Curcumin-like Compound Inhibits Proliferation of Adenocarcinoma Cells by Inducing Cell Cycle Arrest and Senescence
Abstract
1. Introduction
2. Results
2.1. Synthesis of the Target-Compound PQM-214
2.2. Antiproliferative and Cytotoxic Investigation
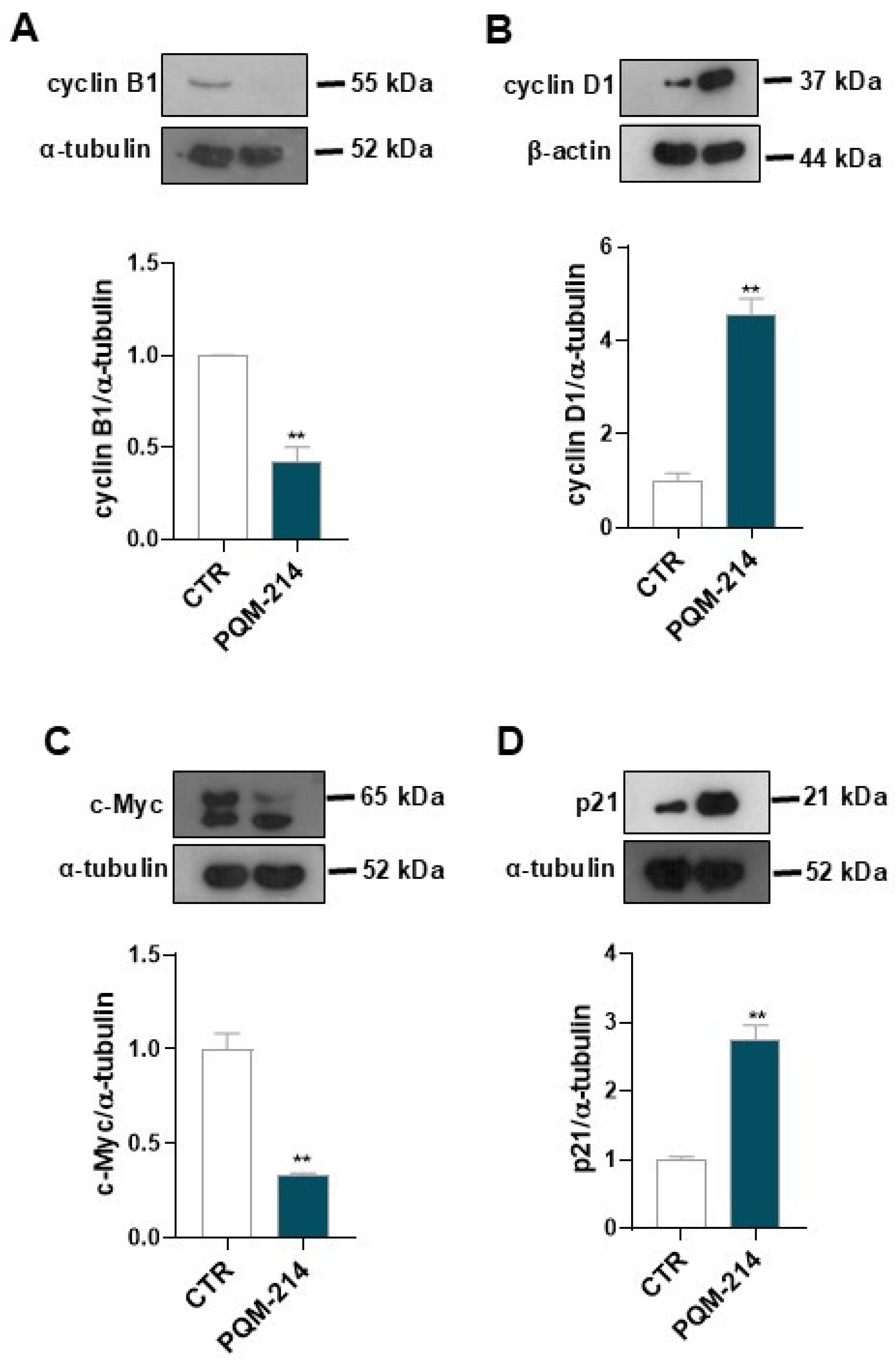
2.3. Pro-Senescent Investigation
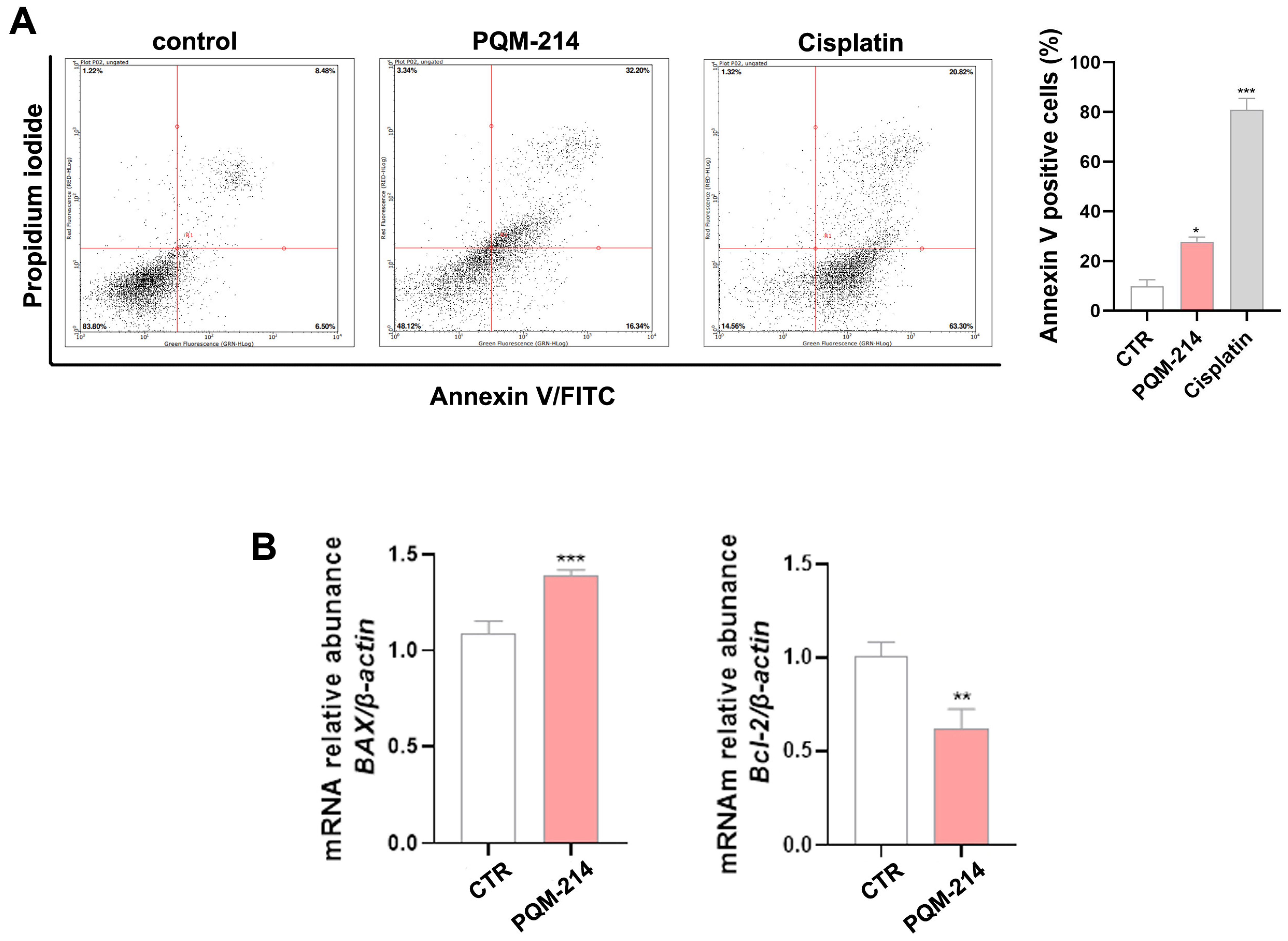
2.4. Apoptosis Investigation
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Compound PQM-214
4.2. Cell Cultures
4.3. Cell Viability Determination
4.4. Clonogenic Assay
4.5. Cell Cycle Analysis
4.6. Gene Expression Analysis Using qPCR
4.7. Protein Expression Analysis by Western Blot
4.8. Annexin V Assay
4.9. Cellular Senescence Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Soler, J.; Reckamp, K.L.; Sankar, K. Emerging Targets in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 10046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Q.; Zhang, M.; Zhang, Y.; Li, F.; Lei, P. Network Analysis in the Identification of Special Mechanisms between Small Cell Lung Cancer and Non-small Cell Lung Cancer. Thorac. Cancer 2014, 5, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-Cell Lung Cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Chang, A. Chemotherapy, Chemoresistance and the Changing Treatment Landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Zullo, L.; Rossi, G.; Grassi, M.; Murianni, V.; Tagliamento, M.; Prelaj, A.; Coco, S.; Longo, L.; Dal Bello, M.G.; et al. Novel Emerging Molecular Targets in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 2625. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Current Advances and Future Trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef]
- Fortney, K.; Jurisica, I. Integrative Computational Biology for Cancer Research. Hum. Genet. 2011, 130, 465–481. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Weng, S.-H.; Kuo, Y.-H.; Chiu, Y.-F.; Lin, Y.-W. Synergistic Effect of Curcumin and Cisplatin via Down-Regulation of Thymidine Phosphorylase and Excision Repair Cross-Complementary 1 (ERCC1). Mol. Pharmacol. 2011, 80, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of Curcuminoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential Anticancer Properties and Mechanisms of Action of Curcumin. Anticancer. Res. 2015, 35, 645–651. [Google Scholar]
- Um, M.Y.; Hwang, K.H.; Choi, W.H.; Ahn, J.; Jung, C.H.; Ha, T.Y. Curcumin Attenuates Adhesion Molecules and Matrix Metalloproteinase Expression in Hypercholesterolemic Rabbits. Nutr. Res. 2014, 34, 886–893. [Google Scholar] [CrossRef]
- Miriyala, S.; Panchatcharam, M.; Rengarajulu, P. Cardioprotective Effects of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 359–377. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.-C. Curcumin in Cancer Therapy: Exploring Molecular Mechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.; Morsy, M.; Boddu, S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Silva, M.; Coelho, L.F.; Guirelli, I.M.; Pereira, R.M.; Ferreira-Silva, G.Á.; Graravelli, G.Y.; Horvath, R.D.O.; Caixeta, E.S.; Ionta, M.; Viegas, C. Synthetic Resveratrol-Curcumin Hybrid Derivative Inhibits Mitosis Progression in Estrogen Positive MCF-7 Breast Cancer Cells. Toxicol. Vitr. 2018, 50, 75–85. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Silva, M.; Juliet Cristancho Ortiz, C.; Ferreira Coelho, L.; Pruccoli, L.; Pagliarani, B.; Pisani, L.; Catto, M.; Poli, G.; Tuccinardi, T.; Cardoso Vilela, F.; et al. Synthesis and Pharmacological Evaluation of Novel N-Aryl-Cinnamoyl-Hydrazone Hybrids Designed as Neuroprotective Agents for the Treatment of Parkinson’s Disease. Bioorganic Chem. 2024, 150, 107587. [Google Scholar] [CrossRef]
- Duarte, C.; Barreiro, E.; Fraga, C. Privileged Structures: A Useful Concept for the Rational Design of New Lead Drug Candidates. Mini-Reviews Med. Chem. 2007, 7, 1108–1119. [Google Scholar] [CrossRef]
- Fraga, C.; Barreiro, E. Medicinal Chemistry of N-Acylhydrazones: New Lead-Compounds of Analgesic, Antiinflammatory and Antithrombotic Drugs. Curr. Med. Chem. 2006, 13, 167–198. [Google Scholar] [CrossRef]
- Maia, R.D.C.; Tesch, R.; Fraga, C.A.M. Acylhydrazone Derivatives: A Patent Review. Expert. Opin. Ther. Pat. 2014, 24, 1161–1170. [Google Scholar] [CrossRef]
- Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E.M.; Dumitru, A.-C.; Nitulescu, G.M.; Sfetea, R.C.; Apostol, T.-V. Acylhydrazones and Their Biological Activity: A Review. Molecules 2022, 27, 8719. [Google Scholar] [CrossRef]
- Alturki, M.S.; Tawfeeq, N.; Alissa, A.; Ahbail, Z.; Gomaa, M.S.; Al Khzem, A.H.; Rants’o, T.A.; Akbar, M.J.; Alharbi, W.S.; Alshehri, B.Y.; et al. Targeting KRAS G12C and G12S Mutations in Lung Cancer: In Silico Drug Repurposing and Antiproliferative Assessment on A549 Cells. Inform. Med. Unlocked 2025, 52, 101612. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS Mutation: From Undruggable to Druggable in Cancer. Sig Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Chen, T.; Ashwood, L.M.; Kondrashova, O.; Strasser, A.; Kelly, G.; Sutherland, K.D. Breathing New Insights into the Role of Mutant P53 in Lung Cancer. Oncogene 2025, 44, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Qin, D.; Hou, X.; Tian, L.; Yu, Y.; Zhang, R.; Lyu, H.; Guo, D.; Chen, X.-Z.; Zhou, C.; et al. Cellular Senescence: A Double-Edged Sword in Cancer Therapy. Front. Oncol. 2023, 13, 1189015. [Google Scholar] [CrossRef] [PubMed]
- Kohli, J.; Wang, B.; Brandenburg, S.M.; Basisty, N.; Evangelou, K.; Varela-Eirin, M.; Campisi, J.; Schilling, B.; Gorgoulis, V.; Demaria, M. Algorithmic Assessment of Cellular Senescence in Experimental and Clinical Specimens. Nat. Protoc. 2021, 16, 2471–2498. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Jha, S.K.; De Rubis, G.; Devkota, S.R.; Zhang, Y.; Adhikari, R.; Jha, L.A.; Bhattacharya, K.; Mehndiratta, S.; Gupta, G.; Singh, S.K.; et al. Cellular Senescence in Lung Cancer: Molecular Mechanisms and Therapeutic Interventions. Ageing Res. Rev. 2024, 97, 102315. [Google Scholar] [CrossRef]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Sieben, C.J.; Sturmlechner, I.; Van De Sluis, B.; Van Deursen, J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Orfanos, C.E.; Geilen, C.C.; Wieder, T.; Sturm, I.; Daniel, P.T. The Bax/Bcl-2 Ratio Determines the Susceptibility of Human Melanoma Cells to CD95/Fas-Mediated Apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef]
- Martin, B.; Paesmans, M.; Berghmans, T.; Branle, F.; Ghisdal, L.; Mascaux, C.; Meert, A.-P.; Steels, E.; Vallot, F.; Verdebout, J.-M.; et al. Role of Bcl-2 as a Prognostic Factor for Survival in Lung Cancer: A Systematic Review of the Literature with Meta-Analysis. Br. J. Cancer 2003, 89, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, B.; Estepa-Fernández, A.; Rovira, M.; Orzáez, M.; Serrano, M.; Martínez-Máñez, R.; Sancenón, F. The Chemistry of Senescence. Nat. Rev. Chem. 2019, 3, 426–441. [Google Scholar] [CrossRef]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The Curcumin Analog EF24 Is a Novel Senolytic Agent. Aging 2019, 11, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Lamartine-Hanemann, S.D.S.; Ferreira-Silva, G.Á.; Horvath, R.D.O.; Soncini, R.; Caixeta, E.S.; Rocha-Sales, B.; Niero, E.L.; Machado-Santelli, G.M.; Dos Santos, M.H.; De Oliveira, J.C.; et al. A Tetraprenylated Benzophenone 7-Epiclusianone Induces Cell Cycle Arrest at G1/S Transition by Modulating Critical Regulators of Cell Cycle in Breast Cancer Cell Lines. Toxicol. Vitr. 2020, 68, 104927. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]





| Cell Lines | IC50 (PQM-214) | GI50 (PQM-214) | IC50 (Cisplatin) |
|---|---|---|---|
| A549 | 23.68 ± 0.66 | 19.67 ± 0.82 | 28.13 ± 1.65 |
| H1299 | 32.15 ± 0.96 | 23.94 ± 0.83 | 34.51 ± 1.20 |
| Primary fibroblasts | ˃150 | ˃150 | 82.95 ± 2.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, R.; Louzano, Y.d.S.; Ortiz, C.J.C.; Silva, M.d.F.; Felix, M.L.V.; Ferreira-Silva, G.Á.; Caixeta, E.S.; Zavan, B.; Viegas, C., Jr.; Ionta, M. Curcumin-like Compound Inhibits Proliferation of Adenocarcinoma Cells by Inducing Cell Cycle Arrest and Senescence. Pharmaceuticals 2025, 18, 914. https://doi.org/10.3390/ph18060914
Fonseca R, Louzano YdS, Ortiz CJC, Silva MdF, Felix MLV, Ferreira-Silva GÁ, Caixeta ES, Zavan B, Viegas C Jr., Ionta M. Curcumin-like Compound Inhibits Proliferation of Adenocarcinoma Cells by Inducing Cell Cycle Arrest and Senescence. Pharmaceuticals. 2025; 18(6):914. https://doi.org/10.3390/ph18060914
Chicago/Turabian StyleFonseca, Rafael, Yasmin dos Santos Louzano, Cindy Juliet Cristancho Ortiz, Matheus de Freitas Silva, Maria Luiza Vieira Felix, Guilherme Álvaro Ferreira-Silva, Ester Siqueira Caixeta, Bruno Zavan, Claudio Viegas, Jr., and Marisa Ionta. 2025. "Curcumin-like Compound Inhibits Proliferation of Adenocarcinoma Cells by Inducing Cell Cycle Arrest and Senescence" Pharmaceuticals 18, no. 6: 914. https://doi.org/10.3390/ph18060914
APA StyleFonseca, R., Louzano, Y. d. S., Ortiz, C. J. C., Silva, M. d. F., Felix, M. L. V., Ferreira-Silva, G. Á., Caixeta, E. S., Zavan, B., Viegas, C., Jr., & Ionta, M. (2025). Curcumin-like Compound Inhibits Proliferation of Adenocarcinoma Cells by Inducing Cell Cycle Arrest and Senescence. Pharmaceuticals, 18(6), 914. https://doi.org/10.3390/ph18060914








