Abstract
Locametz®/Illuccix®/GozellixTM (Novartis AG (Basel, Switzerland) and Telix Pharmaceuticals, Ltd. (Melbourne, Australia), all three [68Ga]Ga-PSMA-11), Pylarify®/Pylclari® (Progenics Pharmaceuticals, Inc. (New York, USA) and Curium PET France SA (Paris, France), both [18F]DCFPyL), Radelumin® (ABX GmbH (Radeberg, Germany), [18F]PSMA-1007), and Posluma® (Blue Earth Diagnostics, Ltd. (Oxford, UK), [18F]rhPSMA-7.3) are four approved PSMA-PET imaging agents that have significantly advanced the diagnosis and management of prostate cancer. These agents offer a new level of precision and accuracy, enabling clinicians to detect prostate cancer with enhanced sensitivity. As a result, they play a critical role in improving detection, staging, and management, ultimately enhancing clinical outcomes for patients. Their use in routine clinical practice is expected to increase diagnostic precision and provide clearer pathways for personalized therapy. This review offers a comprehensive chemical, pharmaceutical, and medicinal overview, discusses comparative studies, and highlights additional highly relevant candidates for prostate cancer detection.
Keywords:
prostate cancer; PSMA; PET diagnostics; fluorine-18; gallium-68; FDA approval; EMA approval; Locametz®; Illuccix®; GozellixTM; Pylarify®; Pylclari®; Radelumin®; Posluma® 1. Introduction
Prostate cancer (PCa) remains a significant public health challenge globally. It is the second most common cancer diagnosed in men worldwide (14.2%), behind lung cancer (15.3%) [1], and it is the most frequently diagnosed cancer in 118 of the world’s 185 countries. Estimates indicate that the numbers of new cases will increase annually from 1.4 million in 2020 to 2.9 million in 2040 [2]. Although PCa is highly prevalent, it ranks fifth among the leading causes of cancer-related deaths (7.3%) worldwide. The impact of PCa varies among different regions. It is the leading cause of cancer-related deaths in 52 countries, with some of the highest mortality rates in the Caribbean and sub-Saharan Africa, highlighting significant disparities in early detection, prevalence of risk factors and treatment availability [1]. Notably, Black men have the highest cancer mortality rates, which are two-to-four times higher than those of other ethnic groups [3,4]. PCa is a complex and heterogeneous disease, characterized by multiple genomic alterations [5,6,7]. In its early stages, it is often asymptomatic, with symptoms typically appearing only when the disease is already advanced, thus limiting treatment options and decreasing the chances of survival. This highlights the importance of early and accurate detection for a more favorable prognosis.
PCa is usually diagnosed by a combination of different methods, digital rectal examination (DRE), a blood test to measure prostate-specific antigen (PSA) levels, and transrectal ultrasound-guided (TRUS) prostate biopsy, which provides the final confirmation. The DRE was the only screening test for PCa until the discovery of PSA [8,9]. However, its performance for the initial detection of PCa is limited and there is a high degree of interobserver variability [8,10]. PSA is a serine protease, discovered in 1971, and its physiological function is to liquefy seminal fluid. High serum PSA levels are associated with PCa, and PSA is widely recognized as a biomarker for the early detection, staging and monitoring of response to treatment. Serum PSA measurement is specific for the prostate but is not specific for PCa, as benign prostate diseases, age, body mass index, and race also significantly influence blood PSA levels [11]. The blood PSA levels cannot be used as a screening test for PCa alone and TRUS prostate biopsy has been the standard diagnostic test to confirm PCa [7,12]. TRUS is widely used due to its accessibility and cost; however, the diagnostic accuracy is highly dependent on the operator and random needle placement relative to the tumor, leading to the risk of sampling error and high rate of false negative results (15 to 46%) [7]. Magnetic resonance imaging (MRI) and MRI-fusion-guided biopsy has been used to improve diagnostic accuracy in PCa with positive results; however, studies have shown that MRI fails in the detection of all cancer sites and, in some cases, underestimates the tumor size, leading to inadequate treatment [12,13]. The lack of accurate diagnostic methods for PCa has resulted in unnecessary biopsies and overdiagnosis, highlighting the urgent need for alternative diagnostic modalities with greater specificity and sensitivity.
The use of positron emission tomography (PET) with prostate-specific membrane antigen (PSMA) imaging is revolutionizing the management of PCa, making diagnosis more accurate and treatment more effective [14,15]. PSMA is a transmembrane glycoprotein, first identified in the late 1980s by Horoszewicz and colleagues [16]. PSMA—also called glutamate carboxypeptidase II (GCPII), N-acetyl-aspartylglutamate (NAAG) peptidase or N-acetyl-l-aspartyl-l-glutamate peptidase I (NAALADase I) and folate hydrolase (FOLH1)—is involved in the enzymatic process of glutamate release, folate hydrolase activity and several enzymatic functions [17]. It is primarily expressed in the prostate, with lower levels found in the salivary gland, brain, small intestine and kidney, and is implicated in PCa and neurological disorders. PSMA is overexpressed in PCa cells, in the primary tumor and in metastases, as well as in the angiogenic vasculature of several solid tumors, including lung, breast, kidney, bladder, ovarian and colon cancers, as well as glioblastomas [18,19]. In the last two decades, PSMA has become a valuable target for the diagnosis and therapy of PCa due to its high expression in malignant prostate cells. Its expression levels correlate with tumor progression, cancer aggressiveness and poor prognosis, making it a biomarker for disease assessment and therapy.
PSMA-targeted imaging using PET allows for the simultaneous detection of primary tumors and metastases with higher accuracy than conventional methods. PET techniques offer high sensitivity and specificity, giving a good overview of the disease and leading to a more accurate diagnosis. These advantages play a critical role in early diagnosis, therapy monitoring, and understanding disease mechanisms, transforming PCa diagnostics. Currently, there are four PSMA-targeting radiotracers approved for the PET imaging of PSMA-positive PCa. The FDA approved three [68Ga]Ga-PSMA-11 kits: Illuccix® in December 2021, Locametz® in March 2022, and GozellixTM in March 2025. Until now, in Europe, only Locametz® has been commercially available (approved by the EMA in December 2022), but the EMA issued a positive opinion for Illuccix® in January 2025 as well. [18F]DCFPyL received FDA approval in May 2021 (Pylarify®), and in July 2023 in Europe (Pylclari®). [18F]rhPSMA-7.3 (Posluma®) was approved by the FDA in May 2023. Finally, [18F]PSMA-1007 (Radelumin®) was initially approved in France in December 2022, with subsequent approvals in several other European countries. In general, PET imaging targeting PSMA is indicated to assess the presence of metastases in patients with PCa before treatment begins, to evaluate PCa recurrence in patients with high PSA levels after treatment and to determine PSMA expression in cancer cells to guide eligibility for PSMA-targeted therapy. The approval of these radiopharmaceuticals for clinical use represents a significant advance in the diagnosis, treatment and management of PCa, enabling earlier detection of metastatic and recurrent disease. PSMA-targeted PET imaging has the potential to greatly influence clinical decisions and optimize treatment planning for patients. In this review, we discuss the four radiotracers approved for clinical use, highlighting the chemical characteristics, radionuclides, clinical applications and future prospects.
2. Chemical Overview
2.1. Names, Chemical Structures and Properties
Before being introduced as commercially available PET radiotracers with trade names, all PSMA inhibitors were initially referred to by working names, many of which are still widely used. Table 1 summarizes these various names, along with their respective manufacturers.

Table 1.
Names and companies of approved PSMA-targeting PET radiotracers.
The IUPAC names of the radiotracers are as follows:
- [18F]PSMA-1007: (3S,10S,14S)-1-[4-[[(2S)-4-carboxy-2-[(2S)-4-carboxy-2-(6-[18F]fluoropyridin-3-amido)butanamido]butanamido]methyl]phenyl]-3-[(naphthalen-2-yl)methyl]-1,4,12-trioxo-2,5,11,13-tetraazahexadecane-10,14,16-tricarboxylic acid
- [68Ga]Ga-PSMA-11: [68Ga]gallium (3S,7S)-22-[3-[[[2-[[[5-(2-carboxyethyl)-2-hydroxyphenyl]-methyl](carboxymethyl)amino]ethyl](carboxymethyl)amino]-methyl]-4-hydroxyphenyl]-5,13,20-trioxo-4,6,12,19-tetraazadocosane-1,3,7-tricarboxylic acid
- [18F]DCFPyL: 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)amino]-pentyl} [18]ureido)-pentanedioic acid
- [18F]rhPSMA-7.3: gallate(6-), [(4S,8S,13R,27R,30R,35S)-35-[4,10-bis[(carboxy-kO)me-thyl]-7-(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl-kN1,kN4,kN7,kN10]-30-[[[4-[bis(1,1dimethylethyl)fluoro-18F-silyl]benzoyl]amino]methyl]-1,36-dihydroxy-1,6,11,18,21,29,32,36-octaoxo-5,7,12,17,22,28,31-heptaazahexatriacontane-4,8,13,27-tetracarboxylato(9-)]-, hydrogen (1:6)
Figure 1 illustrates the chemical structures of the compounds. All four PET ligands are based on a urea motif which plays an important role in binding to PSMA. Although the radiotracers [68Ga]Ga-PSMA-11, [18F]DCFPyL, and [18F]PSMA-1007 share the Glu-NH-CO-NH-Lys binding motif to which, via different linkers, the radiolabeled moiety is attached, the chemical structure of [18F]rhPSMA-7.3 differs significantly. While the difference in the binding motif (Glu-NH-CO-NH-Glu) is slight, the rest of the molecule incorporates a chelator (DOTA-GA: 2-(4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclo-dodecan-1-yl)pentanedioic acid), as well as the silicon–fluoride acceptor (SiFA) moiety, offering two possibilities for radiolabeling: complexation of a radiometal such as gallium-68 (68Ga) or lutetium-177 (177Lu) with the DOTA-GA chelator or by the isotopic exchange reaction of fluorine-18 (18F) against fluorine-19 (19F) in the SiFA moiety [20]. This allows, in particular, for combining a diagnostic radiotracer and a therapeutic radiopharmaceutical in a single molecule, making it, theoretically, an ideal theranostic compound. For therapeutic applications, the radionuclide 177Lu is paired with non-radioactive 19F in the SiFA moiety, while for diagnostic purposes, 18F is combined with non-radioactive 175/176Lu in the chelating moiety. The combination of two radiolabeling possibilities tremendously enhances the complexity of the molecule, as well as its molecular weight (1537.3 g/mol vs. 441.4 g/mol for [18F]DCFPyL).
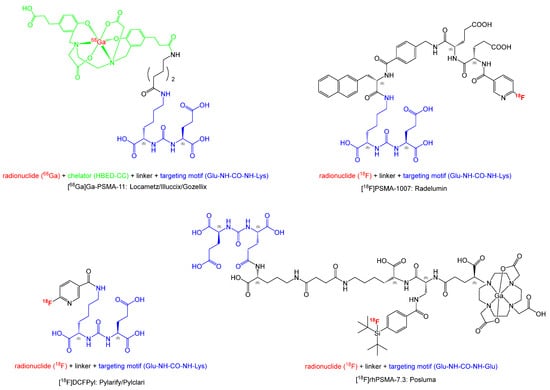
Figure 1.
Chemical structures of approved PSMA-targeting PET radiotracers.
Looking at the radionuclide, three of the four PET radiotracers discussed are labeled with 18F and one with 68Ga. For 68Ga labeling, a chelator is needed, which, in the case of PSMA-11, is known as HBED-CC (N,N′-bis [2-hydroxy-5-(carboxyethyl)benzyl]-ethylenediamine-N,N′-diacetic acid). This acts as a hexadentate chelator with octahedral geometry and coordinates the radiometal to two nitrogen atoms, two hydroxy groups and two carboxylic groups [21,22]. Depending on the reaction conditions, such as temperature, time, and pH of the reaction solution, up to three different diastereomers of [68Ga]Ga-PSMA-11 can be formed. Since preclinical studies have shown that the diastereomers do not bind differently to the PSMA receptor, the PET image should not be influenced by them [22].
Looking at the 18F-labeled radiotracers, [18F]PSMA-1007 and [18F]DCFPyL present many similarities: in both molecules, the 18F label is incorporated into a nicotinic acid moiety. Both radiotracers can be synthesized by the direct fluorination of an unprotected trimethylammonium labeling precursor, where a trimethylammonium-leaving group is substituted by 18F [23,24]. For [18F]rhPSMA-7.3, radiolabeling occurs in a completely different way by an isotopic exchange reaction where the natural 19F is replaced by the radioactive 18F in the SiFA group. This labeling approach enables fluorinations under very mild reaction conditions (e.g., room temperature), thus allowing for access to molecules that may not be stable under the harsher conditions required for classical fluorinations [25]. Furthermore, there is no need to separate the labeling precursor from the product, since it is the same molecule. This lowers the specific activity of the tracer solution and might have an effect on tracer uptake. In the case of [18F]rhPSMA-7, there are four possible isomers which show partially different behavior in vitro and in vivo. Due to high tumor accumulation and low binding in blood, liver and kidneys, [18F]rhPSMA-7.3 has been shown to be the preferred isomer [26].
2.2. Radionuclides
As radionuclides, 68Ga or 18F are employed for the approved PSMA radiotracers. It is not possible to state categorically which PET radionuclide is more suitable, as both have distinct advantages and limitations. When analyzing the physical characteristics of both radionuclides, different parameters can be considered, which impact the image quality [27]. Although both radionuclides decay primarily by positron emission, the positron yield of 68Ga is only 89.1% (decaying to the stable isotope zinc-68 (68Zn)) compared to 96.8% for 18F (decaying to stable oxygen-18 (18O)), resulting in a lower detection sensitivity of 68Ga. This can be counteracted by higher initial activities or longer scan times. Furthermore, the maximum and average β+ energy of both nuclides differ: the maximum β+ energy for 68Ga is 1899.1 keV vs. 633.5 keV for 18F, and the average β+ energy is 836 keV vs. 249.3 keV. These energies directly impact the spatial resolution resulting from the mean positron range in soft tissue which is 1.05 mm for 68Ga compared to 0.27 mm for 18F. Considering these parameters, 18F is the better radionuclide.
Regarding the half-lives of both radionuclides, 67.7 min for 68Ga and 109.7 min for 18F, the considerably shorter half-life of 68Ga has several implications. The synthesis and quality control processes must be performed more rapidly, as the tracer decays continuously during this time, thereby reducing the amount of injectable radiotracer available. As a rule of thumb, the time until the release of the radiotracer should not exceed one half-life. Usually, the synthesis of 68Ga-labeled radiotracers is fast, consisting of the complexation of the radiometal, rarely removal of protecting groups, and most of the time, purification can be accomplished by solid phase extraction. For classical radiofluorinations, the synthesis time, including steps such as drying of the [18F]fluoride, labeling reaction, deprotection and purification, for which frequently high-performance liquid chromatography (HPLC) is necessary, is often longer. Therefore, considering this, there is no major advantage for one or the other radionuclide. However, when it comes to distributing the radiotracer to external hospitals instead of using it exclusively in-house, this is typically not feasible with 68Ga-labeled tracers produced via a 68Ge/68Ga generator, due to the short half-life and limited production capacity. Furthermore, using one batch of radiotracer for more than 1–2 patients is not possible with generator-produced 68Ga-labeled radiotracers, in contrast to 18F-labeled radiotracers, where multiple patients can be imaged with one batch. In recent years, the production of 68Ga with a cyclotron has been under investigation, delivering promising results. The production of 68Ga with a cyclotron can either be carried out by irradiation of a solid or liquid target containing enriched 68Zn. For the solid target, up to 192 GBq 68Ga can be obtained at the end of purification, as well as 72 GBq at end of synthesis (EOS) and for the liquid target up to 3 GBq (EOS) [28]. Especially regarding the activity amount achievable by solid target, a distribution of the final radiotracer is feasible. That the production of 68Ga by cyclotron can be an important route is also demonstrated by the fact that already in 2021 a monograph “gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling” (01/2021:3109) was released in the European Pharmacopoeia [29]. In comparison, depending on the beam energy and beam current, up to 1110 GBq of [18F]fluoride (18 MeV proton beam @300 µA beam current) can be produced by irradiation of enriched H218O [30]. Most of the time, these high amounts of radioactivity are not needed, and by adjusting beam parameters, like beam time or current, a suitable amount can be produced. When using high amounts of 18F, the problem of radiolysis may arise, hampering the stability of the radiotracer [31].
Considering all these facts, 18F seems to be the best choice as a radionuclide. But there are still cases when 68Ga is superior: one case is the possibility that the chosen biomolecule might not be amenable to radiofluorination. Especially considering the relatively harsh reaction conditions required (e.g., high temperatures), the molecule might not be stable and also the introduction of fluorine via nucleophilic or electrophilic substitution into the molecule might not be possible. With the introduction of modern techniques like labeling by isotopic exchange in the SiFA moiety [25] or the introduction of fluorine in the form of an aluminium–[18F]fluoride–chelator complex [32], this limitation might be overcome in the future. But at least when looking at the [Al18F]2+ complexes, there are still a lot of optimizations to be carried out, especially regarding the automation of the synthesis, which is essential for GMP compliance of the production of tracers for human use.
The most significant advantage of ⁶⁸Ga lies in the availability of various authorized 68Ge/68Ga generators on the market which can be used for the synthesis of 68Ga-labeled radiotracers for human use. This is a tremendous benefit for all radiopharmacies without a cyclotron on-site. Since, at least in Europe, the radiolabeling precursor [18F]fluoride for radiolabeling counts as a pharmaceutical and not a starting material, it is not allowed to purchase it commercially from another cyclotron facility, as long as they do not have marketing authorization [33]. Although there are initiatives for a change in the European directive, it is not clear if and when this change will happen. As of now, e.g., in Germany, when applying for a manufacturing authorization for a 18F-labeled radiotracer, the [18F]fluoride must be produced in-house or purchased from a facility holding a marketing authorization, which so far does not exist. And since authorized 68Ge/68Ga generators are commercially available, this problem does not apply to 68Ga. On the generator column, the bound parent nuclide 68Ge (half-life 271 d) continuously decays to its daughter nuclide 68Ga via electron capture. The daughter can be eluted multiple times per day with diluted hydrochloric acid and used in the labeling reactions. Even though authorized generators are quite expensive, they can be used for up to one year, if the decline in radioactivity to less than half is acceptable. As long as the generator is used in compliance with the supplier’s instructions, there is a guarantee that the breakthrough of the parent nuclide 68Ge, which would be critical, will not occur within a year. Therefore, for facilities without a cyclotron, the use of 68Ga-labeled tracers is very beneficial and may represent the only option for GMP-compliant production of radiotracers for human use.
2.3. GMP-Compliant Synthesis
In contrast to radiotracer production for research purposes, the production of radiotracers for human use has to fulfill complete GMP compliance. An important aspect is the appropriate environment, which requires dedicated clean rooms for the complex production of the tracers: all production steps have to be conducted in a class C clean room environment and sterile filtration of the final product, as well as its portioning even in a class A environment. This can be a major problem for smaller facilities, when they do not have appropriate clean rooms. Another part of GMP-compliant production is the automation of the reaction and purification steps. This can be accomplished in automated synthesis units (ASUs) where all steps are conducted with a computer program from outside the hot cell. No manual interference is allowed. There are a lot of different ASUs from various suppliers commercially available. In order to rule out cross contamination, nowadays mostly cassette-based ASUs are used, facilitating the production of more than one tracer on one unit. For many routinely used PET radiotracers, these disposable cassettes are available either by the vendor of the ASU or by special suppliers. All four approved radiotracers can be produced in ASUs and several optimizations have been performed by various groups.
As described above, there are many similarities between [18F]DCFPyL and [18F]PSMA-1007, which also results in similar synthetic routes for their radiolabeling. For both molecules, there are two possible radiolabeling approaches. One of them is the use of the secondary labeling precursor 6-[18F]fluoronicotinic acid tetrafluorophenyl ester ([18F]F-Py-TFP) [23,34]. In a first step, this labeling precursor has to be produced and purified. Then, it can be conjugated to the molecule of interest under relatively mild conditions, followed, if necessary, by a deprotection step and further purification of the final product by HPLC. This complex sequence of reactions results in long production times (128 min for [18F]DCFPyL [34] and 80 min for [18F]PSMA-1007 [23]) and can be difficult to implement in an ASU. Furthermore, optimization concerning the synthesis of [18F]F-Py-TFP still requires an overall production time for [18F]DCFPyL of 56 min [35]. Therefore, whenever possible, a synthetic route using direct radiofluorination of a suitable labeling precursor is preferable. In the case of both radiotracers, this approach is feasible using an unprotected trimethylammonium precursor which has been implemented and optimized on various ASUs (see literature examples for [18F]DCFPyL [24,34,36] and [18F]PSMA-1007 [23,37,38,39]). By enabling purification of the final product using solid phase extraction (SPE) and avoiding time-consuming HPLC purification, total production times can be reduced to as short as 21 min for [18F]DCFPyL [24] and 40 min for [18F]PSMA-1007 [38]. These automated production processes can be easily adopted in suitably equipped radiopharmacies and demonstrate a very high reliability for routine productions.
In contrast to these two 18F-labeled radiotracers, the labeling strategy of [18F]rhPSMA-7.3 differs significantly since the radiofluorine is incorporated into the SiFA moiety by an isotopic exchange reaction. Unlike for the other radiotracers, not a lot of optimizations and automated synthesis strategies have been reported in the literature, only by the group of Wester et al. [40]. These exchange reactions can be conducted at room temperature and azeotropic drying of the [18F]fluoride, as required for classical radiofluorinations to obtain a reactive fluoride complex, can be omitted. The separation of [18F]fluoride from irradiated target water on an SPE cartridge, washing with anhydrous acetonitrile and elution with a kryptofix/potassium hydroxide solution are sufficient. Therefore, a total reaction time of 16 min has been reported for the production of [18F]rhPSMA-7.3. However, this type of reaction can present some pitfalls, as an update on SiFA reactions describes that the elution cocktail of kryptofix/potassium carbonate has a very limited stability which might lead to problems in the production [41]. Furthermore, when taking a look at the achievable specific activities of SiFA-based compounds, they are significantly lower than for 18F-labeled radiotracers from conventional radiofluorinations. This results from the fact, that in SiFA reactions the labeling precursor is the same compound as the labeled tracer, with the only difference being the incorporation of non-radioactive 1⁹F. When comparing the amount of carrier which results in the specific activity of the tracer, it is ≤3.42 µg/GBq for Posluma® (≤20 µg/mL in ≤5846 MBq/mL) [42]) vs. ≤0.27 µg/GBq for Pylarify® [43,44]. This may have an impact on the uptake of the tracers and the image interpretation.
For the production of [68Ga]Ga-PSMA-11, there are two different possibilities: production in an ASU similar to those described for 18F-radiotracers or the use of authorized sterile cold kits, such as those that have been used for radiolabeling with 99mTc for many years. When producing [68Ga]Ga-PSMA-11 in an automated synthesis, all rules for full GMP compliance have to be followed as explained above. This is not necessary for a preparation with sterile kits and an authorized sterile 68Ge/68Ga-generator. Since the synthesis takes place in a closed sterile vial, it only has to be guaranteed that the end product is sterile by preparation in a sterile environment like a laminar flow safety cabinet; a clean room is not mandatory. In typical automated synthesis, the elution and pre-purification of the 68Ga-eluate are followed by reaction with the labeling precursor PSMA-11 at elevated temperatures (e.g., 85 °C) and SPE purification, dilution and sterile filtration [45]. This results in production times of as short as 15 min and an RCY of 85.35 ± 5.78%. For this production route, every local radiopharmacy typically has to apply for some type of authorization with the local authorities since the decentralized production and distribution of the radiotracer are not feasible due to the short half-life of 68Ga. In order to simplify the entire labeling procedure and make it accessible to a larger number of locations, the production of [68Ga]Ga-PSMA-11 by use of sterile cold kits has been developed, and currently, there are three different approved kits on the market: Illuccix®/GozellixTM (both Telix Pharmaceuticals, Inc., Melbourne, Australia) and Locametz® (Novartis AG, Basel, Switzerland). Illuccix® has been the first kit to be approved by the FDA in 2021 [46] as well as in Canada and Australia, and recently, the EMA has given a positive approval statement in 2025 paving the path for use in Europe [47]. In March 2025, GozellixTM, an improved version offering a longer shelf life due to the addition of ascorbic acid as a stabilizer, has also received approval by the FDA [48]. Locametz® has been approved by the FDA and EMA in 2022 [49,50]. While the Locametz® kit consists of one sterile vial including everything needed for a successful production, the Illuccix® and GozellixTM kits have three vials (one containing reaction buffer, one PSMA-11 precursor, and one for elution of the generator) or two vials (reaction buffer, PSMA-11 precursor and an ampule with ascorbic acid), respectively, making their synthesis a bit more complicated. The production of [68Ga]Ga-PSMA-11 is described in the respective prescribing information of the kit [46,48,49,50], and is extremely easy to accomplish since it more or less consists of the elution of the generator, reaction at room temperature for 5 min and quality control of the end product. A very nice comparison of the Illuccix® and Locametz® kits, as well as optimizations and possible pitfalls of the labeling procedures, has been published recently [51]. Since all eluted radioactivity is available in the end product, the RCY is only diminished by the radioactive decay during synthesis and quality control. The required radiochemical purity of >95% is easily achieved. Even though the cold kits are quite expensive with costs well above EUR 1000 per kit, the cold kit production of [68Ga]Ga-PSMA-11 is by far the easiest and most accessible way to get a diagnostic PSMA radiotracer, especially since no manufacturing authorization is necessary but a notification for compassionate use at the local authorities is sufficient.
In contrast to 68Ga-labeled radiotracers, which must be produced on-site or nearby, 18F-labeled radiotracers can be shipped over considerable distances due to the longer half-life of 18F and the ability to produce 18F-labeled radiotracers with much higher activities. Therefore, shipping all three 18F-labeled PSMA tracers to other sites is entirely feasible, and all of the companies involved have multiple production sites to facilitate widespread distribution. While [18F]DCFPyL is available in large parts of the US [52] as well as Europe [53], [18F]rhPSMA-7.3 is distributed only in the US [54] and [18F]PSMA-1007 only in Europe [55].
2.4. End-Product Specifications and Quality Control
For patient safety, it is mandatory to check the quality of each radiotracer batch before application. The amount and type of analytical tests required may differ depending on local authorities as well as the compound and its synthesis route. All analytical methods must be validated according to GMP compliance. In order to pass quality control and be released for patient application, every radiotracer batch has to fulfill certain acceptance criteria (e.g., (radio)chemical purity, pH value, residual solvents and endotoxin content) which are defined in European Pharmacopeia monographs for [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 [56], as well as adequate analytical methods. Until today, for [18F]DCFPyL and [18F]rhPSMA-7.3, no monographs exist but suitable acceptance criteria can be defined in analogy to other 18F-labeled radiotracers, as exemplified in the literature [34,40]. The most important parameter is the radiochemical purity of the tracer which can be determined by radio-HPLC and radio-TLC/iTLC. Using radio-HPLC, the RCP for [68Ga]Ga-PSMA-11 has to be ≥95%, for [18F]PSMA-1007 ≥ 91%. Generally, impurities like non-reacted 68Ga or 18F can be determined better with radio-TLC/iTLC than with radio-HPLC and they should not exceed 3% (68Ga) and 5% (18F), respectively. In addition, the chemical purity of the tracer is of utmost importance and acceptance criteria are specified in the respective monographs and in the literature. Due to the half-life, not all required analytical tests can be performed before release of the batch, e.g., sterility tests and tests on radionuclidic purity.
For [68Ga]Ga-PSMA-11 produced by authorized cold kits, only limited quality control has to be performed for each batch, as described in the label [46,48,49,50]. For radiochemical purity, radio-iTLC is sufficient and the chemical purity does not have to be determined at all. The only other parameters to be analyzed are the appearance (clear, without particles) and pH value (3.2–6.5 (Locametz®) and 4–5 (Illuccix®/GozellixTM)). These tests confirm the quality of each batch and can be performed without the need for a more sophisticated quality control lab.
For 18F-labeled radiotracers, which are produced through much more complex reactions involving a greater number of chemicals, such limited quality control would not be sufficient. Besides tests like appearance, pH value and endotoxin content, a radio-HPLC analysis confirming radiochemical and chemical purity, as well as identity, is essential. When looking at the documentation of the commercially available 18F-labeled radiotracers, not a lot of information about their specifications is given. However, all release specifications have been approved by the respective authorities and must be met for each batch. Parameters which are publicly known are summarized in Table 2:

Table 2.
Release specifications of approved 18F-labeled PSMA-targeting PET radiotracers.
After quality control, which usually takes up to 30 min, the radiotracer batch can be released for patient application. When receiving a released batch of an authorized radiotracer, only the appearance and radioactivity amount have to be checked.
3. Medicinal and Pharmaceutical Overview
3.1. Clinical Indication
All four PSMA-targeting PET radiotracers are used to detect PSMA-positive lesions in adult PCa patients. Their clinical indications [42,43,44,46,48,49,50,55,57] are listed as follows:
- (i)
- Primary staging of patients with high-risk PCa before curative initial treatment
- (ii)
- The detection of suspected PCa recurrence in patients with rising PSA levels after a curative initial treatment (e.g., radical prostatectomy–RP, external beam radiation therapy–EBRT).
In addition, [68Ga]Ga-PSMA-11 has the regulatory indication for selecting patients with metastatic castration-resistant PCa (mCRPC) for potential targeted radionuclide therapy with [177Lu]Lu-PSMA-617 (Pluvicto®, Novartis AG, Basel, Switzerland).
The primary staging of high-risk PCa patients is conducted with PSA levels of ≥20 ng/mL, a Gleason score ≥ 8 and a clinical stage of T3/T4 (extraprostatic extension/seminal vesicle invasion). Despite early intervention, 20–50% of PCa patients will experience biochemical recurrence within ten years after curative treatment [58,59]. For recurrence monitoring, PSA levels are tested regularly and are used to diagnose the biochemical recurrence (BCR) of PCa. Most guideline associations and medical societies agree on base PSA thresholds as a first indication to BCR, which are defined as PSA levels ≥0.2 ng/mL with a confirmed rise (post-RP) and as a PSA increase of ≥2 ng/mL over PSA nadir (post-EBRT) in accordance with the Phoenix criteria [60,61,62,63,64,65]. While PSA levels are crucial in assessing recurrence, additional factors such as Gleason score, pathological stage, surgical margin and lymph node status also need to be taken into consideration [66,67].
3.2. Application
All four described radiotracers share common clinical regulations based on their prescription sheets [42,43,44,46,48,49,50,55,57]. As a safety standard, personnel, trained in radionuclide handling, must ensure drug quality, sterile techniques, radiation shielding, and waste management. If any particles or discolorations to the imaging samples are present, they are not to be used.
The administration is performed intravenously (IV) on PCa patients. To ensure a full dose delivery of [18F]DCFPyL, [18F]PSMA-1007, and [18F]rhPSMA-7.3, a 0.9% sterile saline solution might be used to either dilute the agent sample or flush the IV line post-injection (p.i.). The dilution volume of [68Ga]Ga-PSMA-11 is dependent on the applied generator used for the sterile cold kit preparation. Patients must hydrate well, void frequently p.i. and prior to image acquisition to reduce radiation exposure and imaging artifacts. To improve renal clearance, diuretics may be administered simultaneously to reduce unwanted ureter and bladder activity.
The image acquisition is conducted with patients in supine position with arms above the head and with standardized scanning ranges from the mid-thigh area to the vertex of the skull. Besides their radioisotopes, the different imaging agents have key differences in terms of dose preparation, injection volumes, and p.i. imaging windows. An overview of these parameters for each imaging agent is shown in Table 3.

Table 3.
Comparison of application parameters of the described PSMA-targeting PET radiotracers.
3.3. Pharmacology, Pharmacokinetics and Toxicology
All discussed compounds are synthetic peptidomimetics, featuring a urea-based core moiety (Glu-HN-CO-NH-Lys/Glu) binding with high affinity at PSMA epitopes. The overall pharmacokinetics of these compounds can be additionally influenced through the choice of linkers and radiolabeling sites [68]. The tracers bind to PSMA, significantly overexpressed on most PCa cells (e.g., mCRPC) [69] and are being internalized rapidly [70], enabling a strong uptake to metastases. Since a correlation between PSMA and cancer stage and grade has been revealed, showing enhanced PSMA levels with advanced cancer [71,72,73,74,75], a more efficient diagnosis, or even therapy, using PSMA-based agents might be enabled. However, PSMA is also present on healthy tissue and organs, even though lowly expressed. Still, this might lead to non-specific uptakes giving false positives or making distinct imaging challenging. In difficult cases, using additional CT scans to complement the PET images is advised.
All compounds distribute rapidly via the blood stream and show significant uptake to PCa tumor lesions. Despite these shared traits, the pharmacokinetic profiles of these PET tracers vary significantly. This is most evident in the excretion pathways, which is due to compound properties; e.g., [18F]rhPSMA-7.3, [68Ga]Ga-PSMA-11, and [18F]DCFPyL are renally excreted, while [18F]PSMA-1007 is primarily hepatobiliary excreted [76,77]. As a result, the latter shows less bladder activity, improving imaging results in the pelvic area due to favorable tumor-to-background ratios [78].
However, these structural differences also impact binding kinetics. In general, all aforementioned PSMA-PET agents exhibit irreversible binding kinetics to tumor lesions. Nevertheless, studies revealed that [18F]PSMA-1007 not only binds irreversibly to tumor lesions, but also to organs and tissues [79], resulting in higher mean absorbed doses of non-target tissue. Based on general radiation safety measures, the mean absorbed doses to healthy organs and tissues from PSMA-PET imaging agents are of main concern and are listed in Table 4.

Table 4.
Estimated radiation absorbed doses in organs/tissues in adult PCa patients who received one of the four PSMA-targeting PET radiotracers.
These PSMA-PET imaging agents have no toxicologic safety concerns, as verified in previous animal and clinical studies [42,43,44,46,48,49,50,55,57]. Administered PET tracer concentrations are too low and not intended for continuous use. Hence, doses remain a singular instance with no long-term health effects expected. In less than 1% of patient cases, minor adverse effects, e.g., nausea, diarrhea, dizziness, were observed.
Taking the effective doses of all PSMA-PET imaging agents into consideration, their mean effective doses are in ranges of 0.0121–0.191 mSv/MBq, resulting in effective doses in ranges of 4.1–5.3 mSv per injection, clearly within safety margins of diagnostic routines. Organs absorbing the highest doses oftentimes include the kidneys and the liver (Table 5), with the aim of using the smallest quantity necessary to get the desired information. The applied PSMA-PET imaging agents are designed to deliver the lowest amount of ionizing radiation possible, causing the least risk of cancer and other abnormalities. Overall, delivered radiation doses, at diagnostic levels, are manageable and not a significant safety concern to PCa patients.

Table 5.
Effective and critical radiation doses of each PSMA-targeting PET radiotracer.
3.4. Comparative Studies
Regarding the biodistribution of the described PSMA-PET radiotracers in healthy volunteers, [68Ga]Ga-PSMA-11, [18F]rhPSMA-7.3, and [18F]DCFPyL presented high uptake in the kidneys and salivary glands, as well as moderate uptake in the liver, bladder, spleen and duodenum. This observation is in correlation with the natural known expression of PSMA in these healthy tissues. These three radiotracers present a similar biodistribution profile which is quite different from the one of [18F]PSMA-1007. [18F]PSMA-1007 presents high uptake in the kidneys, salivary glands, liver and duodenum, and moderate uptake in the bladder and the spleen. A fast renal clearance for [68Ga]Ga-PSMA-11, [18F]rhPSMA-7.3, and [18F]DCFPyL was also observed. [18F]rhPSMA-7.3 has a lower average urinary excretion than [18F]DCFPyL and [68Ga]Ga-PSMA-11, which can improve imaging in the pelvic region [80]. Penny et al. confirmed this observation, as they measured a lower bladder uptake compared to [18F]DCFPyL and [68Ga]Ga-PSMA-11 [81]. Nevertheless, the rapid excretion in the urine can also be seen as a drawback, as it causes substantial accumulation in the urinary bladder which can prevent a good visualization of lesions in the pelvic region [82]. In this case, a diuretic agent can be proposed to patients. In comparison, [18F]PSMA-1007 presents hepatobiliary clearance which causes a longer residential time and consequently a higher dose in the organs with high uptake. This gives the advantage of having a lower uptake in the pelvic region and allows for a better visualization of small lesions. For all four radiotracers, the uptake in the liver, intestines and kidneys can prevent the visualization of small lesions in these regions. The moderate-to-high uptake in the liver can prevent the detection of liver metastases which indicate a bad prognosis. In comparison with [18F]fluorocholine and [11C]choline that have high liver uptake, the moderate liver uptake of [18F]rhPSMA-7.3 and [68Ga]Ga-PSMA-11 allow for a better visualization of liver metastases. A visual comparison of the biodistribution of [18F]PSMA-1007 and [18F]rhPSMA-7.3 in healthy volunteers is presented Figure 2.

Figure 2.
Biodistribution in healthy volunteers utilizing (A): [18F]PSMA-1007 PET (220 MBq). Originally published in Eur. J. Nucl. Med. Mol. Imaging © Springer Nature [78]. (B): [18F]rhPSMA-7.3 PET (220 MBq). Originally published in J. Nucl. Med. © SNMMI [80].
Clinical trials of the four PSMA-PET agents are summarized in Table 6 which presents the major observations during these trials. These four radiotracers have successfully shown their safety at injected doses between 2–4 MBq/kg and their abilities to detect primary PCa lesions and metastases. Examples of successful detection of PCa lesions are presented in Figure 3. It is important to note that each study has been made in different centers with different activities applied and different machines and protocols of imaging used.

Figure 3.
Comparison of PET imaging outcomes in PCa patients using the four approved PSMA-targeting PET radiotracers. (A): A 72-y-old patient with high-risk PCa (iPSA, 44 ng/mL) who underwent [18F]rhPSMA-7.3 PET/CT (335 MBq). Images showed primary tumor (blue arrow) and pelvic metastases (red arrows) histologically confirmed after radical prostatectomy. Originally published in J. Nucl. Med., © SNMMI [83]. (B): A patient with high risk PCa (iPSA, 13.68 ng/mL, and biopsy Gleason score, 4D5) who underwent [18F]DCFPyL PET/CT (333 MBq). Images showed multifocal osseous lesions involving spine, ribs, pelvis and right clavicle. On subsequent biopsy of transverse process of L3, osseous metastatic (M1b) disease was confirmed. Originally published in J. Urol. © American Urological Association [84]. (C): A patient with metastatic PCa (Gleason Score 7a, PSA 12 ng/mL) who underwent [18F]PSMA-1007 PET/CT (217 MBq, 90 min p.i.). Image showed PSMA avid multifocal primary tumor and several moderate to intensive PSMA avid lymph nodes, no bone or distant metastases detected [85]. (D): A patient referred for primary staging of PCa who underwent [68Ga]Ga-PSMA-11 PET/CT (2 MBq/kg). Image showed primary disease (black arrow). Originally published in EJNMMI Res. © SpringerOpen [79].
At high PSA level (>5 ng/mL), Afshar-Oromieh et al. demonstrate a detection rate of 90% for [68Ga]Ga-PSMA-11 in a study involving 319 PCa patients with similar rates for the three other PSMA-PET tracers [86]. For the four PSMA-PET agents, a correlation between PSA level and detection rate was observed: the lower the PSA level, the lower the detection rate. For example, Fendler et al. reported these following values for [68Ga]Ga-PSMA-11: 38% for PSA < 0.5 ng/mL (n = 136), 57% for 0.5 ≤ PSA < 1.0 ng/mL (n = 79), 84% for 1.0 ≤ PSA < 2.0 ng/mL (n = 89), 86% for 2.0≤ PSA < 5.0 ng/mL (n = 158) and 97% for PSA > 5.0 ng/mL (n = 173, p < 0.001) [87]. In a study with [68Ga]Ga-PSMA-11, Eiber et al. reported different values compared to Fendler et al.: 96.8% (120/124) for PSA < 2 ng/mL, 93.0% (67/72) for 1 < PSA < 2 ng/mL, 72.7% (24/33) for 0.5 < PSA < 1 ng/mL, and 57.9% (11/19) for 0.2 < PSA < 0.5 ng/mL) [88]. This observation can be due to the heterogeneity of the PCa patients. On the other hand, Morris et al. reported these values for [18F]DCFPyL: 36.2% for PSA < 0.5 ng/mL to 96.7% for PSA ≥ 5 ng/mL [89]. For [18F]PSMA-1007, these values have been reported: 60% for PSA < 0.2 ng/mL, 70–65% for 0.6 ≤ PSA < 1.2 ng/mL and 80% for PSA >1.2 ng/mL [90]. In comparison, [18F]rhPSMA-7.3 and [18F]PSMA-1007 present higher detection rates (64% and 60%, respectively) compared to [18F]DCFPyl (36%) and [68Ga]Ga-PSMA-11 (38%) for patients with very low PSA level (<0.5 ng/mL). As one third of PCa patients will experience BCR within 5 years of treatment, being able to detect lesion in BCR patients is of high importance [91].
Unspecific bone uptake (UBU) has been observed in some patients, which complicates the diagnosis of bone metastasis. Rizzo et al. describe the UBU of these four PSMA-PET radiotracers via the use of homunculus [92]. The three main regions of UBU are the ribs (57.5%, 202/348 patients), the pelvis (24.8%, 87/348 patients) and the spine (9.7%, 34/348 patients) [93]. [18F]PSMA-1007 presents significatively more UBU than the three other approved agents, especially in the pelvic region. Due to the recurrence of these benign lesion detections using [18F]PSMA-1007, patients need to undertake another PET exam using one of the three other radiotracers, notably [68Ga]Ga-PSMA-11. In fact, in a retrospective study, Dias et al. have observed no UBU for the patients imaged with [68Ga]Ga-PSMA-11 and UBU notably in the ribs for the patients imaged with [18F]PSMA-1007 [79]. A retrospective study of dual time point biphasic PET/CT (1 and 3 h p.i.) shows that biphasic images help in the differentiation between malign and benign lesions [94].
Before the approval of PSMA-PET tracers, PET scans were already conducted using the approved radiotracers [18F]FDG, [18F]fluorocholine and [11C]choline to detect PCa lesions. These three radiotracers can be used to diagnose a large panel of cancer types and other pathologies and are not targeting specifically PSMA. Multiple studies have been conducted to compare these gold standards and more general tracers with these four PSMA-PET tracers. For example, PET/CT scans of 37 patients with BCR PCa injected with both [68Ga]Ga-PSMA-11 and [18F]fluorocholine showed a significant improvement of image contrast with [68Ga]Ga-PSMA-11 compared to [18F]fluorocholine. This study concluded in significantly higher lesion detection, mostly for metastases, even in small lymph nodes or liver metastases due to lower background uptake in these regions even at low PSA level [95]. Similarly, a retrospective study on 257 BCR patients showed that [68Ga]Ga-PSMA-11 outperformed [18F]FDG for low PSA levels (≤1 ng/mL). Nevertheless, for higher PSA level, the superiority of [68Ga]Ga-PSMA-11 is less evident [96]. Regarding [18F]FDG, a meta-analysis of 27 literature studies including 2891 patients concluded that [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 outperformed [18F]FDG for lesion detections [97].
In parallel, [18F]fluciclovine, a clinically approved non-PSMA-based PET imaging agent is also used for PCa detection [98]. Comparative studies with PSMA-PET tracers were also performed to evaluate their relative efficiency of detection. Multiple studies concluded a higher performance of the PSMA-PET tracers compared to [18F]fluciclovine in the detection of primary tumors, small lesions and metastases even at a low PSA level (<0.5 ng/mL) [99]. These comparative studies confirm one more time the power of PSMA-PET agents in PCa detection. A non-exhaustive list of comparative clinical trials is provided in Table 7.

Table 6.
Clinical characteristics and outcome of the clinical trials of the four approved PSMA-targeting PET radiotracers (non-exhaustive list).
Table 6.
Clinical characteristics and outcome of the clinical trials of the four approved PSMA-targeting PET radiotracers (non-exhaustive list).
| Imaging Agent | Clinical Trial | Phase | Publication Year | Number of Patients | Type of Patient | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Posluma®: [18F]rhPSMA-7.3 | NCT03995888 | 1 | 2021 | 6 | Healthy volunteers | No clinically detectable pharmacological effects were observed after injection of 220 MBq. The mean effective dose received by the patients was 0.0141 mSv/MBq which is favorable for diagnostics by PET and lower than for other PET PSMA agents. | [80] |
| LIGHTHOUSE NCT04186819 | 3 | 2021 | 335 | High risk PCa | Confirmation of the safety and accuracy of detection of lesions in high risk PCa in patients with a large range of PSA levels. Confirmation of the safety and accuracy of detection of lesions in recurrent PCa in patients with a large range of PSA levels. | [81] | |
| SPOTLIGHT NCT04186845 | 3 | 2021 | 389 | Recurrent PCa | [100] [101] | ||
| Radelumin®: [18F]PSMA-1007 | 1/2 | 2017 | 3 10 | Healthy volunteers PCa patients | Comparable performance of [18F]PSMA-1007 and [68Ga]Ga-PSMA-11. | [102] | |
| 1/2 | 2023 | 3 13 | Healthy volunteers PCa patients | Safe and well tolerated tracer, high accuracy in the diagnoses for primary and metastatic lesions. | [103] | ||
| 2 | 2019 | 40 | After RP or radiation beam therapy | Scan positivity is dependent on PSA level. Moderate detection of tumor lesion (60%) at low PSA levels < 0.2 ng/mL. | [90] | ||
| 3 | 2021 | 175 | PCa | Detection efficiency of 80%. | [104] | ||
| Locametz®/ Illuccix®/GozellixTM: [68Ga]Ga-PSMA-11 | NCT03001869 | 2023 | Completed–No results posted. | ||||
| NCT02659527 | 3 | 2018 | 145 | BCR PCa patients after RP | Detection efficiency of 85%. | [105] | |
| NCT03353740 and NCT02940262 | 3 | 2020 | 635 | BCR PCa patients after RP and/or radiation therapy | High detection rates of 75% and positive prediction values between 84 and 92%. | [87] | |
| 3 | 2017 | 1007 | Recurrent PCa | No adverse clinical side effects observed. Same conclusions, significantly higher detection of lesions compared to other already approved tracers, detection rate increases as the PSA level increases. | [106] | ||
| NCT03582774 PSMA-SRT | 3 | 2025 | 193 | Recurrent PCa after RP | Actively recruiting for [68Ga]Ga-PSMA-11 PET/CT for PCa salvage radiotherapy planning. | [107] | |
| 2015 | 70 | Restaging PCa | Good detection rate even at low PSA level (85%). | [108] | |||
| 2015 | 248 | BCR PCa patients after RP | PSA level ≥ 0.2 ng/mL. [68Ga]Ga-PSMA-11 surpasses other imaging modalities for restaging PCa detection and higher detection efficacy compared to other tracers. 89.5% of detection when PSA level ≥ 1.0 ng/mL. | [88] | |||
| BCR PCa patients after RP | [68Ga]Ga-PSMA-11 surpasses other imaging modalities for restaging PCa detection and higher detection efficacy compared to other tracers as concluded by Eiber et al. [88]. | [109] | |||||
| Pylarify®/Pylclari®: [18F]DCFPyL | NCT02523924 | 2 | 2020 | 31 | RP BCa patients | Prospective study, proved good detection of lesions even at low PSA level. | [110] |
| 2 | 2018 | 248 | BCR PCa patients | Early detection even at low PSA level (<0.5 ng/mL). Results comparable to those of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007. | [111] | ||
| NCT03181867 | 2 | 2019 | 800 | BCR patients | 90% detection rate for PSA level > 0.5 ng/mL. | [112] | |
| NCT02899312 | 3 | 2019 | 2244 | BCR after RP | This study confirmed the safety of use and the good sensitivity of this tracer for small lesion detection | [113] | |
| OSPREY NCT02981368 | 2/3 | 2021 | 385 | high risk and recurrent/metastatic PCa. | High positive prediction values (78.1–90.5%). | [84] | |
| CONDOR NCT03739684 | 3 | 2021 | 208 | BCR PCa after RP of radiotherapy | Confirmation of the accuracy of recurrence or metastases lesion detection from previous clinical trials, study even with patients with low PSA levels. [18F]DCFPyL has similar safety and performance compared to [68Ga]Ga-PSMA-11. | [89] | |
| NCT03232164 | 3 | 2024 | 167 | Pilot studies for PET/CT and PET/MRI. No results posted. |

Table 7.
Comparative clinical studies between approved PSMA- and non-PSMA-based PET radiotracers (non-exhaustive list).
Table 7.
Comparative clinical studies between approved PSMA- and non-PSMA-based PET radiotracers (non-exhaustive list).
| Imaging Agent | Clinical Trials | Phase | Year | Number of Patients | Type of Patient | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| [68Ga]Ga-PSMA-11 and [18F]fluorocholine | 2016 | 32 | BCR PCa patient after RP | 43% of patients with a negative [18F]fluorocholine PET/CT scan have been diagnosed with PCa lesions using [68Ga]Ga-PSMA-11 PET/CT and confirmed with other diagnosis techniques. [68Ga]Ga-PSMA-11 outperformed [18F]fluorocholine. | [91] | ||
| [18F]PSMA-1007 and [18F]fluorocholine | NCT04102553 | 3 | 2022 | 190 | BCR PCa patients | Significantly higher detection rate for [18F]PSMA-1007 than [18F]fluorocholine especially at low PSA levels. | [114] |
| NCT04742361 | 3 | 2024 | Actively recruiting. | ||||
| [18F]PSMA-1007 and [18F]FDG | 2024 | 42 | RP PCa | [18F]PSMA-1007 outperformed the detection of primary tumors in the prostate glands. | [115] | ||
| 2021 | 21 | Significantly more tumor lesion and benign lesion uptake for [18F]PSMA-1007. | [116] | ||||
| [68Ga]Ga-PSMA-11 and [18F]fluciclovine | NCT03515577 | 2020 | 50 | BCR PCa | Detection rate in patients with low PSA level (≤2.0 ng/mL) is significantly lower for [18F]fluciclovine than [68Ga]Ga-PSMA-11 and up to twice lower for lesions in the pelvic lymph nodes region. | [117] | |
| [18F]PSMA-1007 and [18F]fluciclovine | NCT04239742 | 2 | 2020 | 50 | PCa | [18F]PSMA-1007 outperformed [18F]fluciclovine to detect lesions (68% vs 42%). | [118] |
| [18F]PSMA-1007 and [68Ga]Ga-PSMA-11 | NCT05079828 | 3 | 2024 | 100 | BCR PCa patient after RP | No results posted. | [119] |
| [18F]PSMA-1007 and [18F]DCFPyL | 2018 | 12 | Drug-naive or before surgery PCa patients | Equivalence of efficacy for both tracers. [18F]PSMA-1007 clearance pathway is an advantage for pelvic metastases detection. | [120] |
4. Further Candidates and Outlook
Several other PSMA-PET ligands have been developed and undertaken for clinical evaluation. [18F]DCFBC is a 18F-PSMA targeting agent based on the recognition pattern Glu-NH-O-NH-Ser which has been evaluated in clinical trials before its second generation [18F]DCFPyL [121,122]. This latter has outperformed [18F]DCFBC, notably with a lower background uptake, more tumor uptake and better correlation between uptake and the Gleason score. Due to the significantly better performance of [18F]DCFPyL, no further clinical studies were conducted. Neumaier and coworkers have developed [18F]JK-PSMA-7 derived from [18F]DCFPyL. It has a highly similar biodistribution pattern compared to [18F]DCFPyL and [68Ga]Ga-PSMA-11, but PCa patient phase I studies have highlighted the higher edge-contrast, resolution, and signal-to-noise ratio of [18F]DFCPyL notably for small lesions [123,124]. Piron et al. have successfully assessed [18F]PSMA-11 during phase II clinical trials (NCT03573011) [125]. Its safety usage was confirmed (injection of 2–4 MBq/kg) as well as the detection of PCa lesions. Nevertheless, a diuretic agent was used to evaluate lesions in the proximity of ureters. The NOTA equivalent of PSMA-617, called PSMA-BCH, has been radiolabelled with 18F-Al and clinically assessed in 11 PCa patients [126]. Renal clearance was observed and a good detection of small lesions showed a good suitability of this 18F-tracer for PCa lesion detection. Nonetheless, for now, no 18F-PSMA-PET agents have outperformed the three clinically approved tracers.
[124I]MIP-1095 has been assessed in PET/CT on 16 mCRPC patients in a theranostic study in combination with [131I]MIP-1095. SPECT and PET/CT have been recorded after injection and to monitor the treatment, respectively. Good safety of the PET tracer has been confirmed for an injected dose of 67.4 MBq as no side effects have been observed. High uptake in the kidney and salivary glands and moderate uptake in the liver, intestines and bladder have been observed as well as a renal clearance. This biodistribution is similar to the one of [68Ga]Ga-PSMA-11 and clear visualization of the metastases has been demonstrated. The monitoring of treatment has also been successful with the measurement of the reduction in number and size of the metastases [127].
[68Ga]Ga-NGUL is a NOTA-PSMA-based PET agent that has successfully passed a phase I clinical trial presenting good safety, fast renal clearance and good capacity to visualize metastases and primary tumors in PCa patients. These promising results could lead to phase II/III clinical trials in the future [128].
For the treatment of mCRPC, [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T are currently used in clinical routine as therapeutic agents in combination with [68Ga]Ga-PSMA-11 as diagnostic agent. Multiple clinical trials have been conducted with [68Ga]Ga-PSMA-617 [129,130,131] and [68Ga]Ga-PSMA-I&T to develop theranostic pairs with identical ligand structure in order to develop pairs with more similar biodistribution and pharmacological profiles [132,133,134,135]. They showed minor deviations in the biodistribution (lower liver uptake, higher bone and background uptake for [68Ga]Ga-PSMA-617 and [68Ga]Ga-PSMA-I&T compared to [68Ga]Ga-PSMA-11) and no significant difference in tumor uptake [136]. This comparative study confirmed the good suitability of these two tracers to be used in clinical routine for theranostic applications.
Privé et al. have conducted a first-in-human study with [89Zr]Zr-PSMA-617 and [89Zr]Zr-PSMA-I&T in a BCR PCa patient. Biodistributions were observed to be really similar to their 177Lu-analog and cancerous lesions were correctly identified. Thanks to the promising diagnostic capacity of these two 89Zr-PSMA tracers, a clinical study is being planned [137].
Similarly, [64Cu]Cu-PSMA-617 and [64Cu]Cu-PSMA-I&T have been clinically evaluated in the PET detection of lesions in PCa patients. Phase I/II clinical trials showed good safety and excellent capacity to detect PCa lesions for both of these tracers with similar precision in the detection [138,139,140]. A phase III trial is currently ongoing to confirm these promising results (Solar-stage: NCT06235151) [141]. With the development of more efficient production routes for both 64Cu and 67Cu and the development of personalized medicine thanks to radiotheranostics, it is likely that [67Cu]Cu-PSMA-617 and [67Cu]Cu-PSMA-I&T will undergo clinical trials in the next decades.
[61Cu]Cu-PSMA-I&T and its NODA-GA analog [61Cu]Cu-NODA-GA-PSMA-I&T have been preclinically assessed in a PCa rodent model. The NODA-GA analog presented a better biodistribution and excretion profile and higher tumor uptake compared to the DOTA-GA analog. Moreover, the performance of [61Cu]Cu-NODA-GA-PSMA-I&T was similar to those of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 with the advantage of performing PET at later timepoints. A first-in-human study has been further conducted and has shown good safety of the tracer as well as its ability to detect metastases in mCRPC patients [142]. Considering these promising results, a phase I study has been announced [143].
Yang et al. have assessed [64Cu]Cu-PSMA-BCH similarly to their previous [18F]F-Al-PSMA-BCH study. Both tracers showed similar biodistribution and a renal excretion pathway with a good capacity in the detection of PCa lesions. Compared to its 18F-analog, [64Cu]Cu-PSMA-BCH allowed PET at later timepoints (24 h p.i.) after renal excretion which led to better visualization of pelvic lesions [144].
Zhang and coworkers developed a series of PSMA-617-derived tracers for 64Cu-PET. The naphthyl moiety of PSMA-617 has been replaced by a quinoline and they have proposed a NOTA version [145,146]. A study with 29 PCa patients has been conducted to compare [64Cu]Cu-DOTA-PSMA-3Q and [64Cu]Cu-NOTA-PSMA-3Q. Significantly higher salivary glands uptake and lower liver uptake have been observed for the NOTA tracer. This comparison highlights the importance of the choice of the chelating agent toward the biodistribution properties of the tracer which are of high importance for PET or targeted radionuclide therapy applications. The group is currently working on a 18F-equivalent of this NOTA tracer.
[64Cu]Cu-sar-bisPSMA has been used in clinical trials (Cobra: NCT05249127), notably in a phase II trial in comparison with [68Ga]Ga-PSMA-11 (Propeller: NCT04839367) [147,148]. This study demonstrated its good safety and a better efficiency of the 64Cu tracer compared to [68Ga]Ga-PSMA-11. This is notably due to the higher tumor uptake that leads to the better detection of smaller lesions. The tracer is currently under phase III trials to confirm its efficiency in a larger cohort (clarify: NCT06056830) [148]. The therapeutic analog [67Cu]Cu-sar-bisPSMA is currently under clinical trials of phase I/IIa (SECuRE: NCT04868604) [149]. In the case of success in these clinical trials, [64/67Cu]Cu-sar-bisPSMA could become the first true theranostic pair for PCa.
A first-in-human application of [152Tb]Tb-PSMA-617 has been conducted on one PCa patient [150]. The use of [152Tb]Tb-PSMA-617 has been determined as safe and successful in the detection of cancer lesions as [68Ga]Ga-PSMA-11, yet with lower image quality. Moreover, [152Tb]Tb-PSMA-617 has a similar biodistribution profile compared to [177Lu]Lu-PSMA-617, already approved for clinical treatment. The potential use of the terbium sisters in theranostics is huge thanks to their physical properties adapted for clinical purposes and high versatility (152Tb: PET, 155Tb: SPECT, 149Tb: targeted alpha therapy, and 161Tb: β–- targeted radionuclide therapy). There is a great chance that the first clinical approval of these four terbium sisters will be conducted using a PSMA- or TATE-based ligand.
In the same manner, a first-in-human study has been conducted with [44Sc]Sc-PSMA-617 and has shown a similar biodistribution and the same capacity in lesion detection as [68Ga]Ga-PSMA-11 [151,152]. Due to the longer half-life of 44Sc (4.3 h), this radioligand could be used in image-guided radiosurgery using positron probes intraoperatively. The perspective of this work is to assess mCRPC targeted radionuclide therapy using [47Sc]Sc-PSMA-617 to see the potential clinical application of the matched pair 44/47Sc in PCa treatment.
Another strategy to increase the diagnostic efficiency for PCa patients and their differentiation is to conduct imaging with a PSMA-PET agent and [18F]FDG. Kepenek et al. reported a case study with 93 patients with high risk CRPC, where significantly higher tumor uptake and lesion detection were observed with [18F]FDG compared to [68Ga]Ga-PSMA-11 [153]. Within these PSMA-/FDG+ patients with high Gleason scores (≥8) and PSA levels (≥7.9 ng/mL), the glucose metabolism of tumors is increased significantly which allows a better diagnosis for this radiotracer combination and better choice of the following treatment, especially for [177Lu]Lu- or [225Ac]Ac-PSMA-617, that have a poor prognosis in PSMA-/FDG+ patients. This strategy exemplifies so called personalized medicine and is also used to indicate the most adapted treatment for the patients. These observations have been confirmed by the retrospective analysis studies of Khreish et al., Chen et al. and Pan et al. on 29, 56 and 298 CRPC patients, respectively, that underwent both [18F]FDG and [68Ga]Ga-PSMA-11 PET/CT [154,155,156].
Also, other studies have investigated the dual-tracer approach for PCa diagnostics, notably with [68Ga]Ga-PSMA-11 and [68Ga]Ga-DOTA-RM2. A case study on 22 BCR PCa patients proved their complementarity and synergy for the detection of small lesions which reflect the heterogeneity of PCa during the staging phase [157].
5. Conclusions
This review highlights the significant impact of approved PSMA-PET imaging agents [68Ga]Ga-PSMA-11 (Locametz®/Illuccix®/GozellixTM), [18F]DCFPyL (Pylarify®/Pylclari®), [18F]PSMA-1007 (Radelumin®), and [18F]rhPSMA-7.3 (Posluma®) on the landscape of PCa detection and management. These agents have revolutionized clinical practice by offering enhanced sensitivity and accuracy in detecting PCa, leading to improved staging, treatment planning and patient outcomes.
Each radiotracer possesses unique chemical and pharmaceutical characteristics, influencing its application and utility in specific clinical scenarios. [68Ga]Ga-PSMA-11, with its established role and wide availability, remains a cornerstone while the 18F-labeled agents [18F]DCFPyL, [18F]PSMA-1007 and [18F]rhPSMA-7.3 offer advantages in terms of imaging properties and potential for radiotheranostic applications.
The approval and increasing adoption of these PSMA-targeted PET imaging agents mark a pivotal advancement in personalized medicine for PCa. As clinical experience expands and new data emerge, their role in guiding treatment decisions and improving patient care will continue to evolve, promising a future of more precise and effective interventions in the fight against PCa.
Author Contributions
Conceptualization, U.H. and M.B.-S.; writing—original draft preparation, U.H., L.W., H.T., L.K. and M.B.-S.; writing—review and editing, U.H., L.W., H.T., L.K. and M.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors wish to thank Antonia Dimitrakopoulou-Strauss for providing a MIP PET/CT scan to include in this article. We sincerely thank Claudia and Hans-Peter Opitz for their generous financial support of our group. Their contribution plays a crucial role in enabling our research and fostering scientific progress. We are deeply grateful for their commitment and encouragement. During the preparation of this manuscript, the authors used OpenAI’s ChatGPT (GPT-4, June 2025 version) to generate the PET imaging human silhouette depicted in the graphical abstract figure. The authors have reviewed and edited the output, and take full responsibility for the content of this publication.
Conflicts of Interest
Patent applications for various PSMA ligands have been filed by the German Cancer Research Center (DKFZ) in Heidelberg, Germany. M.B.S. is listed as a co-inventor in those patents.
Correction Statement
This article has been republished with a minor correction to resolve spelling and grammatical errors and the references 43 and 44. This change does not affect the scientific content of the article.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Lowder, D.; Rizwan, K.; McColl, C.; Paparella, A.; Ittmann, M.; Mitsiades, N.; Kaochar, S. Racial disparities in prostate cancer: A complex interplay between socioeconomic inequities and genomics. Cancer Lett. 2022, 531, 71–82. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate Cancer Disparities by Race and Ethnicity: From Nucleotide to Neighborhood. Cold Spring Harb. Perspect. Med. 2018, 8, a030387. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Descotes, J.L. Diagnosis of prostate cancer. Asian J. Urol. 2019, 6, 129–136. [Google Scholar] [CrossRef]
- Okotie, O.T.; Roehl, K.A.; Han, M.; Loeb, S.; Gashti, S.N.; Catalona, W.J. Characteristics of prostate cancer detected by digital rectal examination only. Urology 2007, 70, 1117–1120. [Google Scholar] [CrossRef]
- Morote, J.; Paesano, N.; Picola, N.; Munoz-Rodriguez, J.; Ruiz-Plazas, X.; Munoz-Rivero, M.V.; Celma, A.; Garcia-de Manuel, G.; Miro, B.; Abascal, J.M.; et al. The Role of Digital Rectal Examination for Early Detection of Significant Prostate Cancer in the Era of Magnetic Resonance Imaging. Life 2024, 14, 1359. [Google Scholar] [CrossRef]
- Gosselaar, C.; Kranse, R.; Roobol, M.J.; Roemeling, S.; Schroder, F.H. The interobserver variability of digital rectal examination in a large randomized trial for the screening of prostate cancer. Prostate 2008, 68, 985–993. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef]
- Benelli, A.; Vaccaro, C.; Guzzo, S.; Nedbal, C.; Varca, V.; Gregori, A. The role of MRI/TRUS fusion biopsy in the diagnosis of clinically significant prostate cancer. Ther. Adv. Urol. 2020, 12, 1756287220916613. [Google Scholar] [CrossRef]
- Borofsky, S.; George, A.K.; Gaur, S.; Bernardo, M.; Greer, M.D.; Mertan, F.V.; Taffel, M.; Moreno, V.; Merino, M.J.; Wood, B.J.; et al. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology 2018, 286, 186–195. [Google Scholar] [CrossRef]
- Chow, K.M.; So, W.Z.; Lee, H.J.; Lee, A.; Yap, D.W.T.; Takwoingi, Y.; Tay, K.J.; Tuan, J.; Thang, S.P.; Lam, W.; et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2023, 84, 36–48. [Google Scholar] [CrossRef]
- Islam, R.; Desai, S.; Moran, M.; Golombos, D.M. The Role of PSMA PET Imaging in Prostate Cancer: Current Applications and Future Directions. Curr. Urol. Rep. 2025, 26, 46. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar]
- Evans, J.C.; Malhotra, M.; Cryan, J.F.; O’Driscoll, C.M. The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br. J. Pharmacol. 2016, 173, 3041–3079. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Chang, S.S.; O’Keefe, D.S.; Bacich, D.J.; Reuter, V.E.; Heston, W.D.; Gaudin, P.B. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999, 5, 2674–2681. [Google Scholar]
- Wurzer, A.; Di Carlo, D.; Schmidt, A.; Beck, R.; Eiber, M.; Schwaiger, M.; Wester, H.J. Radiohybrid Ligands: A Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA Inhibitors. J. Nucl. Med. 2020, 61, 735–742. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Klivenyi, G.; Hull, W.E.; Matys, R.; Hauser, H.; Kalthoff, H.; Schmiegel, W.H.; Maier-Borst, W.; Matzku, S. A bifunctional HBED-derivative for labeling of antibodies with 67Ga, 111In and 59Fe. Comparative biodistribution with 111In-DPTA and 131I-labeled antibodies in mice bearing antibody internalizing and non-internalizing tumors. Int. J. Rad. Appl. Instrum. B 1992, 19, 809–824. [Google Scholar] [CrossRef]
- Eder, M.; Neels, O.; Muller, M.; Bauder-Wust, U.; Remde, Y.; Schafer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Cardinale, J.; Martin, R.; Remde, Y.; Schafer, M.; Hienzsch, A.; Hubner, S.; Zerges, A.M.; Marx, H.; Hesse, R.; Weber, K.; et al. Procedures for the GMP-Compliant Production and Quality Control of [18F]PSMA-1007: A Next Generation Radiofluorinated Tracer for the Detection of Prostate Cancer. Pharmaceuticals 2017, 10, 77. [Google Scholar] [CrossRef]
- Dornan, M.H.; Simard, J.M.; Leblond, A.; Juneau, D.; Delouya, G.; Saad, F.; Menard, C.; DaSilva, J.N. Simplified and robust one-step radiosynthesis of [18F]DCFPyL via direct radiofluorination and cartridge-based purification. J. Label. Comp. Radiopharm. 2018, 61, 757–763. [Google Scholar] [CrossRef]
- Gower-Fry, L.; Kronemann, T.; Dorian, A.; Pu, Y.; Jaworski, C.; Wangler, C.; Bartenstein, P.; Beyer, L.; Lindner, S.; Jurkschat, K.; et al. Recent Advances in the Clinical Translation of Silicon Fluoride Acceptor (SiFA) 18F-Radiopharmaceuticals. Pharmaceuticals 2021, 14, 701. [Google Scholar] [CrossRef]
- Wurzer, A.; Parzinger, M.; Konrad, M.; Beck, R.; Gunther, T.; Felber, V.; Farber, S.; Di Carlo, D.; Wester, H.J. Preclinical comparison of four [18F,(nat)Ga]rhPSMA-7 isomers: Influence of the stereoconfiguration on pharmacokinetics. EJNMMI Res. 2020, 10, 149. [Google Scholar] [CrossRef]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Ashhar, Z.; Ahmad Fadzil, M.F.; Othman, M.F.; Yusof, N.A.; Abdul Onny, M.A.; Mat Ail, N.; Abd Rahman, S.F. Cyclotron Production of Gallium-68 Radiopharmaceuticals Using the 68Zn(p,n)68Ga Reaction and Their Regulatory Aspects. Pharmaceutics 2022, 15, 70. [Google Scholar] [CrossRef]
- EDQM. European Pharmacopeia Monograph. In “Gallium (68Ga) Chloride (Accelerator-Produced) Solution for Radiolabelling” (01/2021:3109), 11th ed.; EDQM—European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2023. [Google Scholar]
- IAEA TECDOC “Production and Quality Control of Fluorine-18 Labelled Radiopharmaceuticals”. Available online: https://www.iaea.org/publications/14925/production-and-quality-control-of-fluorine-18-labelled-radiopharmaceuticals (accessed on 25 April 2025).
- Scott, P.J.; Hockley, B.G.; Kung, H.F.; Manchanda, R.; Zhang, W.; Kilbourn, M.R. Studies into radiolytic decomposition of fluorine-18 labeled radiopharmaceuticals for positron emission tomography. Appl. Radiat. Isot. 2009, 67, 88–94. [Google Scholar] [CrossRef]
- Schmitt, S.; Moreau, E. Radiochemistry with {Al18F}2+: Current status and optimization perspectives for efficient radiofluorination by complexation. Coordin. Chem. Rev. 2023, 480, 215028. [Google Scholar] [CrossRef]
- Patt, M.; Decristoforo, C.; de Martini, A.; Koole, M.; Oyen, W.J.G.; Kiss, O.C. The revision of the pharmaceutical legislation—It is time to act for nuclear medicine in Europe. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 20–24. [Google Scholar] [CrossRef]
- Ravert, H.T.; Holt, D.P.; Chen, Y.; Mease, R.C.; Fan, H.; Pomper, M.G.; Dannals, R.F. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor, [18F]DCFPyL. J. Label. Comp. Radiopharm. 2016, 59, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, R.; Bach-Gansmo, T.; Cumming, P.; Olberg, D.E. A new automated and putatively versatile synthesis of the PSMA-ligand derivative [18F]DCFPyL using the FASTlab(TM) synthesizer. EJNMMI Radiopharm. Chem. 2022, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, V.; Wuest, M.; Jans, H.S.; Janzen, N.; Genady, A.R.; Valliant, J.F.; Benard, F.; Wuest, F. Automated synthesis of [18F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Shamni, O.; Nebeling, B.; Grievink, H.; Mishani, E. Fine-tuning of the automated [18F]PSMA-1007 radiosynthesis. J. Label. Comp. Radiopharm. 2019, 62, 252–258. [Google Scholar] [CrossRef]
- Maisto, C.; Morisco, A.; de Marino, R.; Squame, E.; Porfidia, V.; D’Ambrosio, L.; Di Martino, D.; Gaballo, P.; Aurilio, M.; Buonanno, M.; et al. On site production of [18F]PSMA-1007 using different [18F]fluoride activities: Practical, technical and economical impact. EJNMMI Radiopharm. Chem. 2021, 6, 36. [Google Scholar] [CrossRef]
- Naka, S.; Watabe, T.; Kurimoto, K.; Uemura, M.; Soeda, F.; Neels, O.C.; Kopka, K.; Tatsumi, M.; Kato, H.; Nonomura, N.; et al. Automated [18F] PSMA-1007 production by a single use cassette-type synthesizer for clinical examination. EJNMMI Radiopharm. Chem. 2020, 5, 18. [Google Scholar] [CrossRef]
- Wurzer, A.; Di Carlo, D.; Herz, M.; Richter, A.; Robu, S.; Schirrmacher, R.; Mascarin, A.; Weber, W.; Eiber, M.; Schwaiger, M.; et al. Automated synthesis of [18F]Ga-rhPSMA-7/ -7.3: Results, quality control and experience from more than 200 routine productions. EJNMMI Radiopharm. Chem. 2021, 6, 4. [Google Scholar] [CrossRef]
- Blok, S.; Wängler, C.; Bartenstein, P.; Jurkschat, K.; Schirrmacher, R.; Lindner, S. Good practices for the automated production of 18F-SiFA radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2023, 8, 25. [Google Scholar] [CrossRef]
- FDA Drug Approval Package for “POSLUMA (Flotufolastat F 18) Injection”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=216023 (accessed on 6 May 2025).
- Prescribing Information for PYLARIFY®. Available online: https://www.pylarify.com/sites/default/files/resources/prescribing-information.pdf (accessed on 21 April 2025).
- FDA Drug Approval Package for PYLARIFY®. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214793 (accessed on 6 May 2025).
- Fuscaldi, L.L.; Sobral, D.V.; Durante, A.C.R.; Mendonca, F.F.; Miranda, A.C.C.; da Cunha, M.L.; Malavolta, L.; Mejia, J.; de Barboza, M.F. Standardization of the [68Ga]Ga-PSMA-11 Radiolabeling Protocol in an Automatic Synthesis Module: Assessments for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 385. [Google Scholar] [CrossRef]
- FDA Drug Approval Package for “ILLUCCIX® (Kit for the Preparation of Gallium Ga 68 Gozetotide Injection)”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214032 (accessed on 14 April 2025).
- Press Release Telix Pharmaceuticals. Available online: https://telixpharma.com/news-views/illuccix-receives-european-approval/#_ftn1 (accessed on 14 April 2025).
- FDA Drug Approval Package for “GOZELLIXTM (Kit for the Preparation of Gallium Ga 68 Gozetotide Injection)”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=219592. (accessed on 6 May 2025).
- FDA Drug Approval Package for “LOCAMETZ® (Kit for the Preparation of Gallium Ga 68 Gozetotide Injection”. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=215841 (accessed on 14 April 2025).
- Product Information—LOCAMETZ®. Available online: https://www.ema.europa.eu/en/documents/product-information/locametz-epar-product-information_en.pdf. (accessed on 21 April 2025).
- Wang, I.E.; Morrissette, L.J.; Wong, K.K.; Brooks, A.F.; Dakanali, M.; Scott, P.J.H. A comparison of routine [68Ga]Ga-PSMA-11 preparation using Locametz and Illuccix kits. EJNMMI Radiopharm. Chem. 2024, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Distribution Sites of Pylarify in the US. Available online: https://www.pylarify.com/support/imaging-site-locator (accessed on 15 April 2025).
- Distribution Sites of Pylclari in Europe. Available online: https://www.curiumpharma.com/product/pylclari/ (accessed on 15 April 2025).
- Distribution Sites of Poslumay in the US. Available online: https://www.siemens-healthineers.com/en-us/molecular-imaging/petnet (accessed on 15 April 2025).
- Product Information—RADELUMIN®. Available online: https://abx.de/_Resources/Persistent/4/d/7/f/4d7ff1ffb656143c2db949b262ef2872fdf19c03/SmPC%20Radelumin%202000%20Germany.pdf (accessed on 21 April 2025).
- EDQM. European Pharmacopeia Monograph. In “Gallium 68 PSMA-11 Injection Solution” (04/2021:3044) and “PSMA-1007 (18F) Injection” (07/2021:3116), 11th ed.; EDQM—European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2023. [Google Scholar]
- Product Information—Pylclari®. Available online: https://www.ema.europa.eu/en/documents/product-information/pylclari-epar-product-information_en.pdf. (accessed on 21 April 2025).
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, P.A.; Buchsbaum, J.C.; Elshaikh, M.; Reddy, C.A.; Zippe, C.; Klein, E.A. Factors affecting recurrence rates after prostatectomy or radiotherapy in localized prostate carcinoma patients with biopsy Gleason score 8 or above. Cancer 2002, 95, 2302–2307. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; Meerleer, G.d.; Santis, M.d.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.B.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1963–1996. [Google Scholar] [CrossRef]
- Virgo, K.S.; Rumble, R.B.; Wit, R.d.; Mendelson, D.S.; Smith, T.J.; Taplin, M.-E.; Wade, J.L.; Bennett, C.L.; Scher, H.I.; Nguyen, P.L.; et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 1274–1305. [Google Scholar] [CrossRef]
- Shore, N.D.; Moul, J.W.; Pienta, K.J.; Czernin, J.; King, M.T.; Freedland, S.J. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: Treatment based on risk stratification. Prostate Cancer Prostatic Dis. 2024, 27, 192–201. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jin, W.; Wang, R.; Zhao, R.; Zhu, L.; Kung, H.F. 68Ga/177Lu-Labeled Bivalent Agents for Targeting Hypoxia and PSMA-Binding in Prostate Cancer. J. Med. Chem. 2024, 67, 13491–13506. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.R.A.; Nanabala, R.; Joy, A.; Sasikumar, A.; Russ Knapp, F.F. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: Effective theranostic tools for imaging and therapy of prostate cancer. Nucl. Med. Biol. 2016, 43, 692–720. [Google Scholar] [CrossRef]
- Davis, M.I.; Bennett, M.J.; Thomas, L.M.; Bjorkman, P.J. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc. Natl. Acad. Sci. USA 2005, 102, 5981–5986. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Annabattula, C.; Lewis, J.; Shetty, D.V.; Kam, J.; Maclean, F.; Arianayagam, M.; Canagasingham, B.; Ferguson, R.; Khadra, M.; et al. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: Correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018, 21, 204–211. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in prostate-specific membrane antigen PET of prostate cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Corfield, J.; Perera, M.; Bolton, D.; Lawrentschuk, N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: A systematic review. World J. Urol. 2018, 36, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, V.; Di Stasio, G.D.; Mansi, L. Nuclear Medicine in Prostate Cancer: A New Era for Radiotracers. World J. Nucl. Med. 2018, 17, 70–78. [Google Scholar] [CrossRef]
- Li, R.; Ravizzini, G.C.; Gorin, M.A.; Maurer, T.; Eiber, M.; Cooperberg, M.R.; Alemozzaffar, M.; Tollefson, M.K.; Delacroix, S.E.; Chapin, B.F. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 4–21. [Google Scholar] [CrossRef]
- Allach, Y.; Banda, A.; van Gemert, W.; Groot, M.d.; Derks, Y.; Schilham, M.; Hoepping, A.; Perk, L.; Gotthardt, M.; Janssen, M.; et al. An Explorative Study of the Incidental High Renal Excretion of [18F]PSMA-1007 for Prostate Cancer PET/CT Imaging. Cancers 2022, 14, 2076. [Google Scholar] [CrossRef]
- Strauss, D.S.; Sachpekidis, C.; Kopka, K.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Pharmacokinetic studies of [68Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: Detection, differences in temporal distribution and kinetic modelling by tissue type. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.H.; Jochumsen, M.R.; Zacho, H.D.; Munk, O.L.; Gormsen, L.C. Multiparametric dynamic whole-body PSMA PET/CT using [68Ga]Ga-PSMA-11 and [18F]PSMA-1007. EJNMMI Res. 2023, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Tolvanen, T.; Kalliokoski, K.; Malaspina, S.; Kuisma, A.; Lahdenpohja, S.; Postema, E.J.; Miller, M.P.; Scheinin, M. Safety, Biodistribution, and Radiation Dosimetry of 18F-rhPSMA-7.3 in Healthy Adult Volunteers. J. Nucl. Med. 2021, 62, 679–684. [Google Scholar] [CrossRef]
- Penny, R.; Fongenie, B.; Davis, P.; Sykes, J. Normal-organ distribution of PSMA-targeting PET radiopharmaceutical 18F-flotufolastat: A post hoc analysis of the LIGHTHOUSE and SPOTLIGHT studies. Am. J. Nucl. Med. Mol. Imaging 2024, 14, 337–344. [Google Scholar] [CrossRef]
- Hope, T.A.; Jadvar, H. PSMA PET AUC Updates: Inclusion of rh-PSMA-7.3. J. Nucl. Med. 2024, 65, 540. [Google Scholar] [CrossRef]
- Langbein, T.; Wang, H.; Rauscher, I.; Kroenke, M.; Knorr, K.; Wurzer, A.; Schwamborn, K.; Maurer, T.; Horn, T.; Haller, B.; et al. Utility of 18F-rhPSMA-7.3 PET for Imaging of Primary Prostate Cancer and Preoperative Efficacy in N-Staging of Unfavorable Intermediate- to Very High-Risk Patients Validated by Histopathology. J. Nucl. Med. 2022, 63, 1334–1342. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Dimitrakopoulou-Strauss, A. (German Cancer Research Center, Heidelberg, Germany). Personal Communication, 2025.
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2015, 42, 197–209. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-P.; Kübler, H.; Haberkorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.; Wong, J.Y.C.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Patena, E.; Giżewska, A.; Dziuk, M.; Miśko, J.; Budzyńska, A.; Walęcka-Mazur, A. Diagnostic performance of 18F-PSMA-1007 PET/CT in biochemically relapsed patients with prostate cancer with PSA levels ≤ 2.0 ng/ml. Prostate Cancer Prostatic Dis. 2020, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Krebs, M.; Polat, B.; Linke, F.; Eiber, M.; Samnick, S.; Lapa, C.; Lassmann, M.; Riedmiller, H.; Czernin, J.; et al. 68Ga-PSMA-PET/CT in Patients with Biochemical Prostate Cancer Recurrence and Negative 18F-Choline-PET/CT. Clin. Nucl. Med. 2016, 41, 515. [Google Scholar] [CrossRef]
- Rizzo, A.; Morbelli, S.; Albano, D.; Fornarini, G.; Cioffi, M.; Laudicella, R.; Dondi, F.; Grimaldi, S.; Bertagna, F.; Racca, M.; et al. The Homunculus of unspecific bone uptakes associated with PSMA-targeted tracers: A systematic review-based definition. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3753–3764. [Google Scholar] [CrossRef]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef]
- Sahlmann, C.-O.; Meller, B.; Bouter, C.; Ritter, C.O.; Ströbel, P.; Lotz, J.; Trojan, L.; Meller, J.; Hijazi, S. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 898–905. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a [69]Ga-labelled PSMA ligand and [18]F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2014, 41, 11–20. [Google Scholar] [CrossRef]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; Oikonomou, E.; De Dosso, S.; Jermini, F.; Prior, J.O.; Roggero, E.; et al. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: A meta-analysis. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 127–139. [Google Scholar]
- Yu, W.; Zhao, M.; Deng, Y.; Liu, S.; Du, G.; Yan, B.; Zhao, Z.; Sun, N.; Guo, J. Meta-analysis of 18 F-PSMA-1007 PET/CT, 18 F-FDG PET/CT, and 68Ga-PSMA PET/CT in diagnostic efficacy of prostate Cancer. Cancer Imaging 2023, 23, 77. [Google Scholar] [CrossRef]
- Scarsbrook, A.F.; Bottomley, D.; Teoh, E.J.; Bradley, K.M.; Payne, H.; Afaq, A.; Bomanji, J.; Van As, N.; Chua, S.; Hoskin, P.; et al. Effect of 18F-Fluciclovine Positron Emission Tomography on the Management of Patients With Recurrence of Prostate Cancer: Results from the FALCON Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 316–324. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer with Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.B.; Ravizzini, G.C.; Gartrell, B.A.; Siegel, B.A.; Twardowski, P.; Saltzstein, D.; Fleming, M.T.; Chau, A.; Davis, P.; Chapin, B.F.; et al. Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men with Suspected Prostate Cancer Recurrence: Results from a Phase 3, Prospective, Multicenter Study (SPOTLIGHT). J. Urol. 2023, 210, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lowentritt, B.H.; Jani, A.B.; Helfand, B.T.; Uchio, E.M.; Morris, M.A.; Michalski, J.M.; Chau, A.; Davis, P.; Chapin, B.F.; Schuster, D.M. Impact of Clinical Factors on 18F-Flotufolastat Detection Rates in Men with Recurrent Prostate Cancer: Exploratory Analysis of the Phase 3 SPOTLIGHT Study. Adv. Radiat. Oncol. 2024, 9, 101532. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of 18F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef]
- Tateishi, U.; Kimura, K.; Tsuchiya, J.; Kano, D.; Watabe, T.; Nonomura, N.; Saito, K.; Yokoyama, K.; Yamagiwa, K.; Adachi, T.; et al. Phase I/IIa trial of 18F-prostate specific membrane antigen (PSMA) 1007 PET/CT in healthy volunteers and prostate cancer patients. Jpn. J. Clin. Oncol. 2024, 54, 282–291. [Google Scholar] [CrossRef]
- Ahmadi Bidakhvidi, N.; Laenen, A.; Jentjens, S.; Deroose, C.M.; Van Laere, K.; De Wever, L.; Mai, C.; Berghen, C.; De Meerleer, G.; Haustermans, K.; et al. Parameters predicting [18F]PSMA-1007 scan positivity and type and number of detected lesions in patients with biochemical recurrence of prostate cancer. EJNMMI Res. 2021, 11, 41. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baltzer, P.; D’Andrea, D.; Korn, S.; Haug, A.R.; Hacker, M.; Grubmüller, K.H.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy—Diagnostic performance and impact on therapeutic decision-making. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 235–242. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging. 2017, 44, 1258–1268. [Google Scholar] [CrossRef]
- Calais, J.; Czernin, J.; Fendler, W.P.; Elashoff, D.; Nickols, N.G. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019, 19, 18. [Google Scholar] [CrossRef]
- Ceci, F.; Uprimny, C.; Nilica, B.; Geraldo, L.; Kendler, D.; Kroiss, A.; Bektic, J.; Horninger, W.; Lukas, P.; Decristoforo, C.; et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Dyankova, M.; Dancheva, Z.; Dokova, K.; Klisarova, A. Evaluation of hybrid PET/CT imaging with the 68Ga-labelled PSMA ligand in patients with prostate cancer and biochemical progression in the low-range values of PSA after radical prostatectomy. Scr. Sci. Med. 2022, 54, 29–39. [Google Scholar] [CrossRef]
- Rowe, S.P.; Campbell, S.P.; Mana-Ay, M.; Szabo, Z.; Allaf, M.E.; Pienta, K.J.; Pomper, M.G.; Ross, A.E.; Gorin, M.A. Prospective Evaluation of PSMA-Targeted 18F-DCFPyL PET/CT in Men with Biochemical Failure After Radical Prostatectomy for Prostate Cancer. J. Nucl. Med. 2020, 61, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Wondergem, M.; Jansen, B.H.E.; Van Der Zant, F.M.; Van Der Sluis, T.M.; Knol, R.J.J.; Van Kalmthout, L.W.M.; Hoekstra, O.S.; Van Moorselaar, R.J.A.; Oprea-Lager, D.E.; Vis, A.N. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1911–1918. [Google Scholar] [CrossRef]
- Mena, E.; Lindenberg, M.L.; Turkbey, I.B.; Shih, J.H.; Harmon, S.A.; Lim, I.; Lin, F.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. 18F-DCFPyL PET/CT Imaging in Patients with Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J. Nucl. Med. 2020, 61, 881–889. [Google Scholar] [CrossRef]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Bénard, F. A Prospective Study on 18F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef]
- Olivier, P.; Giraudet, A.-L.; Skanjeti, A.; Merlin, C.; Weinmann, P.; Rudolph, I.; Hoepping, A.; Gauthé, M. Phase III Study of 18F-PSMA-1007 Versus 18F-Fluorocholine PET/CT for Localization of Prostate Cancer Biochemical Recurrence: A Prospective, Randomized, Crossover Multicenter Study. J. Nucl. Med. 2023, 64, 579–585. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, D.; Kim, H.J.; Oh, K.T.; Kim, S.J.; Choi, Y.D.; Giesel, F.L.; Kopka, K.; Hoepping, A.; et al. Combination of [18F]FDG and [18F]PSMA-1007 PET/CT predicts tumour aggressiveness at staging and biochemical failure postoperatively in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1763–1772. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Jiang, X.; Wang, X.; Chen, S.; Shen, T.; You, J.; Lu, H.; Liao, H.; Li, Z.; et al. Intra-Individual Comparison of 18F-PSMA-1007 and 18F-FDG PET/CT in the Evaluation of Patients With Prostate Cancer. Front. Oncol. 2021, 10, 585213. [Google Scholar] [CrossRef]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Loeff, C.C.; van Gemert, W.; Privé, B.M.; van Oort, I.M.; Hermsen, R.; Somford, D.M.; Nagarajah, J.; Heijmen, L.; Janssen, M.J.R. [18F]PSMA-1007 PET for biochemical recurrence of prostate cancer, a comparison with [18F]Fluciclovine. EJNMMI Rep. 2024, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Bütikofer, L.; Rominger, A.; Afshar-Oromieh, A. A randomised, prospective and head-to-head comparison of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 for the detection of recurrent prostate cancer in PSMA-ligand PET/CT-Protocol design and rationale. PLoS ONE 2022, 17, e0270269. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual Comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Gage, K.L.; Faraj, S.F.; Macura, K.J.; Cornish, T.C.; Gonzalez-Roibon, N.; Guner, G.; Munari, E.; Partin, A.W.; Pavlovich, C.P.; et al. 18F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J. Nucl. Med. 2015, 56, 1003–1010. [Google Scholar] [CrossRef]
- Turkbey, B.; Mena, E.; Lindenberg, L.; Adler, S.; Bednarova, S.; Berman, R.; Ton, A.T.; McKinney, Y.; Eclarinal, P.; Hill, C.; et al. 18F-DCFBC Prostate-Specific Membrane Antigen-Targeted PET/CT Imaging in Localized Prostate Cancer: Correlation With Multiparametric MRI and Histopathology. Clin. Nucl. Med. 2017, 42, 735–740. [Google Scholar] [CrossRef]
- Dietlein, F.; Hohberg, M.; Kobe, C.; Zlatopolskiy, B.D.; Krapf, P.; Endepols, H.; Täger, P.; Hammes, J.; Heidenreich, A.; Neumaier, B.; et al. An 18F-Labeled PSMA Ligand for PET/CT of Prostate Cancer: First-in-Humans Observational Study and Clinical Experience with 18F-JK-PSMA-7 During the First Year of Application. J. Nucl. Med. 2020, 61, 202–209. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Endepols, H.; Krapf, P.; Guliyev, M.; Urusova, E.A.; Richarz, R.; Hohberg, M.; Dietlein, M.; Drzezga, A.; Neumaier, B. Discovery of 18F-JK-PSMA-7, a PET Probe for the Detection of Small PSMA-Positive Lesions. J. Nucl. Med. 2019, 60, 817–823. [Google Scholar] [CrossRef]
- Piron, S.; De Man, K.; Schelfhout, V.; Van Laeken, N.; Kersemans, K.; Achten, E.; De Vos, F.; Ost, P. Optimization of PET protocol and interrater reliability of 18F-PSMA-11 imaging of prostate cancer. EJNMMI Res. 2020, 10, 14. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Xu, X.; Liu, F.; Guo, X.; Li, N.; Wang, X.; Yang, J.; Yang, X.; Zhu, H.; et al. Preclinical Evaluation and Pilot Clinical Study of Al18F-PSMA-BCH for Prostate Cancer PET Imaging. J. Nucl. Med. 2019, 60, 1284–1292. [Google Scholar] [CrossRef]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef]
- Suh, M.; Ryoo, H.G.; Kang, K.W.; Jeong, J.M.; Jeong, C.W.; Kwak, C.; Cheon, G.J. Phase I Clinical Trial of Prostate-Specific Membrane Antigen-Targeting 68Ga-NGUL PET/CT in Healthy Volunteers and Patients with Prostate Cancer. Korean J. Radiol. 2022, 23, 911. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zang, J.; Wang, H.; Liu, Q.; Li, F.; Lin, Y.; Huo, L.; Jacobson, O.; Niu, G.; Fan, X.; et al. Pretherapeutic 68Ga-PSMA-617 PET May Indicate the Dosimetry of 177Lu-PSMA-617 and 177Lu-EB-PSMA-617 in Main Organs and Tumor Lesions. Clin. Nucl. Med. 2019, 44, 431. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Gai, Y.; Liu, Q.; Ruan, W.; Liu, F.; Hu, F.; Zhang, X.; Lan, X. Optimized Application of 68Ga-Prostate-Specific Membrane Antigen-617 Whole-Body PET/CT and Pelvic PET/MR in Prostate Cancer Initial Diagnosis and Staging. Front. Med. 2021, 8, 657619. [Google Scholar] [CrossRef]
- Frenzel, T.; Tienken, M.; Abel, M.; Berliner, C.; Klutmann, S.; Beyersdorff, D.; Schwarz, R.; Krüll, A.; Bannas, P. The impact of [68Ga]PSMA I&T PET/CT on radiotherapy planning in patients with prostate cancer. Strahlenther. Onkologie 2018, 194, 646–654. [Google Scholar] [CrossRef]
- Schmuck, S.; Klot, C.A.v.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial Experience with Volumetric 68Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Cytawa, W.; Seitz, A.K.; Kircher, S.; Fukushima, K.; Tran-Gia, J.; Schirbel, A.; Bandurski, T.; Lass, P.; Krebs, M.; Połom, W.; et al. 68Ga-PSMA I&T PET/CT for primary staging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 168–177. [Google Scholar] [CrossRef]
- Gühne, F.; Radke, S.; Winkens, T.; Kühnel, C.; Greiser, J.; Seifert, P.; Drescher, R.; Freesmeyer, M. Differences in Distribution and Detection Rate of the [68Ga]Ga-PSMA Ligands PSMA-617, -I&T and -11—Inter-Individual Comparison in Patients with Biochemical Relapse of Prostate Cancer. Pharmaceuticals 2022, 15, 9. [Google Scholar] [CrossRef]
- Privé, B.M.; Derks, Y.H.W.; Rosar, F.; Franssen, G.M.; Peters, S.M.B.; Khreish, F.; Bartholomä, M.; Maus, S.; Gotthardt, M.; Laverman, P.; et al. 89Zr-labeled PSMA ligands for pharmacokinetic PET imaging and dosimetry of PSMA-617 and PSMA-I&T: A preclinical evaluation and first in man. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2064–2076. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. 64Cu-PSMA-617 PET/CT Imaging of Prostate Adenocarcinoma: First In-Human Studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Hoberück, S.; Wunderlich, G.; Michler, E.; Hölscher, T.; Walther, M.; Seppelt, D.; Platzek, I.; Zöphel, K.; Kotzerke, J. Dual-time-point 64Cu-PSMA-617-PET/CT in patients suffering from prostate cancer. J. Label. Compd. Radiopharm. 2019, 62, 523–532. [Google Scholar] [CrossRef]
- Cantiello, F.; Crocerossa, F.; Russo, G.I.; Gangemi, V.; Ferro, M.; Vartolomei, M.D.; Lucarelli, G.; Mirabelli, M.; Scafuro, C.; Ucciero, G.; et al. Comparison Between 64Cu-PSMA-617 PET/CT and 18F-Choline PET/CT Imaging in Early Diagnosis of Prostate Cancer Biochemical Recurrence. Clin. Genitourin. Cancer 2018, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- SOLAR: Copper Cu64 PSMA I&T Injection in Patients with Histologically Proven Metastatic Prostate Cancer. Available online: https://www.curiumpharma.com/resources/current-clinical-trials/solar-clinical-trial/ (accessed on 19 April 2025).
- Bernabeu, T.B.; Mansi, R.; Pozzo, L.D.; Zanger, S.; Gaonkar, R.H.; McDougall, L.; Rose, F.D.; Jaafar-Thiel, L.; Herz, M.; Eiber, M.; et al. 61Cu-PSMA–Targeted PET for Prostate Cancer: From Radiotracer Development to First-in-Human Imaging. J. Nucl. Med. 2024, 65, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- NUCLIDIUM Announces First Patient Imaged in Phase 1 Study Evaluating 61Cu-based Radiotracer in Patients with PSMA-positive Prostate Cancer—Nuclidium. Available online: https://nuclidium.com/nuclidium-announces-first-patient-imaged-in-phase-1-study-evaluating-61cu-based-radiotracer-in-patients-with-psma-positive-prostate-cancer/ (accessed on 19 April 2025).
- Yang, N.; Guo, X.-y.; Ding, J.; Wang, F.; Liu, T.-l.; Zhu, H.; Yang, Z. Copper-64 Based PET-Radiopharmaceuticals: Ways to Clinical Translational. Semin. Nucl. Med. 2024, 54, 792–800. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, J.; Pan, Y.; Wen, H.; Xu, X.; Wu, S.; Wang, Y.; Zhang, C.; Ma, G.; et al. Comparison of 64Cu-DOTA-PSMA-3Q and 64Cu-NOTA-PSMA-3Q utilizing NOTA and DOTA as bifunctional chelators in prostate cancer: Preclinical assessment and preliminary clinical PET/CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2792–2803. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhan, Y.; Huang, X.; He, Z.; Ma, D.; Tang, T.; Li, S. Preclinical and clinical evaluation of [64Cu]Cu-PSMA-Q PET/CT for prostate cancer detection and its comparison with [18F]FDG imaging. Sci. Rep. 2025, 15, 14431. [Google Scholar] [CrossRef] [PubMed]
- Clarity Receives, U.S. FDA Fast Track Designation for Cu-64 SAR-bisPSMA in Biochemical Recurrence of Prostate Cancer. Available online: https://www.claritypharmaceuticals.com/news/ftd-2/ (accessed on 19 April 2025).
- Registrational Phase III CLARIFY Trial in Prostate Cancer Commences. Available online: https://www.claritypharmaceuticals.com/news/clarifyphase3/ (accessed on 11 June 2025).
- 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for Identification and Treatment of PSMA-Expressing Metastatic Castrate Resistant Prostate Cancer (SECuRE). Available online: https://clinicaltrials.gov/study/NCT04868604 (accessed on 19 April 2025).
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.R.; Johnston, K.; Benešová, M.; Senftleben, S.; Müller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical Investigations and First-in-Human Application of 152Tb-PSMA-617 for PET/CT Imaging of Prostate Cancer. EJNMMI Res. 2019, 9, 68. [Google Scholar] [CrossRef]
- Eppard, E.; de la Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical Translation and First In-Human Use of [44Sc]Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef]
- Khawar, A.; Eppard, E.; Sinnes, J.P.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. [44Sc]Sc-PSMA-617 Biodistribution and Dosimetry in Patients With Metastatic Castration-Resistant Prostate Carcinoma. Clin. Nucl. Med. 2018, 43, 323. [Google Scholar] [CrossRef]
- Kepenek, F.; Can, C.; Kömek, H.; Kaplan, İ.; Gündoğan, C.; Ebinç, S.; Güzel, Y.; Agüloglu, N.; Karaoglan, H.; Taşdemir, B. Combination of [68Ga]Ga-PSMA PET/CT and [18F]FDG PET/CT in demonstrating dedifferentiation in castration-resistant prostate cancer. Médecine Nucléaire 2023, 47, 193–199. [Google Scholar] [CrossRef]
- Khreish, F.; Ribbat, K.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Schreckenberger, M.; Ezziddin, S.; Rosar, F. Value of Combined PET Imaging with [18F]FDG and [68Ga]Ga-PSMA-11 in mCRPC Patients with Worsening Disease during [177Lu]Lu-PSMA-617 RLT. Cancers 2021, 13, 4134. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; Liu, J. The Added Value of 18F-FDG PET/CT Compared with 68Ga-PSMA PET/CT in Patients with Castration-Resistant Prostate Cancer. J. Nucl. Med. 2022, 63, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, T.; Chen, S.; Bu, T.; Zhao, J.; Ni, X.; Shi, B.; Gan, H.; Wei, Y.; Wang, Q.; et al. Nomogram to predict the presence of PSMA-negative but FDG-positive lesion in castration-resistant prostate cancer: A multicenter cohort study. Ther. Adv. Med. Oncol. 2024, 16, 17588359231220506. [Google Scholar] [CrossRef]
- Mapelli, P.; Ghezzo, S.; Samanes Gajate, A.M.; Preza, E.; Brembilla, G.; Cucchiara, V.; Ahmed, N.; Bezzi, C.; Presotto, L.; Bettinardi, V.; et al. Preliminary Results of an Ongoing Prospective Clinical Trial on the Use of 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in Staging of High-Risk Prostate Cancer Patients. Diagnostics 2021, 11, 2068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).