Comparing the Impact of Different Antiarrhythmic Classes on Clinical Outcomes Following Atrial Fibrillation Catheter Ablation
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Procedural Characteristics
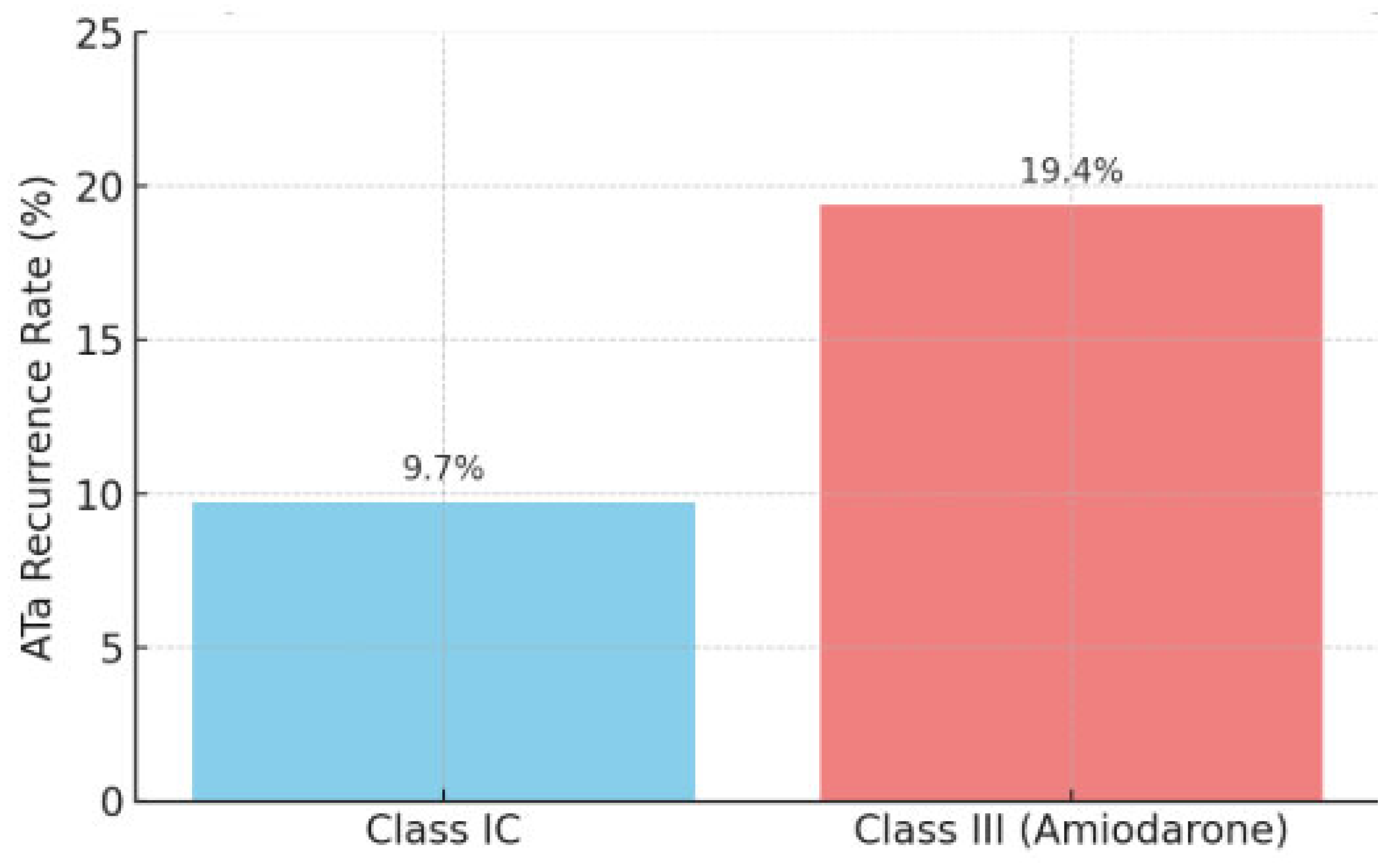
2.3. Atrial Tachycardia Recurrence
2.4. Safety Endpoints
3. Discussion
Strengths and Limitations
4. Materials and Methods
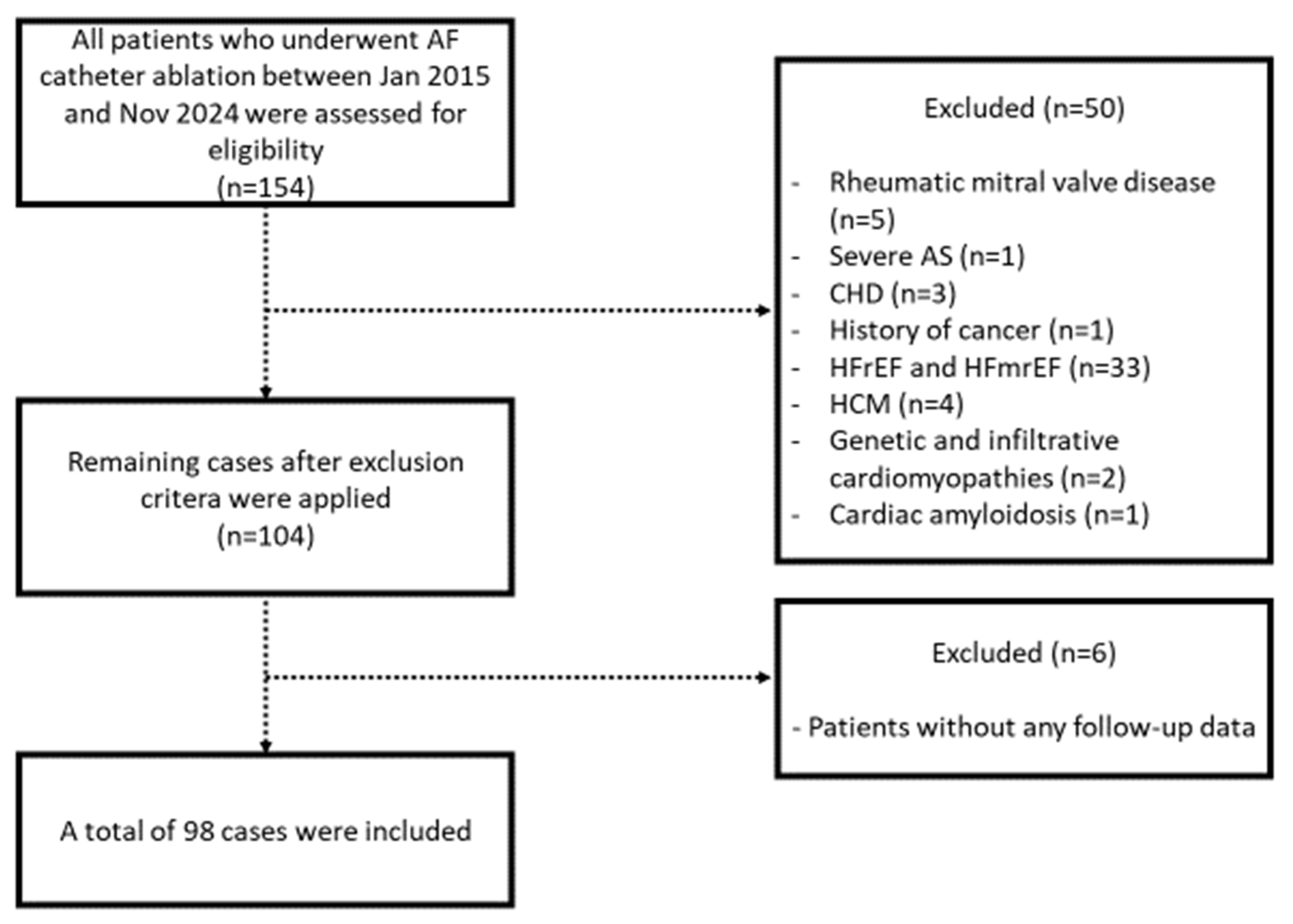
4.1. Study Population
4.2. Pre-Procedural Management
4.3. Cryoablation Procedure
4.4. Radiofrequency (RF) Ablation Procedure
4.5. Post-Procedural Management
4.6. Outcomes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Khan, S.; Sheikh, M.A.; Khuder, S.; Grubb, B.; Moukarbel, G.V. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: Systematic review and meta-analysis. Circ. Arrhythm. Electrophysiol. 2014, 7, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Al-Kaisey, A.M.; Kalman, J.M. Catheter ablation for atrial fibrillation: Current indications and evolving technologies. Nat. Rev. Cardiol. 2021, 18, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.F.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Marchlinski, F.E.; Bala, R.; Dixit, S.; Riley, M.; Russo, A.M.; et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation 2009, 120, 1036–1040. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Ling, Z.; Xu, Y.; Fan, J.; Du, H.; Xiao, P.; Su, L.; Liu, Z.; Lan, X.; et al. Efficacy of Short-Term Antiarrhythmic Drugs Use after Catheter Ablation of Atrial Fibrillation-A Systematic Review with Meta-Analyses and Trial Sequential Analyses of Randomized Controlled Trials. PLoS ONE 2016, 11, e0156121. [Google Scholar] [CrossRef]
- Echt, D.S.; Ruskin, J.N. Use of Flecainide for the Treatment of Atrial Fibrillation. Am. J. Cardiol. 2020, 125, 1123–1133. [Google Scholar] [CrossRef]
- Zylla, M.M.; Wolfes, J.; Schleberger, R.; Lawin, D.; Kieser, M.; Reinke, F.; Eckardt, L.; Rillig, A.; Stellbrink, C.; Thomas, D.; et al. Use of class IC antiarrhythmic drugs in patients with structural heart disease and implantable cardioverter defibrillator. Clin. Res. Cardiol. 2024, 113, 933–941. [Google Scholar] [CrossRef]
- Ad, N.; Holmes, S.D.; Shuman, D.J.; Pritchard, G.; Miller, C.E. Amiodarone after surgical ablation for atrial fibrillation: Is it really necessary? A prospective randomized controlled trial. J. Thorac. Cardiovasc. Surg. 2016, 151, 798–803. [Google Scholar] [CrossRef]
- Kaitani, K.; Inoue, K.; Kobori, A.; Nakazawa, Y.; Ozawa, T.; Kurotobi, T.; Morishima, I.; Miura, F.; Watanabe, T.; Masuda, M.; et al. EAST-AFTrial Investigators Efficacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation (EAST-AF) trial. Eur. Heart J. 2016, 37, 610–618. [Google Scholar] [CrossRef]
- Darkner, S.; Chen, X.; Hansen, J.; Pehrson, S.; Johannessen, A.; Nielsen, J.B.; Svendsen, J.H. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: A double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur. Heart J. 2014, 35, 3356–3364. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Antiarrhythmic Drugs After Ablation: Atrial Fibrillation: Diagnosis Management: Evidence Review K; (NICE Guideline, No. 196.); National Institute for Health and Care Excellence (NICE): London, UK, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK571342/ (accessed on 20 May 2025).
- National Institute for Health and Care Excellence (NICE). Atrial Fibrillation: Diagnosis and Management; NICE: London, UK, 2021; Contract No.: 978-1-4731-4043-1.
- Barekatain, A.; Razavi, M. Antiarrhythmic therapy in atrial fibrillation: Indications, guidelines, and safety. Tex. Heart Inst. J. 2012, 39, 532–534. [Google Scholar] [PubMed]
- Rudo, T.; Kowey, P. Atrial fibrillation: Choosing an antiarrhythmic drug. Curr. Cardiol. Rep. 2006, 8, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2024, 83, 109–279, Erratum in J. Am. Coll. Cardiol. 2024, 83, 959. Erratum in J. Am. Coll. Cardiol. 2024, 83, 2714. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Van Houten, H.K.; Sangaralingham, L.R.; Deshmukh, A.J.; Kapa, S.; Mulpuru, S.K.; McLeod, C.J.; Asirvatham, S.J.; Friedman, P.A.; Sha, N.D.; et al. Effect of Antiarrhythmic Drug Initiation on Readmission After Catheter Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2015, 1, 238–244. [Google Scholar] [CrossRef]
- Leong-Sit, P.; Roux, J.F.; Zado, E.; Callans, D.J.; Garcia, F.; Lin, D.; Marchlinski, F.E.; Bala, R.; Dixit, S.; Riley, M.; et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): Six-month follow-up study. Circ. Arrhythm. Electrophysiol. 2011, 4, 11–14. [Google Scholar] [CrossRef]
- Duytschaever, M.; Demolder, A.; Phlips, T.; Sarkozy, A.; El Haddad, M.; Taghji, P.; Knecht, S.; Tavernier, R.; Vandekerckhove, Y.; De Potter, T. PulmOnary vein isolation with vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): Results from a multicentre randomized trial. Eur. Heart J. 2018, 39, 1429–1437. [Google Scholar] [CrossRef]
- Schleberger, R.; Metzner, A.; Kuck, K.H.; Andresen, D.; Willems, S.; Hoffmann, E.; Deneke, T.; Eckardt, L.; Brachmann, J.; Hochadel, M.; et al. Antiarrhythmic drug therapy after catheter ablation for atrial fibrillation-Insights from the German Ablation Registry. Pharmacol. Res. Perspect. 2021, 9, e00880. [Google Scholar] [CrossRef]
- Şener, Y.Z.; Okşul, M.; Akkaya, F. Predictors of recurrence after atrial fibrillation catheter ablation. Acta Cardiol. 2020, 75, 810. [Google Scholar] [CrossRef]
- Heist, E.K.; Chalhoub, F.; Barrett, C.; Danik, S.; Ruskin, J.N.; Mansour, M. Predictors of atrial fibrillation termination and clinical success of catheter ablation of persistent atrial fibrillation. Am. J. Cardiol. 2012, 110, 545–551. [Google Scholar] [CrossRef]
- Themistoclakis, S.; Schweikert, R.A.; Saliba, W.I.; Bonso, A.; Rossillo, A.; Bader, G.; Wazni, O.; Burkhardt, D.J.; Raviele, A.; Natale, A. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008, 5, 679–685. [Google Scholar] [CrossRef]
- Cai, L.; Yin, Y.; Ling, Z.; Su, L.; Liu, Z.; Wu, J.; Du, H.; Lan, X.; Fan, J.; Chen, W.; et al. Predictors of late recurrence of atrial fibrillation after catheter ablation. Int. J. Cardiol. 2013, 164, 82–87. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.W.; Smedley, T.; Lambiase, P.D.; Ahsan, S.Y.; Segal, O.R.; Rowland, E.; Lowe, M.D.; Chow, A.W. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace 2011, 13, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, W.; Zhou, L.; Gu, J.; Wang, Y.; Liu, Y.; Zhang, X.; Wu, S.; Liu, X. Why atrial fibrillation recurs in patients who obtained current ablation endpoints with longstanding persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2013, 37, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dan, G.A.; Martinez-Rubio, A.; Agewall, S.; Boriani, G.; Borggrefe, M.; Gaita, F.; van Gelder, I.; Gorenek, B.; Kaski, J.C.; Kjeldsen, K.; et al. Antiarrhythmic drugs-clinical use and clinical decision making: A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace 2018, 20, 731–732. [Google Scholar]
- You, H.S.; Yoon, J.H.; Cho, S.B.; Choi, Y.D.; Kim, Y.H.; Choi, W.; Kang, H.-C.; Choi, S.K. Amiodarone-Induced Multi-Systemic Toxicity Involving the Liver, Lungs, Thyroid, and Eyes: A Case Report. Front. Cardiovasc. Med. 2022, 9, 839441. [Google Scholar] [CrossRef]
- Torres, D.; Parrinello, G.; Paterna, S.; Bellanca, M.; Licata, G. Severe Brochostenosis by Oral Propafenone Immediately After Commencing Treatment. Am. J. Ther. 2011. online ahead of print. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Wu, Y. Sinus node dysfunction after radiofrequency catheter ablation of atrial flutter: A case report. Ann. Noninvasive Electrocardiol. 2023, 28, e13010. [Google Scholar] [CrossRef]
- Barton, A.K.; McGowan, M.; Smyth, A.; Wright, G.A. Classification and choice of antiarrhythmic therapies. Prescriber 2021, 31, 11–17. [Google Scholar] [CrossRef]
- Proietti, M.; Popescu, R. Antiarrhythmic Drugs in Elderly and Frail Patients. In Antiarrhythmic Drugs; Martínez-Rubio, A., Tamargo, J., Dan, G.A., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. ESCScientific Document Group 2024 ESCGuidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Steinberg, J.S.; O’Connell, H.; Li, S.; Ziegler, P.D. Thirty-Second Gold Standard Definition of Atrial Fibrillation and Its Relationship with Subsequent Arrhythmia Patterns: Analysis of a Large Prospective Device Database. Circ. Arrhythm. Electrophysiol. 2018, 11, e006274. [Google Scholar] [CrossRef]

| All Patients n = 98 | Class IC AAD n = 62 | Class III AAD n = 36 | p-Value | |
|---|---|---|---|---|
| Age, years | 54.2 ± 14.0 | 51.1 ± 15.3 | 59.5 ± 9.7 | 0.001 ** |
| Male sex, n (%) | 54 (55.1) | 37 (59.7) | 17 (47.2) | 0.232 |
| AF type, paroxysmal | 65 (66.3) | 43 (69.4) | 22 (61.1) | 0.405 |
| Prior DCCV | 34 (34.7) | 17 (24.4) | 17 (47.2) | 0.047 * |
| History of AF ablation | 11 (11.2) | 6 (9.7) | 5 (13.9) | 0.524 |
| Duration of AF (months) | 8 (1–36) | 7 (2–36) | 8 (1–24) | 0.703 |
| CHADS-VA | 1 (0–6) | 1 (0–6) | 2 (0–5) | 0.004 ** |
| HAS-BLED | 1 (0–4) | 1 (0–4) | 1 (0–3) | 0.012 * |
| Comorbidities | ||||
| Hypertension | 69 (70.4) | 39 (62.9) | 30 (83.3) | 0.033 * |
| Diabetes mellitus | 22 (22.4) | 9 (14.5) | 13 (36.1) | 0.014 * |
| Coronary artery disease | 52 (53.1) | 26 (41.9) | 26 (72.2) | 0.004 ** |
| Ischemic stroke | 9 (9.2) | 3 (4.8) | 6 (16.7) | 0.071 |
| Medications, n (%) | ||||
| Beta blockers | 77 (78.6) | 47 (75.8) | 30 (83.3) | 0.381 |
| RAAS inhibitors | 53 (54.1) | 26 (41.9) | 27 (75.0) | 0.002 ** |
| Digoxin | 13 (13.3) | 8 (12.9) | 5 (13.9) | 0.890 |
| Anticoagulants | 87 (88.8) | 54 (87.1) | 33 (91.7) | 0.490 |
| Antiarrhythmic drugs | 64 (65.3) | 41 (66.1) | 23 (63.9) | 0.822 |
| Echocardiographic parameters | ||||
| LA diameter, mm | 38.7 ± 5.1 | 37.7 ± 4.2 | 40.5 ± 6.0 | 0.026 * |
| LVEF, % | 57.6 ± 5.4 | 58.0 ± 5.4 | 56.8 ± 5.4 | 0.336 |
| Moderate and severe MR, n (%) | 21 (23.3) | 10 (17.9) | 11 (32.4) | 0.115 |
| Laboratory values | ||||
| Hemoglobin, g/dL | 14.0 ± 1.9 | 14.1 ± 1.8 | 13.8 ± 2.0 | 0.396 |
| GFR, mL/min/m2 | 90 (23–125) | 90 (23–125) | 90 (54–110) | 0.468 |
| All Patients n = 98 | Class IC AAD n = 62 | Class III AAD n = 36 | p-Value | |
|---|---|---|---|---|
| Baseline rhythm, n (%) | 0.067 | |||
| - Sinus | 73 (74.5) | 50 (80.6) | 23 (63.9) | |
| - AF | 25 (25.5) | 12 (19.4) | 13 (36.1) | |
| Ablation technique, n (%) | 0.243 | |||
| - Cryoablation | 77 (78.6) | 51 (82.3) | 26 (72.2) | |
| - RF ablation | 21 (21.4) | 11 (17.7) | 10 (27.8) | |
| Ablation type, n (%) | 0.309 | |||
| - PVI only | 86 (87.8) | 56 (90.3) | 630 (83.3) | |
| - PVI plus | 12 (12.2) | 6 (9.7) | 6 (16.7) | |
| Common ostium, n (%) | 2 (2) | 2 (3.2) | 0 (0.0) | 2.530 |
| Procedural success, n (%) | 96 (98) | 61 (98.4) | 35 (97.2) | 1.000 |
| ATa recurrence, n (%) | 13 (13.3) | 6 (9.7) | 7 (19.4) | 0.169 |
| Follow-up (months) | 17.5 (1.5–120.0) | 19.1 (1.8–120.0) | 14.1 (1.5–84.0) | 0.446 |
| Variables | Univariable HR (95%CI) | p-Value | Multivariable HR (95%CI) | p-Value |
|---|---|---|---|---|
| Age, years | 1.02 (0.98–1.06) | 0.222 | ||
| Male sex | 0.53 (0.17–1.63) | 0.270 | ||
| Hypertension | 0.92 (0.28–3.00) | 0.925 | ||
| Diabetes mellitus | 1.46 (0.45–4.76) | 0.526 | ||
| Coronary artery disease | 0.77 (0.26–2.30) | 0.645 | ||
| Type of AF | ||||
| Persistent vs. Paroxysmal | 5.38 (1.65–17.50) | 0.005 * | 1.76 (0.32–9.76) | 0.513 |
| Prior DCCV | 5.41 (1.66–17.62) | 0.005 * | 5.86 (1.44–23.82) | 0.013 * |
| AF duration (months) | 1.13 (1.06–1.20) | <0.001 * | 1.07 (0.99–1.16) | 0.082 |
| PVI plus vs. PVI only | 0.85 (0.11–6.57) | 0.879 | ||
| Baseline rhythm | ||||
| AF vs. Sinus rhythm | 3.99 (1.34–11.9) | 0.013 * | 2.06 (0.40–10.69) | 0.386 |
| Ablation type | ||||
| RF vs. Cryoablation | 0.88 (0.19–4.06) | 0.889 | ||
| LA diameter, mm | 1.14 (1.03–1.27) | 0.008 * | 1.17 (1.04–1.31) | 0.008 ** |
| LVEF, % | 1.01 (0.91–1.11) | 0.829 | ||
| AAD at discharge | ||||
| Class III vs. Class IC AAD | 2.17 (0.73–6.48) | 0.162 | 1.57 (0.43–5.64) | 0.489 |
| All Patients n = 98 | Class IC AAD n = 62 | Class III AAD n = 36 | p-Value | |
|---|---|---|---|---|
| Symptomatic bradycardia (<60 bpm) | 6 (6.1) | 3 (4.8) | 3 (8.3) | 0.487 |
| Dyspnea | 3 (3.1) | 3 (4.8) | 0 (0.0) | 0.296 |
| Stroke/TIA | 1 (1) | 0 (0.0) | 1 (2.8) | 0.367 |
| Increase in liver transaminases (x3 ULN) | 1 (1) | 0 (0.0) | 1 (2.8) | 0.367 |
| Thyroid dysfunction | 1 (1) | 0 (0.0) | 1 (2.8) | 0.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belančić, A.; Sener, Y.Z.; Oksul, M.; Ozturk, C.; Soner, S.; Comert, A.D.; Arslan, G.Y.; Vitezić, D.; Jelaković, B.; Baysal, E. Comparing the Impact of Different Antiarrhythmic Classes on Clinical Outcomes Following Atrial Fibrillation Catheter Ablation. Pharmaceuticals 2025, 18, 1022. https://doi.org/10.3390/ph18071022
Belančić A, Sener YZ, Oksul M, Ozturk C, Soner S, Comert AD, Arslan GY, Vitezić D, Jelaković B, Baysal E. Comparing the Impact of Different Antiarrhythmic Classes on Clinical Outcomes Following Atrial Fibrillation Catheter Ablation. Pharmaceuticals. 2025; 18(7):1022. https://doi.org/10.3390/ph18071022
Chicago/Turabian StyleBelančić, Andrej, Yusuf Ziya Sener, Metin Oksul, Cansu Ozturk, Serdar Soner, Adnan Duha Comert, Gamze Yeter Arslan, Dinko Vitezić, Bojan Jelaković, and Erkan Baysal. 2025. "Comparing the Impact of Different Antiarrhythmic Classes on Clinical Outcomes Following Atrial Fibrillation Catheter Ablation" Pharmaceuticals 18, no. 7: 1022. https://doi.org/10.3390/ph18071022
APA StyleBelančić, A., Sener, Y. Z., Oksul, M., Ozturk, C., Soner, S., Comert, A. D., Arslan, G. Y., Vitezić, D., Jelaković, B., & Baysal, E. (2025). Comparing the Impact of Different Antiarrhythmic Classes on Clinical Outcomes Following Atrial Fibrillation Catheter Ablation. Pharmaceuticals, 18(7), 1022. https://doi.org/10.3390/ph18071022









