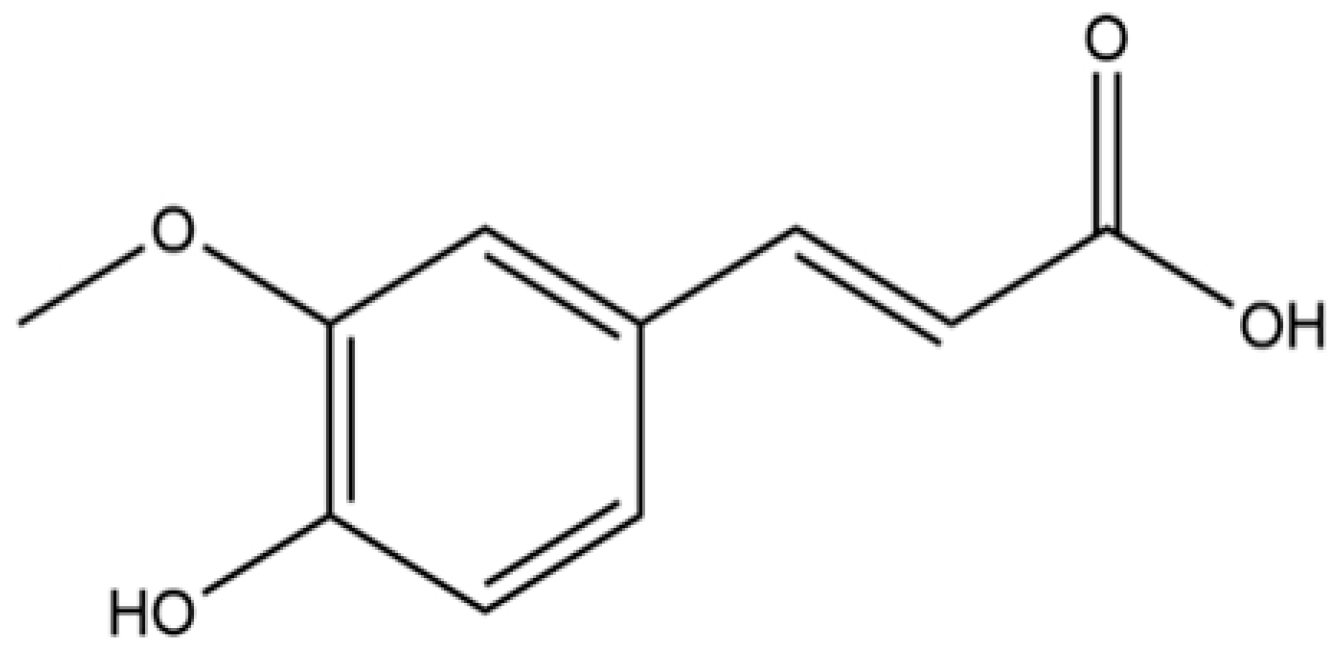
Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications
Abstract
1. Introduction
2. Anti-Inflammatory Pharmacological Properties of Ferulic Acid
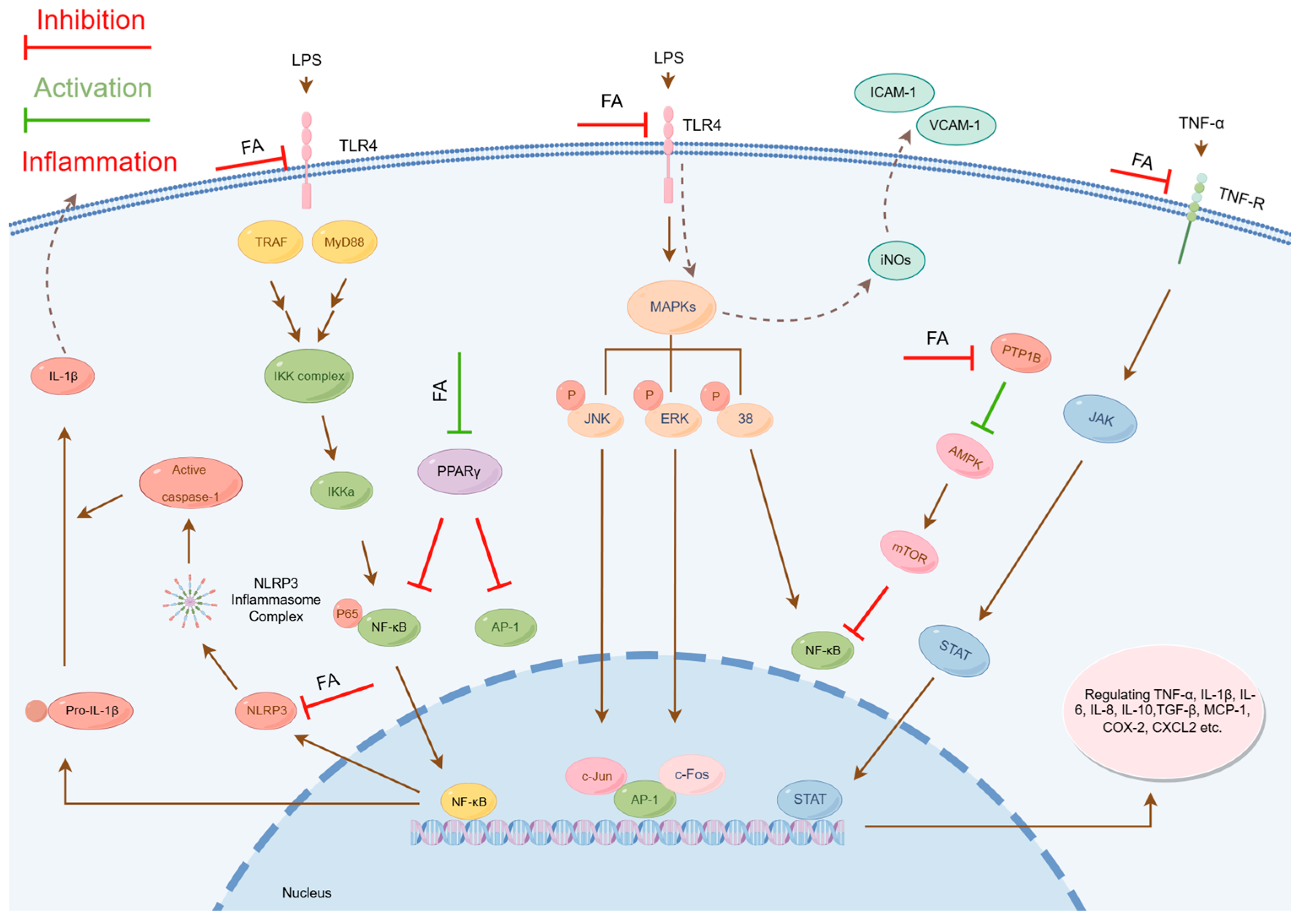
2.1. Modulation of Inflammation-Related Signaling Pathways
2.1.1. Inhibition of Nuclear Factor Kappa B Signaling Pathway Activation
- Inhibition of pNF-κB Phosphorylation: The phosphorylation of critical residues (e.g., Serine 536) in the p65 subunit of pNF-κB serves as a key marker of inflammatory activation. Studies have demonstrated that FA significantly reduces the phosphorylation level at this site [35,65,66,67,68,69]. This mechanism effectively prevents the nuclear translocation of NF-κB.
- Inhibition of IkappaB kinase (IKK) Activity: IKK, a pivotal kinase, is essential for the phosphorylation of IκBα and the activation of the NF-κB signaling pathway. FA directly inhibits IKK activity in the cytoplasm, disrupting the IKK/IκBα phosphorylation cascade and blocking the nuclear entry of NF-κB [60].
- Inhibition of Transcriptional Activity: Within the nucleus, FA suppresses the activity of NF-κB, leading to a downregulation of proinflammatory cytokines and chemokines [61].
2.1.2. Inhibition of Mitogen-Activated Protein Kinase Signaling Pathway Activation
2.1.3. Inhibition of Janus Kinase/Signal Transducer and Activator of Transcription Signaling Pathway Activation
- Inhibition of JAK/STAT Pathway Activation: Studies reveal that in arthritis models, FA effectively suppresses aberrant activation of this pathway and reduces the expression of proinflammatory cytokines, exhibiting potent antiarthritic activity [31]. Additionally, FA shows protective effects against radiation-induced acute liver injury, which is also mediated through JAK/STAT pathway inhibition [41].
- Suppression of STAT1 Phosphorylation: In vitro experiments confirm that FA treatment inhibits LPS-stimulated STAT1 phosphorylation in BV-2 microglial cells [18]. As a pivotal transcription factor in the JAK/STAT pathway, STAT1 regulates multiple immune-inflammatory responses. By specifically blocking STAT1 activation, FA plays a crucial role in immunomodulation.
2.1.4. Inhibition of NOD-like Receptor Protein 3 Inflammasome Activation
2.1.5. Modulation of Peroxisome Proliferator-Activated Receptor Gamma Activity
- Upregulation of PPARγ Expression: FA enhances both gene and protein expression levels of PPARγ, thereby augmenting its activity. In a study on sodium arsenite-induced glucose intolerance and hepatotoxicity, Daryagasht M. et al. found that FA (30–100 mg/kg) upregulated hepatic PPARγ and GLUT2 protein expression in exposed mice, consequently improving glucose metabolism [77].
- Direct Binding to PPARγ: FA may function as an endogenous PPARγ ligand, directly activating PPARγ by binding to its ligand-binding domain and inducing structural changes that regulate gene expression [78]. Notably, in gentamicin-induced nephrotoxicity models, FA exhibited renal effects of protection by enhancing PPARγ gene expression and catalase (CAT) activity [49].
2.1.6. Activation of the AMP-Activated Protein Kinase Signaling Pathway
- Inhibition of protein tyrosine phosphatase 1B (PTP1B): PTP1B is a key phosphatase that dephosphorylates critical protein kinases. FA specifically inhibits PTP1B activity, thereby preventing AMPK dephosphorylation. Wu J. et al. demonstrated that in carbon tetrachloride-induced hepatic inflammation and fibrosis, FA directly binds to and suppresses PTP1B, promoting AMPK phosphorylation [42].
- Direct AMPK Activation: In palmitate-induced hepatocyte models of metabolic syndrome (MetS), FA activates AMPK signaling, reducing ROS levels and ameliorating oxidative stress [45]. This metabolic regulation is closely linked to its anti-inflammatory effects.
2.1.7. Activation of the Nuclear Factor Erythroid 2-Related Factor 2 Signaling Pathway
2.1.8. Activation of the Phosphoinositide 3-Kinase/Protein Kinase B Signaling Pathway
2.1.9. Pathway Interaction
2.2. Inhibition of Oxidative Stress
2.3. Regulation of Cell Adhesion Molecule Expression
2.4. Immunomodulatory Effects
3. Application of Ferulic Acid in the Treatment of Excessive Inflammatory Reactions
3.1. Role of Ferulic Acid in the Treatment of Neurodegenerative Diseases
3.2. Role of Ferulic Acid in the Treatment of Osteoarthrosis
3.2.1. Rheumatoid Arthritis
3.2.2. Acute Gouty Arthritis
3.3. Effects of Ferulic Acid on Respiratory Diseases
3.4. Effects of Ferulic Acid on Cardiovascular Health
3.5. FA for Ulcerative Colitis
3.6. Therapeutic Effects of Ferulic Acid on Skin Inflammation
4. Pharmacokinetics
5. Toxicity and Safety
6. Challenges of Ferulic Acid in the Treatment of Excessive Inflammatory Response
- Drug delivery and bioavailability issues: As previously mentioned, its low oral bioavailability remains a critical bottleneck.
- Balance between therapeutic dose and safety: The effective dose of FA may differ significantly from its safe dose. Prolonged high-dose intake could cause gastrointestinal discomfort, liver and kidney dysfunction, and other adverse reactions. In addition, significant interindividual metabolic differences further complicate dose adjustment and the difficulty of accurate drug administration.
- Uncertainty of therapeutic efficacy: The efficacy of FA on different types of inflammatory reactions may vary, and its mechanism of action is still incompletely elucidated. At present, most of the studies on FA are still limited to animal models and cell experiments, with insufficient large-scale and high-quality clinical research data to back it up, which brings uncertainty to its clinical application.
- Complexity of multitarget regulation: Inflammation regulation involves complex signaling networks, making it difficult for a single drug to fully cover all key targets. Although FA demonstrates potential for multitarget regulation, its specific mechanisms—particularly cross-pathway synergistic regulations—require in-depth exploration. For example, crosstalk between NF-κB and AMPK pathways requires balancing immunosuppression and metabolic regulation [45,61,131], necessitating systems biology approaches to decipher synergistic mechanisms.
7. Prospects of Ferulic Acid in the Treatment of Excessive Inflammatory Response
- Development of high-performance FA derivatives: Researchers can design and synthesize FA derivatives with higher purity, bioactivity, and stability via structural optimization, chemical modification, and nanotechnology. These derivatives are expected to target pleiotropic inflammation-related signaling axes concurrently, enhancing their efficacy in treating inflammatory diseases.
- Optimizing efficacy and safety: In order to maximize the therapeutic potential of FA and minimize potential adverse effects, it is critical to thoroughly investigate the optimal dosage and route of administration as well as individualized treatment regimens. In addition, exploring the efficacy differences across diverse populations will also furnish an important basis for clinical application.
- Exploring synergistic drug combinations: FA combined with other anti-inflammatory agents (e.g., immunomodulators) has shown synergistic effects. For instance, the co-administration of FA (10 mg/kg) and metformin reduces metformin’s effective dose by 75% (from 50 mg/kg to 12.5 mg/kg) and significantly mitigates the adverse effects associated with metformin monotherapy [132].
- Integration of modern science and technology: Combining modern pharmacology and biotechnology to further explore the mechanisms of action of FA in TCM formulas will forge new avenues for its modernization.
8. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FA | Ferulic acid |
| RA | Rheumatoid arthritis |
| UC | Ulcerative colitis |
| NF-κB | Nuclear factor kappa B |
| MAPK | Mitogen-activated protein kinase |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| TCMs | Traditional Chinese medicines |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| CAM | Cell adhesion molecule |
| NLRP3 | NOD-like receptor protein 3 |
| AMPK | AMP-activated protein kinase |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ROS | Reactive oxygen species |
| IKK | IkappaB kinase |
| LPS | Lipopolysaccharide |
| CCI | Chronic constriction injury |
| JNK | c-Jun N-terminal kinase |
| ERK | Extracellular regulated kinase |
| CAT | Catalase |
| PTP1B | Protein tyrosine phosphatase 1B |
| MetS | Metabolic syndrome |
| Nrf2/HO-1 | Nuclear factor erythroid 2-related factor 2/heme oxygenase-1 |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| PIP2 | Phosphatidylinositol-4,5-bisphosphate |
| TLR | Toll-like receptor |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| NO | Nitric oxide |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| RA-FLS | Rheumatoid arthritis fibroblast-like synoviocytes |
| AA-FLS | Adjuvant arthritis fibroblast-like synoviocytes |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| ARDS | Acute respiratory distress syndrome |
| Th2 | T-helper type 2 |
| HIMECs | Human intestinal microvascular endothelial cells |
| IFN-γ | Interferon-gamma |
| iNOs | Inducible nitric oxide synthase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| CUMS | Chronic unpredictable mild stress |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| CIA | Collagen-induced arthritis |
| ALI | Acute lung injury |
| AS | Atherosclerosis |
| ARVM | Adult rat ventricular myocytes |
| ALD | Alcoholic liver disease |
| DN | Diabetic nephropathy |
| AKI | Acute kidney injury |
| IgE | Immunoglobulin E |
| KD | Kawasaki disease |
| CXCL | C-X-C Chemokine Ligand |
| BMECs | Bovine mammary epithelial cells |
| SMEDDS | Self-microemulsifying drug delivery system |
| AUC0−t | Area under the concentration–time curve |
References
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Cain, D.; Kondo, M.; Chen, H.; Kelsoe, G. Effects of acute and chronic inflammation on B-cell development and differentiation. J. Investig. Dermatol. 2009, 129, 266–277. [Google Scholar] [CrossRef]
- Alderton, G.; Scanlon, S.T. Inflammation. Science 2021, 374, 1068–1069. [Google Scholar] [CrossRef]
- Mueller, K. Inflammation. Inflammation’s yin-yang. Introduction. Science 2013, 339, 155. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Sinniah, A.; Yazid, S.; Flower, R.J. From NSAIDs to Glucocorticoids and Beyond. Cells. 2021, 10, 3524. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Xu, B.; Huang, S.W.; Luo, X.; Deng, X.L.; Luo, S.; Liu, C.; Wang, Q.; Chen, J.Y.; Zhou, L. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol. Sin. 2021, 42, 88–96. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a natural anti-inflammatory agent: An Update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef]
- Su, H.; Cui, X.; Zhao, Y.; Li, M.; Wei, J.; Paré, P.W. Light-Regulated Growth, Anatomical, Metabolites Biosynthesis and Transcriptional Changes in Angelica sinensis. Plants 2024, 13, 2744. [Google Scholar] [CrossRef]
- Amalraj, A.; Gopi, S. Biological activities and medicinal properties of Asafoetida: A review. J. Tradit. Complement. Med. 2017, 7, 347–359. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, T.; Fu, Y.; Yu, T.; Ding, Y.; Nie, H. Ferulic Acid: A Review of Pharmacology, Toxicology, and Therapeutic Effects on Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 8011. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Iloki Assanga, S.B.; Lewis Luján, L.; O’Keefe, J.H.; DiNicolantonio, J.J. Nutraceutical Strategies for Suppressing NLRP3 Inflammasome Activation: Pertinence to the Management of COVID-19 and Beyond. Nutrients 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yasukawa, K.; Yamaura, M.; Ukiya, M.; Kimura, Y.; Shimizu, N.; Arai, K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J. Agric. Food Chem. 2000, 48, 2313–2319. [Google Scholar] [CrossRef]
- Sudheer, A.R.; Muthukumaran, S.; Devipriya, N.; Devaraj, H.; Menon, V.P. Influence of ferulic acid on nicotine-induced lipid peroxidation, DNA damage and inflammation in experimental rats as compared to N-acetylcysteine. Toxicology 2008, 243, 317–329. [Google Scholar] [CrossRef]
- Ou, L.; Kong, L.Y.; Zhang, X.M.; Niwa, M. Oxidation of ferulic acid by Momordica charantia peroxidase and related anti-inflammation activity changes. Biol. Pharm. Bull. 2003, 26, 1511–1516. [Google Scholar] [CrossRef]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010, 59, 587–595. [Google Scholar] [CrossRef]
- Sun, X.; Sun, P.; Liu, L.; Jiang, P.; Li, Y. Ferulic acid attenuates microglia-mediated neuroinflammation in retinal degeneration. BMC Ophthalmol. 2021, 21, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577. [Google Scholar] [CrossRef]
- Liu, Y.M.; Shen, J.D.; Xu, L.P.; Li, H.B.; Li, Y.C.; Yi, L.T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, Q.; Xie, Y.; Tao, Z.; Jin, K.; Chen, S.; Bai, Y.; Yang, J.; Shan, S. Ferulic acid attenuates oxidative DNA damage and inflammatory responses in microglia induced by benzo(a)pyrene. Int. Immunopharmacol. 2019, 77, 105980. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Nie, Y.; Huang, C.; Zhu, G.; Zhang, X.; Hu, C.; Li, Z.; Gao, Y.; Ma, Z. Ferulic acid produces neuroprotection against radiation-induced neuroinflammation by affecting NLRP3 inflammasome activation. Int. J. Radiat. Biol. 2022, 98, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.; Cheng, X.; Lin, L.; Wang, Q.; Zhan, R.; Wu, Q.; Liu, S. Protective Effect of Ferulic Acid on Lipopolysaccharide-Induced BV2 Microglia Inflammation via AMPK/mTOR Signaling Pathway. Molecules 2023, 28, 3482. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Moghimi-Khorasgani, A.; Homayouni Moghadam, F.; Nasr-Esfahani, M.H. Ferulic Acid reduces amyloid beta mediated neuroinflammation through modulation of Nurr1 expression in microglial cells. PLoS ONE 2023, 18, e0290249. [Google Scholar] [CrossRef] [PubMed]
- Kinra, M.; Ranadive, N.; Nampoothiri, M.; Arora, D.; Mudgal, J. Involvement of NLRP3 inflammasome pathway in the protective mechanisms of ferulic acid and p-coumaric acid in LPS-induced sickness behavior and neuroinflammation in mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2024, 397, 1829–1839. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Z.; Zhang, J.; Huo, L.; Gao, J.; Gao, W. Ferulic acid ameliorates memory impairment in d-galactose-induced aging mouse model. Int. J. Food Sci. Nutr. 2016, 67, 806–817. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Cheng, X.; Lin, L.; Quan, S.; Li, S.; Zhan, R.; Wu, Q.; Liu, S. Paeoniflorin, ferulic acid, and atractylenolide III improved LPS-induced neuroinflammation of BV2 microglia cells by enhancing autophagy. J. Pharmacol. Sci. 2023, 152, 151–161. [Google Scholar] [CrossRef]
- Youssef, O.M.; Lashine, N.H.; El-Nablaway, M.; El-Yamany, M.I.; Youssef, M.M.; Arida, D.A. Ferulic acid mitigated rotenone toxicity -Evoked Parkinson in rat model by featuring apoptosis, oxidative stress, and neuroinflammation signaling. Tissue Cell 2024, 91, 102614. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.; Li, X.; Shi, H.; Zheng, Y.; Chang, S.; Zhao, G. Ferulic acid alleviates sciatica by inhibiting peripheral sensitization through the RhoA/p38MAPK signalling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2022, 106, 154420. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Z.; Xia, N.; Zhang, W.; Wei, Y.; Huang, J.; Ren, Z.; Meng, F.; Yang, L. Anti-arthritic activity of ferulic acid in complete Freund’s adjuvant (CFA)-induced arthritis in rats: JAK2 inhibition. Inflammopharmacology 2020, 28, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Rasool, M. Ferulic acid inhibits interleukin 17-dependent expression of nodal pathogenic mediators in fibroblast-like synoviocytes of rheumatoid arthritis. J. Cell. Biochem. 2019, 120, 1878–1893. [Google Scholar] [CrossRef]
- Doss, H.M.; Samarpita, S.; Ganesan, R.; Rasool, M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κB signalling pathway. Life Sci. 2018, 207, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, X.; Wang, X.; Liu, Z.; Zheng, G.; Zhang, X.; Schiöth, H.B.; Sun, C.; Wang, H.; Zhang, Y. Hidden liver-joint axis: HBV infection causes rheumatoid arthritis via TRAFD1 with imbalance of HBV X protein and trans-ferulic acid. Virulence 2024, 15, 2422540. [Google Scholar] [CrossRef]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting inflammatory mediators with ferulic acid, a dietary polyphenol, for the suppression of monosodium urate crystal-induced inflammation in rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Yao, S.; Zheng, J.; Gong, X.; Xiao, B. Ferulic acid alleviates alveolar epithelial barrier dysfunction in sepsis-induced acute lung injury by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis. Pharm. Biol. 2022, 60, 2286–2294. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Zhao, P.; Wang, D.; Zhang, Y.; Wang, J.; Chen, L.; Guo, W.; Gao, H.; Jiao, Y. Pretreatment of ferulic acid attenuates inflammation and oxidative stress in a rat model of lipopolysaccharide-induced acute respiratory distress syndrome. Int. J. Immunopathol. Pharmacol. 2018, 32, 394632017750518. [Google Scholar] [CrossRef]
- Hong, K.; Wang, J.; Kang, X.; Xue, H.; Gao, Y.; Liang, H.; Huang, W.; Zhan, J.; You, Y. Ferulic acid and protocatechuic acid alleviate atherosclerosis by promoting UCP1 expression to inhibit the NLRP3-IL-1β signaling pathway. Food Funct. 2025, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic Acid Ameliorates Atherosclerotic Injury by Modulating Gut Microbiota and Lipid Metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Monceaux, K.; Gressette, M.; Karoui, A.; Pires Da Silva, J.; Piquereau, J.; Ventura-Clapier, R.; Garnier, A.; Mericskay, M.; Lemaire, C. Ferulic Acid, Pterostilbene, and Tyrosol Protect the Heart from ER-Stress-Induced Injury by Activating SIRT1-Dependent Deacetylation of eIF2α. Int. J. Mol. Sci. 2022, 23, 6628. [Google Scholar] [CrossRef]
- Gawish, R.A.; Samy, E.M.; Aziz, M.M. Ferulic acid protects against gamma-radiation induced liver injury via regulating JAK/STAT/Nrf2 pathways. Arch. Biochem. Biophys. 2024, 753, 109895. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xue, X.; Fan, G.; Gu, Y.; Zhou, F.; Zheng, Q.; Liu, R.; Li, Y.; Ma, B.; Li, S.; et al. Ferulic Acid Ameliorates Hepatic Inflammation and Fibrotic Liver Injury by Inhibiting PTP1B Activity and Subsequent Promoting AMPK Phosphorylation. Front. Pharmacol. 2021, 12, 754976. [Google Scholar] [CrossRef]
- Nouri, A.; Ghatreh-Samani, K.; Amini-Khoei, H.; Najafi, M.; Heidarian, E. Ferulic acid exerts a protective effect against cyclosporine-induced liver injury in rats via activation of the Nrf2/HO-1 signaling, suppression of oxidative stress, inflammatory response, and halting the apoptotic cell death. J. Biochem. Mol. Toxicol. 2023, 37, e23427. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, C. Ferulic acid from Angelica sinensis (Oliv.) Diels ameliorates lipid metabolism in alcoholic liver disease via AMPK/ACC and PI3K/AKT pathways. J. Ethnopharmacol. 2025, 338, 119118. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Sair, A.T.; Li, T.; Liu, R.H. Ferulic acid restores mitochondrial dynamics and autophagy via AMPK signaling pathway in a palmitate-induced hepatocyte model of metabolic syndrome. Sci. Rep. 2024, 14, 18970. [Google Scholar] [CrossRef]
- Li, Y.; Sair, A.T.; Zhao, W.; Li, T.; Liu, R.H. Ferulic Acid Mediates Metabolic Syndrome via the Regulation of Hepatic Glucose and Lipid Metabolisms and the Insulin/IGF-1 Receptor/PI3K/AKT Pathway in Palmitate-Treated HepG2 Cells. J. Agric. Food Chem. 2022, 70, 14706–14717. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Ma, R.; He, Y.; Fang, Q.; Xie, G.; Qi, M. Ferulic acid ameliorates renal injury via improving autophagy to inhibit inflammation in diabetic nephropathy mice. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 153, 113424. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Helal, S.A. Upregulation of PPAR-γ mediates the renoprotective effect of omega-3 PUFA and ferulic acid in gentamicin-intoxicated rats. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 99, 504–510. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Mir, S.M.; Ravuri, H.G.; Pradhan, R.K.; Narra, S.; Kumar, J.M.; Kuncha, M.; Kanjilal, S.; Sistla, R. Ferulic acid protects lipopolysaccharide-induced acute kidney injury by suppressing inflammatory events and upregulating antioxidant defenses in Balb/c mice. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 100, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Huang, M.; Mei, Y.; Hu, C.; Huang, C.; Zhang, H.; Wei, X.; Gao, Y.; Ma, Z. Ferulic Acid Interferes with Radioactive Intestinal Injury Through the DJ-1-Nrf2 and Sirt1-NF-κB-NLRP3 Pathways. Molecules 2024, 29, 5072. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; AnjomShoa, M.; Rafieian-Kopaei, M. Ferulic acid ameliorates ulcerative colitis in a rat model via the inhibition of two LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways and thus alleviating the inflammatory, oxidative and apoptotic conditions in the colon tissue. Inflammopharmacology 2023, 31, 2587–2597. [Google Scholar] [CrossRef]
- Yu, S.; Qian, H.; Zhang, D.; Jiang, Z. Ferulic acid relieved ulcerative colitis by inhibiting the TXNIP/NLRP3 pathway in rats. Cell Biol. Int. 2023, 47, 417–427. [Google Scholar] [CrossRef]
- Sadar, S.S.; Vyawahare, N.S.; Bodhankar, S.L. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI J. 2016, 15, 482–499. [Google Scholar] [PubMed]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Song, J.; Wang, N.; Liu, Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2019, 35, 112–121. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, T.; Hou, J.; Li, M. Ferulic acid alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Immunopharmacol. Immunotoxicol. 2020, 42, 156–164. [Google Scholar] [CrossRef]
- Lo, H.Y.; Li, C.C.; Cheng, H.M.; Liu, I.C.; Ho, T.Y.; Hsiang, C.Y. Ferulic acid altered IL-17A/IL-17RA interaction and protected against imiquimod-induced psoriasis-like skin injury in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 129, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. Improving the Effect of Ferulic Acid on Inflammation and Insulin Resistance by Regulating the JNK/ERK and NF-κB Pathways in TNF-α-Treated 3T3-L1 Adipocytes. Nutrients 2024, 16, 294. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Tan, P.; Ran, Y.; Guan, Y.; Qian, S.; Feng, X.; Jiang, Y.; Peng, Y.; Sheng, K.; et al. Ferulic acid suppresses the inflammation and apoptosis in Kawasaki disease through activating the AMPK/mTOR/NF-κB pathway. Front. Pharmacol. 2024, 15, 1420602. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Xu, X.; Zhao, X.; Han, Z.; Liu, D.; Bo, R.; Li, J.; Liu, Z. Ferulic acid inhibits LPS-induced apoptosis in bovine mammary epithelial cells by regulating the NF-κB and Nrf2 signalling pathways to restore mitochondrial dynamics and ROS generation. Vet. Res. 2021, 52, 104. [Google Scholar] [CrossRef]
- Mei, Z.; Hong, Y.; Yang, H.; Cai, S.; Hu, Y.; Chen, Q.; Yuan, Z.; Liu, X. Ferulic acid alleviates high fat diet-induced cognitive impairment by inhibiting oxidative stress and apoptosis. Eur. J. Pharmacol. 2023, 946, 175642. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.N.; Li, X.; Shi, H.M.; Zheng, Y.C.; Chang, S.Q.; Gao, L.; Zhao, G.P. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Z.; Yang, Z.; Wang, M.; Zhang, W.; Ren, Y.; Li, L.; Hu, J.; Sun, Z.; Nie, S. Ferulic acid positively modulates the inflammatory response to septic liver injury through the GSK-3β/NF-κB/CREB pathway. Life Sci. 2021, 277, 119584. [Google Scholar] [CrossRef]
- Ermis, A.; Aritici Colak, G.; Acikel-Elmas, M.; Arbak, S.; Kolgazi, M. Ferulic Acid Treats Gastric Ulcer via Suppressing Oxidative Stress and Inflammation. Life 2023, 13, 388. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 7910–7921. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.Y.; Wang, X.T.; Xu, H.L.; Yang, Z.L.; Cheng, Y.; Zhou, B. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats. Food Funct. 2020, 11, 3706–3718. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, X.; Yang, Z.; Hu, Z.; Li, Q.; Dong, J.; Fu, M. Ferulic acid attenuates difenoconazole exposure induced liver injury in carp by modulating oxidative damage, inflammation and apoptosis. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2024, 280, 109885. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, M.; Pan, E.; Li, Y.; Yan, W.; Li, Y.; Ji, G.; Dong, J. Protective effect of feed additive ferulic acid on respiratory depression and oxidation imbalance of carp induced by pesticide difenoconazole via ROS/NF-κB/NLRP3 axis. Fish Shellfish Immunol. 2024, 151, 109659. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Daryagasht, M.; Moosavi, M.; Khorsandi, L.; Azadnasab, R.; Khodayar, M.J. Hepatoprotective and anti-hyperglycemic effects of ferulic acid in arsenic-exposed mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 178, 113924. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, D.H.; Ahn, J.; Lee, H.; Choi, W.H.; Jang, Y.J.; Ha, T.Y. γ-Oryzanol Enhances Adipocyte Differentiation and Glucose Uptake. Nutrients 2015, 7, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Xiang, H.C.; Lin, L.X.; Hu, X.F.; Zhu, H.; Li, H.P.; Zhang, R.Y.; Hu, L.; Liu, W.T.; Zhao, Y.L.; Shu, Y.; et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J. Neuroinflamm. 2019, 16, 34. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Ma, H.; Meng, Z.; Zhou, L.; Feng, H.; Wu, X.; Xin, Y.; Dong, J.; Li, Y. Ferulic acid attenuated difenoconazole-induced immunotoxicity in carp by inhibiting TRAF/TAK1/NF-κB, Nrf2 and p53 pathways. Ecotoxicol. Environ. Saf. 2023, 262, 115339. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Tang, F.; Wang, Y.; Hemmings, B.A.; Rüegg, C.; Xue, G. PKB/Akt-dependent regulation of inflammation in cancer. Semin. Cancer Biol. 2018, 48, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Antioxidant Properties, Metabolism, Application and Mechanism of Ferulic Acid in Medicine, Food, Cosmetics, Livestock and Poultry. Antioxidants 2024, 13, 853. [Google Scholar] [CrossRef]
- Sun, X.; Ma, L.; Li, X.; Wang, J.; Li, Y.; Huang, Z. Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-κB axis. Front. Immunol. 2022, 13, 976729. [Google Scholar] [CrossRef] [PubMed]
- Reglero-Real, N.; Colom, B.; Bodkin, J.V.; Nourshargh, S. Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2048–2057. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H. Ferulic acid confers protection on islet β cells and placental tissues of rats with gestational diabetes mellitus. Cell. Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, Z.; Zhang, G.; Zhang, Z.; Sun, F.; Han, D.; Wang, J.; Zhao, J. Ferulic acid mediates microbial fermentation of arabinoxylan to enhance host immunity by suppressing TLR4/NF-κB signaling. Int. J. Biol. Macromol. 2025, 298, 139810. [Google Scholar] [CrossRef]
- Huang, M.; Ye, A.; Zhang, H.; Chen, J.; Yang, T.; Wei, X.; Gao, Y.; Ma, Z. Ferulic Acid Alleviates Radiation-Induced Immune Damage by Acting on JAK/STAT Signaling Pathway. Pharmaceuticals 2024, 17, 1175. [Google Scholar] [CrossRef]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Kopp, K.O.; Glotfelty, E.J.; Li, Y.; Greig, N.H. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol. Res. 2022, 186, 106550. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Temple, S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Mhillaj, E.; Catino, S.; Miceli, F.M.; Santangelo, R.; Trabace, L.; Cuomo, V.; Mancuso, C. Ferulic Acid Improves Cognitive Skills Through the Activation of the Heme Oxygenase System in the Rat. Mol. Neurobiol. 2018, 55, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y.; Li, J.N.; Liu, W.L.; Huang, Q.; Li, W.X.; Tan, Y.H.; Liu, F.; Song, Z.H.; Wang, M.Y.; Xie, N.; et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurother. J. Am. Soc. Exp. Neurother. 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, J.E.; Lee, S.Y.; Lee, E.J.; Kim, D.S.; Moon, S.J.; Lee, J.; Kwok, S.K.; Park, S.H.; Cho, M.L. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, N.; Fang, K.; Chang, X. 2-Deoxy-D-glucose Alleviates Collagen-Induced Arthritis of Rats and Is Accompanied by Metabolic Regulation of the Spleen and Liver. Front. Immunol. 2021, 12, 713799. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Jiang, W.; Zhou, M.; Li, H.; Chen, Y.; Jiang, F.; Hu, Q. Targeting BRD4 prevents acute gouty arthritis by regulating pyroptosis. Int. J. Biol. Sci. 2020, 16, 3163–3173. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Sin Singer Brugiolo, A.; Carvalho Gouveia, A.C.; de Souza Alves, C.C.; de Castro, E.S.F.M.; Esteves de Oliveira, É.; Ferreira, A.P. Ferulic acid supresses Th2 immune response and prevents remodeling in ovalbumin-induced pulmonary allergy associated with inhibition of epithelial-derived cytokines. Pulm. Pharmacol. Ther. 2017, 45, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Abulaiti, K.; Aikepa, M.; Ainaidu, M.; Wang, J.; Yizibula, M.; Aikemu, M. Metabolomics combined with network pharmacology reveals anti-asthmatic effects of Nepeta bracteata on allergic asthma rats. Chin. Herb. Med. 2024, 16, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.L.; Dungan, M.M.; Smart, C.D.; Madhur, M.S.; Doran, A.C. Inflammation Resolution in the Cardiovascular System: Arterial Hypertension, Atherosclerosis, and Ischemic Heart Disease. Antioxid. Redox signaling 2024, 40, 292–316. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Figueira, M.E.; Minniti, G.; de Carvalho, G.M.; de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; de Góes Corrêa, N.; Dogani Rodrigues, V.; de Alvares Goulart, R.; et al. Medicinal Plants, Phytochemicals and Regulation of the NLRP3 Inflammasome in Inflammatory Bowel Diseases: A Comprehensive Review. Metabolites 2023, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Amić, A.; Dimitrić Marković, J.M.; Marković, Z.; Milenković, D.; Milanović, Ž.; Antonijević, M.; Mastiľák Cagardová, D.; Rodríguez-Guerra Pedregal, J. Theoretical Study of Radical Inactivation, LOX Inhibition, and Iron Chelation: The Role of Ferulic Acid in Skin Protection against UVA Induced Oxidative Stress. Antioxidants 2021, 10, 1303. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Wang, X.; Yan, X.; Guo, Q.; Yue, Y.; Yue, T.; Yuan, Y. The bioaccessibility, bioavailability, bioactivity, and prebiotic effects of phenolic compounds from raw and solid-fermented mulberry leaves during in vitro digestion and colonic fermentation. Food Res. Int. 2023, 165, 112493. [Google Scholar] [CrossRef]
- Bayer, J.; Högger, P. Review of the pharmacokinetics of French maritime pine bark extract (Pycnogenol(®)) in humans. Front. Nutr. 2024, 11, 1389422. [Google Scholar] [CrossRef]
- Jiang, C.; Liang, G.; Ren, Y.; Xu, T.; Song, Y.; Jin, W. An UPLC-MS/MS Method for Simultaneous Quantification of the Components of Shenyanyihao Oral Solution in Rat Plasma. BioMed Res. Int. 2020, 2020, 4769267. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, Y.; Mi, S.; Wang, N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011, 137, 562–567. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Tang, Y.; Wang, H.; Huang, M.; Qian, D.; Duan, J.A. Pharmacokinetic comparison of ferulic acid in normal and blood deficiency rats after oral administration of Angelica sinensis, Ligusticum chuanxiong and their combination. Int. J. Mol. Sci. 2012, 13, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Tang, Z.; Xu, Y.; Li, X.; Wang, Q. Pharmacokinetic Study of Ferulic Acid Following Transdermal or Intragastric Administration in Rats. AAPS PharmSciTech 2020, 21, 169. [Google Scholar] [CrossRef]
- Achour, M.; Saguem, S.; Sarriá, B.; Bravo, L.; Mateos, R. Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems. J. Sci. Food Agric. 2018, 98, 3741–3751. [Google Scholar] [CrossRef]
- Kishida, K.; Matsumoto, H. Urinary excretion rate and bioavailability of chlorogenic acid, caffeic acid, p-coumaric acid, and ferulic acid in non-fasted rats maintained under physiological conditions. Heliyon 2019, 5, e02708. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhu, Y.; Li, Y.; Chang, X.; Lin, J.; Chen, L.; Lyu, Q.; Chen, X.; Ding, W. Examination of the Bioavailability and Bioconversion of Wheat Bran-Bound Ferulic Acid: Insights into Gastrointestinal Processing and Colonic Metabolites. J. Agric. Food Chem. 2025, 73, 1331–1344. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huangfu, C.; Bai, Z.; Zhu, L.; Shen, P.; Wang, N.; Li, G.; Deng, H.; Ma, Z.; Zhou, W.; et al. Multifunctional carbomer based ferulic acid hydrogel promotes wound healing in radiation-induced skin injury by inactivating NLRP3 inflammasome. J. Nanobiotechnol. 2024, 22, 576. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, L.; Hu, Y.N.; Dai, J.L.; Ma, B.; Tang, Q.F.; Tan, X.M. Self-Microemulsifying Drug Delivery System for Improved Oral Delivery and Hypnotic Efficacy of Ferulic Acid. Int. J. Nanomed. 2020, 15, 2059–2070. [Google Scholar] [CrossRef]
- Du, K.; Fang, X.; Li, Z. Ferulic acid suppresses interleukin-1β-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1α signaling pathway. Immun. Inflamm. Dis. 2021, 9, 710–720. [Google Scholar] [CrossRef]
- Singh, S.S.B.; Patil, K.N. trans-ferulic acid attenuates hyperglycemia-induced oxidative stress and modulates glucose metabolism by activating AMPK signaling pathway in vitro. J. Food Biochem. 2022, 46, e14038. [Google Scholar] [CrossRef] [PubMed]
- Nankar, R.; Prabhakar, P.K.; Doble, M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomed. Int. J. Phytother. Phytopharm. 2017, 37, 10–13. [Google Scholar] [CrossRef] [PubMed]

| Diseases | Models | Targets | Reference |
|---|---|---|---|
| Retinal Degeneration | In vitro BV-2 microglial cells vivo RD10 mice | STAT-1, TNF-α, IL-1β, NO, iNOS ↓ | [18] |
| Retinal Degeneration | In vivo retinal degeneration pigmented rabbits | Activating Nrf2/HO-1 pathway MCP-1, IL-8, NF-κB ↓ | [19] |
| Depression | In vivo CUMS mice | Inhibiting NF-κB pathway NLRP3, IL-1β, IL-6, TNF-α ↓ | [20] |
| Neuroinflammation | In vitro BV-2 microglial cells | NLRP3, iNOS, NO, COX-2, ROS, IL-6, IL-1β ↓ | [21] |
| Neuroinflammation | In vitro BV-2 microglial cells vivo brain injury mice | NLRP3, IL-1β ↓ | [22] |
| Neuroinflammation | In vitro BV-2 microglial cells | Activating AMPK/mTOR pathway NLRP3, IL-1β, IL-6, TNF-α, ROS ↓ | [23] |
| Neuroinflammation | In vitro BV-2 microglial cells vivo neuroinflammation mice | NF-κB, iNOS, COX-2, TNF-α, IL-1β, ROS ↓ | [24] |
| Neuroinflammation | In vitro microglial cells | IL-10 ↑ IL-1β ↓ | [25] |
| Neuroinflammation | In vivo neuroinflammation mice | NLRP3, IL-6, TNF-α, IL-1β ↓ | [26] |
| Neurodegeneration | In vivo aging mice | NF-κB, IL-1β, NO ↓ | [27] |
| AD | In vitro BV-2 microglial cells | IL-1β, IL-6, TNF-α ↓ | [28] |
| PD | In vivo PD rats | NF-κB, NO ↓ | [29] |
| Sciatica | In vitro GMI-R1 cells vivo CCI rats | Inhibiting RhoA/p38MAPK pathway Reduced inflammatory cell infiltration PGE2, IL-1β, IL-6, TNF-α, iNOS ↓ IL-10 ↑ | [30] |
| Arthritic | In vivo arthritic rats | Inhibiting JAK/STAT pathway TNF-α ↓ TGF-β ↑ | [31] |
| RA | In vitro AA-FLS and BMCs | IL-17, IL-23 ↓ | [32] |
| RA | In vitro BMCs and RAW264.7 cells | Inhibiting NF-κB pathway | [33] |
| RA | In vivo CIA mice | Inhibiting NF-κB pathway | [34] |
| Acute Gouty Arthritis | In vivo acute gouty arthritis rats | NLRP3, NF-κB p65, TNF-α, IL-1β, NO ↓ | [35] |
| ALI | In vitro MLE-12 cells vivo ALI mice | Activating Nrf2/HO-1 pathway | [36] |
| ARDS | In vivo ARDS rats | Inhibiting MAPK pathway TNF-α, IL-1β, IL-6 ↓ IL-10 ↑ | [37] |
| AS | In vitro C3H10T1/2 cell line, RAW264.7, EA.hy926 cells vivo AS mice | NLRP3, TNF-α, IL-1β, IL-6 ↓ | [38] |
| AS | In vivo AS mice | Activating AMPK | [39] |
| Cardiac Damage | In vitro H9c2 cell line and ARVM vivo cardiac dysfunction mice | Activating SIRT1 | [40] |
| Acute Liver Injury | In vivo acute liver injury rats | Inhibiting JAK/STAT pathway Activating Nrf2 pathway ROS ↓ | [41] |
| Liver Fibrosis | In vitro MPHs, RAW264.7 cells, LX-2 cells vivo fibrotic mice | Inhibiting NF-κB pathway Activating AMPK PTP1B, TNF-α, IL-1β ↓ | [42] |
| Hepatic Injury | In vivo hepatic injury rats | Activating Nrf2/HO-1 pathway NF-κB, TNF-α, IL-1β ↓ | [43] |
| ALD | In vitro HepG2 cells vivo ALD mice | Activating AMPK and PI3K/AKT pathway | [44] |
| MetS | In vitro HepG2 cells | Activating AMPK pathway | [45] |
| MetS | In vitro HepG2 cells | Activating PI3K/AKT pathway PPARγ ↑ | [46] |
| DN | In vitro NRK-52E cells vivo DN rats | Inhibiting MAPK and NF-κB pathway ROS, NO, IL-1β, IL-6, TNF-α, COX-2, iNOS↓ | [47] |
| DN | In vivo DN mice | NLRP3, TNF-α ↓ | [48] |
| Nephrotoxicity | In vivo nephrotoxicity rats | PPARγ ↑ | [49] |
| Nephrotoxicity | In vivo nephrotoxicity rats | Activating Nrf2/ARE/HO-1 pathway NF-κB, NLRP3, ROS ↓ PPARγ ↑ | [50] |
| AKI | In vivo AKI mice | Inhibiting NF-κB pathway Activating Nrf2/HO-1 pathway TNF-α, IL-1β, iNOS, COX-2 ↓ Reduced inflammatory cell infiltration | [51] |
| Intestinal Injury | In vivo intestinal injury mice | NF-κB NLRP3 IL-18 IL-1β ↓ | [52] |
| UC | In vivo UC rats | Inhibiting NF-κB pathway iNOS NO ↓ | [53] |
| UC | In vitro HIMECs vivo UC rats | NLRP3 IL-6 IL-12 IL-1β ↓ | [54] |
| UC | In vivo UC rats | TNF-α IL-1β IL-6 COX-2 iNOs ↓ | [55] |
| Intestinal Epithelial Barrier Dysfunction | In vitro IEC-6 cells | Activating Nrf2/HO-1 pathway ROS, NO ↓ | [56] |
| Atopic Dermatitis | In vitro THP-1 cells vivo atopic dermatitis mice | Inhibiting NF-κB pathway IgE TNF-α IL-6 ↓ | [57] |
| Psoriasis | In vivo psoriasis-like skin injury mice | IL-23 IL-1β ↓ | [58] |
| Endometritis | In vitro BEECs | Inhibiting NF-κB and MAPK pathway IL-1β, IL-6, TNF-α, IL-8 ↓ | [59] |
| Inflammation | In vitro 3T3-L1 adipocytes and RAW264.7 cells | Inhibiting JNK/ERK and NF-κB pathway TNF-α, IL-6, IL-1β, MCP-1↓ | [60] |
| KD | In vitro HUVECs vivo KD mice | Activating AMPK/mTOR pathway Inhibiting NF-κB pathway IL-1β, IL-6, TNF-α, CXCL10 ↓ | [61] |
| Mastitis | In vitro BMECs | Activating Nrf2 IL-1β, IL-6, TNF-α, ROS, COX-2, NF-κB ↓ | [62] |
| Cognitive Impairment | In vitro HT22 cells vivo cognitive impairment mice | Activating IRS1/PI3K/AKT/GSK-3β pathway | [63] |
| Diseases | Models | Concentration | Cytotoxicity | Assay | Reference |
|---|---|---|---|---|---|
| Neuroinflammation | BV-2 microglial cells | 19, 38, 76, 152 μM | Nontoxicity | CCK-8 | [21] |
| Neuroinflammation | BV-2 microglial cells | 2.5, 5, 10 μM | Nontoxicity | CCK-8 | [22] |
| Neuroinflammation | BV-2 microglial cells | 40, 80, 160μM | Nontoxicity | MTT | [23] |
| Neuroinflammation | BV-2 microglial cells | 10, 100 μM | Not mentioned | __ | [24] |
| AD | BV-2 microglial cells | 55 μM | Nontoxicity | MTT | [28] |
| Sciatica | GMI-R1 cells | 2 μM | Nontoxicity | CCK-8 | [30] |
| RA | AA-FLS | 25, 50, 100 μM | ≥100 μM | MTT | [32] |
| RA | RAW264.7 cells | 25, 50, 100 μM | ≥100 μM | MTT | [33] |
| Osteoarthritis | Primary chondrocytes patients | 5, 10 μM | ≥30 μM | CCK-8 | [130] |
| ALI | MLE-12 cells | 0.1 μM | Not mentioned | __ | [36] |
| Liver Fibrosis | MPHs | 25 μM | ≥100 μM | CCK-8 | [42] |
| RAW264.7 cells | 100 μM | Nontoxicity | CCK-8 | ||
| LX-2 cells | 25 μM | ≥50 μM | CCK-8 | ||
| ALD | HepG2 cells | 50, 100 μM | ≥200 μM | MTT | [44] |
| MetS | HepG2 cells | 50, 100, 200 μM | Not mentioned | __ | [45] |
| MetS | HepG2 cells | 50, 100, 200 μM | ≥1 mM | methylene blue | [46] |
| DN | NRK-52E cells | 75 μM | ≥100 μM | MTT | [47] |
| UC | HIMECs | 125, 250, 500 μM | Not mentioned | __ | [54] |
| Intestinal Epithelial Barrier Dysfunction | IEC-6 cells | 5, 10, 20 μM | Not mentioned | __ | [56] |
| Cardiac Damage | H9c2 cell line | 5 μM | Nontoxicity | FDA | [40] |
| Atopic Dermatitis | THP-1 cells | 5, 10 μM | Nontoxicity | TUNEL | [57] |
| Endometritis | BEECs | 40, 80, 120 μM | Nontoxicity | MTT | [59] |
| Inflammation | 3T3-L1 adipocytes | 1, 10, 50 μM | Nontoxicity | MTT | [60] |
| KD | HUVECs | 20 μM | Nontoxicity | CCK-8 | [61] |
| Cognitive Impairment | HT22 cells | 150, 300, 600 μM | Nontoxicity | CCK-8 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Guan, Y.; Yang, L.; Fang, H.; Sun, H.; Sun, Y.; Yan, G.; Kong, L.; Wang, X. Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications. Pharmaceuticals 2025, 18, 912. https://doi.org/10.3390/ph18060912
Liu J, Guan Y, Yang L, Fang H, Sun H, Sun Y, Yan G, Kong L, Wang X. Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications. Pharmaceuticals. 2025; 18(6):912. https://doi.org/10.3390/ph18060912
Chicago/Turabian StyleLiu, Jiaying, Yu Guan, Le Yang, Heng Fang, Hui Sun, Ye Sun, Guangli Yan, Ling Kong, and Xijun Wang. 2025. "Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications" Pharmaceuticals 18, no. 6: 912. https://doi.org/10.3390/ph18060912
APA StyleLiu, J., Guan, Y., Yang, L., Fang, H., Sun, H., Sun, Y., Yan, G., Kong, L., & Wang, X. (2025). Ferulic Acid as an Anti-Inflammatory Agent: Insights into Molecular Mechanisms, Pharmacokinetics and Applications. Pharmaceuticals, 18(6), 912. https://doi.org/10.3390/ph18060912







