In Vivo Antidiabetic and Antilipidemic Effect of Thiazolidine-2,4-Dione Linked Heterocyclic Scaffolds in Obesity-Induced Zebrafish Model
Abstract
1. Introduction
2. Results
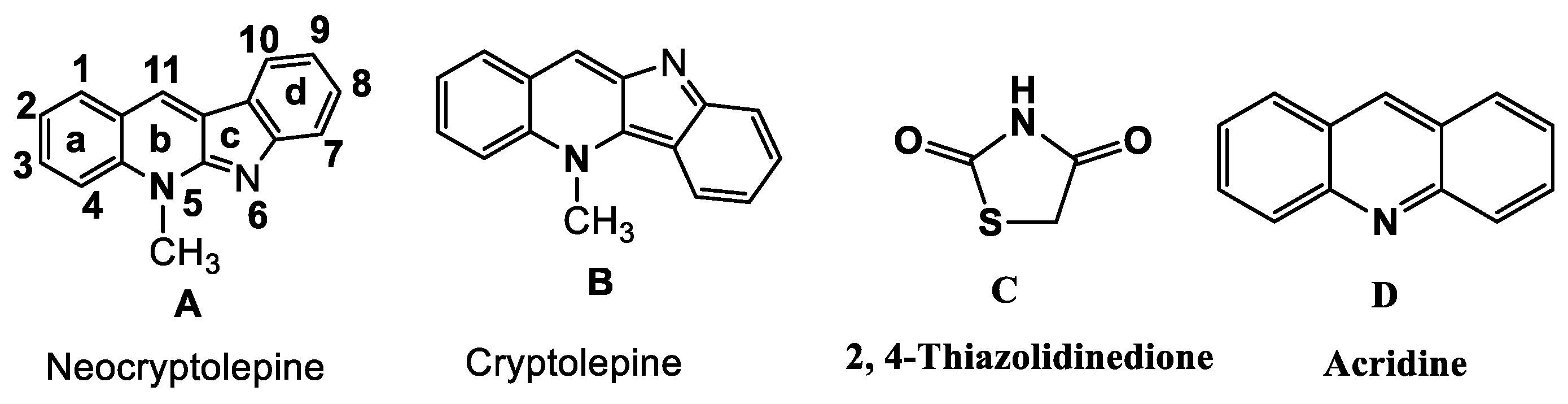
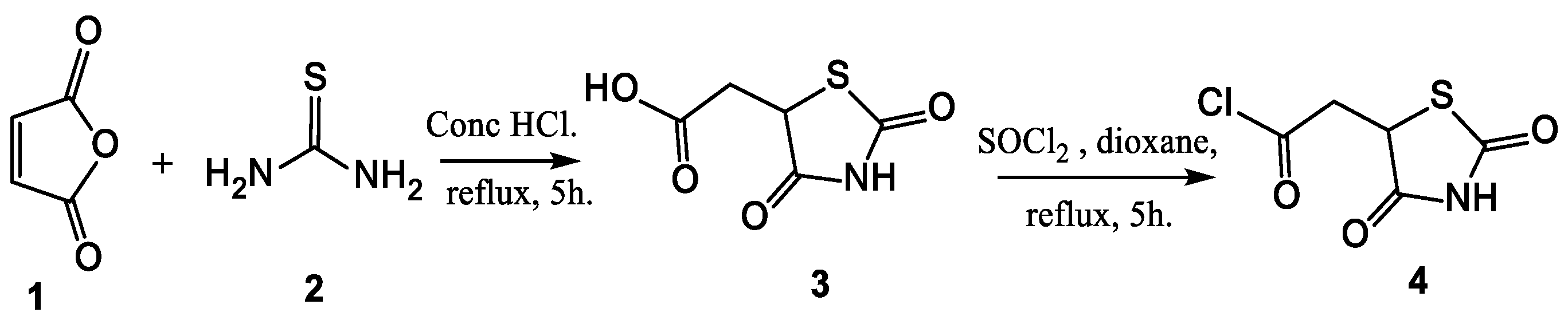
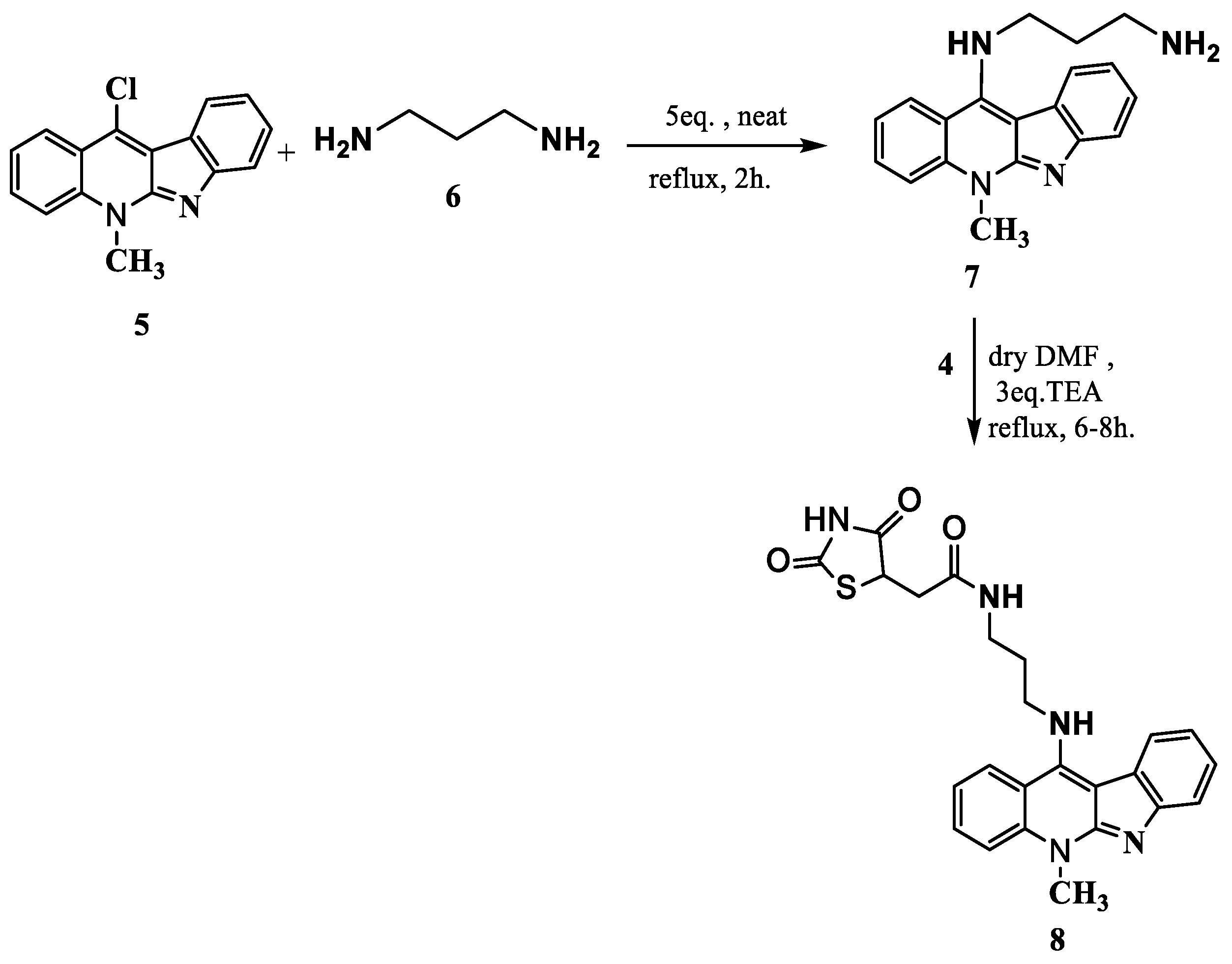
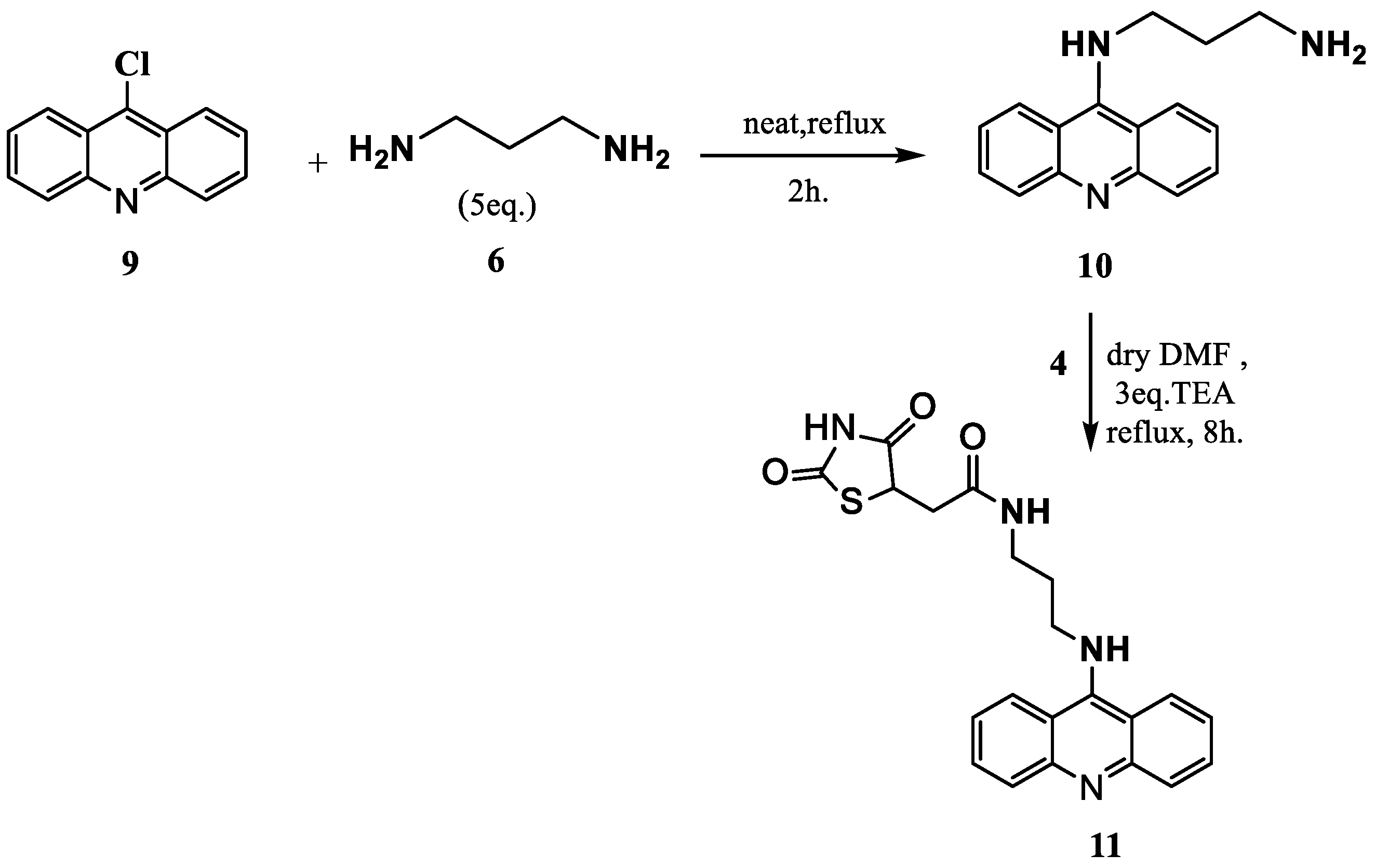
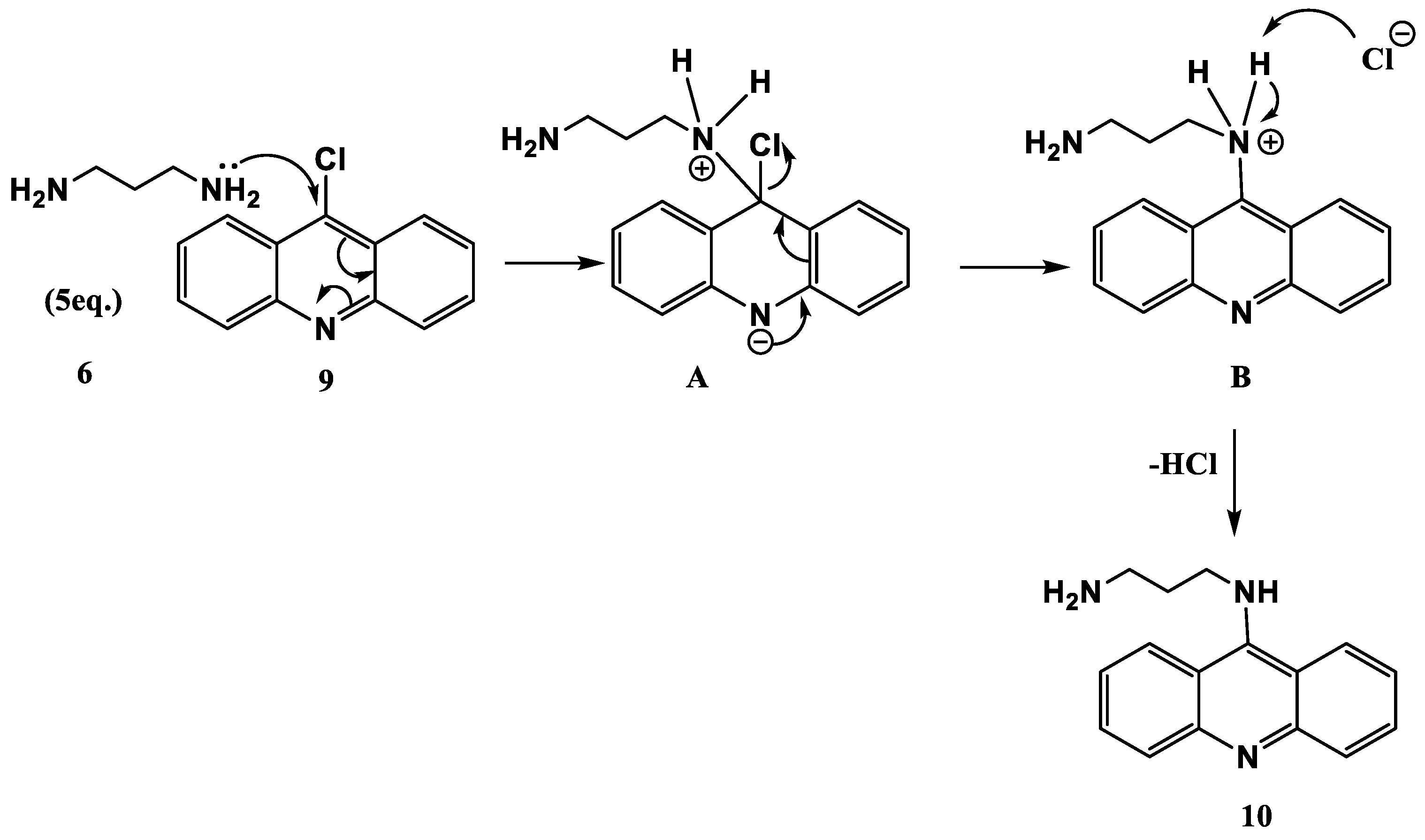
2.1. Synthesis and Elucidation of the New Compounds
Formation of Neocryptolepine–thiazolidinedione (NC-TZD) 8 and Acridine–thiazolidinedione (AC-TZD) 11
2.2. In Vivo Effects of the Compound on Zebrafish
2.2.1. Assessment of Glucose (a) and Triglycerides (b) in Zebrafish Livers
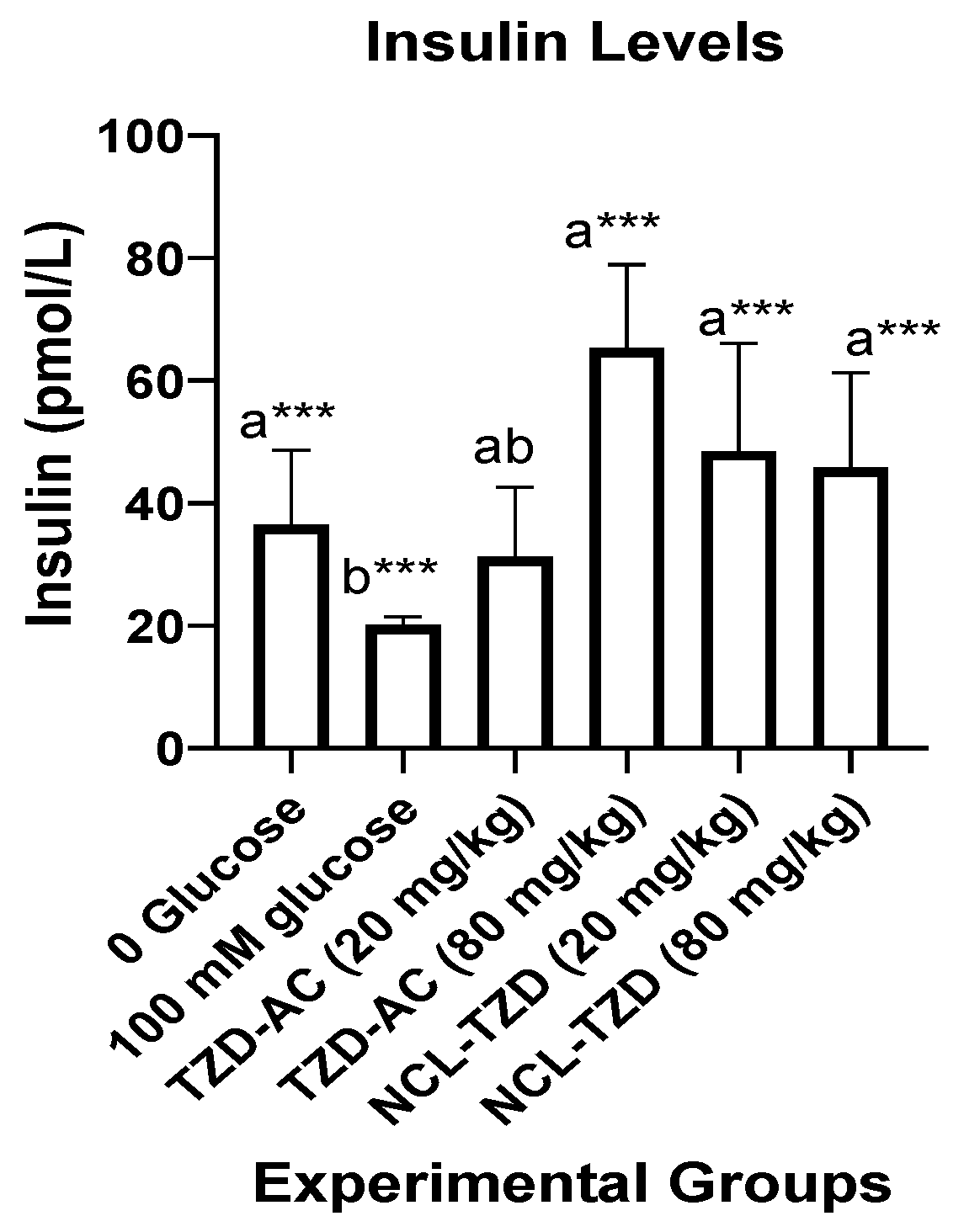
2.2.2. Assessment of Levels of Insulin in Zebrafish Liver Homogenates
2.2.3. Assessment of mRNA Levels (∆∆Ct) of Nuclear Factor Kappa Beta (nfκβ) in Zebrafish Liver Homogenates
2.2.4. Assessment of mRNA Levels (∆∆Ct) of the Peroxisomal Acyl-Coenzyme A Oxidase 1 (acox1) in Zebrafish Liver Homogenates
2.2.5. Assessment of mRNA Levels (∆∆Ct) of the Acetyl-CoA Carboxylase Alpha (accα) in Zebrafish Liver Homogenates
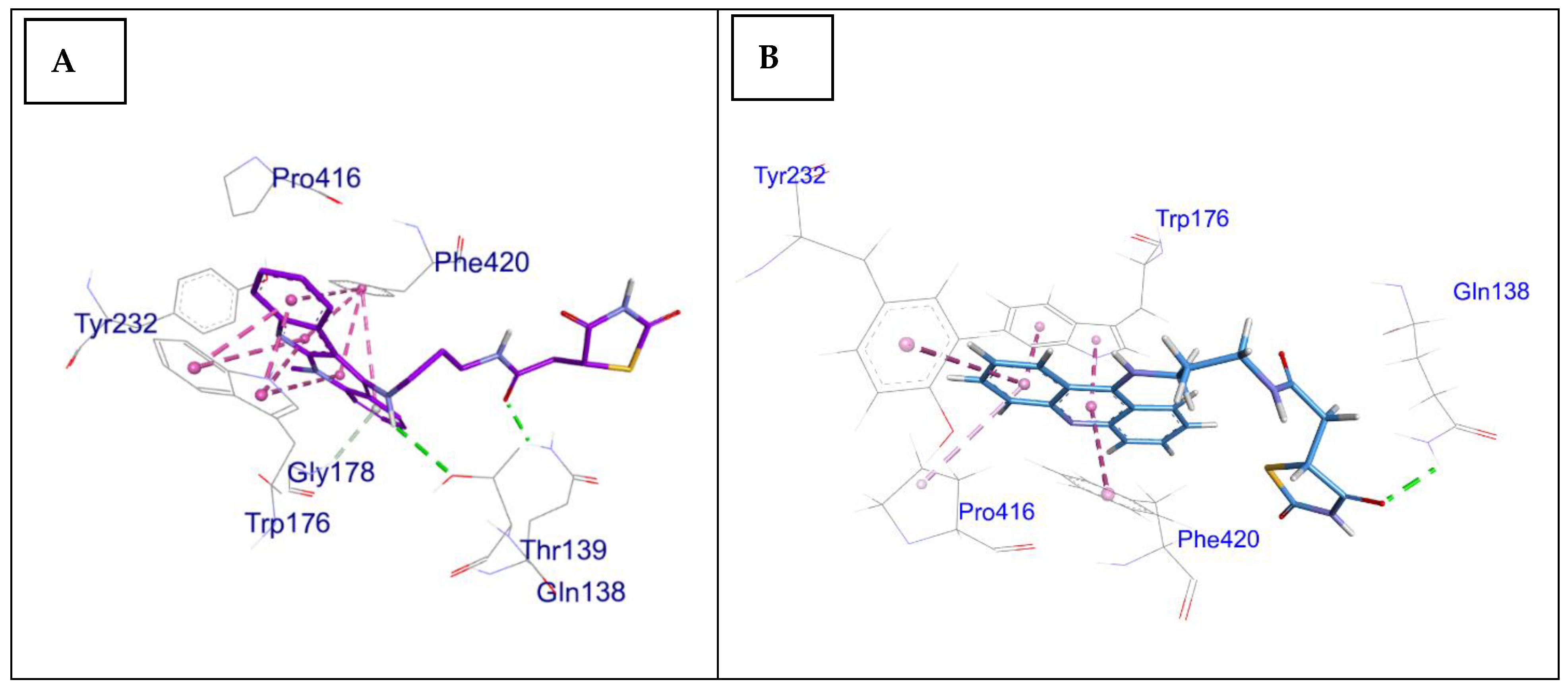
2.3. Docking Study
2.4. In Silico ADMET Analysis for Compounds 8 and 11
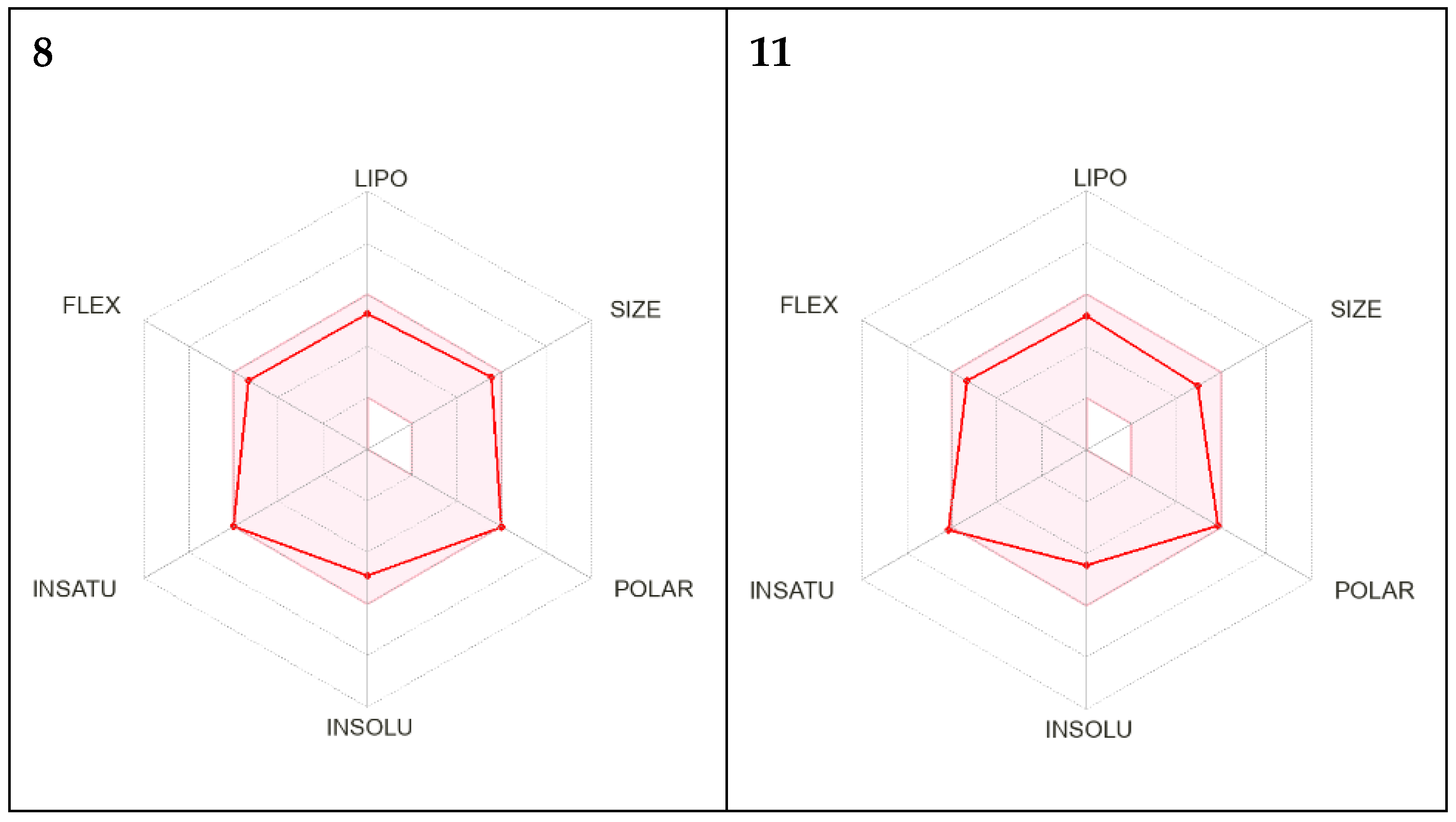
2.5. In Silico Investigation of Physicochemical Properties for Oral Bioavailability and Drug Likeness for Compounds 8 and 11
3. Discussion
4. Materials and Methods
4.1. Characterization Techniques and Chemicals
4.2. Synthesis of Neocryptolepine and Acridine Analogues
4.3. In Vivo Testing of Target Compounds
4.3.1. Laboratory Acclimation of Experimental Fish
4.3.2. In Vivo Experimental Procedures
4.3.3. Assessment of Energetic Metabolites Levels
4.3.4. Assessment of Insulin Levels
4.3.5. RNA Extraction and QPCR
4.4. Applied Data and Programs for Molecular Docking
4.5. Data Analysis
4.6. In Silico Physicochemical and Pharmacokinetics Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ali, M.K.; Galaviz, K.I.; Weber, M.B.; Narayan, K.V. The global burden of diabetes. In Textbook of Diabetes; Wiley: Hoboken, NJ, USA, 2017; pp. 65–83. [Google Scholar]
- Zorena, K.; Michalska, M.; Kurpas, M.; Jaskulak, M.; Murawska, A.; Rostami, S. Environmental factors and the risk of developing type 1 diabetes—Old disease and new data. Biology 2022, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Imagawa, A. Pancreatic β-cells express major histocompatibility complex class II: Do diabetic β-cells have the capacity of antigen-presenting cells? J. Diabetes Investig. 2019, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Krentz, N.A.; Gloyn, A.L. Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nat. Rev. Endocrinol. 2020, 16, 202–212. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar]
- Sugimoto, K.; Imai, T.; Ozeki, T.; Osawa, T.; Furukawa, Y.; Fukui, M.; Maegawa, H.; Kadowaki, T.; Ueki, K.; Araki, E. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The Multicenter Study for Clarifying Evidence for Sarcopenia in Patients with Diabetes Mellitus. J. Diabetes Investig. 2019, 10, 1471–1479. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- Tudu, C.K.; Bandyopadhyay, A.; Kumar, M.; Radha Das, T.; Nandy, S.; Ghorai, M.; Gopalakrishnan, A.V.; Proćków, J.; Dey, A. Unravelling the pharmacological properties of cryptolepine and its derivatives: A mini-review insight. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 229–238. [Google Scholar] [CrossRef]
- Wang, N.; Świtalska, M.; Wang, L.; Shaban, E.; Hossain, M.I.; El Sayed, I.E.; Wietrzyk, J.; Inokuchi, T. Structural modifications of nature-inspired indoloquinolines: A mini review of their potential antiproliferative activity. Molecules 2019, 24, 2121. [Google Scholar] [CrossRef]
- Abd Elrahman, S.F.; Ahmed, A.A.; Abd Elsatar, D.; Elkady, S.; Elgendy, A.; Alnakeeb, F.; Elmongy, E.I.; Henidi, H.A.; El-Gendy, S.M.; El Sayed, I.E.; et al. Cytotoxic Potential of Novel Quinoline Derivative: 11-(1,4-Bisaminopropylpiperazinyl)5-methyl-5H-indolo[2,3-b]quinoline against Different Cancer Cell Lines via Activation and Deactivation of the Expression of Some Proteins. Int. J. Mol. Sci. 2023, 24, 14336. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.T.; Ahmed, A.A.; Elmongy, E.I.; El-Gendy, S.M.; Elmadbouh, I.; El Sayed, I.E.; Abd Eldaim, M.A.; El-Gokha, A.A. Design and cytotoxic evaluation via apoptotic and antiproliferative activity for novel 11 (4-aminophenylamino) neocryptolepine on hepatocellular and colorectal cancer cells. Apoptosis 2023, 28, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Bierer, D.E.; Dubenko, L.G.; Zhang, P.; Lu, Q.; Imbach, P.A.; Garofalo, A.W.; Phuan, P.W.; Fort, D.M.; Litvak, J.; Gerber, R.E.; et al. Antihyperglycemic activities of cryptolepine analogues: An ethnobotanical lead structure isolated from Cryptolepis sanguinolenta. J. Med. Chem. 1998, 41, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, R.; Monga, V.; Mehan, S. Thiazolidine-2, 4-dione hybrids as dual alpha-amylase and alpha-glucosidase inhibitors: Design, synthesis, in vitro and in vivo anti-diabetic evaluation. RSC Med. Chem. 2024, 15, 2826–2854. [Google Scholar] [CrossRef]
- Thabet, H.K.; Ragab, A.; Imran, M.; Helal, M.H.; Alaqel, S.I.; Alshehri, A.; Mohd, A.A.; Alshammari, M.R.; Abusaif, M.S.; Ammar, Y.A. Discovery of new anti-diabetic potential agents based on paracetamol incorporating sulfa-drugs: Design, synthesis, α-amylase, and α-glucosidase inhibitors with molecular docking simulation. Eur. J. Med. Chem. 2024, 275, 116589. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Vijayakumar, V.; Sarveswari, S.; Gayathri, G.A.; Gayathri, M. Synthesis of new thiazolidine-2,-4-dione-azole derivatives and evaluation of their α-amylase and α-glucosidase inhibitory activity. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 735–745. [Google Scholar] [CrossRef]
- Bansal, G.; Thanikachalam, P.V.; Maurya, R.K.; Chawla, P.; Ramamurthy, S. An overview on medicinal perspective of thiazolidine-2, 4-dione: A remarkable scaffold in the treatment of type 2 diabetes. J. Adv. Res. 2020, 23, 163–205. [Google Scholar] [CrossRef]
- Sharma, A.; Piplani, P. Design and synthesis of some acridine-piperazine hybrids for the improvement of cognitive dysfunction. Chem. Biol. Drug Des. 2017, 90, 926–935. [Google Scholar] [CrossRef]
- El-Zahabi, M.A.; Bamanie, F.H.; Ghareeb, S.; Alshaeri, H.K.; Alasmari, M.M.; Moustafa, M.; Al-Marzooki, Z.; Zayed, M.F. Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of quinazoline-sulfonylurea hybrids as peroxisome proliferator-activated receptor gamma (PPARγ) and sulfonylurea receptor (SUR) agonists. Int. J. Mol. Sci. 2022, 23, 9605. [Google Scholar] [CrossRef]
- Patil, S.; Alegaon, S.G.; Gharge, S.; Ranade, S.D.; Khatib, N.A. Molecular hybridization, synthesis, in vitro α-glucosidase inhibition, in vivo antidiabetic activity and computational studies of isatin based compounds. Bioorg. Chem. 2024, 153, 107783. [Google Scholar] [CrossRef]
- Jörgens, K.; Hillebrands, J.-L.; Hammes, H.-P.; Kroll, J. Zebrafish: A Model for Understanding Diabetic Complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2, 4-dione derivatives. J. Enzym. Inhib. Med. Chem. 2018, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.; Blundell, T.L.; Ascher, D. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Hu, C.Q.; Zhang, X.; Ma, B.; Zhang, P. In silico ADME and toxicity prediction of ceftazidime and its impurities. Front. Pharmacol 2019, 10, 434. [Google Scholar] [CrossRef]
- El-Tantawy, A.I.; Elmongy, E.I.; Elsaeed, S.M.; Abdel Aleem, A.A.H.; Binsuwaidan, R.; Eisa, W.H.; Salman, A.U.; Elharony, N.E.; Attia, N.F. Synthesis, Characterization, and Docking Study of Novel Thioureidophosphonate-Incorporated Silver Nanocomposites as Potent Antibacterial Agents. Pharmaceutics 2023, 15, 1666. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1508–1512. [Google Scholar] [CrossRef]
- Taïlé, J.; Patché, J.; Veeren, B.; Gonthier, M.P. Hyperglycemic condition causes pro-inflammatory and permeability alterations associated with monocyte recruitment and deregulated nfκb/pparγ pathways on cerebral endothelial cells: Evidence for polyphenols uptake and protective effect. Int. J. Mol. Sci. 2021, 22, 1385. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Models Mech. 2019, 12, dmm036004. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, K.K.; Bai, W.; Khan, B.V.; Medford, R.M.; Natarajan, R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes 1999, 48, 855–864. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Alhazmi, H.A.; Anwer, M.K.; Bhatia, S.; Bungau, S.G. Alkaloidal phytoconstituents for diabetes management: Exploring the unrevealed potential. Molecules 2022, 27, 5851. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol circumvents obesity induced inflammation and insulin resistance by down-regulating IKKβ/NF-κB and JNK signaling pathway in adipocytes of type 2 diabetic rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Heiss, E.H.; Schachner, D.; Wright, C.W.; Vollmar, A.M.; Dirsch, V.M. Synthetic cryptolepine inhibits DNA binding of NF-κB. Bioorg. Med. Chem. 2007, 15, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ameyaw, E.O.; Koffuor, G.A.; Asare, K.K.; Konja, D.; Du-Bois, A.; Kyei, S.; Forkuo, A.D.; Mensah, R.N. Cryptolepine, an indoloquinoline alkaloid, in the management of diabetes mellitus and its associated complications. J. Intercult. Ethnopharmacol. 2016, 5, 263. [Google Scholar] [CrossRef]
- Feng, M.; Liang, B.; Sun, J.; Min, X.; Wang, S.H.; Lu, Y.; Xu, X. Synthesis, anti-α-glucosidase activity, inhibition interaction, and anti-diabetic activity of novel cryptolepine derivatives. J. Mol. Struct. 2024, 1310, 138311. [Google Scholar] [CrossRef]
- Min, X.; Guo, S.; Lu, Y.; Xu, X. Investigation on the inhibition mechanism and binding behavior of cryptolepine to α-glucosidase and its hypoglycemic activity by multi-spectroscopic method. J. Lumin. 2024, 269, 120437. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Li, W.; Ma, X.C.; Qiu, F.; Sun, C.P. IκB kinase β (IKKβ): Structure, transduction mechanism, biological function, and discovery of its inhibitors. Int. J. Biol. Sci. 2023, 19, 4181. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Liang, B.; Min, X.; Hu, J.; Wu, R.; Xu, X. Thiazolidine-2, 4-dione derivatives as potential α-glucosidase inhibitors: Synthesis, inhibitory activity, binding interaction and hypoglycemic activity. Bioorg. Chem. 2024, 144, 107177. [Google Scholar] [CrossRef]
- Dahiya, L.; Kumar, R.; Baidya, A.T.; Kumar, S.; Kumar, R.; Pawar, S.V.; Yadav, A.K. Design, synthesis, biological evaluations and in silico studies of N-substituted 2, 4-thiazolidinedione derivatives as potential a-glucosidase inhibitors. J. Biomol. Struct. Dyn. 2025, 43, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Gummidi, L.; Kerru, N.; Ebenezer, O.; Awolade, P.; Sanni, O.; Islam, M.S.; Singh, P. Multicomponent reaction for the synthesis of new 1, 3, 4-thiadiazole-thiazolidine-4-one molecular hybrids as promising antidiabetic agents through α-glucosidase and α-amylase inhibition. Bioorg. Chem. 2021, 115, 105210. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Iqbal, S.; Khan, M.; Rehman, W.; Shah, M.; Hussain, R.; Rasheed, L.; Khan, Y.; Dera, A.A.; Pashameah, R.A.; et al. Design, synthesis, in silico testing, and in vitro evaluation of thiazolidinone-based benzothiazole derivatives as inhibitors of α-amylase and α-glucosidase. Pharmaceuticals 2022, 15, 1164. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Molecular mechanism in α-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- Shihana, F.; Cholan, P.M.; Fraser, S.; Oehlers, S.H.; Seth, D. Investigating the role of lipid genes in liver disease using fatty liver models of alcohol and high fat in zebrafish (Danio rerio). Liver Int. 2023, 43, 2455–2468. [Google Scholar] [CrossRef]
- Thangavel, N.; Al Bratty, M.; Akhtar Javed, S.; Ahsan, W.; Alhazmi, H.A. Targeting peroxisome proliferator-activated receptors using thiazolidinediones: Strategy for design of novel antidiabetic drugs. Int. J. Med. Chem. 2017, 2017, 1069718. [Google Scholar] [CrossRef]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The role of lipid sensing nuclear receptors (PPARs and LXR) and metabolic lipases in obesity, diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef]
- Kersten, S.; Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 2017, 136, 75–84. [Google Scholar] [CrossRef]
- Duszka, K.; Gregor, A.; Guillou, H.; König, J.; Wahli, W. Peroxisome proliferator-activated receptors and caloric restriction—Common pathways affecting metabolism, health, and longevity. Cells 2020, 9, 1708. [Google Scholar] [CrossRef]
- Raas, Q.; Gondcaille, C.; Hamon, Y.; Leoni, V.; Caccia, C.; Ménétrier, F.; Lizard, G.; Trompier, D.; Savary, S. CRISPR/Cas9-mediated knockout of Abcd1 and Abcd2 genes in BV-2 cells: Novel microglial models for X-linked Adrenoleukodystrophy. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Sheng, R.; Fan, J.; Wu, W.; Guo, R. Structure-activity relationships of natural and synthetic indole-derived scaffolds as α-glucosidase inhibitors: A mini-review. Mini Rev. Med. Chem. 2020, 20, 1791–1818. [Google Scholar] [CrossRef]
- Sharchil, C.; Vijay, A.; Ramachandran, V.; Bhagavatheeswaran, S.; Devarajan, R.; Koul, B.; Yadav, D.; Balakrishnan, A. Zebrafish: A model to study and understand the diabetic nephropathy and other microvascular complications of type 2 diabetes mellitus. Vet. Sci. 2022, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Zimenkovskii, B.S.; Kutsyk, R.V.; Lesyk, R.B.; Matyichuk, V.S.; Obushak, N.D.; Klyufinska, T.I. Synthesis and antimicrobial activity of 2, 4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J. 2006, 40, 303–306. [Google Scholar] [CrossRef]
- Okada, M.; Mei, Z.W.; Imran Hossain, M.; Wang, L.; Tominaga, T.; Takebayashi, T.; Murakami, M.; Yasuda, M.; Shigehiro, T.; Kasai, T.; et al. Synthesis and in vitro cancer cell growth inhibition evaluation of 11-amino-modified 5-Me-indolo[2,3-b]quinolines and their COMPARE analyses. Med. Chem. Res. 2016, 25, 879–892. [Google Scholar] [CrossRef]
- Sheir, S.K.; Elmongy, E.I.; Mohamad, A.H.; Osman, G.Y.; Bendary, S.E.; Ahmed, A.A.; Binsuwaidan, R.; El-Sayed, I.E. Molluscicidal and Larvicidal Potency of N-Heterocylic Analogs against Biomophalaria alexandrina Snails and Schistosoma mansoni Larval Stages. Pharmaceutics 2023, 15, 1200. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; El Sayed, I.E.; Abd Elmaksoud, A.Z.; Ahmed, A.A. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma–induced hepatorenal injury in mice. Environ. Sci. Pollut. Res. 2021, 28, 21835–21850. [Google Scholar] [CrossRef]
- Olajide, O.A.; Ajayi, A.M.; Wright, C.W. Anti-inflammatory properties of cryptolepine. Phytother. Res. 2009, 23, 1421–1425. [Google Scholar] [CrossRef]
- Olajide, O.A.; Bhatia, H.S.; De Oliveira, A.C.; Wright, C.W.; Fiebich, B.L. Inhibition of neuroinflammation in LPS-activated microglia by cryptolepine. Evid.-Based Complement. Altern. Med. 2013, 2013, 459723. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Mello, T.; Ceni, E.; Surrenti, E.; Surrenti, C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin. Investig. Drugs 2006, 15, 1039–1049. [Google Scholar] [CrossRef]
- Haroun, M. Novel Hybrids of pyrazolidinedione and benzothiazole as TZD analogues. rationale design, synthesis and in vivo anti-diabetic evaluation. Med. Chem. 2019, 15, 624–633. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Abdelgawad, M.A.; Shalaby, K.; Ghoneim, M.M.; AboulMagd, A.M.; Abdelwahab, N.S.; Hassan, H.M.; Othman, A.M. Pioglitazone synthetic analogue ameliorates streptozotocin-induced diabetes mellitus through modulation of ACE 2/angiotensin 1–7 via PI3K/AKT/mTOR signaling pathway. Pharmaceuticals 2022, 15, 341. [Google Scholar] [CrossRef]
- Galal-Khallaf, A.; Al-Awthan, Y.S.; Al-Duais, M.A.; Mohammed-Geba, K. Nile crab Potamonautes niloticus shell extract: Chromatographic and molecular elucidation of potent antioxidant and anti-inflammatory capabilities. Bioorg. Chem. 2022, 127, 106023. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.S.; Sarras Jr, M.P.; Leontovich, A.; Intine, R.V. Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression. Diabetes 2012, 61, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Knopp, J.L.; Holder-Pearson, L.; Chase, J.G. Insulin units and conversion factors: A story of truth, boots, and faster half-truths. J. Diabetes Sci. Technol. 2019, 13, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.S.; Badshah, Y.; Shabbir, M.; Rafiq, M. Molecular docking using chimera and autodock vina software for nonbioinformaticians. JMIR Bioinform. Biotechnol. 2020, 1, e14232. [Google Scholar] [CrossRef]
- Badr, M.; Elmongy, E.I.; Elkhateeb, D.; Moemen, Y.S.; Khalil, A.; Ali, H.; Binsuwaidan, R.; Awadallah, F.; El Sayed, I.E. In Silico and In Vitro Investigation of Cytotoxicity and Apoptosis of Acridine/Sulfonamide Hybrids Targeting Topoisomerases I and II. Pharmaceuticals 2024, 17, 1487. [Google Scholar] [CrossRef]






| Compound No. | PDB ID | ΔG (kcal/mol) | Ki (µM) | RMSD (Å) | Residue of Interaction | Type of Interaction |
|---|---|---|---|---|---|---|
| 8 | 2ZNN | −9.17 | 0.27 | 1.3 | MET 355 SER 280 PHE 273 CYS 276 LYS 358 TYR 464 | H-bond H-bond H-pi pi-H pi-H pi-H |
| 7Q86 | −8.51 | 0.575 | 1.3 | THR 139 TYP 176 PHE 420 GLN 138 PRO 177 | H-bond Pi-pi stack Pi-pi stack H-bond Pi-H | |
| 11 | 2ZNN | −7.89 | 1.89 | 1.8 | CYS 276 MET 330 | H-bond H-bond |
| 7Q86 | −7.15 | 5.60 | 1.9 | GLN 138 TRP 176 TYR 232 PHE 420 PRO 416 | H-bond Pi-pi stack Pi-pi stack Pi-pi stack Pi-alkyl |
| Property | Model Name (Unit) | Predicted Value | |
|---|---|---|---|
| Compound 8 | Compound 11 | ||
| Absorption | Intestinal absorption (human) (% absorbed) | 92.6 | 83.729 |
| Caco2 permeability (log Papp in 10−6 cm/s) | 0.606 | 1.088 | |
| Water solubility (log mol/L) | −4.615 | −4.173 | |
| Skin permeability (log Kp) | −2.768 | −2.752 | |
| P-glycoprotein substrate | Yes | Yes | |
| P-glycoprotein I inhibitor | Yes | Yes | |
| P-glycoprotein II inhibitor | Yes | Yes | |
| Distribution | VDss (human) (log L/kg) | 0.165 | 0.35 |
| Fraction unbound (human) (Fu) | 0.056 | 0.134 | |
| BBB permeability (log BB) | −0.987 | −1.102 | |
| CNS permeability (log PS) | −2.607 | −2.649 | |
| Metabolism | CYP2D6 substrate | No | No |
| CYP3A4 substrate | Yes | Yes | |
| CYP1A2 inhibitor | No | Yes | |
| CYP2C19 inhibitor | Yes | Yes | |
| CYP2C9 inhibitor | Yes | Yes | |
| CYP2D6 inhibitor | No | No | |
| CYP3A4 inhibitor | Yes | Yes | |
| Excretion | Total clearance (log mL/min/kg) | 0.416 | 0.285 |
| Renal OCT2 substrate | No | No | |
| Toxicity | AMES toxicity | No | Yes |
| Max. tolerated dose (human) (log mg/kg/day) | −0.473 | 0.322 | |
| hERG I inhibitor | No | No | |
| hERG II inhibitor | Yes | Yes | |
| Oral rat acute toxicity (LD50) (mol/kg) | 2.446 | 2.502 | |
| Oral rat chronic toxicity (LOAEL) (log mg/kg_bw/day) | 1.129 | 1.229 | |
| Hepatotoxicity | Yes | Yes | |
| Skin sensitization | No | No | |
| T. pyriformis toxicity | 0.444 | 0.387 | |
| Minnow toxicity | 4.187 | 2.79 | |
| Compound 8 | Compound 11 | |
|---|---|---|
| Physicochemical Properties | ||
| Formula | C24H23N5O3S | C21H20N4O3S |
| Molecular weight | 461.54 g/mol | 408.47 g/mol |
| Num. heavy atoms | 33 | 29 |
| Num. arom. heavy atoms | 17 | 14 |
| Fraction Csp3 | 0.25 | 0.24 |
| Num. rotatable bonds | 8 | 8 |
| Num. H-bond acceptors | 4 | 4 |
| Num. H-bond donors | 3 | 3 |
| Molar refractivity | 134.95 | 118.19 |
| TPSA | 130.42 Å2 | 125.49 Å2 |
| Lipophilicity | ||
| Log Po/w (iLOGP) | 2.30 | 1.82 |
| Log Po/w (XLOGP3) | 3.73 | 3.55 |
| Hydrophilicity | ||
| Log S (ESOL) | −4.90 | −4.44 |
| Solubility | 5.75 × 10−3 mg/mL; 1.25 × 10−5 mol/L | 1.49 × 10−2 mg/mL; 3.65 × 10−5 mol/L |
| Class | Moderately soluble | Moderately soluble |
| Drug likeness | ||
| Lipinski | Yes; 0 violation | Yes; 0 violation |
| Ghose | 1 violation: MR > 130 | Yes |
| Veber | Yes | Yes |
| Egan | Yes | Yes |
| Muegge | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 |
| Locus ID | Gene | Amplicon Size (bp) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| NM_001003414 | nf-κB | 202 | GGTCGGACAGAGATCACGGATT | TGCTGTTCTTCACGTCCTCT |
| NM_001271308.1 | accα | 194 | AGAGAGGGCAGGTTTTACCA | GCCATCATACGAGAGCAACA |
| BC097101.1 | acox1 | 162 | GCACGGATGTGTGTACCGTGC | GCGTCCAGAGCCCCTTGACCT |
| NM_181601.3 | actb | 197 | ACTGTATTGTCTGGTGGTAC | TACTCCTGCTTGCTAATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galal-Khallaf, A.; Mousa, D.; Atyah, A.; El-Bahnsawye, M.; Hussein, M.K.A.; El Sayed, I.E.T.; Elmongy, E.I.; Binsuwaidan, R.; El-Torgoman, A.M.A.K.; Abdel-Bary, H.; et al. In Vivo Antidiabetic and Antilipidemic Effect of Thiazolidine-2,4-Dione Linked Heterocyclic Scaffolds in Obesity-Induced Zebrafish Model. Pharmaceuticals 2025, 18, 1023. https://doi.org/10.3390/ph18071023
Galal-Khallaf A, Mousa D, Atyah A, El-Bahnsawye M, Hussein MKA, El Sayed IET, Elmongy EI, Binsuwaidan R, El-Torgoman AMAK, Abdel-Bary H, et al. In Vivo Antidiabetic and Antilipidemic Effect of Thiazolidine-2,4-Dione Linked Heterocyclic Scaffolds in Obesity-Induced Zebrafish Model. Pharmaceuticals. 2025; 18(7):1023. https://doi.org/10.3390/ph18071023
Chicago/Turabian StyleGalal-Khallaf, Asmaa, Dawlat Mousa, Aml Atyah, Mohamed El-Bahnsawye, Mona K. Abo Hussein, Ibrahim El Tantawy El Sayed, Elshaymaa I. Elmongy, Reem Binsuwaidan, Abdel Moneim A. K. El-Torgoman, Hamed Abdel-Bary, and et al. 2025. "In Vivo Antidiabetic and Antilipidemic Effect of Thiazolidine-2,4-Dione Linked Heterocyclic Scaffolds in Obesity-Induced Zebrafish Model" Pharmaceuticals 18, no. 7: 1023. https://doi.org/10.3390/ph18071023
APA StyleGalal-Khallaf, A., Mousa, D., Atyah, A., El-Bahnsawye, M., Hussein, M. K. A., El Sayed, I. E. T., Elmongy, E. I., Binsuwaidan, R., El-Torgoman, A. M. A. K., Abdel-Bary, H., & Mohammed-Geba, K. (2025). In Vivo Antidiabetic and Antilipidemic Effect of Thiazolidine-2,4-Dione Linked Heterocyclic Scaffolds in Obesity-Induced Zebrafish Model. Pharmaceuticals, 18(7), 1023. https://doi.org/10.3390/ph18071023







