New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives
Abstract
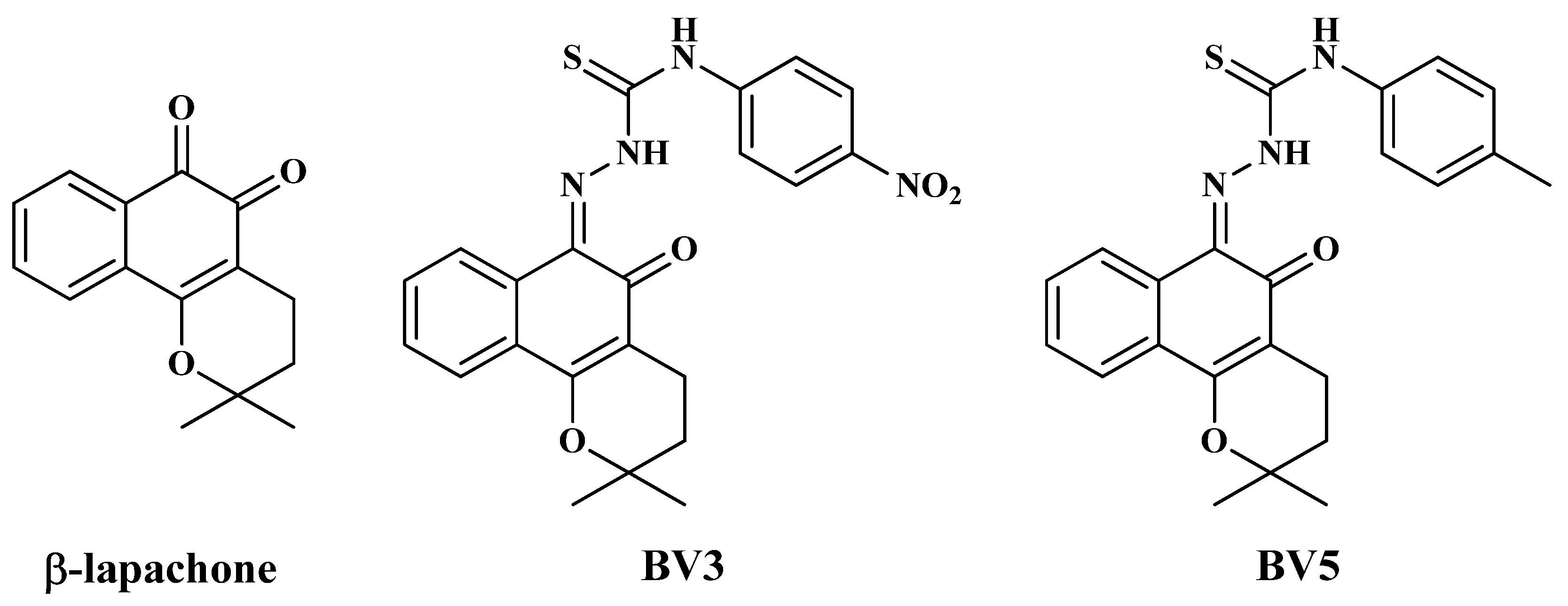
1. Introduction
2. Results
2.1. Cellular Cytotoxicity
2.2. Morphological Alterations in HeLa
2.3. Flow Cytometry Analysis
2.4. In Vitro and In Silico Analyses of Kinase Inhibition
2.5. Toxicogenomic Safety
3. Discussion
3.1. Cellular Cytotoxic
3.2. BV3 and BV5 Increased Apoptosis in HeLa Cells
3.3. BV3 and BV5 Can Inhibit Kinases
3.4. BV3 and BV5 Have Toxicogenetic Safety
4. Materials and Methods
4.1. Reagents
4.2. Molecules
4.3. Cell Lines
4.4. Cell Viability and Determination of IC50
4.5. Selectivity Index (SI)
4.6. Morphological Analyses
4.7. Evaluation of the Induction of Apoptosis or Necrosis
4.8. Protein Kinase Assays
4.9. Molecular Docking
4.10. In Vivo Assessment of Toxicogenetic Safety
4.10.1. Groups and Administration Protocol
4.10.2. Alkaline Comet Assay
4.10.3. Micronucleus Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Li, C.J.; Yu, D.; Pardee, A.B. Potent Induction of Apoptosis by β-Lapachone in Human Multiple Myeloma Cell Lines and Patient Cells. Mol. Med. 2000, 6, 1008–1015. [Google Scholar] [CrossRef]
- Gomes, C.L.; de Albuquerque Wanderley Sales, V.; Gomes de Melo, C.; Ferreira da Silva, R.M.; Vicente Nishimura, R.H.; Rolim, L.A.; Rolim Neto, P.J. Beta-Lapachone: Natural Occurrence, Physicochemical Properties, Biological Activities, Toxicity and Synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef]
- Khong, H.T.; Dreisbach, L.; Kindler, H.L.; Trent, D.F.; Jeziorski, K.G.; Bonderenko, I.; Popiela, T.; Yagovane, D.M.; Dombal, G. A Phase 2 Study of ARQ 501 in Combination with Gemcitabine in Adult Patients with Treatment Naïve, Unresectable Pancreatic Adenocarcinoma. J. Clin. Oncol. 2007, 25, 15017. [Google Scholar] [CrossRef]
- Kawecki, A.; Adkins, D.R.; Cunningham, C.C.; Vokes, E.; Yagovane, D.M.; Dombal, G.; Koralewski, P.; Hotko, Y.; Vladimirov, V. A Phase II Study of ARQ 501 in Patients with Advanced Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2007, 25, 16509. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; LaMont, J.T.; Pardee, A.B.; Li, C.J. Selective Killing of Cancer Cells by β-Lapachone: Direct Checkpoint Activation as a Strategy against Cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 2674–2678. [Google Scholar] [CrossRef]
- Gerber, D.E.; Beg, M.S.; Fattah, F.; Frankel, A.E.; Fatunde, O.; Arriaga, Y.; Dowell, J.E.; Bisen, A.; Leff, R.D.; Meek, C.C.; et al. Phase 1 Study of ARQ 761, a β-Lapachone Analogue That Promotes NQO1-Mediated Programmed Cancer Cell Necrosis. Br. J. Cancer 2018, 119, 928–936. [Google Scholar] [CrossRef]
- de Andrade, J.K.F.; da Silva Góes, A.J.; Barbosa, V.X.; de Lima Silva, M.S.; Matos Donato, M.A.; Peixoto, C.A.; Militão, G.C.G.; da Silva, T.G. Anticancer Activity of β-Lapachone Derivatives on Human Leukemic Cell Lines. Chem. Biol. Interact. 2022, 365, 110057. [Google Scholar] [CrossRef]
- Feun, L.; Modiano, M.; Lee, K.; Mao, J.; Marini, A.; Savaraj, N.; Plezia, P.; Almassian, B.; Colacino, E.; Fischer, J.; et al. Phase I and Pharmacokinetic Study of 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone (3-AP) Using a Single Intravenous Dose Schedule. Cancer Chemother. Pharmacol. 2002, 50, 223–229. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Khaleefathullah, S.; Kaveri, K.; Palani, G.; Ramanathan, G.; Thennarasu, S.; Tirichurapalli Sivagnanam, U. Antiviral Activity of Thiosemicarbazones Derived from A-amino Acids against Dengue Virus. J. Med. Virol. 2017, 89, 546–552. [Google Scholar] [CrossRef]
- Ressler, A.J.; Frate, M.; Hontoria, A.; Ream, A.; Timms, E.; Li, H.; Stettler, L.D.; Bollinger, A.; Poor, J.E.; Parra, M.A.; et al. Synthesis, Anti-Ferroptosis, Anti-Quorum Sensing, Antibacterial and DNA Interaction Studies of Chromene-Hydrazone Derivatives. Bioorg. Med. Chem. 2023, 90, 117369. [Google Scholar] [CrossRef]
- da Costa, V.d.C.M.; de Lima, M.d.C.A.; da Cruz Filho, I.J.; Galdino, L.V.; Pereira, M.C.; de Oliveira Silva, B.; de Barros Albuquerque, A.P.; da Rosa, M.M.; da Rocha Pitta, M.G.; de Melo Rêgo, M.J.B. 5-Nitro-Thiophene-Thiosemicarbazone Derivative Induces Cell Death, Cell Cycle Arrest, and Phospho-Kinase Shutdown in Pancreatic Ductal Adenocarcinoma Cells. Eur. J. Pharmacol. 2024, 983, 176963. [Google Scholar] [CrossRef]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid. Redox Signal. 2019, 30, 1062–1082. [Google Scholar] [CrossRef]
- Halder, A.K.; Saha, A.; Saha, K.D.; Jha, T. Stepwise Development of Structure–Activity Relationship of Diverse PARP-1 Inhibitors through Comparative and Validated in Silic o Modeling Techniques and Molecular Dynamics Simulation. J. Biomol. Struct. Dyn. 2015, 33, 1756–1779. [Google Scholar] [CrossRef]
- Vanni, A.; Fiore, M.; De Salvia, R.; Cundari, E.; Ricordy, R.; Ceccarelli, R.; Degrassi, F. DNA Damage and Cytotoxicity Induced by β-Lapachone: Relation to Poly(ADP-Ribose) Polymerase Inhibition. Mutat. Res. Mol. Mech. Mutagen. 1998, 401, 55–63. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Barros, F.W.A.; Cabral, I.O.; Ferreira, J.R.O.; Magalhães, H.I.F.; Júnior, H.V.N.; da Silva Júnior, E.N.; de Abreu, F.C.; Costa, C.O.; Goulart, M.O.F.; et al. Preclinical Genotoxicology of Nor-β-Lapachone in Human Cultured Lymphocytes and Chinese Hamster Lung Fibroblasts. Chem. Res. Toxicol. 2011, 24, 1560–1574. [Google Scholar] [CrossRef]
- Lima, K.M.M.; Calandrini de Azevedo, L.F.; Rissino, J.D.; Vale, V.V.; Costa, E.V.S.; Dolabela, M.F.; Nagamachi, C.Y.; Pieczarka, J.C. Anticancer Potential and Safety Profile of β-Lapachone In Vitro. Molecules 2024, 29, 1395. [Google Scholar] [CrossRef]
- Gupta, A.; Dagar, G.; Chauhan, R.; Sadida, H.Q.; Almarzooqi, S.K.; Hashem, S.; Uddin, S.; Macha, M.A.; Akil, A.S.A.-S.; Pandita, T.K.; et al. Cyclin-Dependent Kinases in Cancer: Role, Regulation, and Therapeutic Targeting. Adv. Protein Chem. Struct. Biol. 2023, 135, 21–55. [Google Scholar]
- Kumar, V.; Kaur, N.; Sahu, S.; Sharma, V.; Kumar, D.; Sharma, A.; Wadhwa, P. Role of Tyrosine Kinases and Their Inhibitors in Cancer Therapy: A Comprehensive Review. Curr. Med. Chem. 2023, 30, 1464–1481. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Warren, G.L.; Skillman, A.G.; Nicholls, A. How to Do an Evaluation: Pitfalls and Traps. J. Comput. Aided. Mol. Des. 2008, 22, 179–190. [Google Scholar] [CrossRef]
- Carlson, H.A.; Smith, R.D.; Damm-Ganamet, K.L.; Stuckey, J.A.; Ahmed, A.; Convery, M.A.; Somers, D.O.; Kranz, M.; Elkins, P.A.; Cui, G.; et al. CSAR 2014: A Benchmark Exercise Using Unpublished Data from Pharma. J. Chem. Inf. Model. 2016, 56, 1063–1077. [Google Scholar] [CrossRef]
- Ma, Y.; Kong, J.; Yan, G.; Ren, X.; Jin, D.; Jin, T.; Lin, L.; Lin, Z. NQO1 Overexpression Is Associated with Poor Prognosis in Squamous Cell Carcinoma of the Uterine Cervix. BMC Cancer 2014, 14, 414. [Google Scholar] [CrossRef]
- Osman, N.A.T.A.G.; Abd El-Maqsoud, N.M.R.; El Gelany, S.A.A. Correlation of NQO1 and Nrf2 in Female Genital Tract Cancer and Their Precancerous Lesions (Cervix, Endometrium and Ovary). World J. Oncol. 2015, 6, 364–374. [Google Scholar] [CrossRef]
- Chakrabarti, G.; Silvers, M.A.; Ilcheva, M.; Liu, Y.; Moore, Z.R.; Luo, X.; Gao, J.; Anderson, G.; Liu, L.; Sarode, V.; et al. Tumor-Selective Use of DNA Base Excision Repair Inhibition in Pancreatic Cancer Using the NQO1 Bioactivatable Drug, β-Lapachone. Sci. Rep. 2015, 5, 17066. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, K.S.; Kwon, T.K. β-Lapachone-Induced Reactive Oxygen Species (ROS) Generation Mediates Autophagic Cell Death in Glioma U87 MG Cells. Chem. Biol. Interact. 2011, 189, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Shan, Y.; Liu, F.; Xu, X.; Yang, Y.; Zhang, Q.; Zhang, Y.; Kuang, H.; Wang, Z.; et al. Design, Synthesis and Anticancer Activity of Novel Nopinone-Based Thiosemicarbazone Derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2360–2363. [Google Scholar] [CrossRef]
- Nicolás, Á.; Quero, J.G.; Barroso, M.; Gándara, Z.; Gude, L. DNA Interactions and Biological Activity of 2,9-Disubstituted 1,10-Phenanthroline Thiosemicarbazone-Based Ligands and a 4-Phenylthiazole Derivative. Biology 2024, 13, 60. [Google Scholar] [CrossRef]
- Marković, V.; Janićijević, A.; Stanojković, T.; Kolundžija, B.; Sladić, D.; Vujčić, M.; Janović, B.; Joksović, L.; Djurdjević, P.T.; Todorović, N.; et al. Synthesis, Cytotoxic Activity and DNA-Interaction Studies of Novel Anthraquinone–Thiosemicarbazones with Tautomerizable Methylene Group. Eur. J. Med. Chem. 2013, 64, 228–238. [Google Scholar] [CrossRef]
- Zhao, L.; Miao, H.; Quan, M.; Wang, S.; Zhang, Y.; Zhou, H.; Zhang, X.; Lin, Z.; Piao, J. β-Lapachone Induces Ferroptosis of Colorectal Cancer Cells via NCOA4-Mediated Ferritinophagy by Activating JNK Pathway. Chem. Biol. Interact. 2024, 389, 110866. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Li, W.; Zhang, B.; Wang, A.J.; Sun, J.; Mikule, K.; Jiang, Z.; Li, C.J. Selective Induction of Necrotic Cell Death in Cancer Cells by β-Lapachone through Activation of DNA Damage Response Pathway. Cell Cycle 2006, 5, 2029–2035. [Google Scholar] [CrossRef]
- Bey, E.A.; Reinicke, K.E.; Srougi, M.C.; Varnes, M.; Anderson, V.E.; Pink, J.J.; Li, L.S.; Patel, M.; Cao, L.; Moore, Z.; et al. Catalase Abrogates β-Lapachone–Induced PARP1 Hyperactivation–Directed Programmed Necrosis in NQO1-Positive Breast Cancers. Mol. Cancer Ther. 2013, 12, 2110–2120. [Google Scholar] [CrossRef]
- Gupta, D.; Podar, K.; Tai, Y.-T.; Lin, B.; Hideshima, T.; Akiyama, M.; LeBlanc, R.; Catley, L.; Mitsiades, N.; Mitsiades, C.; et al. β-Lapachone, a Novel Plant Product, Overcomes Drug Resistance in Human Multiple Myeloma Cells. Exp. Hematol. 2002, 30, 711–720. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.; Saddler-Shawnette, S.; Aller, A.; Brown, J. Potential Mechanism of Phytochemical-Induced Apoptosis in Human Prostate Adenocarcinoma Cells: Therapeutic Synergy in Genistein and β-Lapachone Combination Treatment. Cancer Cell Int. 2004, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Pink, J.J.; Planchon, S.M.; Tagliarino, C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H:Quinone Oxidoreductase Activity Is the Principal Determinant of β-Lapachone Cytotoxicity. J. Biol. Chem. 2000, 275, 5416–5424. [Google Scholar] [CrossRef]
- Planchon, S.M.; Pink, J.J.; Tagliarino, C.; Bornmann, W.G.; Varnes, M.E.; Boothman, D.A. β-Lapachone-Induced Apoptosis in Human Prostate Cancer Cells: Involvement of NQO1/Xip3. Exp. Cell Res. 2001, 267, 95–106. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Zhao, J.; Zhang, H.; Fu, J.; Luo, P.; Ma, Y.; Zou, D.; Gao, H.; Hu, J.; et al. Dp44mT, an Iron Chelator, Suppresses Growth and Induces Apoptosis via RORA-Mediated NDRG2-IL6/JAK2/STAT3 Signaling in Glioma. Cell. Oncol. 2020, 43, 461–475. [Google Scholar] [CrossRef]
- Krishan, S.; Sahni, S.; Leck, L.Y.W.; Jansson, P.J.; Richardson, D.R. Regulation of Autophagy and Apoptosis by Dp44mT-Mediated Activation of AMPK in Pancreatic Cancer Cells. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165657. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-Targeted Cancer Therapies: Progress, Challenges and Future Directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Nairismägi, M.-L.; Gerritsen, M.E.; Li, Z.M.; Wijaya, G.C.; Chia, B.K.H.; Laurensia, Y.; Lim, J.Q.; Yeoh, K.W.; Yao, X.S.; Pang, W.L.; et al. Oncogenic Activation of JAK3-STAT Signaling Confers Clinical Sensitivity to PRN371, a Novel Selective and Potent JAK3 Inhibitor, in Natural Killer/T-Cell Lymphoma. Leukemia 2018, 32, 1147–1156. [Google Scholar] [CrossRef]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Domoto, T.; Uehara, M.; Bolidong, D.; Minamoto, T. Glycogen Synthase Kinase 3β in Cancer Biology and Treatment. Cells 2020, 9, 1388. [Google Scholar] [CrossRef]
- Ryu, Y.-K.; Park, H.-Y.; Go, J.; Lee, I.-B.; Choi, Y.-K.; Lee, C.-H.; Kim, K.-S. Β-Lapachone Ameliorates L-DOPA-induced Dyskinesia in a 6-OHDA-induced Mouse Model of Parkinson’s Disease. Mol. Med. Rep. 2021, 23, 217. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Lai, S.-Y.; Su, Y.-T.; Yang, K.-C.; Chau, Y.-P.; Don, M.-J.; Lu, K.-H.; Shy, H.-T.; Lai, S.-M.; Kung, H.-N. β-Lapachone, an NQO1 Activator, Alleviates Diabetic Cardiomyopathy by Regulating Antioxidant Ability and Mitochondrial Function. Phytomedicine 2022, 104, 154255. [Google Scholar] [CrossRef]
- Mokarizadeh, N.; Karimi, P.; Erfani, M.; Sadigh-Eteghad, S.; Fathi Maroufi, N.; Rashtchizadeh, N. β-Lapachone Attenuates Cognitive Impairment and Neuroinflammation in Beta-Amyloid Induced Mouse Model of Alzheimer’s Disease. Int. Immunopharmacol. 2020, 81, 106300. [Google Scholar] [CrossRef]
- Oh, G.-S.; Kim, H.-J.; Choi, J.-H.; Shen, A.; Choe, S.-K.; Karna, A.; Lee, S.H.; Jo, H.-J.; Yang, S.-H.; Kwak, T.H.; et al. Pharmacological Activation of NQO1 Increases NAD+ Levels and Attenuates Cisplatin-Mediated Acute Kidney Injury in Mice. Kidney Int. 2014, 85, 547–560. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- de Santana, T.I.; de Oliveira Barbosa, M.; de Moraes Gomes, P.A.T.; da Cruz, A.C.N.; da Silva, T.G.; Leite, A.C.L. Synthesis, Anticancer Activity and Mechanism of Action of New Thiazole Derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Rel. Methodol. 2020, 46, 273–307. [Google Scholar] [CrossRef]
- Zegzouti, H.; Zdanovskaia, M.; Hsiao, K.; Goueli, S.A. ADP-Glo: A Bioluminescent and Homogeneous ADP Monitoring Assay for Kinases. Assay Drug Dev. Technol. 2009, 7, 560–572. [Google Scholar] [CrossRef]
- Ibrahim, N.; Bonnet, P.; Brion, J.-D.; Peyrat, J.-F.; Bignon, J.; Levaique, H.; Josselin, B.; Robert, T.; Colas, P.; Bach, S.; et al. Identification of a New Series of Flavopiridol-like Structures as Kinase Inhibitors with High Cytotoxic Potency. Eur. J. Med. Chem. 2020, 199, 112355. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Arruda, M.G.M.; da Silva, N.F.; da Cruz, R.C.D.; de Barros, S.C.L.; da Silva, M.S.; Santos Souza, T.G.D.; de Oliveira, E.B.; Chagas, C.A.; Santos Aguiar, J.D.; et al. Cytotoxic, Acute Oral Toxicity, Genotoxic and Mutagenic Assessment of the Essential Oil from Fresh Leaves of Croton Argyrophyllus (Kunth.). J. Ethnopharmacol. 2024, 330, 118206. [Google Scholar] [CrossRef]
- Silva, M.A.; Souza, T.G.; Melo, M.E.G.; Silva, J.M.; Lima, J.R.; Lira, A.F.A.; de Aguiar-Júnior, F.C.A.; Martins, R.D.; Jorge, R.J.B.; Chagas, C.A.; et al. Tityus Stigmurus Venom Causes Genetic Damage in Blood and Testicular Cells and Affects the Number and Morphology of Gametogenic Lineage Cells in Mice. Toxicon 2020, 185, 114–119. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The Comet Assay: Topical Issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Eiji, Y.; Haruyoshi, H.; Masaki, T.; Motoyoshi, K.; Setsuko, A. The Micronucleus Assay with Mouse Peripheral Blood Reticulocytes Using Acridine Orange-Coated Slides with Triethylenemelamine. Mutat. Res. Toxicol. 1992, 278, 127–130. [Google Scholar] [CrossRef]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M. The Micronucleus Assay with Mouse Peripheral Blood Reticulocytes Using Acridine Orange-Coated Slides. Mutat. Res. Lett. 1990, 245, 245–249. [Google Scholar] [CrossRef]







| Compounds and Kinases’ Inhibition (% of Residual Activity) | ||||||
|---|---|---|---|---|---|---|
| BV3 | BV5 | β-lap | ||||
| Kinase | 1 µM | 10 µM | 1 µM | 10 µM | 1 µM | 10 µM |
| CDK5/p25 | ≥100 | 44 | ≥100 | 62 | 74 | 88 |
| CDK9/CyclinT | ≥100 | 67 | ≥100 | 78 | ≥100 | 72 |
| HASPIN | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 | 91 |
| PIM1 | ≥100 | 18 | 94 | 42 | 56 | 42 |
| GSK3β | 58 | 7 | 64 | 24 | 67 | 43 |
| CK1ε | ≥100 | 98 | 98 | 86 | ≥100 | 94 |
| ABL1 | 52 | 35 | 83 | 56 | 83 | 81 |
| JAK3 | −3 | −9 | 64 | 4 | 65 | 69 |
| Compound | JAK3 (3LXK) | GSK3β (1Q4L) | ||
|---|---|---|---|---|
| Free Binding Energy (kcal/mol) | Amino Acids Interactions | Free Binding Energy (kcal/mol) | Amino Acids Interactions | |
| BV3 | −10.25 | Leu828, Gly829, Lys830, Gly831, Asn832 Gly834, Val836, Ala853, Val884, Met902, Arg953, Leu956, Ala966, Asp967 and Gly969 | −9.40 | Ile62, Gly63, Asn64, Val70, Ala83, Lys85, Glu97, Val110, Leu132, Asp133, Thr134, Val135, Pro136, Thr138, Arg141, Gln185, Leu188, Cys199, Asp200 and Phe201 |
| BV5 | −10.08 | Leu828, Gly829, Lys830, Gly831, Val836, Ala853, Val884, Met902, Arg911, Arg953, Leu956 and Ala966 | −9.48 | Ile62, Val70, Ala83, Lys85, Met101, Leu132, Thr134, Gln185, Leu188, Cys199 and Asp200 |
| β-Lapachone | −8.21 | Leu828, Val836, Ala853, Leu905, Leu956 and Ala966 | −7.32 | Val70, Ala83, Val110, Leu132, Leu188, Cys199 and Asp200 |
| Co-crystallized ligand * | −8.99 | Leu828, Ala853, Val884, Met902, Leu905, Cys909, Arg953, Asn954, Leu956 and Ala966 | −9.94 | Ile62, Val70, Ala83, Lys85, Val110, Leu132, Val135, Arg141, Gln185, Leu188 and Cys199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lima, J.R.; Da Silva Góes, A.J.; De Oliveira Borba, E.F.; da Silva, M.A.; Caiana, R.R.A.; Rodrigues, M.d.D.; De Lima Silva, M.S.; Chagas, C.A.; Baratte, B.; Robert, T.; et al. New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives. Pharmaceuticals 2025, 18, 837. https://doi.org/10.3390/ph18060837
De Lima JR, Da Silva Góes AJ, De Oliveira Borba EF, da Silva MA, Caiana RRA, Rodrigues MdD, De Lima Silva MS, Chagas CA, Baratte B, Robert T, et al. New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives. Pharmaceuticals. 2025; 18(6):837. https://doi.org/10.3390/ph18060837
Chicago/Turabian StyleDe Lima, José Rivaldo, Alexandre José Da Silva Góes, Elizabeth Fernanda De Oliveira Borba, Meykson Alexandre da Silva, Rodrigo Ribeiro Alves Caiana, Maria do Desterro Rodrigues, Mariza Severina De Lima Silva, Cristiano Aparecido Chagas, Blandine Baratte, Thomas Robert, and et al. 2025. "New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives" Pharmaceuticals 18, no. 6: 837. https://doi.org/10.3390/ph18060837
APA StyleDe Lima, J. R., Da Silva Góes, A. J., De Oliveira Borba, E. F., da Silva, M. A., Caiana, R. R. A., Rodrigues, M. d. D., De Lima Silva, M. S., Chagas, C. A., Baratte, B., Robert, T., Bach, S., Ourliac-Garnier, I., Marchand, P., & Da Silva, T. G. (2025). New Insights into the Anticancer Effects and Toxicogenomic Safety of Two β-Lapachone Derivatives. Pharmaceuticals, 18(6), 837. https://doi.org/10.3390/ph18060837










