Combined Network Pharmacology, Transcriptomics and Metabolomics Strategies Reveal the Mechanism of Action of Lang Chuang Wan to Ameliorate Lupus Nephritis in MRL/lpr Mice
Abstract
1. Introduction
2. Results
2.1. Active Components of LCW
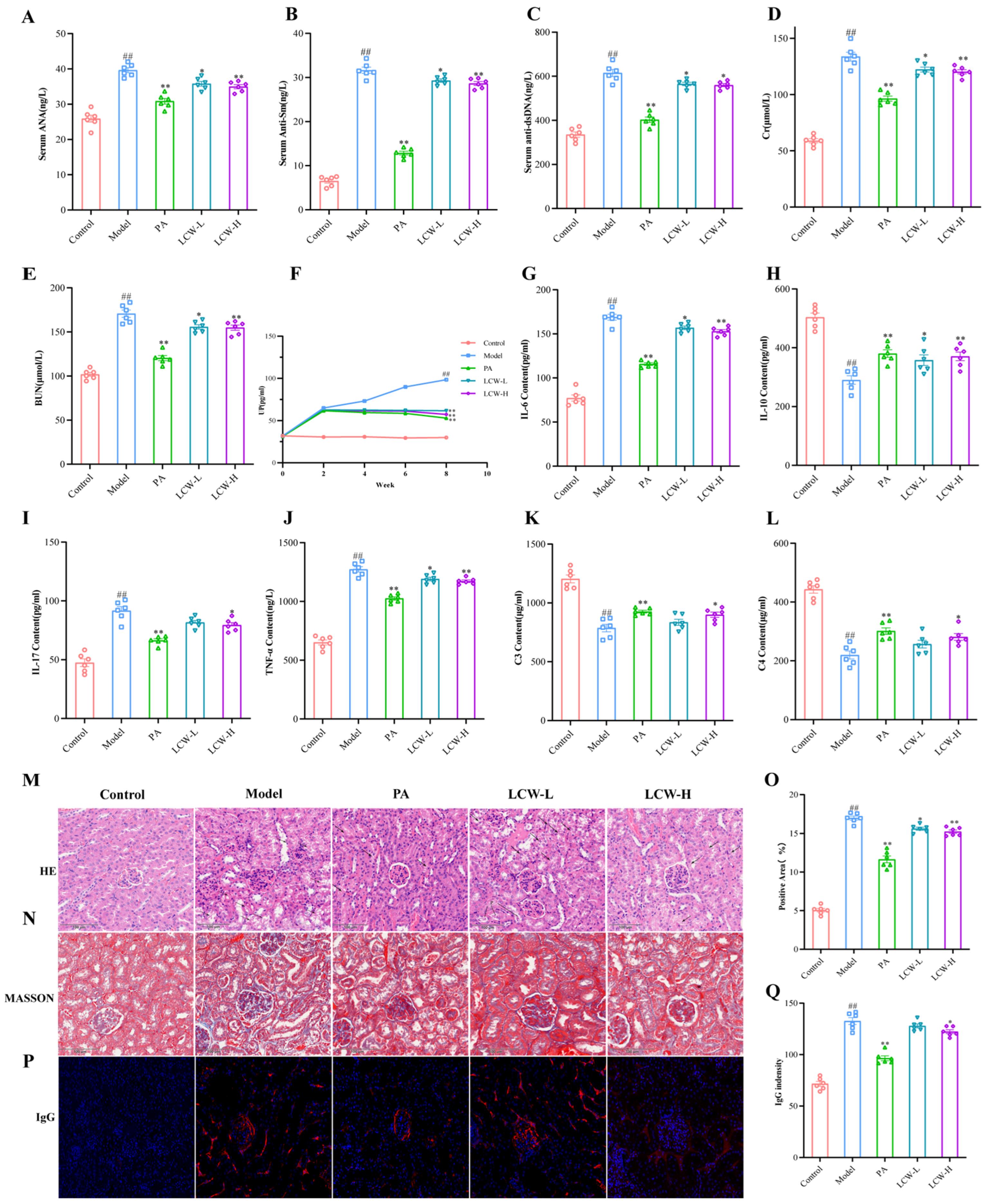
2.2. LCW Regulates the Levels of Serum Autoantibodies and Inflammatory Cytokines in MRL/lpr Mice, Enhancing Renal Function and Reducing Kidney Damage
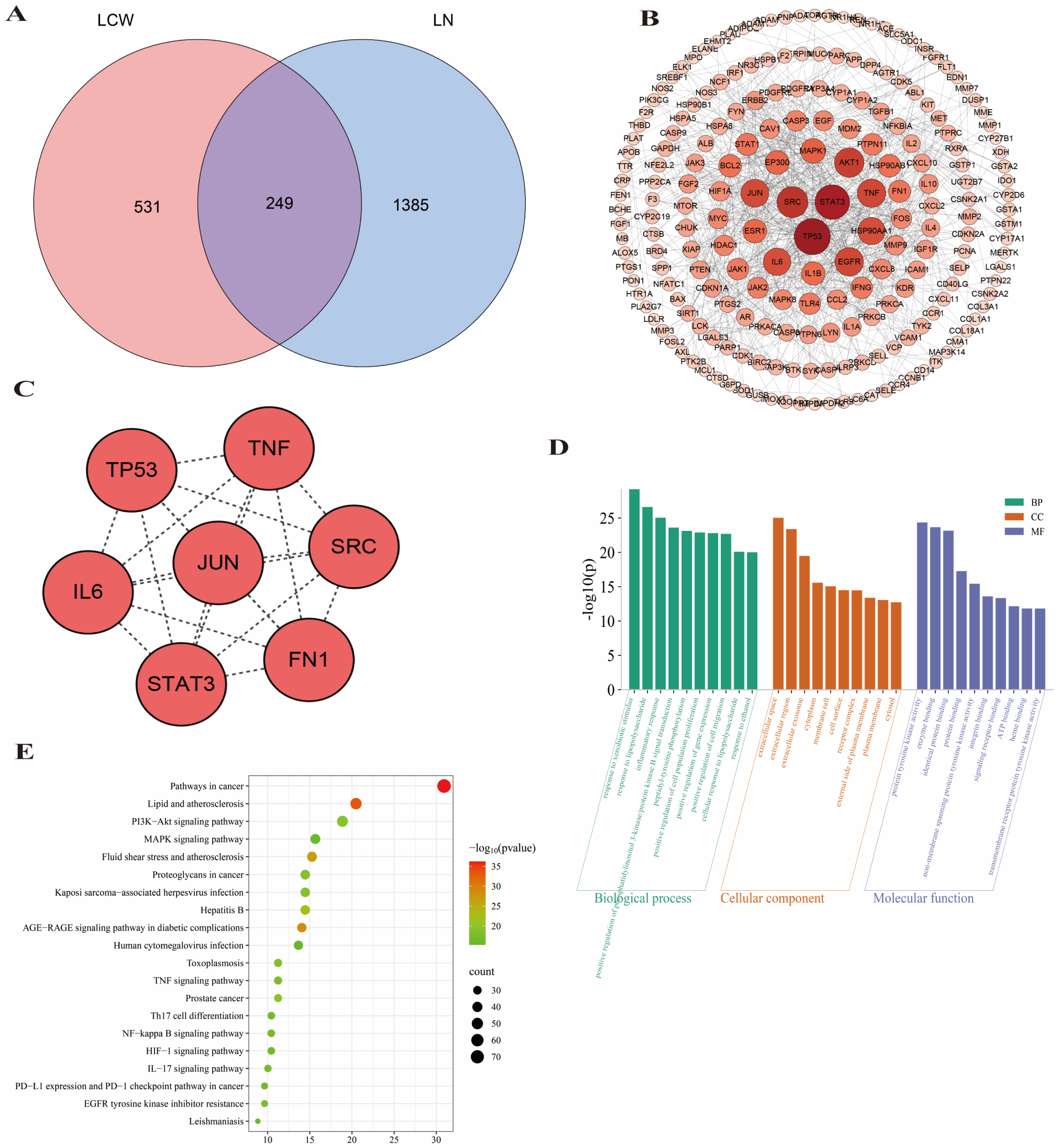
2.3. Network Pharmacology Prediction for LCW Treatment of LN
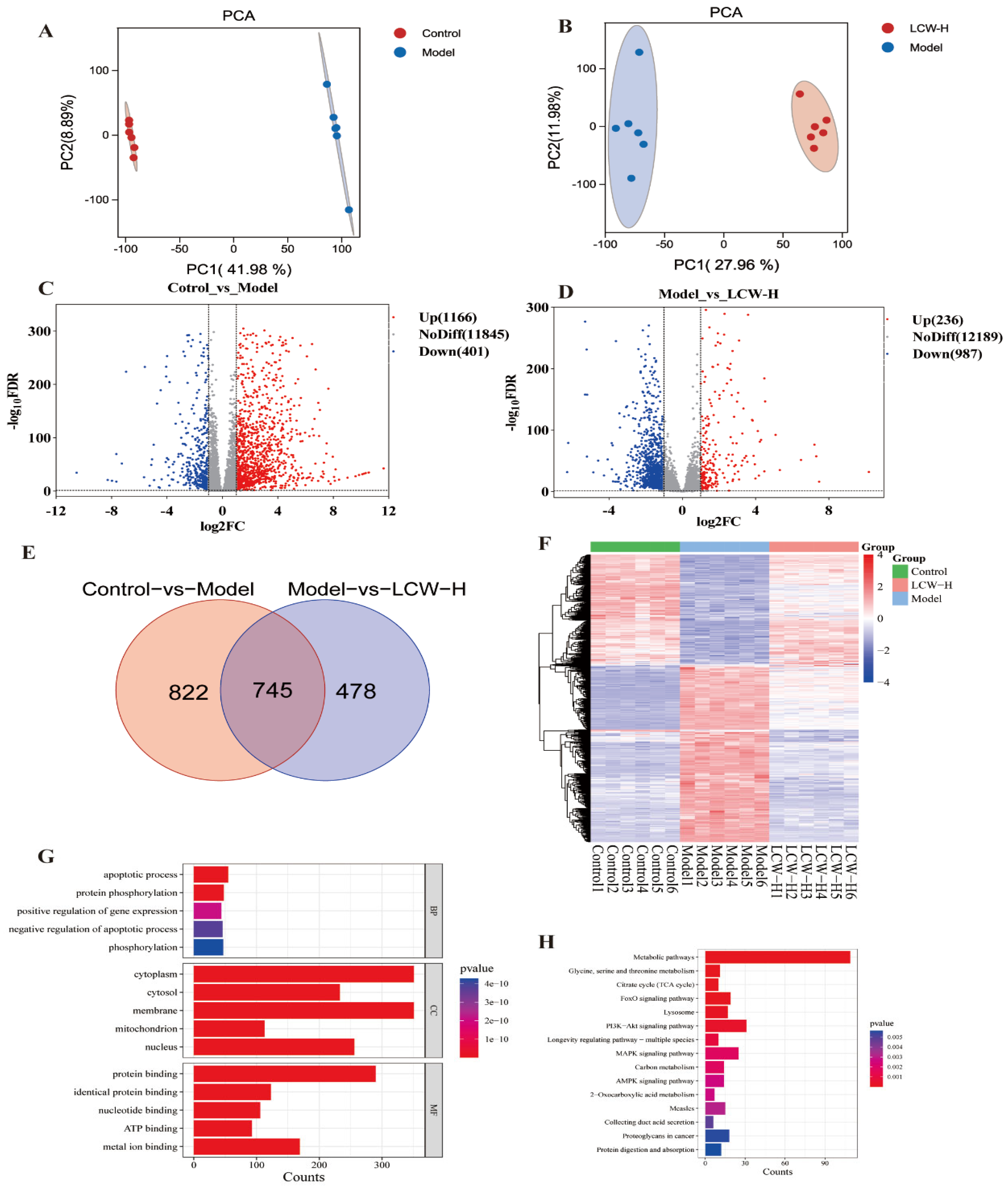
2.4. Transcriptomics Analysis Results
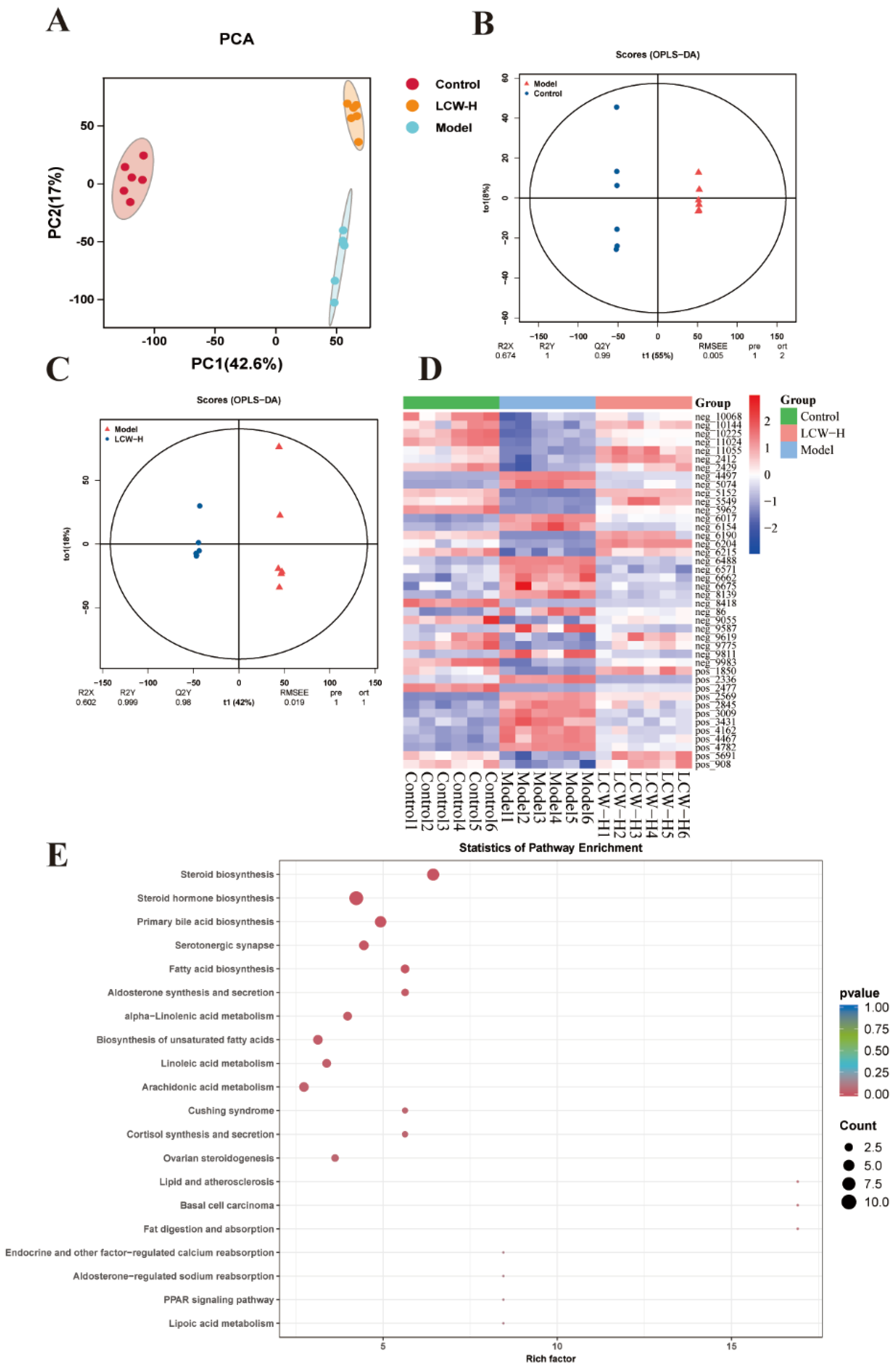
2.5. Results of Metabolomics Analysis
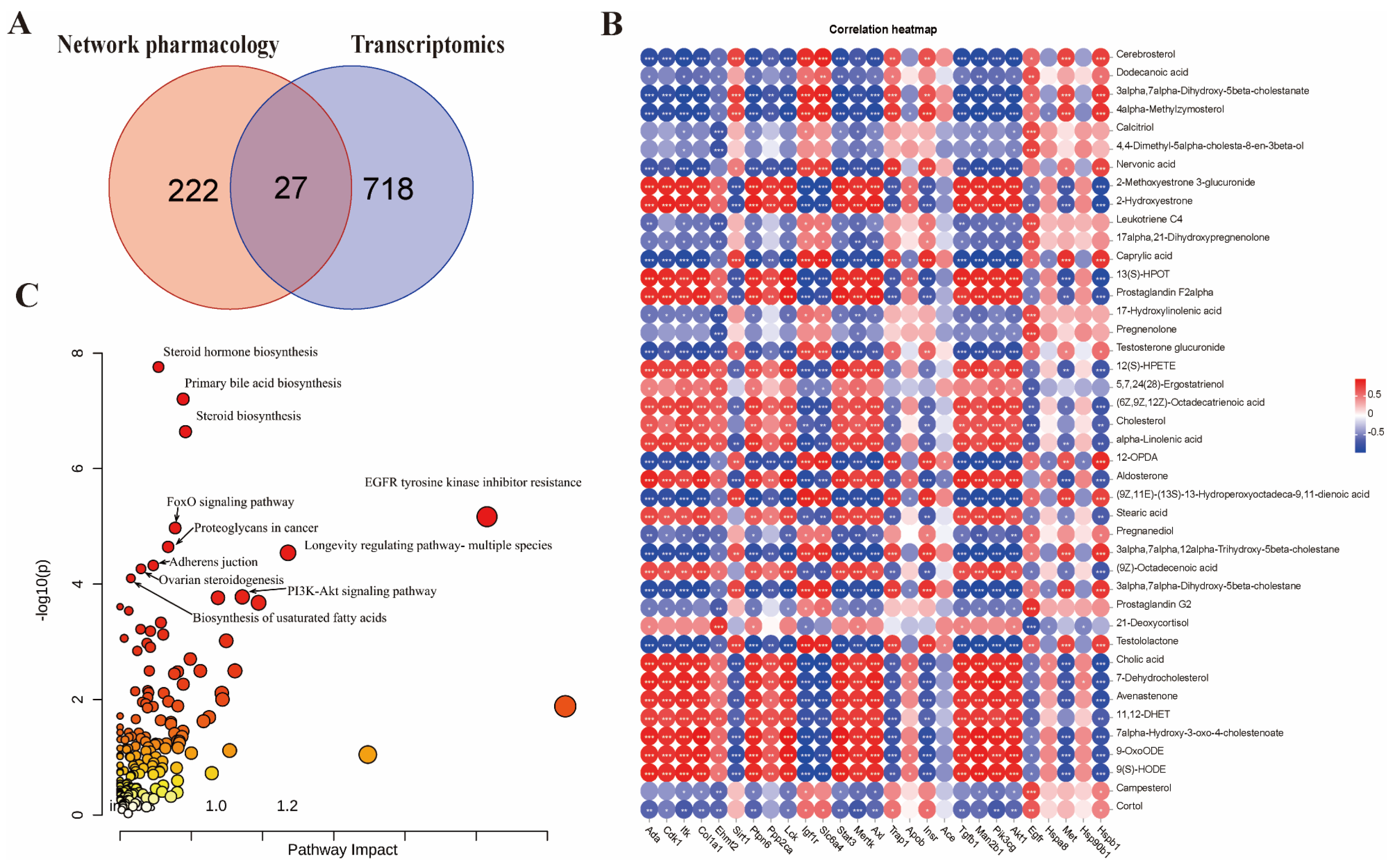
2.6. Integrated Multi-Omics Analysis of LCW Treatment in MRL/lpr Mice
2.7. LCW Inhibits Key Proteins in the PI3K/AKT/mTOR Pathway to Improve LN
3. Discussion
3.1. Key Bioactive Components of LCW in LN Therapy
3.2. LCW Ameliorates LN via Immune Regulation, Anti-Inflammatory Effects, and Renal Protection
3.3. Network Pharmacology Reveals LCW’s Anti-LN Mechanisms
3.4. Transcriptomic Analysis
3.5. Metabolic Profiling Analysis
3.6. Integrated Multi-Omics Analysis
3.7. LCW Ameliorates LN via Suppression of PI3K/AKT/mTOR Signaling
3.8. Limitations and Prospects
4. Materials and Methods
4.1. Drugs and Reagents
4.2. UPLC–MS/MS Analysis of LCW Components
4.3. Animal and Experimental Design
4.4. ELISA Analysis
4.5. Histopathological Analysis of Kidney Tissue
4.6. Network Pharmacology
4.7. Kidney Tissue Transcriptomic Analysis
4.8. Serum Metabolomic Analysis
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLE | Systemic lupus erythematosus |
| LN | Lupus nephritis |
| LCW | Lang Chuang Wan |
| ANA | Anti-nuclear antibodies |
| ANOVA | One-way analysis of variance |
| anti-dsDNA | Anti-double-stranded DNA antibodies |
| anti-Sm | Anti-Sm antibody |
| BP | Biological processes |
| CC | Cellular components |
| MF | Molecular functions |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BPC | Base peak chromatograms |
| BUN | Blood urea nitrogen |
| DAVID | Database for annotation, visualization and integrated discovery |
| DEGs | Differentially expressed genes |
| DEM | Differentially expressed metabolites |
| ELISA | Enzyme-linked immunosorbent assay |
| HMDB | Human metabolome database |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PCA | Principal component analysis |
| SEM | Standard error of the mean |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| UP | Urinary protein |
| VIP | Variable importance for the projection |
References
- Voelker, R. What Is Lupus? JAMA 2024, 332, 1312. [Google Scholar] [CrossRef] [PubMed]
- Halfon, M.; Tankeu, A.T.; Ribi, C. Mitochondrial Dysfunction in Systemic Lupus Erythematosus with a Focus on Lupus Nephritis. Int. J. Mol. Sci. 2024, 25, 6162. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, Q.; Li, X.; Gao, X.; Zhang, L.; Xu, K. Urinary Biomarkers Associated with Podocyte Injury in Lupus Nephritis. Front. Pharmacol. 2024, 15, 1324540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, W. Experimental Study of Nanostructured Lipid Carrier in the Treatment of Immune Lupus Nephritis. J. Nanosci. Nanotechnol. 2021, 21, 1099–1106. [Google Scholar] [CrossRef]
- Sharafaldin, E.N.K.; Sim, M.S.; Lim, S.K.; Alhussieni, K.; Huri, H.Z. Precision Medicine in Lupus Nephritis. Clin. Chim. Acta 2024, 562, 119894. [Google Scholar] [CrossRef]
- Dall’Era, M. Treatment of Lupus Nephritis: Current Paradigms and Emerging Strategies. Curr. Opin. Rheumatol. 2017, 29, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Ayoub, I.M.; Chan, T.M.; Liu, Z.-H.; Mejía-Vilet, J.M.; Floege, J. KDIGO 2024 Clinical Practice Guideline for the Management of LUPUS NEPHRITIS. Kidney Int. 2024, 105, S1–S69. [Google Scholar] [CrossRef]
- Anders, H.-J.; Saxena, R.; Zhao, M.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus Nephritis. Nat. Rev. Dis. Prim. 2020, 6, 7. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Y.; Lu, C.; Li, Y.; Zhou, B.; Qin, X.; Du, G.; Gao, L.; Guan, D.; Lu, A. Uncovering the Complexity Mechanism of Different Formulas Treatment for Rheumatoid Arthritis Based on a Novel Network Pharmacology Model. Front. Pharmacol. 2020, 11, 1035. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; Wang, P.; Li, X.; Chen, J.; Zhou, B.; Tian, J.; Guan, D.; Qin, X.; Lu, A. A Novel Network Pharmacology Strategy to Decode Mechanism of Lang Chuang Wan in Treating Systemic Lupus Erythematosus. Front. Pharmacol. 2020, 11, 512877. [Google Scholar] [CrossRef]
- Ji, G.; Liu, Y.; Yang, X.; Wang, R.; Bu, H.; Wang, S. Therapeutic Effect of Lupus Pills on Pristane-induced Systemic Lupus Erythematosus in Mice. Spec. Wild Econ. Anim. Plant Res. 2022, 44, 16–23. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, T.; Wang, B.; Guo, H.; Liu, Q. Application of Network Pharmacology in the Study of the Mechanism of Action of Traditional Chinese Medicine in the Treatment of COVID-19. Front. Pharmacol. 2022, 13, 926901. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sui, X.; Dong, X.; Li, Z.; Lun, S.; Wang, S. Low Beauvericin Concentrations Promote PC-12 Cell Survival under Oxidative Stress by Regulating Lipid Metabolism and PI3K/AKT/mTOR Signaling. Ecotoxicol. Environ. Saf. 2024, 269, 115786. [Google Scholar] [CrossRef]
- Mishra, S.; Kell, P.; Scherrer, D.; Dietzen, D.J.; Vite, C.H.; Berry-Kravis, E.; Davidson, C.; Cologna, S.M.; Porter, F.D.; Ory, D.S.; et al. Accumulation of Alkyl-Lysophosphatidylcholines in Niemann-Pick Disease Type C1. J. Lipid Res. 2024, 65, 100600. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Alarcón-Riquelme, M.E. Transcriptome Studies in Lupus Nephritis. Arch. Immunol. Ther. Exp. 2022, 70, 15. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Boumendjel, A.; Seteyen, A.-L.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the High Therapeutic Potentials of Quercetin and Its Derivatives. Phytomedicine Plus 2022, 2, 100220. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Chiang, Y.-F.; Hong, Y.-H.; Shieh, T.-M.; Huang, T.-C.; Ali, M.; Chang, H.-Y.; Wang, K.-L.; Hsia, S.-M. Quercetin Ameliorates Renal Injury and Pyroptosis in Lupus Nephritis through Inhibiting IL-33/ST2 Pathway In Vitro and In Vivo. Antioxidants 2022, 11, 2238. [Google Scholar] [CrossRef]
- Ding, T.; Yi, T.; Li, Y.; Zhang, W.; Wang, X.; Liu, J.; Fan, Y.; Ji, J.; Xu, L. Luteolin Attenuates Lupus Nephritis by Regulating Macrophage Oxidative Stress via HIF-1α Pathway. Eur. J. Pharmacol. 2023, 953, 175823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Z.; Cai, B.; Chen, Q. Berberine as a Potential Multi-Target Agent for Metabolic Diseases: A Review of Investigations for Berberine. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 971–979. [Google Scholar] [CrossRef]
- Gao, R.; Lu, Y.; Zhang, W.; Zhang, Z. The Application of Berberine in Fibrosis and the Related Diseases. Am. J. Chin. Med. 2024, 52, 753–773. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.X.; Fan, X.P.; Li, M.; Liu, M.J.; Sun, Q.L. MiR-146a Relieves Kidney Injury in Mice with Systemic Lupus Erythematosus through Regulating NF-κB Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7024–7032. [Google Scholar] [PubMed]
- Nashi, R.A.; Shmerling, R.H. Antinuclear Antibody Testing for the Diagnosis of Systemic Lupus Erythematosus. Med. Clin. N. Am. 2021, 105, 387–396. [Google Scholar] [CrossRef]
- Solé, C.; Royo, M.; Sandoval, S.; Moliné, T.; Gabaldón, A.; Cortés-Hernández, J. Precise Targeting of Autoantigen-Specific B Cells in Lupus Nephritis with Chimeric Autoantibody Receptor T Cells. Int. J. Mol. Sci. 2024, 25, 4226. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Zeeshan, B.; Dissanayake, G.; Zergaw, M.; Elgendy, M.; Billey, A. Anti-Smith Antibodies as a Predictive Factor for Developing Lupus Nephritis in Systemic Lupus Erythematosus Patients: A Systematic Review. Cureus 2024, 16, e66270. [Google Scholar] [CrossRef]
- Hokamp, J.A.; Nabity, M.B. Renal Biomarkers in Domestic Species. Vet. Clin. Pathol. 2016, 45, 28–56. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Huang, J.; Gu, C.; Liu, Q.; Zhang, W.; Gao, F.; Tian, Y.; Miao, X.; Zhu, Z.; et al. TRIM27 Contributes to Glomerular Endothelial Cell Injury in Lupus Nephritis by Mediating the FoxO1 Signaling Pathway. Lab. Investig. 2021, 101, 983–997. [Google Scholar] [CrossRef]
- Schmeisser, K.; Parker, J.A. Pleiotropic Effects of mTOR and Autophagy During Development and Aging. Front. Cell Dev. Biol. 2019, 7, 192. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Wu, Y.; Cheng, L.; Yan, Y.; Yang, X.; Zhu, F.; He, S.; Tang, W.; Zuo, J. (5R)-5-Hydroxytriptolide Ameliorates Lupus Nephritis in MRL/Lpr Mice by Preventing Infiltration of Immune Cells. Am. J. Physiol.-Ren. Physiol. 2017, 312, F769–F777. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-Producing Cells in Protection versus Pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Hendriks, R.W. Interleukin-10 Multitasking in Allergic Airway Inflammation. Cell. Mol. Immunol. 2023, 20, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, V.; Artero, M.R.; Petkova, M.; Mihaylova, G.; Dragon-Durey, M.-A.; Radanova, M.; Roumenina, L.T. Clinical Relevance of Anti-C3 and Anti-C4 Autoantibodies in Lupus Nephritis. Kidney Int. Rep. 2024, 9, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, W.; Zhu, M.; Zheng, L.; Xie, L.; Yao, Z.; Zhang, H.; Cao, D.; Lu, B. Lupus Nephritis Pathology Prediction with Clinical Indices. Sci. Rep. 2018, 8, 10231. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Cozzi, M.; Barinotti, A.; Radin, M.; Cecchi, I.; Fenoglio, R.; Mancardi, D.; Wilson Jones, G.; Rossi, D.; Roccatello, D. Renal Fibrosis in Lupus Nephritis. Int. J. Mol. Sci. 2022, 23, 14317. [Google Scholar] [CrossRef]
- Adhya, Z.; Borozdenkova, S.; Karim, M.Y. The Role of Cytokines as Biomarkers in Systemic Lupus Erythematosus and Lupus Nephritis. Nephrol. Dial. Transplant. 2011, 26, 3273–3280. [Google Scholar] [CrossRef]
- Qing, X.; Chinenov, Y.; Redecha, P.; Madaio, M.; Roelofs, J.J.; Farber, G.; Issuree, P.D.; Donlin, L.; Mcllwain, D.R.; Mak, T.W.; et al. iRhom2 Promotes Lupus Nephritis through TNF-α and EGFR Signaling. J. Clin. Investig. 2018, 128, 1397. [Google Scholar] [CrossRef]
- Yu, J.; Fan, S.; Li, X.; Hou, R.; Hu, X.; Wang, J.; Shan, R.; Dong, Z.; Xie, M.; Dong, Y.; et al. Novel Insights into STAT3 in Renal Diseases. Biomed. Pharmacother. 2023, 165, 115166. [Google Scholar] [CrossRef]
- Stylianou, K.; Petrakis, I.; Mavroeidi, V.; Stratakis, S.; Vardaki, E.; Perakis, K.; Stratigis, S.; Passam, A.; Papadogiorgaki, E.; Giannakakis, K.; et al. The PI3K/Akt/mTOR Pathway Is Activated in Murine Lupus Nephritis and Downregulated by Rapamycin. Nephrol. Dial. Transplant. 2010, 26, 498–508. [Google Scholar] [CrossRef]
- Zhao, C.; Gu, Y.; Chen, L.; Su, X. Upregulation of FoxO3a Expression through PI3K/Akt Pathway Attenuates the Progression of Lupus Nephritis in MRL/Lpr Mice. Int. Immunopharmacol. 2020, 89, 107027. [Google Scholar] [CrossRef]
- Feng, X.; Wu, C.; Yan, G.; Liu, Q.; Liu, J.; Hao, J.; Xing, L.; Yang, M.; Liu, S. TLR2 Plays a Critical Role in HMGB1-Induced Glomeruli Cell Proliferation Through the FoxO1 Signaling Pathway in Lupus Nephritis. J. Interferon Cytokine Res. 2016, 36, 258–266. [Google Scholar] [CrossRef]
- Ji, G.; Liu, Y.; Yang, X.; Wang, R.; Bu, H.; Wang, S. Network Pharmacology to Unveil the Mechanism of Astragali Radix in the Treatment of Lupus Nephritis via PI3K/AKT/mTOR Pathway. Sci. Rep. 2024, 14, 25983. [Google Scholar] [CrossRef]
- Hu, C.; Du, Y.; Xu, X.; Li, H.; Duan, Q.; Xie, Z.; Wen, C.; Han, X. Lipidomics Revealed Aberrant Metabolism of Lipids Including FAHFAs in Renal Tissue in the Progression of Lupus Nephritis in a Murine Model. Metabolites 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An Update on the Lipid Nephrotoxicity Hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yao, X.; Li, X.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Farnesoid X Receptor Regulates PI3K/AKT/mTOR Signaling Pathway, Lipid Metabolism, and Immune Response in Hybrid Grouper. Fish Physiol. Biochem. 2022, 48, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, P.; Zhang, Q.; Fu, Y.; Hou, Y.; Wei, Y.; Zheng, X.; Feng, W. Taxifolin Improves Disorders of Glucose Metabolism and Water-Salt Metabolism in Kidney via PI3K/AKT Signaling Pathway in Metabolic Syndrome Rats. Life Sci. 2020, 263, 118713. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, L.; Sun, Q.; Jia, L.; Liu, D. Increased Levels of IL-17 and Autoantibodies Following Bisphenol A Exposure Were Associated with Activation of PI3K/AKT/mTOR Pathway and Abnormal Autophagy in MRL/Lpr Mice. Ecotoxicol. Environ. Saf. 2023, 255, 114788. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.-L. mTOR: A Pharmacologic Target for Autophagy Regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Dong, L.; Song, Z. Cordyceps Cicadae Ameliorates Inflammatory Responses, Oxidative Stress, and Fibrosis by Targeting the PI3K/mTOR-mediated Autophagy Pathway in the Renal of MRL/Lpr Mice. Immun. Inflamm. Dis. 2024, 12, e1168. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Qing, M.-J.; Liu, X.-K.; Peng, L. Complement Factor B Inhibitor LNP023 Mediates the Effect and Mechanism of AMPK/mTOR on Autophagy and Oxidative Stress in Lupus Nephritis. Kaohsiung J. Med. Sci. 2024, 40, 996–1005. [Google Scholar] [CrossRef]
- Tsay, A.; Wang, J.-C. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. Int. J. Mol. Sci. 2023, 24, 12665. [Google Scholar] [CrossRef]
- Ruby, M.; Gifford, C.C.; Pandey, R.; Raj, V.S.; Sabbisetti, V.S.; Ajay, A.K. Autophagy as a Therapeutic Target for Chronic Kidney Disease and the Roles of TGF-Β1 in Autophagy and Kidney Fibrosis. Cells 2023, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Chen, A.; Ye, Y.; Ren, Y.; Lu, J.; Xuan, F.; Zhou, W. Effect of Berberine Combined with Metformin on Autophagy in Polycystic Ovary Syndrome by Regulating AMPK/AKT/mTOR Pathway. Mol. Reprod. Dev. 2024, 91, e23768. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Hu, S.L.; Wang, S.S.; Guo, X.; Zhang, X.N.; Sun, B.; Chen, L.M. Effects and underlying mechanism of berberine on renal tubulointerstitial injury in diabetic rats. Zhonghua Yi Xue Za Zhi 2016, 96, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, C.; Wei, C.; Wang, C.; Du, H.; Liu, R.; Wang, X.; Hong, Q.; Chen, X. Fufang Shenhua Tablet Inhibits Renal Fibrosis by Inhibiting PI3K/AKT. Phytomedicine 2023, 116, 154873. [Google Scholar] [CrossRef]
- Xu, X.; Yu, Z.; Han, B.; Li, S.; Sun, Y.; Du, Y.; Wang, Z.; Gao, D.; Zhang, Z. Luteolin Alleviates Inorganic Mercury-Induced Kidney Injury via Activation of the AMPK/mTOR Autophagy Pathway. J. Inorg. Biochem. 2021, 224, 111583. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A Flavonoid with a Multifaceted Anticancer Potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.; Boccard, J.; Lang, G.; Grömping, U.; Fischer, R.; Goepfert, S.; Rudaz, S.; Schillberg, S. Structured Plant Metabolomics for the Simultaneous Exploration of Multiple Factors. Sci. Rep. 2016, 6, 37390. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
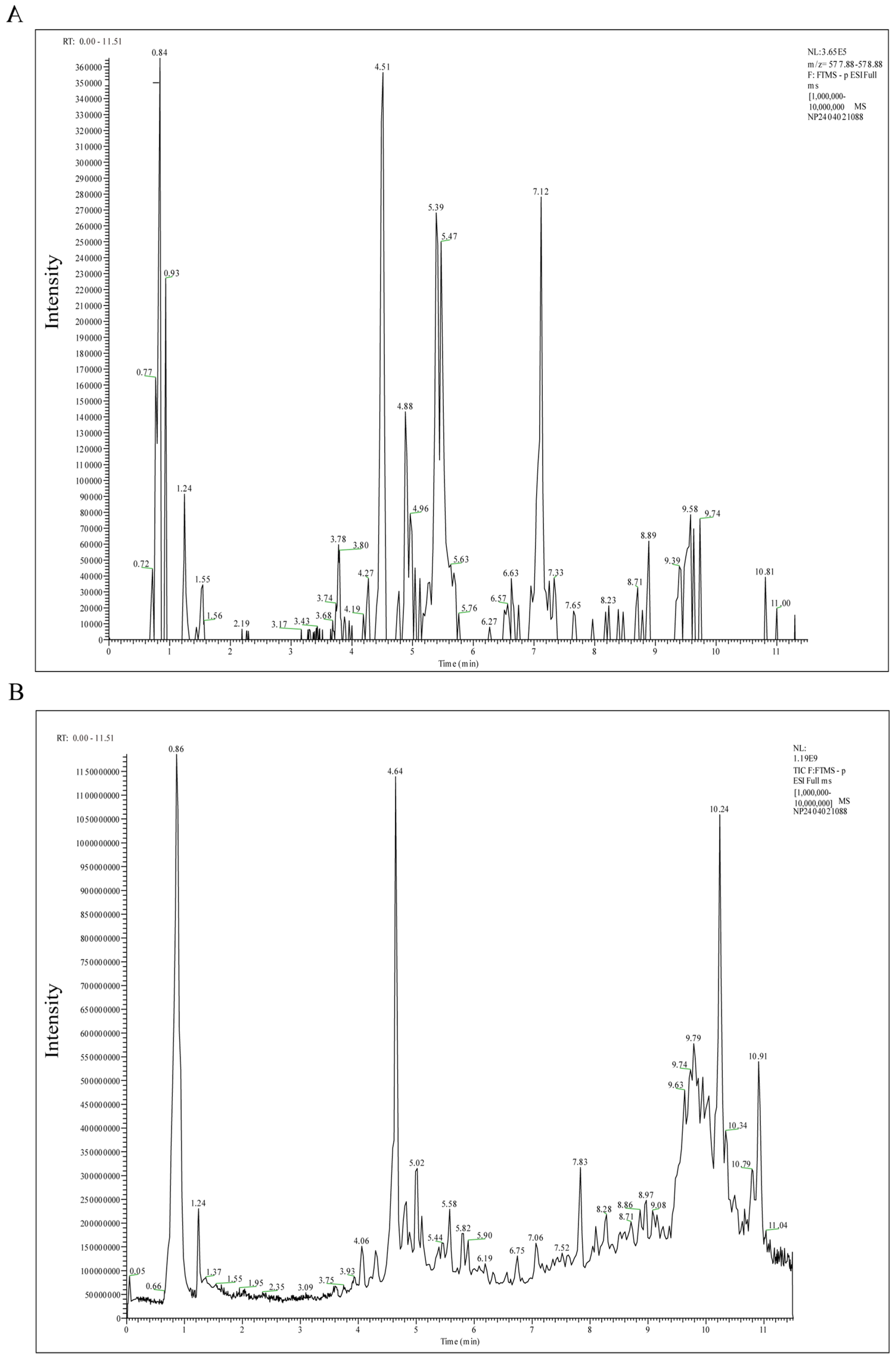

| No. | Compounds | Formula | Precursor Type | Exact Mass | Class | Peak Area |
|---|---|---|---|---|---|---|
| 1 | Berberine | [C20H18NO4]+ | [M + H]+ | 336.12358 | Alkaloids | 765,955,338 |
| 2 | Chrysin | C15H10O4 | [M − H]− | 254.05791 | Flavonoids | 959,729,637.3 |
| 3 | Licoisoflavone a | C20H18O6 | [M + H]+ | 354.11033 | Flavonoids | 343,842,417.9 |
| 4 | Desmethylxanthohumol | C20H20O5 | [M + H]+ | 340.13107 | Flavonoids | 234,716,721.9 |
| 5 | Rutin | C27H30O16 | [M − H]− | 610.15338 | Flavonoids | 192,697,182 |
| 6 | 2,4,6-trihydroxydihydrochalcone | C15H14O4 | [M − H]− | 258.0892 | Flavonoids | 73,232,107.88 |
| 7 | Baicalein | C15H10O5 | [M + H]+ | 270.05282 | Flavonoids | 28,482,364.63 |
| 8 | 2′,7-dihydroxy-4′,5′-dimethoxyisoflavone | C17H14O6 | [M − H]− | 314.07903 | Flavonoids | 18,475,453.99 |
| 9 | Daidzin | C21H20O9 | [M − H2O − H]− | 416.11073 | Flavonoids | 7,967,367.338 |
| 10 | Acacetin | C16H12O5 | [M + H]+ | 284.06847 | Flavonoids | 6,688,437.089 |
| 11 | Luteolin | C15H10O6 | [M − H2O + H]+ | 286.04774 | Flavonoids | 4,144,178.487 |
| 12 | Tibolone | C21H28O2 | [M − H]− | 312.20892 | Hormone class | 24,691,286.41 |
| 13 | Syringic acid | C9H10O5 | [M − H]− | 198.05282 | Phenolic acids | 1,025,933,162 |
| 14 | 3-o-feruloylquinic acid | C17H20O9 | [M − H]− | 368.11073 | Phenolic acids | 353,099,163.3 |
| 15 | 5-caffeoylquinic acid | C16H18O9 | [M − H]− | 354.09508 | Phenolic acids | 272,904,467.6 |
| 16 | 5-feruloylquinic acid | C17H20O9 | [M − H]− | 368.11073 | Phenolic acids | 177,445,299.3 |
| 17 | Quinic acid | C7H12O6 | [M − H]− | 192.06339 | Phenolic acids | 112,171,658.5 |
| 18 | Caffeic acid | C9H8O4 | [M − H2O + H]+ | 180.04226 | Phenolic acids | 21,905,035.5 |
| 19 | Beta-sitosterol | C29H50O | [M − H2O + H]+ | 414.38614 | phytosterols | 41,671,858.23 |
| 20 | Oleanoic acid | C30H48O3 | [M − H]− | 456.36033 | Terpenoids | 2,895,688,889 |
| 21 | Hederagenin | C30H48O4 | [M − H]− | 472.35524 | Terpenoids | 1,355,770,073 |
| 22 | Glycyrrhetinic acid | C30H46O4 | [M + H]+ | 470.33959 | Terpenoids | 379,591,568.6 |
| 23 | Albiflorin | C23H28O11 | [M + H]+ | 480.16315 | Terpenoids | 178,862,591.3 |
| 24 | Licoricesaponin g2 | C42H62O17 | [M + H]+ | 838.39868 | Terpenoids | 27,520,043.37 |
| 25 | Dihydrotanshinone i | C18H14O3 | [M − H]− | 278.09429 | Terpenoids | 15,131,118.85 |
| No. | Metabolite Name | Molecular Formula | HMDB ID | Model/Control | LCW-H/Model |
|---|---|---|---|---|---|
| 1 | Cerebrosterol | C27H46O2 | HMDB0001419 | ↓ | ↑ |
| 2 | Dodecanoic acid | C12H24O2 | HMDB0000638 | ↓ | ↑ |
| 3 | 3alpha,7alpha-Dihydroxy-5beta-cholestanate | C27H46O4 | HMDB0000359 | ↓ | ↑ |
| 4 | 4alpha-Methylzymosterol | C28H46O | HMDB0001217 | ↓ | ↑ |
| 5 | Calcitriol | C27H44O3 | HMDB0001903 | ↓ | ↑ |
| 6 | 4,4-Dimethyl-5alpha-cholesta-8-en-3beta-ol | C29H50O | HMDB0006840 | ↓ | ↑ |
| 7 | Nervonic acid | C24H46O2 | HMDB0002368 | ↓ | ↑ |
| 8 | 2-Methoxyestrone 3-glucuronide | C25H32O9 | HMDB0004482 | ↑ | ↓ |
| 9 | 2-Hydroxyestrone | C18H22O3 | HMDB0000343 | ↑ | ↓ |
| 10 | Leukotriene C4 | C30H47N3O9S | HMDB0001198 | ↓ | ↑ |
| 11 | 17alpha,21-Dihydroxypregnenolone | C21H32O4 | HMDB0006762 | ↓ | ↑ |
| 12 | Caprylic acid | C8H16O2 | HMDB0000482 | ↓ | ↑ |
| 13 | 13(S)-HPOT | C18H30O4 | HMDB0301803 | ↑ | ↓ |
| 14 | Prostaglandin F2alpha | C20H34O5 | HMDB0001139 | ↑ | ↓ |
| 15 | 17-Hydroxylinolenic acid | C18H30O3 | HMDB0011108 | ↓ | ↑ |
| 16 | Pregnenolone | C21H32O2 | HMDB0000253 | ↓ | ↑ |
| 17 | Testosterone glucuronide | C25H36O8 | HMDB0003193 | ↓ | ↑ |
| 18 | 12(S)-HPETE | C20H32O4 | HMDB0004243 | ↑ | ↓ |
| 19 | 5,7,24(28)-Ergostatrienol | C28H44O | HMDB0060404 | ↑ | ↓ |
| 20 | (6Z,9Z,12Z)-Octadecatrienoic acid | C18H30O2 | HMDB0003073 | ↓ | ↑ |
| 21 | Cholesterol | C27H46O | HMDB0000067 | ↑ | ↓ |
| 22 | alpha-Linolenic acid | C18H30O2 | HMDB0001388 | ↑ | ↓ |
| 23 | 12-OPDA | C18H28O3 | HMDB0301804 | ↓ | ↑ |
| 24 | Aldosterone | C21H28O5 | HMDB0000037 | ↑ | ↓ |
| 25 | (9Z,11E)-(13S)-13-Hydroperoxyoctadeca-9,11-dienoic acid | C18H32O4 | HMDB0003871 | ↓ | ↑ |
| 26 | Stearic acid | C18H36O2 | HMDB0000827 | ↑ | ↓ |
| 27 | Pregnanediol | C21H36O2 | HMDB0004025 | ↓ | ↑ |
| 28 | 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholestane | C27H48O3 | HMDB0001457 | ↓ | ↑ |
| 29 | (9Z)-Octadecenoic acid | C18H34O2 | HMDB0000207 | ↑ | ↓ |
| 30 | 3alpha,7alpha-Dihydroxy-5beta-cholestane | C27H48O2 | HMDB0006893 | ↓ | ↑ |
| 31 | Prostaglandin G2 | C20H32O6 | HMDB0003235 | ↓ | ↑ |
| 32 | 21-Deoxycortisol | C21H30O4 | HMDB0004030 | ↓ | ↑ |
| 33 | Testololactone | C19H26O3 | HMDB0258855 | ↓ | ↑ |
| 34 | Cholic acid | C24H40O5 | HMDB0000619 | ↑ | ↑ |
| 35 | 7-Dehydrocholesterol | C27H44O | HMDB0000032 | ↑ | ↓ |
| 36 | Avenastenone | C29H46O | HMDB0304267 | ↑ | ↓ |
| 37 | 11,12-DHET | C20H34O4 | HMDB0002314 | ↑ | ↓ |
| 38 | 7alpha-Hydroxy-3-oxo-4-cholestenoate | C27H42O4 | HMDB0012458 | ↑ | ↓ |
| 39 | 9-OxoODE | C18H30O3 | HMDB0004669 | ↑ | ↓ |
| 40 | 9(S)-HODE | C18H32O3 | HMDB0004670 | ↑ | ↓ |
| 41 | Campesterol | C28H48O | HMDB0002869 | ↓ | ↑ |
| 42 | Cortol | C21H36O5 | HMDB0003180 | ↓ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ji, G.; Zhang, X.; Yu, H.; Li, Z.; Yang, B.; Yao, Z.; Wang, S.; Jiang, T.; Wang, S. Combined Network Pharmacology, Transcriptomics and Metabolomics Strategies Reveal the Mechanism of Action of Lang Chuang Wan to Ameliorate Lupus Nephritis in MRL/lpr Mice. Pharmaceuticals 2025, 18, 916. https://doi.org/10.3390/ph18060916
Li C, Ji G, Zhang X, Yu H, Li Z, Yang B, Yao Z, Wang S, Jiang T, Wang S. Combined Network Pharmacology, Transcriptomics and Metabolomics Strategies Reveal the Mechanism of Action of Lang Chuang Wan to Ameliorate Lupus Nephritis in MRL/lpr Mice. Pharmaceuticals. 2025; 18(6):916. https://doi.org/10.3390/ph18060916
Chicago/Turabian StyleLi, Cuicui, Guoxin Ji, Xinru Zhang, Hang Yu, Zhimeng Li, Bo Yang, Zhuangzhuang Yao, Shilei Wang, Tongwei Jiang, and Shumin Wang. 2025. "Combined Network Pharmacology, Transcriptomics and Metabolomics Strategies Reveal the Mechanism of Action of Lang Chuang Wan to Ameliorate Lupus Nephritis in MRL/lpr Mice" Pharmaceuticals 18, no. 6: 916. https://doi.org/10.3390/ph18060916
APA StyleLi, C., Ji, G., Zhang, X., Yu, H., Li, Z., Yang, B., Yao, Z., Wang, S., Jiang, T., & Wang, S. (2025). Combined Network Pharmacology, Transcriptomics and Metabolomics Strategies Reveal the Mechanism of Action of Lang Chuang Wan to Ameliorate Lupus Nephritis in MRL/lpr Mice. Pharmaceuticals, 18(6), 916. https://doi.org/10.3390/ph18060916






