Ethanolic Extract of Ganoderma mexicanum Pat. Mycelium: A Source of Bioactive Compounds with Antiproliferative Activity and Potential PPAR-γ Natural Ligands
Abstract
1. Introduction
2. Results
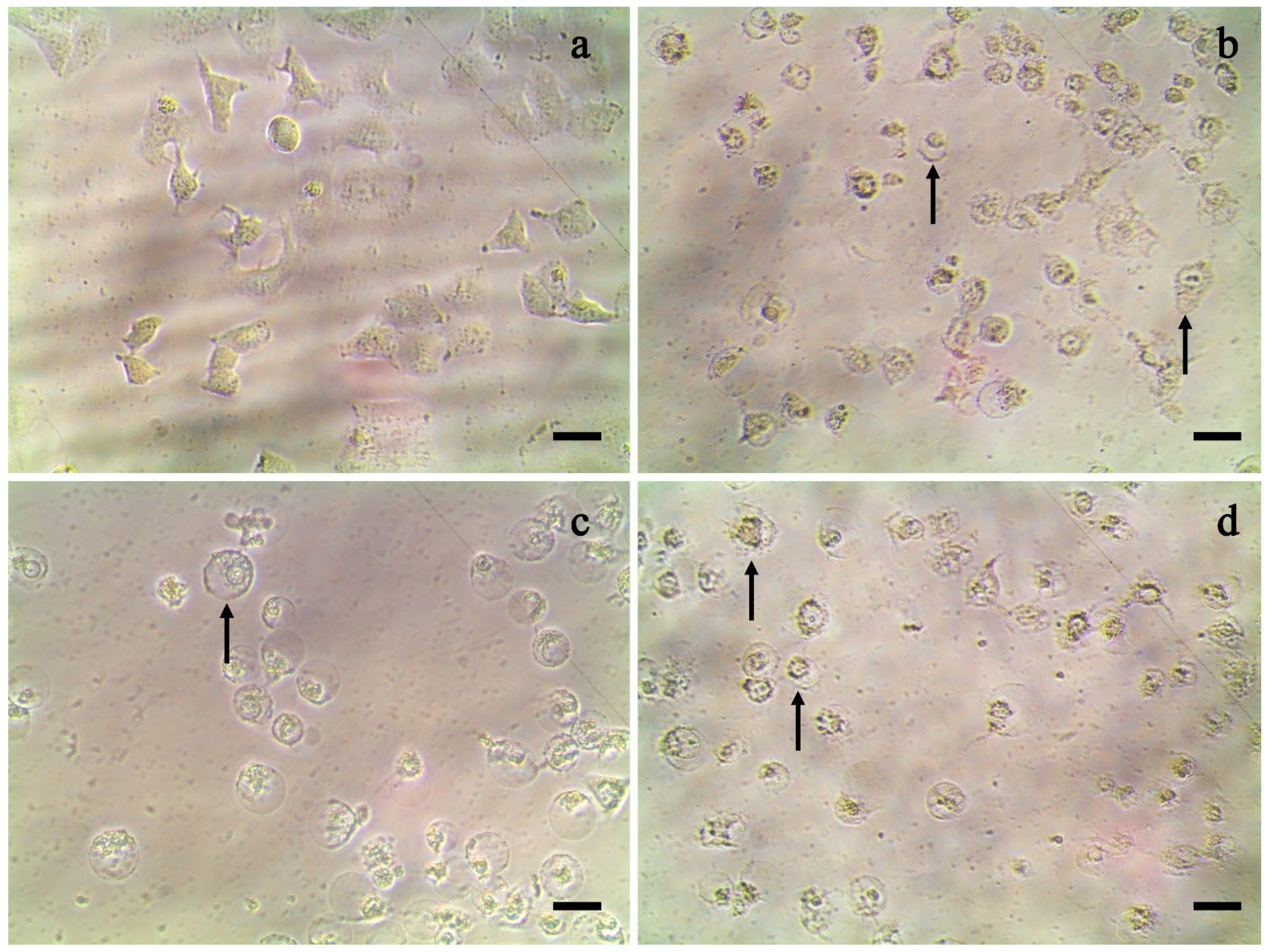
2.1. Antiproliferative Activity of Ethanolic Extract
2.2. Antiproliferative Activity of Hexane Fraction
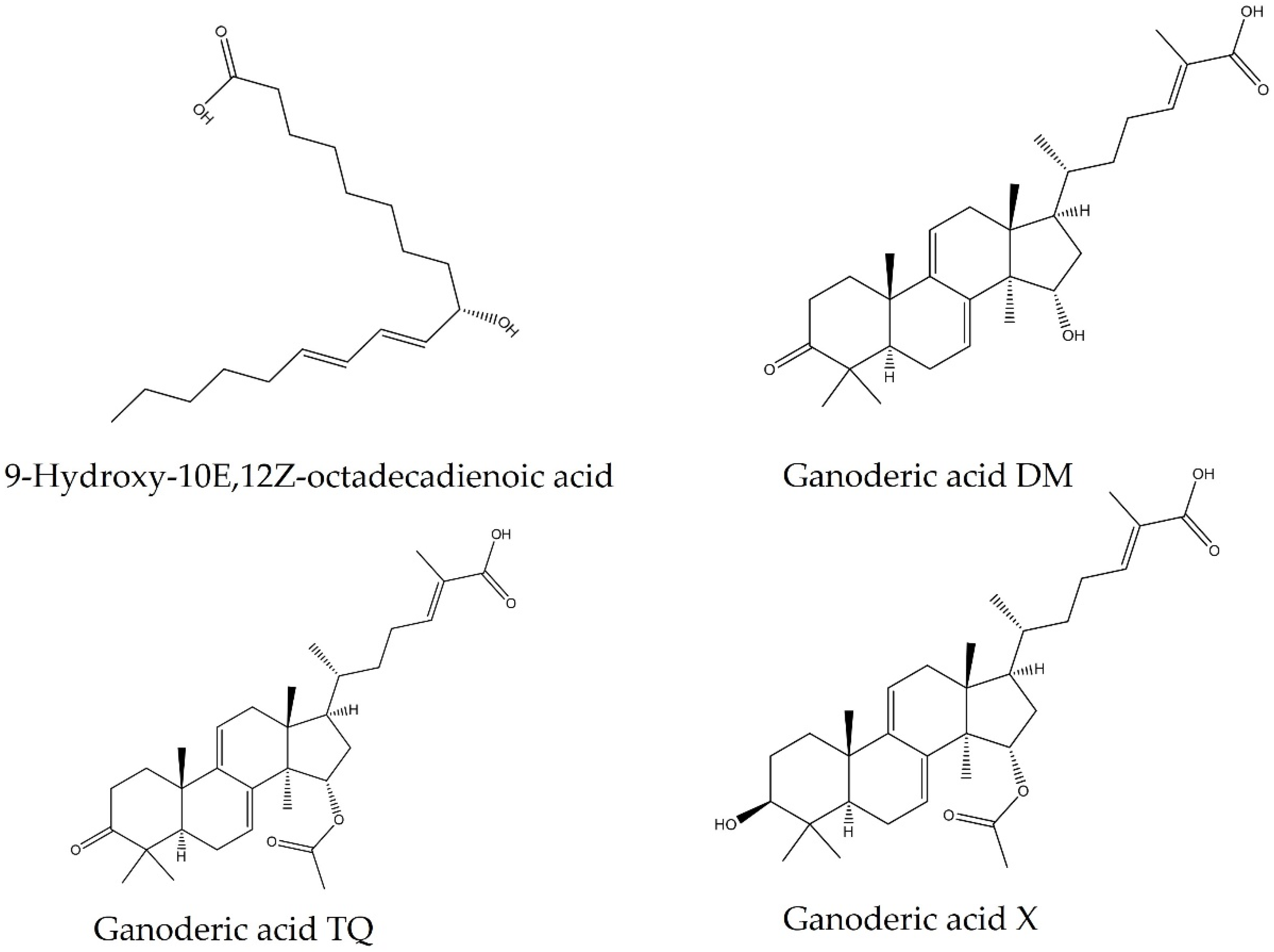
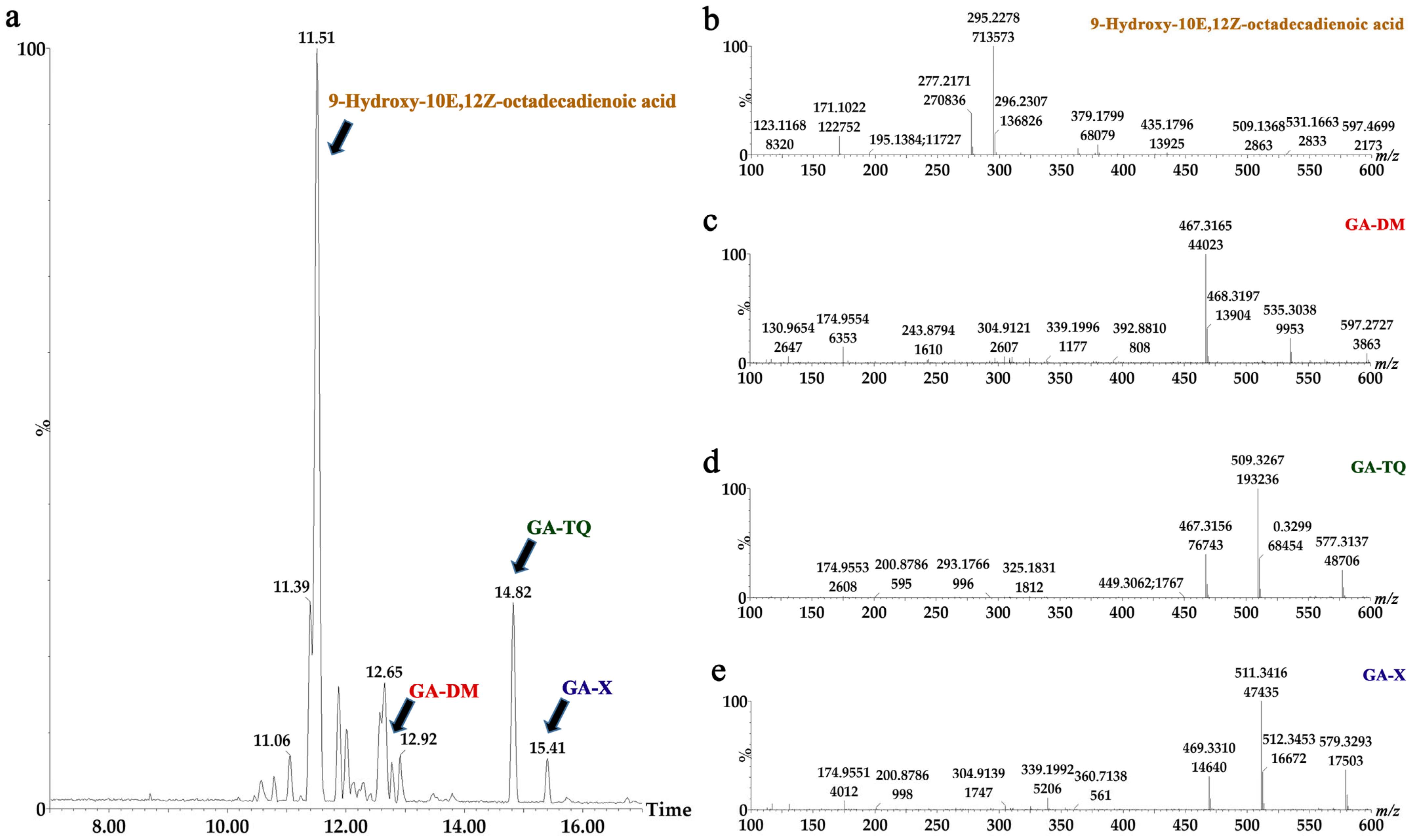
2.3. Characterization of Hexane Fraction
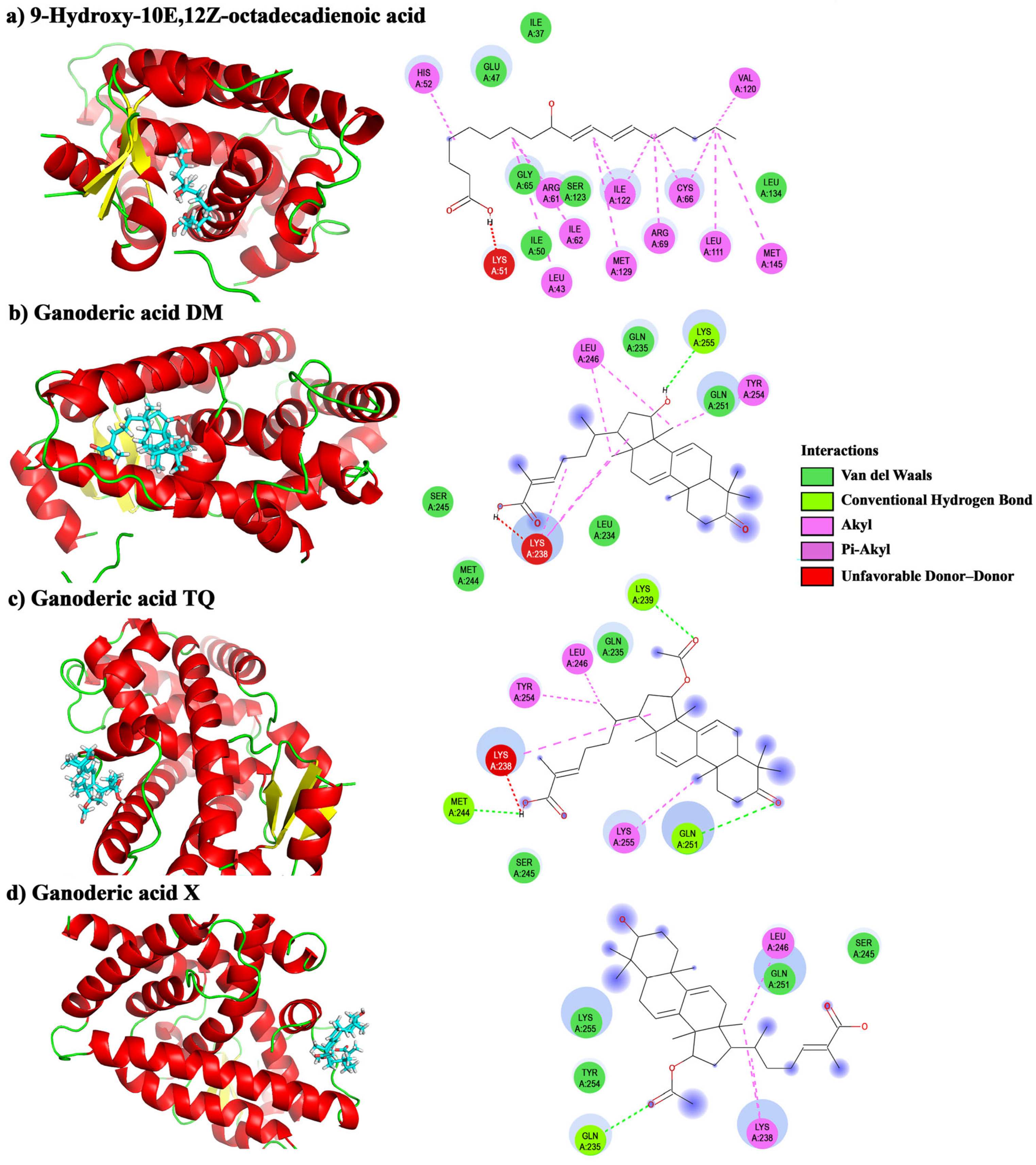
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Fungal Culture
4.2. Extraction and Isolation
4.3. UHPLC-ESI-TOF-MS
4.4. Cell Lines and Culture Conditions
4.5. Antiproliferative Activity
4.6. In Silico PPAR-γ Interaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STAT3 | signal transducer and activator of transcription 3 |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| VPW | vineyard pruning waste |
| FH | hexane fraction |
| FEA | ethyl acetate fraction |
| GA | ganoderic acid |
| GSDME | gasdermin E |
| Caspase | cysteine aspartate specific proteases |
| Ganooil | Ganoderma lucidum spore oil |
| NF-κB | nuclear factor—kappa B |
| IL-6/IL-6R | interleukina-6/interleukina-6 receptor |
| NSCLC | non-small-cell lung cancer |
| PD-L1 | programmed death ligand 1 |
| TLR4 | Toll-like receptor 4 |
| COX-2 | cyclooxygenase-2 |
| DMSO | dimethyl sulfoxide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MeJa | methyl jasmonate |
References
- MYCOBANK. Mycobank Database. Available online: https://www.mycobank.org/Simple%20names%20search (accessed on 14 December 2024).
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2023, 13, 24. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhang, X.; Xu, J.; Wang, H.; Zhao, L.; Wang, Y. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo zebrafish assays. Front. Pharmacol. 2020, 11, e287. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Guo, Y.; Lai, H.; Cheng, S.; Chen, Z.; Li, H.; Li, Q.; Mao, X. Water extract of sporoderm-broken spores of Ganoderma lucidum elicits dual antitumor effects by inhibiting p-STAT3/PD-L1 and promoting ferroptosis in castration-resistant prostate cancer. J. Funct. Foods 2024, 113, e106018. [Google Scholar] [CrossRef]
- López-Peña, D.; Torres-Moreno, H.; Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Gutiérrez, A.; Esqueda, M. Antiproliferative activity of mycelium vs. fruiting body: Ganoderma subincrustatum and G. weberianum from Sonora, Mexico. Microbiol. Res. 2023, 14, 1534–1544. [Google Scholar] [CrossRef]
- Cheng, C.R.; Yue, Q.X.; Wu, Z.Y.; Song, X.Y.; Tao, S.J.; Wu, X.H.; Xu, P.P.; Liu, X.; Guan, S.H.; Guo, D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry 2010, 71, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Wang, C.; Cui, B.; Zhang, J.; Zhou, S.; Pan, X.; Pan, F.; Dai, Y.; Feng, N. Lanostane triterpenoids from mycelia-associated Ganoderma sinense and their anti-inflammatory activity. Phytochemistry 2023, 215, e113870. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, J.W.; Deng, Y.Y.; Wong, L.Y.; Wang, C.; Liang, Y.L.; Leung, Y.T.; Tian, J.Y.; Wu, Y.; Leung, K.S.Y.; et al. Identification of sedative-hypnotic compounds shared by five medicinal Polyporales mushrooms using UPLC-QTOF-MS/MS-based untargeted metabolomics. Phytomedicine 2024, 128, e155355. [Google Scholar] [CrossRef]
- Chafouz, R.; Karavergou, S.; Tsiftsoglou, O.S.; Maskovic, P.; Lazari, D. Ganoderma adspersum (Ganodermataceae): Investigation of its secondary metabolites and the antioxidant, antimicrobial, and cytotoxic potential of its extracts. Int. J. Mol. Sci. 2024, 25, 516. [Google Scholar] [CrossRef]
- Yangchum, A.; Fujii, R.; Choowong, W.; Rachtawee, P.; Pobkwamsuk, M.; Boonpratuang, T.; Mori, S.; Isaka, M. Phytochemistry lanostane triterpenoids from cultivated fruiting bodies of basidiomycete Ganoderma mbrekobenum. Phytochemistry 2022, 196, e113075. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Van Le, V.; Nguyen, B.T.T.; Nguyen, H.T.T.; Tran, A.D.; Ngo, N.X. Optimization of mycelial growth and cultivation of wild Ganoderma sinense. BioTechnologia 2023, 104, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gurbilek, M.; Deniz, C.D.; Eroglu Gunes, C.; Kurar, E.; Reisli, I.; Kursunel, M.A.; Topcu, C.; Koc, M. Anticancer activity of thymoquinone in non-small cell lung cancer and possible involvement of PPAR-γ pathway. Int. J. Radiat. Biol. 2025, 101, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Reka, A.K.; Goswami, M.T.; Krishnapuram, R.; Standiford, T.J.; Keshamouni, V.G. Molecular cross-regulation between PPAR-γ and other signaling pathways: Implications for lung cancer therapy. Lung Cancer 2011, 72, 154–159. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, M.; Shang, J. PPARγ modulators in lung cancer: Molecular mechanisms, clinical prospects, and challenges. Biomolecules 2024, 14, 190. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Yang, J.; Sheng, L.; Tang, X.; Zhang, X.; Hua, H. Deciphering the chemical composition of Ganoderma lucidum from different geographical origins by mass spectrometry molecular networking coupled with multivariate analysis. Biomed. Chromatogr. 2023, 37, e5506. [Google Scholar] [CrossRef]
- Liu, W.Y.; Guo, H.B.; Yang, R.H.; Xu, A.G.; Zhao, J.C.; Yang, Z.Q.; Han, W.J.; Yu, X.D. UPLC-ESI-MS/MS-based widely targeted metabolomics reveals differences in metabolite composition among four Ganoderma species. Front. Nutr. 2024, 11, 1335538. [Google Scholar] [CrossRef]
- Sanmanoch, W.; Surapat, W.; Phosri, S.; Yaraksa, N. Antioxidant activity and cytotoxicity against the cervical epithelial carcinoma (HeLa) cell line of crude Ganoderma lucidum mycelial extracts. Creat. Sci. 2024, 16, e254094. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Long, G.; Yang, Y.; Chen, G.; Hou, G.; Huo, X.; Jia, J.; Wang, A.; Hu, G. Lanostane-type triterpenoids from the mycelial mat of Ganoderma lucidum and their hepatoprotective activities. Phytochemistry 2022, 198, e113131. [Google Scholar] [CrossRef]
- Zhang, R.R.; Zhang, J.; Guo, X.; Chen, Y.Y.; Sun, J.Y.; Miao, J.L.; Carpena, M.; Prieto, M.A.; Li, N.Y.; Zhou, Q.X.; et al. Molecular mechanisms of the chemical constituents from anti-inflammatory and antioxidant active fractions of Ganoderma neo-japonicum Imazeki. Curr. Res. Food Sci. 2023, 6, e100441. [Google Scholar] [CrossRef]
- Chinthanom, P.; Choowong, W.; Thummarukcharoen, T.; Chen, H.P.; Liu, J.K.; Isaka, M. Lanostane triterpenoids from mycelial cultures of the basidiomycete Ganoderma weberianum. Phytochem. Lett. 2022, 51, 12–17. [Google Scholar] [CrossRef]
- Gouvêa, P.R.; de Oliveira, S.D.; Alves, V.; Cruz, C.L.; Sales-Campos, C.; Ramos, L. Agro-wastes bioconversion by an Amazonian isolate of Ganoderma sp. and a commercial strain of Ganoderma lingzhi. Biocatal. Agric. Biotechnol. 2023, 54, e102959. [Google Scholar] [CrossRef]
- Harris-Valle, C.; Valenzuela-Soto, E.; Sánchez, A.; Gaitán-Hernández, R.; Esqueda, M. Variability in the ligninolytic enzymes activity by Lentinula edodes in submerged culture with lignin and glucose. Am. J. Agric. Biol. Sci. 2014, 9, 369–378. [Google Scholar] [CrossRef]
- SIAP. Table Grape Production Shows Positive Progress: Agriculture. Available online: https://www.gob.mx/agricultura/prensa/registra-produccion-de-uva-de-mesa-avance-positivo-agricultura (accessed on 10 November 2023).
- Cruz-Félix, M.; Angulo-Sanchez, L.; Vargas, G.; Gutiérrez, A.; Orozco, A.; Ramos-Clamont, G.; Esqueda, M. Enhancement of biomass production of Ganoderma spp. (Polyporaceae) native strains from the Sonoran desert, Mexico, grown in liquid culture with vineyard pruning extracts. Acta Bot. Mex. 2024, 131, e2258. [Google Scholar] [CrossRef]
- Xu, J.W.; Xu, Y.N.; Zhong, J.J. Production of individual ganoderic acids and expression of biosynthetic genes in liquid static and shaking cultures of Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2010, 85, 941–948. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Galanty, A.; Szewczyk, A.; Paśko, P.; Kała, K.; Apola, A.; Podolak, I.; Muszyńska, B. Effect of methyl jasmonate elicitation on triterpene production and evaluation of cytotoxic activity of mycelial culture extracts of Ganoderma applanatum (Pers.) Pat. Plants 2023, 12, 294. [Google Scholar] [CrossRef]
- Angulo-Sanchez, L.T.; Cruz-Félix, M.C.; Vidal-Gutiérrez, M.; Torres-Moreno, H.; Muñoz-Bernal, O.A.; Álvarez-Parrilla, E.; Robles-Zepeda, R.E.; Álvarez-Bajo, O.; Gutiérrez, A.; Esqueda, M. Ganoderma tuberculosum liquid culture with vineyard pruning extracts for bioactive composite production with antiproliferative activity. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 5245451. [Google Scholar] [CrossRef]
- Bacallao-Escudero, A.; Guerrero-Germán, P.; Torres-Moreno, H.; Vidal-Gutiérrez, M.; López-Romero, J.C.; Tejeda-Mansir, A.; Esqueda, M.; Robles-Zepeda, R.E. Biological activity of Ganoderma species (Agaricomycetes) from Sonoran desert, Mexico. Int. J. Med. Mush. 2023, 25, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miranda, X.; López-Cruz, R.; Gutiérrez, A.; Álvarez-Bajo, O.; Esqueda, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. Metabolism of Ganoderma spp. native strains from the Sonoran Desert with vineyard pruning extracts by isothermal microcalorimetry. J. Therm. Anal. Calorim. 2023, 148, 6845–6853. [Google Scholar] [CrossRef]
- Cabarroi-Hernández, M.; Villalobos-Arámbula, A.R.; Torres-Torres, M.G.; Decock, C.; Guzmán-Dávalos, L. The Ganoderma weberianum-resinaceum lineage: Multilocus phylogenetic analysis and morphology confirm G. mexicanum and G. parvulum in the Neotropics. MycoKeys 2019, 59, 95–131. [Google Scholar] [CrossRef]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef]
- Zhong, C.; Li, Y.; Li, W.; Lian, S.; Li, Y.; Wu, C.; Zhang, K.; Zhou, G.; Wang, W.; Xu, H.; et al. Ganoderma lucidum extract promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem. Toxicol. 2023, 174, e113654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, Z. Cancer-associated pyroptosis: A new license to kill tumor. Front. Immunol. 2023, 14, 1082165. [Google Scholar] [CrossRef]
- Yu, L.; Xu, Y.; Pu, Z.; Kang, H.; Li, M.; Sessler, J.L.; Kim, J.S. Photocatalytic superoxide radical generator that induces pyroptosis in cancer cells. J. Am. Chem. Soc. 2022, 144, 11326–11337. [Google Scholar] [CrossRef]
- Huang, C.; Li, J.; Wu, R.; Li, Y.; Zhang, C. Targeting pyroptosis for cancer immunotherapy: Mechanistic insights and clinical perspectives. Mol. Cancer 2025, 24, 131. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Chen, Y.; Huang, C.; Wu, S.; Wang, X.; Zhao, X.; Xu, Q.; Sun, R.; Kong, X.; Jiang, X.; et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci. Transl. Med. 2023, 15, eabl7895. [Google Scholar] [CrossRef]
- Xia, J.; Dai, L.; Wang, L.; Zhu, J. Ganoderic acid DM induces autophagic apoptosis in non-small cell lung cancer cells by inhibiting the PI3K/Akt/mTOR activity. Chem. Biol. Interact. 2020, 316, e108932. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yuan, L.; Du, M.; Chen, Y.; Zhang, M.H.; Gu, J.F.; He, J.J.; Wang, Y.; Cao, W. Anti-lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.) Karst. Molecules 2013, 18, 9966–9981. [Google Scholar] [CrossRef]
- Hayshi, Y.; Nishikawa, Y.; Mori, H.; Tamura, H.; Matsushita, Y.I.; Matsui, T. Antitumor activity of (10E,12Z)-9-hydroxy-10,12-octadecadienoic acid from rice bran. J. Ferment. Bioeng. 1998, 86, 149–153. [Google Scholar] [CrossRef]
- Beccaccioli, M.; Reverberi, M.; Scala, V. Fungal lipids: Biosynthesis and signaling during plant-pathogen interaction. Front. Biosci. 2019, 24, 172–185. [Google Scholar] [CrossRef]
- Lian, S.; Li, W.; Zhong, C.; Li, Y.; Wu, C.; Zhang, K.; Lin, J.; Wang, W.; Katanaev, V.; Xie, X.; et al. Ganoderma lucidum spore oil synergistically enhances the function of cyclophosphamide in the prevention of breast cancer metastasis. J. Chin. Med. Assoc. 2024, 87, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Xie, Y.; Yun, H.; Liang, H.; He, C.; Jiang, A.; Wu, Q.; Yang, B.B. The effect of Ganoderma lucidum spore oil in early wound healing: Interactions of skin microbiota and inflammation. Aging 2020, 12, 14125–14140. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Sawai, T. Neuraminidase Inhibitor, Anti-Influenza Agent, Food Product and Agent Comprising the Same, and Method for Producing the Same. Patent JP-2019163292-A, 26 September 2019. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Shang, J.; Mosure, S.A.; Zheng, J.; Brust, R.; Bass, J.; Nichols, A.; Solt, L.A.; Griffin, P.R.; Kojetin, D.J. A molecular switch regulating transcriptional repression and activation of PPARγ. Nat. Commun. 2020, 11, e956. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Sharma, P.; Kumar, R.; Kumar, S. Misconstrued versatility of Ganoderma lucidum: A key player in multi-targeted cellular signaling. Tumor Biol. 2016, 37, 2789–2804. [Google Scholar] [CrossRef]
- Chang, T.H.; Szabo, E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Res. 2000, 60, 1129–1138. [Google Scholar]
- Muzio, G.; Trombetta, A.; Maggiora, M.; Martinasso, G.; Vasiliou, V.; Lassen, N.; Canuto, R.A. Arachidonic acid suppresses growth of human lung tumor A549 cells through down-regulation of ALDH3A1 expression. Free Radic. Biol. Med. 2006, 40, 1929–1938. [Google Scholar] [CrossRef]
- Lin, G.; Lin, L.; Chen, X.; Chen, L.; Yang, J.; Chen, Y.; Qian, D.; Zeng, Y.; Xu, Y. PPAR-γ/NF-kB/AQP3 axis in M2 macrophage orchestrates lung adenocarcinoma progression by upregulating IL-6. Cell Death Dis. 2024, 15, 532. [Google Scholar] [CrossRef]
- Gou, Q.; Che, S.; Chen, M.; Chen, H.; Shi, J.; Hou, Y. PPARγ inhibited tumor immune escape by inducing PD-L1 autophagic degradation. Cancer Sci. 2023, 114, 2871–2881. [Google Scholar] [CrossRef]
- Fu, Q.; Shen, N.; Fang, T.; Zhang, H.; Di, Y.; Liu, X.; Du, C.; Guo, J. ACT001 alleviates inflammation and pyroptosis through the PPAR-γ/NF-κB signaling pathway in LPS-induced alveolar macrophages. Genes Genom. 2024, 46, 323–332. [Google Scholar] [CrossRef]
- Wang, N.; Kong, R.; Han, W.; Bao, W.; Shi, Y.; Ye, L.; Lu, J. Honokiol alleviates ulcerative colitis by targeting PPAR-γ-TLR4-NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int. Immunopharmacol. 2022, 111, 109058. [Google Scholar] [CrossRef]
- Gallorini, M.; Di Valerio, V.; Bruno, I.; Carradori, S.; Amoroso, R.; Cataldi, A.; Ammazzalorso, A. Phenylsulfonimide PPARα antagonists enhance Nrf2 activation and promote oxidative stress-induced apoptosis/pyroptosis in MCF7 breast cancer cells. Int. J. Mol. Sci. 2023, 24, 1316. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Peebles, K.A.; Sharma, S.; Mao, J.T.; Dubinett, S.M. The role of PPARγ in the cyclooxygenase pathway in lung cancer. PPAR Res. 2008, 2008, 790568. [Google Scholar] [CrossRef]
- Li, H.; Sorenson, A.L.; Poczobutt, J.; Amin, J.; Joyal, T.; Sullivan, T.; Crossno, J.T., Jr.; Weiser-Evans, M.C.; Nemenoff, R.A. Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis. PLoS ONE 2011, 6, e28133. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ding, Z.Y.; Qian, Z.; Zhao, C.X.; Zhang, K.C. Improved production of mycelial biomass and ganoderic acid by submerged culture of Ganoderma lucidum SB97 using complex media. Enzyme Microb. Technol. 2008, 42, 325–331. [Google Scholar] [CrossRef]
- Báez-Vallejo, N.; Camarena-Pozos, D.A.; Monribot-Villanueva, J.L.; Ramírez-Vázquez, M.; Carrión-Villarnovo, G.L.; Guerrero-Analco, J.A.; Partida-Martínez, L.P.; Reverchon, F. Forest tree associated bacteria for potential biological control of Fusarium solani and of Fusarium kuroshium, causal agent of Fusarium dieback. Microbiol. Res. 2020, 235, e126440. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; López-Romero, J.C.; Vázquez-Solorio, J.Y.; Velázquez-Contreras, C.A.; Garibay-Escobar, A.; Díaz-López, R.; Robles-Zepeda, R.E. Antioxidant, anti-inflammatory and antiproliferative properties of Ibervillea sonorae. S. Afr. J. Bot. 2019, 125, 207–213. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; Velázquez, C.A.; Garibay-Escobar, A.; Curini, M.; Marcotullio, M.C.; Robles-Zepeda, R.E. Antiproliferative and apoptosis induction of cucurbitacin-type triterpenes from Ibervillea sonorae. Ind. Crops Prod. 2015, 77, 895–900. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Ibrahim, M.H.; Brinza, I.; Boiangiu, R.S.; Honceriu, I.; Amin, A.; Ben-Attia, M.; Hritcu, L. Anxiolytic and antidepressant effects of Tribulus terrestris ethanolic extract in scopolamine-induced amnesia in zebrafish: Supported by molecular docking investigation targeting monoamine oxidase A. Pharmaceuticals 2024, 17, 1208. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]

| Treatments | A549 | HeLa | ARPE-19 |
|---|---|---|---|
| Ethanolic crude extract | 144.96 ± 9.4 | >200 | >200 |
| Hexane fraction | 140.84 ± 17.9 | >200 | >200 |
| Ethyl acetate fraction | 107.842 ± 9.4 | >200 | >200 |
| Residual fraction | >200 | >200 | >200 |
| Fraction | A549 | HeLa | MDA-MB-231 | ARPE-19 |
|---|---|---|---|---|
| FH1–FH10 | >200 | >200 | >200 | >200 |
| FH11 | 78.4 ± 7.1 | 106.5 ± 2.9 | 162 ± 9.8 | >200 |
| FH12 | 96.1 ±12.8 | 148.8 ± 8.5 | >200 | >200 |
| FH13 | 89.3 ± 4.2 | 136.6 ± 10.4 | 181.5 ± 9.6 | >200 |
| Fraction | Molecules | Formula | Mass | m/z | RT |
|---|---|---|---|---|---|
| FH11 | 9-Hydroxy-10E,12Z-octadecadienoic acid | C18H31O3 | 296.64 | 295.22 | 11.39 |
| Ganoderic acid TQ | C32H46O5 | 510.70 | 503.32 | 14.82 | |
| Ganoderic acid X | C32H48O5 | 512.35 | 511.34 | 15.40 | |
| FH12 | 9-Hydroxy-10E,12Z-octadecadienoic acid | C18H31O3 | 296.64 | 295.22 | 11.52 |
| Ganoderic acid DM | C30H44O4 | 468.70 | 467.31 | 12.78 | |
| Ganoderic acid TQ | C32H46O5 | 510.70 | 509.32 | 14.82 | |
| Ganoderic acid X | C32H48O5 | 512.35 | 511.34 | 15.39 | |
| FH13 | 9-Hydroxy-10E,12Z-octadecadienoic acid | C18H31O3 | 296.64 | 295.22 | 11.51 |
| Ganoderic acid DM | C30H44O4 | 468.70 | 467.31 | 12.77 | |
| Ganoderic acid X | C32H48O5 | 512.35 | 511.34 | 15.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo-Sanchez, L.T.; Vidal-Gutiérrez, M.; Torres-Moreno, H.; Esqueda, M.; Gutiérrez, A.; Vargas, G.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Muñoz-Bacasehua, C.; Robles-Zepeda, R.E. Ethanolic Extract of Ganoderma mexicanum Pat. Mycelium: A Source of Bioactive Compounds with Antiproliferative Activity and Potential PPAR-γ Natural Ligands. Pharmaceuticals 2025, 18, 909. https://doi.org/10.3390/ph18060909
Angulo-Sanchez LT, Vidal-Gutiérrez M, Torres-Moreno H, Esqueda M, Gutiérrez A, Vargas G, Monribot-Villanueva JL, Guerrero-Analco JA, Muñoz-Bacasehua C, Robles-Zepeda RE. Ethanolic Extract of Ganoderma mexicanum Pat. Mycelium: A Source of Bioactive Compounds with Antiproliferative Activity and Potential PPAR-γ Natural Ligands. Pharmaceuticals. 2025; 18(6):909. https://doi.org/10.3390/ph18060909
Chicago/Turabian StyleAngulo-Sanchez, Lucia T., Max Vidal-Gutiérrez, Heriberto Torres-Moreno, Martín Esqueda, Aldo Gutiérrez, Georgina Vargas, Juan Luis Monribot-Villanueva, José A. Guerrero-Analco, César Muñoz-Bacasehua, and Ramón Enrique Robles-Zepeda. 2025. "Ethanolic Extract of Ganoderma mexicanum Pat. Mycelium: A Source of Bioactive Compounds with Antiproliferative Activity and Potential PPAR-γ Natural Ligands" Pharmaceuticals 18, no. 6: 909. https://doi.org/10.3390/ph18060909
APA StyleAngulo-Sanchez, L. T., Vidal-Gutiérrez, M., Torres-Moreno, H., Esqueda, M., Gutiérrez, A., Vargas, G., Monribot-Villanueva, J. L., Guerrero-Analco, J. A., Muñoz-Bacasehua, C., & Robles-Zepeda, R. E. (2025). Ethanolic Extract of Ganoderma mexicanum Pat. Mycelium: A Source of Bioactive Compounds with Antiproliferative Activity and Potential PPAR-γ Natural Ligands. Pharmaceuticals, 18(6), 909. https://doi.org/10.3390/ph18060909









