Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade
Abstract
1. Introduction
2. Relationship Between Inflammation and Depression
2.1. Mechanisms of Inflammation
2.2. Neuroinflammation
2.2.1. Microglia
2.2.2. Astrocytes
2.2.3. Lymphocyte Infiltration
2.2.4. Neuroplasticity
2.2.5. Oxidative Stress
2.2.6. Hypothalamic–Adrenal Axis
2.2.7. The Kynurenine Pathway
2.2.8. Gut–Brain Axis
3. Results
3.1. Anti-Inflammatory Effect of Antidepressants
3.1.1. IL-1β
3.1.2. IL-1ra
3.1.3. IL-2
3.1.4. IL-4
3.1.5. IL-5
3.1.6. IL-6
3.1.7. IL-7
3.1.8. IL-8
3.1.9. IL-9
3.1.10. IL-10
3.1.11. IL-12
3.1.12. IL-13
3.1.13. IL-15
3.1.14. IL-17
3.1.15. IFN-γ
3.1.16. TNF-α
3.1.17. CRP
| Name | Class | Effect | Cytokines | Model | Citation |
|---|---|---|---|---|---|
| Escitalopram | SSRI | ↓ | IL-1β, IL-2, IL-5, IL-6, IL-7, IL-8, IL-9, IL-17, TNF-α, IFN-γ | Moderate depression, patients were their own control, serum, Luminex xMAP multiplexing technology, 26 weeks of antidepressant treatment, 90 participants (71% women) with a mean age of 38 years. | [191] |
| ↓ | IL-1ra, IL-4, IL-10, IL-13 | [191] | |||
| ↑ | IL-12, IL-15 | [191] | |||
| ↔ | IL-10, IL-1ra, IL-6, IL-8, TNF-α | Depression, randomized double-blinded trial, whole blood stimulated with LPS or PHA (in vitro), Luminex 100 platform, 4 weeks of antidepressant treatment, 44 participants (28 women), with a mean age of 32.7 years. | [198] | ||
| ↓ | IL-2, IL-6 and TNF-a | MDD, patients were their own control, cytokines were analyzed in serum by ELISA test, 6 weeks of antidepressant treatment, 65 participants (35 women), with a mean age of 36 years. | [203] | ||
| ↓ | IL-6 | MDD, open-label part-randomized multicenter pharmacogenetic study with two active pharmacological treatment arms, leukocyte mRNA levels were measured, 8 weeks of antidepressant treatment, 38 participants with a mean age of 38 years. | [215] | ||
| Fluoxetine | ↔ | IL-1β, IL-6 | MDD, patients were their own control, cytokines were analyzed in serum by ELISA test, 8 weeks of study, 14 participants (10 women), with a mean age of 37 years. | [196] | |
| ↓ | IL-2, IL-6 and TNF-a | MDD, patients were their own control, cytokines were analyzed in serum by ELISA test, 6 weeks of study, 65 participants (36 women), with a mean age of 36 years. | [203] | ||
| ↑ | IL-10 | Crohn’s disease, presence of a separate control group that received a placebo, cytokines were analyzed by flow cytometry, 6 months of antidepressant treatment, 26 participants (12 women), with a mean age of 37 years. | [231] | ||
| ↓ | TNF-a | Depression, patients were their own control, cytokines were analyzed in serum by ELISA test, 12 weeks of antidepressant treatment, 30 participants (20 women), with a mean age of 36 years. | [252] | ||
| ↓ | IL-1β | Depression, the effect of fluoxetine was compared to the untreated group, cytokines were analyzed in serum by ELISA test, 12 weeks of antidepressant treatment, 32 participants (18 women), with a mean age of 34 years. | [192] | ||
| ↔ | IL-2, CRP | Depression | [205] | ||
| ↓ | IL-1ra | Depression, cytokine measurement in the 4th week of therapy, presence of a separate control group, cytokines were analyzed in serum by multiplex bead-based immunoassays, 8 weeks of antidepressant treatment, 22 participants (18 women), with a mean age of 17 years. | [193] | ||
| ↓ | IFN-γ, IL-1β, TNF-α, IL-6, IL-12, IL-15 | [193] | |||
| ↑ | IL-4 | Depression, cytokine measurement in the 8th week of therapy. | [193] | ||
| ↑ | IL-12 and IL-5 | [193] | |||
| ↔ | IL-10 and IL-13 | Depression | [193] | ||
| ↓ | CRP | COVID-19; during a mid-hospital stay, a double-blind randomized, placebo-controlled clinical trial; 4 weeks of antidepressant treatment, 72 participants (35 women), with a mean age of 52 years. | [253] | ||
| ↓ | NLRP3, IL-1β, and IL-18 | First episode, moderate to severe MDD, patients were their own control, cytokines were analyzed in serum by ELISA test, 12 weeks of antidepressant treatment, 48 participants (30 women), with a mean age of 17 years. | [194] | ||
| ↑ | IL-4 | [194] | |||
| Sertraline | ↔ | hsCRP, IL-6 | MDD and CKD, randomized, double-blind placebo-controlled trial, cytokines were analyzed in serum by ELISA test, 12 weeks of antidepressant treatment, 201 participants (61 women), with a mean age of 58 years. | [222] | |
| ↓ | IL-6 | Hemodialysis and depression, randomized double-blind, placebo-controlled clinical trial, cytokines were analyzed in serum by ELISA test, 12 weeks of antidepressant treatment, 43 participants (16 women), with a mean age of 63 years. | [216] | ||
| ↔ | TNF-α | [216] | |||
| ↔ | IL-10 | [216] | |||
| ↓ | IL-4, IL-10 | Unipolar depression, double-blind, placebo-controlled trial, blood cytokines were measured by flow cytometry, 6 weeks of antidepressant treatment, 120 participants (82 women), with a mean age of 42 years. | [204] | ||
| ↓ | IL-2, IL-6, IL-17a, IFN-γ | [204] | |||
| ↔ | TNF-α | [204] | |||
| ↔ | hsCRP, IL-6, and TNF-α | CHD and MDD, randomized, double-blind, placebo-controlled trial, cytokines were analyzed in serum by ELISA test, 10 weeks of antidepressant treatment, 122 participants (41 women), with a mean age of 59 years. | [223] | ||
| ↓ | CRP and IL-6 | CHD and MDD, randomized double-blind, placebo-controlled clinical trial, cytokines were analyzed in serum by ELISA test, 20 weeks of antidepressant treatment, 95 participants (48 women), with a mean age of 57 years. | [217] | ||
| Paroxetine | ↑ | IL-10 | MDD, two randomized placebo-controlled clinical studies, cytokines were analyzed in serum by ELISA test, 10 weeks of antidepressant treatment, 106 participants (72 women), with a mean age of 46 years. | [220] | |
| ↑ | TNF-α, IL-6 and CRP | [220] | |||
| Venlafaxine | SNRI | ↓ | CRP | MDD, two randomized placebo-controlled clinical studies, cytokines were analyzed in serum by ELISA test, 10 weeks of antidepressant treatment, 104 participants (64 women), with a mean age of 45 years. | [220] |
| Ketamine | ↓ | CRP | Post-operative cognitive dysfunction, patients randomly received placebo or an i.v. bolus of ketamine (0.5 mg/kg) during anesthetic induction. Anesthesia was maintained with isoflurane and fentanyl. A nonsurgical group was also included as control, serum C-reactive protein (CRP) concentrations were determined before surgery and on the first post-operative day, 96 participants with a mean age of 66 years. | [251] | |
| ↓ | IL-8/IL-10 ratio | MDD, presence of a separate control group that received a placebo, plasma concentrations of cytokines were analyzed by ELISA test 24 h after ketamine infusion (0.5 mg/kg), 25 participants (7 women) with a mean age of 46 years. | [254] | ||
| ↓ | IL-4, and IL-10 | TRD, cytokines were analyzed in plasma by multiplex bead-based immunoassays on days 13 and 26, TRD patients received intravenous ketamine (0.5 mg/kg) three times weekly for 2 weeks, 66 participants (37 women) with a mean age of 36 years. | [195] | ||
| ↓ | IFN-γ, IL-17α, IL-1β, IL-2, IL-23, IL-5, IL-6, IL-7, and TNF-α | [195] | |||
| ↓ | IL-8 | Depression, presence of a separate control group that received a placebo, depressed patients (n = 46, female, n = 17), cytokines were analyzed in plasma by multiplex bead-based immunoassays 24 h after infusion of ketamine (0.5 mg/kg). | [226] | ||
| ↓ | TNF-α | TRD, randomized, double-blind control study, patients were randomized into three groups according to the treatment received: 0.5 mg/kg ketamine, 0.2 mg/kg ketamine, and normal saline infusion, cytokines were analyzed in plasma by ELISA test at baseline and at 40 min, 240 min, day 3, and day 7 post-infusion, 71 participants (53 women), with a mean age of 46 years. | [246] | ||
| ↑ | IL-6 | MDD, double-blind, placebo-controlled studies, cytokines were analyzed in plasma by multiplex bead-based immunoassays, blood samples were received at 60 min before ketamine infusion and at 230 min, one day, and three days post-infusion ketamine (0.5 mg/kg) or saline placebo, 80 participants (61 women), with a mean age of 45 years. | [221] | ||
| ↑ | TNF-α | TRD, randomized placebo-controlled and open-label trials, cytokines were analyzed in plasma by ELISA test, 78 patients were allocated to receive two ketamine infusions (n = 30, days 1 and 4), a single ketamine (0.5 mg/kg) infusion (n = 24, only day 1), or normal saline placebo infusion (n = 24, only day 1). | [247] | ||
| Esketamine | ↓ | IL-6 and CRP | Labor and postpartum depression, a randomized, double-blinded controlled trial, cytokines were analyzed in plasma by ELISA test, a total of 120 women who underwent labor analgesia by epidural analgesia pump were enrolled and divided into two groups randomly. Esketamine at a dose of 0.2 mg/kg was intravenously injected after fetal disengagement in the test group and placebo was administered in the control group. | [218] | |
| ↑ | IL-10 | [218] | |||
| ↓ | IL-6 | Elective non-cardiac thoracic surgery under general anesthesia, randomized controlled trial. During the operation, patients received 0.2 mg/kg (low-esketamine group) or 0.5 mg/kg esketamine (high-esketamine group) vs. placebo, cytokines were analyzed in plasma by ELISA test before surgery, post-operative day 1 and day 3, 129 participants (56 women), with a mean age of 65 years. | [219] |
4. Discussion
4.1. Mechanisms of Anti-Inflammatory Action
4.1.1. NF-κB
4.1.2. NLRP3 Inflammasome Complex
4.1.3. SSRIs
Fluoxetine
Escitalopram
Sertraline
Paroxetine
4.1.4. SNRIs: Venlafaxine
4.1.5. SSRIs vs. SNRIs
4.1.6. Ketamine/Esketamine
4.2. Limitations of Evidence
5. Materials and Methods
5.1. Literature Search
5.2. Eligibility Criteria
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 9 April 2024).
- Andrews, G.; Issakidis, C.; Sanderson, K.; Corry, J.; Lapsley, H. Utilising survey data to inform public policy: Comparison of the cost-effectiveness of treatment of ten mental disorders. Br. J. Psychiatry J. Ment. Sci. 2004, 184, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Roose, S.P.; Schatzberg, A.F. The efficacy of antidepressants in the treatment of late-life depression. J. Clin. Psychopharmacol. 2005, 25, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, D.; Sanderson, K.; Ayuso-Mateos, J.L.; Saxena, S. Reducing the global burden of depression: Population-level analysis of intervention cost-effectiveness in 14 world regions. Br. J. Psychiatry J. Ment. Sci. 2004, 184, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.M.; Harkness, K.L. Recurrence in major depression: A conceptual analysis. Psychol. Rev. 2011, 118, 655–674. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. IHME The Lancet: COVID-19 Pandemic Led to Stark Rise in Depressive and Anxiety Disorders Globally in 2020, with Women and Younger People Most Affected. Available online: https://www.healthdata.org/news-events/newsroom/news-releases/lancet-covid-19-pandemic-led-stark-rise-depressive-and-anxiety (accessed on 9 April 2024).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Lamers, F.; Berk, M.; Penninx, B.W.J.H. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol. Psychiatry 2020, 88, 369–380. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Bunney, W.E.; Davis, J.M. Norepinephrine in depressive reactions. A review. Arch. Gen. Psychiatry 1965, 13, 483–494. [Google Scholar] [CrossRef]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 7–11. [Google Scholar] [PubMed]
- Hirschfeld, R.M.A. History and Evolution of the Monoamine Hypothesis of Depression. J. Clin. Psychiatry 2000, 61, 8272. [Google Scholar]
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef]
- Wong, D.T.; Bymaster, F.P.; Engleman, E.A. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sci. 1995, 57, 411–441. [Google Scholar] [CrossRef]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. Case history: The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef]
- Papakostas, G.I. Serotonin Norepinephrine Reuptake Inhibitors: Spectrum of Efficacy in Major Depressive Disorder. Prim. Psychiatry 2009, 16, 16. [Google Scholar]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Hyman, S.E.; Nestler, E.J. Initiation and adaptation: A paradigm for understanding psychotropic drug action. Am. J. Psychiatry 1996, 153, 151–162. [Google Scholar] [CrossRef]
- Mill, J.; Petronis, A. Molecular studies of major depressive disorder: The epigenetic perspective. Mol. Psychiatry 2007, 12, 799–814. [Google Scholar] [CrossRef]
- Charney, D.S. Monoamine dysfunction and the pathophysiology and treatment of depression. J. Clin. Psychiatry 1998, 59 (Suppl. 14), 11–14. [Google Scholar] [PubMed]
- Heninger, G.R.; Delgado, P.L.; Charney, D.S. The revised monoamine theory of depression: A modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 1996, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.A.; Mauri, M.C.; Ferrara, A.; Moro, A.R.; D’Andrea, G.; Zamberlan, F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am. J. Psychiatry 1993, 150, 1731–1733. [Google Scholar] [CrossRef]
- Küçükibrahimoğlu, E.; Saygin, M.Z.; Calişkan, M.; Kaplan, O.K.; Unsal, C.; Gören, M.Z. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 2009, 65, 571–577. [Google Scholar] [CrossRef]
- Mauri, M.C.; Ferrara, A.; Boscati, L.; Bravin, S.; Zamberlan, F.; Alecci, M.; Invernizzi, G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998, 37, 124–129. [Google Scholar] [CrossRef]
- Levine, J.; Panchalingam, K.; Rapoport, A.; Gershon, S.; McClure, R.J.; Pettegrew, J.W. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 2000, 47, 586–593. [Google Scholar] [CrossRef]
- Domino, E.F. Taming the ketamine tiger. 1965. Anesthesiology 2010, 113, 678–684. [Google Scholar] [CrossRef]
- Lord, B.; Wintmolders, C.; Langlois, X.; Nguyen, L.; Lovenberg, T.; Bonaventure, P. Comparison of the ex vivo receptor occupancy profile of ketamine to several NMDA receptor antagonists in mouse hippocampus. Eur. J. Pharmacol. 2013, 715, 21–25. [Google Scholar] [CrossRef]
- Yamakura, T.; Shimoji, K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 1999, 59, 279–298. [Google Scholar] [CrossRef]
- Yamakura, T.; Mori, H.; Masaki, H.; Shimoji, K.; Mishina, M. Different sensitivities of NMDA receptor channel subtypes to non-competitive antagonists. Neuroreport 1993, 4, 687–690. [Google Scholar] [CrossRef]
- Mendelsohn, L.G.; Kalra, V.; Johnson, B.G.; Kerchner, G.A. Sigma opioid receptor: Characterization and co-identity with the phencyclidine receptor. J. Pharmacol. Exp. Ther. 1985, 233, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hashimoto, Y.; Lambert, D.G. Interaction of intravenous anesthetics with recombinant human M1-M3 muscarinic receptors expressed in chinese hamster ovary cells. Anesth. Analg. 2002, 95, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Bouchal, R.L.; deSanctis, C.A.; Monroe, P.J.; Amedro, J.B.; Perrotti, J.M.; Crisp, T. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology 1987, 26, 1253–1260. [Google Scholar] [CrossRef]
- Nishimura, M.; Sato, K.; Okada, T.; Yoshiya, I.; Schloss, P.; Shimada, S.; Tohyama, M. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 1998, 88, 768–774. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N.; Liu, R.-J.; Duric, V.; Aghajanian, G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Daly, E.J.; Singh, J.B.; Fedgchin, M.; Cooper, K.; Lim, P.; Shelton, R.C.; Thase, M.E.; Winokur, A.; Van Nueten, L.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 139–148. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients with Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef]
- Cristea, I.A.; Naudet, F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6, 975–977. [Google Scholar] [CrossRef]
- Lee, C.-H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 372–387.e14. [Google Scholar] [CrossRef] [PubMed]
- Biltz, R.G.; Sawicki, C.M.; Sheridan, J.F.; Godbout, J.P. The neuroimmunology of social-stress-induced sensitization. Nat. Immunol. 2022, 23, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Nadjar, A.; Bluthé, R.-M.; May, M.J.; Dantzer, R.; Parnet, P. Inactivation of the Cerebral NFκB Pathway Inhibits Interleukin-1β-Induced Sickness Behavior and c-Fos Expression in Various Brain Nuclei. Neuropsychopharmacology 2005, 30, 1492–1499. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Fan, N.; Luo, Y.; Ou, Y.; He, H. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum. Psychopharmacol. 2017, 32, e2588. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain. Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Renault, P.F.; Hoofnagle, J.H.; Park, Y.; Mullen, K.D.; Peters, M.; Jones, D.B.; Rustgi, V.; Jones, E.A. Psychiatric complications of long-term interferon alfa therapy. Arch. Intern. Med. 1987, 147, 1577–1580. [Google Scholar] [CrossRef]
- Buter, J.; de Vries, E.G.; Sleijfer, D.T.; Willemse, P.H.; Mulder, N.H. Neuropsychiatric symptoms during treatment with interleukin-2. Lancet 1993, 341, 628. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Brouwer, J.T.; van der Mast, R.C.; Schalm, S.W. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J. Hepatol. 1994, 21, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Ravaud, A.; Miller, A.H.; Dantzer, R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain. Behav. Immun. 2004, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dieperink, E.; Ho, S.B.; Tetrick, L.; Thuras, P.; Dua, K.; Willenbring, M.L. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen. Hosp. Psychiatry 2004, 26, 237–240. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H.; de Beaurepaire, R. Cytokines as mediators of depression: What can we learn from animal studies? Neurosci. Biobehav. Rev. 2005, 29, 891–909. [Google Scholar] [CrossRef]
- Loftis, J.M.; Huckans, M.; Morasco, B.J. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol. Dis. 2010, 37, 519–533. [Google Scholar] [CrossRef]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef]
- Maes, M.; Meltzer, H.Y.; Bosmans, E.; Bergmans, R.; Vandoolaeghe, E.; Ranjan, R.; Desnyder, R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J. Affect. Disord. 1995, 34, 301–309. [Google Scholar] [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef]
- Jones, K.A.; Thomsen, C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013, 53, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.W.; Miller, A.H. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann. N. Y. Acad. Sci. 2009, 1179, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, J.; Olsson, S.K.; Samuelsson, M.; Walther-Jallow, L.; Johansson, C.; Erhardt, S.; Landén, M.; Engberg, G. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J. Psychiatry Neurosci. JPN 2011, 36, 114–118. [Google Scholar] [CrossRef]
- Smith, K.J.; Au, B.; Ollis, L.; Schmitz, N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp. Gerontol. 2018, 102, 109–132. [Google Scholar] [CrossRef]
- Korczak, D.J.; Pereira, S.; Koulajian, K.; Matejcek, A.; Giacca, A. Type 1 diabetes mellitus and major depressive disorder: Evidence for a biological link. Diabetologia 2011, 54, 2483–2493. [Google Scholar] [CrossRef]
- Dickens, C.; McGowan, L.; Clark-Carter, D.; Creed, F. Depression in rheumatoid arthritis: A systematic review of the literature with meta-analysis. Psychosom. Med. 2002, 64, 52–60. [Google Scholar] [CrossRef]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Bialek, K.; Czarny, P.; Strycharz, J.; Sliwinski, T. Major depressive disorders accompanying autoimmune diseases—Response to treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109678. [Google Scholar] [CrossRef]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.; Grannemann, B.; Soyombo, A.; Mayes, T.L.; Rush, A.J.; Trivedi, M.H. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 2017, 78, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primer 2023, 9, 44. [Google Scholar] [CrossRef]
- Patil, C.R.; Suryakant Gawli, C.; Bhatt, S. Targeting inflammatory pathways for treatment of the major depressive disorder. Drug Discov. Today 2023, 28, 103697. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory Cytokines in Depression: Neurobiological Mechanisms and Therapeutic Implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- Chang, D.; Xing, Q.; Su, Y.; Zhao, X.; Xu, W.; Wang, X.; Dong, C. The Conserved Non-coding Sequences CNS6 and CNS9 Control Cytokine-Induced Rorc Transcription during T Helper 17 Cell Differentiation. Immunity 2020, 53, 614–626.e4. [Google Scholar] [CrossRef]
- Müller, N.; Ackenheil, M. Psychoneuroimmunology and the cytokine action in the CNS: Implications for psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 1998, 22, 1–33. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Hong, H.; Kim, B.S.; Im, H.-I. Pathophysiological Role of Neuroinflammation in Neurodegenerative Diseases and Psychiatric Disorders. Int. Neurourol. J. 2016, 20, S2–S7. [Google Scholar] [CrossRef]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2014, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Holtman, I.R.; Boddeke, E.W.G.M.; Möller, T.; Eggen, B.J.L. Next generation transcriptomics and genomics elucidate biological complexity of microglia in health and disease. Glia 2016, 64, 197–213. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- Lemaitre, P.; Tareen, S.H.; Pasciuto, E.; Mascali, L.; Martirosyan, A.; Callaerts-Vegh, Z.; Poovathingal, S.; Dooley, J.; Holt, M.G.; Yshii, L.; et al. Molecular and cognitive signatures of ageing partially restored through synthetic delivery of IL2 to the brain. EMBO Mol. Med. 2023, 15, e16805. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, M.; Pan, R.; Wang, Z.; Tao, X.; Li, C.; Xia, T.; Liu, X.; Chang, Q. Radix Polygalae extract exerts antidepressant effects in behavioral despair mice and chronic restraint stress-induced rats probably by promoting autophagy and inhibiting neuroinflammation. J. Ethnopharmacol. 2021, 265, 113317. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, G.; Wang, L.; Tan, Y.; Song, Z. Lactobacillus delbrueckii alleviates depression-like behavior through inhibiting toll-like receptor 4 (TLR4) signaling in mice. Ann. Transl. Med. 2021, 9, 366. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Yu, H.; Tian, Y.; Chen, X.; Chen, C.; Ren, Y.; Chen, Z.; Ren, Y.; Gong, X.; et al. Protective effect of Nr4a2 (Nurr1) against LPS-induced depressive-like behaviors via regulating activity of microglia and CamkII neurons in anterior cingulate cortex. Pharmacol. Res. 2023, 191, 106717. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflamm. 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Singhal, G.; Baune, B.T. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front. Cell. Neurosci. 2017, 11, 270. [Google Scholar] [CrossRef]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, Y.-K. The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef]
- Brites, D.; Fernandes, A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 2015, 9, 476. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, Y.; Won, S.J.; Neumann, M.; Hu, D.; Zhou, L.; Weinstein, P.R.; Liu, J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 2007, 38, 146–152. [Google Scholar] [CrossRef]
- Cabezas, R.; Avila-Rodriguez, M.; Vega-Vela, N.E.; Echeverria, V.; González, J.; Hidalgo, O.A.; Santos, A.B.; Aliev, G.; Barreto, G.E. Growth Factors and Astrocytes Metabolism: Possible Roles for Platelet Derived Growth Factor. Med. Chem. 2016, 12, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Han, R.T.; Kim, R.D.; Molofsky, A.V.; Liddelow, S.A. Astrocyte-immune cell interactions in physiology and pathology. Immunity 2021, 54, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xie, X.; Yao, L.; Wang, W.; Zhang, H.; Chen, M.; Sun, S.; Nie, Z.; Nagy, C.; Liu, Z. Human in vivo evidence of reduced astrocyte activation and neuroinflammation in patients with treatment-resistant depression following electroconvulsive therapy. Psychiatry Clin. Neurosci. 2023, 77, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Schlaaff, K.; Dobrowolny, H.; Frodl, T.; Mawrin, C.; Gos, T.; Steiner, J.; Bogerts, B. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain. Behav. Immun. 2020, 88, 497–506. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Song, N.-N.; Ding, Y.-Q.; Zhang, L. Neural plasticity and depression treatment. IBRO Neurosci. Rep. 2023, 14, 160–184. [Google Scholar] [CrossRef]
- Fuchs, E.; Flügge, G. Adult Neuroplasticity: More Than 40 Years of Research. Neural Plast. 2014, 2014, 541870. [Google Scholar] [CrossRef]
- Toda, T.; Gage, F.H. Review: Adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2018, 373, 693–709. [Google Scholar] [CrossRef]
- Albert, P.R. Adult neuroplasticity: A new “cure” for major depression? J. Psychiatry Neurosci. JPN 2019, 44, 147–150. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.Y.; Harmer, C.J.; Norbury, R.; O’Sullivan, U.; Goodwin, G.M.; Portella, M.J. Hippocampal volume in vulnerability and resilience to depression. J. Affect. Disord. 2016, 189, 199–202. [Google Scholar] [CrossRef]
- Savitz, J.B.; Drevets, W.C. Imaging phenotypes of major depressive disorder: Genetic correlates. Neuroscience 2009, 164, 300–330. [Google Scholar] [CrossRef]
- Cobb, J.A.; Simpson, J.; Mahajan, G.J.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Herbst, N.; May, W.; Rajkowska, G.; Stockmeier, C.A. Hippocampal volume and total cell numbers in major depressive disorder. J. Psychiatr. Res. 2013, 47, 299–306. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- McEwen, B.S.; Eiland, L.; Hunter, R.G.; Miller, M.M. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012, 62, 3–12. [Google Scholar] [CrossRef]
- Kuhn, M.; Höger, N.; Feige, B.; Blechert, J.; Normann, C.; Nissen, C. Fear Extinction as a Model for Synaptic Plasticity in Major Depressive Disorder. PLoS ONE 2014, 9, e115280. [Google Scholar] [CrossRef]
- Malberg, J.E.; Duman, R.S. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 1562–1571. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Manji, H.K.; Moore, G.J.; Rajkowska, G.; Chen, G. Neuroplasticity and cellular resilience in mood disorders. Mol. Psychiatry 2000, 5, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; Deslandes, A.; McEwen, B.S. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur. J. Pharmacol. 1999, 371, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Butt, T.H.; Santiago, A.N.; Tamir, H.; Dwork, A.J.; Rosoklija, G.B.; Arango, V.; Hen, R.; Mann, J.J. Benzodiazepines and the Potential Trophic Effect of Antidepressants on Dentate Gyrus Cells in Mood Disorders. Int. J. Neuropsychopharmacol. Off. Sci. J. Coll. Int. Neuropsychopharmacol. CINP 2014, 17, 1923–1933. [Google Scholar] [CrossRef]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochem. Biokhimiia 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Bajpai, A. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.-M. ROS and Brain Diseases: The Good, the Bad, and the Ugly. Oxid. Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- McCracken, E.; Valeriani, V.; Simpson, C.; Jover, T.; McCulloch, J.; Dewar, D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J. Cereb. Blood Flow Metab. 2000, 20, 1529–1536. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Michel, T.M.; Frangou, S.; Thiemeyer, D.; Camara, S.; Jecel, J.; Nara, K.; Brunklaus, A.; Zoechling, R.; Riederer, P. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder--a postmortem study. Psychiatry Res. 2007, 151, 145–150. [Google Scholar] [CrossRef]
- Michel, T.M.; Thome, J.; Martin, D.; Nara, K.; Zwerina, S.; Tatschner, T.; Weijers, H.G.; Koutsilieri, E. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J. Neural Transm. 2004, 111, 1191–1201. [Google Scholar] [CrossRef]
- Michel, T.M.; Sybille, C.; Thomas, T.; Sophia, F.; Jane, S.A.; Peter, R.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry 2010, 11, 314–320. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxid. Med. Cell. Longev. 2009, 2, 623654. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Alexander, M.Y.; Tutar, Y.; Wilkinson, F.L.; Venditti, A. Oxidative Stress in Metabolic Disorders and Drug-Induced Injury: The Potential Role of Nrf2 and PPARs Activators. Oxid. Med. Cell. Longev. 2017, 2017, 2508909. [Google Scholar] [CrossRef]
- Murphy, B.E. Steroids and depression. J. Steroid Biochem. Mol. Biol. 1991, 38, 537–559. [Google Scholar] [CrossRef]
- Anders, S.; Tanaka, M.; Kinney, D.K. Depression as an evolutionary strategy for defense against infection. Brain. Behav. Immun. 2013, 31, 9–22. [Google Scholar] [CrossRef]
- Milne, A.M.B.; MacQueen, G.M.; Hall, G.B.C. Abnormal hippocampal activation in patients with extensive history of major depression: An fMRI study. J. Psychiatry Neurosci. JPN 2012, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.G.; Mazurka, R.; Bond, L.; Wynne-Edwards, K.E.; Harkness, K.L. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. J. Abnorm. Child Psychol. 2013, 41, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Nikkheslat, N.; Zunszain, P.A.; Horowitz, M.A.; Barbosa, I.G.; Parker, J.A.; Myint, A.-M.; Schwarz, M.J.; Tylee, A.T.; Carvalho, L.A.; Pariante, C.M. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain. Behav. Immun. 2015, 48, 8–18. [Google Scholar] [CrossRef]
- Kokras, N.; Hodes, G.E.; Bangasser, D.A.; Dalla, C. Sex differences in the hypothalamic–pituitary–adrenal axis: An obstacle to antidepressant drug development? Br. J. Pharmacol. 2019, 176, 4090–4106. [Google Scholar] [CrossRef]
- Frank, M.G.; Hershman, S.A.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology 2014, 40, 191–200. [Google Scholar] [CrossRef]
- Chai, Y.; Cai, Y.; Fu, Y.; Wang, Y.; Zhang, Y.; Zhang, X.; Zhu, L.; Miao, M.; Yan, T. Salidroside Ameliorates Depression by Suppressing NLRP3-Mediated Pyroptosis via P2X7/NF-κB/NLRP3 Signaling Pathway. Front. Pharmacol. 2022, 13, 812362. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. Off. J. Soc. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, T.; Wu, M.; Zhu, A.; Zhu, G. Botanicals as modulators of depression and mechanisms involved. Chin. Med. 2019, 14, 24. [Google Scholar] [CrossRef]
- Wong, M.L.; Lewis, M.; Licinio, J. Translational research in endocrinology and neuroimmunology applied to depression. In Biomedical Chemistry; De Gruyter: Berlin, Germany, 2015; pp. 119–131. ISBN 978-3-11-046874-8. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. IJTR 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Pariante, C.M.; Palacios, J.E.; Tylee, A.; Carvalho, L.A.; Viganò, C.A.; Nikkheslat, N. Inflammation associated with coronary heart disease predicts onset of depression in a three-year prospective follow-up: A preliminary study. Brain. Behav. Immun. 2019, 81, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Macedo e Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the kynurenine metabolism pathway in inflammation-induced depression—Preclinical approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Mándi, Y.; Stone, T.W.; Guillemin, G.J.; Vécsei, L.; Williams, R.O. Editorial: Multiple Implications of the Kynurenine Pathway in Inflammatory Diseases: Diagnostic and Therapeutic Applications. Front. Immunol. 2022, 13, 860867. [Google Scholar] [CrossRef]
- Bay-Richter, C.; Linderholm, K.R.; Lim, C.K.; Samuelsson, M.; Träskman-Bendz, L.; Guillemin, G.J.; Erhardt, S.; Brundin, L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain. Behav. Immun. 2015, 43, 110–117. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- Mohammadi, G.; Dargahi, L.; Peymani, A.; Mirzanejad, Y.; Alizadeh, S.A.; Naserpour, T.; Nassiri-Asl, M. The Effects of Probiotic Formulation Pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.-Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Tillmann, S.; Awwad, H.M.; Eskelund, A.R.; Treccani, G.; Geisel, J.; Wegener, G.; Obeid, R. Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression. Mol. Nutr. Food Res. 2018, 62, e1701070. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Karbownik, M.S.; Sokołowska, P.; Kowalczyk, E. Gut Microbiota Metabolites Differentially Release Gliotransmitters from the Cultured Human Astrocytes: A Preliminary Report. Int. J. Mol. Sci. 2023, 24, 6617. [Google Scholar] [CrossRef]
- Bleibel, L.; Dziomba, S.; Waleron, K.F.; Kowalczyk, E.; Karbownik, M.S. Deciphering psychobiotics’ mechanism of action: Bacterial extracellular vesicles in the spotlight. Front. Microbiol. 2023, 14, 1211447. [Google Scholar] [CrossRef]
- Rathour, D.; Shah, S.; Khan, S.; Singh, P.K.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Role of gut microbiota in depression: Understanding molecular pathways, recent research, and future direction. Behav. Brain Res. 2023, 436, 114081. [Google Scholar] [CrossRef]
- Hao, W.; Ma, Q.; Wang, L.; Yuan, N.; Gan, H.; He, L.; Li, X.; Huang, J.; Chen, J. Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3. Microbiome 2024, 12, 34. [Google Scholar] [CrossRef]
- Rudzki, L.; Maes, M. The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. The cytokine hypothesis of depression: Inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol. Lett. 2008, 29, 287–291. [Google Scholar] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Spiller, R.; Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 2009, 136, 1979–1988. [Google Scholar] [CrossRef]
- Clouse, R.E.; Lustman, P.J.; Geisman, R.A.; Alpers, D.H. Antidepressant therapy in 138 patients with irritable bowel syndrome: A five-year clinical experience. Aliment. Pharmacol. Ther. 1994, 8, 409–416. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Huang, R.; Ning, H.; Yang, L.; Jia, C.; Yang, F.; Xu, G.; Tan, H. Efficacy of Probiotics on Anxiety: A Meta-analysis of Randomized Controlled Trials. Neuropsychiatry 2017, 7, 862–871. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Goh, K.K.; Liu, Y.-W.; Kuo, P.-H.; Chung, Y.-C.E.; Lu, M.-L.; Chen, C.-H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Liu, C.; Sutthawongwadee, S.; Li, Y.; Lv, W.; Chen, W.; Yu, L.; Zhou, J.; Guo, A.; Li, Z.; et al. Effects of Probiotics on Depressive or Anxiety Variables in Healthy Participants Under Stress Conditions or With a Depressive or Anxiety Diagnosis: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, K.; Basso, M.; Knytl, P.; Johnstone, N.; Lau, J.Y.F.; Gibson, G.R. Psychobiotic interventions for anxiety in young people: A systematic review and meta-analysis, with youth consultation. Transl. Psychiatry 2021, 11, 352. [Google Scholar] [CrossRef]
- El Dib, R.; Periyasamy, A.G.; de Barros, J.L.; França, C.G.; Senefonte, F.L.; Vesentini, G.; Alves, M.G.O.; Rodrigues, J.V.d.S.; Gomaa, H.; Gomes Júnior, J.R.; et al. Probiotics for the treatment of depression and anxiety: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 75–90. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Kofod, J.; Elfving, B.; Nielsen, E.H.; Mors, O.; Köhler-Forsberg, O. Depression and inflammation: Correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur. Neuropsychopharmacol. 2022, 54, 116–125. [Google Scholar] [CrossRef]
- Song, C.; Halbreich, U.; Han, C.; Leonard, B.E.; Luo, H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: The effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry 2009, 42, 182–188. [Google Scholar] [CrossRef]
- Pérez-Sánchez, G.; Becerril-Villanueva, E.; Arreola, R.; Martínez-Levy, G.; Hernández-Gutiérrez, M.E.; Velasco-Velásquez, M.A.; Alvarez-Herrera, S.; Cruz-Fuentes, C.; Palacios, L.; de la Peña, F.; et al. Inflammatory Profiles in Depressed Adolescents Treated with Fluoxetine: An 8-Week Follow-up Open Study. Mediators Inflamm. 2018, 2018, 4074051. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Chen, W.; Tan, J.; Li, X.; Zheng, A.; Fu, Y.; Qiu, T. The relationship between serum resolvin D1, NLRP3, cytokine levels, and adolescents with first-episode medication-naïve major depressive disorder. BMC Psychiatry 2024, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, C.; Lan, X.; Li, H.; Chao, Z.; Ning, Y. Plasma inflammatory cytokines and treatment-resistant depression with comorbid pain: Improvement by ketamine. J. Neuroinflamm. 2021, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Chamari, M.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Thompson, R.C. Blocking IL-1: Interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today 1991, 12, 404–410. [Google Scholar] [CrossRef]
- Haastrup, E.; Knorr, U.; Erikstrup, C.; Kessing, L.V.; Ullum, H. No evidence for an anti-inflammatory effect of escitalopram intervention in healthy individuals with a family history of depression. J. Neuroimmunol. 2012, 243, 69–72. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S.; Subramaniam, S.; Haining, W.N.; Smith, K.A.; Ahmed, R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 2010, 32, 91–103. [Google Scholar] [CrossRef]
- Pipkin, M.E.; Sacks, J.A.; Cruz-Guilloty, F.; Lichtenheld, M.G.; Bevan, M.J.; Rao, A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 2010, 32, 79–90. [Google Scholar] [CrossRef]
- Williams, M.A.; Tyznik, A.J.; Bevan, M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef]
- Malek, T.R.; Castro, I. Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity 2010, 33, 153–165. [Google Scholar] [CrossRef]
- Xiaoling, Z.; Yunping, H.; Yingdong, L. Analysis of curative effect of fluoxetine and escitalopram in the depression treatment based on clinical observation. Pak. J. Pharm. Sci. 2018, 31, 1115–1118. [Google Scholar]
- Brunoni, A.R.; Machado-Vieira, R.; Zarate, C.A.; Valiengo, L.; Vieira, E.L.; Benseñor, I.M.; Lotufo, P.A.; Gattaz, W.F.; Teixeira, A.L. Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): Results from a factorial, randomized, controlled trial. Psychopharmacology 2014, 231, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Mackay, G.M.; Forrest, C.M.; Christofides, J.; Bridel, M.A.; Mitchell, S.; Cowlard, R.; Stone, T.W.; Darlington, L.G. Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling. Clin. Exp. Pharmacol. Physiol. 2009, 36, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Hural, J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 1997, 17, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef]
- Fenton, M.J.; Buras, J.A.; Donnelly, R.P. IL-4 reciprocally regulates IL-1 and IL-1 receptor antagonist expression in human monocytes. J. Immunol. 1992, 149, 1283–1288. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma—The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Kishimoto, T. Factors affecting B-cell growth and differentiation. Annu. Rev. Immunol. 1985, 3, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Gennarelli, M.; Uher, R.; Breen, G.; Farmer, A.; Aitchison, K.J.; Craig, I.W.; Anacker, C.; Zunsztain, P.A.; McGuffin, P.; et al. Candidate Genes Expression Profile Associated with Antidepressants Response in the GENDEP Study: Differentiating between Baseline ‘Predictors’ and Longitudinal ‘Targets’. Neuropsychopharmacology 2013, 38, 377–385. [Google Scholar] [CrossRef]
- Taraz, M.; Khatami, M.-R.; Dashti-Khavidaki, S.; Akhonzadeh, S.; Noorbala, A.-A.; Ghaeli, P.; Taraz, S. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: Results of a randomized double-blind, placebo-controlled clinical trial. Int. Immunopharmacol. 2013, 17, 917–923. [Google Scholar] [CrossRef]
- Pizzi, C.; Mancini, S.; Angeloni, L.; Fontana, F.; Manzoli, L.; Costa, G. Effects of Selective Serotonin Reuptake Inhibitor Therapy on Endothelial Function and Inflammatory Markers in Patients with Coronary Heart Disease. Clin. Pharmacol. Ther. 2009, 86, 527–532. [Google Scholar] [CrossRef]
- Ling, B.; Zhu, Y.; Yan, Z.; Chen, H.; Xu, H.; Wang, Q.; Yu, W.; Wang, W. Effect of single intravenous injection of esketamine on postpartum depression after labor analgesia and potential mechanisms: A randomized, double-blinded controlled trial. BMC Pharmacol. Toxicol. 2023, 24, 66. [Google Scholar] [CrossRef]
- Luo, T.; Deng, Z.; Ren, Q.; Mu, F.; Zhang, Y.; Wang, H. Effects of esketamine on postoperative negative emotions and early cognitive disorders in patients undergoing non-cardiac thoracic surgery: A randomized controlled trial. J. Clin. Anesth. 2024, 95, 111447. [Google Scholar] [CrossRef]
- Carboni, L.; McCarthy, D.J.; Delafont, B.; Filosi, M.; Ivanchenko, E.; Ratti, E.; Learned, S.M.; Alexander, R.; Domenici, E. Biomarkers for response in major depression: Comparing paroxetine and venlafaxine from two randomised placebo-controlled clinical studies. Transl. Psychiatry 2019, 9, 182. [Google Scholar] [CrossRef]
- Park, M.; Newman, L.E.; Gold, P.W.; Luckenbaugh, D.A.; Yuan, P.; Machado-Vieira, R.; Zarate, C.A. Change in Cytokine Levels is Not Associated with Rapid Antidepressant Response to Ketamine in Treatment-Resistant Depression. J. Psychiatr. Res. 2017, 84, 113–118. [Google Scholar] [CrossRef]
- Gregg, L.P.; Carmody, T.; Le, D.; Bharadwaj, N.; Trivedi, M.H.; Hedayati, S.S. Depression and the Effect of Sertraline on Inflammatory Biomarkers in Patients with Nondialysis CKD. Kidney360 2020, 1, 436–446. [Google Scholar] [CrossRef]
- Bot, M.; Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Mann, D.L. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. J. Psychosom. Res. 2011, 71, 13–17. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Kruse, J.L.; Vasavada, M.M.; Olmstead, R.; Hellemann, G.; Wade, B.; Breen, E.C.; Brooks, J.O.; Congdon, E.; Espinoza, R.; Narr, K.L.; et al. Depression treatment response to ketamine: Sex-specific role of interleukin-8, but not other inflammatory markers. Transl. Psychiatry 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Siegel, R. 9—Cytokines and cytokine receptors. In Clinical Immunology, 4th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier: London, UK, 2013; pp. 108–135. ISBN 978-0-7234-3691-1. [Google Scholar]
- Rojas-Zuleta, W.G.; Sanchez, E. IL-9: Function, Sources, and Detection. In Th9 Cells: Methods and Protocols; Goswami, R., Ed.; Springer: New York, NY, USA, 2017; pp. 21–35. ISBN 978-1-4939-6877-0. [Google Scholar]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Hughes, P.A.; Bampton, P.; Gordon, A.; Campaniello, M.A.; Mavrangelos, C.; Stewart, B.J.; Esterman, A.; Andrews, J.M. Fluoxetine for Maintenance of Remission and to Improve Quality of Life in Patients with Crohn’s Disease: A Pilot Randomized Placebo-Controlled Trial. J. Crohns Colitis 2016, 11, 509. [Google Scholar] [CrossRef]
- Horowitz, M.C.; Lorenzo, J.A. Chapter 57—Local Regulators of Bone: IL-1, TNF, Lymphotoxin, Interferon-γ, the LIF/IL-6 Family, and Additional Cytokines. In Principles of Bone Biology, 3rd ed.; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1209–1234. ISBN 978-0-12-373884-4. [Google Scholar]
- Aste-Amezaga, M.; D’Andrea, A.; Kubin, M.; Trinchieri, G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell. Immunol. 1994, 156, 480–492. [Google Scholar] [CrossRef]
- Perussia, B.; Chan, S.H.; D’Andrea, A.; Tsuji, K.; Santoli, D.; Pospisil, M.; Young, D.; Wolf, S.F.; Trinchieri, G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J. Immunol. 1992, 149, 3495–3502. [Google Scholar] [CrossRef]
- Salcedo, T.W.; Azzoni, L.; Wolf, S.F.; Perussia, B. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J. Immunol. 1993, 151, 2511–2520. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.E. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998, 102, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.-Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of Interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. Inst. Pasteur 2011, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Billiau, A. Interferon-gamma: Biology and role in pathogenesis. Adv. Immunol. 1996, 62, 61–130. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Beyaert, R.; Fiers, W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity. What we do understand and what we do not. FEBS Lett. 1994, 340, 9–16. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Chen, M.-H.; Li, C.-T.; Lin, W.-C.; Hong, C.-J.; Tu, P.-C.; Bai, Y.-M.; Cheng, C.-M.; Su, T.-P. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018, 269, 207–211. [Google Scholar] [CrossRef]
- Chen, M.-H.; Wu, H.-J.; Li, C.-T.; Lin, W.-C.; Tsai, S.-J.; Hong, C.-J.; Tu, P.-C.; Bai, Y.-M.; Mao, W.-C.; Su, T.-P. Is one or two infusions better in the first week of low-dose ketamine treatment for medication-resistant depression? A post hoc pooled analysis of randomized placebo-controlled and open-label trials. J. Psychiatr. Res. 2021, 144, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Yano, T. 3—The Nonspecific Immune System: Humoral Defense. In Fish Physiology; Iwama, G., Nakanishi, T., Eds.; Organism, Pathogen, and Environment; Academic Press: Cambridge, MA, USA, 1996; Volume 15, pp. 105–157. [Google Scholar]
- Du Clos, T.W.; Mold, C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Byrne, A.J.; Hudetz, A.G.; Pagel, P.S.; Warltier, D.C. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol. Scand. 2009, 53, 864–872. [Google Scholar] [CrossRef]
- Gupta, K.; Gupta, R.; Bhatia, M.S.; Tripathi, A.K.; Gupta, L.K. Effect of Agomelatine and Fluoxetine on HAM-D Score, Serum Brain-Derived Neurotrophic Factor, and Tumor Necrosis Factor-α Level in Patients with Major Depressive Disorder with Severe Depression. J. Clin. Pharmacol. 2017, 57, 1519–1526. [Google Scholar] [CrossRef]
- Sedighi, F.; Zarghami, M.; Alizadeh Arimi, F.; Moosazadeh, M.; Ala, S.; Ghasemian, R.; Mehravaran, H.; Elyasi, F. Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non-critical COVID-19 pneumonia: A double-blind randomized, placebo-controlled clinical trial. Neuropsychopharmacol. Rep. 2023, 43, 202–212. [Google Scholar] [CrossRef]
- Langhein, M.; Seitz-Holland, J.; Lyall, A.E.; Pasternak, O.; Chunga, N.; Cetin-Karayumak, S.; Kubicki, A.; Mulert, C.; Espinoza, R.T.; Narr, K.L.; et al. Association between peripheral inflammation and free-water imaging in Major Depressive Disorder before and after ketamine treatment—A pilot study. J. Affect. Disord. 2022, 314, 78–85. [Google Scholar] [CrossRef]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef]
- Caldiroli, A.; Capuzzi, E.; Tagliabue, I.; Capellazzi, M.; Marcatili, M.; Mucci, F.; Colmegna, F.; Clerici, M.; Buoli, M.; Dakanalis, A. Augmentative Pharmacological Strategies in Treatment-Resistant Major Depression: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 13070. [Google Scholar] [CrossRef]
- Sokołowska, P.; Seweryn Karbownik, M.; Jóźwiak-Bębenista, M.; Dobielska, M.; Kowalczyk, E.; Wiktorowska-Owczarek, A. Antidepressant mechanisms of ketamine’s action: NF-κB in the spotlight. Biochem. Pharmacol. 2023, 218, 115918. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-Y.; Guo, Y.-X.; Lian, W.-W.; Yan, Y.; Ma, B.-Z.; Cheng, Y.-C.; Xu, J.-K.; He, J.; Zhang, W.-K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased Stress-Induced Inflammatory Responses in Male Patients with Major Depression and Increased Early Life Stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Mincheva-Tasheva, S.; Soler, R.M. NF-κB Signaling Pathways: Role in Nervous System Physiology and Pathology. Neuroscientist 2013, 19, 175–194. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular Basis of NF-κB Signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Karin, M. The Beginning of the End: IκB Kinase (IKK) and NF-κB Activation. J. Biol. Chem. 1999, 274, 27339–27342. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Z.; Xu, J.; Liu, Z.; Wang, Y. Ketamine reduces NFkappaB activation and TNFalpha production in rat mononuclear cells induced by lipopolysaccharide in vitro. Ann. Clin. Lab. Sci. 2002, 32, 292–298. [Google Scholar]
- Sun, J.; Zhou, Z.Q.; Lv, R.; Li, W.Y.; Xu, J.G. Ketamine inhibits LPS-induced calcium elevation and NF-kappa B activation in monocytes. Inflamm. Res. 2004, 53, 304–308. [Google Scholar] [CrossRef]
- Chang, H.-C.; Lin, K.-H.; Tai, Y.-T.; Chen, J.-T.; Chen, R.-M. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation oF ERK1/2 and NFκB. Shock Augusta Ga 2010, 33, 485–492. [Google Scholar] [CrossRef]
- Welters, I.D.; Hafer, G.; Menzebach, A.; Mühling, J.; Neuhäuser, C.; Browning, P.; Goumon, Y. Ketamine Inhibits Transcription Factors Activator Protein 1 and Nuclear Factor-κB, Interleukin-8 Production, as well as CD11b and CD16 Expression: Studies in Human Leukocytes and Leukocytic Cell Lines. Anesth. Analg. 2010, 110, 934. [Google Scholar] [CrossRef]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef]
- Wang, W.-F.; Liu, S.; Xu, B. A study of the protective effect and mechanism of ketamine on acute lung injury induced by mechanical ventilation. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1362–1367. [Google Scholar] [PubMed]
- Tian, M.; Yang, M.; Li, Z.; Wang, Y.; Chen, W.; Yang, L.; Li, Y.; Yuan, H. Fluoxetine suppresses inflammatory reaction in microglia under OGD/R challenge via modulation of NF-κB signaling. Biosci. Rep. 2019, 39, BSR20181584. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.A.; Wertz, J.; Zhu, D.; Cattaneo, A.; Musaelyan, K.; Nikkheslat, N.; Thuret, S.; Pariante, C.M.; Zunszain, P.A. Antidepressant Compounds Can Be Both Pro- and Anti-Inflammatory in Human Hippocampal Cells. Int. J. Neuropsychopharmacol. 2015, 18, pyu076. [Google Scholar] [CrossRef]
- Thomas, P.G.; Dash, P.; Aldridge, J.R.; Ellebedy, A.H.; Reynolds, C.; Funk, A.J.; Martin, W.J.; Lamkanfi, M.; Webby, R.J.; Boyd, K.L.; et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009, 30, 566–575. [Google Scholar] [CrossRef]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P.-Y. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschläger, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Body-Malapel, M.; Amer, A.; Park, J.-H.; Whitfield, J.; Franchi, L.; Taraporewala, Z.F.; Miller, D.; Patton, J.T.; Inohara, N.; et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006, 281, 36560–36568. [Google Scholar] [CrossRef]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Chen, M.; Chen, J.; Chen, J. Safflower extract improves depression in mice by inhibiting the TLR4-NLRP3 inflammation signaling pathway. Ann. Palliat. Med. 2021, 10, 8015023–8018023. [Google Scholar] [CrossRef]
- Sahin, C.; Albayrak, O.; Akdeniz, T.F.; Akbulut, Z.; Yanikkaya Demirel, G.; Aricioglu, F. Agmatine Reverses Sub-chronic Stress induced Nod-like Receptor Protein 3 (NLRP3) Activation and Cytokine Response in Rats. Basic Clin. Pharmacol. Toxicol. 2016, 119, 367–375. [Google Scholar] [CrossRef]
- Pandey, G.N.; Zhang, H.; Sharma, A.; Ren, X. Innate immunity receptors in depression and suicide: Upregulated NOD-like receptors containing pyrin (NLRPs) and hyperactive inflammasomes in the postmortem brains of people who were depressed and died by suicide. J. Psychiatry Neurosci. 2021, 46, E538–E547. [Google Scholar] [CrossRef] [PubMed]
- Taene, A.; Khalili-Tanha, G.; Esmaeili, A.; Mobasheri, L.; Kooshkaki, O.; Jafari, S.; Shokouhifar, A.; Sarab, G.A. The Association of Major Depressive Disorder with Activation of NLRP3 Inflammasome, Lipid Peroxidation, and Total Antioxidant Capacity. J. Mol. Neurosci. 2020, 70, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain. Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Chamera, K.; Bryniarska, N.; Kotarska, K.; Leśkiewicz, M.; Regulska, M.; Basta-Kaim, A. Role of Chronic Administration of Antidepressant Drugs in the Prenatal Stress-Evoked Inflammatory Response in the Brain of Adult Offspring Rats: Involvement of the NLRP3 Inflammasome-Related Pathway. Mol. Neurobiol. 2019, 56, 5365–5380. [Google Scholar] [CrossRef]
- Li, J.-M.; Liu, L.-L.; Su, W.-J.; Wang, B.; Zhang, T.; Zhang, Y.; Jiang, C.-L. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology 2019, 146, 149–153. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Cao, Q.; Liu, C. Targeting NLRP3 Inflammasome in the Treatment of CNS Diseases. Front. Mol. Neurosci. 2018, 11, 320. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry Rev. Can. Psychiatr. 2016, 61, 540–560. [Google Scholar] [CrossRef]
- Stokes, P.E. Ten years of fluoxetine. Depress. Anxiety 1998, 8 (Suppl. 1), 1–4. [Google Scholar] [CrossRef]
- Stokes, P.E.; Holtz, A. Fluoxetine tenth anniversary update: The progress continues. Clin. Ther. 1997, 19, 1135–1250. [Google Scholar] [CrossRef]
- Tranter, R.; O’Donovan, C.; Chandarana, P.; Kennedy, S. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci. JPN 2002, 27, 241–247. [Google Scholar]
- Mulrow, C.D.; Williams, J.W.; Chiquette, E.; Aguilar, C.; Hitchcock-Noel, P.; Lee, S.; Cornell, J.; Stamm, K. Efficacy of newer medications for treating depression in primary care patients. Am. J. Med. 2000, 108, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kirino, E. Escitalopram for the management of major depressive disorder: A review of its efficacy, safety, and patient acceptability. Patient Prefer. Adherence 2012, 6, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Ferla, T.L.; Furukawa, T.A.; Signoretti, A.; Nakagawa, A.; Churchill, R.; McGuire, H.; Barbui, C. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009, CD006117. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Geddes, J.R.; Malvini, L.; Signoretti, A.; McGuire, H.; Churchill, R.; Nakagawa, A.; Barbui, C. MANGA Study Group Does randomized evidence support sertraline as first-line antidepressant for adults with acute major depression? A systematic review and meta-analysis. J. Clin. Psychiatry 2008, 69, 1732–1742. [Google Scholar] [CrossRef]
- Gunasekara, N.S.; Noble, S.; Benfield, P. Paroxetine. An update of its pharmacology and therapeutic use in depression and a review of its use in other disorders. Drugs 1998, 55, 85–120. [Google Scholar] [CrossRef]
- Sáiz-Ruiz, J.; Ibáñez, A.; Diaz-Marsá, M.; Arias, F.; Padin, J.; Martin-Carrasco, M.; Montes, J.M.; Ferrando, L.; Carrasco, J.L.; Martin-Ballesteros, E.; et al. Efficacy of venlafaxine in major depression resistant to selective serotonin reuptake inhibitors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 1129–1134. [Google Scholar] [CrossRef]
- Sanders, B.; Brula, A.Q. Intranasal esketamine: From origins to future implications in treatment-resistant depression. J. Psychiatr. Res. 2021, 137, 29–35. [Google Scholar] [CrossRef]
- Chen-Li, D.; Lui, L.M.W.; Rosenblat, J.D.; Lipsitz, O.; Teopiz, K.M.; Ho, R.; Vinberg, M.; Golts, M.; Jawad, M.Y.; Lee, Y.; et al. Ketamine as potential treatment for postpartum depression: A narrative review. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2022, 34, 264–274. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Dywel, A.; Cubała, W.J. Safety and Tolerability of the Acute Ketamine Treatment in Treatment-Resistant Depression: Focus on Comorbidities Interplay with Dissociation and Psychomimetic Symptoms. Pharmaceuticals 2023, 16, 173. [Google Scholar] [CrossRef]
- Vasiliu, O. Esketamine for treatment-resistant depression: A review of clinical evidence (Review). Exp. Ther. Med. 2023, 25, 111. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Jin, B.; Hou, N.; Yang, H. Safety and efficacy of low-dose esketamine in laparoscopic cholecystectomy: A prospective, double-blind randomized controlled trial. BMC Anesthesiol. 2024, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
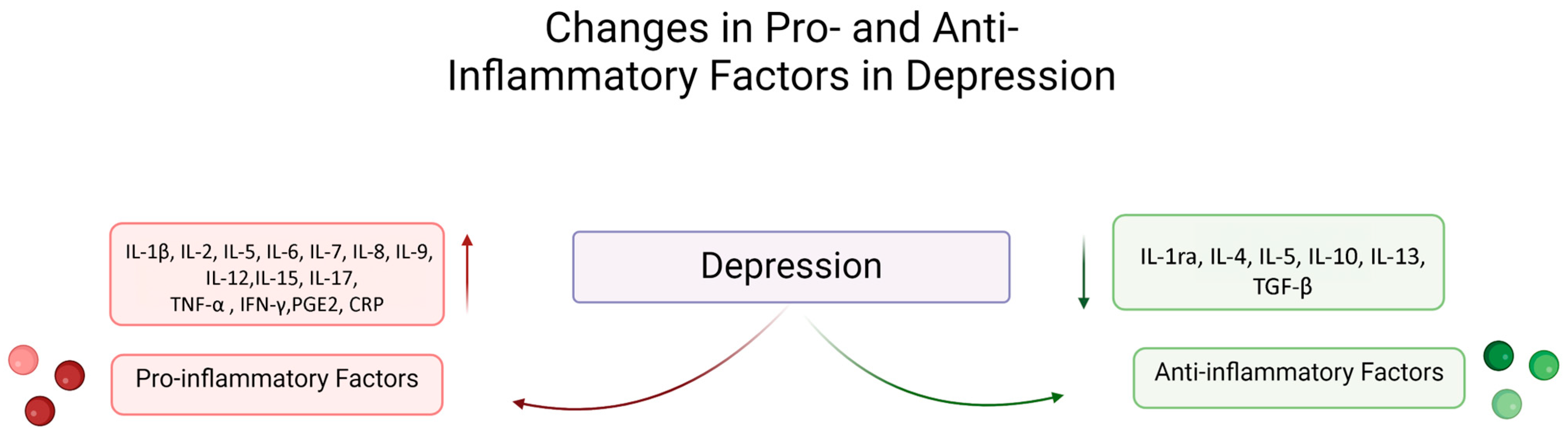
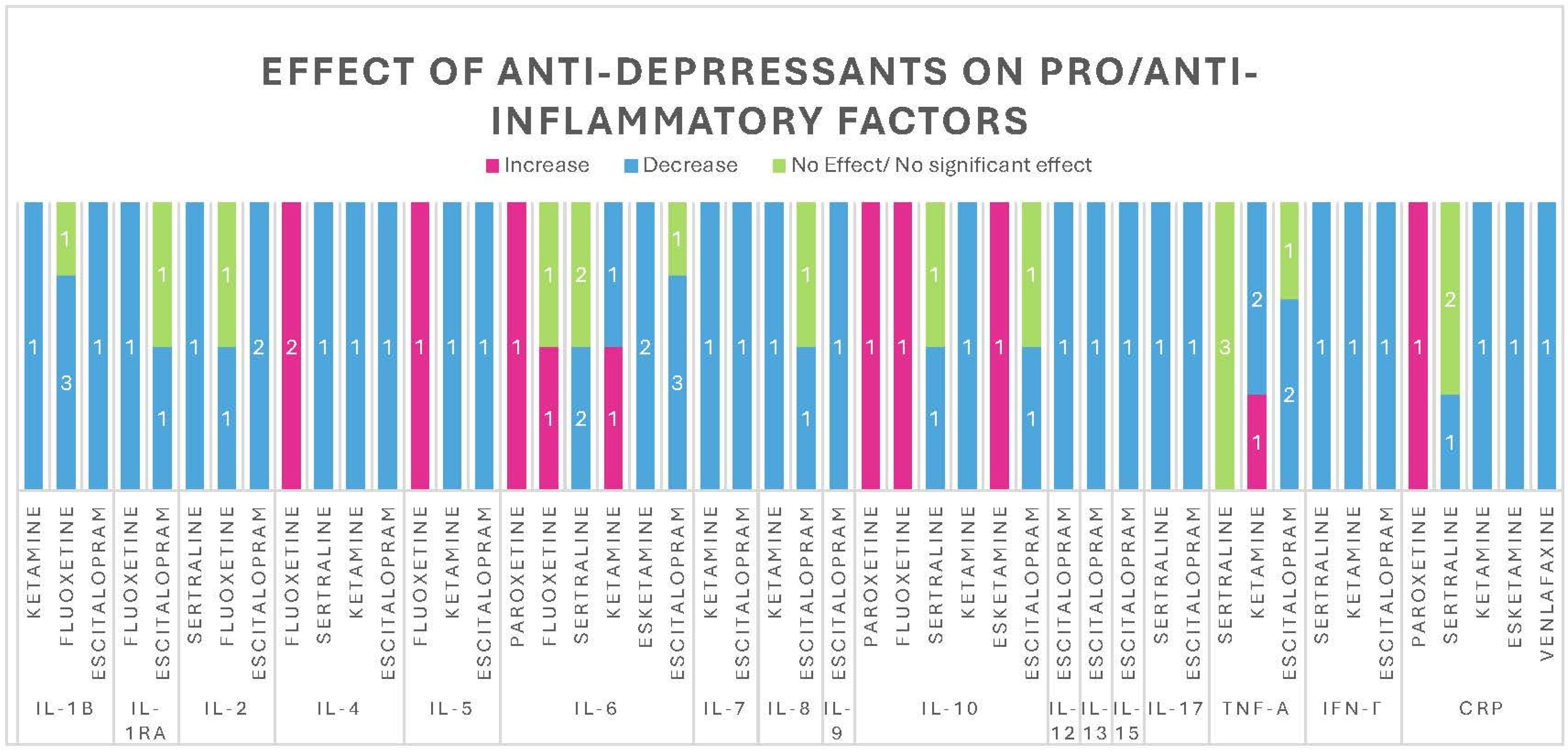
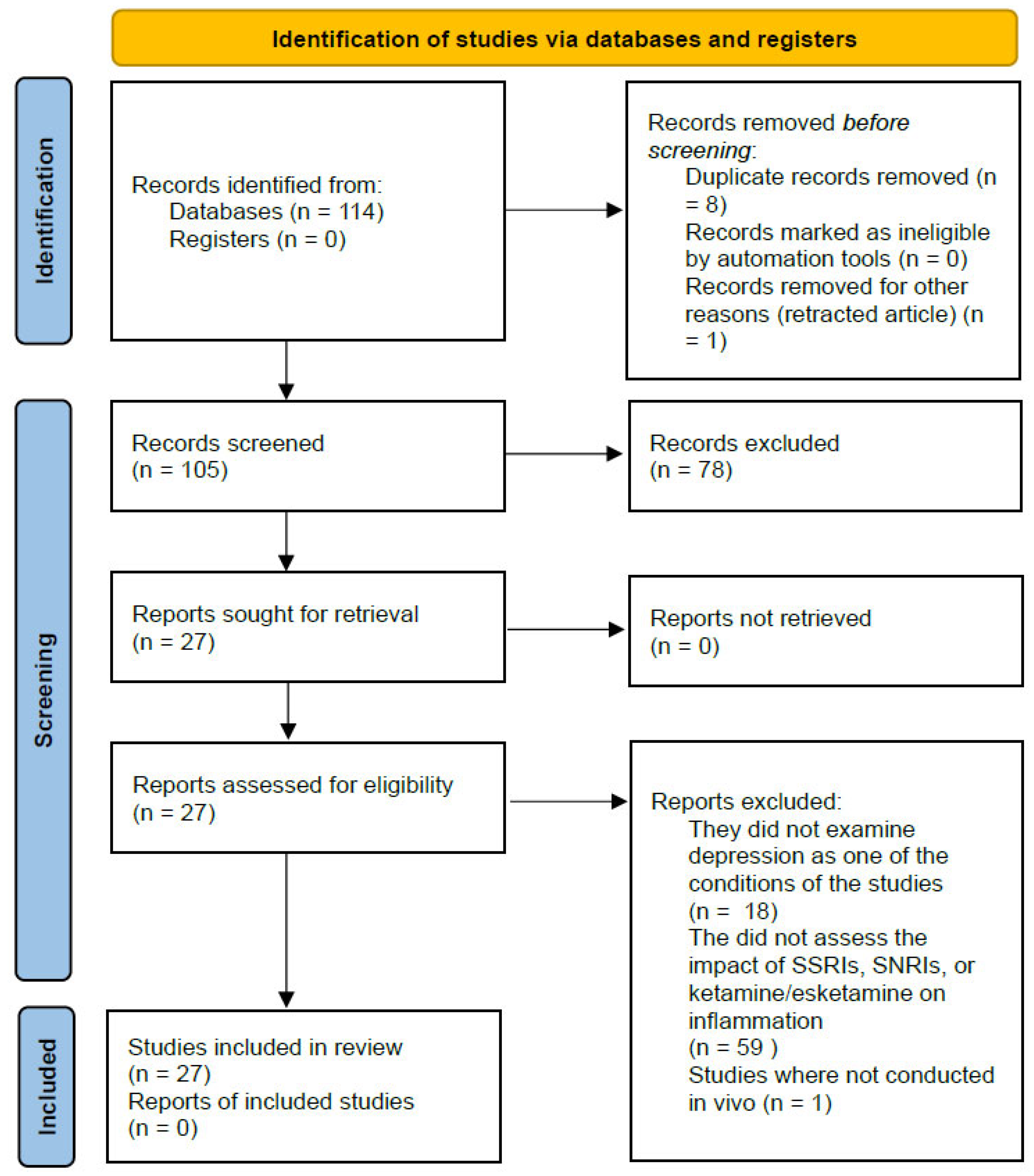
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleibel, L.; Sokołowska, P.; Henrykowska, G.; Owczarek, J.; Wiktorowska-Owczarek, A. Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade. Pharmaceuticals 2025, 18, 867. https://doi.org/10.3390/ph18060867
Bleibel L, Sokołowska P, Henrykowska G, Owczarek J, Wiktorowska-Owczarek A. Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade. Pharmaceuticals. 2025; 18(6):867. https://doi.org/10.3390/ph18060867
Chicago/Turabian StyleBleibel, Layla, Paulina Sokołowska, Gabriela Henrykowska, Jacek Owczarek, and Anna Wiktorowska-Owczarek. 2025. "Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade" Pharmaceuticals 18, no. 6: 867. https://doi.org/10.3390/ph18060867
APA StyleBleibel, L., Sokołowska, P., Henrykowska, G., Owczarek, J., & Wiktorowska-Owczarek, A. (2025). Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade. Pharmaceuticals, 18(6), 867. https://doi.org/10.3390/ph18060867







