1. Introduction
The human brain is highly sensitive to hypoxia. In plateau regions, the unique hypobaric hypoxia environment leads to a significant reduction in systemic oxygen levels, which can readily trigger brain damage and negatively affect cognitive function [
1,
2]. Brief exposure to such environments often induces acute mountain sickness (AMS), characterized by symptoms such as headache and fatigue. This condition not only interferes with daily activities but also significantly impairs cognitive function. Specifically, short-term hypoxia exposure can lead to changes in cerebral blood flow and disorders in neurotransmitter metabolism, which in turn can weaken working and learning memory capacity, reduce attention span, and slow reaction times. The higher the altitude, the more pronounced the decline in attention and work capacity and the more severe the impairment of memory function [
3,
4]. Cognitive deficits become more severe with prolonged exposure to hypobaric hypoxia environments. Long-term exposure to high altitudes not only exacerbates cognitive dysfunction but also induces structural brain changes, including hippocampal volume reduction, cortical thickness decrease, neuronal atrophy and necrosis, and synaptic structural alterations. These structural changes are closely associated with cognitive dysfunction, suggesting that long-term plateau exposure may have significant detrimental effects on brain plasticity and neuroregenerative capacity [
5]. With increasing economic prosperity and tourism development, the number of people migrating to plateau regions is gradually rising. This trend heightens the demand for effective health protection measures in plateau areas and underscores the critical need for the exploration of drugs to prevent and treat plateau cognitive impairment.
Numerous studies have demonstrated that components derived from medicinal plants, used either in the form of pharmaceuticals or nutraceuticals, can exert anti-cognitive disorder effects in humans [
6,
7]. Qi Jing Wan (QJW) consists of three food–medicine dual-use herbs:
Angelicae Sinensis Radix (ASR),
Astragalus Membranaceus (AM), and
Polygonati Rhizoma (PR). These herbs possess the effects of nourishing and invigorating blood, replenishing qi, and elevating yang, as well as strengthening the spleen and benefiting the kidneys as per the traditional Chinese medicine theory. Pharmacological studies have demonstrated that certain active ingredients in ASR possess potent antioxidant and anti-inflammatory properties. These properties enable the ingredients to alleviate oxidative damage to nerve cells by scavenging free radicals and inhibiting oxidative stress. They also promote the elevated expression of neurotrophic factors, which supports neuronal survival and synaptic plasticity. Furthermore, these active ingredients inhibit the activity of acetylcholinesterase (AChE), thereby enhancing learning and memory abilities [
8,
9]. The primary active constituents of AM encompass saponins, flavonoids, and polysaccharides. These compounds work synergistically and exhibit a range of pharmacological effects, including anti-inflammatory, antioxidant, immunomodulatory, and neuroprotective pharmacological effects through synergistic interactions [
10]. Additionally, AM may enhance cognitive function by reducing neuroinflammation, alleviating oxidative stress, inhibiting ferroptosis, and promoting neural regeneration and synaptic plasticity [
11,
12,
13]. The chemical composition of PR is rich and diverse, primarily encompassing polysaccharides, saponins, flavonoids, amino acids, and volatile compounds. Notably, PR and its extracts have demonstrated significant potential in enhancing cognitive functions, particularly in improving learning and memory abilities, as supported by preclinical studies [
14,
15]. The aforementioned findings indicate that QJW holds considerable potential for the treatment of cognitive impairment. However, its specific role and the underlying mechanisms governing its action in plateau-induced cognitive impairment remain to be fully elucidated through further research.
In this study, we investigated the effects of QJW and its active ingredient, diosgenin (Dio), on preventing and treating plateau cognitive impairment using mouse models of acute and chronic plateau cognitive impairment. We also explored the specific mechanisms via a hypoxic brain organoid damage model. Initially, an acute plateau cognitive impairment model was established with a hypobaric oxygen chamber to assess QJW’s pharmacological effects and determine the optimal dose for the chronic study. Then, the chronic effects of QJW were examined by observing hippocampal neurons and synapses and by measuring the levels of microglia and inflammatory factors. The chemical composition of QJW was analyzed, with blood–brain barrier-crossing components identified for further evaluation. Transcriptomics revealed the gene PDE4C to be linked to Dio’s effects on hypoxic brain organoid damage, a finding validated using immunofluorescence and molecular docking.
3. Discussion
Exposure to hypobaric hypoxia environments elicits a range of uncomfortable symptoms in humans. The brain is highly sensitive to hypoxia and is prone to hypoxic injury, resulting in cognitive decline [
5]. This study established a hypobaric hypoxia mouse model and evaluated QJW’s protective effects through behavioral tests, histopathology, hippocampal ultrastructure analysis, and inflammation assays. Dio, identified as a blood–brain barrier-penetrating compound, was further studied for its mechanism in alleviating hypoxic neurological injury using both animal models and brain organoids. In summary, our study explored the beneficial effects of QJW on plateau-induced cognitive impairment and provided a foundation for its further research.
The Noval Object Recognition Test (NOR) and Morris Water Maze Test (MWM) are widely used to evaluate learning and memory abilities in rodents [
16,
17]. During learning, hippocampal neurons encode and store information via synaptic plasticity mechanisms. Behavioral experiments revealed that QJW administration can effectively ameliorate the learning and spatial memory deficits induced by hypobaric hypoxia environments in plateau model mice. The hippocampus is a critical region for memory and spatial cognition. Neurons, as the fundamental units of the nervous system, are essential for maintaining cognitive functions. In learning and memory processes, hippocampal neurons are responsible for receiving, processing, and transmitting information, and their functional integrity directly impacts cognitive performance [
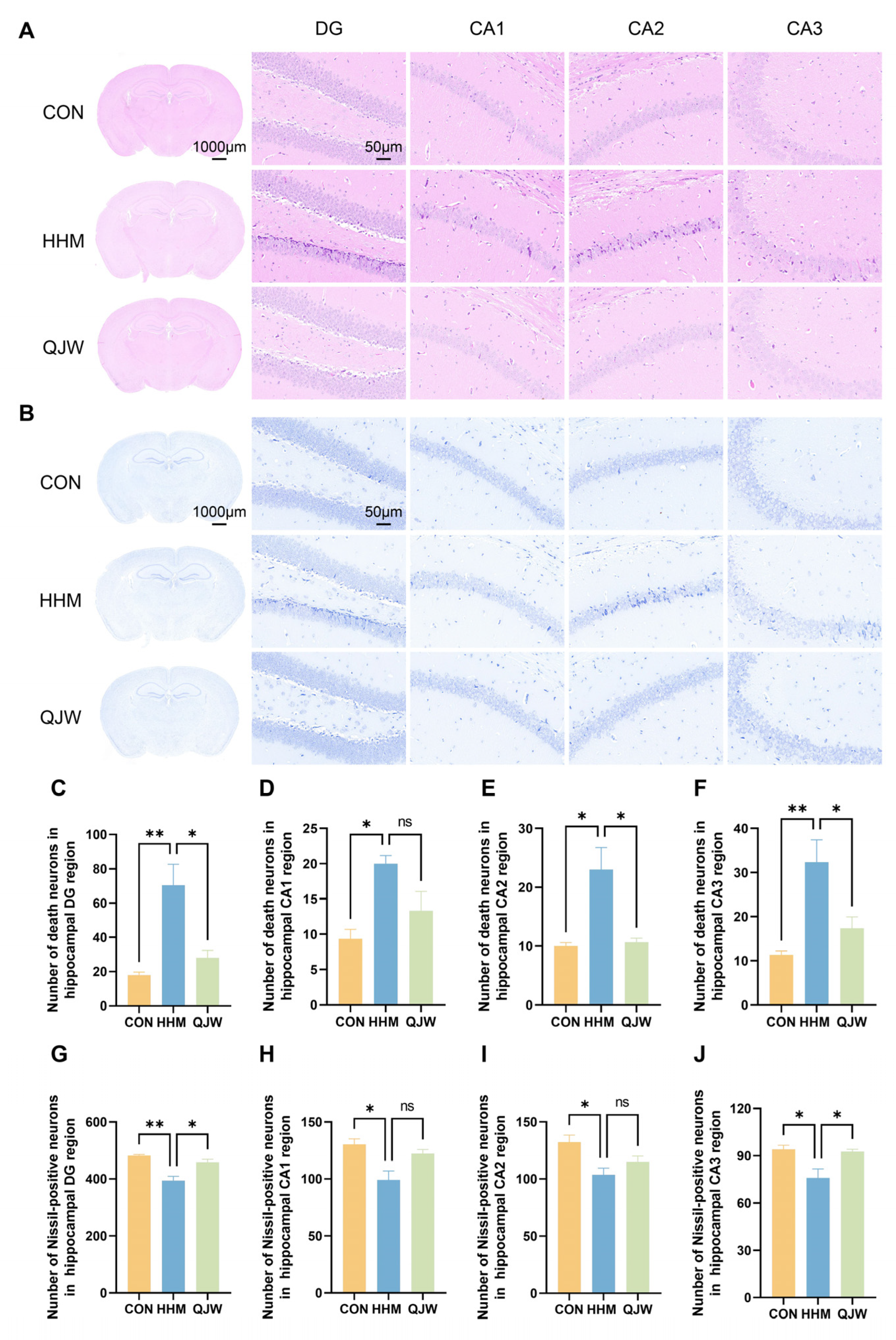
18]. Histological findings confirmed the neuroprotective and repair-promoting effects of QJW in plateau model mice.
The development of cognitive dysfunction is closely associated with abnormal levels of systemic inflammation [
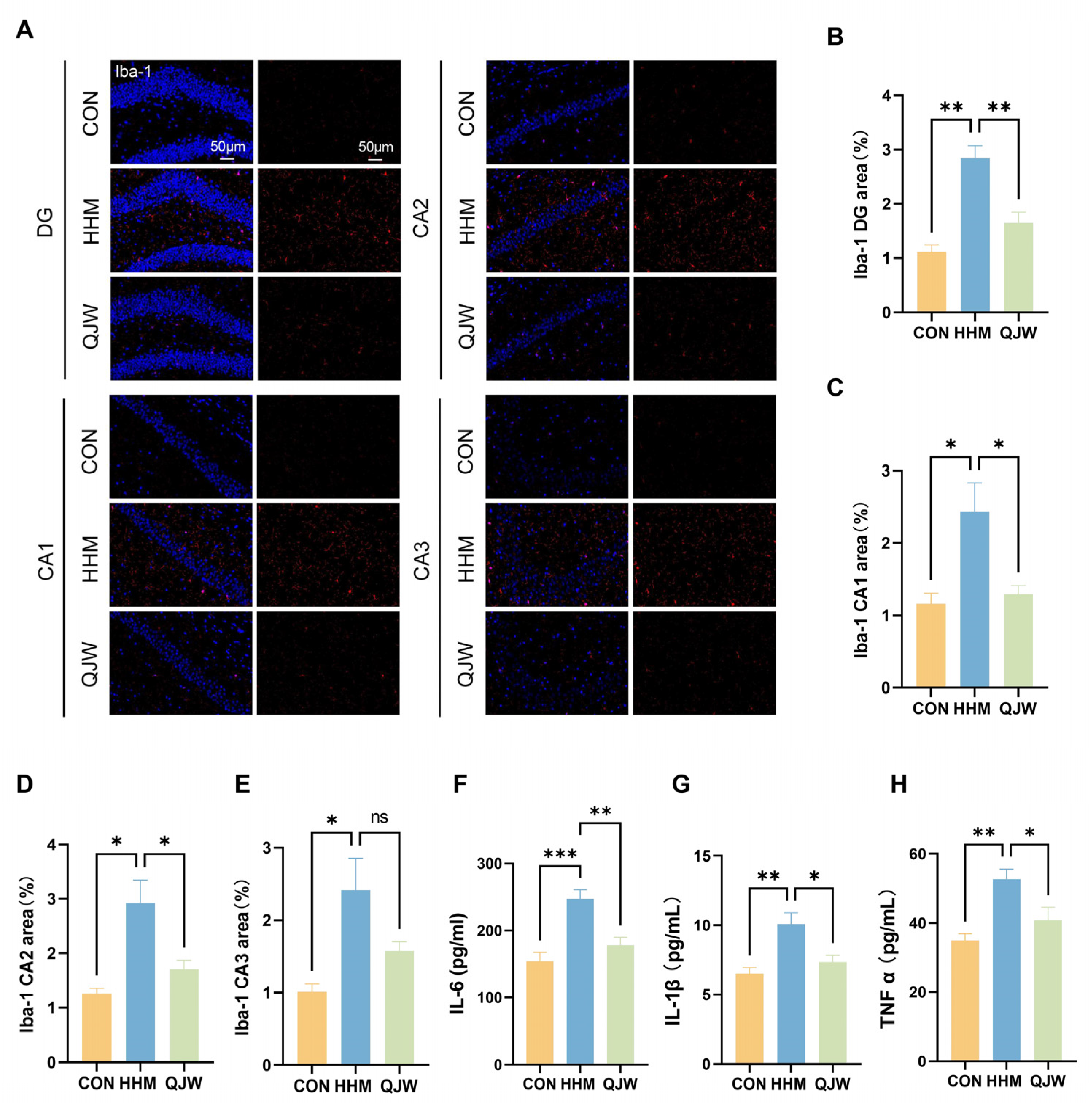
19]. In this study, we observed that QJW administration mitigated the increased levels of inflammatory factors induced by the low-pressure hypoxic environment. Elevated inflammatory markers in vivo often indicate the activation of microglia. Abnormal activation of the brain’s immune microenvironment is considered a key driver of cognitive impairment. Persistent activation of astrocytes and microglia not only disrupts normal neuronal communication but also alters the blood–brain barrier permeability, leading to abnormal infiltration of peripheral immune cells and creating a persistent neuroinflammatory microenvironment that further exacerbates neuroinflammation [
20,
21]. Iba-1 immunofluorescence staining indicated that QJW may mitigate neuroinflammation by inhibiting microglial activation, thereby improving cognitive impairment.
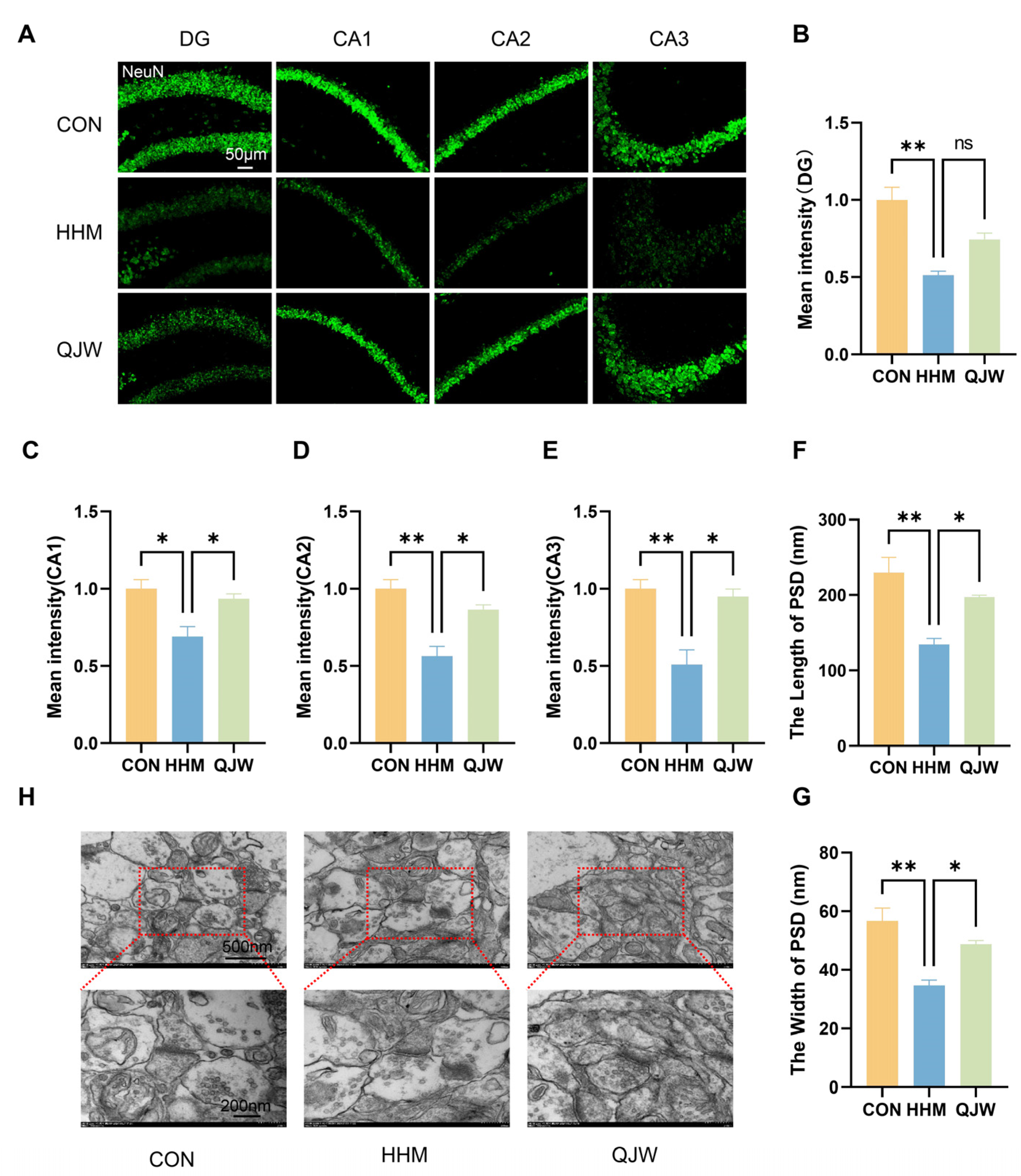
Synapses are critical sites for information transfer and functional activity within the interneuronal system, and their coordinated expression of synaptic plasticity underpins learning and memory processes, being closely related to cognitive function [
22,
23]. Synaptic plasticity refers to the capacity of synaptic efficacy to change in response to neuronal activity, a property that constitutes the neurological basis of learning and memory [
24]. Damage to hippocampal neurons and synapses leads to severe cognitive impairment [
25,
26]. Inhibition of synaptic plasticity is closely associated with microglial activation and the release of inflammatory factors [
27]. These findings suggest that cognitive impairment is associated with various factors, such as neuroinflammation and synaptic damage; however, the specific gene regulatory pathways remain unclear. Microglia, as core cells of the central nervous system, are closely associated with inflammatory signaling networks through their dynamic interactions with synapses. Under normal physiological conditions, these cells participate in the dynamic regulation of the synaptic structure with the help of low levels of inflammatory molecules. However, under pathological conditions, excessive inflammatory signaling becomes a significant pathological basis for the development of neurodegenerative and psychiatric diseases [
28]. Studies have shown that microglia are involved in synaptic pruning processes via the classical complement pathway. For example, in Alzheimer’s disease (AD), the aberrant accumulation of β-amyloid specifically activates intracellular inflammasomes, contributing to the overproduction of the pro-inflammatory factors IL-1β and TNF-α, which impair synaptic signaling efficiency [
29]. In this study, we found that QJW administration reduced the elevated inflammation levels induced by exposure to the hypobaric hypoxia environment of the plateau, attenuated the abnormal synaptic modifications induced by microglial activation, and protected hippocampal neurons and synaptic structures, findings that are consistent with those of a previously reported study [
30].
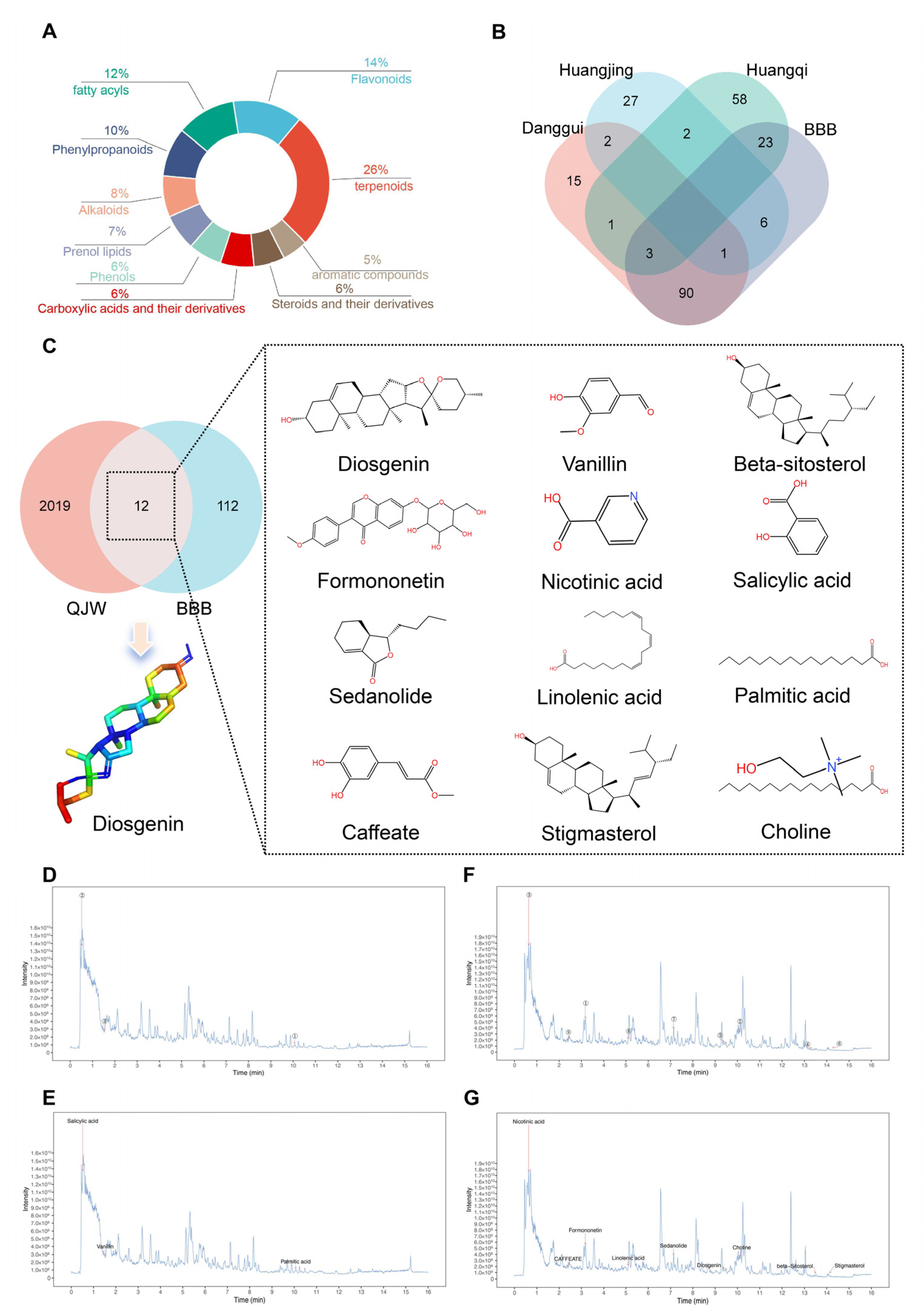
UPLC-MS/MS was employed to identify and analyze bioactive compounds within QJW and further investigate the active components that exert preventive effects [
31]. In order to elucidate the specific components of QJW that play a role in preventing and treating cognitive impairment in plateau environments, the chemical composition of QJW lyophilized powder was examined in this study. By comparing with TCMSP, a database of traditional Chinese medicines, we screened out Dio, a potential active ingredient that passes through the blood–brain barrier, and verified the screened Dio in animal experiments in the acute plateau model mice. Behavioral experiments and histological analyses demonstrate that Dio effectively prevents hypoxia-induced neuronal damage and protects cognitive function. This study demonstrates the preventative and therapeutic effects of QJW and its potential brain-penetrating constituent, Dio, on high-altitude cognitive impairment. Future research could further investigate the synergistic effects of QJW and Dio, evaluating their potential to prevent and treat cognitive deficits associated with high-altitude exposure.
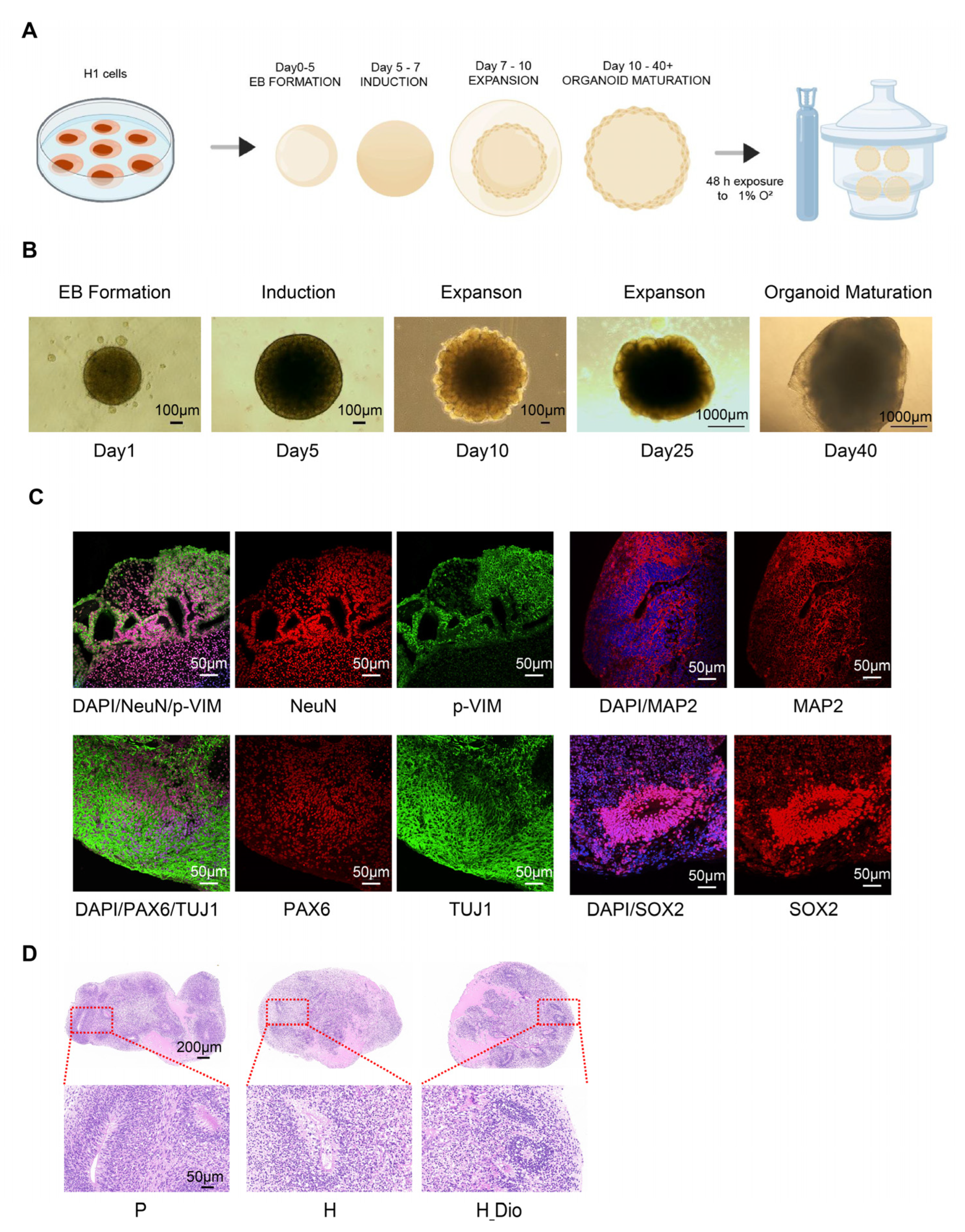
Brain organoids are three-dimensional cellular models differentiated from induced pluripotent stem cells or embryonic stem cells that are capable of replicating the developmental process and some functional features of the human brain [
32]. In recent years, researchers have increasingly used brain organoids in neurodegenerative disease research. These organoids can effectively reproduce the core pathological features of many cognitive disorders, including AD models and neuroinflammation, as well as other neurodegenerative pathways [
33,
34]. For example, in AD models, brain organoids can recapitulate the formation of Aβ amyloid plaques and tau protein neurofibrillary tangles, thereby revealing the potential impact of these pathological changes on neuronal and synaptic functions [
35]. Brain organoids have thus opened new avenues for investigating the pathogenesis of cognitive disorders. For instance, by analyzing the gene expression and epigenetic changes in organoids, researchers have gained insights into the molecular basis of cognitive disorders [
36]. Other studies have demonstrated that hypoxia causes significant damage to neurons in brain organoids [
37,
38]. This finding is particularly relevant for studying the combination of plateau hypoxic brain damage and brain organoids. In this study, we used mature brain organoids and referred to the hypoxic brain injury conditions described in previous studies to establish a hypoxic injury model in them [
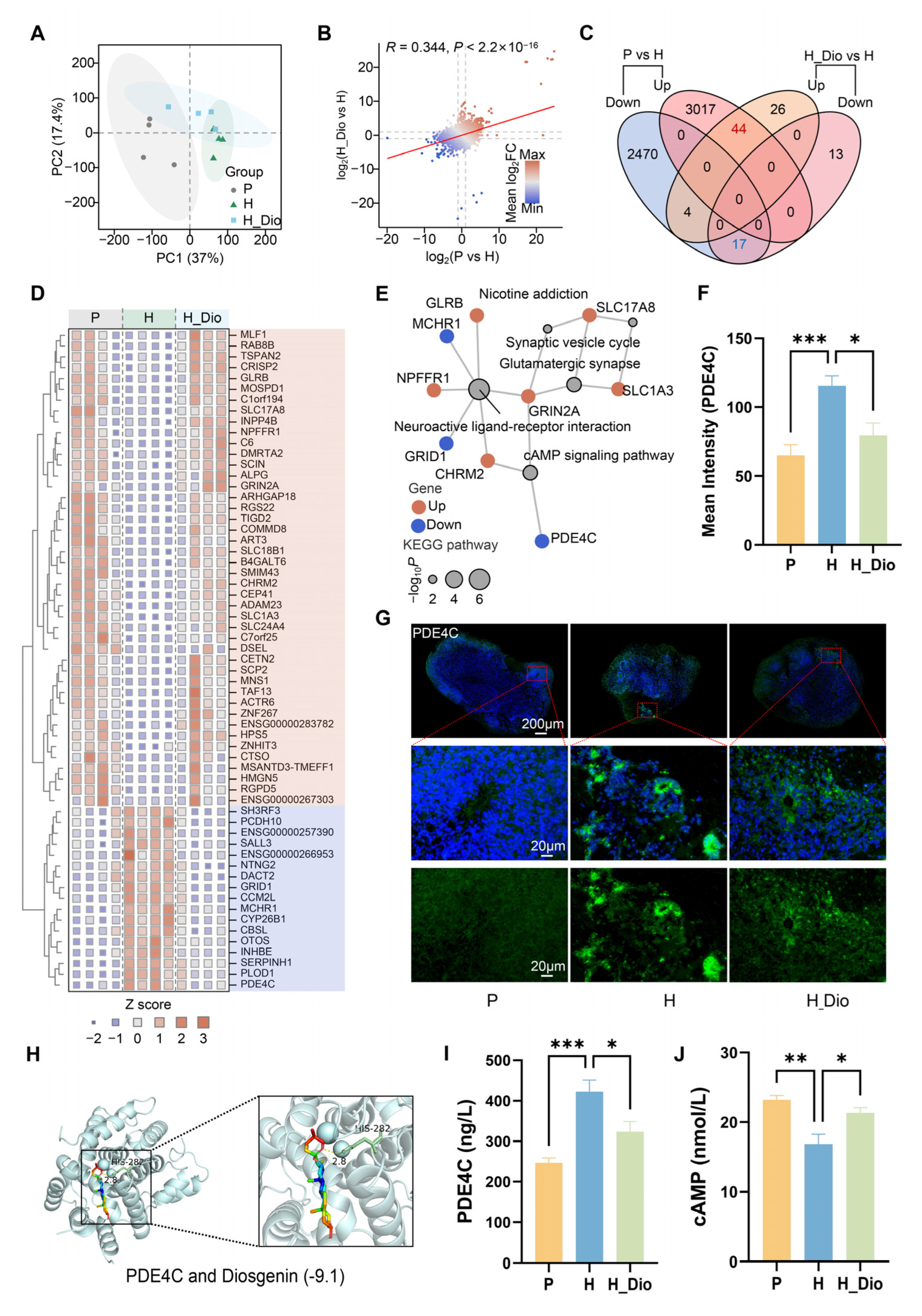
36]. We then pharmacologically intervened using Dio and conducted transcriptome analyses to explore its mechanism of action. The PCA results showed a significant separation between groups, indicating that Dio exerted a protective effect on hypoxia-induced transcriptional changes in human brain organoids.
GO enrichment is associated with functions related to DNA damage repair, hypoxia response pathways, and neurotransmitter-related processes. Hypoxia may impede the proliferation and differentiation of neuronal cells, influencing the growth and development of the nervous system, the maturation of brain structure and function, and consequently, cognitive function [
39]. Hypoxia induces an increase in intracellular oxidative stress, leading to DNA damage. The activation of DNA damage repair mechanisms is crucial for preserving the integrity and function of neuronal cells [
40,
41]. The hypoxia-inducible factor signaling pathway is activated under hypoxic conditions, regulating the expression of a variety of genes, including those involved in angiogenesis, erythropoiesis, and cellular metabolism. These responses aid the body in adapting to hypoxic environments but may also be linked to the onset of cognitive impairment [
42]. Changes in hypoxia-associated signaling pathways may impact neurotransmitter release and reuptake, thereby affecting cognitive function [
43]. KEGG enrichment analysis revealed that the differential genes were enriched in signaling pathways such as neuroactive ligand–receptor interactions, glutamatergic synapses, and the cAMP signaling pathway. The cAMP signaling pathway is involved in the regulation of neural stem cell proliferation and differentiation, as well as the growth and regeneration of neuronal axons, and is closely related to the development of brain organoids. Proteins within this pathway, including protein kinase A (PKA), cAMP response element-binding protein (CREB), phosphodiesterase (PDE), and cAMP direct activation exchange protein (Epac), regulate the activity of the signaling pathway, thereby influencing neuronal excitability, synaptic transmission, and neuroplasticity, all of which are closely associated with cognitive dysfunction. The key differential gene PDE4C is enriched in the cAMP signaling pathway, suggesting that it is closely related to Dio’s protective effects against hypoxic brain organoid damage and may play a crucial role in preventing hypoxic cognitive impairment.
Phosphodiesterase 4 (PDE4) inhibition has been demonstrated to significantly ameliorate cognitive dysfunction in various neurodegenerative disorders [
35]. PDE4 regulates cognitive function by modulating intracellular cyclic adenosine monophosphate (cAMP) levels, which subsequently influences cognitive performance. cAMP acts as a key second messenger involved in the regulation of synaptic plasticity and neuronal excitability. PDE4C, an isoform of the PDE4 family, can lead to decreased cAMP levels when its activity is elevated, thereby inhibiting protein kinase A (PKA) activity and ultimately affecting synaptic plasticity and memory formation [
44]. Additionally, PDE4 inhibition has been shown to reduce microglial activation and inflammatory factor release, thereby attenuating neuroinflammation [
45], findings that are consistent with our observations in the chronic plateau cognitive impairment study presented here. Our study revealed that Dio significantly inhibited the hypoxia-induced elevation in PDE4C expression and significantly restored the hypoxia-induced reduction in cAMP expression. Additionally, Dio exhibited high affinity for PDE4C. These results suggest that Dio is closely associated with the expression of PDE4C and cAMP and may serve as an important factor in alleviating hypoxia-induced brain damage and combating cognitive impairment.
4. Materials and Methods
4.1. Preparation of QJW Extract
ASR, AM, and PR were purchased from Beijing Tong Ren Tang (Beijing, China). PR was washed, sliced, and mixed with yellow rice wine (1:1 water-to-wine ratio) at a 5:1 ratio. The mixture was soaked at room temperature for 4 h, sterilized at 121 °C for 2.5 h, and then dried at 70 °C. ASR, AM, and PR were ground into fine powders and mixed in a 1:1:1 ratio. The mixture was refluxed with 10 times its volume of water for two 1 h cycles. The combined extracts were concentrated under reduced pressure at 65 °C using a Heidolph rotary evaporator (Beijing, China) and then freeze-dried into a powder using a Songyuan vacuum freeze-dryer (Beijing, China) for storage.
4.2. Grouping of Experimental Animals
4.2.1. QJW Intervention in the Acute Plateau Model Mouse Experiment
Forty 7-week-old male C57BL/6J mice were acclimated for one week and then randomly assigned to the following groups: blank control (CON), acute plateau model (HHJ), and acute plateau model treated with low, medium, and high doses of QJW (Q-L, Q-M, Q-H). Mice from the HHJ, Q-L, Q-M, and Q-H groups were placed in a hypobaric hypoxic chamber to simulate exposure at an altitude of 7000 m for 72 h according to a previous study [
46]. Mice in the CON group were housed in an environment with similar light, humidity, and temperature conditions but at an altitude of 100 m above sea level. Before being placed in a hypobaric hypoxic chamber, mice underwent continuous gavage of QJW for 11 days. The doses of QJW administered in the acute plateau model were determined based on the results of our team’s previous study [
47]. Specifically, the Q-L, Q-M, and Q-H groups received 1.8 g/kg/day, 3.6 g/kg/day, and 7.2 g/kg/day of QJW, respectively. The lyophilized powder of QJW was prepared with saline, and the CON and HHJ groups were gavaged with equivalent volumes of saline. Body weights were measured every two days. After 72 h of hypobaric hypoxia exposure, mice were removed from the chamber for behavioral experiments and organ sampling. Following anesthesia, the experimental mice underwent surgical exposure of the brain. The brains of three mice were promptly excised and immersed in a 4% paraformaldehyde solution for fixation to maintain tissue integrity. The brains of the remaining mice were rapidly transferred to an ice-cold environment for dissection of the hippocampus and subsequent storage.
4.2.2. QJW Intervention in Chronic Plateau Model Mice
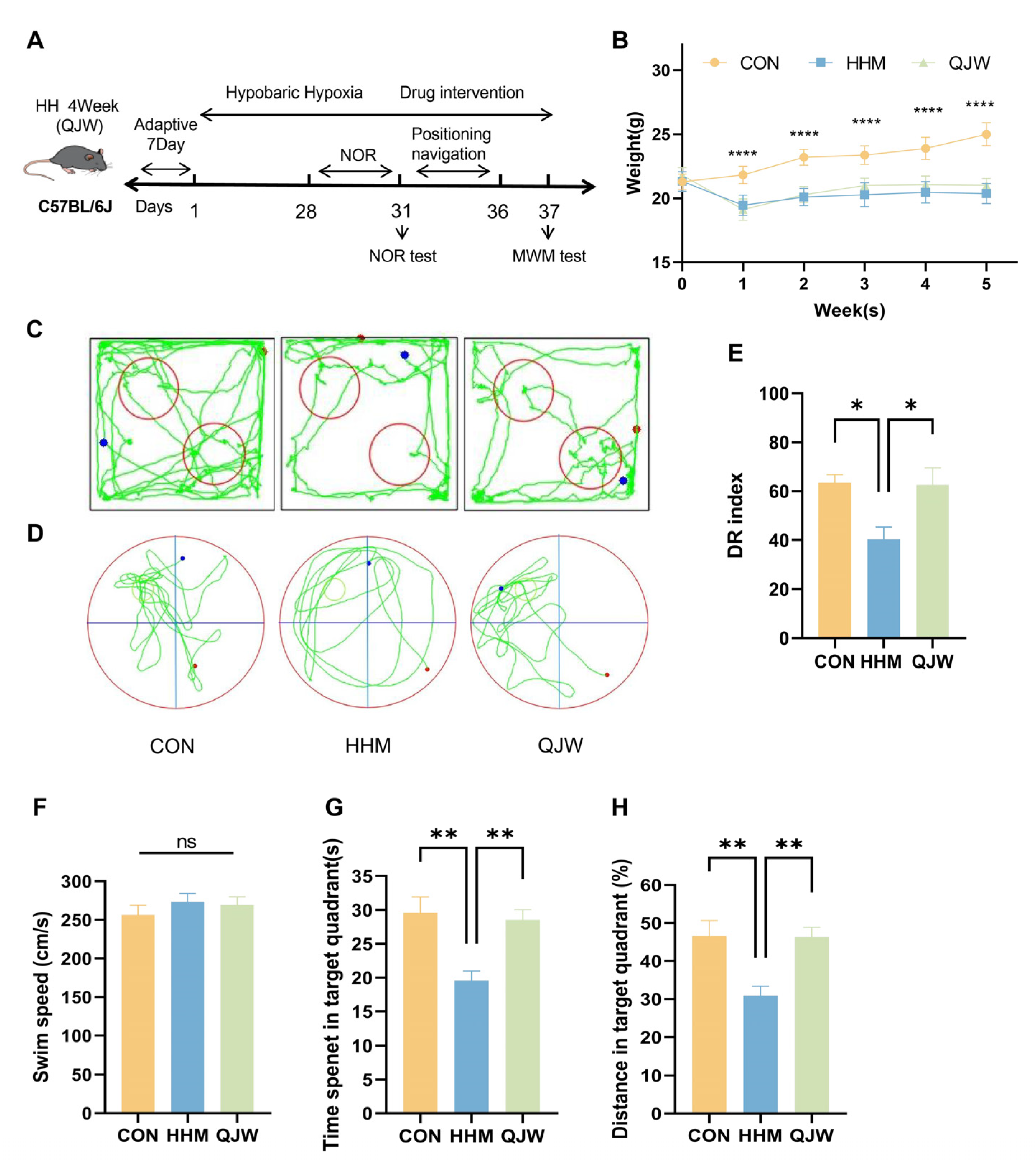
Twenty-four 7-week-old male C57BL/6J mice were acclimated for one week. The optimal dose of QJW was determined by the previous experiments, and the mice were randomly assigned to the following groups: control (CON), chronic plateau model (HHM), and chronic plateau model with the QJW intervention (QJW). The HHM and QJW groups were placed in a hypobaric hypoxic chamber to simulate exposure at an altitude of 6000 m for 4 weeks. Mice in the CON group were housed in an environment with similar light, humidity, and temperature conditions but at an altitude of 100 m above sea level. Gavage administration of the drug commenced upon the mice’s entry into the hypobaric hypoxia chamber and continued until the conclusion of the experiment. With reference to prior studies, the QJW intervention was adjusted to a dosing regimen of once every two days at a dose of 7.2 g/kg [
48]. The lyophilized powder of QJW was configured with saline, and the control and model groups were gavaged with equal doses of saline. Body weights were measured weekly, and mice were removed from the chamber for behavioral experiments as well as organ sampling after 4 weeks of hypobaric hypoxia exposure.
4.2.3. Diosgenin (Dio) Intervention in Acute Plateau Model Mice
Twenty-four 7-week-old male C57BL/6J mice were acclimated for one week and randomly assigned to three groups: blank control (CON), acute plateau model (HHJ), and acute plateau model with the Dio intervention (Dio). The HHJ and Dio groups were placed in a hypobaric hypoxic chamber to simulate exposure at an altitude of 7000 m for 72 h. Mice in the CON group were housed in an environment with similar light, humidity, and temperature conditions (100 m above sea level). Diosgenin (HY-N0177) was purchased from MCE (Monmouth Junction, NJ, USA). The compound was dissolved in ethanol and then thoroughly mixed with corn oil to prepare the dosing solution. Prior to exposure in the hypobaric hypoxia chamber, mice in the acute plateau model received intraperitoneal injections of Dio at a dosage of 60 mg/kg/day for 11 consecutive days, referencing the optimal dose identified in the previous literature [
49], while the control and model groups were given equivalent volumes of solvent. Body weights were measured every two days, and mice were removed from the chamber for behavioral experiments and organ sampling after 72 h of hypobaric hypoxia exposure.
4.3. Novel Object Recognition Test
The Novel Object Recognition Test (NOR) is a classical behavioral paradigm for assessing learning and cognitive memory abilities based on animal exploratory behavior. For acclimatization, mice were placed in an empty chamber for 5 min to eliminate the interference of environmental unfamiliarity on subsequent exploratory behaviors. In the learning stage, two identical objects (A and B) were placed symmetrically in the chamber, 10 cm away from the wall and fixed to avoid odor interference. Mice were released from the starting position (a point equidistant from the two objects) and their exploratory behaviors were recorded for 10 min using a video tracking system. In the test phase, one of the familiar objects (A or B) was replaced with a new object (C), maintaining positional symmetry. The system was then activated for a 5 min test. Immediately after each test, the objects were cleaned with 75% alcohol. Time spent exploring the old and new objects was recorded. The discrimination index (DR) was calculated as follows: DR = (time spent exploring the new object/(time spent exploring the new object + time spent exploring the old object)) × 100%. For the acute plateau model, both the QJW and Dio intervention groups underwent adaptation on day 11 after drug administration, learning on day 13, and testing on day 14 (
Figure 1A and
Figure 7A). For the chronic high-altitude model, the QJW intervention group underwent adaptation on day 28 after drug administration, learning on day 30, and testing on day 31 (
Figure 2A).
4.4. Morris Water Maze Test
The Morris Water Maze Test (MWM) was used to assess rodents’ spatial learning and memory. The setup featured a circular pool with a 120 cm diameter, a 50 cm height, and an opaque black interior. A 10 cm diameter platform with a mesh surface was placed 1 cm below the water in the first quadrant’s center. A visual tracking system with an infrared camera and EthoVision XT 18 software recorded movement trajectories. The pool, filled with 25 ± 1 °C water mixed with milk powder for visibility, was divided into four quadrants with wall cues. Mice underwent five days of training, with four daily sessions from different quadrants. Each trial lasted 60 s; escape latency was recorded or capped at 60 s if the platform was not found. Intervals between sessions were ≥30 min. Post-training, a probe test removed the platform, and 24 h later, mice were released from a random quadrant for a 60 s free swim. Measurements including time in the target quadrant and swimming speed were analyzed to evaluate long-term memory. For the acute plateau model, both the QJW and Dio intervention groups underwent orientation training from day 6 to day 10 after drug administration, with testing conducted on day 14. For the chronic plateau model, the QJW intervention group underwent orientation training from day 32 to day 36 after drug administration, with testing conducted on day 37 (
Figure 2A).
4.5. Hematoxylin and Eosin (HE) Staining
Brain tissues were fixed, embedded, and sectioned. Subsequently, the sections were subjected to xylene deparaffinization and gradient ethanol hydration. The sections were then stained with hematoxylin and eosin. The hippocampal tissue structure was examined at 400× magnification under a microscope. Three mice per group were selected, and four fields of view in the hippocampal region were photographed for each mouse. Image analysis software was employed to analyze the collected images and quantify the number of necrotic neurons in specific regions.
4.6. Nissl Staining
Paraffin sections were deparaffinized with xylene and hydrated using gradient ethanol. Nissl staining was performed and the chromogenic effect optimized through differential differentiation. Sections were then dehydrated with gradient ethanol, cleared with xylene, and sealed with neutral resin. The hippocampal tissue structure was examined at 400× magnification under a microscope. Three mice per group were selected, and four fields of view in the hippocampal region were photographed for each mouse. Image J 1.48v was used to analyze the collected images and quantify the number of Nissl-positive neurons in specific regions.
4.7. Immunofluorescence (IF) Detection
Coronal sections of the mouse hippocampus and sections of human brain organoids were prepared; primary antibodies were applied to cover the tissue, followed by overnight incubation in a humidified chamber at 4 °C. Subsequently, the corresponding secondary antibodies were added under light-avoidance conditions and incubated at 37 °C for 60 min. Unbound secondary antibodies were removed by washing with PBS, and sections were then sealed using an anti-fluorescence quenching mounting medium containing DAPI for nuclear staining. Sections were stored at 4° C in the dark. The antibodies used for IF included the following: anti-NeuN (1:5000, GB11138, Servicebio, Wuhan, China), anti-Iba-1 (1:500, GB113502, Servicebio), anti-p-VIM (1:300, D076-3, MBL International, Woburn, MA, USA), anti-MAP2 (1:250, 17490-1-AP, Proteintech, Wuhan, China), anti-PAX6 (1:300, 20932-1-AP, Proteintech), anti-TUJ1 (1:300, MMS-435P, BioLegend, San Diego, CA, USA), anti-SOX2 (1:200, 11064-1-AP, Proteintech), anti-PDE4C (1:300, PA5-106624, Invitrogen, Carlsbad, CA, USA), anti-HRP-labeled goat anti-rabbit secondary antibody (1:500, GB23303, Servicebio), and CY3-labeled goat anti-rabbit secondary antibody (1:300, GB21303, Proteintech). Non-specific binding was blocked with 10% goat serum prior to primary antibody application. At the end of the staining process, the hippocampal and brain organoid tissues were examined using a confocal laser scanning microscope at magnifications of 400× and 40×. For each experimental group, three mice were selected, and four fluorescent images of the hippocampal region were captured per mouse. Similarly, three brain organoids per group were selected, and three fluorescent images were captured per organoid. Image analysis software was employed to analyze the collected images and quantify the fluorescence intensity of proteins in specific regions.
4.8. Ultrastructural Observation of Hippocampal Tissue
Mouse hippocampi were collected and immediately fixed in fresh pre-cooled 2.5% glutaraldehyde solution at 4 °C overnight. Subsequently, the samples were rinsed with 0.1 M phosphate buffer for 10 min three times. Next, samples were sequentially dehydrated with gradient ethanol, followed by infiltration and embedding with epoxy resin. Finally, 70 nm thick slices were prepared using an ultrathin slicer and double-stained with uranyl acetate and lead citrate, and hippocampal synaptic structures were observed using transmission electron microscopy.
4.9. Multifactor Assay and Enzyme-Linked Immunosorbent Assay (ELISA)
A cytokine and chemokine kit from Leitz Biotechnology Co., Ltd. (Beijing, China). was used to detect the levels of IL-1β, IL-6, and TNF-α in mouse serum. The antigen standard was prepared and then diluted. Magnetic beads were added to the diluted standard and vortexed for 30 s, and 50 μL of the bead mixture was added to each well. After removing the liquid, 25 μL of the serum samples was added to the wells. The plate was sealed and incubated with shaking at room temperature for 120 min. The beads were then washed three times. Following this, 25 μL of a detection antibody mixture was added to the wells, which were then sealed and incubated with shaking for 30 min. The beads were washed three times again before was adding streptavidin. The beads were incubated with shaking for another 30 min and washed three times. Finally, 120 μL of buffer was added to resuspend the beads, which were then sealed and shaken for 5 min before being assayed on the machine to collect data. The expression levels of PDE4C and cAMP in brain organoids were determined using an enzyme-linked immunosorbent assay (ELISA, Wuxi, China) kit. The organoids were homogenized thoroughly using a homogenizer and then centrifuged at 2–8 °C for 20 min at 2000–3000 rpm. The supernatant was collected and the prepared samples and standards added. The mixture was incubated at 37 °C for 30 min. After washing the plate five times, a second incubation was performed at 37 °C for 30 min. Following another five washes, chromogenic substrates A and B were added and the plate was incubated at 37 °C for 10 min. The reaction was terminated by adding the stop solution. The absorbance of each well was measured sequentially at 450 nm using a microplate reader within 15 min.
4.10. UPLC-MS/MS Detection of QJW and Screening of Potential Active Ingredients
QJW lyophilized powder was dissolved in 1 mL of methanol–water (4:1, v/v) containing 10 μg/mL internal standard. After vortexing for 30 s and sonication in an ice–water bath for 1 h, the sample was stored at −40 °C for 1 h to induce precipitation. Then, centrifugation was performed at 12,000 rpm for 15 min at 4 °C, and the supernatant (500 μL) was filtered through a 0.22 μm membrane for analysis. UHPLC was performed on a Waters UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) at 55 °C with a 5 μL injection volume and a 0.5 mL/min flow rate. Mobile phases were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Gradient elution: 85% A to 25% A over 0–11 min, 2% A at 11–14 min, and re-equilibration to 85% A by 16 min. The UHPLC was coupled to a Q Exactive Focus mass spectrometer with ESI in an alternating positive/negative mode. Parameters: spray voltage: ±4.0/3.6 kV; sheath gas: 45 Arb; auxiliary gas: 15 Arb; capillary temperature: 400 °C. Full MS scan (m/z 100–1500, 70,000 resolution), with top 3 ions fragmented (DDA mode, MS/MS resolution 17,500, NCE steps 15/30/45). Raw data were processed using XCMS, and components were identified via a secondary MS database, retention time, and fragment ion matching. A chemical composition database was established. Using the TCMSP database, QJW ingredients that could cross the blood–brain barrier were selected and cross-referenced with the in-house database. Ingredients were further screened based on oral bioavailability and druglikeness for potential cognitive improvement effects.
4.11. Brain Organoid Culture, Characterization, and Intervention
Brain organoid models were generated using H1 human embryonic stem cells and the STEMdiff™ Brain Organoid Kit (STEMCELL Technologies, Vancouver, Canada). The protocol involved multi-stage induction: H1 cells were first expanded in Matrigel-coated 6-well plates with the mTeSR1 medium until 70–80% confluence, then dissociated into single cells and seeded into low-adhesion 96-well plates at 9 × 10
3 cells per well. After centrifugation (200×
g, 5 min), embryoid bodies (EBs) were formed in the EB medium (mTeSR1, STEMdiff™ Basal Medium 1, Supplement A, and Y-27632). From day 1 to day 4, the medium was refreshed every other day to stabilize EB structure. On day 5, cells were transferred to low-adhesion 24-well plates and cultured in Neuroinduction Medium (Basal Medium 1 and Supplement B) for 48 h to initiate neural differentiation. On day 7, EBs were embedded in Matrigel drops and cultured in the expansion medium (Basal Medium 2, Supplements C and D) to promote neuroepithelial growth. From day 10, maturation medium (Basal Medium 2 and Supplement E) was used, and cultures were shaken at 60 rpm until day 40 to facilitate cortical layering and synaptic network formation. Cultures were maintained at 37 °C with 5% CO
2, and organoid diameters were monitored weekly. After maturation, immunofluorescence was used to detect markers such as PAX6, SOX2, TUJ1, MAP2, p-VIM, and NeuN. Mature organoids were randomized into three groups: blank control (P), hypoxic model (H), and hypoxic model with the diosgenin (Dio) intervention (H_Dio). Groups H and H_Dio were exposed to 1% O
2 for 48 h in a hypoxic incubator [
36,
50], while the H_Dio group received 5 μM Dio concurrently. At the end of the experiment, part of the organoids was snap-frozen in liquid nitrogen for storage at −80 °C, and the remaining part was fixed in paraformaldehyde for embedding and sectioning.
4.12. Brain Organoid Transcriptome Sequencing
Brain organoids from each group were snap-frozen in liquid nitrogen for sequencing. Total RNA was extracted and used to construct sequencing libraries. Oligo(dT)-modified magnetic beads specifically captured eukaryotic mRNAs to exclude non-target RNAs. After quality control, mRNAs were fragmented chemically and reverse transcription was initiated with random hexamer primers to synthesize single-stranded cDNA, followed by double-stranded cDNA synthesis using DNA polymerase I and RNase H. The cDNA was purified via magnetic bead adsorption to remove enzyme residues and by-products. Purified cDNA underwent end repair, adenine overhang addition, and adapter ligation. Size selection and PCR amplification were performed to generate standardized libraries, which were sequenced after quality control. Raw data were processed with fastp to remove adapters and low-quality reads [
51], and clean reads were aligned to the hg38 genome using Salmon [
52]. Differential expression analysis was conducted with DESeq2 [
53], identifying DEGs with |log
2(fold change)| > 1 and adjusted
p < 0.05. These DEGs were classified as up-regulated or down-regulated and analyzed for biological processes and KEGG pathways using clusterProfiler to explore their functional roles [
54].
4.13. Molecular Docking
The three-dimensional structure of PDE4C (in the PDB format) was obtained from the PDB database (
http://www.rcsb.org) and saved in the PDB format after removing water molecules and small-molecule ligands using the software Pymol (
https://pymol.org/). Subsequently, the processed structures were hydrogenated using the software Autodock (
https://autodock.scripps.edu/) and converted to the PDBQT format for subsequent use. The two-dimensional structure of Dio (in the SDF format) was retrieved from the PubChem database (
https://pubchem.ncbi.nlm.nih.gov), optimized for minimum free energy using the software ChemBio3D Ultra 14.0.0.117, and temporarily stored in the MOL2 format. It was then converted into the PDBQT format using the software Autodock. Finally, molecular docking experiments were conducted using the software Autodock and the minimum binding energy was recorded.
4.14. Statistical Analysis
All data are presented as the mean ± standard deviation (Mean ± SD). Statistical analysis was conducted using GraphPad Prism version 9.5. Intergroup comparisons were made using one-way ANOVA, followed by Tukey HSD post hoc testing to ascertain specific pairwise differences. A p-value less than 0.05 was considered statistically significant.