Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. WA Improves Cognitive Deficits in 5xFAD Mice
- To assess whether chronic WA treatment improves cognitive function in 5xFAD mice, we conducted a series of behavioral tests in transgenic AD amyloidosis mouse models. Female 5xFAD mice were treated with WA (2 mg/kg every 2 days) or 1% (v/v) dimethyl sulfoxide (DMSO) as control for 14 days.
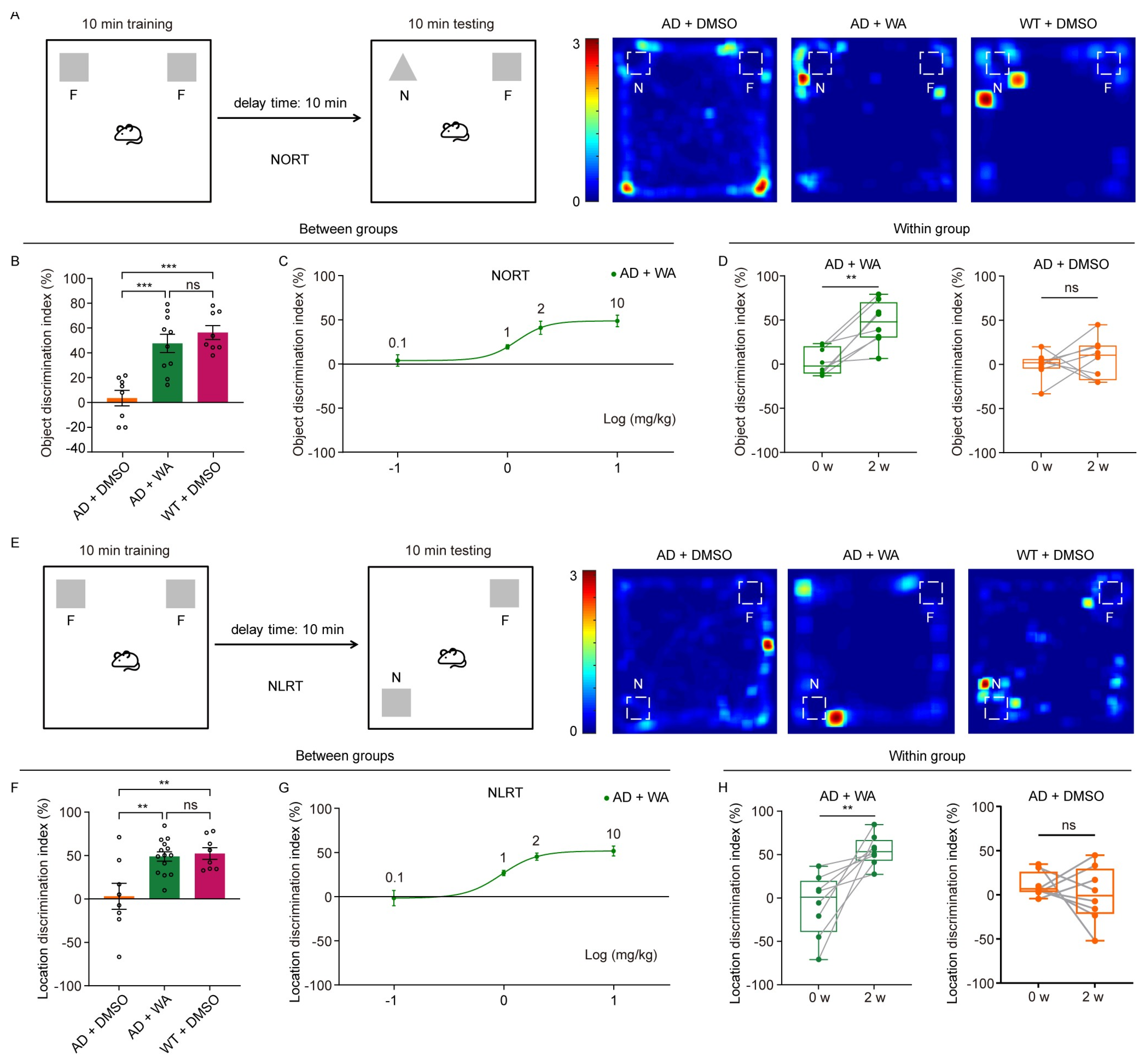
- To assess the effect of WA on short-term memory in 5xFAD mice, we first performed the Novel Object Recognition Test (NORT) and Novel Location Recognition Test (NLRT) (Figure 1). The mice were placed in an open-field box with two identical objects and allowed to explore freely for 10 min (Figure 1A,E). After a 10 min delay, the same mice were reintroduced to the box, where one object was replaced with a novel object (NORT) or relocated to a new location (NLRT), and exploration was recorded for another 10 min. The discrimination index, calculated as the difference in time spent exploring novel versus familiar objects (NORT) or locations (NLRT), was used to assess cognitive function.
- Compared to age-matched AD control and wild-type (WT) mice, WA-treated mice exhibited significantly improved performance in both NORT and NLRT, as indicated by an increased discrimination index for novel objects (Figure 1B,C) and novel locations (Figure 1F,G). In contrast, AD control mice showed no significant improvement (Figure 1D,H).
- To evaluate the dose-dependent effects of WA, we tested multiple doses (0.1, 1, 2, and 10 mg/kg) in 5xFAD mice (Figure 1C,G). While lower doses (0.1–1 mg/kg) had no significant impact on non-spatial or spatial memory, higher doses (2 and 10 mg/kg) significantly enhanced cognitive performance, suggesting a dose-dependent effect of WA on cognitive function.
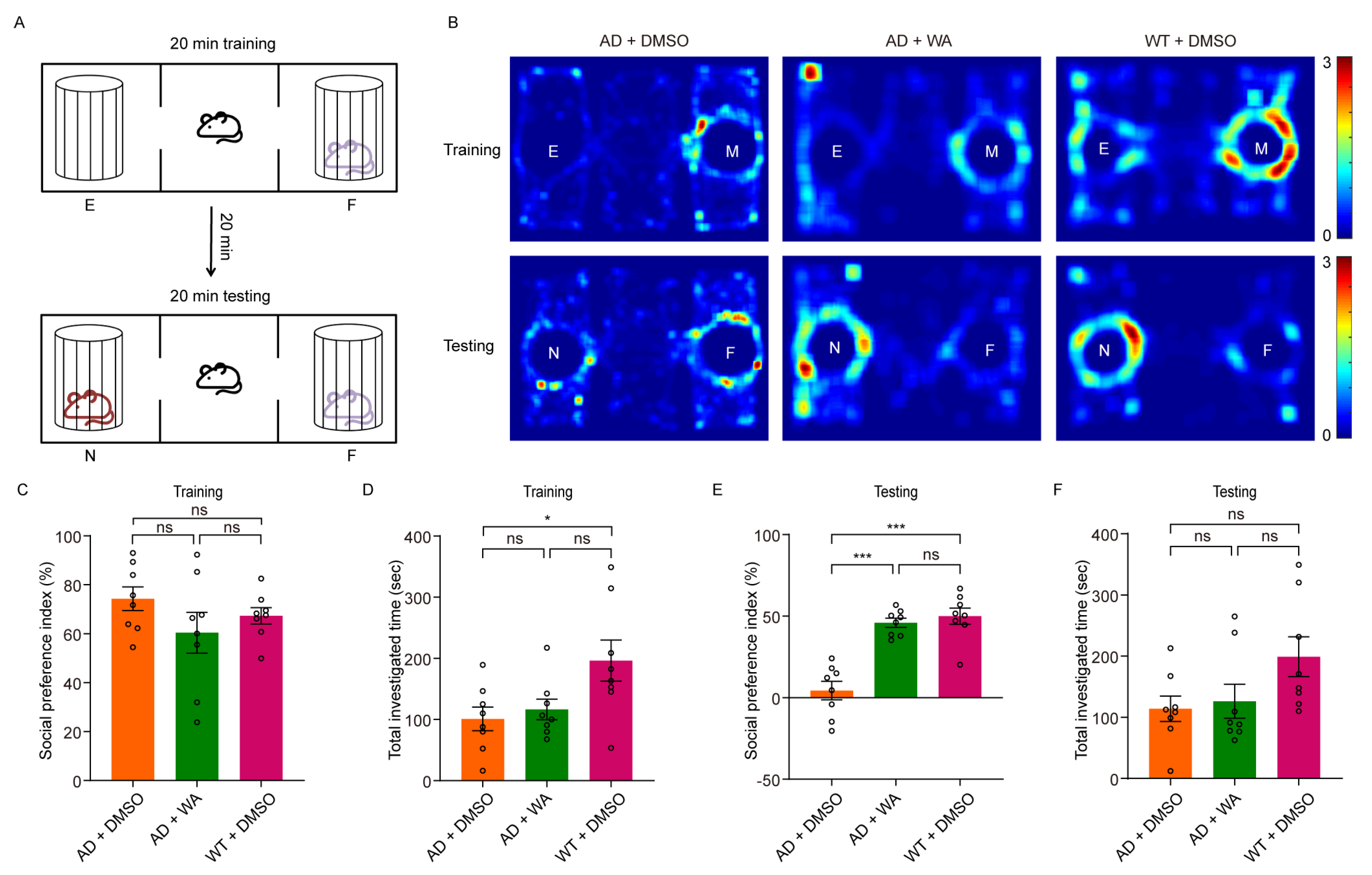
- Next, we assessed social memory using the Three-Chamber Social Test (TCST) after 14 days of WA treatment. During the training phase, mice were placed in a chamber with one compartment containing a stimulus mouse and another empty compartment, allowing free exploration for 20 min (Figure 2A,B). No differences were observed in social preference or investigation time between the WA-treated and AD control groups during this phase (Figure 2C,D). However, WA-treated mice demonstrated a significant preference for the chamber containing an unfamiliar mouse during the testing phase (Figure 2E). Despite this, no significant difference was observed in the overall investigation time (Figure 2F).
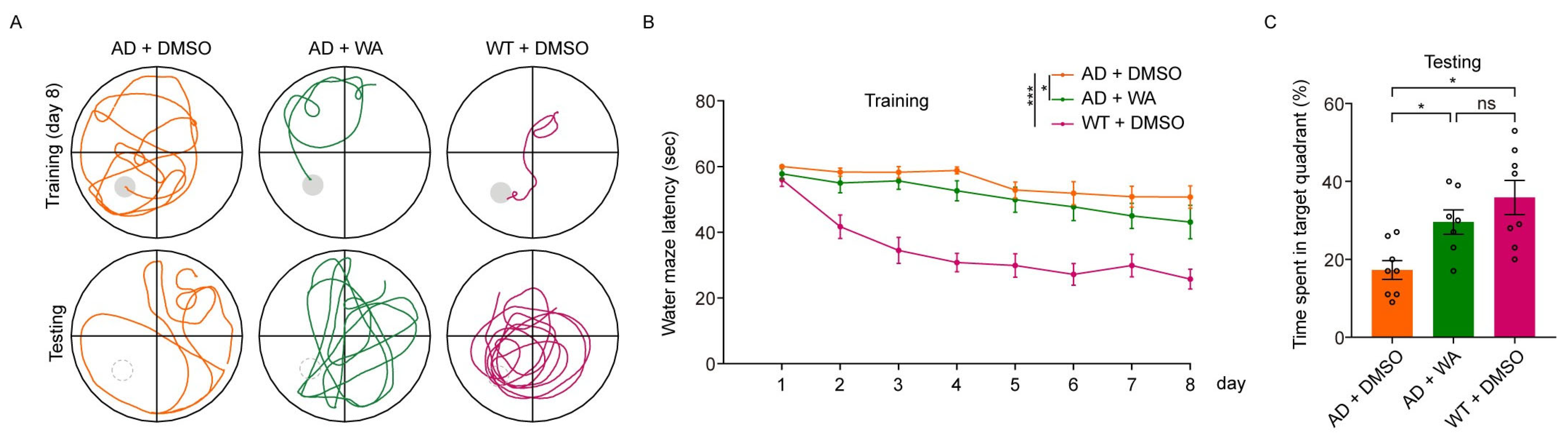
- To assess long-term memory, we conducted the Morris Water Maze Test (MWMT). As shown in Figure 3B, WA treatment ameliorated learning deficits in 5xFAD mice, with a notable reduction in escape latency compared to AD control mice. In the probe test on day 9, WA-treated mice crossed the target quadrant more frequently and spent more time in the target quadrant than AD control mice (Figure 3A,C), indicating improved spatial memory function.
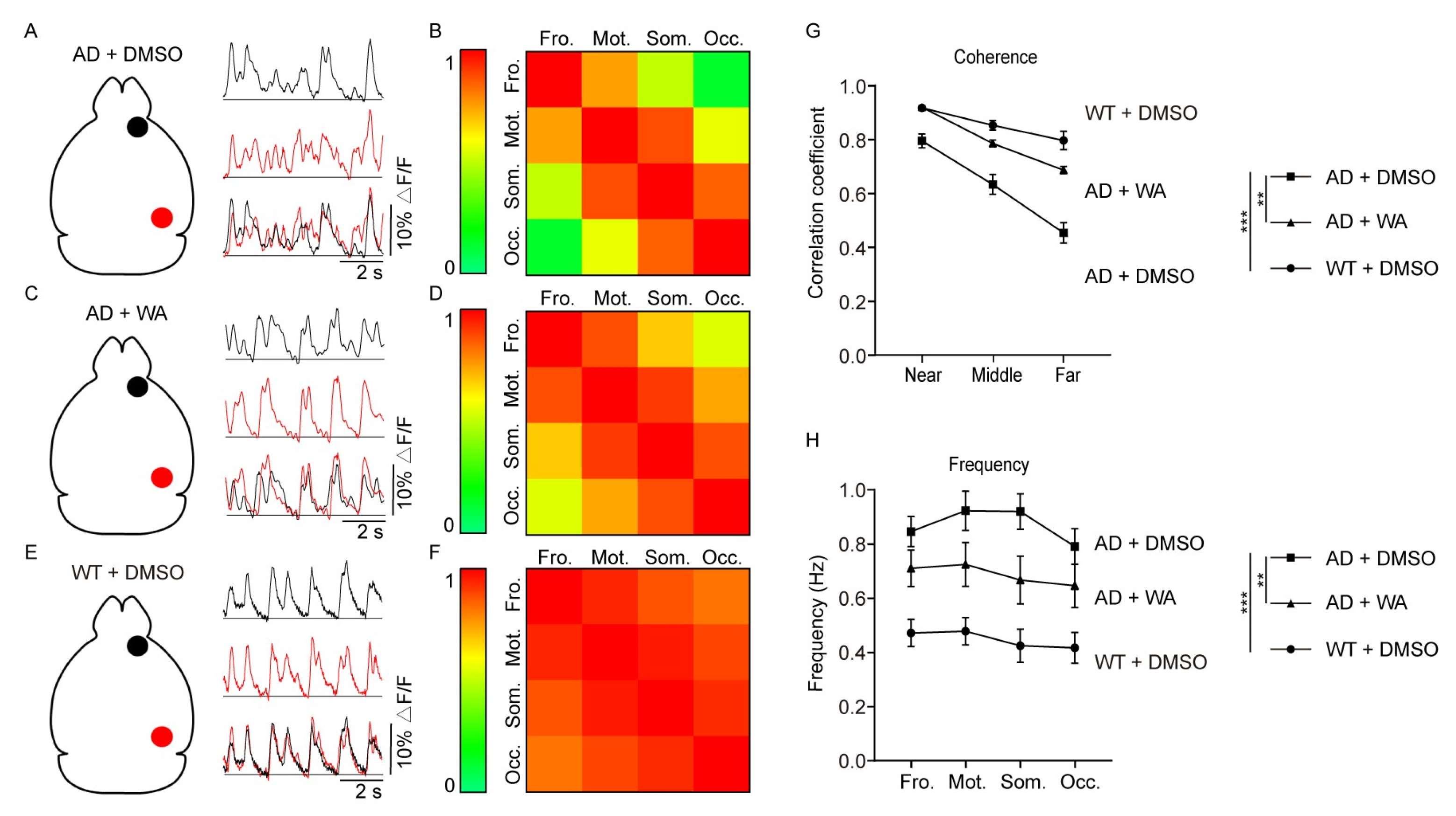
2.2. WA Treatment Improves Long-Range Coherence of Slow-Wave Activity
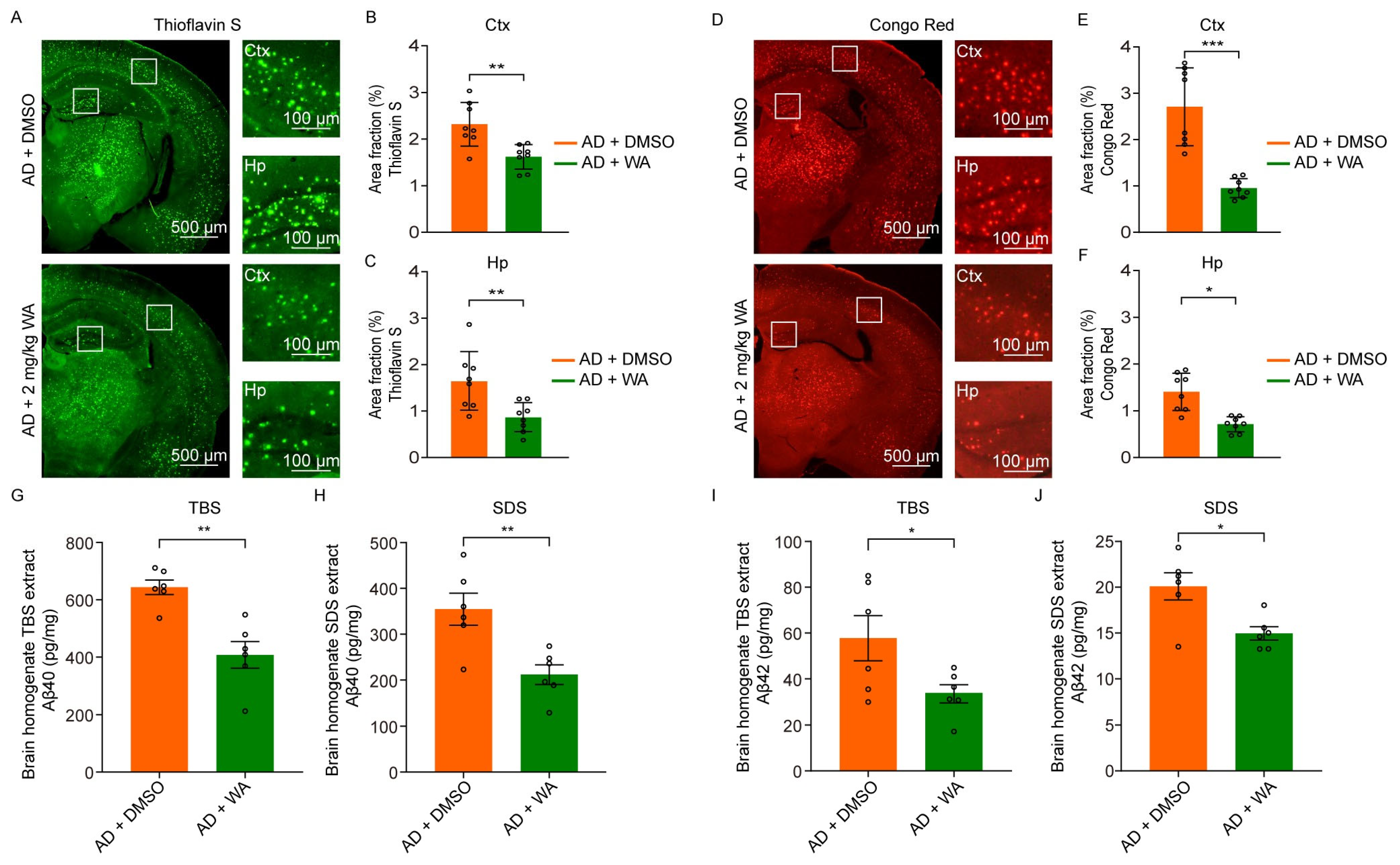
2.3. WA Reduces Aβ Deposition in 5xFAD Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. WA Treatment
4.3. Novel Object Recognition/Novel Location Recognition Test (NORT/NLRT)
4.4. Three-Chamber Social Test (TCST)
4.5. Morris Water Maze Test (MWMT)
4.6. Wide-Field Fluorescence Imaging
4.7. Imaging Data Analysis
4.8. Thioflavin S Staining
4.9. Congo Red Staining
4.10. Aβ Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WA | Withaferin A |
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| NORT | Novel Object Recognition Test |
| NLRT | Novel Location Recognition Test |
| TCST | Three-Chamber Social Test |
| MWMT | Morris Water Maze Test |
| ELISA | Enzyme-linked immunosorbent assay |
| DMSO | Dimethyl sulfoxide |
| WT | Wild type |
| 2-CAFE | 2-Channel Alternating exposure wide-Field Explorer |
| TBS | Tris-buffered saline |
| SDS | Sodium dodecyl sulfate |
| non-REM | Non-rapid eye movement |
| ROS | Reactive oxygen species |
| Ctx | Cortex |
| Hp | Hippocampus |
References
- Logie, E.; Vanden Berghe, W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Gueorguieva, I.; Willis, B.A.; Chua, L.; Chow, K.; Ernest, C.S.; Shcherbinin, S.; Ardayfio, P.; Mullins, G.R.; Sims, J.R. Donanemab Population Pharmacokinetics, Amyloid Plaque Reduction, and Safety in Participants with Alzheimer’s Disease. Clin. Pharmacol. Ther. 2023, 113, 1258–1267. [Google Scholar] [CrossRef]
- Vitek, G.E.; Decourt, B.; Sabbagh, M.N. Lecanemab (BAN2401): An anti-beta-amyloid monoclonal antibody for the treatment of Alzheimer disease. Expert Opin. Investig. Drugs 2023, 32, 89–94. [Google Scholar] [CrossRef]
- Høilund-Carlsen, P.F.; Revheim, M.E.; Costa, T.; Alavi, A.; Kepp, K.P.; Sensi, S.L.; Perry, G.; Robakis, N.K.; Barrio, J.R.; Vissel, B. Passive Alzheimer’s immunotherapy: A promising or uncertain option? Ageing Res. Rev. 2023, 90, 101996. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic Mono-Carbonyl Curcumin Analogues Attenuate Oxidative Stress in Mouse Models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Baazaoui, N.; Iqbal, K. Alzheimer’s Disease: Challenges and a Therapeutic Opportunity to Treat It with a Neurotrophic Compound. Biomolecules 2022, 12, 1409. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Gerashchenko, D.; Timofeev, I.; Bacskai, B.J.; Kastanenka, K.V. Slow Wave Sleep Is a Promising Intervention Target for Alzheimer’s Disease. Front. Neurosci. 2020, 14, 705. [Google Scholar] [CrossRef]
- Massimini, M.; Huber, R.; Ferrarelli, F.; Hill, S.; Tononi, G. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004, 24, 6862–6870. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Kekuš, M.; Adelsberger, H.; Noda, T.; Förstl, H.; Nelken, I.; Konnerth, A. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci. 2015, 18, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Vetere, L.M.; Galas, A.M.; Vaughan, N.; Feng, Y.; Wick, Z.C.; Philipsberg, P.A.; Liobimova, O.; Fernandez-Ruiz, A.; Cai, D.J.; Shuman, T. Medial entorhinal-hippocampal desynchronization parallels the emergence of memory impairment in a mouse model of Alzheimer’s disease pathology. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Xing, Z.; Su, A.; Mi, L.; Zhang, Y.; He, T.; Qiu, Y.; Wei, T.; Li, Z.; Zhu, J.; Wu, W. Withaferin A: A Dietary Supplement with Promising Potential as an Anti-Tumor Therapeutic for Cancer Treatment—Pharmacology and Mechanisms. Drug Des. Devel. Ther. 2023, 17, 2909–2929. [Google Scholar] [CrossRef]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Patil, D.; Gautam, M.; Mishra, S.; Karupothula, S.; Gairola, S.; Jadhav, S.; Pawar, S.; Patwardhan, B. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: Application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J. Pharm. Biomed. Anal. 2013, 80, 203–212. [Google Scholar] [CrossRef]
- Vaidya, V.G.; Naik, N.N.; Ganu, G.; Parmar, V.; Jagtap, S.; Saste, G.; Bhatt, A.; Mulay, V.; Girme, A.; Modi, S.J.; et al. Clinical pharmacokinetic evaluation of Withania somnifera (L.) Dunal root extract in healthy human volunteers: A non-randomized, single dose study utilizing UHPLC-MS/MS analysis. J. Ethnopharmacol. 2024, 322, 117603. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.J.; Tiwari, A.; Ghule, C.; Pawar, S.; Saste, G.; Jagtap, S.; Singh, R.; Deshmukh, A.; Girme, A.; Hingorani, L. Pharmacokinetic Study of Withanosides and Withanolides from Withania somnifera Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS). Molecules 2022, 27, 1476. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.S.R.; Yndart Arias, A.; Jayant, R.D.; Kaushik, A.; Geiger, J.; Nair, M.N. Withaferin A Suppresses Beta Amyloid in APP Expressing Cells: Studies for Tat and Cocaine Associated Neurological Dysfunctions. Front. Aging Neurosci. 2018, 10, 291. [Google Scholar] [CrossRef]
- Hu, H.; Cao, B.; Huang, D.; Lin, Y.; Zhou, B.; Ying, J.; Huang, L.; Zhang, L. Withaferin a modulation of microglia autophagy mitigates neuroinflammation and enhances cognitive function in POCD. Sci. Rep. 2024, 14, 26112. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, B.; Zhang, C.; Su, Z.; Guo, B.; Zhao, Y.; Zheng, R. The DJ1-Nrf2-STING axis mediates the neuroprotective effects of Withaferin A in Parkinson’s disease. Cell Death Differ. 2021, 28, 2517–2535. [Google Scholar] [CrossRef]
- Mishra, P.S.; Phaneuf, D.; Boutej, H.; Picher-Martel, V.; Dupre, N.; Kriz, J.; Julien, J.P. Inhibition of NF-κB with an Analog of Withaferin-A Restores TDP-43 Homeostasis and Proteome Profiles in a Model of Sporadic ALS. Biomedicines 2024, 12, 1017. [Google Scholar] [CrossRef]
- Cardin, J.A.; Crair, M.C.; Higley, M.J. Mesoscopic Imaging: Shining a Wide Light on Large-Scale Neural Dynamics. Neuron 2020, 108, 33–43. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, L.; Zhu, J.; Fan, J.; Zheng, H.; Liao, X.; Yang, Z.; Zhang, K.; Jia, H.; Konnerth, A.; et al. Pitolisant alleviates brain network dysfunction and cognitive deficits in a mouse model of Alzheimer’s disease. Transl. Psychiatry 2025, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Balado, J.; Eich, T.S. GABAergic dysfunction, neural network hyperactivity and memory impairments in human aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021, 116, 146–159. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflammation 2023, 20, 165. [Google Scholar] [CrossRef]
- Gallego-Rudolf, J.; Wiesman, A.I.; Pichet Binette, A.; Villeneuve, S.; Baillet, S. Synergistic association of Aβ and tau pathology with cortical neurophysiology and cognitive decline in asymptomatic older adults. Nat. Neurosci. 2024, 27, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Terao, I.; Kodama, W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 2024, 94, 102203. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Cheng, Y.F.; Wu, J.G.; Wang, C.M.; Wang, H.T.; Zhang, C.; Qiu, Z.K.; Xu, J.P. Donepezil improves learning and memory deficits in APP/PS1 mice by inhibition of microglial activation. Neuroscience 2015, 290, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Grienberger, C.; Rochefort, N.L.; Adelsberger, H.; Henning, H.A.; Hill, D.N.; Reichwald, J.; Staufenbiel, M.; Konnerth, A. Staged decline of neuronal function in vivo in an animal model of Alzheimer’s disease. Nat. Commun. 2012, 3, 774. [Google Scholar] [CrossRef]
- Zott, B.; Nästle, L.; Grienberger, C.; Unger, F.; Knauer, M.M.; Wolf, C.; Keskin-Dargin, A.; Feuerbach, A.; Busche, M.A.; Skerra, A.; et al. β-amyloid monomer scavenging by an anticalin protein prevents neuronal hyperactivity in mouse models of Alzheimer’s Disease. Nat. Commun. 2024, 15, 5819. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.Q.; Shim, S.S.; Pourkhodadad, S.; Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 2023, 18, 43. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef]
- Wunderlin, M.; Züst, M.A.; Hertenstein, E.; Fehér, K.D.; Schneider, C.L.; Klöppel, S.; Nissen, C. Modulating overnight memory consolidation by acoustic stimulation during slow-wave sleep: A systematic review and meta-analysis. Sleep 2021, 44, zsaa296. [Google Scholar] [CrossRef]
- Facchin, L.; Schöne, C.; Mensen, A.; Bandarabadi, M.; Pilotto, F.; Saxena, S.; Libourel, P.A.; Bassetti, C.L.A.; Adamantidis, A.R. Slow Waves Promote Sleep-Dependent Plasticity and Functional Recovery after Stroke. J. Neurosci. 2020, 40, 8637–8651. [Google Scholar] [CrossRef]
- Li, S.; Jin, M.; Koeglsperger, T.; Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Zott, B.; Busche, M.A.; Sperling, R.A.; Konnerth, A. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annu. Rev. Neurosci. 2018, 41, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Raziya Banu, M.; Ibrahim, M.; Prabhu, K.; Rajasankar, S. Withaferin-A Protects the Nigral Dopamine Neuron and Recovers Motor Activity in Aged Rats. Cells Tissues Organs 2019, 208, 59–65. [Google Scholar] [CrossRef]
- Li, Y.D.; Luo, Y.J.; Xie, L.; Tart, D.S.; Sheehy, R.N.; Zhang, L.; Coleman, L.G., Jr.; Chen, X.; Song, J. Activation of hypothalamic-enhanced adult-born neurons restores cognitive and affective function in Alzheimer’s disease. Cell Stem Cell 2023, 30, 415–432.e416. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, M.; Zhao, J.; Zhao, S.; Zhu, W.; Wu, X.; Li, F.; Liu, W.; Wang, Z.; Gao, M.; et al. Sulindac selectively induces autophagic apoptosis of GABAergic neurons and alters motor behaviour in zebrafish. Nat. Commun. 2023, 14, 5351. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, F.; Moursi, S.; Mahmoud, M.R.; Shahid, S.M.A.; Khatoon, F.; Shahid Khan, M.; Khan, M.A.; Alam, M.J.; Saleem, M.; Syed Khaja, A.S. Withaferin A alleviates inflammation in animal models of arthritis by inhibiting the NF-κB pathway and cytokine release. Chem. Biol. Interact. 2024, 398, 111114. [Google Scholar] [CrossRef]
- Atteeq, M. Evaluating anticancer properties of Withaferin A—A potent phytochemical. Front. Pharmacol. 2022, 13, 975320. [Google Scholar] [CrossRef]
- Li, J.; Yao, D.; Zhang, T.; Tong, T.; Shen, J.; Yan, S.; Zeng, J.; Aslam, M.S.; Li, M.; You, Z.; et al. GABA(B) modulate NF-κB/NLRP3 pathways in electroacupuncture prevention of depression in CUMS rats. Brain Res. Bull. 2024, 218, 111108. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Neog, M.K.; Sultana, F.; Rasool, M. Targeting RAW 264.7 macrophages (M1 type) with Withaferin-A decorated mannosylated liposomes induces repolarization via downregulation of NF-κB and controlled elevation of STAT-3. Int. Immunopharmacol. 2018, 61, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, K.; Yap, S.; Tsuji, M.; Kawamura, A. Molecular Mechanism behind the Safe Immunostimulatory Effect of Withania somnifera. Biomolecules 2023, 13, 828. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Daniele, S.; Giacomelli, C.; Loprinzi, P.D.; Franzoni, F. Editorial: Physical Activity: Epigenetic and Metabolic Regulation of Brain Aging. Front. Aging Neurosci. 2020, 12, 195. [Google Scholar] [CrossRef]
- Rajesh, Y.; Kanneganti, T.D. Innate Immune Cell Death in Neuroinflammation and Alzheimer’s Disease. Cells 2022, 11, 1885. [Google Scholar] [CrossRef]
- Puntambekar, S.S.; Moutinho, M.; Lin, P.B.; Jadhav, V.; Tumbleson-Brink, D.; Balaji, A.; Benito, M.A.; Xu, G.; Oblak, A.; Lasagna-Reeves, C.A.; et al. CX3CR1 deficiency aggravates amyloid driven neuronal pathology and cognitive decline in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Stazi, M.; Wirths, O. Chronic Memantine Treatment Ameliorates Behavioral Deficits, Neuron Loss, and Impaired Neurogenesis in a Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, J.H.; Jeong, Y.J.; Hwang, J.W.; Lee, S.; Lee, H.; Seol, E.; Kim, I.W.; Cha, B.Y.; Seo, J.; et al. Donepezil ameliorates Aβ pathology but not tau pathology in 5xFAD mice. Mol. Brain 2022, 15, 63. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Park, S.K.; Park, J.H.; Jeong, H.R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.S. Donepezil Regulates LPS and Aβ-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Couly, S.; Denus, M.; Bouchet, M.; Rubinstenn, G.; Maurice, T. Anti-Amnesic and Neuroprotective Effects of Fluoroethylnormemantine in a Pharmacological Mouse Model of Alzheimer’s Disease. Int. J. Neuropsychopharmacol. 2021, 24, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Cader, Z. Human Blood-Brain-Barrier In Vitro Models: Overview and Applications. Handb. Exp. Pharmacol. 2022, 273, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Cataldo, K.; Gozal, D. Cognitive Impairments, Neuroinflammation and Blood-Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. Int. J. Mol. Sci. 2023, 24, 9880. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, P.; Mukherjee, S.; Sreedhar, B.; Banerjee, R. Glucocorticoid receptor-mediated delivery of nano gold-withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine 2016, 11, 2529–2546. [Google Scholar] [CrossRef]
- Sari, A.N.; Dhanjal, J.K.; Elwakeel, A.; Kumar, V.; Meidinna, H.N.; Zhang, H.; Ishida, Y.; Terao, K.; Sundar, D.; Kaul, S.C.; et al. A Low Dose Combination of Withaferin A and Caffeic Acid Phenethyl Ester Possesses Anti-Metastatic Potential In Vitro: Molecular Targets and Mechanisms. Cancers 2022, 14, 787. [Google Scholar] [CrossRef]
- Liu, J.; van Beusekom, H.; Bu, X.L.; Chen, G.; Henrique Rosado de Castro, P.; Chen, X.; Chen, X.; Clarkson, A.N.; Farr, T.D.; Fu, Y.; et al. Preserving cognitive function in patients with Alzheimer’s disease: The Alzheimer’s disease neuroprotection research initiative (ADNRI). Neuroprotection 2023, 1, 84–98. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Mielke, M.M. Sex Differences in Alzheimer’s Disease. Neurol. Clin. 2023, 41, 343–358. [Google Scholar] [CrossRef]
- Blasco Tavares Pereira Lopes, F.; Schlatzer, D.; Wang, R.; Li, X.; Feng, E.; Koyutürk, M.; Qi, X.; Chance, M.R. Temporal and Sex-Linked Protein Expression Dynamics in a Familial Model of Alzheimer’s Disease. Mol. Cell. Proteomics 2022, 21, 100280. [Google Scholar] [CrossRef]
- Wang, M.; Yan, C.; Li, X.; Yang, T.; Wu, S.; Liu, Q.; Luo, Q.; Zhou, F. Non-invasive modulation of meningeal lymphatics ameliorates ageing and Alzheimer’s disease-associated pathology and cognition in mice. Nat. Commun. 2024, 15, 1453. [Google Scholar] [CrossRef]
- Qin, H.; Fu, L.; Jian, T.; Jin, W.; Liang, M.; Li, J.; Chen, Q.; Yang, X.; Du, H.; Liao, X.; et al. REM sleep-active hypothalamic neurons may contribute to hippocampal social-memory consolidation. Neuron 2022, 110, 4000–4014.e4006. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Fu, L.; Hu, B.; Liao, X.; Lu, J.; He, W.; Liang, S.; Zhang, K.; Li, R.; Yao, J.; et al. A Visual-Cue-Dependent Memory Circuit for Place Navigation. Neuron 2018, 99, 47–55.e44. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zou, Y.; Fan, J.; Yin, P.; Qin, H.; Li, Z.; Wu, F.; Li, X.; Teng, H.; Zhang, Y.; et al. Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2025, 18, 816. https://doi.org/10.3390/ph18060816
Yang L, Zou Y, Fan J, Yin P, Qin H, Li Z, Wu F, Li X, Teng H, Zhang Y, et al. Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Pharmaceuticals. 2025; 18(6):816. https://doi.org/10.3390/ph18060816
Chicago/Turabian StyleYang, Linhan, Yang Zou, Jihua Fan, Pu Yin, Han Qin, Zhen Li, Fengjuan Wu, Xingyi Li, Huaijin Teng, Yun Zhang, and et al. 2025. "Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease" Pharmaceuticals 18, no. 6: 816. https://doi.org/10.3390/ph18060816
APA StyleYang, L., Zou, Y., Fan, J., Yin, P., Qin, H., Li, Z., Wu, F., Li, X., Teng, H., Zhang, Y., Chen, X., & Li, S. C. (2025). Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. Pharmaceuticals, 18(6), 816. https://doi.org/10.3390/ph18060816





