Triple Non-Statin Therapy with Ezetimibe, Inclisiran, and Bempedoic Acid in Patients with Genetically Confirmed Statin-Induced Rhabdomyolysis: A Dual Case Report
Abstract
1. Introduction
2. Case Presentation
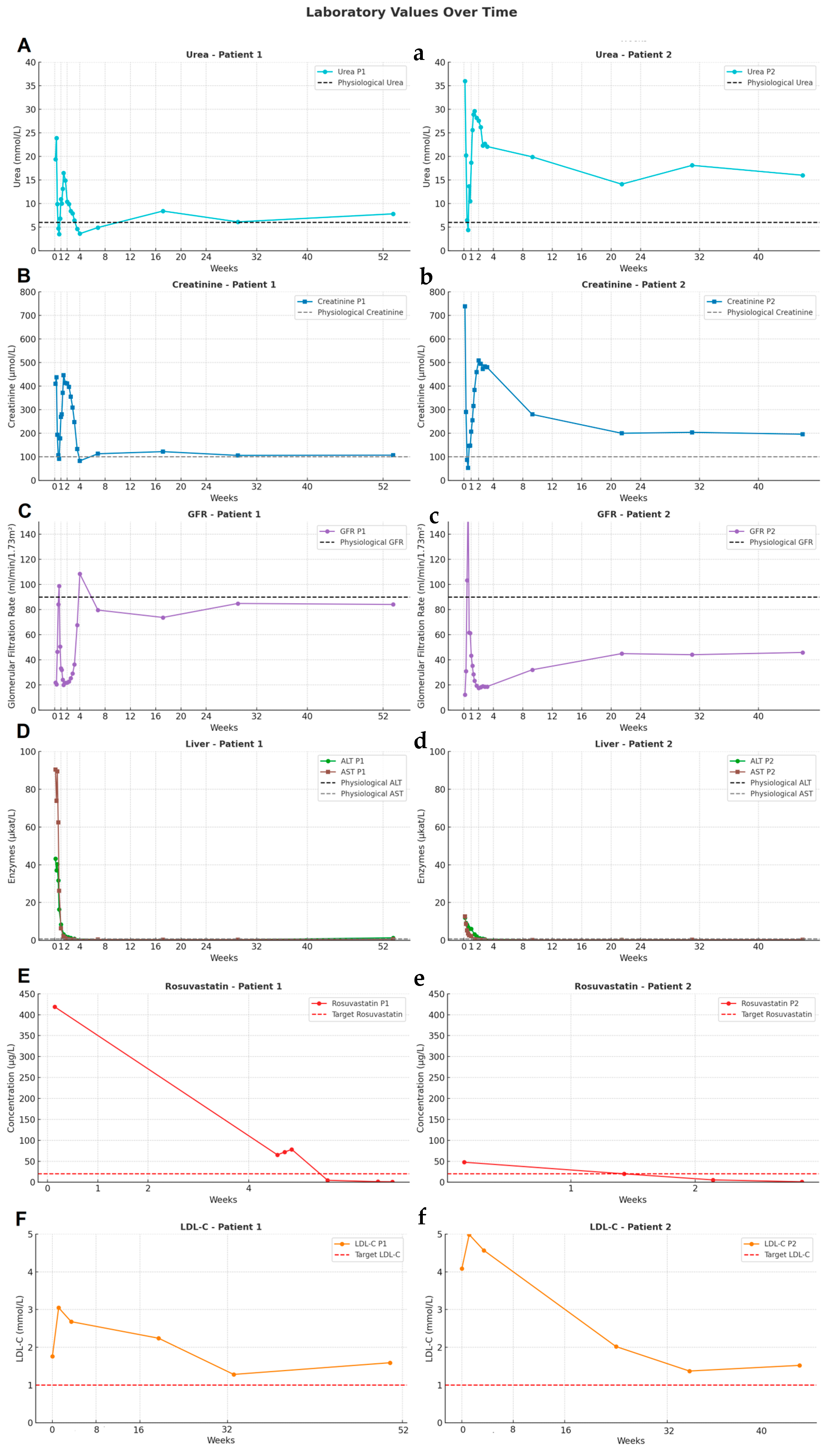
2.1. Patient 1
2.2. Patient 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarrarapu, S.N.S.; Goyal, A.; Venkata, V.S.; Panchal, V.; Sivasubramanian, B.P.; Du, D.T.; Jakulla, R.S.; Pamulapati, H.; Afaq, M.A.; Owens, S.; et al. Comprehensive review of statin-intolerance and the practical application of Bempedoic Acid. J. Cardiol. 2024, 84, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC Scientific Document Group, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Guyton, J.R. Benefit versus risk in statin treatment. Am. J. Cardiol. 2006, 97, 95C–97C. [Google Scholar] [CrossRef] [PubMed]
- Naritaka, H.; Aoki, Y.; Obata, Y.; Mimuro, S.; Nakajima, Y. Rhabdomyolysis in a Long-Term Statin User Without Traditional Risk Factors: A Case Report. Cureus 2023, 15, e46069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, E.M.; Davidson, M.H. PCSK9 Inhibitors: Mechanism of Action, Efficacy, and Safety. Rev. Cardiovasc. Med. 2018, 19, S31–S46. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, L.B.; Johnson, S.G.; Caudle, K.E.; Haidar, C.E.; Voora, D.; Wilke, R.A.; Maxwell, W.D.; McLeod, H.L.; Krauss, R.M.; Roden, D.M.; et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin. Pharmacol. Ther. 2014, 96, 423–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J. The safety of statins in clinical practice. Lancet 2007, 370, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M.; CLEAR Harmony Trial. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Burnett, H.; Cichewicz, A.; Natani, H.; Bhowmik, D.; Buesch, K.; Fahrbach, K.; Reichelt, A.; Neupane, B.; Pierre, V.; Jindal, R. Comparative Efficacy of Non-Statin Lipid-Lowering Therapies in Patients With Hypercholesterolemia at Increased Cardiovascular Risk: An Updated Network Meta-Analysis. J Cardiovasc. Pharmacol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- SEARCH Collaborative Group; Link, E.; Parish, S.; Armitage, J.; Bowman, L.; Heath, S.; Matsuda, F.; Gut, I.; Lathrop, M.; Collins, R. SLCO1B1 variants and statin-induced myopathy—A genomewide study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, R.C.; Smith, S.C., Jr.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C.; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J. Am. Coll. Cardiol. 2002, 40, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Sirtori, C.R.; Ferri, N.; Corsini, A. New players in the treatment of hypercholesterolaemia: Focus on bempedoic acid and inclisiran. Eur. Heart J. Suppl. 2021, 23 (Suppl. E), E59–E62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lütjohann, D.; Stellaard, F. Degree of serum LDL cholesterol reduction by simvastatin and ezetimibe is dependent on baseline LDL cholesterol concentration but not on baseline values and changes in cholesterol synthesis and absorption parameters. Int. J. Clin. Pharmacol. Ther. 2024, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B. Safety of Statins and Nonstatins for Treatment of Dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2022, 51, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
| (A) Patient 1 | (B) Patient 2 | |||
|---|---|---|---|---|
| Parameter | Before (mmol/L) | After (mmol/L) | Before (mmol/L) | After (mmol/L) |
| TC | 4.02 | 2.63 | 6.99 | 3.48 |
| HDL-C | 1.04 | 0.89 | 1.21 | 1.6 |
| Non-HDL-C | 2.98 | 1.74 | 5.78 | 1.88 |
| LDL-C | 3.05 | 1.59 | 4.99 | 1.52 |
| TAG | 1.4 | 1.07 | 2.57 | 1.25 |
| APOA1 | 1.24 | 1.05 | 1.04 | 1.58 |
| APOB | 0.86 | 0.51 | 1.36 | 0.57 |
| Lp(a) | 0.804 | 0.859 | 1.54 | 1.11 |
| (A) Patient 1 | (B) Patient 2 | |||
|---|---|---|---|---|
| Parameter | Before (mg/dL) | After (mg/dL) | Before (mg/dL) | After (mg/dL) |
| TC | 155.5 | 101.7 | 270.3 | 134.6 |
| HDL-C | 40.2 | 34.4 | 46.8 | 61.9 |
| Non-HDL-C | 115.2 | 67.3 | 223.5 | 72.7 |
| LDL-C | 124 | 94.8 | 227.6 | 110.7 |
| TAG | 48 | 40.6 | 40.2 | 61.1 |
| APOA1 | 33.3 | 19.7 | 52.6 | 22 |
| APOB | 31.1 | 33.2 | 59.8 | 42.9 |
| Lp(a) | 117.9 | 61.5 | 193 | 58.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodulík, J.; Plášek, J.; Kacířová, I.; Uřinovská, R.; Vrtal, J.; Václavík, J. Triple Non-Statin Therapy with Ezetimibe, Inclisiran, and Bempedoic Acid in Patients with Genetically Confirmed Statin-Induced Rhabdomyolysis: A Dual Case Report. Pharmaceuticals 2025, 18, 818. https://doi.org/10.3390/ph18060818
Dodulík J, Plášek J, Kacířová I, Uřinovská R, Vrtal J, Václavík J. Triple Non-Statin Therapy with Ezetimibe, Inclisiran, and Bempedoic Acid in Patients with Genetically Confirmed Statin-Induced Rhabdomyolysis: A Dual Case Report. Pharmaceuticals. 2025; 18(6):818. https://doi.org/10.3390/ph18060818
Chicago/Turabian StyleDodulík, Jozef, Jiří Plášek, Ivana Kacířová, Romana Uřinovská, Jiří Vrtal, and Jan Václavík. 2025. "Triple Non-Statin Therapy with Ezetimibe, Inclisiran, and Bempedoic Acid in Patients with Genetically Confirmed Statin-Induced Rhabdomyolysis: A Dual Case Report" Pharmaceuticals 18, no. 6: 818. https://doi.org/10.3390/ph18060818
APA StyleDodulík, J., Plášek, J., Kacířová, I., Uřinovská, R., Vrtal, J., & Václavík, J. (2025). Triple Non-Statin Therapy with Ezetimibe, Inclisiran, and Bempedoic Acid in Patients with Genetically Confirmed Statin-Induced Rhabdomyolysis: A Dual Case Report. Pharmaceuticals, 18(6), 818. https://doi.org/10.3390/ph18060818








