(-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms
Abstract
1. Introduction
2. Results
2.1. Effect of (-)-Fenchone in Intestinal Acute Inflammation in a TNBS-Induced Colitis Model
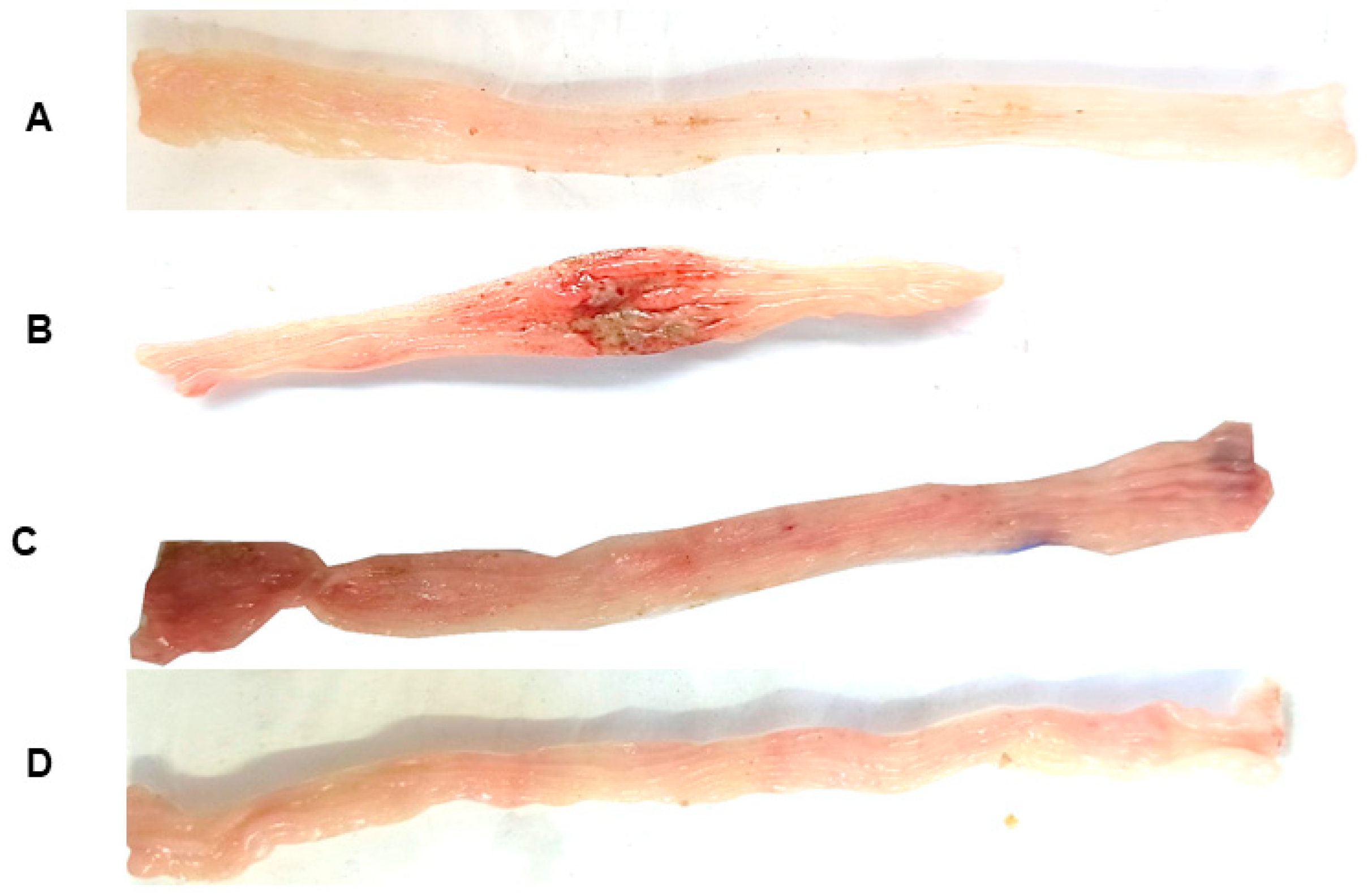
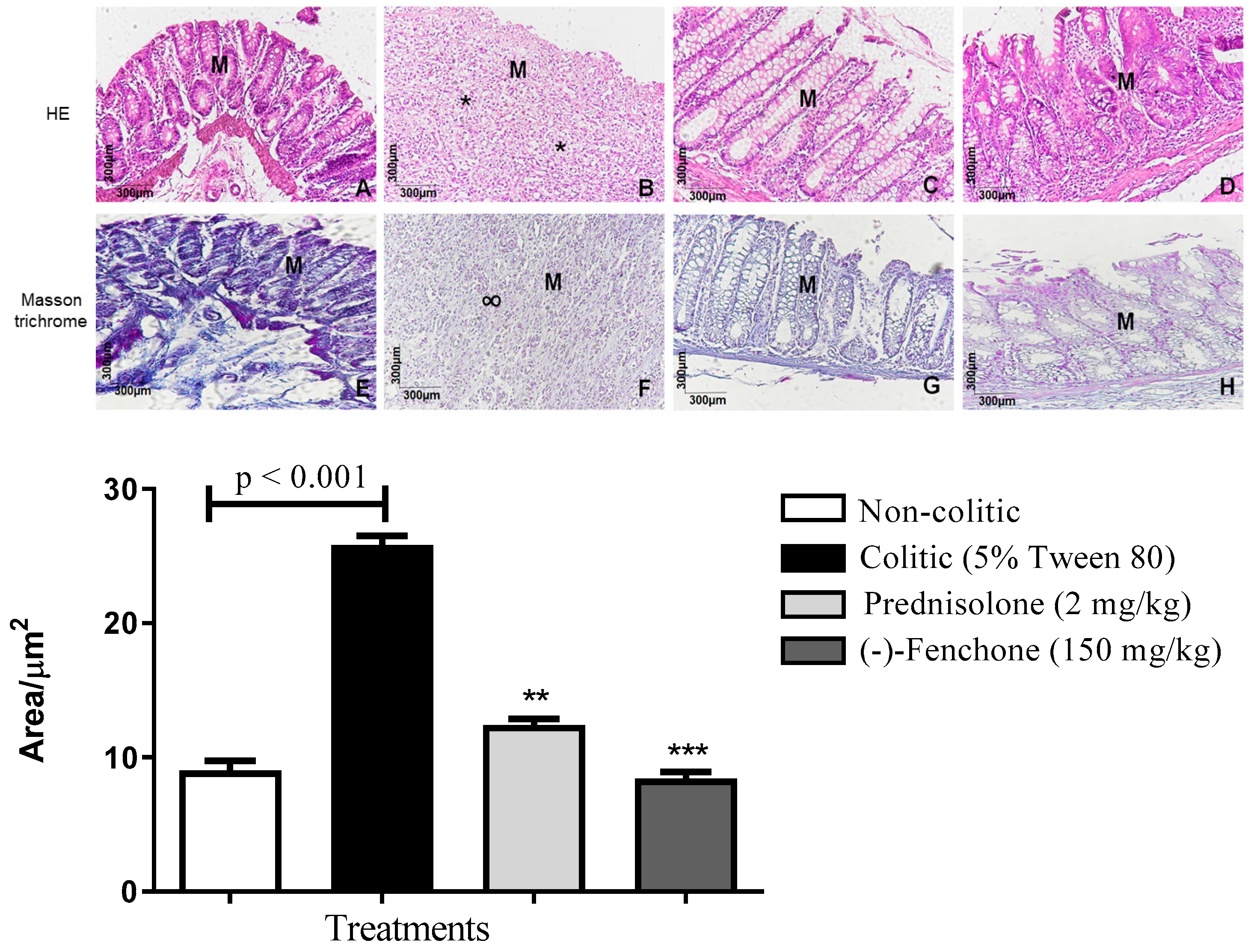
2.1.1. Histological Analysis
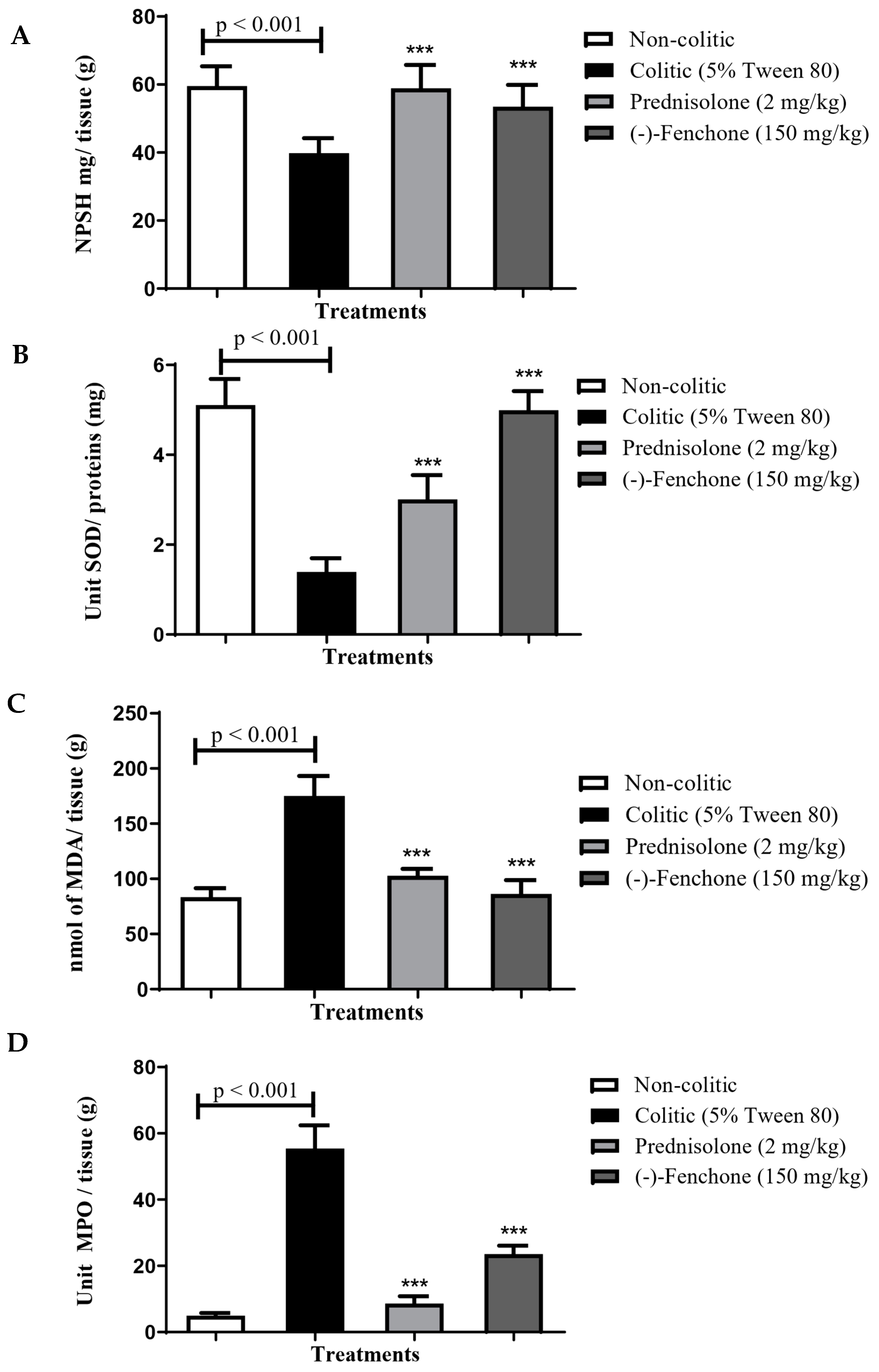
2.1.2. Effect of (-)-Fenchone in the Modulation of Antioxidant and Anti-Inflammatory Properties in Acute Colitis
GSH
SOD
MDA
MPO
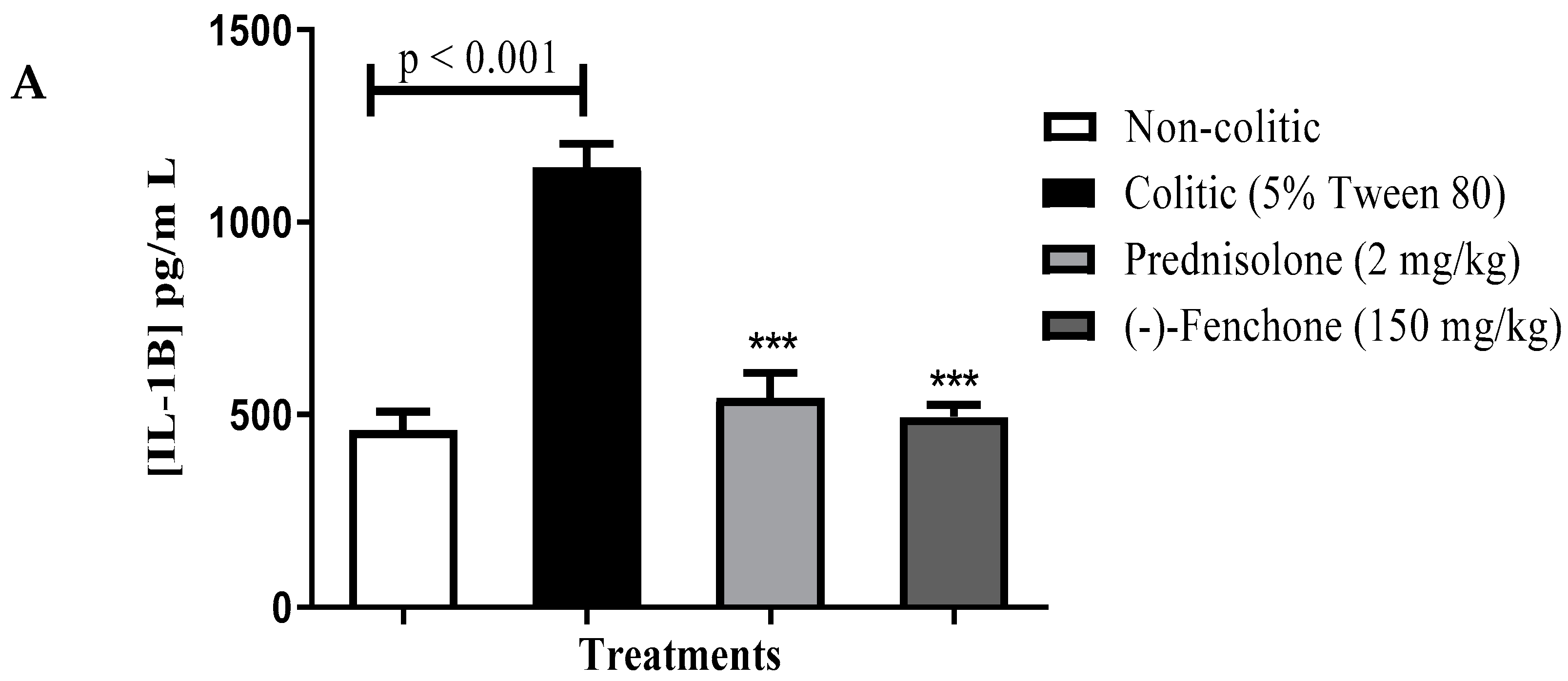
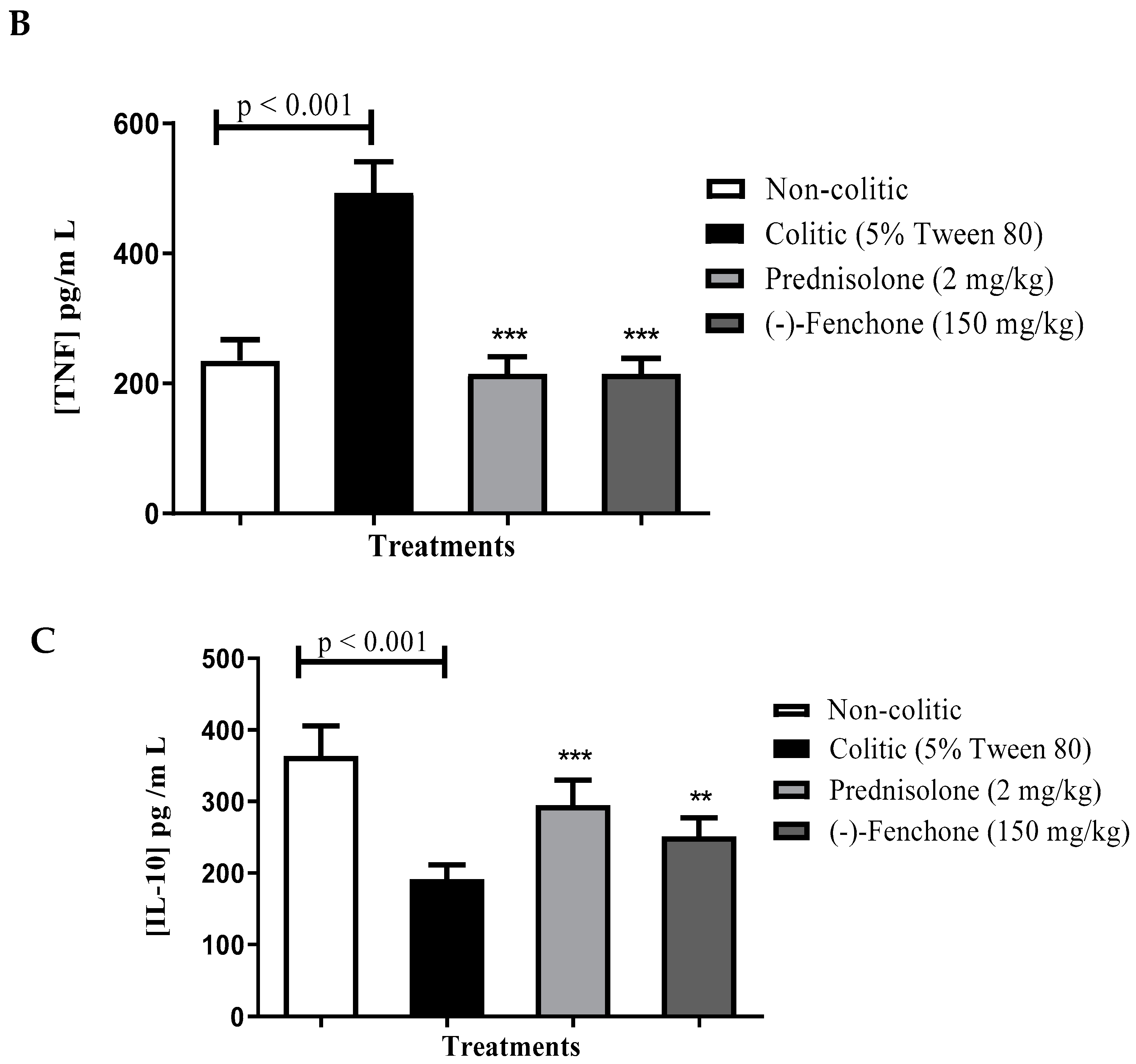
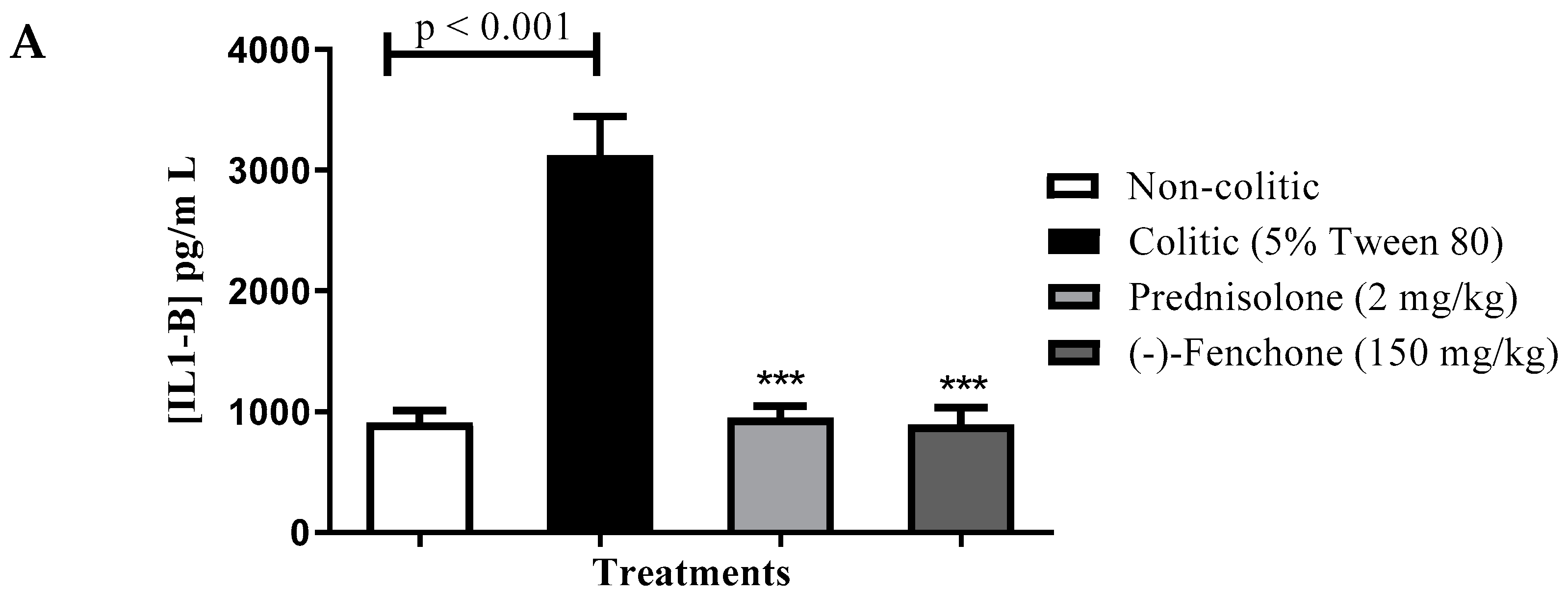
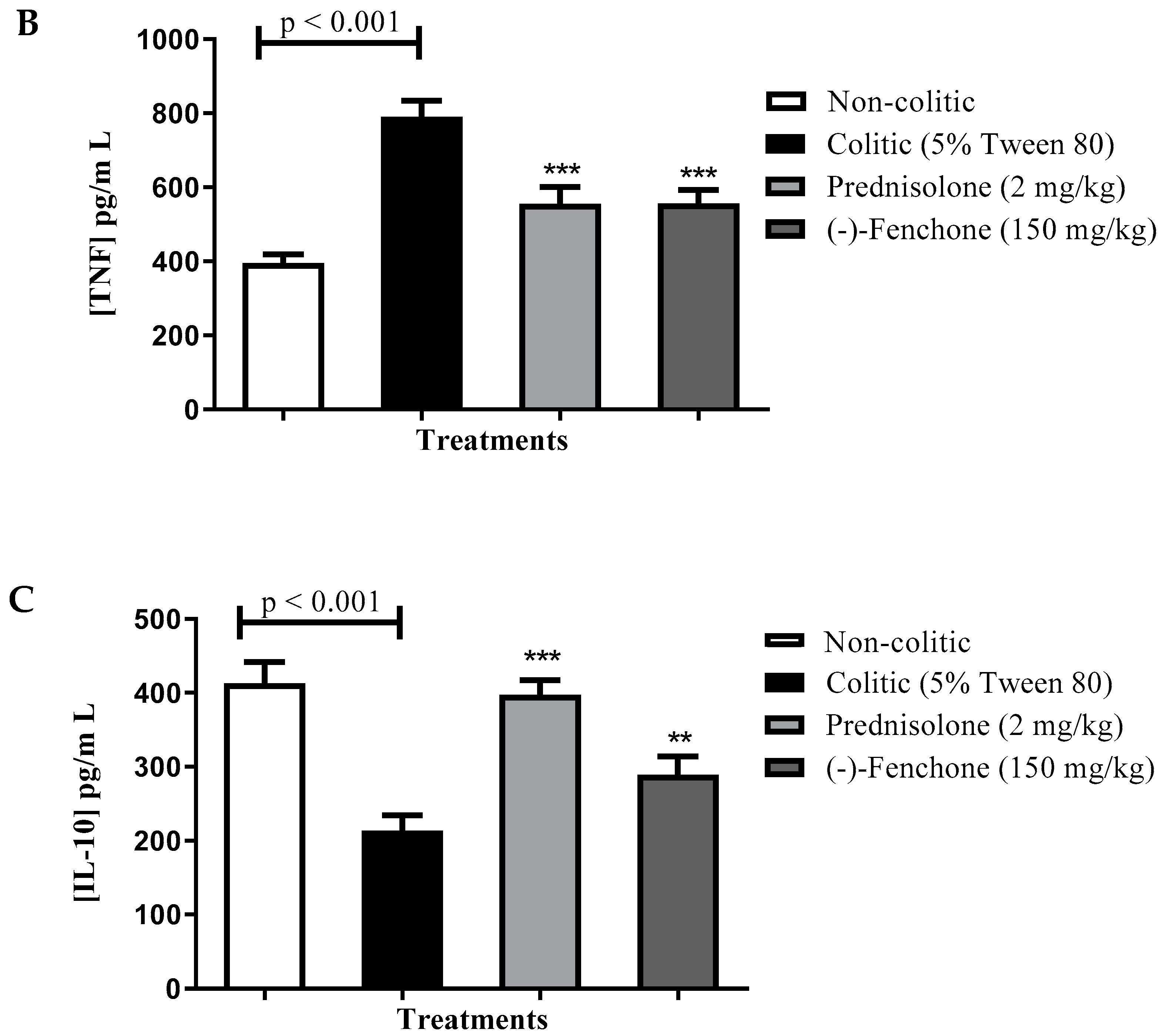
Levels of IL-1β, TNF-α and IL-10
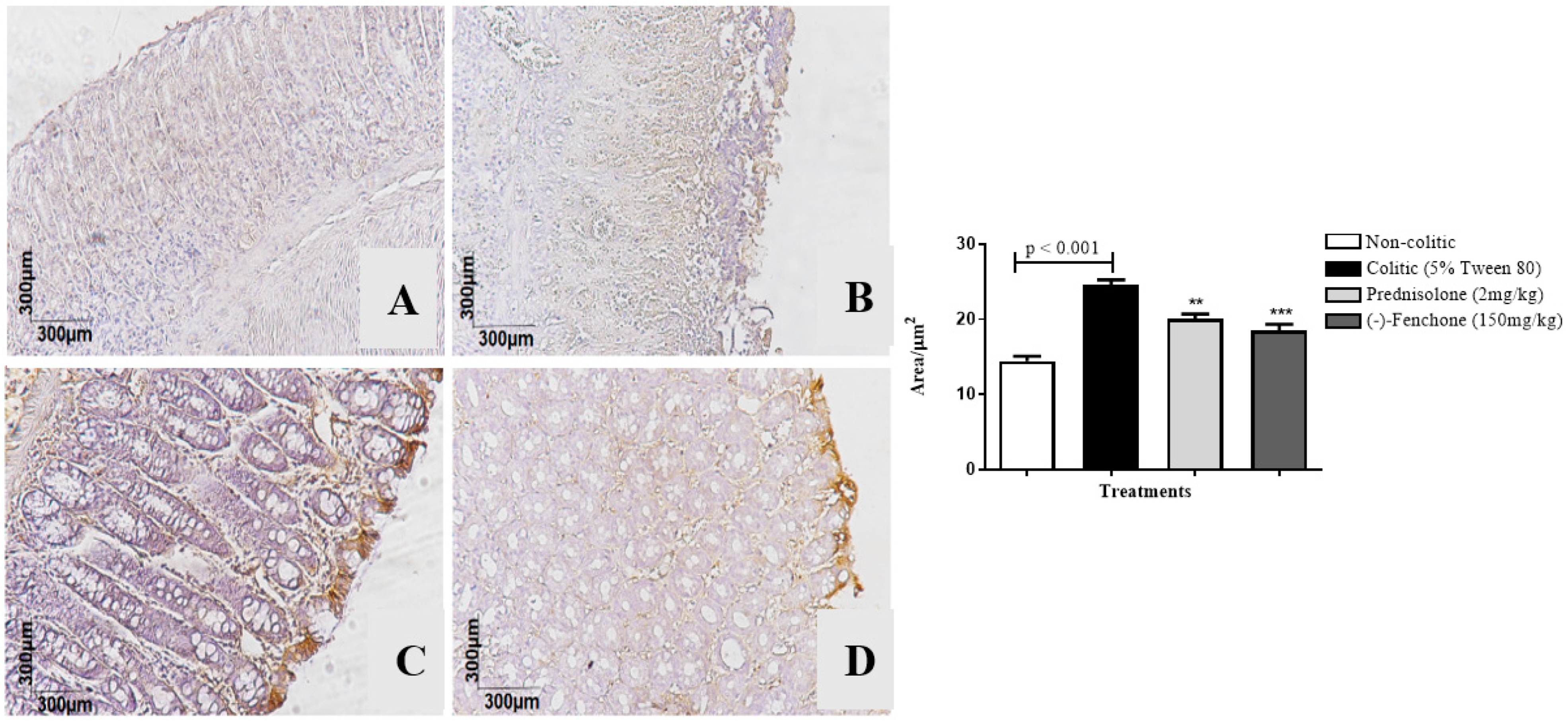
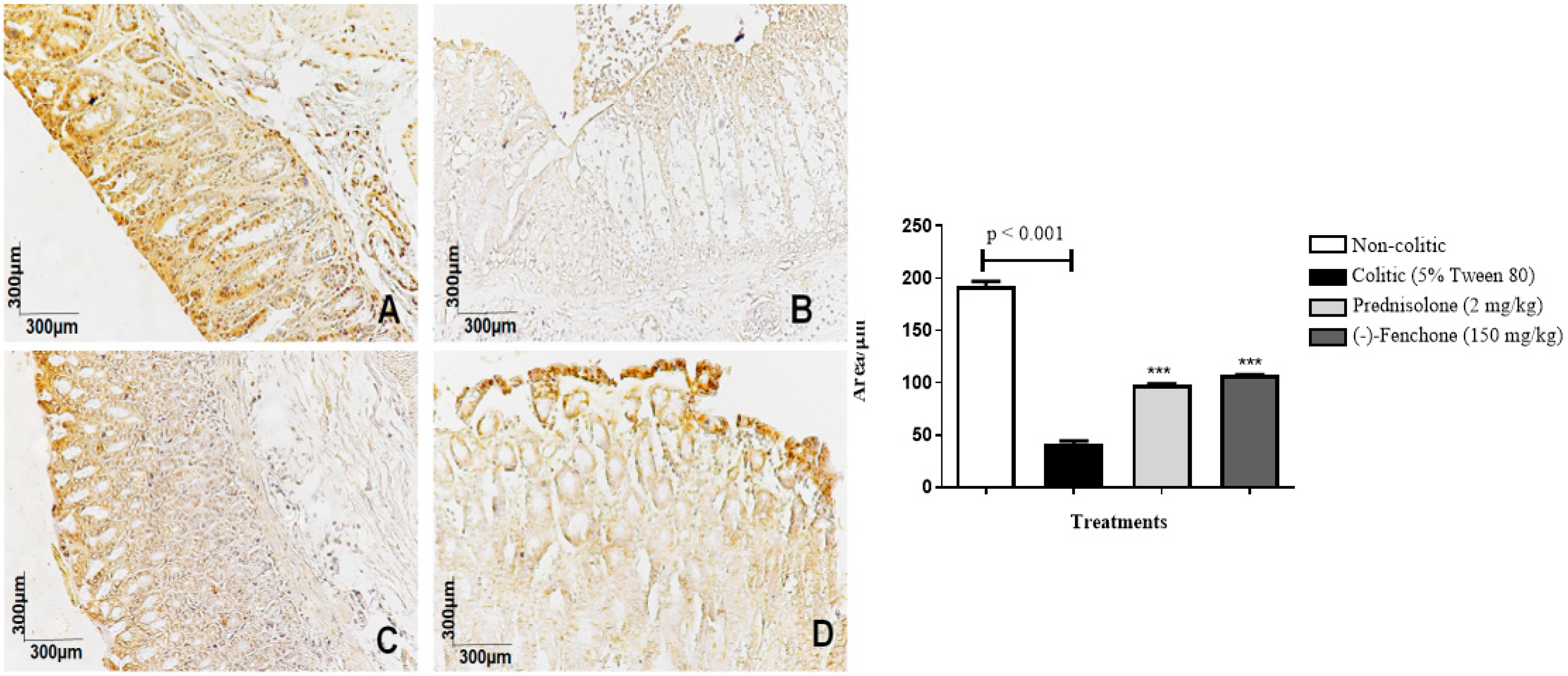
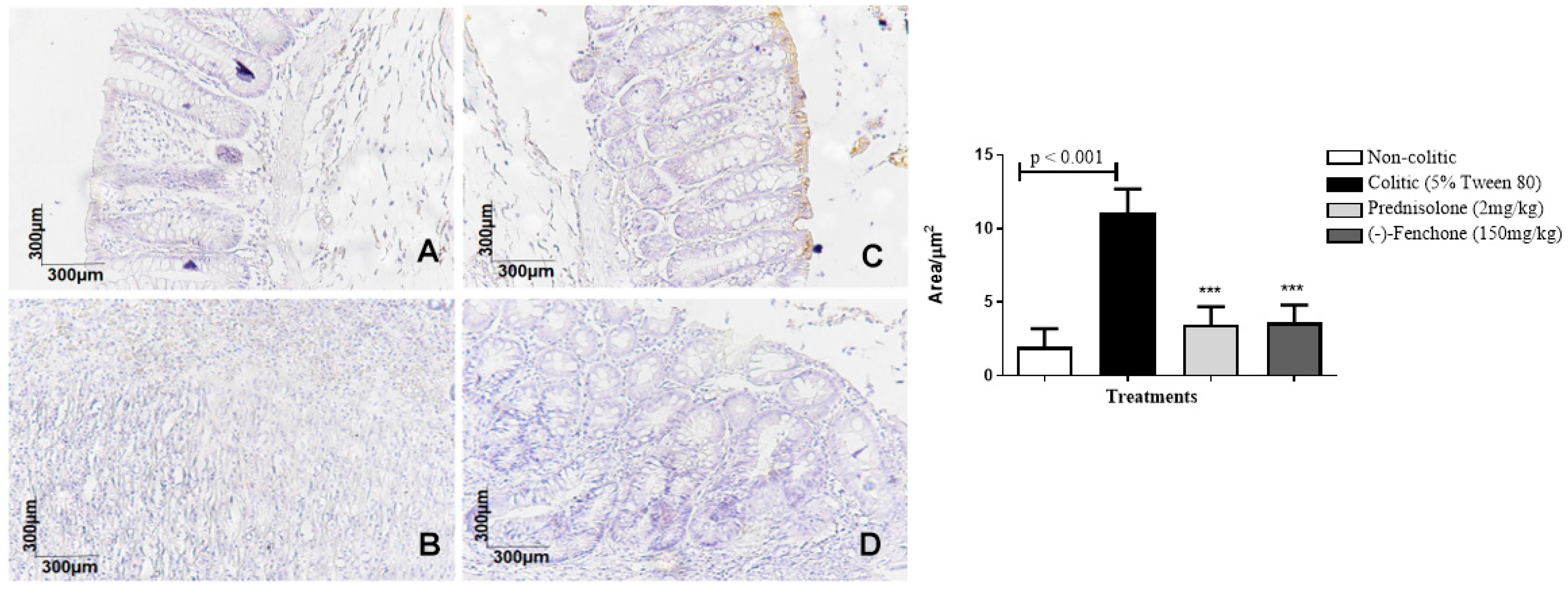
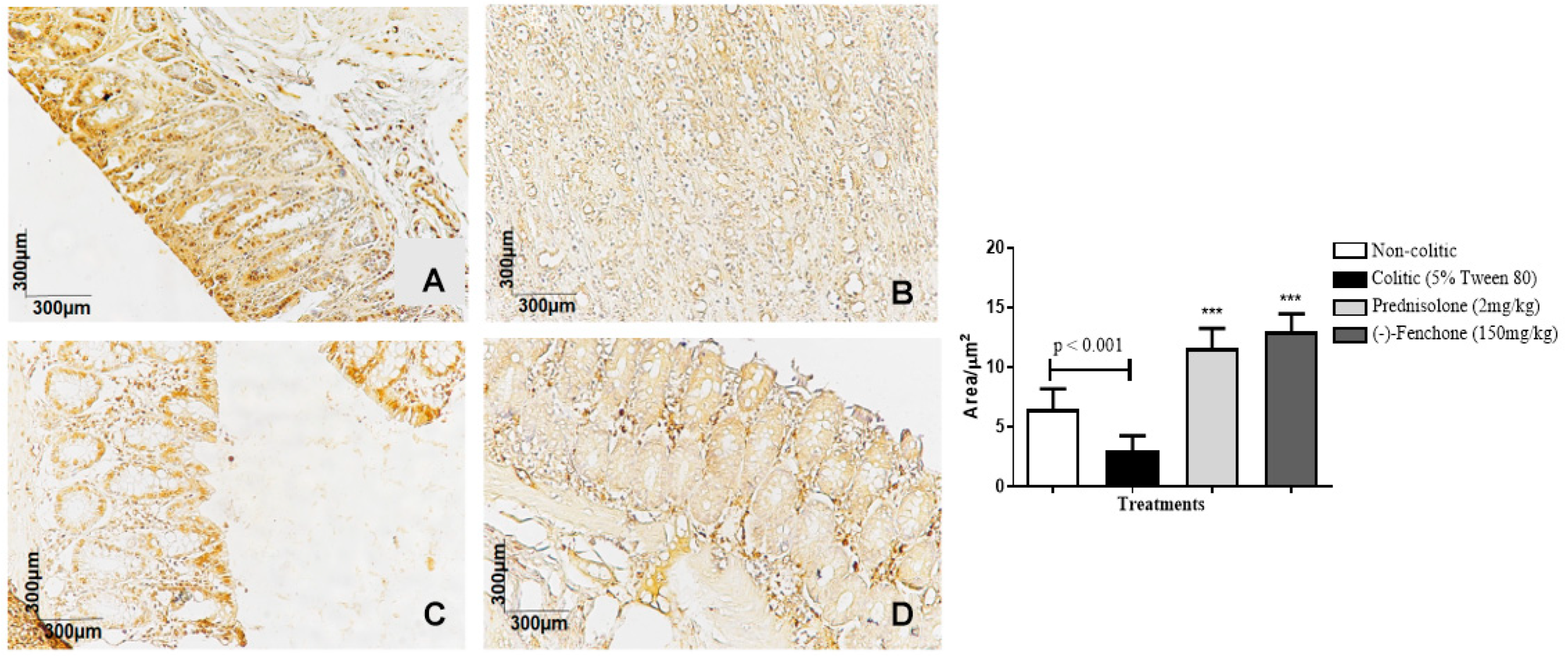
2.1.3. Immunohistochemical Analysis for NF-κB, TGF-β, and ZO-1 in Colon Samples Submitted to Acute Inflammation in a TNBS-Induced Colitis Model
2.2. Effect of (-)-Fenchone in the Chronic Phase with Recurrence of the Intestinal Inflammatory Process in a TNBS-Induced Colitis Model
2.2.1. Histological Analysis
2.2.2. Effect of (-)-Fenchone in the Modulation of Antioxidant and Anti-Inflammatory Properties in Chronic Phase Colitis with Recurrence of the Intestinal Inflammatory Process
GSH
SOD
MDA
MPO
Levels of IL-1β, TNF-α, and IL-10
2.2.3. Immunohistochemical Analysis for NF-κB, TGF-β, and ZO-1 in Colon Samples Submitted to Chronic Phase Colitis with Recurrence of the Intestinal Inflammatory Process
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. TNBS-Induced Intestinal Acute Inflammation in Rats
4.4. Chronic Phase with Recurrence of the Intestinal Inflammatory Process in TNBS-Induced Intestinal Inflammation in Rats
4.4.1. Histological Analysis and Morphometric Analysis
4.4.2. Determination of Reduced Glutathione (GSH), Malondialdehyde (MDA), Myeloperoxidase (MPO), and Superoxide Dismutase (SOD)
GSH Determination
MDA Determination
MPO Determination
SOD Determination
4.4.3. Immunomodulatory Activity
Determination of IL-1, TNF-α, and IL-10 Levels
4.4.4. Immunohistochemical Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, J.M.; Subedi, S.; Leleiko, N.S. Inflammatory bowel disease. Pediatr. Rev. 2016, 37, 337–347. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Huo, L.L.; Sun, Z.R. MiR-128-3p alleviates TNBS-induced colitis through inactivating TRAF6/NF-κB signaling pathway in rats. Kaohsiung J. Med. Sci. 2021, 37, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 769–778. [Google Scholar] [CrossRef]
- Windsor, J.W.; Kaplan, G.G. Evolving epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019, 156, 1345–1353. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 e fatores bacterianos relacionados: Alvos terapêuticos para colite ulcerativa. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; El jurdi, K.; Zmeter, N.; Rubin, D.T. Emerging therapies for inflammatory bowel disease. Adv. Ther. 2018, 35, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Triantafillidis, J. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Dev. Ther. 2011, 5, 185–210. [Google Scholar] [CrossRef]
- Liverani, E. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017. [Google Scholar] [CrossRef]
- Actis, G.C.; Pellicano, R. Inflammatory bowel disease: Efficient remission maintenance is crucial for cost containment. World J. Gastrointest. Pharmacol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- Khan, R.A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Roaa, M.H. A review article: The importance of the major groups of plants secondary metabolism phenols, alkaloids, and terpenes. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 354–358. [Google Scholar] [CrossRef]
- Sá, S.C.R.; Andrade, L.N.; De sousa, D.P. A Review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimarães, A.G.; Quintans-Junior, L.J.; Araújo, A.A.d.S.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Venzon, L.; Mariano, L.N.B.; Somensi, L.B.; Boeing, T.; de Souza, P.; Wagner, T.M.; de Andrade, S.F.; Nesello, L.A.N.; da Silva, L.M. Essential oil of Cymbopogon citratus (lemongrass) and geraniol, but not citral, promote gastric healing activity in mice. Biomed. Pharmacother. 2018, 98, 118–124. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; González-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Contreras, M.d.M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and antimycotic activities of two native lavandula species from portugal. Evid. Based Complement. Altern. Med. 2015, 2015, 570521. [Google Scholar] [CrossRef] [PubMed]
- Slavchev, I.; Dobrikov, G.M.; Valcheva, V.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Antimycobacterial activity generated by the amide coupling of (-)-Fenchone derived aminoalcohol with cinnamic acids and analogues. Bioorgan. Med. Chem. Lett. 2014, 24, 5030–5033. [Google Scholar] [CrossRef]
- Araruna, E.; Júnior, E.B.A.; Serafim, C.A.d.L.; Pessoa, M.M.B.; Pessôa, M.L.d.S.; Alves, V.P.; da Silva, M.S.; Sobral, M.V.; Alves, A.F.; Nunes, M.K.d.S.; et al. (-)-Fenchone Prevents Cysteamine-Induced Duodenal Ulcers and Accelerates Healing Promoting Re-Epithelialization of Gastric Ulcers in Rats via Antioxidant and Immunomodulatory Mechanisms. Pharmaceuticals 2024, 17, 641. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, M.L.S.; Silva, L.M.O.; Araruna, M.E.C.; Serafim, C.A.d.L.; Júnior, E.B.A.; Silva, A.O.; Pessoa, M.M.B.; Neto, H.D.; Lima, E.d.O.; Batista, L.M. Antifungal activity and antidiarrheal activity via antimotility mechanisms of (-)-Fenchone in experimental models. World J. Gastroenterol. 2020, 26, 6795–6809. [Google Scholar] [CrossRef]
- Agrawal, M.; Colombel, J.F. Treat-to-target in inflammatory bowel diseases, what is the target and how do we treat? Gastrointest. Endosc. Clin. 2019, 29, 421–436. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Solas, J.; Pinto, R.; Mateus, V. Chronic experimental model of TNBS-induced colitis to study inflammatory bowel disease. Int. J. Mol. Sci. 2022, 23, 4739. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Binder, H.J. Mechanisms of diarrhea in inflammatory bowel diseases. Ann. N. Y. Acad. Sci. 2009, 1165, 285–293. [Google Scholar] [CrossRef]
- Barmeyer, C.; Fromm, M.; Schulzke, J.D. Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflug. Arch. 2017, 469, 15–26. [Google Scholar] [CrossRef]
- Formiga, R.O.; Júnior, E.B.A.; Vasconcelos, R.C.; Guerra, G.C.B.; de Araújo, A.A.; de Carvalho, T.G.; Garcia, V.B.; Junior, R.F.d.A.; Gadelha, F.A.A.F.; Vieira, G.C.; et al. p-Cymene and rosmarinic acid ameliorate TNBS-induced intestinal inflammation upkeeping ZO-1 and MUC-2: Role of antioxidant system and immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef] [PubMed]
- Soubh, A.A.; Abdallah, D.M.; El-abhar, H.S. Geraniol ameliorates TNBS-induced colitis: Involvement of Wnt/β-catenin, p38MAPK, NF-βB, and PPAR signaling pathways. Life Sci. 2015, 136, 142–150. [Google Scholar] [CrossRef]
- Yang, X.L.; Guo, T.-K.; Wang, Y.-H.; Gao, M.-T.; Qin, H.; Wu, Y.-J. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch. Pharm. Res. 2012, 35, 1231–1239. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Bigagli, E.; Agostiniani, S.; Giudici, F.; Zambonin, D.; Scaringi, S.; Ficari, F.; Lodovici, M.; Malentacchi, C. Analysis of oxidative stress-related markers in crohn’s disease patients at surgery and correlations with clinical findings. Antioxidants 2019, 8, 378. [Google Scholar] [CrossRef]
- Kivrak, E.G.; Yurt, K.; Kaplan, A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 2010, 167–176. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- De santana souza, M.T.; Teixeira, D.F.; de Oliveira, J.P.; Oliveira, A.S.; Quintans-Júnior, L.J.; Correa, C.B.; Camargo, E.A. Protective effect of carvacrol on acetic acid-induced colitis. Biomed. Pharmacother. 2017, 96, 313–319. [Google Scholar] [CrossRef]
- Ghasemi-pirbaluti, M.; Motaghi, E.; Bozorgi, H. The effect of menthol on acute experimental colitis in rats. Eur. J. Pharmacol. 2017, 805, 101–107. [Google Scholar] [CrossRef]
- Song, X.; Zhang, W.; Wang, T.; Jiang, H.; Zhang, Z.; Fu, Y.; Yang, Z.; Cao, Y.; Zhang, N. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 2014, 37, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.S.; Kim, I.-S.; Ahn, D.; Tae, H.-J.; Nam, H.-H.; Choo, B.-K.; Kim, K.; Park, B.-Y. Anti-inflammatory and gastroprotective roles of rabdosia inflexa through downregulation of pro-inflammatory cytokines and MAPK/NF-κB signaling pathways. Int. J. Mol. Sci. 2018, 19, 584. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Hellstern, P.; Panaccione, R.; Rogler, G.; Fraser, G.; Kohn, A.; Desreumaux, P.; Leong, R.W.; Comer, G.M.; et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 2017, 68, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 2195–2205. [Google Scholar] [CrossRef]
- D’alessio, P.A.; Ostan, R.; Bisson, J.-F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-Limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef]
- Vargas-robles, H.; Castro-Ochoa, K.F.; Citalán-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal, barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Bell, C.J.; Gall, D.G.; Wallace, J.L. Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 268, G622–G630. [Google Scholar] [CrossRef] [PubMed]
- Faure, P.; Lafond, J.-L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Birkhäuser Basel: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; pp. 407–421. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Kendall, A.C.; Keys, A.J.; Turner, J.C.; Lea, P.J.; Miflin, B.J. The isolation and characterisation of a catalase-deficient mutant of barley (Hordeum vulgare L.). Planta 2018, 159, 505–511. [Google Scholar] [CrossRef] [PubMed]
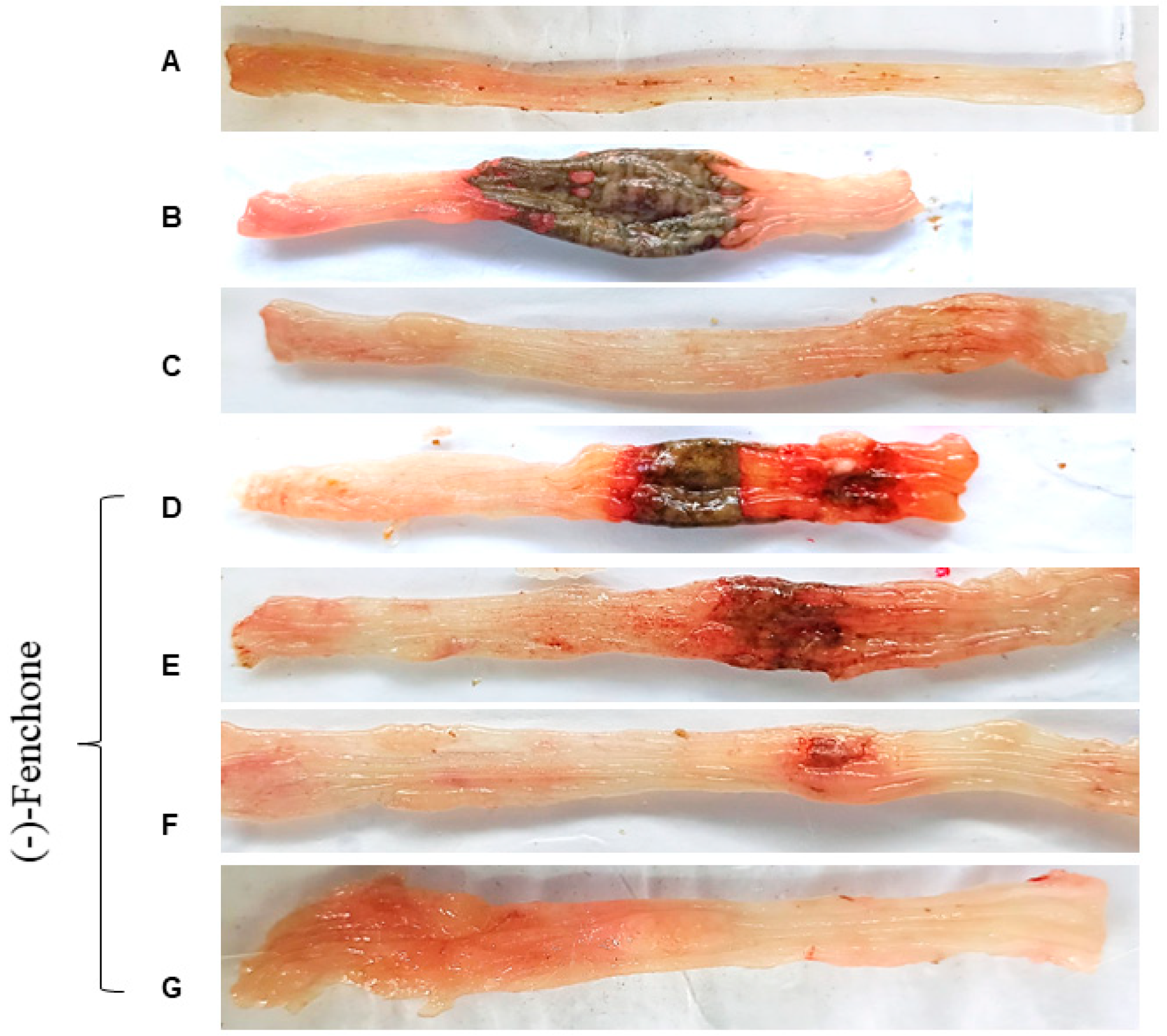
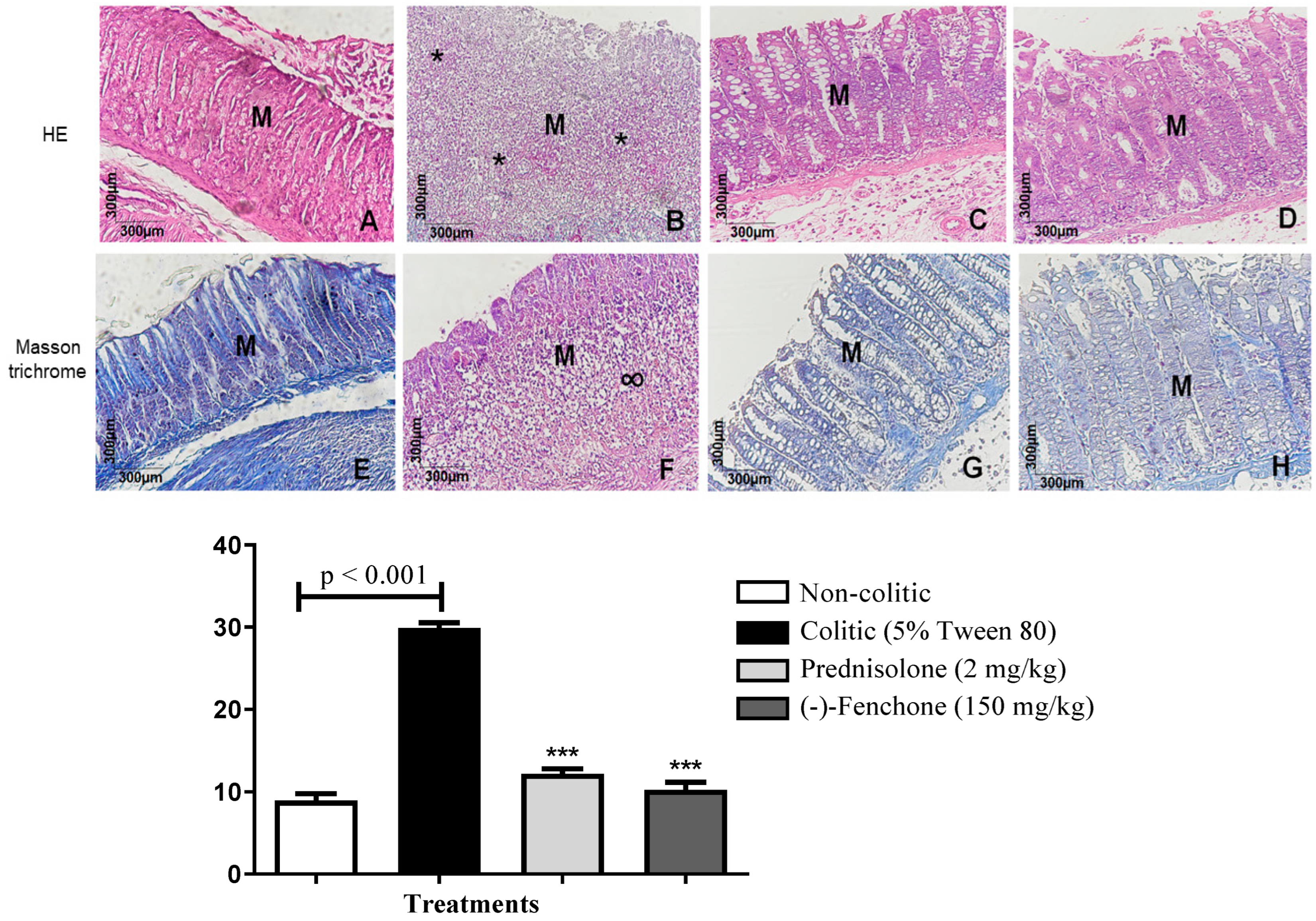



| Group | Dose (mg/kg) | ULA (mm2) | % Lesion Inhibition | Lesion Score | Weight/ Length (mg/cm) | Evacuation Index (EI) |
|---|---|---|---|---|---|---|
| Non-colitic | - | ND | ND | ND | 74.9 ± 5.9 | 12.0 ± 3.0 |
| Colitic (5% tween 80) | - | 286.6 ± 5.7 ### | - | 6.0 ± 0.9 ### | 162.7 ± 22.4 ### | 20.0 ± 2.4 # |
| Prednisolone | 2 | 36.7 ± 5.7 *** | 87% | 3.0 ± 0.5 *** | 107.5 ± 15.0 *** | 7.0 ± 3.0 *** |
| (-)-Fenchone | 37.5 | 240.9 ± 24.9 * | 16% | 4.5 ± 1.2 * | 126.7 ± 10.9 * | 9.0 ± 1.1 * |
| 75 | 181.4 ± 22.7 *** | 37% | 4.0 ± 1.5 * | 118.5 ± 32.9 ** | 7.0 ± 0.7 *** | |
| 150 | 72.2 ± 10.0 *** | 75% | 3.0 ± 0.7 ** | 113.1± 18.7 ** | 6.0 ± 1.1 *** | |
| 300 | 73.8 ± 10.8 *** | 74% | 3.0 ± 0.8 ** | 112.8 ± 9.7 ** | 4.0 ± 1.3 *** |
| Groups | ULA (mm2) | % Lesion Inhibition | Lesion Score | Weight/Length (mg/cm) |
|---|---|---|---|---|
| Non-colitic | ND | ND | ND | 91.4 ± 3.5 |
| Colitic (5% tween 80) | 57.17 ± 7.5 ### | 0 | 3.0 ± 0.7 ### | 148.9 ± 10.5 ### |
| Prednisolone (2 mg/kg) | 11.00 ± 2.1 *** | 81% | 1.0 ± 0.6 *** | 127.5 ± 13.7 ** |
| (-)-fenchone (150 mg/kg) | 11.50 ±2.9 *** | 80% | 1.5± 0.7 ** | 130.6 ± 8.3 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araruna, M.E.C.; Alves Júnior, E.B.; de Lima Serafim, C.A.; Pessoa, M.M.B.; de Souza Pessôa, M.L.; Alves, V.P.; Sobral, M.V.; da Silva, M.S.; Alves, A.F.; de Paiva Sousa, M.C.; et al. (-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms. Pharmaceuticals 2025, 18, 18. https://doi.org/10.3390/ph18010018
Araruna MEC, Alves Júnior EB, de Lima Serafim CA, Pessoa MMB, de Souza Pessôa ML, Alves VP, Sobral MV, da Silva MS, Alves AF, de Paiva Sousa MC, et al. (-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms. Pharmaceuticals. 2025; 18(1):18. https://doi.org/10.3390/ph18010018
Chicago/Turabian StyleAraruna, Maria Elaine Cristina, Edvaldo Balbino Alves Júnior, Catarina Alves de Lima Serafim, Matheus Marley Bezerra Pessoa, Michelle Liz de Souza Pessôa, Vitória Pereira Alves, Marianna Vieira Sobral, Marcelo Sobral da Silva, Adriano Francisco Alves, Maria Carolina de Paiva Sousa, and et al. 2025. "(-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms" Pharmaceuticals 18, no. 1: 18. https://doi.org/10.3390/ph18010018
APA StyleAraruna, M. E. C., Alves Júnior, E. B., de Lima Serafim, C. A., Pessoa, M. M. B., de Souza Pessôa, M. L., Alves, V. P., Sobral, M. V., da Silva, M. S., Alves, A. F., de Paiva Sousa, M. C., Araújo, A. A., & Batista, L. M. (2025). (-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms. Pharmaceuticals, 18(1), 18. https://doi.org/10.3390/ph18010018







