Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors
Abstract
1. Introduction
2. Methods
2.1. Database Searches and Study Identification
2.2. Inclusion and Exclusion Criteria
2.3. Methodological Quality Appraisal
2.4. Outcome Definition
- Canagliflozin: low: 100 mg; high: 300 mg.
- Efpeglenatide: low: 4 mg; high: 6 mg.
- Ertugliflozin: low: 5 mg; high: 15 mg.
- Injectable Semaglutide: low: 0.5 mg; Medium: 1.0 mg; high: 2.4 mg.
- Empagliflozin: low: 1–10 mg; high: 25–50 mg.
- Tirzepatide: low: 5 mg; Medium: 10 mg; high: 15 mg.
- Dulaglutide: low: 0.75 mg; high: 1.5 mg
2.5. Data Extraction, Management and Conversion
2.6. Statistical Analyses
2.7. Sensitivity Analyses
2.8. General Declaration
3. Results
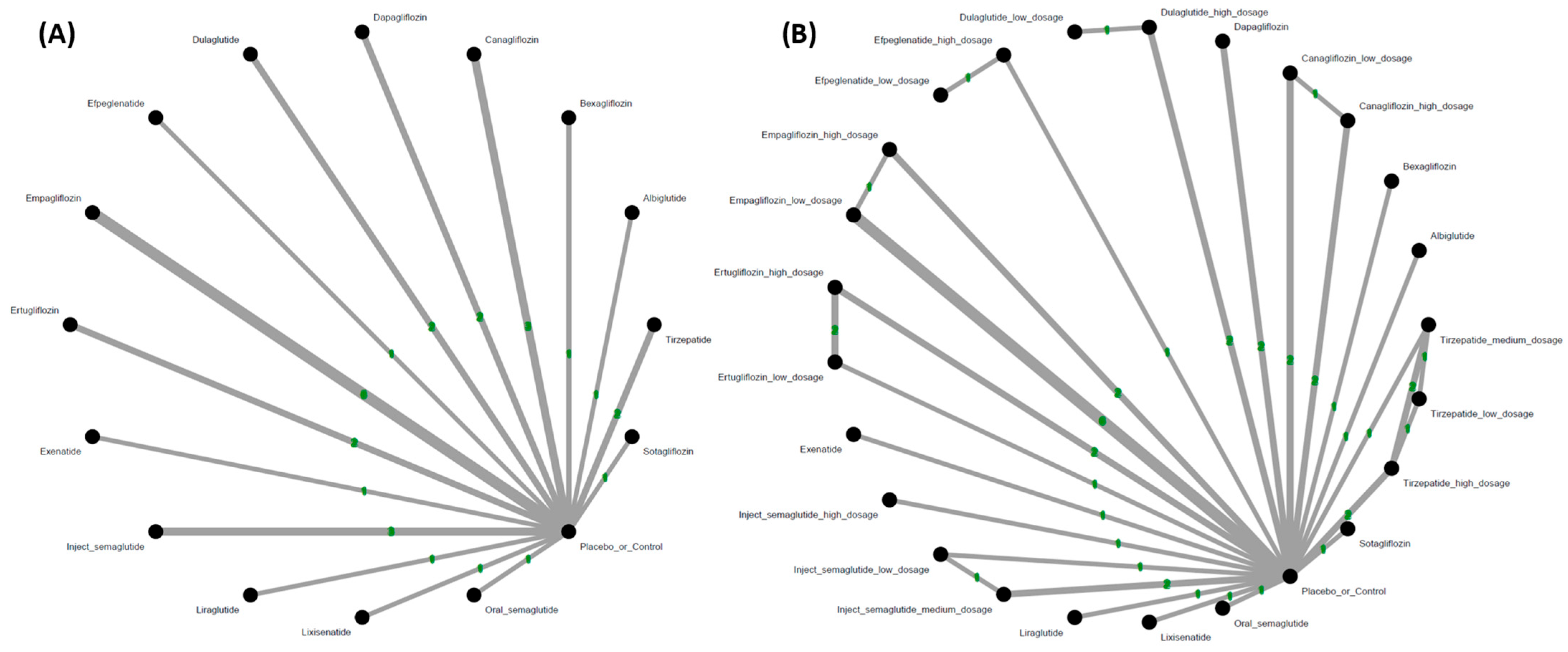
3.1. Eligibility of the Studies
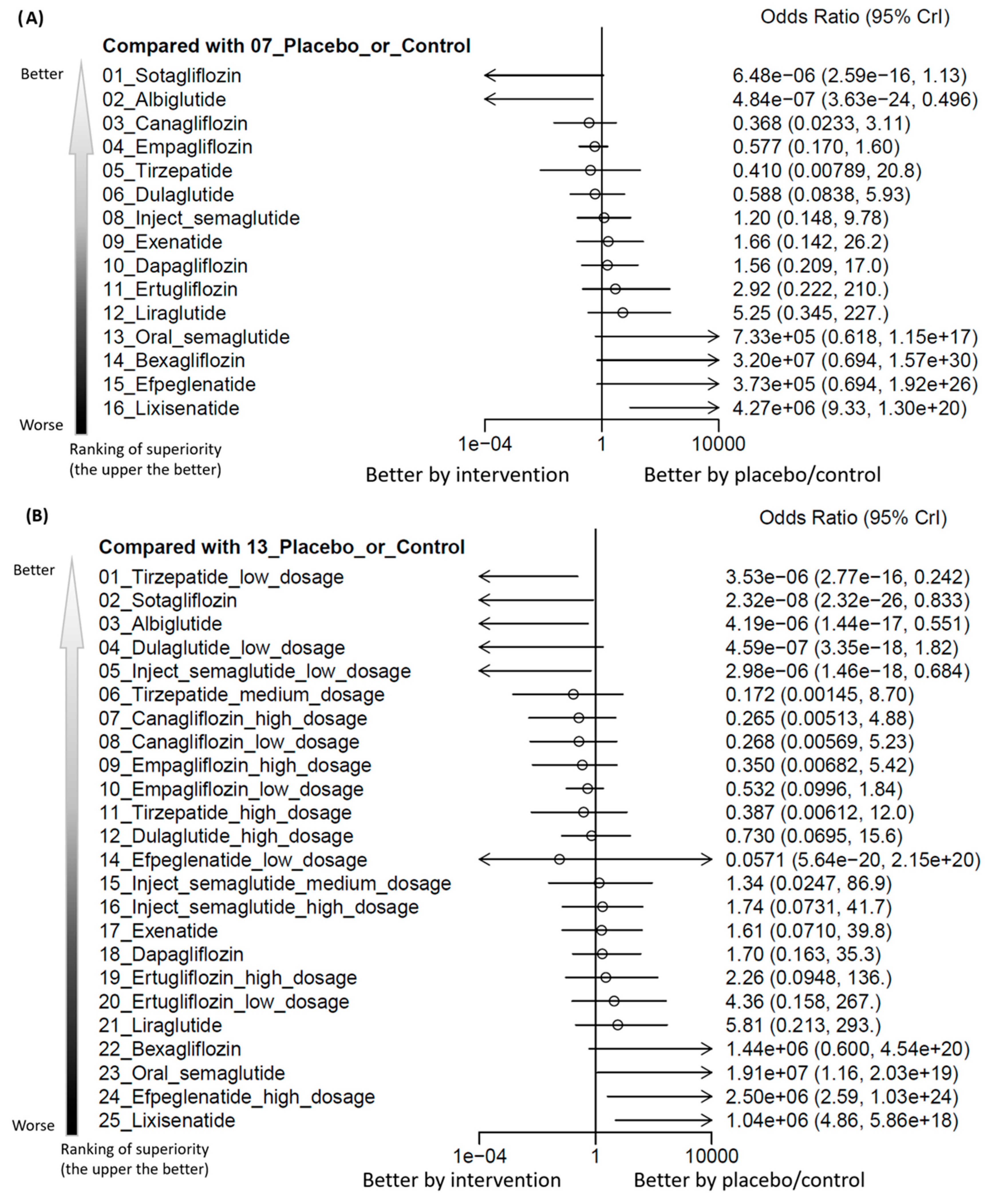
3.2. Primary Outcome: Incidence of Hearing Loss
3.3. Subgroup Analyses of Various Dosage
3.4. Sensitivity Analysis with Re-Validation by RR
3.5. Subgroup Analysis of RCTs Recruiting Subjects with Baseline Illness of Diabetes
3.6. Drop-Out Rate
3.7. Sensitivity Analysis with Deviation-Model Evaluation
3.8. Risk of Bias and Inconsistency
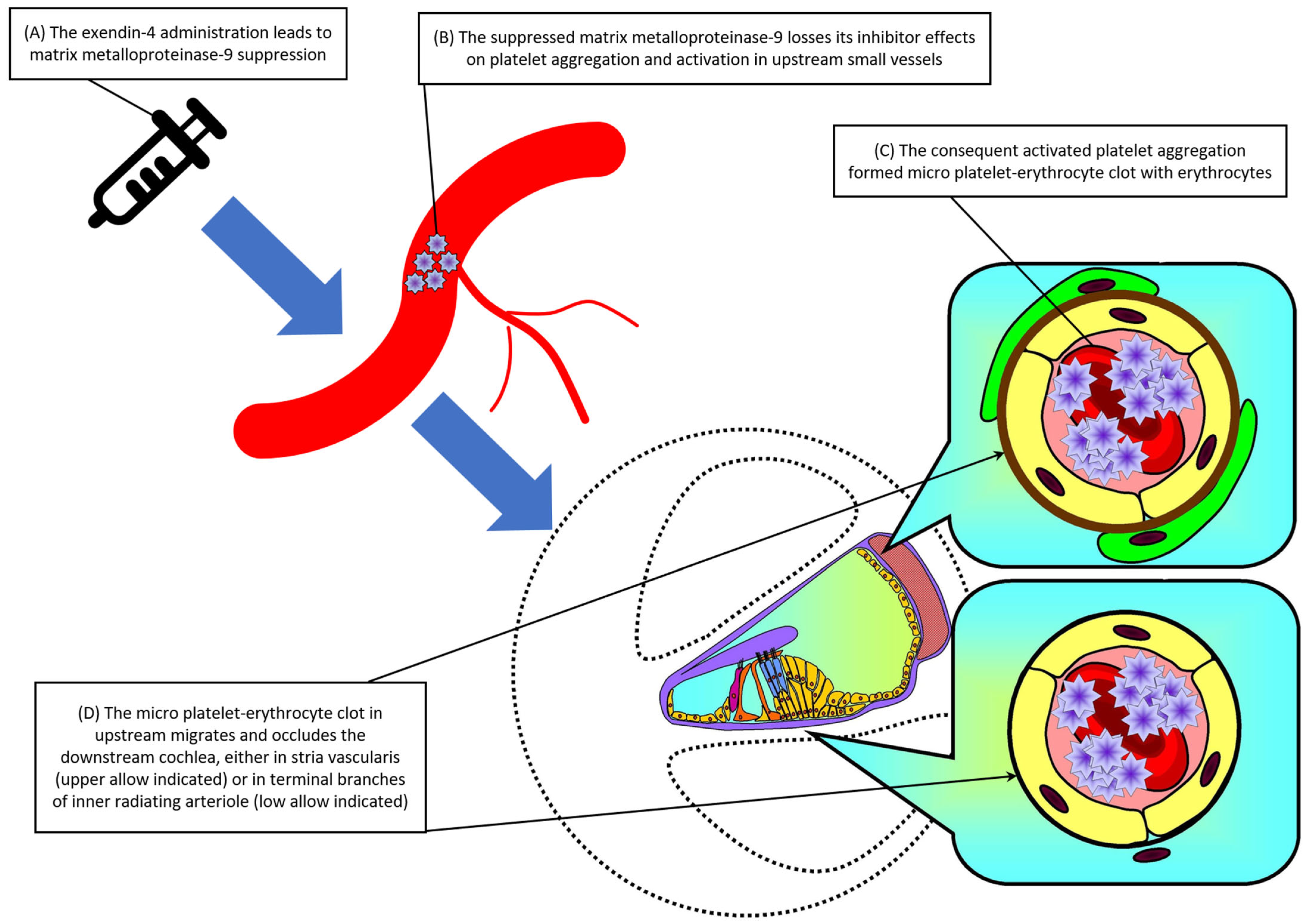
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avogaro, A.; de Kreutzenberg, S.V.; Morieri, M.L.; Fadini, G.P.; Del Prato, S. Glucose-lowering drugs with cardiovascular benefits as modifiers of critical elements of the human life history. Lancet Diabetes Endocrinol. 2022, 10, 882–889. [Google Scholar] [CrossRef]
- Hathaway, J.T.; Shah, M.P.; Hathaway, D.B.; Zekavat, S.M.; Krasniqi, D.; Gittinger, J.W., Jr.; Cestari, D.; Mallery, R.; Abbasi, B.; Bouffard, M.; et al. Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide. JAMA Ophthalmol. 2024, 142, 732–739. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, B.; Han, K.; Lee, J.H.; Kim, M.; Kim, M.K.; Baek, K.H.; Song, K.H.; Kwon, H.S.; Roh, Y.J. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Retinal Vein Occlusion Among Patients with Type 2 Diabetes: A Propensity Score-Matched Cohort Study. Diabetes Care 2021, 44, 2419–2426. [Google Scholar] [CrossRef]
- Kuroki, T.; Tanaka, R.; Shimada, Y.; Yamashiro, K.; Ueno, Y.; Shimura, H.; Urabe, T.; Hattori, N. Exendin-4 Inhibits Matrix Metalloproteinase-9 Activation and Reduces Infarct Growth After Focal Cerebral Ischemia in Hyperglycemic Mice. Stroke 2016, 47, 1328–1335. [Google Scholar] [CrossRef]
- Mastenbroek, T.G.; Feijge, M.A.; Kremers, R.M.; van den Bosch, M.T.; Swieringa, F.; De Groef, L.; Moons, L.; Bennett, C.; Ghevaert, C.; Johnson, J.L.; et al. Platelet-Associated Matrix Metalloproteinases Regulate Thrombus Formation and Exert Local Collagenolytic Activity. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2554–2561. [Google Scholar] [CrossRef]
- Quirk, W.S.; Avinash, G.; Nuttall, A.L.; Miller, J.M. The influence of loud sound on red blood cell velocity and blood vessel diameter in the cochlea. Hear. Res. 1992, 63, 102–107. [Google Scholar] [CrossRef]
- Chen, J.J.; Hsu, C.W.; Chen, Y.W.; Chen, T.Y.; Zeng, B.S.; Tseng, P.T. Audiovestibular Dysfunction in Systemic Lupus Erythematosus Patients: A Systematic Review. Diagnostics 2024, 14, 1670. [Google Scholar] [CrossRef]
- Chen, J.J.; Hsu, C.W.; Chen, Y.W.; Chen, T.Y.; Zeng, B.Y.; Tseng, P.T. Audiovestibular Dysfunction Related to Anti-Phospholipid Syndrome: A Systematic Review. Diagnostics 2024, 14, 2522. [Google Scholar] [CrossRef]
- Chen, J.J.; Hsu, C.W.; Chen, Y.W.; Chen, T.Y.; Zeng, B.S.; Tseng, P.T. Audiological Features in Patients with Rheumatoid Arthritis: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 13290. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Kober, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Welton, N.J. Network meta-analysis: A norm for comparative effectiveness? Lancet 2015, 386, 628–630. [Google Scholar] [CrossRef]
- Tseng, P.T.; Zeng, B.Y.; Hsu, C.W.; Hung, C.M.; Carvalho, A.F.; Stubbs, B.; Chen, Y.W.; Chen, T.Y.; Lei, W.T.; Chen, J.J.; et al. The pharmacodynamics-based prophylactic benefits of GLP-1 receptor agonists and SGLT2 inhibitors on neurodegenerative diseases: Evidence from a network meta-analysis. BMC Med. 2025, 23, 197. [Google Scholar] [CrossRef]
- Peryer, G.; Golder, S.; Junqueira, D.; Vohra, S.; Loke, Y.K.; Group, C.A.E.M. Chapter 19: Adverse effects. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. [Google Scholar]
- Hinkle, J.T.; Graziosi, M.; Nayak, S.M.; Yaden, D.B. Adverse Events in Studies of Classic Psychedelics: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2024, 81, 1225–1235. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Panfili, E.; Frontino, G.; Pallotta, M.T. GLP-1 receptor agonists as promising disease-modifying agents in WFS1 spectrum disorder. Front. Clin. Diabetes Healthc. 2023, 4, 1171091. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Wu, B.; Ryugo, D.K. Diabetes mellitus and hearing loss: A review. Ageing Res. Rev. 2021, 71, 101423. [Google Scholar] [CrossRef]
- Phillips, R.; Hazell, L.; Sauzet, O.; Cornelius, V. Analysis and reporting of adverse events in randomised controlled trials: A review. BMJ Open 2019, 9, e024537. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2; The Cochrane Collaboration: London, UK, 2009. [Google Scholar]
- Tseng, P.T.; Yang, C.P.; Su, K.P.; Chen, T.Y.; Wu, Y.C.; Tu, Y.K.; Lin, P.Y.; Stubbs, B.; Carvalho, A.F.; Matsuoka, Y.J.; et al. The association between melatonin and episodic migraine: A pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J. Pineal Res. 2020, 69, e12663. [Google Scholar] [CrossRef]
- Tseng, P.T.; Zeng, B.Y.; Chen, Y.W.; Yang, C.P.; Su, K.P.; Chen, T.Y.; Wu, Y.C.; Tu, Y.K.; Lin, P.Y.; Carvalho, A.F.; et al. The dose- and duration-dependent association between melatonin treatment and overall cognition in Alzheimer’s dementia: A network meta-analysis of randomized placebo-controlled trials. Curr. Neuropharmacol. 2022, 20, 1816–1833. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Tseng, P.T.; Pei-Chen Chang, J.; Su, H.; Satyanarayanan, S.K.; Su, K.P. Melatonergic agents in the prevention of delirium: A network meta-analysis of randomized controlled trials. Sleep Med. Rev. 2020, 50, 101235. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodriguez, A. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef]
- Gallo, S.; Charbonnel, B.; Goldman, A.; Shi, H.; Huyck, S.; Darekar, A.; Lauring, B.; Terra, S.G. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes. Metab. 2019, 21, 1027–1036. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Ryden, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Ji, L.; Lu, Y.; Li, Q.; Fu, L.; Luo, Y.; Lei, T.; Li, L.; Ye, S.; Shi, B.; Li, X.; et al. Efficacy and safety of empagliflozin in combination with insulin in Chinese patients with type 2 diabetes and insufficient glycaemic control: A phase III, randomized, double-blind, placebo-controlled, parallel study. Diabetes Obes. Metab. 2023, 25, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Chin, R.; Ozeki, A.; Imaoka, T.; Ogawa, Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): A multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Cho, Y.M.; Kim, S.G.; Ko, S.H.; Lim, S.; Dahaoui, A.; Jeong, J.S.; Lim, H.J.; Yu, J.M. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as Metformin Add-on in a Korean Population with Type 2 Diabetes. Diabetes Ther. 2024, 15, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Natale, P.; Tunnicliffe, D.J.; Toyama, T.; Palmer, S.C.; Saglimbene, V.M.; Ruospo, M.; Gargano, L.; Stallone, G.; Gesualdo, L.; Strippoli, G.F. Sodium-glucose co-transporter protein 2 (SGLT2) inhibitors for people with chronic kidney disease and diabetes. Cochrane Database Syst. Rev. 2024, 5, CD015588. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M.; et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults with Type 2 Diabetes Uncontrolled with Metformin Alone or with Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019, 321, 1466–1480. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- A Study of Tirzepatide (LY3298176) in Participants with Obesity Disease (SURMOUNT-J). Available online: https://clinicaltrials.gov/study/NCT04844918?cond=NCT04844918&rank=1 (accessed on 28 October 2024).
- EMPA-Kidney Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Mori-Anai, K.; Takahashi, A.; Matsui, T.; Inagaki, M.; Iida, M.; Maruyama, K.; Tsuda, H. Effect of canagliflozin on the decline of estimated glomerular filtration rate in chronic kidney disease patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III study in Japan. J. Diabetes Investig. 2022, 13, 1981–1989. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.Q.; Xu, X.H.; Kong, X.C.; Sun, R.; Jing, T.; Ye, L.; Su, X.F.; Ma, J.H. The Effects of Once-Weekly Dulaglutide and Insulin Glargine on Glucose Fluctuation in Poorly Oral-Antidiabetic Controlled Patients with Type 2 Diabetes Mellitus. Biomed. Res. Int. 2019, 2019, 2682657. [Google Scholar] [CrossRef]
- Wason, S. Efficacy and Bone Safety of Sotagliflozin 400 and 200 mg Versus Placebo in Participants with Type 2 Diabetes Mellitus Who Have Inadequate Glycemic Control (SOTA-BONE). Available online: https://clinicaltrials.gov/study/NCT03386344?cond=NCT03386344&rank=1 (accessed on 28 October 2024).
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2018; Volume 7. [Google Scholar]
- Lee, C.C.; Ju, T.R.; Lai, P.C.; Lin, H.T.; Huang, Y.T. Should We Use High-Flow Nasal Cannula in Patients Receiving Gastrointestinal Endoscopies? Critical Appraisals through Updated Meta-Analyses with Multiple Methodologies and Depiction of Certainty of Evidence. J. Clin. Med. 2022, 11, 3860. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Pullenayegum, E.; Marshall, J.K.; Iorio, A.; Thabane, L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: A simulation study. BMJ Open 2016, 6, e010983. [Google Scholar] [CrossRef]
- Brockhaus, A.C.; Bender, R.; Skipka, G. The Peto odds ratio viewed as a new effect measure. Stat. Med. 2014, 33, 4861–4874. [Google Scholar] [CrossRef]
- Dias, S.; Ades, A.E.; Welton, N.J.; Jansen, J.P.; Sutton, A.J. Chapter 3: Model fit, model comparison and outlier detection. In Network Meta-Anlaysis for Decision-Making; Scott, M., Barnett, V., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002; pp. 59–92. [Google Scholar]
- Brignardello-Petersen, R.; Izcovich, A.; Rochwerg, B.; Florez, I.D.; Hazlewood, G.; Alhazanni, W.; Yepes-Nunez, J.; Santesso, N.; Guyatt, G.H.; Schunemann, H.J.; et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 2020, 371, m3907. [Google Scholar] [CrossRef]
- Nakashima, T.; Naganawa, S.; Sone, M.; Tominaga, M.; Hayashi, H.; Yamamoto, H.; Liu, X.; Nuttall, A.L. Disorders of cochlear blood flow. Brain Res. Rev. 2003, 43, 17–28. [Google Scholar] [CrossRef]
- Sheu, J.R.; Fong, T.H.; Liu, C.M.; Shen, M.Y.; Chen, T.L.; Chang, Y.; Lu, M.S.; Hsiao, G. Expression of matrix metalloproteinase-9 in human platelets: Regulation of platelet activation in in vitro and in vivo studies. Br. J. Pharmacol. 2004, 143, 193–201. [Google Scholar] [CrossRef]
- Fernandez-Patron, C.; Martinez-Cuesta, M.A.; Salas, E.; Sawicki, G.; Wozniak, M.; Radomski, M.W.; Davidge, S.T. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb. Haemost. 1999, 82, 1730–1735. [Google Scholar] [CrossRef]
- Arpornchayanon, W.; Canis, M.; Suckfuell, M.; Ihler, F.; Olzowy, B.; Strieth, S. Modeling the measurements of cochlear microcirculation and hearing function after loud noise. Otolaryngol. Head Neck Surg. 2011, 145, 463–469. [Google Scholar] [CrossRef]
- Aktas, G.; Atak Tel, B.M.; Tel, R.; Balci, B. Treatment of type 2 diabetes patients with heart conditions. Expert Rev. Endocrinol. Metab. 2023, 18, 255–265. [Google Scholar] [CrossRef]
- Aktas, G.; Taslamacioglu Duman, T. Current usage of long-acting insulin analogs in patients with type 2 diabetes mellitus. Expert Rev. Endocrinol. Metab. 2024, 19, 155–161. [Google Scholar] [CrossRef]
- Chen, J.J.; Chen, Y.W.; Zeng, B.Y.; Hung, C.M.; Zeng, B.S.; Stubbs, B.; Carvalho, A.F.; Thompson, T.; Roerecke, M.; Su, K.P.; et al. Efficacy of pharmacologic treatment in tinnitus patients without specific or treatable origin: A network meta-analysis of randomised controlled trials. eClinicalMedicine 2021, 39, 101080. [Google Scholar] [CrossRef]
- Wang, S.D. Opportunities and challenges of clinical research in the big-data era: From RCT to BCT. J. Thorac. Dis. 2013, 5, 721–723. [Google Scholar] [CrossRef]
- Zhang, Z. Big data and clinical research: Perspective from a clinician. J. Thorac. Dis. 2014, 6, 1659–1664. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects with Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef]
- Ahren, B.; Masmiquel, L.; Kumar, H.; Sargin, M.; Karsbol, J.D.; Jacobsen, S.H.; Chow, F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): A 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Bain, S.C.; Cariou, B.; Piletic, M.; Rose, L.; Axelsen, M.; Rowe, E.; DeVries, J.H. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): A randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Frias, J.P.; Ji, L.; Niemoeller, E.; Nguyen-Pascal, M.L.; Denkel, K.; Espinasse, M.; Guo, H.; Baek, S.; Choi, J.; et al. Efficacy and safety of once-weekly efpeglenatide in people with suboptimally controlled type 2 diabetes: The AMPLITUDE-D, AMPLITUDE-L and AMPLITUDE-S randomized controlled trials. Diabetes Obes. Metab. 2023, 25, 2084–2095. [Google Scholar] [CrossRef]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Morales Villegas, E.C.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzik, M.; et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison with Placebo in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment with Tirzepatide for Maintenance of Weight Reduction in Adults with Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Aronson, R.; Frias, J.; Goldman, A.; Darekar, A.; Lauring, B.; Terra, S.G. Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes. Metab. 2018, 20, 1453–1460. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Barnett, A.H.; Mithal, A.; Manassie, J.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C.; EMPA-REG RENAL Trial Investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 369–384. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Bliddal, H.; Bays, H.; Czernichow, S.; Udden Hemmingsson, J.; Hjelmesaeth, J.; Hoffmann Morville, T.; Koroleva, A.; Skov Neergaard, J.; Velez Sanchez, P.; Wharton, S.; et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N. Engl. J. Med. 2024, 391, 1573–1583. [Google Scholar] [CrossRef]
- Blonde, L.; Jendle, J.; Gross, J.; Woo, V.; Jiang, H.; Fahrbach, J.L.; Milicevic, Z. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): A randomised, open-label, phase 3, non-inferiority study. Lancet 2015, 385, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Garg, S.K.; Rosenstock, J.; Bailey, T.S.; Banks, P.; Bode, B.W.; Danne, T.; Kushner, J.A.; Lane, W.S.; Lapuerta, P.; et al. Sotagliflozin in Combination with Optimized Insulin Therapy in Adults with Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care 2018, 41, 1970–1980. [Google Scholar] [CrossRef]
- Buse, J.B.; Nordahl Christensen, H.; Harty, B.J.; Mitchell, J.; Soule, B.P.; Zacherle, E.; Cziraky, M.; Willey, V.J. Study design and baseline profile for adults with type 2 diabetes in the once-weekly subcutaneous SEmaglutide randomized PRAgmatic (SEPRA) trial. BMJ Open Diabetes Res. Care 2023, 11, e003206. [Google Scholar] [CrossRef]
- Buse, J.B.; Rosenstock, J.; Sesti, G.; Schmidt, W.E.; Montanya, E.; Brett, J.H.; Zychma, M.; Blonde, L.; Group, L.-S. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009, 374, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Leiter, L.A.; de Bruin, T.W.; Gause-Nilsson, I.; Sugg, J.; Parikh, S.J. Dapagliflozin’s Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients with Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study with a 28-Week Extension. Diabetes Care 2015, 38, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, B.; Steinberg, H.; Eymard, E.; Xu, L.; Thakkar, P.; Prabhu, V.; Davies, M.J.; Engel, S.S. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: A randomised clinical trial. Diabetologia 2013, 56, 1503–1511. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Ferrannini, E.; Umpierrez, G.E.; Peters, A.L.; Rosenstock, J.; Powell, D.R.; Davies, M.J.; Banks, P.; Agarwal, R. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes Obes. Metab. 2023, 25, 1646–1657. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Danne, T.; Cariou, B.; Banks, P.; Brandle, M.; Brath, H.; Franek, E.; Kushner, J.A.; Lapuerta, P.; McGuire, D.K.; Peters, A.L.; et al. HbA(1c) and Hypoglycemia Reductions at 24 and 52 Weeks with Sotagliflozin in Combination with Insulin in Adults with Type 1 Diabetes: The European inTandem2 Study. Diabetes Care 2018, 41, 1981–1990. [Google Scholar] [CrossRef]
- Davies, M.; Faerch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Davies, M.J.; Bain, S.C.; Atkin, S.L.; Rossing, P.; Scott, D.; Shamkhalova, M.S.; Bosch-Traberg, H.; Syren, A.; Umpierrez, G.E. Efficacy and Safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients with Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): A Randomized Clinical Trial. Diabetes Care 2016, 39, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjoth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Micheli, M.M.; Aldigeri, R.; Gardini, S.; Ferrari-Pellegrini, F.; Perini, M.; Messa, G.; Antonini, M.; Spigoni, V.; Cinquegrani, G.; et al. Long-acting exenatide does not prevent cognitive decline in mild cognitive impairment: A proof-of-concept clinical trial. J. Endocrinol. Investig. 2024, 47, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Dungan, K.M.; Povedano, S.T.; Forst, T.; Gonzalez, J.G.; Atisso, C.; Sealls, W.; Fahrbach, J.L. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet 2014, 384, 1349–1357. [Google Scholar] [CrossRef]
- Dungan, K.M.; Weitgasser, R.; Perez Manghi, F.; Pintilei, E.; Fahrbach, J.L.; Jiang, H.H.; Shell, J.; Robertson, K.E. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8). Diabetes Obes. Metab. 2016, 18, 475–482. [Google Scholar] [CrossRef]
- Feng, P.; Sheng, X.; Ji, Y.; Urva, S.; Wang, F.; Miller, S.; Qian, C.; An, Z.; Cui, Y. A Phase 1 Multiple Dose Study of Tirzepatide in Chinese Patients with Type 2 Diabetes. Adv. Ther. 2023, 40, 3434–3445. [Google Scholar] [CrossRef]
- Ferrannini, E.; Berk, A.; Hantel, S.; Pinnetti, S.; Hach, T.; Woerle, H.J.; Broedl, U.C. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: An active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013, 36, 4015–4021. [Google Scholar] [CrossRef]
- Fox, C.K.; Clark, J.M.; Rudser, K.D.; Ryder, J.R.; Gross, A.C.; Nathan, B.M.; Sunni, M.; Dengel, D.R.; Billington, C.J.; Bensignor, M.O.; et al. Exenatide for weight-loss maintenance in adolescents with severe obesity: A randomized, placebo-controlled trial. Obesity 2022, 30, 1105–1115. [Google Scholar] [CrossRef]
- Frias, J.P.; Choi, J.; Rosenstock, J.; Popescu, L.; Niemoeller, E.; Muehlen-Bartmer, I.; Baek, S. Efficacy and Safety of Once-Weekly Efpeglenatide Monotherapy Versus Placebo in Type 2 Diabetes: The AMPLITUDE-M Randomized Controlled Trial. Diabetes Care 2022, 45, 1592–1600. [Google Scholar] [CrossRef]
- Frias, J.P.; Davies, M.J.; Rosenstock, J.; Perez Manghi, F.C.; Fernandez Lando, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Frias, J.P.; Hsia, S.; Eyde, S.; Liu, R.; Ma, X.; Konig, M.; Kazda, C.; Mather, K.J.; Haupt, A.; Pratt, E.; et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: A multicentre, randomised, dose-response, phase 2 study. Lancet 2023, 402, 472–483. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Gallwitz, B.; Bohmer, M.; Segiet, T.; Molle, A.; Milek, K.; Becker, B.; Helsberg, K.; Petto, H.; Peters, N.; Bachmann, O. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: A randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care 2011, 34, 604–606. [Google Scholar] [CrossRef]
- Gallwitz, B.; Guzman, J.; Dotta, F.; Guerci, B.; Simo, R.; Basson, B.R.; Festa, A.; Kiljanski, J.; Sapin, H.; Trautmann, M.; et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): An open-label, randomised controlled trial. Lancet 2012, 379, 2270–2278. [Google Scholar] [CrossRef]
- Gao, L.; Lee, B.W.; Chawla, M.; Kim, J.; Huo, L.; Du, L.; Huang, Y.; Ji, L. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: The SURPASS-AP-Combo trial. Nat. Med. 2023, 29, 1500–1510. [Google Scholar] [CrossRef]
- Garber, A.; Henry, R.; Ratner, R.; Garcia-Hernandez, P.A.; Rodriguez-Pattzi, H.; Olvera-Alvarez, I.; Hale, P.M.; Zdravkovic, M.; Bode, B.; the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009, 373, 473–481. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jodar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Lee, S.F.; Pare, G.; Bethel, M.A.; Colhoun, H.M.; Hoover, A.; Lakshmanan, M.; Lin, Y.; Pirro, V.; Qian, H.R.; et al. Biomarker Changes Associated with Both Dulaglutide and Cardiovascular Events in the REWIND Randomized Controlled Trial: A Nested Case-Control Post Hoc Analysis. Diabetes Care 2023, 46, 1046–1051. [Google Scholar] [CrossRef]
- Giorgino, F.; Benroubi, M.; Sun, J.H.; Zimmermann, A.G.; Pechtner, V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients with Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care 2015, 38, 2241–2249. [Google Scholar] [CrossRef]
- Grunberger, G.; Camp, S.; Johnson, J.; Huyck, S.; Terra, S.G.; Mancuso, J.P.; Jiang, Z.W.; Golm, G.; Engel, S.S.; Lauring, B. Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther. 2018, 9, 49–66. [Google Scholar] [CrossRef]
- Hadjadj, S.; Rosenstock, J.; Meinicke, T.; Woerle, H.J.; Broedl, U.C. Initial Combination of Empagliflozin and Metformin in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 1718–1728. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Heise, T.; Mari, A.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Coskun, T.; Thomas, M.K.; Mather, K.J.; Haupt, A.; et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: A multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022, 10, 418–429. [Google Scholar] [CrossRef]
- Heller, S.R.; Geybels, M.S.; Iqbal, A.; Liu, L.; Wagner, L.; Chow, E. A higher non-severe hypoglycaemia rate is associated with an increased risk of subsequent severe hypoglycaemia and major adverse cardiovascular events in individuals with type 2 diabetes in the LEADER study. Diabetologia 2022, 65, 55–64. [Google Scholar] [CrossRef]
- Home, P.D.; Ahren, B.; Reusch, J.E.B.; Rendell, M.; Weissman, P.N.; Cirkel, D.T.; Miller, D.; Ambery, P.; Carr, M.C.; Nauck, M.A. Three-year data from 5 HARMONY phase 3 clinical trials of albiglutide in type 2 diabetes mellitus: Long-term efficacy with or without rescue therapy. Diabetes Res. Clin. Pract. 2017, 131, 49–60. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Inagaki, N.; Takeuchi, M.; Oura, T.; Imaoka, T.; Seino, Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): A double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 623–633. [Google Scholar] [CrossRef]
- Flat-Sugar Trial Investigators. Glucose Variability in a 26-Week Randomized Comparison of Mealtime Treatment with Rapid-Acting Insulin Versus GLP-1 Agonist in Participants with Type 2 Diabetes at High Cardiovascular Risk. Diabetes Care 2016, 39, 973–981. [Google Scholar] [CrossRef]
- Jagomae, T.; Seppa, K.; Reimets, R.; Pastak, M.; Plaas, M.; Hickey, M.A.; Kukker, K.G.; Moons, L.; De Groef, L.; Vasar, E.; et al. Early Intervention and Lifelong Treatment with GLP1 Receptor Agonist Liraglutide in a Wolfram Syndrome Rat Model with an Emphasis on Visual Neurodegeneration, Sensorineural Hearing Loss and Diabetic Phenotype. Cells 2021, 10, 3193. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Butler, J.; Jarolim, P.; Sattar, N.; Vijapurkar, U.; Desai, M.; Davies, M.J. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults with Type 2 Diabetes. J. Am. Coll. Cardiol. 2017, 70, 704–712. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Kadowaki, T.; Isendahl, J.; Khalid, U.; Lee, S.Y.; Nishida, T.; Ogawa, W.; Tobe, K.; Yamauchi, T.; Lim, S.; investigators, S. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): A randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022, 10, 193–206. [Google Scholar] [CrossRef]
- Kaku, K.; Yamada, Y.; Watada, H.; Abiko, A.; Nishida, T.; Zacho, J.; Kiyosue, A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes. Metab. 2018, 20, 1202–1212. [Google Scholar] [CrossRef]
- Kellerer, M.; Kaltoft, M.S.; Lawson, J.; Nielsen, L.L.; Strojek, K.; Tabak, O.; Jacob, S. Effect of once-weekly semaglutide versus thrice-daily insulin aspart, both as add-on to metformin and optimized insulin glargine treatment in participants with type 2 diabetes (SUSTAIN 11): A randomized, open-label, multinational, phase 3b trial. Diabetes Obes. Metab. 2022, 24, 1788–1799. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Esterline, R.; Furtado, R.H.M.; Oscarsson, J.; Gasparyan, S.B.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Chopra, V.; et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021, 9, 586–594. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Seshiah, V.; Merker, L.; Christiansen, A.V.; Roux, F.; Salsali, A.; Kim, G.; Stella, P.; Woerle, H.J.; Broedl, U.C.; et al. Empagliflozin as Add-on Therapy to Pioglitazone with or Without Metformin in Patients with Type 2 Diabetes Mellitus. Clin. Ther. 2015, 37, 1773–1788.e1. [Google Scholar] [CrossRef] [PubMed]
- Koychev, I.; Reid, G.; Nguyen, M.; Mentz, R.J.; Joyce, D.; Shah, S.H.; Holman, R.R. Inflammatory proteins associated with Alzheimer’s disease reduced by a GLP1 receptor agonist: A post hoc analysis of the EXSCEL randomized placebo controlled trial. Alzheimer’s Res. Ther. 2024, 16, 212. [Google Scholar] [CrossRef]
- Lavalle-Gonzalez, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Cefalu, W.T.; de Bruin, T.W.; Xu, J.; Parikh, S.; Johnsson, E.; Gause-Nilsson, I. Long-term maintenance of efficacy of dapagliflozin in patients with type 2 diabetes mellitus and cardiovascular disease. Diabetes Obes. Metab. 2016, 18, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Catarig, A.M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef]
- Lock, J.P. Bexagliflozin Efficacy and Safety Trial (BEST). Available online: https://clinicaltrials.gov/study/NCT02558296?cond=NCT02558296&rank=1 (accessed on 28 October 2024).
- Ludvik, B.; Frias, J.P.; Tinahones, F.J.; Wainstein, J.; Jiang, H.; Robertson, K.E.; Garcia-Perez, L.E.; Woodward, D.B.; Milicevic, Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): A 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 370–381. [Google Scholar] [CrossRef]
- Ludvik, B.; Giorgino, F.; Jodar, E.; Frias, J.P.; Fernandez Lando, L.; Brown, K.; Bray, R.; Rodriguez, A. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Mathieu, C.; Ranetti, A.E.; Li, D.; Ekholm, E.; Cook, W.; Hirshberg, B.; Chen, H.; Hansen, L.; Iqbal, N. Randomized, Double-Blind, Phase 3 Trial of Triple Therapy with Dapagliflozin Add-on to Saxagliptin Plus Metformin in Type 2 Diabetes. Diabetes Care 2015, 38, 2009–2017. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Mellander, A.; Billger, M.; Johnsson, E.; Traff, A.K.; Yoshida, S.; Johnsson, K. Hypersensitivity Events, Including Potentially Hypersensitivity-Related Skin Events, with Dapagliflozin in Patients with Type 2 Diabetes Mellitus: A Pooled Analysis. Clin. Drug Investig. 2016, 36, 925–933. [Google Scholar] [CrossRef]
- Meneilly, G.S.; Roy-Duval, C.; Alawi, H.; Dailey, G.; Bellido, D.; Trescoli, C.; Manrique Hurtado, H.; Guo, H.; Pilorget, V.; Perfetti, R.; et al. Lixisenatide Therapy in Older Patients with Type 2 Diabetes Inadequately Controlled on Their Current Antidiabetic Treatment: The GetGoal-O Randomized Trial. Diabetes Care 2017, 40, 485–493. [Google Scholar] [CrossRef]
- Mosenzon, O.; Blicher, T.M.; Rosenlund, S.; Eriksson, J.W.; Heller, S.; Hels, O.H.; Pratley, R.; Sathyapalan, T.; Desouza, C.; PIONEER 5 Investigators. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): A placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019, 7, 515–527. [Google Scholar] [CrossRef]
- Mu, Y.; Bao, X.; Eliaschewitz, F.G.; Hansen, M.R.; Kim, B.T.; Koroleva, A.; Ma, R.C.W.; Yang, T.; Zu, N.; Liu, M.; et al. Efficacy and safety of once weekly semaglutide 2.4 mg for weight management in a predominantly east Asian population with overweight or obesity (STEP 7): A double-blind, multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2024, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef]
- Nauck, M.; Frid, A.; Hermansen, K.; Shah, N.S.; Tankova, T.; Mitha, I.H.; Zdravkovic, M.; During, M.; Matthews, D.R.; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Rizzo, M.; Johnson, A.; Bosch-Traberg, H.; Madsen, J.; Cariou, B. Once-Daily Liraglutide Versus Lixisenatide as Add-on to Metformin in Type 2 Diabetes: A 26-Week Randomized Controlled Clinical Trial. Diabetes Care 2016, 39, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Stewart, M.W.; Perkins, C.; Jones-Leone, A.; Yang, F.; Perry, C.; Reinhardt, R.R.; Rendell, M. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016, 59, 266–274. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Pieber, T.R.; Bode, B.; Mertens, A.; Cho, Y.M.; Christiansen, E.; Hertz, C.L.; Wallenstein, S.O.R.; Buse, J.B.; PIONEER 7 Investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): A multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019, 7, 528–539. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Polidori, D.; Mari, A.; Ferrannini, E. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia 2014, 57, 891–901. [Google Scholar] [CrossRef]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J.; PIONEER 4 Investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3a trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Ludemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A.; SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Pratley, R.E.; Nauck, M.A.; Barnett, A.H.; Feinglos, M.N.; Ovalle, F.; Harman-Boehm, I.; Ye, J.; Scott, R.; Johnson, S.; Stewart, M.; et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): A randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014, 2, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ridderstrale, M.; Andersen, K.R.; Zeller, C.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG H2H-SU Trial Investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: A 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014, 2, 691–700. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Rosenstock, J.; Canani, L.H.; Deerochanawong, C.; Gumprecht, J.; Lindberg, S.O.; Lingvay, I.; Sondergaard, A.L.; Treppendahl, M.B.; Montanya, E.; et al. Oral Semaglutide Versus Empagliflozin in Patients with Type 2 Diabetes Uncontrolled on Metformin: The PIONEER 2 Trial. Diabetes Care 2019, 42, 2272–2281. [Google Scholar] [CrossRef]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG MONO Trial Investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.; Migdal, A.L.; Rodriguez, T.G.; Chen, Z.Z.; Nath, A.K.; Gerszten, R.E.; Kasid, N.; Toschi, E.; Tripaldi, J.; Heineman, B.; et al. Weight Loss Outcomes Among Early High Responders to Exenatide Treatment: A Randomized, Placebo Controlled Study in Overweight and Obese Women. Front. Endocrinol. 2021, 12, 742873. [Google Scholar] [CrossRef]
- Rosenstock, J.; Fonseca, V.A.; Gross, J.L.; Ratner, R.E.; Ahren, B.; Chow, F.C.; Yang, F.; Miller, D.; Johnson, S.L.; Stewart, M.W.; et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: A comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care 2014, 37, 2317–2325. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frias, J.P.; Rodbard, H.W.; Tofe, S.; Sears, E.; Huh, R.; Fernandez Lando, L.; Patel, H. Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631–1640. [Google Scholar] [CrossRef]
- Rosenstock, J.; Raccah, D.; Koranyi, L.; Maffei, L.; Boka, G.; Miossec, P.; Gerich, J.E. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: A 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013, 36, 2945–2951. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frias, J.P.; Kaneko, S.; Lee, C.J.; Fernandez Lando, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sorrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Schechter, M.; Wiviott, S.D.; Raz, I.; Goodrich, E.L.; Rozenberg, A.; Yanuv, I.; Murphy, S.A.; Zelniker, T.A.; Fredriksson, M.; Johansson, P.A.; et al. Effects of dapagliflozin on hospitalisations in people with type 2 diabetes: Post-hoc analyses of the DECLARE-TIMI 58 trial. Lancet Diabetes Endocrinol. 2023, 11, 233–241. [Google Scholar] [CrossRef]
- Schernthaner, G.; Gross, J.L.; Rosenstock, J.; Guarisco, M.; Fu, M.; Yee, J.; Kawaguchi, M.; Canovatchel, W.; Meininger, G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: A 52-week randomized trial. Diabetes Care 2013, 36, 2508–2515. [Google Scholar] [CrossRef]
- Scully, K.J.; Wolfsdorf, J.I. Efficacy of GLP-1 Agonist Therapy in Autosomal Dominant WFS1-Related Disorder: A Case Report. Horm. Res. Paediatr. 2020, 93, 409–414. [Google Scholar] [CrossRef]
- Spertus, J.A.; Birmingham, M.C.; Nassif, M.; Damaraju, C.V.; Abbate, A.; Butler, J.; Lanfear, D.E.; Lingvay, I.; Kosiborod, M.N.; Januzzi, J.L. The SGLT2 inhibitor canagliflozin in heart failure: The CHIEF-HF remote, patient-centered randomized trial. Nat. Med. 2022, 28, 809–813. [Google Scholar] [CrossRef]
- Stack, A.G.; Han, D.; Goldwater, R.; Johansson, S.; Dronamraju, N.; Oscarsson, J.; Johnsson, E.; Parkinson, J.; Erlandsson, F. Dapagliflozin Added to Verinurad Plus Febuxostat Further Reduces Serum Uric Acid in Hyperuricemia: The QUARTZ Study. J. Clin. Endocrinol. Metab. 2021, 106, e2347–e2356. [Google Scholar] [CrossRef] [PubMed]
- Stenlof, K.; Cefalu, W.T.; Kim, K.A.; Jodar, E.; Alba, M.; Edwards, R.; Tong, C.; Canovatchel, W.; Meininger, G. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-week CANTATA-M study. Curr. Med. Res. Opin. 2014, 30, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Hauske, S.J.; Canziani, M.E.; Caramori, M.L.; Cherney, D.; Cronin, L.; Heerspink, H.J.L.; Hugo, C.; Nangaku, M.; Rotter, R.C.; et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: A randomised, controlled, phase 2 trial. Lancet 2024, 403, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Levin, A.; Nangaku, M.; Kadowaki, T.; Agarwal, R.; Hauske, S.J.; Elsasser, A.; Ritter, I.; Steubl, D.; Wanner, C.; et al. Safety of Empagliflozin in Patients with Type 2 Diabetes and Chronic Kidney Disease: Pooled Analysis of Placebo-Controlled Clinical Trials. Diabetes Care 2022, 45, 1445–1452. [Google Scholar] [CrossRef]
- Umpierrez, G.; Tofe Povedano, S.; Perez Manghi, F.; Shurzinske, L.; Pechtner, V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014, 37, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Desai, A.S.; Jhund, P.S.; Fang, J.C.; Claggett, B.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; et al. Dapagliflozin and Mode of Death in Heart Failure with Improved Ejection Fraction: A Post Hoc Analysis of the DELIVER Trial. JAMA Cardiol. 2024, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults with Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2023, 29, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Wason, S. Efficacy and Safety of Sotagliflozin Versus Placebo in Participants with Type 2 Diabetes Mellitus Who Have Inadequate Glycemic Control While Taking Insulin Alone or with Other Oral Antidiabetic Agents (SOTA-INS). Available online: https://clinicaltrials.gov/study/NCT03285594?cond=NCT03285594&rank=1 (accessed on 28 October 2024).
- Weinstock, R.S.; Guerci, B.; Umpierrez, G.; Nauck, M.A.; Skrivanek, Z.; Milicevic, Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): A randomized, phase III study. Diabetes Obes. Metab. 2015, 17, 849–858. [Google Scholar] [CrossRef]
- Weissman, P.N.; Carr, M.C.; Ye, J.; Cirkel, D.T.; Stewart, M.; Perry, C.; Pratley, R. HARMONY 4: Randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia 2014, 57, 2475–2484. [Google Scholar] [CrossRef]
- Wilding, J.P.; Woo, V.; Soler, N.G.; Pahor, A.; Sugg, J.; Rohwedder, K.; Parikh, S.; Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann. Intern. Med. 2012, 156, 405–415. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Wysham, C.; Blevins, T.; Arakaki, R.; Colon, G.; Garcia, P.; Atisso, C.; Kuhstoss, D.; Lakshmanan, M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014, 37, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Brunt, K.V.; Milicevic, Z.; Varnado, O.; Boye, K.S. Patient-reported Outcomes in Patients with Type 2 Diabetes Treated with Dulaglutide Added to Titrated Insulin Glargine (AWARD-9). Clin. Ther. 2017, 39, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, Z.; Lu, Y.; Liu, M.; Chen, H.; Zhang, M.; Wang, R.; Yuan, Y.; Li, X. Tirzepatide for Weight Reduction in Chinese Adults with Obesity: The SURMOUNT-CN Randomized Clinical Trial. JAMA 2024, 332, 551–560. [Google Scholar] [CrossRef]

| (A) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01_Sotagliflozin | 0.17 [0.01; 4.10] | |||||||||||||||||||||||||||||
| 0.22 (0, 2286941337189.82) | 02_Albiglutide | 0.20 [0.01; 4.16] | ||||||||||||||||||||||||||||
| 0 (0, 2.56) | 0 (0, 2.39) | 03_Canagliflozin | 0.56 [0.11; 2.90] | |||||||||||||||||||||||||||
| 0 (0, 1.05) | 0 (0, 1.16) | 0.65 (0.04, 7.39) | 04_Empagliflozin | 0.63 [0.32; 1.23] | ||||||||||||||||||||||||||
| 0 (0, 2.87) | 0 (0, 3.35) | 0.81 (0.01, 56.86) | 1.21 (0.02, 65.45) | 05_Tirzepatide | 0.41 [0.04; 3.98] | |||||||||||||||||||||||||
| 0 (0, 1.03) | 0 (0, 1.15) | 0.61 (0.02, 10.65) | 0.96 (0.06, 9.25) | 0.77 (0.01, 70.74) | 06_Dulaglutide | 0.52 [0.12; 2.26] | ||||||||||||||||||||||||
| 0 (0, 0.57) | 0 (0, 0.54) | 0.38 (0.02, 3.16) | 0.57 (0.17, 1.58) | 0.46 (0.01, 21.69) | 0.59 (0.08, 5.9) | 07_Placebo_ or_Control | 0.89 [0.22; 3.62] | 0.66 [0.11; 3.98] | 0.80 [0.19; 3.36] | 0.66 [0.10; 4.21] | 0.25 [0.03; 2.23] | 0.60 [0.03; 12.47] | 0.34 [0.01; 8.30] | 0.40 [0.02; 8.35] | 0.11 [0.01; 2.06] | |||||||||||||||
| 0 (0, 0.5) | 0 (0, 0.62) | 0.3 (0.01, 5.56) | 0.48 (0.04, 4.22) | 0.4 (0.01, 31.86) | 0.48 (0.03, 10.47) | 0.84 (0.11, 5.84) | 08_Inject_ semaglutide | |||||||||||||||||||||||
| 0 (0, 0.53) | 0 (0, 0.57) | 0.24 (0.01, 5.69) | 0.37 (0.02, 5.35) | 0.29 (0, 33.06) | 0.38 (0.02, 11.35) | 0.66 (0.05, 7.81) | 0.8 (0.03, 20.52) | 09_Exenatide | ||||||||||||||||||||||
| 0 (0, 0.5) | 0 (0, 0.52) | 0.24 (0.01, 4.26) | 0.37 (0.02, 3.22) | 0.3 (0, 21.2) | 0.38 (0.02, 7.12) | 0.65 (0.06, 4.68) | 0.8 (0.03, 11.95) | 0.99 (0.03, 23.27) | 10_Dapagliflozin | |||||||||||||||||||||
| 0 (0, 0.28) | 0 (0, 0.33) | 0.12 (0, 2.92) | 0.18 (0, 2.72) | 0.15 (0, 14.28) | 0.19 (0, 5.45) | 0.33 (0.01, 3.85) | 0.39 (0.01, 9.76) | 0.49 (0.01, 17.46) | 0.52 (0.01, 15.02) | 11_Ertugliflozin | ||||||||||||||||||||
| 0 (0, 0.14) | 0 (0, 0.16) | 0.06 (0, 1.78) | 0.1 (0, 1.72) | 0.07 (0, 9.52) | 0.1 (0, 3.85) | 0.18 (0, 2.6) | 0.2 (0, 6.43) | 0.25 (0, 11.63) | 0.26 (0, 10.68) | 0.51 (0.01, 45.3) | 12_Liraglutide | |||||||||||||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0.33) | 0 (0, 0.53) | 0 (0, 0.73) | 0 (0, 0.44) | 0 (0, 0.93) | 0 (0, 1.29) | 0 (0, 2.04) | 0 (0, 1.92) | 0 (0, 4.23) | 0 (0, 5.95) | 13_Oral_ semaglutide | ||||||||||||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0.41) | 0 (0, 0.57) | 0 (0, 1.03) | 0 (0, 0.75) | 0 (0, 0.95) | 0 (0, 1.62) | 0 (0, 1.89) | 0 (0, 1.97) | 0 (0, 5.04) | 0 (0, 10.18) | 145.21 (0, 15638204434817468416) | 14_Bexagliflozin | |||||||||||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0.29) | 0 (0, 0.27) | 0 (0, 0.49) | 0 (0, 0.39) | 0 (0, 0.49) | 0 (0, 0.7) | 0 (0, 0.88) | 0 (0, 1.03) | 0 (0, 3.33) | 0 (0, 5.35) | 1.53 (0, 39759673530850082816) | 0.02 (0, 642665163278770) | 15_Efpeglenatide | ||||||||||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0.05) | 0 (0, 0.06) | 0 (0, 0.07) | 0 (0, 0.07) | 0 (0, 0.1) | 0 (0, 0.18) | 0 (0, 0.2) | 0 (0, 0.21) | 0 (0, 0.38) | 0 (0, 1.4) | 0.52 (0, 41443130473760) | 0 (0, 174818703323.2) | 0.02 (0, 16044177798929.8) | 16_Lixisenatide | |||||||||||||||
| (B) | ||||||||||||||||||||||||||||||
| 01_Tirzepatide_ low_dosage | 0.33 [0.01; 8.14] | 0.34 [0.01; 8.48] | ||||||||||||||||||||||||||||
| 174.27 (0, 10463610754211418112) | 02_Sotagliflozin | 0.17 [0.01; 4.10] | ||||||||||||||||||||||||||||
| 0.94 (0, 23865462628940.6) | 0.02 (0, 83814535670.54) | 03_Albiglutide | 0.20 [0.01; 4.16] | |||||||||||||||||||||||||||
| 7.18 (0, 10384045242332) | 0.02 (0, 76625689170891.9) | 2.79 (0, 226025279542229) | 04_Dulaglutide_ low_dosage | 0.33 [0.01; 8.22] | ||||||||||||||||||||||||||
| 1.17 (0, 93257222307134.8) | 0 (0, 592940298063597) | 1.01 (0, 131811533539368) | 0.7 (0, 181440300040.18) | 05_Inject_ semaglutide_ low_dosage | 0.66 [0.03; 16.34] | 0.34 [0.01; 8.27] | ||||||||||||||||||||||||
| 0 (0, 0.79) | 0 (0, 8.45) | 0 (0, 25.84) | 0 (0, 27.45) | 0 (0, 10.56) | 06_Tirzepatide_ medium_ dosage | 1.01 [0.10; 9.75] | 0.34 [0.01; 8.43] | |||||||||||||||||||||||
| 0 (0, 2.37) | 0 (0, 5.31) | 0 (0, 16.31) | 0 (0, 16.7) | 0 (0, 6.32) | 0.68 (0, 146.15) | 07_Canagliflozin_ high_dosage | 1.00 [0.06; 15.96] | 0.62 [0.08; 5.08] | ||||||||||||||||||||||
| 0 (0, 2.2) | 0 (0, 5.17) | 0 (0, 4.46) | 0 (0, 17.79) | 0 (0, 6.1) | 0.63 (0, 154.69) | 0.94 (0.01, 59.33) | 08_Canagliflozin_ low_dosage | 0.62 [0.08; 5.07] | ||||||||||||||||||||||
| 0 (0, 1.31) | 0 (0, 6.04) | 0 (0, 4.16) | 0 (0, 12.78) | 0 (0, 4.37) | 0.53 (0, 117.71) | 0.76 (0.01, 103.71) | 0.79 (0.01, 100.39) | 09_Empagliflozin _high_dosage | 1.00 [0.06; 15.98] | 0.62 [0.08; 5.07] | ||||||||||||||||||||
| 0 (0, 0.57) | 0 (0, 1.87) | 0 (0, 1.29) | 0 (0, 4.23) | 0 (0, 1.53) | 0.34 (0, 24.29) | 0.51 (0.01, 14.49) | 0.53 (0.01, 14.92) | 0.69 (0.01, 13.22) | 10_Empagliflozin _low_dosage | 0.64 [0.33; 1.26] | ||||||||||||||||||||
| 0 (0, 0.4) | 0 (0, 3.6) | 0 (0, 3.88) | 0 (0, 8.59) | 0 (0, 3.25) | 0.43 (0.01, 11.28) | 0.6 (0, 81.02) | 0.69 (0, 99.71) | 0.87 (0, 102.78) | 1.33 (0.03, 103.38) | 11_Tirzepatide_ high_dosage | 0.99 [0.10; 9.57] | |||||||||||||||||||
| 0 (0, 0.44) | 0 (0, 1.44) | 0 (0, 0.94) | 0 (0, 1.32) | 0 (0, 1.14) | 0.21 (0, 20.54) | 0.33 (0, 16.23) | 0.34 (0, 14.65) | 0.45 (0, 15.63) | 0.72 (0.02, 9.31) | 0.48 (0, 31.08) | 12_Dulaglutide_ high_dosage | 0.61 [0.14; 2.61] | ||||||||||||||||||
| 0 (0, 0.24) | 0 (0, 0.83) | 0 (0, 0.55) | 0 (0, 1.82) | 0 (0, 0.68) | 0.17 (0, 8.7) | 0.26 (0.01, 4.88) | 0.27 (0.01, 5.23) | 0.35 (0.01, 5.42) | 0.53 (0.1, 1.84) | 0.39 (0.01, 12.01) | 0.73 (0.07, 15.56) | 13_Placebo_ or_Control | 0.71 [0.07; 6.80] | 0.67 [0.11; 3.99] | 0.66 [0.11; 3.98] | 0.80 [0.19; 3.36] | 0.99 [0.10; 9.54] | 0.43 [0.06; 2.90] | 0.25 [0.03; 2.23] | 0.34 [0.01; 8.30] | 0.60 [0.03; 12.47] | 0.20 [0.01; 4.17] | 0.11 [0.01; 2.06] | |||||||
| 0 (0, 2749665048400134) | 0 (0, 13835349680732138) | 0 (0, 4106605056265394) | 0 (0, 416046115627.38) | 0 (0, 247794060699236) | 3.36 (0, 1747509744556715776) | 4.57 (0, 3863746537860638720) | 4.97 (0, 5275996480618600448) | 5.3 (0, 4429532891571235840) | 8.65 (0, 7964217336657846272) | 8.04 (0, 6558691788735834112) | 14.84 (0, 11731741958374318080) | 17.51 (0, 17741192217616175104) | 14_Efpeglenatide _low_dosage | 0.20 [0.01; 4.16] | ||||||||||||||||
| 0 (0, 0.44) | 0 (0, 1.6) | 0 (0, 0.88) | 0 (0, 2.9) | 0 (0, 0.57) | 0.1 (0, 38.54) | 0.16 (0, 29.55) | 0.17 (0, 26.76) | 0.23 (0, 30.52) | 0.38 (0, 25.55) | 0.25 (0, 60.04) | 0.52 (0, 78.93) | 0.75 (0.01, 40.49) | 0.04 (0, 80325932675121217536) | 15_Inject_ semaglutide_ medium_dosage | ||||||||||||||||
| 0 (0, 0.24) | 0 (0, 0.64) | 0 (0, 0.54) | 0 (0, 1.75) | 0 (0, 0.65) | 0.1 (0, 14.57) | 0.15 (0, 10.53) | 0.16 (0, 11.04) | 0.2 (0, 12.12) | 0.3 (0.01, 8.21) | 0.22 (0, 24.01) | 0.41 (0.01, 38.67) | 0.57 (0.02, 13.68) | 0.03 (0, 2.01122131702702e+20) | 0.8 (0, 147.22) | 16_Inject_ semaglutide_ high_dosage | |||||||||||||||
| 0 (0, 0.26) | 0 (0, 0.88) | 0 (0, 0.58) | 0 (0, 1.74) | 0 (0, 0.72) | 0.09 (0, 15.53) | 0.15 (0, 12.22) | 0.15 (0, 11.81) | 0.2 (0, 12.92) | 0.32 (0.01, 8.36) | 0.23 (0, 23.58) | 0.46 (0.01, 39.17) | 0.62 (0.03, 14.08) | 0.03 (0, 1.7925843597753e+20) | 0.89 (0.01, 179.63) | 1.06 (0.01, 84.22) | 17_Exenatide | ||||||||||||||
| 0 (0, 0.2) | 0 (0, 0.64) | 0 (0, 0.47) | 0 (0, 1.19) | 0 (0, 0.56) | 0.09 (0, 9.19) | 0.14 (0, 6.29) | 0.15 (0, 6.62) | 0.18 (0, 7.19) | 0.31 (0.01, 3.84) | 0.2 (0, 13.5) | 0.43 (0.01, 19.45) | 0.59 (0.03, 6.15) | 0.03 (0, 1.01388022129584e+20) | 0.81 (0.01, 84.95) | 1.01 (0.01, 46.22) | 0.96 (0.01, 45.76) | 18_Dapagliflozin | |||||||||||||
| 0 (0, 0.18) | 0 (0, 0.63) | 0 (0, 0.37) | 0 (0, 1.15) | 0 (0, 0.54) | 0.06 (0, 13.86) | 0.1 (0, 8.88) | 0.1 (0, 9.66) | 0.13 (0, 10.34) | 0.23 (0, 6.78) | 0.15 (0, 18.82) | 0.29 (0, 27.46) | 0.44 (0.01, 10.54) | 0.02 (0, 54300857840542908416) | 0.55 (0, 99.78) | 0.74 (0, 63.75) | 0.65 (0, 62.92) | 0.73 (0.01, 60.62) | 19_Ertugliflozin_ high_dosage | 0.58 [0.07; 5.15] | |||||||||||
| 0 (0, 0.09) | 0 (0, 0.33) | 0 (0, 0.21) | 0 (0, 0.76) | 0 (0, 0.27) | 0.03 (0, 6.65) | 0.05 (0, 4.94) | 0.05 (0, 5.09) | 0.07 (0, 6.1) | 0.11 (0, 3.75) | 0.08 (0, 10.64) | 0.17 (0, 17.44) | 0.23 (0, 6.33) | 0.01 (0, 27862512272519688192) | 0.29 (0, 67.19) | 0.37 (0, 40.33) | 0.35 (0, 41.08) | 0.37 (0, 39.43) | 0.53 (0.03, 12.71) | 20_Ertugliflozin_ low_dosage | |||||||||||
| 0 (0, 0.09) | 0 (0, 0.2) | 0 (0, 0.19) | 0 (0, 0.59) | 0 (0, 0.23) | 0.03 (0, 5.33) | 0.04 (0, 3.56) | 0.04 (0, 3.63) | 0.06 (0, 4.11) | 0.09 (0, 2.84) | 0.07 (0, 8.4) | 0.13 (0, 13.58) | 0.17 (0, 4.7) | 0.01 (0, 17672406927695316992) | 0.23 (0, 47.84) | 0.29 (0, 29.33) | 0.28 (0, 29.45) | 0.3 (0, 29.33) | 0.4 (0, 73.46) | 0.74 (0, 141.71) | 21_Liraglutide | ||||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0.01) | 0 (0, 0.01) | 0 (0, 0) | 0 (0, 0.53) | 0 (0, 0.89) | 0 (0, 0.63) | 0 (0, 0.58) | 0 (0, 0.89) | 0 (0, 0.98) | 0 (0, 1.67) | 0 (0, 1.67) | 0 (0, 12286429074044.5) | 0 (0, 3.79) | 0 (0, 4.67) | 0 (0, 4.48) | 0 (0, 4.1) | 0 (0, 6.99) | 0 (0, 14.67) | 0 (0, 19.44) | 22_Bexagliflozin | |||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.35) | 0 (0, 0.35) | 0 (0, 0.33) | 0 (0, 0.51) | 0 (0, 0.46) | 0 (0, 0.56) | 0 (0, 0.93) | 0 (0, 0.86) | 0 (0, 120950538640435856) | 0 (0, 4.43) | 0 (0, 2.1) | 0 (0, 2.36) | 0 (0, 2.02) | 0 (0, 7.57) | 0 (0, 12.28) | 0 (0, 8.89) | 1.21 (0, 1747737841683757) | 23_Oral_ semaglutide | ||||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.01) | 0 (0, 0.22) | 0 (0, 0.21) | 0 (0, 0.2) | 0 (0, 0.18) | 0 (0, 0.2) | 0 (0, 0.33) | 0 (0, 0.46) | 0 (0, 0.39) | 0 (0, 0.43) | 0 (0, 1.86) | 0 (0, 0.98) | 0 (0, 1.04) | 0 (0, 0.99) | 0 (0, 1.9) | 0 (0, 4) | 0 (0, 5.11) | 0.09 (0, 25965618197430972) | 0.92 (0, 466713242374613) | 24_Efpeglenatide _high_dosage | |||||||
| 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0.09) | 0 (0, 0.1) | 0 (0, 0.09) | 0 (0, 0.11) | 0 (0, 0.11) | 0 (0, 0.2) | 0 (0, 0.25) | 0 (0, 0.21) | 0 (0, 20563509532733) | 0 (0, 0.82) | 0 (0, 0.56) | 0 (0, 0.68) | 0 (0, 0.61) | 0 (0, 1.08) | 0 (0, 2.14) | 0 (0, 2.52) | 2.06 (0, 6625401557077732) | 6.09 (0, 2608743386738966) | 8.01 (0, 65039373111329704) | 25_Lixisenatide | ||||||
| Medications | Overall | Subgroup of Dosage |
|---|---|---|
| Lixisenatide | Significantly increased events of hearing loss/highest risk | Significantly increased events of hearing loss/highest risk |
| Efpeglenatide | Not significant | High-dose efpeglenatide (i.e., 6 mg/week) significantly increased events of hearing loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Hsu, C.-W.; Hung, C.-M.; Liang, C.-S.; Su, K.-P.; Carvalho, A.F.; Stubbs, B.; Chen, Y.-W.; Chen, T.-Y.; Lei, W.-T.; et al. Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors. Pharmaceuticals 2025, 18, 735. https://doi.org/10.3390/ph18050735
Chen J-J, Hsu C-W, Hung C-M, Liang C-S, Su K-P, Carvalho AF, Stubbs B, Chen Y-W, Chen T-Y, Lei W-T, et al. Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors. Pharmaceuticals. 2025; 18(5):735. https://doi.org/10.3390/ph18050735
Chicago/Turabian StyleChen, Jiann-Jy, Chih-Wei Hsu, Chao-Ming Hung, Chih-Sung Liang, Kuan-Pin Su, Andre F. Carvalho, Brendon Stubbs, Yen-Wen Chen, Tien-Yu Chen, Wei-Te Lei, and et al. 2025. "Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors" Pharmaceuticals 18, no. 5: 735. https://doi.org/10.3390/ph18050735
APA StyleChen, J.-J., Hsu, C.-W., Hung, C.-M., Liang, C.-S., Su, K.-P., Carvalho, A. F., Stubbs, B., Chen, Y.-W., Chen, T.-Y., Lei, W.-T., Zeng, B.-Y., & Tseng, P.-T. (2025). Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors. Pharmaceuticals, 18(5), 735. https://doi.org/10.3390/ph18050735







