Marantodes pumilum var. alata Enhances Fracture Healing Through Gene Regulation in a Postmenopausal Rat Model
Abstract
1. Introduction
2. Results
2.1. Body Weight
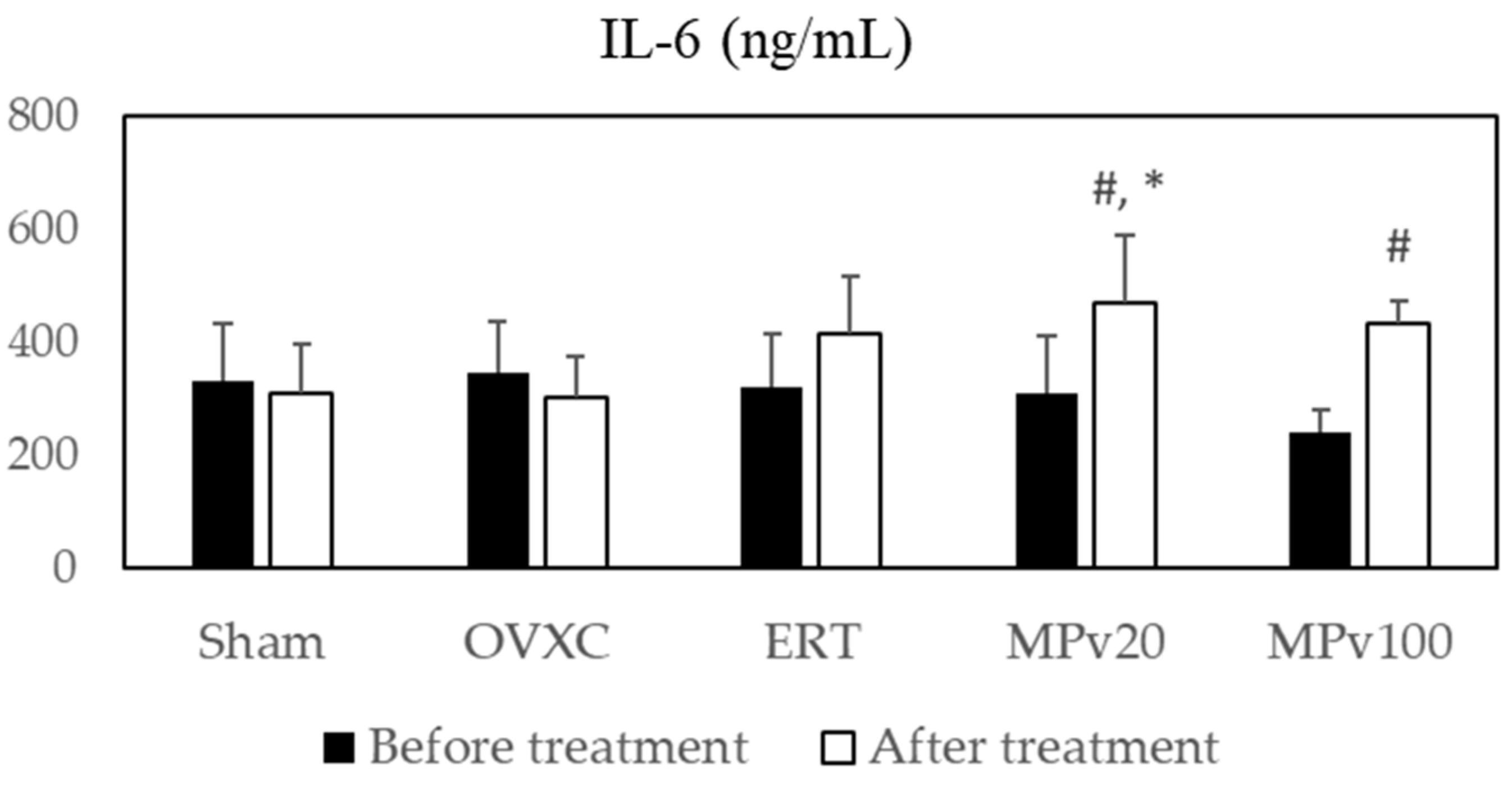
2.2. Pro-Inflammatory Cytokines
2.3. Oxidative Status Markers
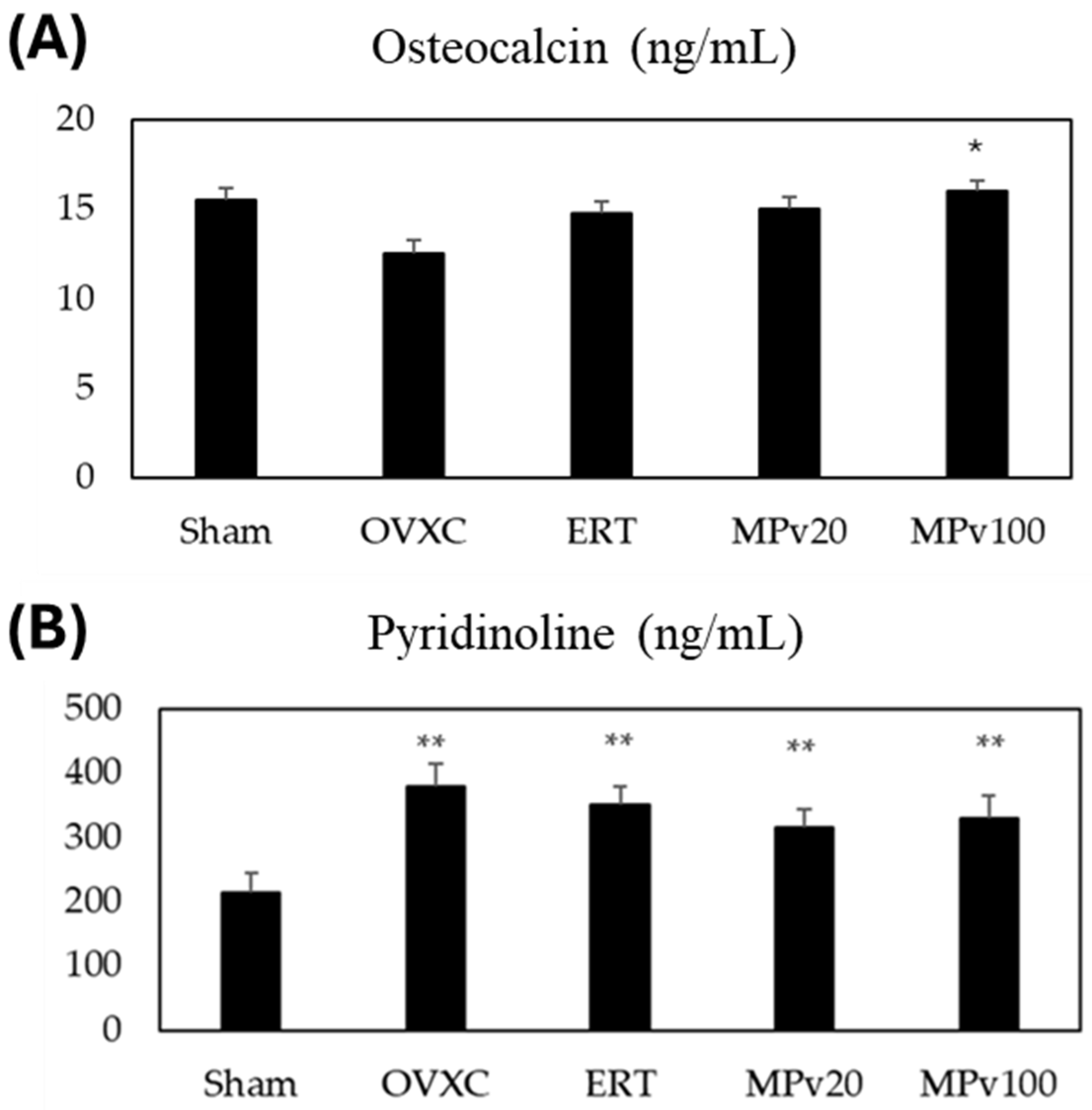
2.4. Bone-Turnover Markers
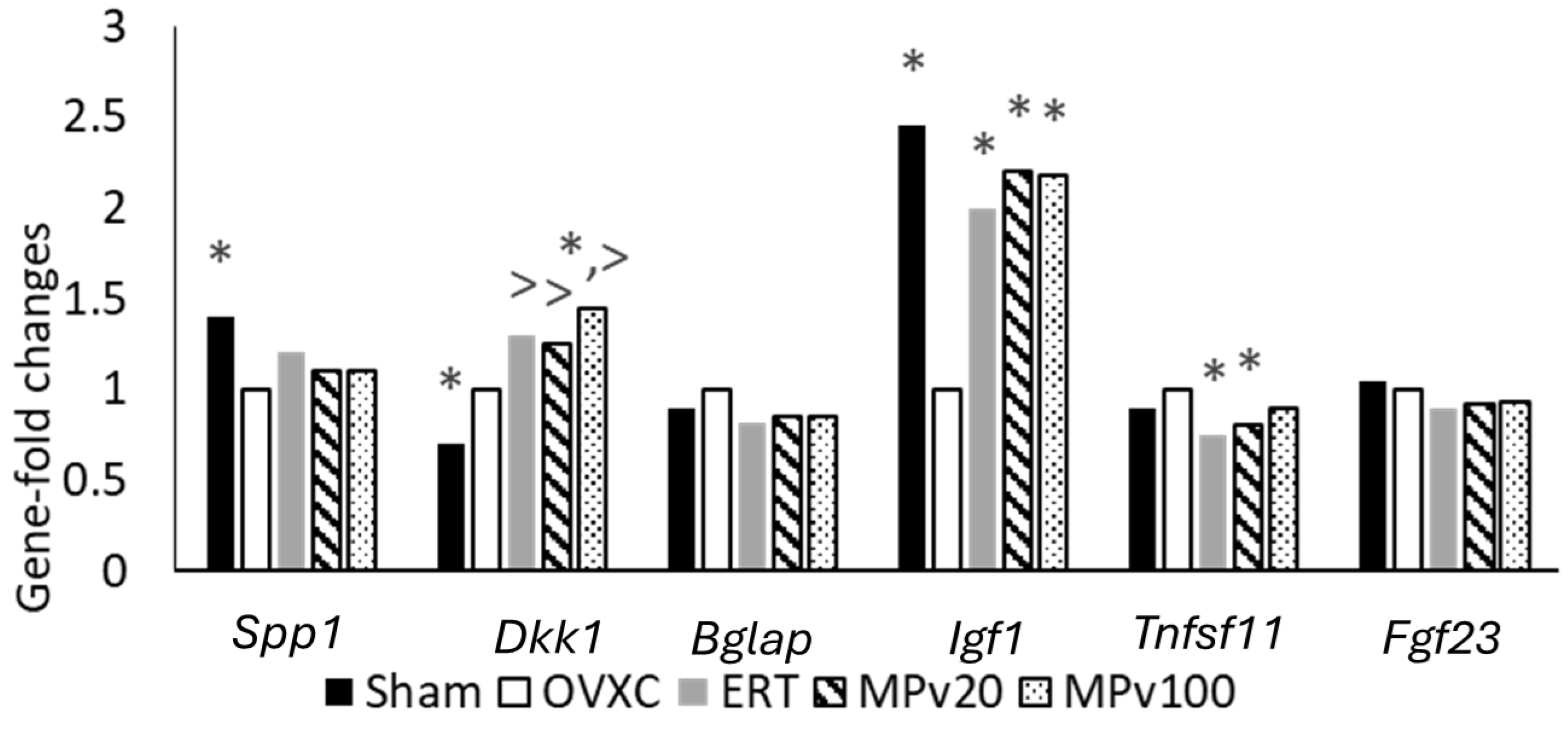
2.5. QuantiGene Plex Assay
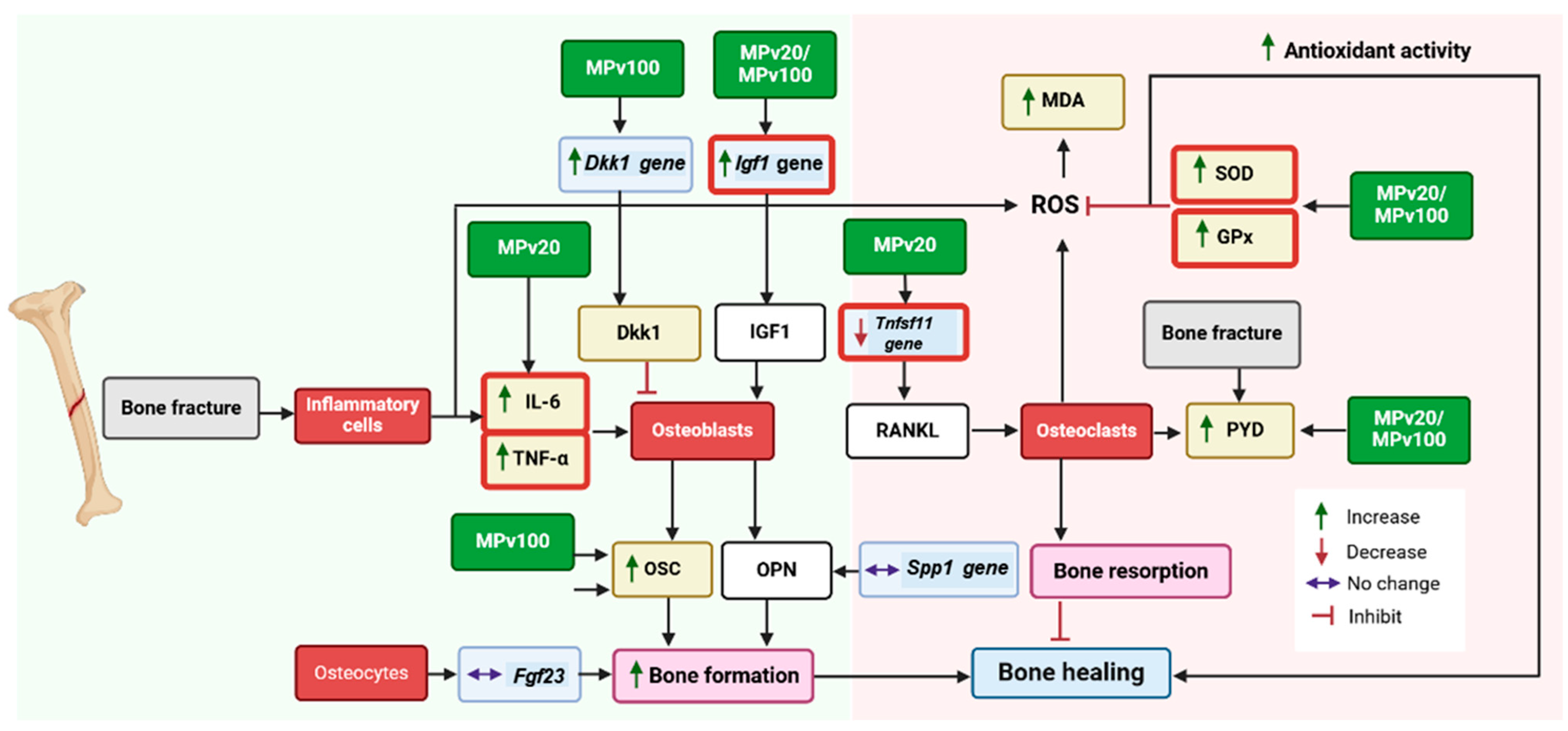
3. Discussion
4. Materials and Methods
4.1. Plant Extract Preparation
4.2. Experimental Animals
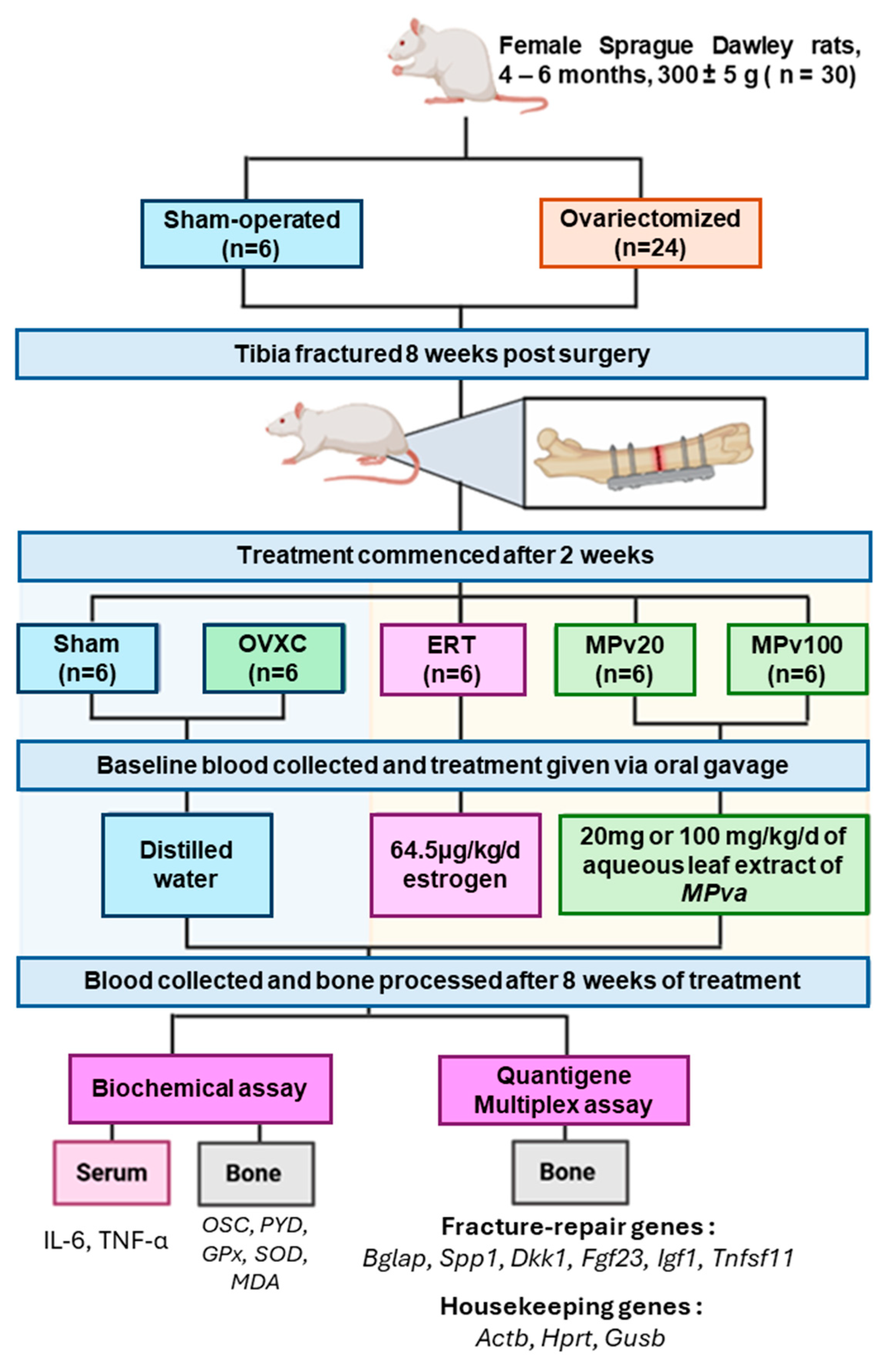
4.3. Study Design
4.4. Blood and Bone Sample Preparation
4.5. Biochemical Assay
4.6. QuantiGene Plex Assay
4.7. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, H.T.; Nguyen, B.T.; Thai, T.H.N.; Tran, A.V.; Nguyen, T.T.; Vo, T.; Mai, L.D.; Tran, T.S.; Nguyen, T.V.; Ho-Pham, L.T. Prevalence, incidence of and risk factors for vertebral fracture in the community: The Vietnam Osteoporosis Study. Sci. Rep. 2024, 14, 32. [Google Scholar] [CrossRef]
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D. Closing the gap. Nature 2017, 550, S194–S195. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Cheung, W.H.; Miclau, T.; Chow, S.K.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47 (Suppl. S2), S21–S26. [Google Scholar] [CrossRef]
- Ortona, E.; Pagano, M.T.; Capossela, L.; Malorni, W. The Role of Sex Differences in Bone Health and Healing. Biology 2023, 12, 993. [Google Scholar] [CrossRef]
- Steppe, L.; Megafu, M.; Tschaffon-Müller, M.E.A.; Ignatius, A.; Haffner-Luntzer, M. Fracture healing research: Recent insights. Bone Rep. 2023, 19, 101686. [Google Scholar] [CrossRef]
- Chow, S.K.; Chim, Y.N.; Wang, J.Y.; Wong, R.M.; Choy, V.M.; Cheung, W.H. Inflammatory response in postmenopausal osteoporotic fracture healing. Bone Jt. Res. 2020, 9, 368–385. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Fischer, V.; Prystaz, K.; Liedert, A.; Ignatius, A. The inflammatory phase of fracture healing is influenced by oestrogen status in mice. Eur. J. Med. Res. 2017, 22, 23. [Google Scholar] [CrossRef]
- Chow, S.K.-H.; Wong, C.H.-W.; Cui, C.; Li, M.M.-C.; Wong, R.M.Y.; Cheung, W.-H. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J. Orthop. Transl. 2022, 36, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Khosla, S. Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocr. Rev. 2022, 43, 984–1002. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.G.; Wood, E. Meta-Analysis of Clinical Fracture Risk Reduction of Antiosteoporosis Drugs: Direct and Indirect Comparisons and Meta-Regressions. Endocr. Pract. 2021, 27, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Imam, B.; Aziz, K.; Khan, M.; Zubair, T.; Iqbal, A. Role of Bisphosphonates in Postmenopausal Women with Osteoporosis to Prevent Future Fractures: A Literature Review. Cureus 2019, 11, e5328. [Google Scholar] [CrossRef]
- Kates, S.L.; Ackert-Bicknell, C.L. How do bisphosphonates affect fracture healing? Injury 2016, 47 (Suppl. S1), S65–S68. [Google Scholar] [CrossRef]
- Fu, L.; Tang, T.; Hao, Y.; Dai, K. Long-term effects of alendronate on fracture healing and bone remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol. Sin. 2013, 34, 387–392. [Google Scholar] [CrossRef]
- Billington, E.; Aghajafari, F.; Skulsky, E.; Kline, G.A. Bisphosphonates. BMJ 2024, 386, e076898. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Fadzil, N.F.D.M.; Raman, V.; Yunus, F.M.; Mark-Lee, W.F.; Chong, Y.Y.; Qian, J.; Zhang, Y.; et al. Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats. Life 2023, 13, 570. [Google Scholar] [CrossRef]
- Karimi, S.M.; Bayat, M.; Rahimi, R. Plant-derived natural medicines for the management of osteoporosis: A comprehensive review of clinical trials. J. Tradit. Complement. Med. 2024, 14, 1–18. [Google Scholar] [CrossRef]
- Tan, N.A.S.; Giribabu, N.; Karim, K.; Nyamathulla, S.; Salleh, N. Intravaginal treatment with Marantodes pumilum (Kacip Fatimah) ameliorates vaginal atrophy in rats with post-menopausal condition. J. Ethnopharmacol. 2019, 236, 9–20. [Google Scholar] [CrossRef]
- Ahmad, S.U.; Azam, A.; Shuid, A.N.; Mohamed, I.N. Phyto-estrogenic effects of Marantodes pumilum (Blume) Kuntze syn. Labisia pumila (Blume) Fern.-Vill. for the prevention and treatment of post-menopausal diseases. Indian J. Tradit. Knowl. 2017, 16, 208–215. [Google Scholar]
- Effendy, N.M.; Abdullah, S.; Yunoh, M.F.M.; Shuid, A.N. Time and dose-dependent effects of Labisia pumila on the bone strength of postmenopausal osteoporosis rat model. BMC Complement. Altern. Med. 2015, 15, 58. [Google Scholar] [CrossRef][Green Version]
- Giaze, T.R.; Shuid, A.N.; Soelaiman, I.N.; Muhammad, N.; Jamal, J.A.; Fauzi, M.B.; Mohamed, N. Comparative anti-osteoporotic properties of the leaves and roots of Marantodes pumilum var. alata in postmenopausal rat model. J. Tradit. Complement. Med 2019, 9, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Giaze, T.R.; Shuid, A.N.; Soelaiman, I.N.; Muhammad, N.; Fauzi, M.B.; Arlamsyah, A.M.; Mohamed, N. Marantodes pumilum leaves promote repair of osteoporotic fracture in postmenopausal Sprague-Dawley rats. Int. J. Pharmacol. 2018, 14, 973–980. [Google Scholar] [CrossRef]
- Husniza, H. Estrogenic and Androgenic Activities of Kacip Fatimah (Labisia pumila); Institute for Medical Research, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2002.
- Chua, L.S.; Lee, S.Y.; Abdullah, N.; Sarmidi, M.R. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia 2012, 83, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Ragipoglu, D.; Bülow, J.; Hauff, K.; Voss, M.; Haffner-Luntzer, M.; Dudeck, A.; Ignatius, A.; Fischer, V. Mast cells drive systemic inflammation and compromised bone repair after trauma. Front. Immunol. 2022, 13, 883707. [Google Scholar] [CrossRef]
- Zhang, E.; Miramini, S.; Patel, M.; Richardson, M.; Ebeling, P.; Zhang, L. Role of TNF-α in early-stage fracture healing under normal and diabetic conditions. Comput. Methods Programs Biomed. 2022, 213, 106536. [Google Scholar] [CrossRef]
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory effects of MSCs in bone healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Glass, G.E.; Ersek, A.; Freidin, A.; Williams, G.A.; Gowers, K.; Santo, A.I.E.; Jeffery, R.; Otto, W.R.; Poulsom, R. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol. Med. 2015, 7, 547–561. [Google Scholar] [CrossRef]
- Karnes, J.M.; Daffner, S.D.; Watkins, C.M. Multiple roles of tumor necrosis factor-alpha in fracture healing. Bone 2015, 78, 87–93. [Google Scholar] [CrossRef]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.; Graves, D.; Einhorn, T. Impaired fracture healing in the absence of TNF-α signaling: The role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef]
- Marques-Carvalho, A.; Kim, H.-N.; Almeida, M. The role of reactive oxygen species in bone cell physiology and pathophysiology. Bone Rep. 2023, 19, 101664. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Liang, S.; Zhu, X.; Dong, Z. The mechanism of pyrroloquinoline quinone influencing the fracture healing process of estrogen-deficient mice by inhibiting oxidative stress. Biomed. Pharmacother. 2021, 139, 111598. [Google Scholar] [CrossRef]
- Keskin, D.; Kiziltunc, A. Reduction of total antioxidant capacity after femoral fracture. Acta Chir. Orthop. Traumatol. Cechoslov. 2015, 82, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Mohamad, S.; Mohamed, N.; Mokhtar, S.A.; Muhammad, N.; Soelaiman, I.N. Bone oxidative changes during early fracture healing of postmenopausal osteoporosis rat model. Asian J. Anim. Vet. Adv. 2011, 6, 1193–1203. [Google Scholar] [CrossRef]
- Mzid, M.; Badraoui, R.; Khedir, S.B.; Sahnoun, Z.; Rebai, T. Protective effect of ethanolic extract of Urtica urens L. against the toxicity of imidacloprid on bone remodeling in rats and antioxidant activities. Biomed. Pharmacother. 2017, 91, 1022–1041. [Google Scholar] [CrossRef]
- Shetty, S.; Kapoor, N.; Bondu, J.D.; Thomas, N.; Paul, T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar]
- Jiang, A.; Liu, Y.; Li, X.; Chen, J.; Wang, H.; Yang, H.; Jiang, W. Serum osteocalcin and urinary free deoxypyridinoline as potential risk factors in predicting the prevalence of bone trauma among the post-menopausal Chinese women. Bangladesh J. Pharmacol. 2018, 13, 231–235. [Google Scholar] [CrossRef]
- Wagner, J.M.; Schmidt, S.V.; Dadras, M.; Huber, J.; Wallner, C.; Dittfeld, S.; Becerikli, M.; Jaurich, H.; Reinkemeier, F.; Drysch, M.; et al. Inflammatory processes and elevated osteoclast activity chaperon atrophic non-union establishment in a murine model. J. Transl. Med. 2019, 17, 416. [Google Scholar] [CrossRef] [PubMed]
- Nurdiana, N.; Mariati, N.; Noorhamdani, N.; Setiawan, B.; Budhiparama, N.; Noor, Z. Effects of Labisia pumila on bone turnover markers and OPG/RANKL system in a rat model of post-menopausal osteoporosis. Clin. Nutr. Exp. 2018, 20, 41–47. [Google Scholar] [CrossRef][Green Version]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Xing, D.; Liu, Z.; Gao, H.; Ma, W.; Nie, L.; Gong, M. Effect of transplantation of marrow mesenchymal stem cells transfected with insulin-like growth factor-1 gene on fracture healing of rats with diabetes. Bratisl. Lek. Listy 2015, 116, 64–68. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- To, T.T.; Witten, P.E.; Renn, J.; Bhattacharya, D.; Huysseune, A.; Winkler, C. Rankl-induced osteoclastogenesis leads to loss of mineralization in a medaka osteoporosis model. Development 2012, 139, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Masaoodi, R.A.; Al-Sallami, A.S.; Al-Baseesee, H. The relation between the RANKL and resistin in menopausal women with osteoporosis. AIP Conf. Proc. 2019, 2144, 040012. [Google Scholar]
- Wang, X.-F.; Zhang, Y.-K.; Yu, Z.-S.; Zhou, J.-L. The role of the serum RANKL/OPG ratio in the healing of intertrochanteric fractures in elderly patients. Mol. Med. Rep. 2013, 7, 1169–1172. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; Wei, Y.; Jing, J.; Li, J. Icariin promotes fracture healing in ovariectomized rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924554-1–e924554-8. [Google Scholar] [CrossRef]
- Zhang, W.; Drake, M.T. Potential role for therapies targeting DKK1, LRP5, and serotonin in the treatment of osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 93–100. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Fouda, N.; Abbas, A.A. Serum dickkopf-1 level in postmenopausal females: Correlation with bone mineral density and serum biochemical markers. J. Osteoporos. 2013, 2013, 460210. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Huang, D.; Zhou, W.; Yan, L.; Yue, J.; Lu, W.; Song, D.; Zhou, X.; Ye, L.; Zhang, L. Dickkopf-1 may regulate bone coupling by attenuating wnt/β-catenin signaling in chronic apical periodontitis. Arch. Oral Biol. 2018, 86, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Morvan, F.; Boulukos, K.; Clément-Lacroix, P.; Roman, S.R.; Suc-Royer, I.; Vayssière, B.; Ammann, P.; Martin, P.; Pinho, S.; Pognonec, P.; et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006, 21, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Fazliana, M.; Gu, H.F.; Östenson, C.-G.; Yusoff, M.M.; Nazaimoon, W.W. Labisia pumila extract down-regulates hydroxysteroid (11-beta) dehydrogenase 1 expression and corticosterone levels in ovariectomized rats. J. Nat. Med. 2012, 66, 257–264. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Fathilah, S.N.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012, 142, 294–299. [Google Scholar] [CrossRef]
- Jamal, J.A.; Sateri, A.A.; Husain, K.; Jantan, I.; Mohamad-Arshad, M. Perbandingan pelbagai kaedah pengekstrakan air Labisia pumila var. alata. Malays. J. Health Sci. 2006, 4, 37–46. [Google Scholar]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Description of a new method of ovariectomy in female rats. Rev. Bras. Reumatol. 2012, 52, 466–470. [Google Scholar] [CrossRef]
- Stuermer, E.K.; Sehmisch, S.; Rack, T.; Wenda, E.; Seidlova-Wuttke, D.; Tezval, M.; Wuttke, W.; Frosch, K.-H.; Stuermer, K.-M. Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbeck’s Arch. Surg. 2010, 395, 163–172. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef]
- Ariffin, S.N.F.A. Branched DNA: A novel technique for molecular diagnostics. Res. Updates Med. Sci. 2013, 1, 27–29. [Google Scholar]

| Group | Weekly Animal Weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Sham | 240 ± 4 | 245 ± 5 | 241 ± 4 * | 250 ± 7 | 259 ± 7 * | 257 ± 7 * | 253 ± 7 * | 255 ± 6 * | 257 ± 6 * | 256 ± 6 * |
| OVXC | 245 ± 5 | 266 ± 7 | 288 ± 9 | 322 ± 9 | 333 ± 9 | 339 ± 11 | 348 ± 12 | 351 ± 13 | 355 ± 13 | 347 ± 7 |
| ERT | 245 ± 4 | 269 ± 7 | 294 ± 10 | 297 ± 10 | 299 ± 10 | 306 ± 9 | 308 ± 10 | 310 ± 11 | 316 ± 11 | 318 ± 11 |
| MPv20 | 248 ± 2 | 274 ± 5 | 306 ± 8 | 312 ± 10 | 320 ± 12 | 328 ± 10 | 327 ± 11 | 328 ± 11 | 336 ± 13 | 338 ± 13 |
| MPv100 | 246 ± 4 | 269 ± 4 | 290 ± 9 | 310 ± 10 | 313 ± 11 | 322 ± 12 | 324 ± 14 | 333 ± 14 | 335 ± 14 | 335 ± 13 |
| Sham | OVXC | ERT | MPv20 | MPv100 | |
|---|---|---|---|---|---|
| GPx activity (mU/mL) | 28 ± 0.4 | 17 ± 0.28 | 32 ± 0.37 * | 38 ± 0.8 * | 34 ± 0.33 * |
| SOD activity (mU/mL) | 2230 ± 70 * | 1540 ± 70 | 1730 ± 100 | 1950 ± 80 * | 2120 ± 50 * |
| MDA | 0.96 ± 0.05 | 1.32 ± 0.11 | 0.97 ± 0.01 | 1.00 ± 0.09 | 0.95 ± 0.02 |
| Genes | HKGs | Sham | OVXC | ERT | MPv20 | MPv100 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spp1 | Actb | 0.8229 * | 🡫 | 1 | 0.8616 | 0.7851 * | 🡫 | 0.7704 * | 🡫 | |
| Hprt1 | 0.0147 * | 🡫 | 1 | 0.0183 * | 🡫 | 0.0162 * | 🡫 | 0.0177 * | 🡫 | |
| Gusb | 0.0513 * | 🡫 | 1 | 0.0636 * | 🡫 | 0.0624 * | 🡫 | 0.0584 * | 🡫 | |
| Bglap | Actb | 1.0925 | 1 | 0.9399 a | 🡫 | 0.9322 a | 🡫 | 0.9115 a | 🡫 | |
| Hprt1 | 0.7605 * | 🡫 | 1 | 0.7661 * | 🡫 | 0.7558 * | 🡫 | 0.8188 | ||
| Gusb | 1.0012 | 1 | 1.0289 | 1.0914 | 1.0146 | |||||
| Igf1 | Actb | 1.4358 * | 🡩 | 1 | 1.0517 a | 🡩 | 1.0881 a | 🡩 | 1.0678 a | 🡩 |
| Hprt1 | 1.0064 | 1 | 0.8524 *,a | 🡫 | 0.8828 | 0.9565 | ||||
| Gusb | 1.3135 * | 🡩 | 1 | 1.1416 a | 🡩 | 1.2832 | 1.1822 | |||
| Dkk1 | Actb | 0.9736 | 1 | 1.2919 | 1.2406 | 1.4449 | ||||
| Hprt1 | 0.6923 | 1 | 1.0554 | 1.0119 | 1.3273 a | 🡩 | ||||
| Gusb | 0.8825 | 1 | 1.3870 | 1.4503 | 1.5658 a | 🡩 | ||||
| Tnfsf11 | Actb | 0.9793 | 1 | 0.7701 *,a | 🡫 | 0.7885 *,a | 🡫 | 0.8353 * | 🡫 | |
| Hprt1 | 0.6706 * | 🡫 | 1 | 0.6215 * | 🡫 | 0.6263 * | 🡫 | 0.7444 * | 🡫 | |
| Gusb | 0.9096 | 1 | 0.8479 | 0.9455 | 0.9516 | |||||
| Fgf23 | Actb | 1 | 1 | 0.7950 *,a | 🡫 | 0.8034 *,a | 🡫 | 0.7990 *,a | 🡫 | |
| Hprt1 | 1 | 1 | 0.9387 | 0.9336 | 1.0331 | |||||
| Gusb | 1 | 1 | 0.9428 | 1.0273 | 0.9713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaze, T.R.; Mohamed, N.; Syed Hashim, S.A.; Shuid, A.N.; Soelaiman, I.N.; Muhammad, N.; Jafar Sidik, F.Z.; Jamal, J.A. Marantodes pumilum var. alata Enhances Fracture Healing Through Gene Regulation in a Postmenopausal Rat Model. Pharmaceuticals 2025, 18, 736. https://doi.org/10.3390/ph18050736
Giaze TR, Mohamed N, Syed Hashim SA, Shuid AN, Soelaiman IN, Muhammad N, Jafar Sidik FZ, Jamal JA. Marantodes pumilum var. alata Enhances Fracture Healing Through Gene Regulation in a Postmenopausal Rat Model. Pharmaceuticals. 2025; 18(5):736. https://doi.org/10.3390/ph18050736
Chicago/Turabian StyleGiaze, Tijjani Rabiu, Norazlina Mohamed, Syed Alhafiz Syed Hashim, Ahmad Nazrun Shuid, Ima Nirwana Soelaiman, Norliza Muhammad, Fadhlullah Zuhair Jafar Sidik, and Jamia Azdina Jamal. 2025. "Marantodes pumilum var. alata Enhances Fracture Healing Through Gene Regulation in a Postmenopausal Rat Model" Pharmaceuticals 18, no. 5: 736. https://doi.org/10.3390/ph18050736
APA StyleGiaze, T. R., Mohamed, N., Syed Hashim, S. A., Shuid, A. N., Soelaiman, I. N., Muhammad, N., Jafar Sidik, F. Z., & Jamal, J. A. (2025). Marantodes pumilum var. alata Enhances Fracture Healing Through Gene Regulation in a Postmenopausal Rat Model. Pharmaceuticals, 18(5), 736. https://doi.org/10.3390/ph18050736







