosan/Starch Polymer Blend. Compos. Interfaces 2014, 21, 95–109. [Google Scholar] [CrossRef]
- Latwal, M.; Arora, S.; Joshi, A.; Irfan, M.; Pandey, G. Sustainable Ceramic Membrane for Decontamination of Water: A Cost-Effective Approach. Heliyon 2023, 9, e13321. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.P.; Akil, H.M.; Nasir, R.M.; Nurdijati, S. Abrasive Wear Performance and Antibacterial Assessment of Untreated and Treated ZnO-Reinforced Polymer Composite. Polym. Compos. 2013, 34, 1020–1032. [Google Scholar] [CrossRef]
- Pozo, C.; Díaz-Visurraga, J.; Contreras, D.; Freer, J.; Rodríguez, J. Characterization of temporal biodegradation of radiata pine by Gloeophyllum trabeum through principal component analysis-based two-dimensional correlation FTIR spectroscopy. J. Chil. Chem. Soc. 2016, 61, 2878–2883. [Google Scholar] [CrossRef]
- Chey, C.O.; Ibupoto, Z.H.; Khun, K.; Nur, O.; Willander, M. Indirect Determination of Mercury Ion by Inhibition of a Glucose Biosensor Based on ZnO Nanorods. Sensors 2012, 12, 15063–15077. [Google Scholar] [CrossRef] [PubMed]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.C. Synthesis and Characterization of Amorphous Mesoporous Silica from Palm Kernel Shell Ash. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 159–164. [Google Scholar] [CrossRef]
- Nallathambi, G.; Ramachandran, T.; Rajendran, V.; Palanivelu, R. Effect of Silica Nanoparticles and BTCA on Physical Properties of Cotton Fabrics. Mater. Res. 2011, 14, 552–559. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. An Alternative and Simple Method for the Preparation of Bare Silica Nanoparticles Using Sugarcane Waste Ash, an Abundant and Despised Residue in the Brazilian Industry. J. Braz. Chem. Soc. 2019, 30, 1524–1533. [Google Scholar] [CrossRef]
- Prasetyo, A.B.; Handayani, M.; Sulistiyono, E.; Syahid, A.N.; Febriana, E.; Mayangsari, W.; Muslih, E.Y.; Nugroho, F.; Firdiyono, F. Development of High Purity Amorphous Silica from Emulsifier Silicon by Pyrolysis Process at Temperature of 700 °C. J. Phys. Conf. Ser. 2022, 2190, 012013. [Google Scholar] [CrossRef]
- Khamis, F.; Arafah, D.E. Thermoluminescence Characteristics of Natural Quartz and Synthesized Silica Glass Prepared by Sol-Gel Technique. Asian J. Phys. Chem. Sci. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Rana, M.; Banerjee, C.; Chowdhury, P. Studies on Optical Signal Due to Oxygen Effect on Hydrogenated Amorphous/Crystalline Silicon Thin Films. Appl. Phys. A Mater. Sci. Process 2021, 127, 192. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of Amorphous Silica Nanoparticles: Status and Prospects. Nanomedicine 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous Silica Nanoparticles for Bioadsorption, Enzyme Immobilisation, and Delivery Carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, X.; Jin, Q.; Yi, D. Concentrically Encapsulated Dual-Enzyme Capsules for Synergistic Metabolic Disorder Redressing and Cytotoxic Intermediates Scavenging. Nanomaterials 2022, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kwak, M.; Kim, J.; Lee, T.G.; Heo, M.B. Comparative Study on Nanotoxicity in Human Primary and Cancer Cells. Nanomaterials 2022, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, K.; Shao, X.; Shi, X.; He, Y.; Liu, Z.; Jacob, J.A.; Deng, L. Nanotoxic Effects of Silver Nanoparticles on Normal HEK-293 Cells in Comparison to Cancerous HeLa Cell Line. Int. J. Nanomed. 2021, 16, 753. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.W.; Austin, S.; Gamma, M.; Cheff, D.M.; Lee, T.D.; Wilson, K.M.; Johnson, J.; Travers, J.; Braisted, J.C.; Guha, R.; et al. Cytotoxic Profiling of Annotated and Diverse Chemical Libraries Using Quantitative High-Throughput Screening. SLAS Discov. 2020, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Tudrej, P.; Kujawa, K.A.; Cortez, A.J.; Lisowska, K.M. Characteristics of in Vitro Model Systems for Ovarian Cancer Studies. Oncol. Clin. Pract. 2019, 15, 246–259. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, H.; Hong, R.; Gong, J.; Wan, Y.; Fu, Q.; Huang, K.; Li, Y.; Wang, N.; Zhao, P.; et al. A self-accelerating ‘copper bomb’ strategy activated innate and adaptive immune response against triple-negative breast cancer. Bioact. Mater. 2025, 49, 193. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.; Ranjan, A.; Ramsey, J.D. Intracellular Delivery of Glucose Oxidase for Enhanced Cytotoxicity toward PSMA-Expressing Prostate Cancer Cells. Macromol. Biosci. 2019, 19, 1900183. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211. [Google Scholar] [CrossRef] [PubMed]
- Adekola, K.; Rosen, S.T.; Shanmugam, M. Glucose Transporters in Cancer Metabolism. Curr. Opin. Oncol. 2012, 24, 650. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Geng, X.; Zhang, C. Cancer Cells and Effects of Glucose Starvation. In Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy; Springer: Cham, Switzerland, 2019; pp. 2169–2184. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.S. From “omics” to Complex Disease: A Systems Biology Approach to Gene-Environment Interactions in Cancer. Cancer Cell Int. 2010, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wong, A.; Sun, H.; Bhatia, V.; Javier, G.; Jana, S.; Wu, Q.; Montgomery, R.B.; Wright, J.L.; Lam, H.M.; et al. A Combinatorial Genetic Strategy for Exploring Complex Genotype–Phenotype Associations in Cancer. Nat. Genet. 2024, 56, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Ittiudomrak, T.; Puthong, S.; Roytrakul, S.; Chanchao, C. α-Mangostin and Apigenin Induced Cell Cycle Arrest and Programmed Cell Death in SKOV-3 Ovarian Cancer Cells. Toxicol. Res. 2019, 35, 167–179. [Google Scholar] [CrossRef]
- AmeliMojarad, M.; Cui, X.; Shariati, P. Pan-Cancer Analysis of CTNNB1 with Potential as a Therapeutic Target for Human Tumorigenesis. Inform. Med. Unlocked 2023, 42, 101331. [Google Scholar] [CrossRef]
- Du, L.; Qian, X.; Dai, C.; Wang, L.; Huang, D.; Wang, S.; Shen, X. Screening the Molecular Targets of Ovarian Cancer Based on Bioinformatics Analysis. Tumori 2015, 101, 384–389. [Google Scholar] [CrossRef]
- Ledinek, Ž.; Sobočan, M.; Knez, J. The Role of CTNNB1 in Endometrial Cancer. Dis. Markers 2022, 2022, 1442441. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cho, U.; Yoo, A.; Jung, C.L.; Kim, B.; Kim, H.; Lee, J.; Jo, H.A.; Han, Y.; Song, M.H.; et al. Wnt/β-Catenin Inhibition by CWP232291 as a Novel Therapeutic Strategy in Ovarian Cancer. Front. Oncol. 2022, 12, 852260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, Y.; Feng, S.; Zou, Y.; Xu, S.; Qiu, S.; Li, L.; Zheng, J. Role of Wnt/β-Catenin, Wnt/c-Jun N-Terminal Kinase and Wnt/Ca2+ Pathways in Cisplatin-Induced Chemoresistance in Ovarian Cancer. Exp. Ther. Med. 2016, 12, 3851–3858. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Raimondo, D.; Reppuccia, S.; Ruggiero, A.; Arena, A.; Casadio, P.; Zullo, F.; Insabato, L.; Seracchioli, R.; et al. Prognostic Significance of CTNNB1 Mutation in Early Stage Endometrial Carcinoma: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2022, 306, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, S.; Zhu, M.; Zhang, H.; Wang, J.; Xu, Q.; Lin, K.; Zhou, X.; Tao, M.; Li, C.; et al. PS341 Inhibits Hepatocellular and Colorectal Cancer Cells through the FOXO3/CTNNB1 Signaling Pathway. Sci. Rep. 2016, 6, 22090. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Ji, S.; Li, Y.; Fu, L.Y.; Jiang, T.; Meng, F.D. β-Catenin Promotes Cell Proliferation, Migration, and Invasion but Induces Apoptosis in Renal Cell Carcinoma. Onco Targets Ther. 2017, 10, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Luo, P.; Wang, L.; Yang, S.; Qin, S.; Wei, Z.; Liu, J. CTNNB1 Knockdown Inhibits Cell Proliferation and Aldosterone Secretion Through Inhibiting Wnt/β-Catenin Signaling in H295R Cells. Technol. Cancer Res. Treat. 2020, 19, 1533033820979685. [Google Scholar] [CrossRef] [PubMed]
- Tuna, M.; Ju, Z.; Yoshihara, K.; Amos, C.I.; Tanyi, J.L.; Mills, G.B. Clinical Relevance of TP53 Hotspot Mutations in High-Grade Serous Ovarian Cancers. Br. J. Cancer 2019, 122, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mandilaras, V.; Garg, S.; Cabanero, M.; Tan, Q.; Pastrello, C.; Burnier, J.; Karakasis, K.; Wang, L.; Dhani, N.C.; Butler, M.O.; et al. TP53 Mutations in High Grade Serous Ovarian Cancer and Impact on Clinical Outcomes: A Comparison of next Generation Sequencing and Bioinformatics Analyses. Int. J. Gynecol. Cancer 2019, 29, 346–352. [Google Scholar] [CrossRef]
- Silwal-Pandit, L.; Langerød, A.; Børresen-Dale, A.L. TP53 Mutations in Breast and Ovarian Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a026252. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.; Sahib, U.I.; Taqi, Z.; Taha, A.; Sulaiman, G.; Albukhaty, S.; Al-Shammari, A.; Alwahibi, M.; Soliman, D.; Dewir, Y.H.; et al. Linalool-Loaded Glutathione-Modified Gold Nanoparticles Conjugated with Calnn Peptide as Apoptosis Inducer and Nf-Κb Translocation Inhibitor in Skov-3 Cell Line. Int. J. Nanomed. 2020, 15, 9025–9047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Huang, F.H.; Zhang, G.L.; Bai, D.P.; de Felici, M.; Huang, Y.F.; Gurunathan, S. Novel Biomolecule Lycopene-Reduced Graphene Oxide-Silver Nanoparticle Enhances Apoptotic Potential of Trichostatin A in Human Ovarian Cancer Cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551–7575. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, N.; Zhao, Y.; Wang, W.; Feng, X.L. Salidroside Induces Apoptosis in Human Ovarian Cancer SKOV3 and A2780 Cells through the P53 Signaling Pathway. Oncol. Lett. 2018, 15, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Kupryjańczyk, J.; Szymańska, T.; Ma̧dry, R.; Timorek, A.; Stelmachów, J.; Karpińska, G.; Rembiszewska, A.; Ziółkowska, I.; Kraszewska, E.; Dȩbniak, J.; et al. Evaluation of Clinical Significance of TP53, BCL-2, BAX and MEK1 Expression in 229 Ovarian Carcinomas Treated with Platinum-Based Regimen. Br. J. Cancer 2003, 88, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Babaei, G.; Gholizadeh-Ghaleh Aziz, S.; Abolhasani, S. Alantolactone and ZnO Nanoparticles Induce Apoptosis Activity of Cisplatin in an Ovarian Cancer Cell Line (SKOV3). Res. Pharm. Sci. 2022, 17, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, L.; Dadashpour, M.; Sadeghzadeh, H.; Mahdavi, M.; Zarghami, N. Enhanced Anti-Proliferative and pro-Apoptotic Effects of Metformin Encapsulated PLGA-PEG Nanoparticles on SKOV3 Human Ovarian Carcinoma Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/β-Catenin Signalling in Ovarian Cancer: Insights into Its Hyperactivation and Function in Tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Krithicaa Narayanaa, Y.; Perumalsamy, N.K.; Warrier, S.; Perumalsamy, L.R.; Dharmarajan, A. Wnt Antagonist as Therapeutic Targets in Ovarian Cancer. Int. J. Biochem. Cell Biol. 2022, 145, 106191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt Signaling in Breast Cancer: Biological Mechanisms, Challenges and Opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, A.B.; Joseph, P.; Kovalenko, O.; Singh, S.; Armstrong, A.; Redline, R.; Resnick, K.; Zanotti, K.; Waggoner, S.; Di Feo, A. Critical Role of Wnt/β-Catenin Signaling in Driving Epithelial Ovarian Cancer Platinum Resistance. Oncotarget 2015, 6, 23720–23734. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, Y.; Lee, J.S.; Gonik, A.M.; Dong, T.; Fung, G.; Sanchez, E.; Xing, L.; Cheng, H.R.; Luo, J.; et al. “OA02” Peptide Facilitates the Precise Targeting of Paclitaxel-Loaded Micellar Nanoparticles to Ovarian Cancer In Vivo. Cancer Res. 2012, 72, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Wlodarczyk, M.T.; Dragulska, S.A.; Poursharifi, M.; Chen, Y.; Martignetti, J.A. PH-Responsive Nanoparticles for Pt (II) Delivery to Ovarian Cancer Cells. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Gralewska, P.; Gajek, A.; Marczak, A.; Rogalska, A. Targeted Nanocarrier-Based Drug Delivery Strategies for Improving the Therapeutic Efficacy of PARP Inhibitors against Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 8304. [Google Scholar] [CrossRef] [PubMed]
- Chuan, D.; Mu, M.; Hou, H.; Zhao, N.; Li, J.; Tong, A.; Zou, B.; Chen, H.; Han, B.; Guo, G. Folic Acid-Functionalized Tea Polyphenol as a Tumor-Targeting Nano-Drug Delivery System. Mater. Des. 2021, 206, 109805. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ding, J.; Han, L.; Guo, X. Anti-Tumor Efficacy of Folate Modified PLGA-Based Nanoparticles for the Co-Delivery of Drugs in Ovarian Cancer. Drug Des. Devel. Ther. 2019, 13, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, S.; Sienkiewicz, J.; Zhou, H.; Berahovich, R.; Sun, J.; Li, M.; Ocampo, A.; Liu, X.; Huang, Y.; et al. HER2-CD3-Fc Bispecific Antibody-Encoding MRNA Delivered by Lipid Nanoparticles Suppresses HER2-Positive Tumor Growth. Vaccines 2024, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, B.; Yue, S.; Zhao, S.; Meng, F.; Zhong, Z. HER-2-Mediated Nano-Delivery of Molecular Targeted Drug Potently Suppresses Orthotopic Epithelial Ovarian Cancer and Metastasis. Int. J. Pharm. 2022, 625, 122126. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, R.; Zhao, E.; Wang, Q.; Lian, W.; Xiong, J. Targeting CD44-Positive Ovarian Cancers via Engineered Paclitaxel Prodrug Nanoparticles for Enhanced Chemotherapeutic Efficacy. Biomed. Pharmacother. 2022, 154, 113655. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Khan, M.; Yibang, Z.; Raza, F.; Hasnat, M.; Cao, J.; Qi, X.; Hussain, A.; Liu, D. Hollow Mesoporous Silica Nanoparticles for Dual Chemo-Starvation Therapy of Hepatocellular Carcinoma. Pharm. Res. 2023, 40, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Shi, G.; Wang, S.; Hu, H.; Zhang, Z.; Li, Z.; Hao, L.; Yin, Q. A Novel PH-Responsive Iron Oxide Core-Shell Magnetic Mesoporous Silica Nanoparticle (M-MSN) System Encapsulating Doxorubicin (DOX) and Glucose Oxidase (Gox) for Pancreatic Cancer Treatment. Int. J. Nanomed. 2023, 18, 7133–7147. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Fan, W.; Wang, W.; Tang, W.; Yang, Z.; Wang, Z.; Liu, Y.; Shen, Z.; Dai, Y.; Cheng, S.; et al. Organosilica-Based Hollow Mesoporous Bilirubin Nanoparticles for Antioxidation-Activated Self-Protection and Tumor-Specific Deoxygenation-Driven Synergistic Therapy. ACS Nano 2019, 13, 8903–8916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Ling, C.; Gu, D.; Gao, Z.; Li, Y.; An, P.; Zhang, Y.; You, C.; Zhang, R.; Sun, B. Multimodal Therapies: Glucose Oxidase-Triggered Tumor Starvation-Induced Synergism with Enhanced Chemodynamic Therapy and Chemotherapy. New J. Chem. 2020, 44, 1524–1536. [Google Scholar] [CrossRef]
- da Costa, J.P.; Oliveira-Silva, R.; Daniel-da-Silva, A.L.; Vitorino, R. Bionanoconjugation for proteomics applications—An overview. Biotechnol. Adv. 2014, 32, 952–970. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, M.; Yu, M.; Yang, Y.; Strounina, E.; Gu, Z.; Huang, X.; Zhang, J.; Song, H.; Yu, C. Tailoring Mesoporous-Silica Nanoparticles for Robust Immobilization of Lipase and Biocatalysis. Nano Res. 2017, 10, 605–617. [Google Scholar] [CrossRef]
- Gustafsson, H. Enzyme Immobilization in Mesoporous Silica. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2013. [Google Scholar]
- Mahran, A.; Howaili, F.; Bhadane, R.; Mathiyalagan, R.; Viitala, T.; Wang, X.; Rosenholm, J.M. Functional Enzyme Delivery via Surface-Modified Mesoporous Silica Nanoparticles in 3D Printed Nanocomposite Hydrogels. Eur. J. Pharm. Sci. 2025, 211, 107132. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, H.; Li, D.; Zhao, W.; Ding, L.; Han, Y. A New Immobilized Glucose Oxidase Using SiO2 Nanoparticles as Carrier. Mater. Sci. Eng. C 2011, 31, 1374–1378. [Google Scholar] [CrossRef]
- Hou, X.; Liu, B.; Deng, X.; Zhang, B.; Chen, H.; Luo, R. Covalent Immobilization of Glucose Oxidase onto Poly(Styrene-Co-Glycidyl Methacrylate) Monodisperse Fluorescent Microspheres Synthesized by Dispersion Polymerization. Anal. Biochem. 2007, 368, 100–110. [Google Scholar] [CrossRef] [PubMed]
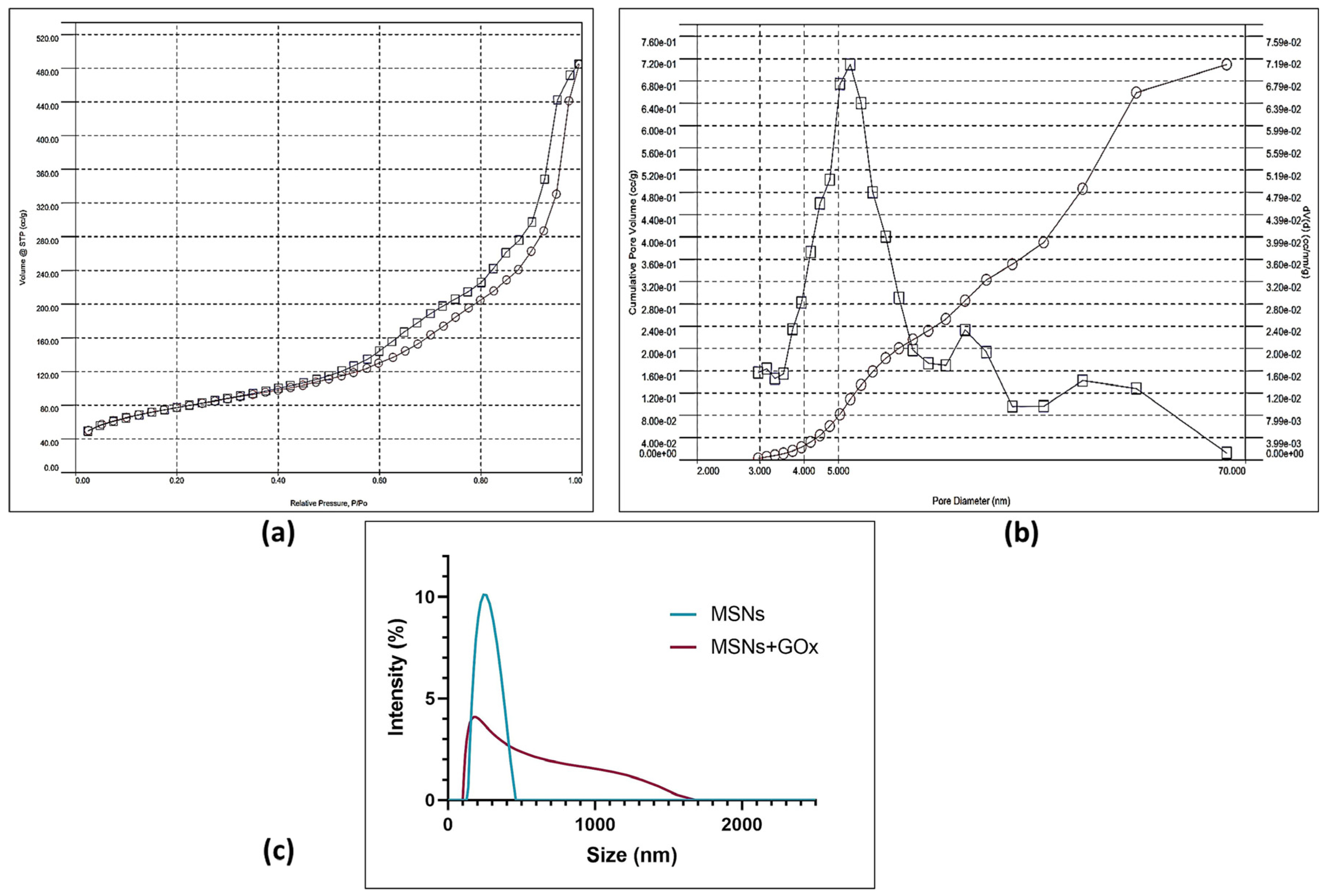
| Specific Surface Area (m2/g) | Average Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|
| 254.153 | 0.710 | 5.397 |
| Free GOx | Immobilized GOx | |
|---|---|---|
| IC50 | 60.77 ng/mL | 111.6 µg/mL |
| Enzymatic activity (U) | 0.0987 ± 0.012 | 0.0018 ± 0.0006 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| BCL-2 | GCCTTCTTTGAGTTCGGTGG | GAAATCAAACAGAGGCCGCA |
| CTNNB1 | TGAGGAGCAGCTTCAGTCCC | CTTGAGTAGCCATTGTCCACG |
| GAPDH | ACAGTTGCCATGTAGACC | TTGAGCACAGGGTACTTTA |
| TP53 | ACCTATGGAAACTACTTCCTG | ACCATTGTTCAATATCGTCC |
| WNT1 | CCCTAACCGGTGCGCCCTGGTGC | AGCGCCCAGAGCCCCATGGCCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uriostegui-Pena, A.G.; Sahare, P.; Luna-Bárcenas, G.; Paul, S. Therapeutic Potential of Glucose Oxidase-Loaded Biogenic Mesoporous Silica Nanoparticles in Ovarian Cancer. Pharmaceuticals 2025, 18, 1060. https://doi.org/10.3390/ph18071060
Uriostegui-Pena AG, Sahare P, Luna-Bárcenas G, Paul S. Therapeutic Potential of Glucose Oxidase-Loaded Biogenic Mesoporous Silica Nanoparticles in Ovarian Cancer. Pharmaceuticals. 2025; 18(7):1060. https://doi.org/10.3390/ph18071060
Chicago/Turabian StyleUriostegui-Pena, Andrea G., Padmavati Sahare, Gabriel Luna-Bárcenas, and Sujay Paul. 2025. "Therapeutic Potential of Glucose Oxidase-Loaded Biogenic Mesoporous Silica Nanoparticles in Ovarian Cancer" Pharmaceuticals 18, no. 7: 1060. https://doi.org/10.3390/ph18071060
APA StyleUriostegui-Pena, A. G., Sahare, P., Luna-Bárcenas, G., & Paul, S. (2025). Therapeutic Potential of Glucose Oxidase-Loaded Biogenic Mesoporous Silica Nanoparticles in Ovarian Cancer. Pharmaceuticals, 18(7), 1060. https://doi.org/10.3390/ph18071060





