An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression
Abstract
1. Introduction
2. Results
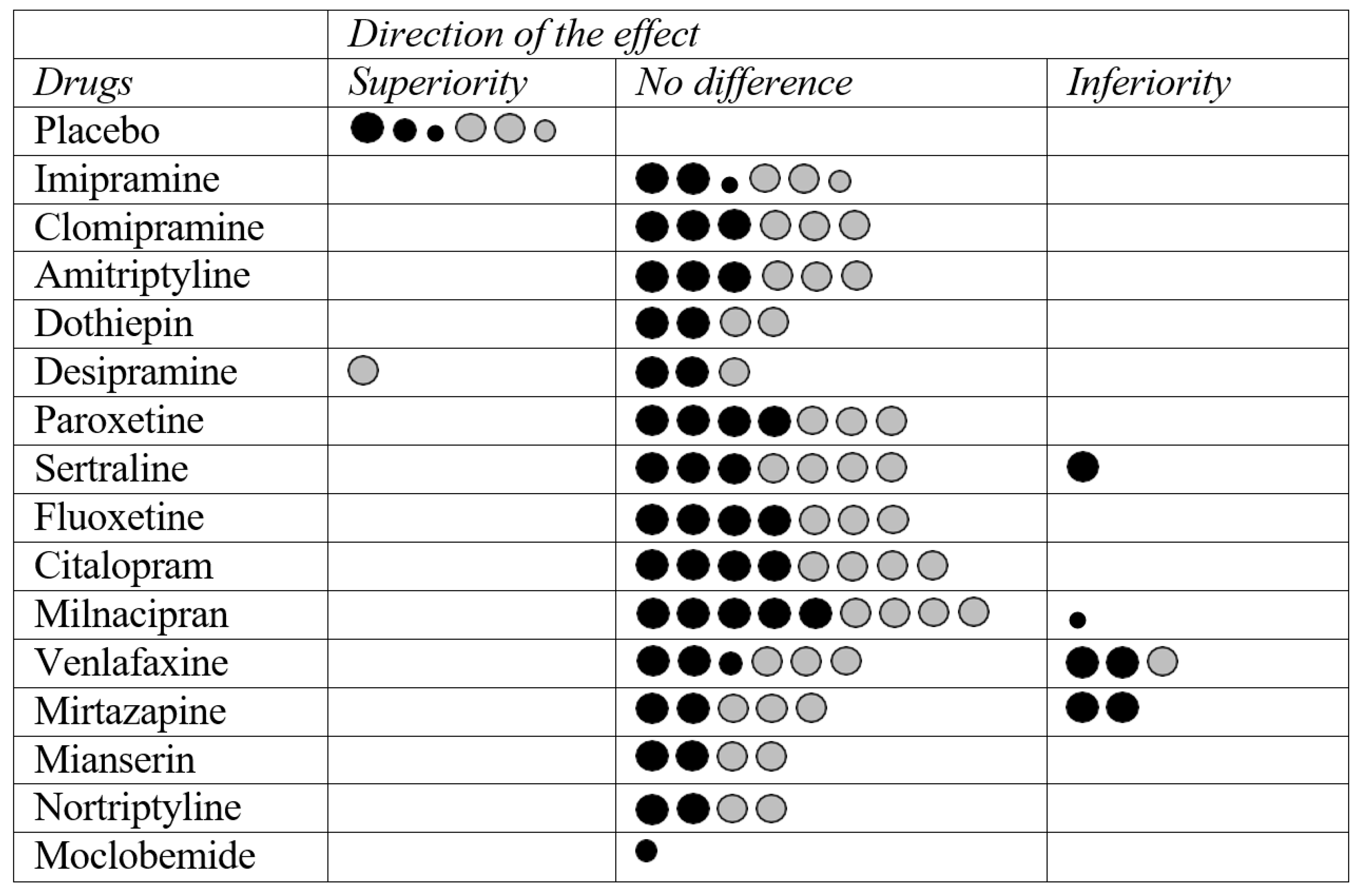
2.1. Fluvoxamine vs. Placebo
2.2. Fluvoxamine vs. Tricyclic Antidepressants (TCAs)
2.3. Fluvoxamine vs. SSRIs
2.4. Fluvoxamine vs. SNRIs
2.5. Fluvoxamine vs. Other Antidepressants
3. Discussion
4. Methods
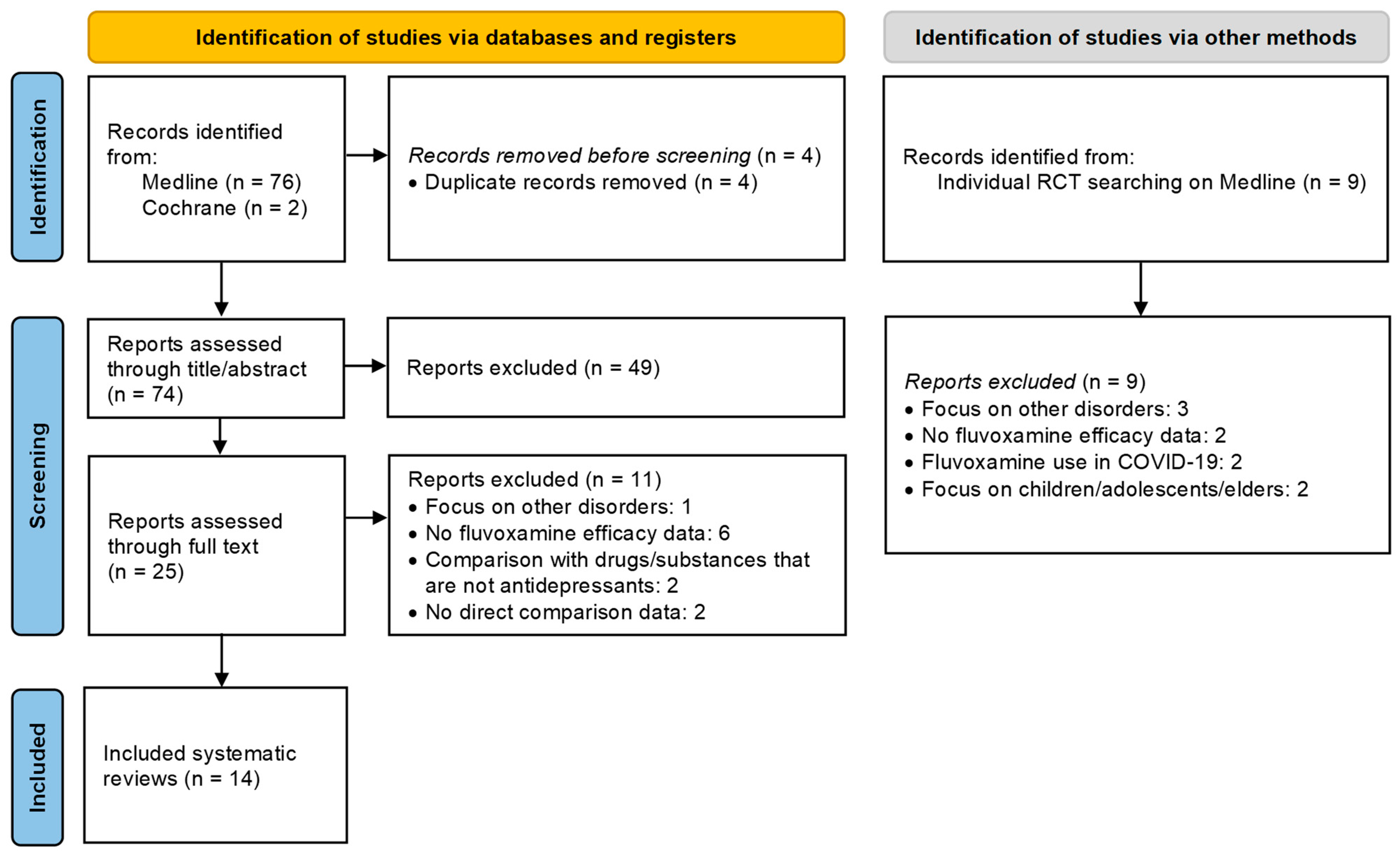
4.1. Search and Selection
4.2. Data Extraction
4.3. Methodological Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Thornicroft, G.; Chatterji, S.; Evans-Lacko, S.; Gruber, M.; Sampson, N.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Andrade, L.; Borges, G.; et al. Undertreatment of people with major depressive disorder in 21 countries. Br. J. Psychiatry 2017, 210, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Ware, M.; Marmorstein, N.; Cipriani, A.; Dendukuri, N.; et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef]
- Zenebe, Y.; Akele, B.; W/Selassie, M.; Necho, M. Prevalence and determinants of depression among old age: A systematic review and meta-analysis. Ann. Gen. Psychiatry 2021, 20, 55. [Google Scholar] [CrossRef]
- Szymkowicz, S.M.; Gerlach, A.R.; Homiack, D.; Taylor, W.D. Biological factors influencing depression in later life: Role of aging processes and treatment implications. Transl. Psychiatry 2023, 13, 160. [Google Scholar] [CrossRef]
- Beijers, L.; Wardenaar, K.J.; van Loo, H.M.; Schoevers, R.A. Data-driven biological subtypes of depression: Systematic review of biological approaches to depression subtyping. Mol. Psychiatry 2019, 24, 888–900. [Google Scholar] [CrossRef]
- Musil, R.; Seemüller, F.; Meyer, S.; Spellmann, I.; Adli, M.; Bauer, M.; Kronmüller, K.T.; Brieger, P.; Laux, G.; Bender, W.; et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int. J. Methods Psychiatr. Res. 2018, 27, e1569. [Google Scholar] [CrossRef]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, G.; Su, Y.; Li, M.; Wolfson, C.; Meng, X.; Schmitz, N. Characterization of depression subtypes and their relationships to stressor profiles among middle-aged and older adults: An analysis of the canadian longitudinal study on aging (CLSA). J. Psychiatr. Res. 2024, 175, 333–342. [Google Scholar] [CrossRef]
- Tozzi, L.; Zhang, X.; Pines, A.; Olmsted, A.M.; Zhai, E.S.; Anene, E.T.; Chesnut, M.; Holt-Gosselin, B.; Chang, S.; Stetz, P.C.; et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat. Med. 2024, 30, 2076–2087. [Google Scholar] [CrossRef]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Zamparelli, A.; Nettis, M.A.; Pariante, C.M. Antidepressant Drugs: Mechanisms of Action and Side Effects. In Encyclopedia of Behavioral Neuroscience, 2nd ed.; Della Sala, S., Ed.; Elsevier: Oxford, UK, 2022; pp. 613–626. [Google Scholar]
- Saletu, B.; Schjerve, M.; Grünberger, J.; Schanda, H.; Arnold, O.H. Fluvoxamine-a new serotonin re-uptake inhibitor: First clinical and psychometric experiences in depressed patients. J. Neural Transm. 1977, 41, 17–36. [Google Scholar] [CrossRef]
- Claassen, V. Review of the animal pharmacology and pharmacokinetics of fluvoxamine. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S3), 349S–355S. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, P.D. Pharmacology of serotonin uptake inhibitors: Focus on fluvoxamine. J. Psychiatry Neurosci. 1991, 16, 10–18. [Google Scholar]
- Ago, Y.; Hasebe, S.; Hiramatsu, N.; Hashimoto, H.; Takuma, K.; Matsuda, T. Psychopharmacology of combined activation of the serotonin. Eur. J. Pharmacol. 2017, 809, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, I.; Hashimoto, K. Cognition and depression: The effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum. Psychopharmacol. 2010, 25, 193–200. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ishiwata, K.; Ishii, K.; Kimura, Y.; Sakata, M.; Naganawa, M.; Oda, K.; Miyatake, R.; Fujisaki, M.; Shimizu, E.; et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: A positron emission tomography study using [11C]SA4503. Biol. Psychiatry 2007, 62, 878–883. [Google Scholar] [CrossRef]
- Ren, P.; Wang, J.; Li, N.; Li, G.; Ma, H.; Zhao, Y.; Li, Y. Sigma-1 Receptors in Depression: Mechanism and Therapeutic Development. Front. Pharmacol. 2022, 13, 925879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Xia, C.Y.; Jia, H.M.; He, J.; Lian, W.W.; Yan, Y.; Wang, W.P.; Zhang, W.K.; Xu, J.K. Sigma-1 receptor: A potential target for the development of antidepressants. Neurochem. Int. 2022, 159, 105390. [Google Scholar] [CrossRef] [PubMed]
- Dobrodeeva, V.; Abdyrahmanova, A.; Astafeva, D.; Smirnova, D.; Cumming, P.; De Sousa, A.; Davydkin, I.; Yashikhina, A.; Shnayder, N.; Nasyrova, R. Pharmacogenetic Aspects of COVID-19 Management and Post-COVID-19 Depression Treatment with Fluvoxamine. Psychiatr. Danub. 2022, 34, 25–30. [Google Scholar]
- Lenze, E.J.; Reiersen, A.M.; Santosh, P.J. Repurposing fluvoxamine, and other psychiatric medications, for COVID-19 and other conditions. World Psychiatry 2022, 21, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Suzuki, T.; Hashimoto, K. Mechanisms of action of fluvoxamine for COVID-19: A historical review. Mol. Psychiatry 2022, 27, 1898–1907. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- McAllister-Williams, R.H.; Arango, C.; Blier, P.; Demyttenaere, K.; Falkai, P.; Gorwood, P.; Hopwood, M.; Javed, A.; Kasper, S.; Malhi, G.S.; et al. The identification, assessment and management of difficult-to-treat depression: An international consensus statement. J. Affect. Disord. 2020, 267, 264–282. [Google Scholar] [CrossRef]
- Gammoh, O.S.; Bashatwah, R. Potential strategies to optimize the efficacy of antidepressants: Beyond the monoamine theory. Electron. J. Gen. Med. 2023, 20, em513. [Google Scholar] [CrossRef]
- Haddad, M.; Dieckmann, L.H.J.; Viola, T.W.; de Araújo, M.R.; da Silva, N.R.; Mari, J.J. The Efficacy of Fluvoxamine in Anxiety Disorders and Obsessive-Compulsive Disorder: An Overview of Systematic Reviews and Meta-Analyses. Pharmaceuticals 2025, 18, 353. [Google Scholar] [CrossRef]
- European Medicines Agency Floxyfral Registration. Available online: https://www.ema.europa.eu/en/documents/referral/summary-information-referral-opinion-following-arbitration-pursuant-article-30-council-directive-200183ec-floxyfral-and-associated-names-international-non-proprietary-name-inn-fluvoxamine-background_en.pdf (accessed on 25 April 2025).
- FDA Label—Fluvoxamine (Luvox®) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022235lbl.pdf (accessed on 25 April 2025).
- Williams, T.; McCaul, M.; Schwarzer, G.; Cipriani, A.; Stein, D.J.; Ipser, J. Pharmacological treatments for social anxiety disorder in adults: A systematic review and network meta-analysis. Acta Neuropsychiatr. 2020, 32, 169–176. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, G.; Pan, Y.; Zhang, Y.; Ni, Y. The efficacy and safety of fluvoxamine in patients with COVID-19: A systematic review and meta-analysis from randomized controlled trials. PLoS ONE 2024, 19, e0300512. [Google Scholar] [CrossRef] [PubMed]
- van Harten, J. Overview of the pharmacokinetics of fluvoxamine. Clin. Pharmacokinet. 1995, 29 (Suppl. 1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Gatti, G.; Spina, E. Clinical pharmacokinetics of fluvoxamine. Clin. Pharmacokinet. 1994, 27, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Burhanuddin, K.; Badhan, R. Optimising Fluvoxamine Maternal/Fetal Exposure during Gestation: A Pharmacokinetic Virtual Clinical Trials Study. Metabolites 2022, 12, 1281. [Google Scholar] [CrossRef]
- Omori, I.M.; Watanabe, N.; Nakagawa, A.; Akechi, T.; Cipriani, A.; Barbui, C.; McGuire, H.; Churchill, R.; Furukawa, T.A.; Meta-Analysis of New Generation Antidepressants (MANGA) Study Group. Efficacy, tolerability and side-effect profile of fluvoxamine for major depression: Meta-analysis. J. Psychopharmacol. 2009, 23, 539–550. [Google Scholar] [CrossRef]
- Omori, I.M.; Watanabe, N.; Nakagawa, A.; Cipriani, A.; Barbui, C.; McGuire, H.; Churchill, R.; Furukawa, T.A. Fluvoxamine versus other anti-depressive agents for depression. Cochrane Database Syst. Rev. 2010, 2010, CD006114. [Google Scholar] [CrossRef]
- Anderson, I.M.; Tomenson, B.M. The efficacy of selective serotonin re-uptake inhibitors in depression: A meta-analysis of studies against tricyclic antidepressants. J. Psychopharmacol. 1994, 8, 238–249. [Google Scholar] [CrossRef]
- Möller, H.J.; Fuger, J.; Kasper, S. Efficacy of new generation antidepressants: Meta-analysis of imipramine-controlled studies. Pharmacopsychiatry 1994, 27, 215–223. [Google Scholar] [CrossRef]
- Lopez-Ibor, J.; Guelfi, J.D.; Pletan, Y.; Tournoux, A.; Prost, J.F. Milnacipran and selective serotonin reuptake inhibitors in major depression. Int. Clin. Psychopharmacol. 1996, 11 (Suppl. S4), 41–46. [Google Scholar] [CrossRef]
- Anderson, I.M. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. J. Affect. Disord. 2000, 58, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Entsuah, R.; Benattia, I.; Demitrack, M.; Sloan, D.M.; Thase, M.E. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol. Psychiatry 2008, 63, 424–434. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Geddes, J.R.; Higgins, J.P.; Churchill, R.; Watanabe, N.; Nakagawa, A.; Omori, I.M.; McGuire, H.; et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet 2009, 373, 746–758. [Google Scholar] [CrossRef]
- Nakagawa, A.; Watanabe, N.; Omori, I.M.; Barbui, C.; Cipriani, A.; McGuire, H.; Churchill, R.; Furukawa, T.A. Milnacipran versus other antidepressive agents for depression. Cochrane Database Syst. Rev. 2009, 2009, CD006529. [Google Scholar] [CrossRef] [PubMed]
- Ramsberg, J.; Asseburg, C.; Henriksson, M. Effectiveness and cost-effectiveness of antidepressants in primary care: A multiple treatment comparison meta-analysis and cost-effectiveness model. PLoS ONE 2012, 7, e42003. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, Z.; Xue, M.; Zhang, J.; Leng, L. Application of antidepressants in depression: A systematic review and meta-analysis. J. Clin. Neurosci. 2020, 80, 169–181. [Google Scholar] [CrossRef]
- Suchting, R.; Tirumalajaru, V.; Gareeb, R.; Bockmann, T.; de Dios, C.; Aickareth, J.; Pinjari, O.; Soares, J.C.; Cowen, P.J.; Selvaraj, S. Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: A systematic review and network meta-analysis. J. Affect. Disord. 2021, 282, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: A systematic review and network meta-analysis. Mol. Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Nematizadeh, M.; Ghorbanzadeh, H.; Moghaddam, H.S.; Shalbafan, M.; Boroon, M.; Keshavarz-Akhlaghi, A.A.; Akhondzadeh, S. L-theanine combination therapy with fluvoxamine in moderate-to-severe obsessive-compulsive disorder: A placebo-controlled, double-blind, randomized trial. Psychiatry Clin. Neurosci. 2023, 77, 478–485. [Google Scholar] [CrossRef]
- Farahani, R.H.; Ajam, A.; Naeini, A.R. Effect of fluvoxamine on preventing neuropsychiatric symptoms of post COVID syndrome in mild to moderate patients, a randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2023, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- She, D.P.; He, Y.; Li, M.Q.; Su, L.; Ren, D.; Huang, X.H.; Zhang, Y.H.; Hu, H.T.; Deng, D.C.; Wu, J.L. Pharmacokinetics and bioequivalence studies of fluvoxamine maleate tablets in healthy Chinese subjects. Biomed. Chromatogr. 2023, 37, e5613. [Google Scholar] [CrossRef]
- Tsujii, T.; Sakurai, H.; Takeuchi, H.; Suzuki, T.; Mimura, M.; Uchida, H. Predictors of response to pharmacotherapy in children and adolescents with psychiatric disorders: A combined post hoc analysis of four clinical trial data. Neuropsychopharmacol. Rep. 2022, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Brar, J.; Sidana, A.; Chauhan, N.; Bajaj, M.K. A randomized, open-label pilot trial of selective serotonin reuptake inhibitors on neuropsychological functions in patients with obsessive compulsive disorder. J. Psychiatr. Res. 2022, 151, 439–444. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Amin, M.M.; Ananth, J.V.; Coleman, B.S.; Darcourt, G.; Farkas, T.; Goldstein, B.; Lapierre, Y.D.; Paykel, E.; Wakelin, J.S. Fluvoxamine: Antidepressant effects confirmed in a placebo-controlled international study. Clin. Neuropharmacol. 1984, 7 (Suppl. S1), S316–S317. [Google Scholar] [CrossRef]
- Brown, W.A.; Arato, M.; Shrivastava, R. Pituitary-adrenocortical hyperfunction and intolerance to fluvoxamine, a selective serotonin uptake inhibitor. Am. J. Psychiatry 1986, 143, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Cassano, G.B.; Conti, L.; Massimetti, G.; Mengali, F.; Waekelin, J.S.; Levine, J. Use of a standardized documentation system (BLIPS/BDP) in the conduct of a multicenter international trial comparing fluvoxamine, imipramine, and placebo. Psychopharmacol. Bull. 1986, 22, 52–58. [Google Scholar] [PubMed]
- Claghorn, J.L.; Earl, C.Q.; Walczak, D.D.; Stoner, K.A.; Wong, L.F.; Kanter, D.; Houser, V.P. Fluvoxamine maleate in the treatment of depression: A single-center, double-blind, placebo-controlled comparison with imipramine in outpatients. J. Clin. Psychopharmacol. 1996, 16, 113–120. [Google Scholar] [CrossRef]
- Dominguez, R.A.; Goldstein, B.J.; Jacobson, A.F.; Steinbook, R.M. A double-blind placebo-controlled study of fluvoxamine and imipramine in depression. J. Clin. Psychiatry 1985, 46, 84–87. [Google Scholar]
- Fabre, L.; Birkhimer, L.J.; Zaborny, B.A.; Wong, L.F.; Kapik, B.M. Fluvoxamine versus imipramine and placebo: A double-blind comparison in depressed patients. Int. Clin. Psychopharmacol. 1996, 11, 119–127. [Google Scholar]
- Feighner, J.P.; Boyer, W.F.; Meredith, C.H.; Hendrickson, G.G. A placebo-controlled inpatient comparison of fluvoxamine maleate and imipramine in major depression. Int. Clin. Psychopharmacol. 1989, 4, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Itil, T.M.; Shrivastava, R.K.; Mukherjee, S.; Coleman, B.S.; Michael, S.T. A double-blind placebo-controlled study of fluvoxamine and imipramine in out-patients with primary depression. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S3), 433S–438S. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, Y.D.; Browne, M.; Horn, E.; Oyewumi, L.K.; Sarantidis, D.; Roberts, N.; Badoe, K.; Tessier, P. Treatment of major affective disorder with fluvoxamine. J. Clin. Psychiatry 1987, 48, 65–68. [Google Scholar] [PubMed]
- Lydiard, R.B.; Laird, L.K.; Morton, W.A.; Steele, T.E.; Kellner, C.; Laraia, M.T.; Ballenger, J.C. Fluvoxamine, imipramine, and placebo in the treatment of depressed outpatients: Effects on depression. Psychopharmacol. Bull. 1989, 25, 68–70. [Google Scholar]
- March, J.S.; Kobak, K.A.; Jefferson, J.W.; Mazza, J.; Greist, J.H. A double-blind, placebo-controlled trial of fluvoxamine versus imipramine in outpatients with major depression. J. Clin. Psychiatry 1990, 51, 200–202. [Google Scholar]
- Norton, K.R.; Sireling, L.I.; Bhat, A.V.; Rao, B.; Paykel, E.S. A double-blind comparison of fluvoxamine, imipramine and placebo in depressed patients. J. Affect. Disord. 1984, 7, 297–308. [Google Scholar] [CrossRef]
- Roth, D.; Mattes, J.; Sheehan, K.H.; Sheehan, D.V. A double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 1990, 14, 929–939. [Google Scholar] [CrossRef]
- Walczak, D.D.; Apter, J.T.; Halikas, J.A.; Borison, R.L.; Carman, J.S.; Post, G.L.; Patrick, R.; Cohn, J.B.; Cunningham, L.A.; Rittberg, B.; et al. The oral dose-effect relationship for fluvoxamine: A fixed-dose comparison against placebo in depressed outpatients. Ann. Clin. Psychiatry 1996, 8, 139–151. [Google Scholar] [CrossRef]
- Terra, J.L.; Montgomery, S.A. Fluvoxamine prevents recurrence of depression: Results of a long-term, double-blind, placebo-controlled study. Int. Clin. Psychopharmacol. 1998, 13, 55–62. [Google Scholar] [CrossRef]
- Guy, W.; Wilson, W.H.; Ban, T.A.; King, D.L.; Manov, G.; Fjetland, O.K. A double-blind clinical trial of fluvoxamine and imipramine in patients with primary depression. Psychopharmacol. Bull. 1984, 20, 73–78. [Google Scholar] [CrossRef]
- Miller, H.L.; Ekstrom, R.D.; Mason, G.A.; Lydiard, R.B.; Golden, R.N. Noradrenergic function and clinical outcome in antidepressant pharmacotherapy. Neuropsychopharmacology 2001, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zohar, J.; Keegstra, H.; Barrelet, L. Fluvoxamine as effective as clomipramine against symptoms of severe depression: Results from a multicentre, double-blind study. Hum. Psychopharmacol. 2003, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, J.E.; Mertens, C.; Wakelin, J.S. Clinical trials of fluvoxamine vs chlorimipramine with single and three times daily dosing. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S3), 427S–431S. [Google Scholar] [CrossRef]
- Ottevanger, E.A. Fluvoxamine and clomipramine in depressed hospitalised patients: Results from a randomised, double-blind study. Encephale 1995, 21, 317–321. [Google Scholar] [PubMed]
- Barge-Schaapveld, D.Q.; Nicolson, N.A.; van der Hoop, R.G.; De Vries, M.W. Changes in daily life experience associated with clinical improvement in depression. J. Affect. Disord. 1995, 34, 139–154. [Google Scholar] [CrossRef]
- Harris, B.; Szulecka, T.K.; Anstee, J.A. Fluvoxamine versus amitriptyline in depressed hospital out-patients: A multicentre double-blind comparative trial. Br. J. Clin. Res. 1991, 2, 89–99. [Google Scholar]
- Kostiukova, E.G.; Granenov, G.M.; Andreĭchik, L.A.; Serditov, O.V.; Mosolov, S.N. Comparative efficacy and tolerance of fluvoxamine and amitriptyline in the treatment of moderate and severe depression in mental hospital. Zh Nevrol Psikhiatr Im S S Korsakova 2003, 103, 24–29. [Google Scholar]
- Remick, R.A.; Reesal, R.; Oakander, M.; Allen, J.; Claman, J.; Ramirez, C.E.; Perry, K.; Keller, F.D. Comparison of fluvoxamine and amitriptyline in depressed outpatients. Curr. Ther. Res. 1994, 55, 243–250. [Google Scholar] [CrossRef]
- Murasaki, M.; Mori, A.; Miura, S. Clinical evaluation of SME3110 (fluvoxamine maleate) in the treatment of depression and depressive state: A double-blind, comparative study with amitriptyline. Rinsho-Iyaku (J. Clin. Ther. Med.) 1998, 14, 951–980. [Google Scholar]
- Mullin, J.M.; Pandita-Gunawardena, V.R.; Whitehead, A.M. A double-blind comparison of fluvoxamine and dothiepin in the treatment of major affective disorder. Br. J. Clin. Pract. 1988, 42, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.K.; Akhtar, M.J.; Savla, N.C.; Sharma, R.R.; Kellett, J.M.; Ashford, J.J. A double-blind, randomised comparison of fluvoxamine with dothiepin in the treatment of depression in elderly patients. Br. J. Clin. Pract. 1991, 45, 255–258. [Google Scholar] [CrossRef]
- Tourigny-Rivard, M.; Nair, N.; Vincent, P. Fluvoxamine versus desipramine in elderly patients with major depression: A double-blind comparison. In Proceedings of the 9th ECNP (European College of Neuropsychopharmacology) Congress, Amsterdam, The Netherlands, 21–25 September 1996. [Google Scholar]
- Otsubo, T.; Akimoto, Y.; Yamada, H.; Koda, R.; Aoyama, H.; Tanaka, K.; Mimura, M.; Nakagome, K.; Kamijima, K. A comparative study of the efficacy and safety profiles between fluvoxamine and nortriptyline in Japanese patients with major depression. Pharmacopsychiatry 2005, 38, 30–35. [Google Scholar] [CrossRef]
- Ansseau, M.; Gabriëls, A.; Loyens, J.; Bartholomé, F.; Evrard, J.L.; De Nayer, A.; Linhart, R.; Wirtz, J.; Bruynooghe, F.; Surinx, K.; et al. Controlled comparison of paroxetine and fluvoxamine in major depression. Hum. Psychopharmacol. Clin. Exp. 1994, 9, 329–336. [Google Scholar] [CrossRef]
- Kato, M.; Fukuda, T.; Wakeno, M.; Fukuda, K.; Okugawa, G.; Ikenaga, Y.; Yamashita, M.; Takekita, Y.; Nobuhara, K.; Azuma, J.; et al. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology 2006, 53, 186–195. [Google Scholar] [CrossRef]
- Kiev, A.; Feiger, A. A double-blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients. J. Clin. Psychiatry 1997, 58, 146–152. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Ninan, P.T.; Ballenger, J.; Lydiard, R.B.; Feighner, J.; Patterson, W.M.; Greist, J.H. Double-blind multicenter comparison of fluvoxamine versus sertraline in the treatment of depressed outpatients. Depression 1995, 3, 163–169. [Google Scholar] [CrossRef]
- Rossini, D.; Serretti, A.; Franchini, L.; Mandelli, L.; Smeraldi, E.; De Ronchi, D.; Zanardi, R. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: A double-blind, randomized trial. J. Clin. Psychopharmacol. 2005, 25, 471–475. [Google Scholar] [CrossRef]
- Haffmans, P.M.; Timmerman, L.; Hoogduin, C.A. Efficacy and tolerability of citalopram in comparison with fluvoxamine in depressed outpatients: A double-blind, multicentre study. The LUCIFER Group. Int. Clin. Psychopharmacol. 1996, 11, 157–164. [Google Scholar] [CrossRef]
- Dalery, J.; Honig, A. Fluvoxamine versus fluoxetine in major depressive episode: A double-blind randomised comparison. Hum. Psychopharmacol. 2003, 18, 379–384. [Google Scholar] [CrossRef]
- Rapaport, M.; Coccaro, E.; Sheline, Y.; Perse, T.; Holland, P.; Fabre, L.; Bradford, D. A comparison of fluvoxamine and fluoxetine in the treatment of major depression. J. Clin. Psychopharmacol. 1996, 16, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Clerc, G.; Group, M.F.S. Antidepressant efficacy and tolerability of milnacipran, a dual serotonin and noradrenaline reuptake inhibitor: A comparison with fluvoxamine. Int. Clin. Psychopharmacol. 2001, 16, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ansseau, M.; von Frenckell, R.; Gérard, M.-A.; Mertens, C.; De Wilde, J.; Botte, L.; Devoitille, J.-M.; Evrard, J.-L.; De Nayer, A.; Darimont, P.; et al. Interest of a loading dose of milnacipran in endogenous depressive inpatients: Comparison with the standard regimen and with fluvoxamine. Eur. Neuropsychopharmacol. 1991, 1, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Hackett, D.; Salinas, E.; Desmet, A. Efficacy and safety of venlafaxine vs. fluvoxamine in outpatients with major depression. Eur. Neuropsychopharmacol. 1998, 8 (Suppl. 2), S209. [Google Scholar] [CrossRef]
- Schoemaker, J.; Gailledreau, J.; Hoyberg, O.J. First, randomized, double-blind comparison of mirtazapine (15–45 mg) and fl uvoxamine (50–150 mg) in the treatment of depression. Int. J. Neuropsychopharmacol. 2002, 5 (Suppl. S1), 140. [Google Scholar]
- Murasaki, M.; Schoemaker, J.H.; Miyake, K.; Gailledreau, J.; Heukels, A.J.; Fennema, H.P.; Sitsen, J.M.A. Comparison of efficacy and safety of mirtazapine versus fluvoxamine in Japanese and Caucasian patients with major depressive disorder. Rinsho-Seishin-Yakuri (Jpn. J. Clin. Psychopharmacol.) 2010, 13, 339–355. (In Japanese) [Google Scholar]
- Moon, C.A.; Jesinger, D.K. The effects of psychomotor performance of fluvoxamine versus mianserin in depressed patients in general practice. Br. J. Clin. Pract. 1991, 45, 259–262. [Google Scholar] [CrossRef]
- Perez, A.; Ashford, J.J. A double-blind, randomized comparison of fluvoxamine with mianserin in depressive illness. Curr. Med. Res. Opin. 1990, 12, 234–241. [Google Scholar] [CrossRef]
- Hieronymus, F.; Emilsson, J.F.; Nilsson, S.; Eriksson, E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol. Psychiatry 2016, 21, 523–530. [Google Scholar] [CrossRef]
- Boschloo, L.; Hieronymus, F.; Lisinski, A.; Cuijpers, P.; Eriksson, E. The complex clinical response to selective serotonin reuptake inhibitors in depression: A network perspective. Transl. Psychiatry 2023, 13, 19. [Google Scholar] [CrossRef]
- Westenberg, H.G.; Sandner, C. Tolerability and safety of fluvoxamine and other antidepressants. Int. J. Clin. Pract. 2006, 60, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Entsuah, A.R.; Rudolph, R.L. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br. J. Psychiatry 2001, 178, 234–241. [Google Scholar] [CrossRef]
- Smith, D.; Dempster, C.; Glanville, J.; Freemantle, N.; Anderson, I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: A meta-analysis. Br. J. Psychiatry 2002, 180, 396–404. [Google Scholar] [CrossRef] [PubMed]
- de Silva, V.A.; Hanwella, R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: A meta-analysis of published studies. Int. Clin. Psychopharmacol. 2012, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, C.; Flores-Ramos, M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J. Affect. Disord. 2006, 95, 119–123. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [CrossRef]
- Naito, S.; Sato, K.; Yoshida, K.; Higuchi, H.; Takahashi, H.; Kamata, M.; Ito, K.; Ohkubo, T.; Shimizu, T. Gender differences in the clinical effects of fluvoxamine and milnacipran in Japanese major depressive patients. Psychiatry Clin. Neurosci. 2007, 61, 421–427. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Althagafy, H.S.; Baraka, M.A.; Abd-alhameed, E.K.; Ibrahim, I.M. Pharmacological update of mirtazapine: A narrative literature review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2603–2619. [Google Scholar] [CrossRef]
- Anttila, S.A.; Leinonen, E.V. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef]
- Van den Eynde, V.; Abdelmoemin, W.R.; Abraham, M.M.; Amsterdam, J.D.; Anderson, I.M.; Andrade, C.; Baker, G.B.; Beekman, A.T.F.; Berk, M.; Birkenhäger, T.K.; et al. The prescriber’s guide to classic MAO inhibitors (phenelzine, tranylcypromine, isocarboxazid) for treatment-resistant depression. CNS Spectr. 2023, 28, 427–440. [Google Scholar] [CrossRef]
- Van den Eynde, V.; Parker, G.; Ruhé, H.G.; Birkenhäger, T.K.; Godet, L.; Shorter, E.; Gillman, P.K. On the Origins of MAOI Misconceptions: Reaffirming their Role in Melancholic Depression. Psychopharmacol. Bull. 2023, 53, 35–54. [Google Scholar] [PubMed]
- Athira, K.V.; Bandopadhyay, S.; Samudrala, P.K.; Naidu, V.G.M.; Lahkar, M.; Chakravarty, S. An Overview of the Heterogeneity of Major Depressive Disorder: Current Knowledge and Future Prospective. Curr. Neuropharmacol. 2020, 18, 168–187. [Google Scholar] [CrossRef]
- Buch, A.M.; Liston, C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology 2021, 46, 156–175. [Google Scholar] [CrossRef]
- Li, X.; Yan, D.; Liao, M.; Zhang, L.; Li, Z.; Liu, B.; Chen, Y.; Zhang, Y.; Liu, J.; Li, L. Effect of fluvoxamine on plasma interleukin-6 in patients with major depressive disorder: A prospective follow-up study. Front. Psychiatry 2023, 14, 1163754. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Z.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; Liu, S.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Zanardi, R.; Palladini, M.; Rovere-Querini, P.; Benedetti, F. Rapid response to selective serotonin reuptake inhibitors in post-COVID depression. Eur. Neuropsychopharmacol. 2022, 54, 1–6. [Google Scholar] [CrossRef]
- Smeraldi, E.; Zanardi, R.; Benedetti, F.; Di Bella, D.; Perez, J.; Catalano, M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol. Psychiatry 1998, 3, 508–511. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, K.; Sato, K.; Takahashi, H.; Kamata, M.; Higuchi, H.; Shimizu, T.; Itoh, K.; Inoue, K.; Tezuka, T.; et al. Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluvoxamine in Japanese depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 383–386. [Google Scholar] [CrossRef]
- Yoshida, K.; Higuchi, H.; Kamata, M.; Takahashi, H.; Inoue, K.; Suzuki, T.; Itoh, K.; Ozaki, N. The G196A polymorphism of the brain-derived neurotrophic factor gene and the antidepressant effect of milnacipran and fluvoxamine. J. Psychopharmacol. 2007, 21, 650–656. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Nickchen, K.; Bauer, M.; Wong, M.L.; Licinio, J.; Roots, I.; Brockmöller, J. Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol. Psychiatry 2004, 9, 442–473. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zastrozhin, M.S.; Grishina, E.A.; Denisenko, N.P.; Skryabin, V.Y.; Markov, D.D.; Savchenko, L.M.; Bryun, E.A.; Sychev, D.A. Effects of CYP2D6 genetic polymorphisms on the efficacy and safety of fluvoxamine in patients with depressive disorder and comorbid alcohol use disorder. Pharmacogenomics Pers. Med. 2018, 11, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Dahl, M.L.; Svensson, J.O.; Alm, C.; Rodríguez, I.; Bertilsson, L. Disposition of fluvoxamine in humans is determined by the polymorphic CYP2D6 and also by the CYP1A2 activity. Clin. Pharmacol. Ther. 1996, 60, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Peng, J.B.; Fu, C.H.; Cao, D.; Li, D.; Tong, L.; Wang, Z.Y. Activation of Sigma-1 receptor ameliorates anxiety-like behavior and cognitive impairments in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2016, 311, 408–415. [Google Scholar] [CrossRef]
- Toyohara, J.; Sakata, M.; Ishiwata, K. Roles of σ1 receptors in the mechanisms of action of CNS drugs. Transl. Neurosci. 2012, 3, 294–299. [Google Scholar] [CrossRef]
- Sałaciak, K.; Pytka, K. Revisiting the sigma-1 receptor as a biological target to treat affective and cognitive disorders. Neurosci. Biobehav. Rev. 2022, 132, 1114–1136. [Google Scholar] [CrossRef]
- Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 25 April 2025).
- Pollock, M.F.R.; Becker, L.A.; Pieper, D.; Hartling, L. Chapter V: Overviews of Reviews. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. [Google Scholar]
- Lin, L.; Aloe, A.M. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 2021, 40, 403–426. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Stein, D.J.; Westenberg, H.G.; Yang, H.; Li, D.; Barbato, L.M. Fluvoxamine CR in the long-term treatment of social anxiety disorder: The 12- to 24-week extension phase of a multicentre, randomized, placebo-controlled trial. Int. J. Neuropsychopharmacol. 2003, 6, 317–323. [Google Scholar] [CrossRef]
- Hudson, J.I.; McElroy, S.L.; Raymond, N.C.; Crow, S.; Keck, P.E.; Carter, W.P.; Mitchell, J.E.; Strakowski, S.M.; Pope, H.G.; Coleman, B.S.; et al. Fluvoxamine in the treatment of binge-eating disorder: A multicenter placebo-controlled, double-blind trial. Am. J. Psychiatry 1998, 155, 1756–1762. [Google Scholar] [CrossRef]
- Amore, M.; Bellini, M.; Berardi, D.; Berlinzani, L.; Cervino, G.; Cremonini, A.; Ferrari, G.; Innamorati, A. Double-blind comparison of fluvoxamine and imipramine in depressed patients. Curr. Ther. Res. 1989, 4, 815–820. [Google Scholar]
- De Wilde, J.E.; Doogan, D.P. Fluvoxamine and chlorimipramine in endogenous depression. J. Affect. Disord. 1982, 4, 249–259. [Google Scholar] [CrossRef]
- Dick, P.; Ferrero, E. A double-blind comparative study of the clinical efficacy of fluvoxamine and chlorimipramine. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S3), 419S–425S. [Google Scholar] [CrossRef] [PubMed]
- Gonella, G.; Baignoli, G.; Ecari, U. Fluvoxamine and imipramine in the treatment of depressive patients: A double-blind controlled study. Curr. Med. Res. Opin. 1990, 12, 177–184. [Google Scholar] [CrossRef]
- Guelfi, J.D.; Dreyfus, J.F.; Pichot, P. A double-blind controlled clinical trial comparing fluvoxamine with imipramine. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S3), 411S–417S. [Google Scholar] [CrossRef] [PubMed]
- Klok, C.J.; Brouwer, G.J.; van Praag, H.M.; Doogan, D. Fluvoxamine and clomipramine in depressed patients. A double-blind clinical study. Acta Psychiatr. Scand. 1981, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.S.; Perel, J.M.; Pollock, B.G.; Kupfer, D.J. The role of neuropharmacologic selectivity in antidepressant action: Fluvoxamine versus desipramine. J. Clin. Psychiatry 1990, 51, 367–372. [Google Scholar]
- Gasperini, M.; Gatti, F.; Bellini, L.; Anniverno, R.; Smeraldi, E. Perspectives in clinical psychopharmacology of amitriptyline and fluvoxamine. A double-blind study in depressed inpatients. Neuropsychobiology 1992, 26, 186–192. [Google Scholar] [CrossRef]
- Kasper, S.; Voll, G.; Vieira, A.; Kick, H. Response to total sleep deprivation before and during treatment with fluvoxamine or maprotiline in patients with major depression-results of a double-blind study. Pharmacopsychiatry 1990, 23, 135–142. [Google Scholar] [CrossRef]
- Conti; Placidi, G.F.; Dell, L.; Lenzi, A.; Cassano, G.B. Therapeutic response in subtypes of major depression. New Trends Exp. Clin. Psychiatry 1987, 101–107. [Google Scholar]
- Pöldinger, W.; Bures, E. Fluvoxamine in patients with depressive disorder. In Proceedings of the International Symposium on Fluvoxamine; Duphar Medical Publications: Bern, Switzerland, 1984; pp. 41–44. [Google Scholar]
- Wagner; Wakelin, J.; Coleman, B.S.; Cimander, K. Therapeutische Ergebnisse mit Fluvoxamin und der Einfluß psychotroper Begleitmedikation auf Wirksamkeit und Verträglichkeit. Adv. Pharrnacother. 1985, 2, 33–65. [Google Scholar]
- Wakelin, J.S. Fluvoxamine in the treatment of the older depressed patient; double-blind, placebo-controlled data. Int. Clin. Psychopharmacol. 1986, 1, 221–230. [Google Scholar] [CrossRef] [PubMed]
- de Jonghe, F.; Swinkels, J.; Tuynman-Qua, H. Randomized double-blind study of fluvoxamine and maprotiline in treatment of depression. Pharmacopsychiatry 1991, 24, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, P.; Ricci, R.M.; Roncari, R.; Bilone, F.; Inga, F.; Teti, V.; DeCristofaro, A.M.; Ceccarelli, G.; DiPerri, R.; Candela, L. An Italian multicentre experience with fluvoxamine, a new antidepressant drug, versus imipramine. Curr. Ther. Res. 1988, 43, 718–724. [Google Scholar]
| Review | Type | Number of Included RCTs (Number of Patients) | Time of Follow-Up (Weeks) | Blinding RCTs | Summary Estimates (ES, 95% CI) | Main Findings |
|---|---|---|---|---|---|---|
| Fluvoxamine vs. Placebo | ||||||
| Cipriani et al., 2018 [49] | NMA | 14 (1799) | 4–6 | db | OR = 1.69 (1.41, 2.02) | Fluvoxamine significantly more effective than placebo |
| Fluvoxamine vs. TCA Unspecified TCA | ||||||
| Omori et al., 2010 [40] | MA | 16 (935) | 6–10 | db | OR = 0.97 (0.73, 1.29) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 16 (872) | 6–10 | db | RR = 0.99 (0.86, 1.14) | Non-significant difference |
| Imipramine | ||||||
| Omori et al., 2010 [40] | MA | 7 (422) | 6 | db | OR = 0.97 (0.59, 1,58) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 6 (282) | 6 | db | RR = 0.95 (0.67, 1.36) | Non-significant difference |
| Clomipramine | ||||||
| Omori et al., 2010 [40] | MA | 2 (159) | 6–8 | db | OR = 0.84 (0.38, 1.85) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (86) | 8 | db | RR = 0.99 (0.68, 1.44) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (83) | 4–6 | db | OR = 1.01 (0.76, 1.32) | Non-significant difference |
| Amitriptyline | ||||||
| Omori et al., 2010 [40] | MA | 4 (185) | 6–7 | ol and db | OR = 0.79 (0.35, 1.75) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 4 (185) | 6–7 | ol and db | RR = 0.91 (0.61, 1.38) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 3 (337) | 4–7 | db | OR = 1.25 (0.99, 1.59) | Non-significant difference |
| Dothiepin | ||||||
| Omori et al., 2009 [39] | MA | 2 (125) | 6 | db | RR = 1.05 (0.65, 1.69) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 2 (125) | 6 | db | OR = 1.11 (0.55, 2.24) | Non-significant difference |
| Desipramine | ||||||
| Omori et al., 2010 [40] | MA | 1 (47) | 10 | db | OR = 4.22 (0.98, 18.13) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (47) | 10 | db | RR = 1.44 (0.90, 2.31) | Non-significant difference |
| Nortriptyline | ||||||
| Omori et al., 2010 [40] | MA | 1 (74) | 8 | ol | OR = 0.91 (0.36, 2.28) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (74) | 8 | ol | OR = 0.96 (0.57, 1.62) | Non-significant difference |
| Fluvoxamine vs. SSRIs Unspecified SSRIs | ||||||
| Omori et al., 2010 [40] | MA | 8 (967) | 6–7 | db | OR = 0.96 (0.74, 1.25) | Non-significant difference |
| Paroxetine | ||||||
| Omori et al., 2010 [40] | MA | 3 (281) | 6–7 | ol and db | OR = 0.83 (0.51, 1.34) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 3 (281) | 6–7 | ol and db | RR = 0.92 (0.70, 1.21) | Non-significant difference |
| Cipriani et al., 2009 [46] | MA | 3 (260) | 6–7 | db | OR = 0.83 (0.51, 1.34) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (180) | 6–7 | db | OR= 0.84 (0.67, 1.04) | Non-significant difference |
| Sertraline | ||||||
| Omori et al., 2010 [40] | MA | 2 (185) | 7 | db | OR = 1.21 (0.53, 2.75) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 2 (185) | 7 | db | RR = 1.10 (0.71, 1.70) | Non-significant difference |
| Cipriani et al., 2009 [46] | NMA | 2 (185) | 7 | db | OR = 1.21 (0.53, 2.75) | Sertraline significantly more effective than fluvoxamine |
| Cipriani et al., 2018 [49] | NMA | 2 (185) | 7 | db | OR = 0.89 (0.70, 1.13) | Non-significant difference |
| Fluoxetine | ||||||
| Omori et al., 2009 [39] | MA | 2 (284) | 6–7 | db | RR = 1.00 (0.78, 1.28) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 2 (284) | 6–7 | db | OR = 1.00 (0.62, 1.61) | Non-significant difference |
| Cipriani et al., 2009 [46] | MA | 2 (284) | 6–7 | db | OR = 1.03 (0.64, 1.66) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (284) | 6–7 | db | OR= 1.00 (0.80, 1.25) | Non-significant difference |
| Citalopram | ||||||
| Omori et al., 2010 [40] | MA | 1 (217) | 6 | db | OR = 0.90 (0.50, 1.62) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (217) | 6 | db | RR = 0.93 (0.54, 1.60) | Non-significant difference |
| Cipriani et al., 2009 [46] | MA | 1 (217) | 6 | db | OR = 0.90 (0.50, 1.62) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 1 (217) | 6 | db | OR = 1.06 (0.82, 1.39) | Non-significant difference |
| Fluvoxamine vs. SNRIs Unspecified SNRIs | ||||||
| Omori et al., 2010 [40] | MA | 3 (258) | 6 | db | OR = 0.48 (0.27, 0.85) | SNRIs significantly more effective than fluvoxamine |
| Omori et al., 2009 [39] | MA | 2 (224) | 6 | db | RR = 0.76 (0.56, 1.04) | Non-significant difference |
| Milnacipran | ||||||
| Omori et al., 2010 [40] | MA | 1 (113) | 6 | db | OR = 0.57 (0.26, 1.23) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (113) | 6 | db | RR = 0.81 (0.56, 1.18) | Non-significant difference |
| Nakagawa et al., 2009 [47] | MA | 1 (113) | 6 | db | OR = 1.76 (0.81, 3.83) | Non-significant difference |
| Cipriani et al., 2009 [46] | MA | 1 (113) | 6 | db | OR = 0.57 (0.26, 1.23) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (239) | 4–6 | db | OR = 0.89 (0.67, 1.17) | Non-significant difference |
| Venlafaxine | ||||||
| Cipriani et al., 2009 [46] | MA | 1 (71) | 6 | db | OR = 0.42 (0.19, 0.96) | Venlafaxine significantly more effective than fluvoxamine |
| Omori et al., 2010 [40] | MA | 2 (145) | 6 | db | OR = 0.40 (0.18, 0.92) | Fluvoxamine less effective than venlafaxine |
| Omori et al., 2009 [39] | MA | 1 (111) | 6 | db | RR = 0.65 (0.37, 1.15) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (111) | 6 | db | OR = 0.84 (0.66, 1.07) | Non-significant difference |
| Fluvoxamine vs. Other Antidepressants Mirtazapine | ||||||
| Omori et al., 2009 [39] | MA | 1 (412) | 6 | db | RR = 0.95 (0.78, 1.16) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 1 (412) | 6 | db | OR = 0.72 (0.47, 1.11) | Non-significant difference |
| Cipriani et al., 2009 [46] | MA | 1 (412) | 6 | db | OR = 0.88 (0.59, 1.31) | Mirtazapine significantly more effective than fluvoxamine |
| Cipriani et al., 2018 [49] | NMA | 2 (412) | 6 | db | OR= 0.78 (0.60, 0.99) | Mirtazapine superior to fluvoxamine |
| Mianserin | ||||||
| Omori et al., 2009 [39] | MA | 2 (125) | 6 | db | RR = 1.09 (0.86, 1.40) | Non-significant difference |
| Omori et al. 2010 [40] | MA | 2 (125) | 6 | db | OR = 1.25 (0.55, 2.87) | Non-significant difference |
| Review | Type | Number of Included RCTs (Number of Patients) | Time of Follow-Up (Weeks) | Blinding RCTs | Summary Estimates (ES, 95% CI) | Main Findings |
|---|---|---|---|---|---|---|
| Fluvoxamine vs. Placebo | ||||||
| Kishi et al., 2023 [52] | NMA | 1 (204) | 52 | db | RR = 0.298 (0.114, 0.686) * | Fluvoxamine significantly more effective than placebo |
| Cipriani et al., 2018 [49] | NMA | 14 (1799) ** | 4–6 | db | OR = 0.58 (0.39, 0.86) | Fluvoxamine significantly more effective than placebo |
| Fluvoxamine vs. TCA Unspecified TCA | ||||||
| Omori et al., 2010 [40] | MA | 16 (965) | 6–10 | db | OR = 1.00 (0.69, 1.45) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 16 (872) | 6–10 | db | RR = 0.98 (0.71, 1.35) | Non-significant difference |
| Imipramine | ||||||
| Omori et al., 2010 [40] | MA | 6 (375) | 6 | db | OR = 1.07 (0.59, 1.94) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 6 (282) | 6 | db | RR = 1.03 (0.53, 2.00) | Non-significant difference |
| Clomipramine | ||||||
| Omori et al., 2010 [40] | MA | 2 (159) | 6–8 | db | OR = 0.64 (0.28, 1.49) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (86) | 8 | db | RR = 0.72 (0.20, 2.56) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (83) ** | 4–6 | db | OR = 1.57 (0.56, 4.57) | Non-significant difference |
| Amitriptyline | ||||||
| Omori et al., 2010 [40] | MA | 4 (185) | 6–7 | ol and db | OR = 0.61 (0.28, 1.31) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 4 (185) | 6–7 | ol and db | RR = 0.74 (0.42, 1.30) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 3 (337) ** | 4–7 | db | OR = 1.07 (0.61, 1.88) | Non-significant difference |
| Dothiepin | ||||||
| Omori et al., 2009 [39] | MA | 2 (125) | 6 | db | RR = 1.05 (0.48, 2.25) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 2 (125) | 6 | db | OR = 1.06 (0.48, 2.35) | Non-significant difference |
| Desipramine | ||||||
| Omori et al., 2010 [40] | MA | 1 (47) | 10 | db | OR = 4.5 (1.31, 15.42) | Fluvoxamine significantly more effective than desipramine |
| Omori et al., 2009 [39] | MA | 1 (47) | 10 | db | RR = 2.27 (0.90, 5.73) | Non-significant difference |
| Nortriptyline | ||||||
| Omori et al., 2010 [40] | MA | 1 (74) | 8 | ol | OR = 1.78 (0.67, 4.77) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (74) | 8 | ol | OR = 1.48 (0.61, 3.57) | Non-significant difference |
| Fluvoxamine vs. SSRIs Unspecified SSRIs | ||||||
| Omori et al., 2010 [40] | MA | 8 (967) | 6–7 | db | OR = 0.98 (0.71, 1.37) | Non-significant difference |
| Paroxetine | ||||||
| Omori et al., 2010 [40] | MA | 3 (281) | 6–7 | ol and db | OR = 0.77 (0.45, 1.33) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 3 (281) | 6–7 | ol and db | RR = 0.83 (0.52, 1.31) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (180) ** | 6–7 | db | OR= 1.13 (0.50, 2.46) | Non-significant difference |
| Sertraline | ||||||
| Omori et al., 2010 [40] | MA | 2 (185) | 7 | db | OR = 1.31 (0.48, 3.57) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 2 (185) | 7 | db | RR = 1.10 (0.63, 2.15) | Non-significant difference |
| Ramsberg et al., 2012 [48] | NMA | 1 (88) | 7 | db | OR = 1.41 (0.92, 2.10) *** | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (185) ** | 7 | db | OR = 0.68 (0.34, 1.36) | Non-significant difference |
| Fluoxetine | ||||||
| Omori et al., 2009 [39] | MA | 2 (284) | 6–7 | db | RR = 1.15 (0.72, 1.82) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 2 (284) | 6–7 | db | OR = 1.24 (0.74, 2.06) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (284) ** | 6–7 | db | OR = 0.85 (0.47, 1.51) | Non-significant difference |
| Citalopram | ||||||
| Omori et al., 2010 [40] | MA | 1 (217) | 6 | db | OR = 0.56 (0.23, 1.34) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (217) | 6 | db | RR = 0.59 (0.21, 1.66) | Non-significant difference |
| Ramsberg et al., 2012 [48] | NMA | 1 (217) | 6 | db | OR = 0.80 (0.51, 1.19) *** | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 1 (217) ** | 6 | db | OR = 1.84 (0.72, 5.00) | Non-significant difference |
| Fluvoxamine vs. SNRIs Unspecified SNRIs | ||||||
| Omori et al., 2010 [40] | MA | 3 (258) | 6 | db | OR = 0.61 (0.34, 1.08) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 2 (224) | 6 | db | RR = 0.73 (0.45, 1.20) | Non-significant difference |
| Milnacipram | ||||||
| Omori et al., 2010 [40] | MA | 1 (113) | 6 | db | OR = 0.68 (0.3, 1.51) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (113) | 6 | db | RR = 0.76 (0.37, 1.59) | Non-significant difference |
| Nakagawa et al., 2009 [47] | MA | 1 (113) | 6 | db | OR = 1.48 (0.66, 3.3) | Non-significant difference |
| Lopez-Ibor et al., 1996 [43] | MA | 1 (113) | 6 | db | Milnacipram = 47% Fluvoxamine = 36% p = 0.20 | Non-significant difference |
| Venlafaxine | ||||||
| Omori et al., 2010 [40] | MA | 2 (145) | 6 | db | OR = 0.54 (0.23, 1.24) | Non-significant difference |
| Omori et al., 2009 [39] | MA | 1 (111) | 6 | db | RR = 0.70 (0.36, 1.37) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (111) ** | 6. | db | OR = 1.93 (0.76, 5.01) | Non-significant difference |
| Fluvoxamine vs. Other Antidepressants Mirtazapine | ||||||
| Omori et al., 2009 [39] | MA | 1 (412) | 6 | db | RR = 1.10 (0.83, 1.45) | Non-significant difference |
| Omori et al., 2010 [40] | MA | 1 (412) | 6 | db | OR = 1.19 (0.81, 1.76) | Non-significant difference |
| Cipriani et al., 2018 [49] | NMA | 2 (412) ** | 6 | db | OR = 0.84 (0.52, 1.36) | Non-significant difference |
| Mianserin | ||||||
| Omori et al., 2009 [39] | MA | 2 (125) | 6 | db | RR = 1.16 (0.93, 1.44) | Non-significant difference |
| Omori et al. 2010 [40] | MA | 2 (125) | 6 | db | OR = 2.02 (0.55, 7.39) | Non-significant difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieckmann, L.H.J.; Haddad, M.; Wendt Viola, T.; Franco Scarante, F.; Rodrigues da Silva, N.; Mari, J.d.J. An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression. Pharmaceuticals 2025, 18, 711. https://doi.org/10.3390/ph18050711
Dieckmann LHJ, Haddad M, Wendt Viola T, Franco Scarante F, Rodrigues da Silva N, Mari JdJ. An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression. Pharmaceuticals. 2025; 18(5):711. https://doi.org/10.3390/ph18050711
Chicago/Turabian StyleDieckmann, Luiz Henrique Junqueira, Michel Haddad, Thiago Wendt Viola, Franciele Franco Scarante, Naielly Rodrigues da Silva, and Jair de Jesus Mari. 2025. "An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression" Pharmaceuticals 18, no. 5: 711. https://doi.org/10.3390/ph18050711
APA StyleDieckmann, L. H. J., Haddad, M., Wendt Viola, T., Franco Scarante, F., Rodrigues da Silva, N., & Mari, J. d. J. (2025). An Overview of the Systematic Reviews About the Efficacy of Fluvoxamine on Depression. Pharmaceuticals, 18(5), 711. https://doi.org/10.3390/ph18050711







