Epigenetic Regulation and Therapeutic Targeting of Alternative Splicing Dysregulation in Cancer
Abstract
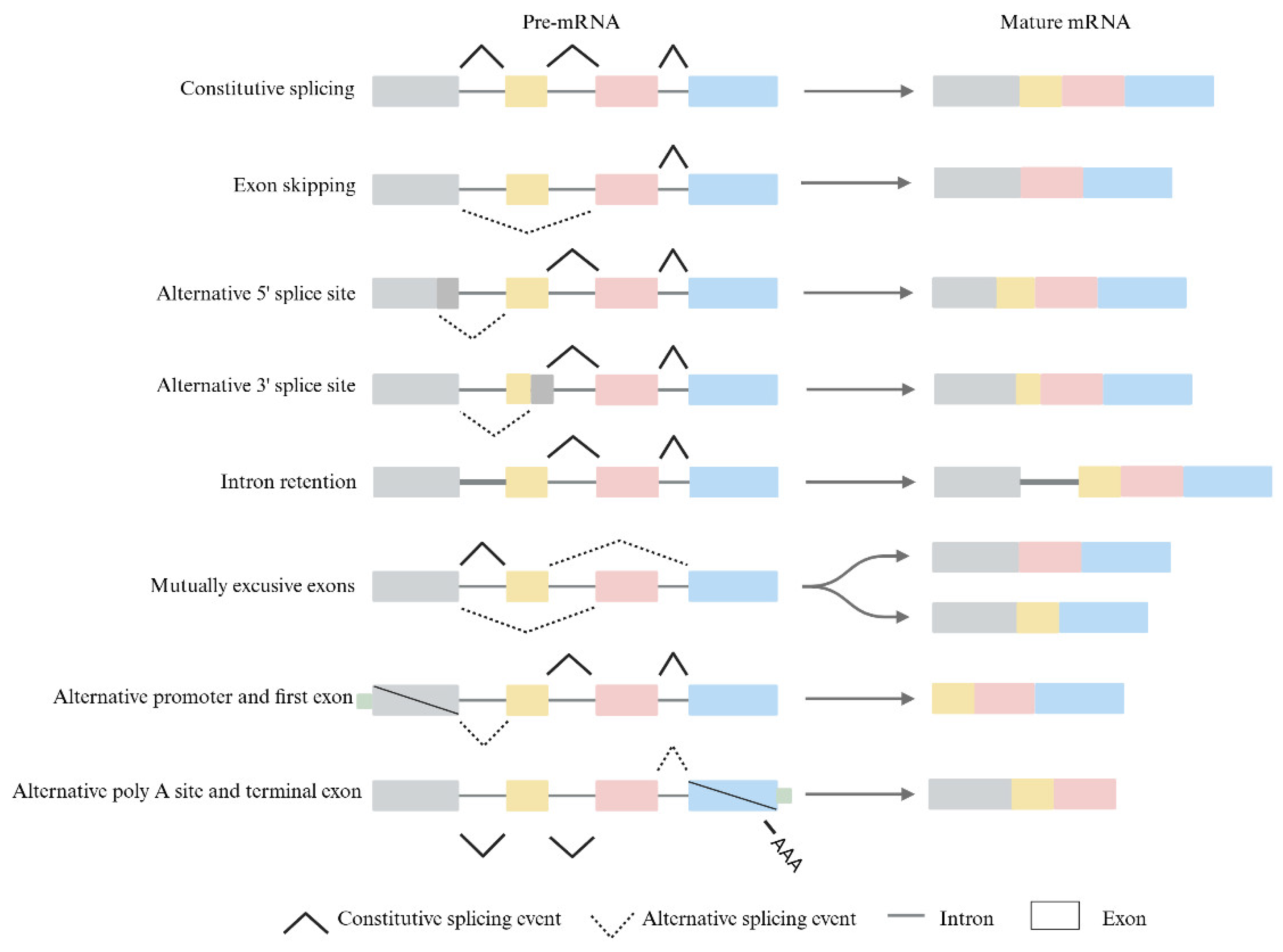
1. Introduction
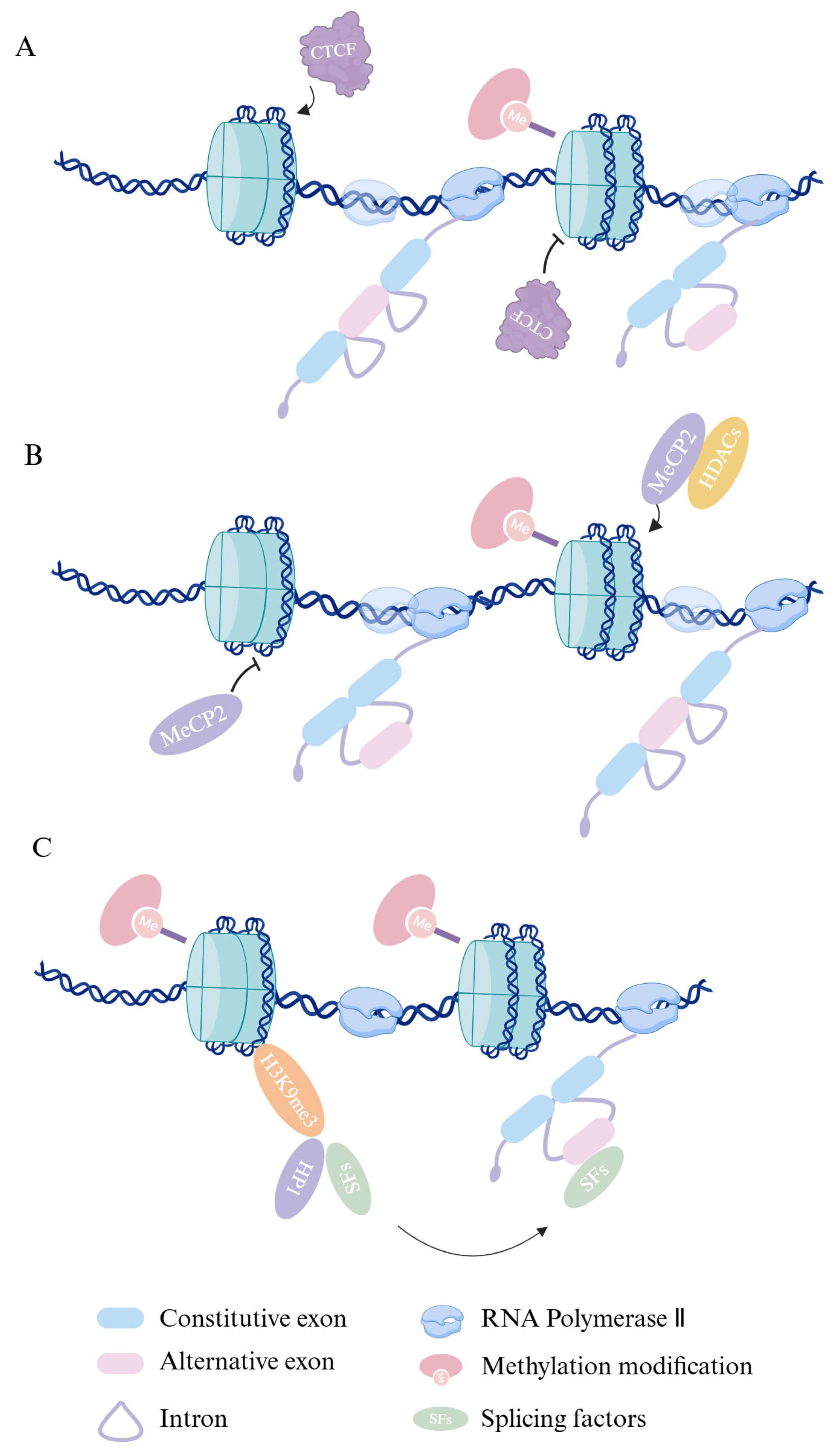
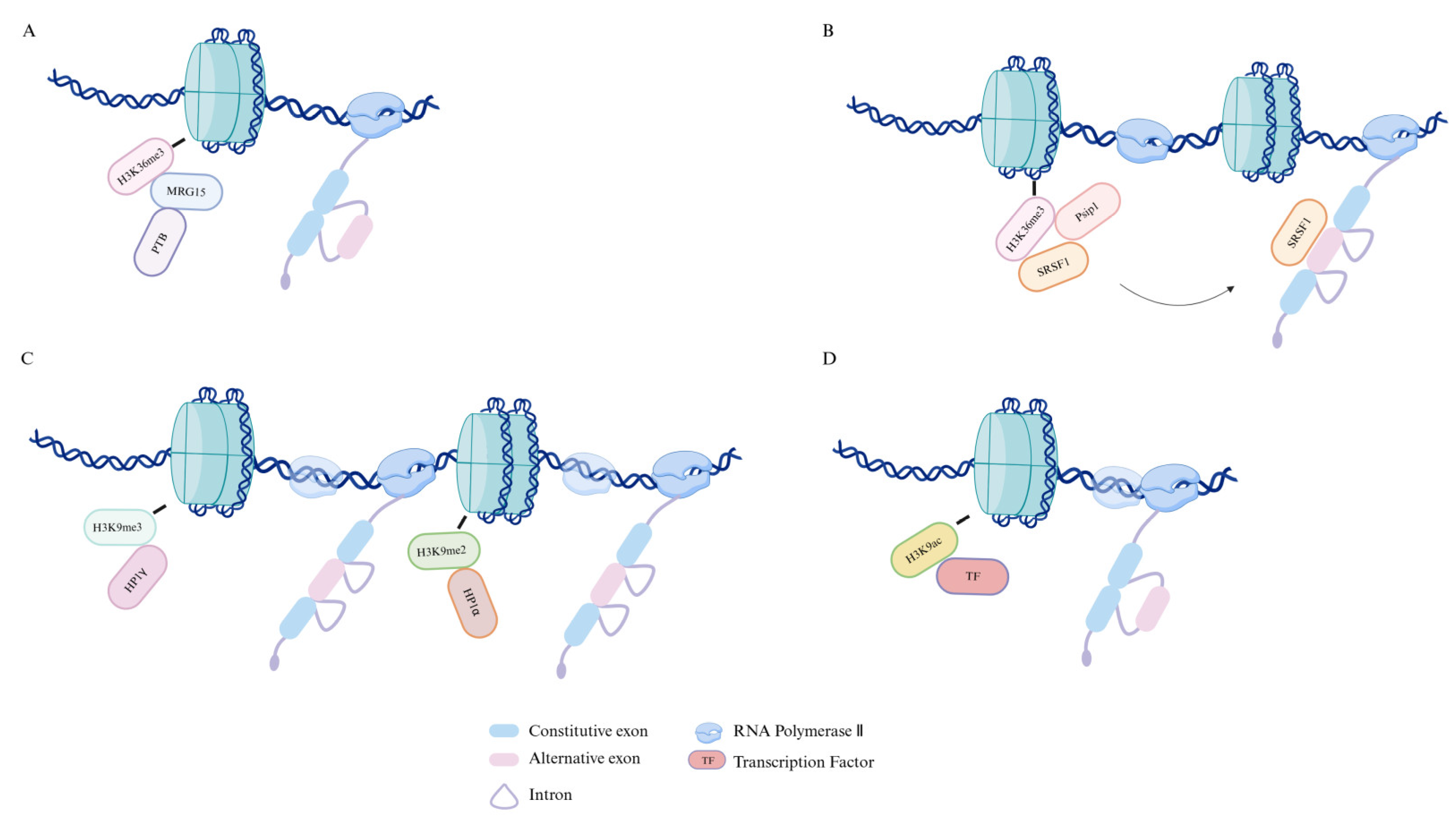
2. How Epigenetics Affects Alternative Splicing
2.1. Dive into DNA Methylation’s Role in Alternative Splicing
2.2. Histone Modifications and Their Impact on Alternative Splicing
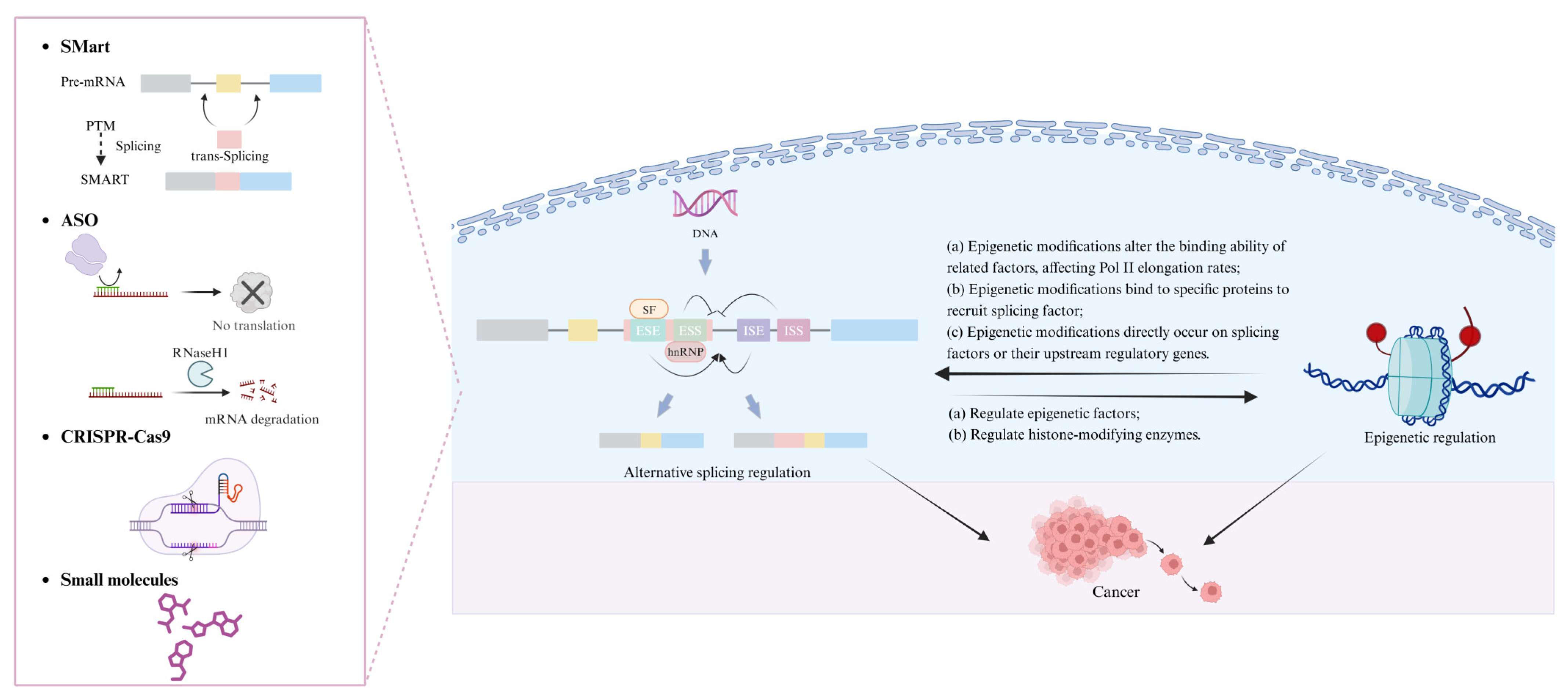
3. Interplay Between Alternative Splicing and Epigenetics
4. Epigenetic Regulation’s Influence on Alternative Splicing in Cancer
5. Therapeutic Approaches Targeting Epigenetics and Alternative Splicing
5.1. Histone Deacetylase Inhibitors (HDACis)
5.2. DNA Methyltransferase Inhibitors (DNMTis)
5.3. Bromodomain and Extra-Terminal Domain Inhibitors (BETis)
6. Exploring Strategies for Targeting Alternative Splicing
6.1. Spliceosome-Mediated RNA Trans-Splicing
6.2. Antisense Oligonucleotides
6.3. CRISPR–Cas9: Advancements in Genome Editing
6.4. Small Molecules
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMaRT | spliceosome-mediated mRNA trans-splicing |
| ASO | antisense oligonucleotides |
| ncRNAs | non-coding RNAs |
| CTCF | CCCTC-binding factor |
| MeCP2 | methyl-CpG-binding protein 2 |
| HP1 | heterochromatin protein 1 |
| MRG15 | MORF-related gene 15 |
| PTB | polypyrimidine tract-binding |
| TSA | trichostatin A |
| HDAC | histone deacetylase |
| VPA | valproic acid |
| AML | acute myeloid leukemia |
| DNMTs | DNA methyltransferases |
| DNMTis | DNMT inhibitors |
| EGCG | epigallocatechin gallate |
| BET | bromodomain and extraterminal |
| CLL | chronic lymphocytic leukemia |
| ASIs | androgen signaling inhibitors |
| ENZ | enzalutamide |
| MCRPC | metastatic castration-resistant prostate carcinoma |
| PTM | pre-mRNA trans-splicing molecule |
| SMN1 | survival motor neuron 1 |
| hATTR-PN | hereditary transthyretin-mediated amyloidosis |
| ALS | amyotrophic lateral sclerosis |
| SOD1 | superoxide dismutase 1 |
| ADRs | adverse drug reactions |
| ZFNs | zinc finger nucleases |
| TALENs | transcription activator-like effector nucleases |
| DSBs | DNA double-strand breaks |
| NHEJ | non-homologous end joining |
| HDR | homology-directed repair |
| iPSCs | induced pluripotent stem cells |
| SVA | SINE-VNTR-Alu |
| CTGexp | CTG repeat expansion |
| DM1 | myotonic dystrophy type 1 |
| CLK | CDC-like kinase |
| SRSFs | serine and arginine-rich splicing factors |
References
- Gilbert, W. Why genes in pieces? Nature 1978, 271, 501. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Verta, J.-P.; Jacobs, A. The role of alternative splicing in adaptation and evolution. Trends Ecol. Evol. 2022, 37, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, B.J. Alternative Splicing: New Insights from Global Analyses. Cell 2006, 126, 37–47. [Google Scholar] [CrossRef]
- Dong, X.; Chen, R. Understanding aberrant RNA splicing to facilitate cancer diagnosis and therapy. Oncogene 2020, 39, 2231–2242. [Google Scholar] [CrossRef]
- Climente-González, H.; Porta-Pardo, E.; Godzik, A.; Eyras, E. The Functional Impact of Alternative Splicing in Cancer. Cell Rep. 2017, 20, 2215–2226. [Google Scholar] [CrossRef]
- Qi, F.; Li, Y.; Yang, X.; Wu, Y.-P.; Lin, L.-J.; Liu, X.-M. Significance of alternative splicing in cancer cells. Chin. Med. J. 2020, 133, 221–228. [Google Scholar] [CrossRef]
- Beyer, A.L.; Osheim, Y.N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988, 2, 754–765. [Google Scholar] [CrossRef]
- Iannone, C.; Valcárcel, J. Chromatin’s thread to alternative splicing regulation. Chromosoma 2013, 122, 465–474. [Google Scholar] [CrossRef]
- Perales, R.; Bentley, D. “Cotranscriptionality”: The Transcription Elongation Complex as a Nexus for Nuclear Transactions. Mol. Cell 2009, 36, 178–191. [Google Scholar] [CrossRef]
- Tardiff, D.F.; Lacadie, S.A.; Rosbash, M. A Genome-Wide Analysis Indicates that Yeast Pre-mRNA Splicing Is Predominantly Posttranscriptional. Mol. Cell 2006, 24, 917–929. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, S.F.; Carmo-Fonseca, M. Design principles of interconnections between chromatin and pre-mRNA splicing. Trends Biochem. Sci. 2012, 37, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Sciarrillo, R.; Wojtuszkiewicz, A.; Assaraf, Y.G.; Jansen, G.; Kaspers, G.J.L.; Giovannetti, E.; Cloos, J. The role of alternative splicing in cancer: From oncogenesis to drug resistance. Drug Resist. Updat. 2020, 53, 100728. [Google Scholar] [CrossRef]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics: Figure 1. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef]
- Rodríguez-Paredes, M.; Esteller, M. Cancer epigenetics reaches mainstream oncology. Nat. Med. 2011, 17, 330–339. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Zhu, Y.-R.; Dai, D.-J.; Wang, X.; Jin, H.-C. Epigenetic regulation of alternative splicing. Am. J. Cancer Res. 2018, 8, 2346. [Google Scholar]
- Maor, G.L.; Yearim, A.; Ast, G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015, 31, 274–280. [Google Scholar] [CrossRef]
- Shukla, S.; Kavak, E.; Gregory, M.; Imashimizu, M.; Shutinoski, B.; Kashlev, M.; Oberdoerffer, P.; Sandberg, R.; Oberdoerffer, S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 2011, 479, 74–79. [Google Scholar] [CrossRef]
- Maunakea, A.K.; Chepelev, I.; Cui, K.; Zhao, K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013, 23, 1256–1269. [Google Scholar] [CrossRef]
- Young, J.I.; Hong, E.P.; Castle, J.C.; Crespo-Barreto, J.; Bowman, A.B.; Rose, M.F.; Kang, D.; Richman, R.; Johnson, J.M.; Berget, S.; et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl. Acad. Sci. USA 2005, 102, 17551–17558. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, L.; Park, J.W.; Lv, R.; Chen, H.; Jiao, F.; Xu, W.; Mu, S.; Wen, H.; Qiu, J.; et al. BS69/ZMYND11 Reads and Connects Histone H3.3 Lysine 36 Trimethylation-Decorated Chromatin to Regulated Pre-mRNA Processing. Mol. Cell 2014, 56, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Saint-André, V.; Batsché, E.; Rachez, C. Muchardt, Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat. Struct. Mol. Biol. 2011, 18, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Luco, R.F.; Pan, Q.; Tominaga, K.; Blencowe, B.J.; Pereira-Smith, O.M.; Misteli, T. Regulation of Alternative Splicing by Histone Modifications. Science 2010, 327, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Enroth, S.; Rada-Iglesias, A.; Wadelius, C.; Komorowski, J. Nucleosomes are well positioned in exons and carry characteristic histone modifications. Genome Res. 2009, 19, 1732–1741. [Google Scholar] [CrossRef]
- Schwartz, S.; Meshorer, E.; Ast, G. Chromatin organization marks exon-intron structure. Nat. Struct. Mol. Biol. 2009, 16, 990–995. [Google Scholar] [CrossRef]
- Wilhelm, B.T.; Marguerat, S.; Aligianni, S.; Codlin, S.; Watt, S.; Bähler, J. Differential patterns of intronic and exonic DNA regions with respect to RNA polymerase II occupancy, nucleosome density and H3K36me3 marking in fission yeast. Genome Biol. 2011, 12, R82. [Google Scholar] [CrossRef]
- Pradeepa, M.M.; Sutherland, H.G.; Ule, J.; Grimes, G.R.; Bickmore, W.A. Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing. PLoS Genet. 2012, 8, e1002717. [Google Scholar] [CrossRef]
- Alló, M.; Buggiano, V.; Fededa, J.P.; Petrillo, E.; Schor, I.; De La Mata, M.; Agirre, E.; Plass, M.; Eyras, E.; Elela, S.A.; et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat. Struct. Mol. Biol. 2009, 16, 717–724. [Google Scholar] [CrossRef]
- Schor, I.E.; Rascovan, N.; Pelisch, F.; Alló, M.; Kornblihtt, A.R. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc. Natl. Acad. Sci. USA 2009, 106, 4325–4330. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Hinman, M.N.; Barron, V.A.; Geng, C.; Zhou, G.; Luo, G.; Siegel, R.E.; Lou, H. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc. Natl. Acad. Sci. USA 2011, 108, E627–E635. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Fong, N.; Erickson, B.; Bentley, D.L. Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13564–13569. [Google Scholar] [CrossRef] [PubMed]
- Iannone, C.; Kainov, Y.; Zhuravskaya, A.; Hamid, F.; Nojima, T.; Makeyev, E.V. PTBP1-activated co-transcriptional splicing controls epigenetic status of pluripotent stem cells. Mol. Cell 2023, 83, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Zardo, G. The Role of H3K4 Trimethylation in CpG Islands Hypermethylation in Cancer. Biomolecules 2021, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Veenstra, G.J.C.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Chen, R.; Wang, L.; Li, B.; Cheng, H.; Duan, X.; Zhu, H.; Wei, W.; Li, J.; et al. Dissecting the precise role of H3K9 methylation in crosstalk with DNA maintenance methylation in mammals. Nat. Commun. 2016, 7, 12464. [Google Scholar] [CrossRef]
- Bannister, A.J.; Zegerman, P.; Partridge, J.F.; Miska, E.A.; Thomas, J.O.; Allshire, R.C.; Kouzarides, T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 2001, 410, 120–124. [Google Scholar] [CrossRef]
- Lachner, M.; O’Carroll, D.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef]
- Nakayama, J.; Rice, J.C.; Strahl, B.D.; Allis, C.D.; Grewal, S.I.S. Role of Histone H3 Lysine 9 Methylation in Epigenetic Control of Heterochromatin Assembly. Science 2001, 292, 110–113. [Google Scholar] [CrossRef]
- Lehnertz, B.; Ueda, Y.; Derijck, A.A.H.A.; Braunschweig, U.; Perez-Burgos, L.; Kubicek, S.; Chen, T.; Li, E.; Jenuwein, T.; Peters, A.H.F.M. Suv39h-Mediated Histone H3 Lysine 9 Methylation Directs DNA Methylation to Major Satellite Repeats at Pericentric Heterochromatin. Curr. Biol. 2003, 13, 1192–1200. [Google Scholar] [CrossRef]
- Jyotsana, N.; Heuser, M. Exploiting differential RNA splicing patterns: A potential new group of therapeutic targets in cancer. Expert Opin. Ther. Targets 2018, 22, 107–121. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.D.; Loukola, A.; Aaltonen, L.A.; Mortensen, N.J.M.; Bodmer, W.F. The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. J. Med. Genet. 2000, 37, 588. [Google Scholar] [CrossRef] [PubMed]
- Parreno, V.; Loubiere, V.; Schuettengruber, B.; Fritsch, L.; Rawal, C.C.; Erokhin, M.; Győrffy, B.; Normanno, D.; Di Stefano, M.; Moreaux, J.; et al. Transient loss of Polycomb components induces an epigenetic cancer fate. Nature 2024, 629, 688–696. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Shen, S.; Wang, Y.; Wang, C.; Wu, Y.N.; Xing, Y. SURVIV for survival analysis of mRNA isoform variation. Nat. Commun. 2016, 7, 11548. [Google Scholar] [CrossRef]
- Tomas, F. Interaction of p53 with the Δ133p53α and Δ160p53α isoforms regulates p53 conformation and transcriptional activity. Cell Death Dis. 2024, 15, 845. [Google Scholar] [CrossRef]
- Shirai, C.L.; White, B.S.; Tripathi, M.; Tapia, R.; Ley, J.N.; Ndonwi, M.; Kim, S.; Shao, J.; Carver, A.; Saez, B.; et al. Mutant U2AF1-expressing cells are sensitive to pharmacological modulation of the spliceosome. Nat. Commun. 2017, 8, 14060. [Google Scholar] [CrossRef]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef]
- Lee, S.C.-W.; Abdel-Wahab, O. Therapeutic targeting of splicing in cancer. Nat. Med. 2016, 22, 976–986. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Paik, P.K.; Drilon, A.; Fan, P.-D.; Yu, H.; Rekhtman, N.; Ginsberg, M.S.; Borsu, L.; Schultz, N.; Berger, M.F.; Rudin, C.M.; et al. Response to MET Inhibitors in Patients with Stage IV Lung Adenocarcinomas Harboring MET Mutations Causing Exon 14 Skipping. Cancer Discov. 2015, 5, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.K.; Thoene, S.; Bietenbeck, A.; Nawroth, R.; Tauber, R.; Thalgott, M.; Schmid, S.; Secci, R.; Retz, M.; Gschwend, J.E.; et al. AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide. Eur. Urol. 2017, 72, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Huang, S.; He, X. Genome-wide analysis reveals that exon methylation facilitates its selective usage in the human transcriptome. Brief. Bioinform. 2018, 19, 754–764. [Google Scholar] [CrossRef]
- Guo, T.; Zambo, K.D.A.; Zamuner, F.T.; Ou, T.; Hopkins, C.; Kelley, D.Z.; Wulf, H.A.; Winkler, E.; Erbe, R.; Danilova, L.; et al. Chromatin structure regulates cancer-specific alternative splicing events in primary HPV-related oropharyngeal squamous cell carcinoma. Epigenetics 2020, 15, 959–971. [Google Scholar] [CrossRef]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone Deacetylase Is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]
- Buggy, J.J.; Cao, Z.A.; Bass, K.E.; Verner, E.; Balasubramanian, S.; Liu, L.; Schultz, B.E.; Young, P.R.; Dalrymple, S.A. CRA-024781: A novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol. Cancer Ther. 2006, 5, 1309–1317. [Google Scholar] [CrossRef]
- Lai, C.-J.; Bao, R.; Tao, X.; Wang, J.; Atoyan, R.; Qu, H.; Wang, D.-G.; Yin, L.; Samson, M.; Forrester, J.; et al. CUDC-101, a Multitargeted Inhibitor of Histone Deacetylase, Epidermal Growth Factor Receptor, and Human Epidermal Growth Factor Receptor 2, Exerts Potent Anticancer Activity. Cancer Res. 2010, 70, 3647–3656. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Abaza, Y.; Takahashi, K.; Medeiros, B.C.; Arellano, M.; Khaled, S.K.; Patnaik, M.; Odenike, O.; Sayar, H.; Tummala, M.; et al. Pracinostat plus azacitidine in older patients with newly diagnosed acute myeloid leukemia: Results of a phase 2 study. Blood Adv. 2019, 3, 508–518. [Google Scholar] [CrossRef]
- Leoni, F.; Fossati, G.; Lewis, E.C.; Lee, J.-K.; Porro, G.; Pagani, P.; Modena, D.; Moras, M.L.; Pozzi, P.; Reznikov, L.L.; et al. The Histone Deacetylase Inhibitor ITF2357 Reduces Production of Pro-Inflammatory Cytokines In Vitro and Systemic Inflammation In Vivo. Mol. Med. 2005, 11, 1–15. [Google Scholar] [CrossRef]
- Li, S.; Fossati, G.; Marchetti, C.; Modena, D.; Pozzi, P.; Reznikov, L.L.; Moras, M.L.; Azam, T.; Abbate, A.; Mascagni, P.; et al. Specific Inhibition of Histone Deacetylase 8 Reduces Gene Expression and Production of Proinflammatory Cytokines in Vitro and in Vivo. J. Biol. Chem. 2015, 290, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-G. Givinostat inhibition of hepatic stellate cell proliferation and protein acetylation. World J. Gastroenterol. 2015, 21, 8326. [Google Scholar] [CrossRef] [PubMed]
- Batlevi, C.L.; Crump, M.; Andreadis, C.; Rizzieri, D.; Assouline, S.E.; Fox, S.; Van Der Jagt, R.H.C.; Copeland, A.; Potvin, D.; Chao, R.; et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br. J. Haematol. 2017, 178, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Boumber, Y.; Younes, A.; Garcia-Manero, G. Mocetinostat (MGCD0103): A review of an isotype-specific histone deacetylase inhibitor. Expert Opin. Investig. Drugs 2011, 20, 823–829. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Podoltsev, N.A.; Othus, M.; Pagel, J.M.; Radich, J.P.; Fang, M.; Rizzieri, D.A.; Marcucci, G.; Strickland, S.A.; Litzow, M.R.; et al. A randomized phase III study of standard versus high-dose cytarabine with or without vorinostat for AML. Leukemia 2024, 38, 58–66. [Google Scholar] [CrossRef]
- Bertino, E.M.; Otterson, G.A. Romidepsin: A novel histone deacetylase inhibitor for cancer. Expert Opin. Investig. Drugs 2011, 20, 1151–1158. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.; Wang, L.; Pei, X.; Kramer, L.B.; Dent, P.; Grant, S. Bortezomib interacts synergistically with belinostat in human acute myeloid leukaemia and acute lymphoblastic leukaemia cells in association with perturbations in NF-κB and Bim. Br. J. Haematol. 2011, 153, 222–235. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, S.; Wang, D.; Edwards, H.; Thibodeau, J.; Kim, S.; Stemmer, P.; Wang, G.; Jin, J.; Savasan, S.; et al. Panobinostat sensitizes AraC-resistant AML cells to the combination of azacitidine and venetoclax. Biochem. Pharmacol. 2024, 228, 116065. [Google Scholar] [CrossRef]
- Gu, S.; Hou, Y.; Dovat, K.; Dovat, S.; Song, C.; Ge, Z. Synergistic effect of HDAC inhibitor Chidamide with Cladribine on cell cycle arrest and apoptosis by targeting HDAC2/c-Myc/RCC1 axis in acute myeloid leukemia. Exp. Hematol. Oncol. 2023, 12, 23. [Google Scholar] [CrossRef]
- Raffoux, E.; Cras, A.; Recher, C.; Boëlle, P.-Y.; De Labarthe, A.; Turlure, P.; Marolleau, J.-P.; Reman, O.; Gardin, C.; Victor, M.; et al. Phase 2 clinical trial of 5-azacitidine, valproic acid, and all-trans retinoic acid in patients with high-risk acute myeloid leukemia or myelodysplastic syndrome. Oncotarget 2010, 1, 34–42. [Google Scholar] [CrossRef]
- Galloway, T.J.; Wirth, L.J.; Colevas, A.D.; Gilbert, J.; Bauman, J.E.; Saba, N.F.; Raben, D.; Mehra, R.; Ma, A.W.; Atoyan, R.; et al. A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized Controlled Trial of Azacitidine in Patients With the Myelodysplastic Syndrome: A Study of the Cancer and Leukemia Group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.-P.J.; Kantarjian, H.M.; Kirkpatrick, P. Azacitidine. Nat. Rev. Drug Discov. 2005, 4, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Daher-Reyes, G.S.; Merchan, B.M.; Yee, K.W.L. Guadecitabine (SGI-110): An investigational drug for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Expert Opin. Investig. Drugs 2019, 28, 835–849. [Google Scholar] [CrossRef]
- Fahy, J.; Jeltsch, A.; Arimondo, P.B. DNA methyltransferase inhibitors in cancer: A chemical and therapeutic patent overview and selected clinical studies. Expert Opin. Ther. Pat. 2012, 22, 1427–1442. [Google Scholar] [CrossRef]
- Candelaria, M.; Gallardo-Rincón, D.; Arce, C.; Cetina, L.; Aguilar-Ponce, J.L.; Arrieta, Ó.; González-Fierro, A.; Chávez-Blanco, A.; De La Cruz-Hernández, E.; Camargo, M.F.; et al. A phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Ann. Oncol. 2007, 18, 1529–1538. [Google Scholar] [CrossRef]
- Plummer, R.; Vidal, L.; Griffin, M.; Lesley, M.; De Bono, J.; Coulthard, S.; Sludden, J.; Siu, L.L.; Chen, E.X.; Oza, A.M.; et al. Phase I Study of MG98, an Oligonucleotide Antisense Inhibitor of Human DNA Methyltransferase 1, Given as a 7-Day Infusion in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2009, 15, 3177–3183. [Google Scholar] [CrossRef]
- Shkreta, L.; Froehlich, U.; Toutant, J.; Elela, S.A.; Chabot, B. Anticancer drugs affect the alternative splicing of Bcl-x and other human apoptotic genes. Mol. Cancer Ther. 2008, 7, 1398–1409. [Google Scholar] [CrossRef]
- Chuang, J.C.; Warner, S.L.; Vollmer, D.; Vankayalapati, H.; Redkar, S.; Bearss, D.J.; Qiu, X.; Yoo, C.B.; Jones, P.A. S110, a 5-Aza-2′-Deoxycytidine–Containing Dinucleotide, Is an Effective DNA Methylation Inhibitor In vivo and Can Reduce Tumor Growth. Mol. Cancer Ther. 2010, 9, 1443–1450. [Google Scholar] [CrossRef]
- Vachhani, P.; Murthy, G.S.G.; Jamy, O.; Bachiashvili, K.; Rangaraju, S.; Cole, T.; Augelli-Szafran, C.E.; Boohaker, R.J.; Moukha-Chafiq, O.; Hanks, L.J.; et al. A phase 1 study of NTX-301, an oral DNMT1 inhibitor, in patients with MDS and AML (trial in progress). J. Clin. Oncol. 2022, 40, TPS7077. [Google Scholar] [CrossRef]
- Choi, W.J.; Chung, H.-J.; Chandra, G.; Alexander, V.; Zhao, L.X.; Lee, H.W.; Nayak, A.; Majik, M.S.; Kim, H.O.; Kim, J.-H.; et al. Fluorocyclopentenyl-cytosine with Broad Spectrum and Potent Antitumor Activity. J. Med. Chem. 2012, 55, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (−)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Yoo, C.B.; Kwan, J.M.; Li, T.W.H.; Liang, G.; Yang, A.S.; Jones, P.A. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2′-deoxycytidine. Mol. Cancer Ther. 2005, 4, 1515–1520. [Google Scholar] [CrossRef]
- Arce, C.; Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Candelaria, M.; Dueñnas-Gonzalez, A. Hydralazine target: From blood vessels to the epigenome. J. Transl. Med. 2006, 4, 10. [Google Scholar] [CrossRef]
- Yang, X.; Lay, F.; Han, H.; Jones, P.A. Targeting DNA methylation for epigenetic therapy. Trends Pharmacol. Sci. 2010, 31, 536–546. [Google Scholar] [CrossRef]
- Wang, N.; Wu, R.; Tang, D.; Kang, R. The BET family in immunity and disease. Signal Transduct. Target. Ther. 2021, 6, 23. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Chiang, C.-M. The Double Bromodomain-containing Chromatin Adaptor Brd4 and Transcriptional Regulation. J. Biol. Chem. 2007, 282, 13141–13145. [Google Scholar] [CrossRef]
- Donati, B.; Lorenzini, E.; Ciarrocchi, A. BRD4 and Cancer: Going beyond transcriptional regulation. Mol. Cancer 2018, 17, 164. [Google Scholar] [CrossRef]
- Asangani, I.A.; Dommeti, V.L.; Wang, X.; Malik, R.; Cieslik, M.; Yang, R.; Escara-Wilke, J.; Wilder-Romans, K.; Dhanireddy, S.; Engelke, C.; et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014, 510, 278–282. [Google Scholar] [CrossRef]
- Chaidos, A.; Caputo, V.; Gouvedenou, K.; Liu, B.; Marigo, I.; Chaudhry, M.S.; Rotolo, A.; Tough, D.F.; Smithers, N.N.; Bassil, A.K.; et al. Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood 2014, 123, 697–705. [Google Scholar] [CrossRef]
- Coudé, M.-M.; Braun, T.; Berrou, J.; Dupont, M.; Bertrand, S.; Masse, A.; Raffoux, E.; Itzykson, R.; Delord, M.; Riveiro, M.E.; et al. BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. Oncotarget 2015, 6, 17698–17712. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Song, S.; Han, H.; Xu, H.; Huang, M.; Qian, C.; Zhang, X.; Ouyang, L.; Hong, Y.; Zhuang, W.; et al. Potent Activity of the Bromodomain Inhibitor OTX015 in Multiple Myeloma. Mol. Pharm. 2018, 15, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, B.K.; Gehling, V.S.; Hewitt, M.C.; Vaswani, R.G.; Côté, A.; Leblanc, Y.; Nasveschuk, C.G.; Bellon, S.; Bergeron, L.; Campbell, R.; et al. Identification of a Benzoisoxazoloazepine Inhibitor (CPI-0610) of the Bromodomain and Extra-Terminal (BET) Family as a Candidate for Human Clinical Trials. J. Med. Chem. 2016, 59, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Rhyasen, G.W.; Hattersley, M.M.; Yao, Y.; Dulak, A.; Wang, W.; Petteruti, P.; Dale, I.L.; Boiko, S.; Cheung, T.; Zhang, J.; et al. AZD5153: A Novel Bivalent BET Bromodomain Inhibitor Highly Active against Hematologic Malignancies. Mol. Cancer Ther. 2016, 15, 2563–2574. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, L.; Xiong, Z.; Huang, F.; Yang, N.; Jiang, F.; Li, H.; Cui, Y.; Ren, J.; Cheng, Z.; et al. Discovery of a Bromodomain and Extra Terminal Domain (BET) Inhibitor with the Selectivity for the Second Bromodomain (BD2) and the Capacity for the Treatment of Inflammatory Diseases. J. Med. Chem. 2023, 66, 10824–10848. [Google Scholar] [CrossRef]
- Gavai, A.V.; Norris, D.; Delucca, G.; Tortolani, D.; Tokarski, J.S.; Dodd, D.; O’Malley, D.; Zhao, Y.; Quesnelle, C.; Gill, P.; et al. Discovery and Preclinical Pharmacology of an Oral Bromodomain and Extra-Terminal (BET) Inhibitor Using Scaffold-Hopping and Structure-Guided Drug Design. J. Med. Chem. 2021, 64, 14247–14265. [Google Scholar] [CrossRef]
- Ozer, H.G.; El-Gamal, D.; Powell, B.; Hing, Z.A.; Blachly, J.S.; Harrington, B.; Mitchell, S.; Grieselhuber, N.R.; Williams, K.; Lai, T.-H.; et al. Lapalombella, BRD4 Profiling Identifies Critical Chronic Lymphocytic Leukemia Oncogenic Circuits and Reveals Sensitivity to PLX51107, a Novel Structurally Distinct BET Inhibitor. Cancer Discov. 2018, 8, 458–477. [Google Scholar] [CrossRef]
- Tontsch-Grunt, U.; Traexler, P.-E.; Baum, A.; Musa, H.; Marzin, K.; Wang, S.; Trapani, F.; Engelhardt, H.; Solca, F. Therapeutic impact of BET inhibitor BI 894999 treatment: Backtranslation from the clinic. Br. J. Cancer 2022, 127, 577–586. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Schweizer, M.T.; Nanus, D.M.; Pantuck, A.J.; Heath, E.I.; Campeau, E.; Attwell, S.; Norek, K.; Snyder, M.; Bauman, L.; et al. A Phase Ib/IIa Study of the Pan-BET Inhibitor ZEN-3694 in Combination with Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2020, 26, 5338–5347. [Google Scholar] [CrossRef]
- Kim, E.; Hacken, E.T.; Sivina, M.; Clarke, A.; Thompson, P.A.; Jain, N.; Ferrajoli, A.; Estrov, Z.; Keating, M.J.; Wierda, W.G.; et al. The BET inhibitor GS-5829 targets chronic lymphocytic leukemia cells and their supportive microenvironment. Leukemia 2020, 34, 1588–1598. [Google Scholar] [CrossRef]
- Aggarwal, R.; Starodub, A.N.; Koh, B.D.; Xing, G.; Armstrong, A.J.; Carducci, M.A. Phase Ib Study of the BET Inhibitor GS-5829 as Monotherapy and Combined with Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022, 28, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Garcia-Manero, G.; Paquette, R.; Dinner, S.; Donnellan, W.B.; Grunwald, M.R.; Ribadeneira, M.D.; Schroeder, P.; Brevard, J.; Wilson, L.; et al. Phase 1 Dose Escalation and Expansion Study to Determine Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the BET Inhibitor FT-1101 As a Single Agent in Patients with Relapsed or Refractory Hematologic Malignancies. Blood 2019, 134, 3907. [Google Scholar] [CrossRef]
- Wally, V.; Murauer, E.M.; Bauer, J.W. Spliceosome-Mediated Trans-Splicing: The Therapeutic Cut and Paste. J. Investig. Dermatol. 2012, 132, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Walsh, C.E. Spliceosome-Mediated RNA Trans-splicing. Mol. Ther. 2005, 12, 1006–1012. [Google Scholar] [CrossRef]
- Berger, A.; Maire, S.; Gaillard, M.; Sahel, J.; Hantraye, P.A. Bemelmans, m RNA trans-splicing in gene therapy for genetic diseases. WIREs RNA 2016, 7, 487–498. [Google Scholar] [CrossRef]
- Davis, R.E.; Hardwick, C.; Tavernier, P.; Hodgson, S.; Singh, H. RNA Trans-splicing in Flatworms. J. Biol. Chem. 1995, 270, 21813–21819. [Google Scholar] [CrossRef]
- Murphy, W.J.; Agabian, N. Identification of a Novel Y Branch Structure as an Intermediate in Trypanosome mRNA Processing: Evidence for Trans Splicing. Cell 1986, 47, 517–525. [Google Scholar] [CrossRef]
- Dallinger, G.; Puttaraju, M.; Mitchell, L.G.; Yancey, K.B.; Yee, C.; Klausegger, A.; Hintner, H.; Bauer, J.W. Development of spliceosome-mediated RNA trans-splicing (SMaRTTM) for the correction of inherited skin diseases. Exp. Dermatol. 2003, 12, 37–46. [Google Scholar] [CrossRef]
- Puttaraju, M.; DiPasquale, J.; Baker, C.C.; Mitchell, L.G.; Garcia-Blanco, M.A. Messenger RNA Repair and Restoration of Protein Function by Spliceosome-Mediated RNA Trans-Splicing. Mol. Ther. 2001, 4, 105–114. [Google Scholar] [CrossRef]
- Chao, H.; Mansfield, S.G.; Bartel, R.C.; Hiriyanna, S.; Mitchell, L.G.; Garcia-Blanco, M.A.; Walsh, C.E. Phenotype correction of hemophilia A mice by spliceosome-mediated RNA trans-splicing. Nat. Med. 2003, 9, 1015–1019. [Google Scholar] [CrossRef]
- Coady, T.H.; Shababi, M.; Tullis, G.E.; Lorson, C.L. Restoration of SMN Function: Delivery of a Trans-splicing RNA Re-directs SMN2 Pre-mRNA Splicing. Mol. Ther. 2007, 15, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Coady, T.H.; Lorson, C.L. Trans -Splicing-Mediated Improvement in a Severe Mouse Model of Spinal Muscular Atrophy. J. Neurosci. 2010, 30, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, T.; Garcia-Blanco, M.A.; Mansfield, S.G.; Grover, A.C.; Hutton, M.; Yu, Q.; Zhou, J.; Anderton, B.H.; Gallo, J.-M. Reprogramming of tau alternative splicing by spliceosome-mediated RNA trans-splicing: Implications for tauopathies. Proc. Natl. Acad. Sci. USA 2005, 102, 15659–15664. [Google Scholar] [CrossRef]
- Woess, K.; Sun, Y.; Morio, H.; Stierschneider, A.; Kaufmann, A.; Hainzl, S.; Trattner, L.; Kocher, T.; Tockner, B.; Leb-Reichl, V.; et al. Evaluating a Targeted Cancer Therapy Approach Mediated by RNA trans-Splicing In Vitro and in a Xenograft Model for Epidermolysis Bullosa-Associated Skin Cancer. Int. J. Mol. Sci. 2022, 23, 575. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Havens, M.A.; Duelli, D.M.; Hastings, M.L. Targeting RNA splicing for disease therapy. WIREs RNA 2013, 4, 247–266. [Google Scholar] [CrossRef]
- Levin, A.A. Treating Disease at the RNA Level with Oligonucleotides. N. Engl. J. Med. 2019, 380, 57–70. [Google Scholar] [CrossRef]
- Tao, Y.-J.; Li, Y.; Zheng, W.; Zhao, J.; Guo, M.; Zhou, Y.; Qin, N.; Zheng, J.; Xu, L. Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int. 2015, 15, 77. [Google Scholar] [CrossRef]
- Burghes, A.H.M.; McGovern, V.L. Antisense oligonucleotides and spinal muscular atrophy: Skipping along: Figure 1. Genes Dev. 2010, 24, 1574–1579. [Google Scholar] [CrossRef][Green Version]
- Cartegni, L.; Hastings, M.L.; Calarco, J.A.; De Stanchina, E.; Krainer, A.R. Determinants of Exon 7 Splicing in the Spinal Muscular Atrophy Genes, SMN1 and SMN2. Am. J. Hum. Genet. 2006, 78, 63–77. [Google Scholar] [CrossRef]
- Wan, L.; Kral, A.J.; Voss, D.; Schäfer, B.; Sudheendran, K.; Danielsen, M.; Caruthers, M.H.; Krainer, A.R. Screening Splice-Switching Antisense Oligonucleotides in Pancreas-Cancer Organoids. Nucleic Acid Ther. 2024, 34, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Inotersen: First Global Approval. Drugs 2018, 78, 1371–1376. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef]
- Heo, Y.-A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. [Google Scholar] [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875–879. [Google Scholar] [CrossRef]
- Blair, H.A. Tofersen: First Approval. Drugs 2023, 83, 1039–1043. [Google Scholar] [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- Alhamadani, F.; Zhang, K.; Parikh, R.; Wu, H.; Rasmussen, T.P.; Bahal, R.; Zhong, X.; Manautou, J.E. Adverse Drug Reactions and Toxicity of the Food and Drug Administration–Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022, 50, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Rodino-Klapac, L.R.; Sahenk, Z.; Roush, K.; Bird, L.; Lowes, L.P.; Alfano, L.; Gomez, A.M.; Lewis, S.; Kota, J.; et al. The Eteplirsen Study Group, Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013, 74, 637–647. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Frank, D.E.; Schnell, F.J.; Akana, C.; El-Husayni, S.H.; Desjardins, C.A.; Morgan, J.; Charleston, J.S.; Sardone, V.; Domingos, J.; Dickson, G.; et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 2020, 94, e2270–e2282. [Google Scholar] [CrossRef]
- Clemens, P.R.; Rao, V.K.; Connolly, A.M.; Harper, A.D.; Mah, J.K.; Smith, E.C.; McDonald, C.M.; Zaidman, C.M.; Morgenroth, L.P.; Osaki, H.; et al. Safety, Tolerability, and Efficacy of Viltolarsen in Boys With Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping. JAMA Neurol. 2020, 77, 982–991. [Google Scholar] [CrossRef]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef]
- Karimian, A.; Azizian, K.; Parsian, H.; Rafieian, S.; Shafiei, V.; Kheyrollah, M.; Yousefi, M.; Majidinia, M.; Yousefi, B. CRISPR/Cas9 technology as a potent molecular tool for gene therapy. J. Cell. Physiol. 2019, 234, 12267–12277. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Huang, X. Genome modification by CRISPR/Cas9. FEBS J. 2014, 281, 5186–5193. [Google Scholar] [CrossRef]
- Foltz, L.P.; Howden, S.E.; Thomson, J.A.; Clegg, D.O. Functional Assessment of Patient-Derived Retinal Pigment Epithelial Cells Edited by CRISPR/Cas9. Int. J. Mol. Sci. 2018, 19, 4127. [Google Scholar] [CrossRef] [PubMed]
- Aneichyk, T.; Hendriks, W.T.; Yadav, R.; Shin, D.; Gao, D.; Vaine, C.A.; Collins, R.L.; Domingo, A.; Currall, B.; Stortchevoi, A.; et al. Dissecting the Causal Mechanism of X-Linked Dystonia-Parkinsonism by Integrating Genome and Transcriptome Assembly. Cell 2018, 172, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.; Majumdar, D.; Tipanee, J.; Singh, K.; Klein, A.F.; Furling, D.; Chuah, M.K.; VandenDriessche, T. Comprehensive transcriptome-wide analysis of spliceopathy correction of myotonic dystrophy using CRISPR-Cas9 in iPSCs-derived cardiomyocytes. Mol. Ther. 2022, 30, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chen, Z.-H.; Effenberger, K.A.; Jurica, M.S. Enantioselective Total Syntheses of FR901464 and Spliceostatin A and Evaluation of Splicing Activity of Key Derivatives. J. Org. Chem. 2014, 79, 5697–5709. [Google Scholar] [CrossRef]
- Nakajima, H.; Hori, Y.; Terano, H.; Okuhara, M.; Manda, T.; Matsumoto, S.; Shimomura, K. New Antitumor Substances, FR901463, FR901464 and FR901465. II. Activities against Experimental Tumors in Mice and Mechanism of Action. J. Antibiot. 1996, 49, 1204–1211. [Google Scholar] [CrossRef]
- Kaida, D.; Motoyoshi, H.; Tashiro, E.; Nojima, T.; Hagiwara, M.; Ishigami, K.; Watanabe, H.; Kitahara, T.; Yoshida, T.; Nakajima, H.; et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 2007, 3, 576–583. [Google Scholar] [CrossRef]
- Larrayoz, M.; Blakemore, S.J.; Dobson, R.C.; Blunt, M.D.; Rose-Zerilli, M.J.J.; Walewska, R.; Duncombe, A.; Oscier, D.; Koide, K.; Forconi, F.; et al. The SF3B1 inhibitor spliceostatin A (SSA) elicits apoptosis in chronic lymphocytic leukaemia cells through downregulation of Mcl-1. Leukemia 2016, 30, 351–360. [Google Scholar] [CrossRef]
- Albert, B.J.; Sivaramakrishnan, A.; Naka, T.; Czaicki, N.L.; Koide, K. Total Syntheses, Fragmentation Studies, and Antitumor/Antiproliferative Activities of FR901464 and Its Low Picomolar Analogue. J. Am. Chem. Soc. 2007, 129, 2648–2659. [Google Scholar] [CrossRef]
- Albert, B.J.; McPherson, P.A.; O’Brien, K.; Czaicki, N.L.; DeStefino, V.; Osman, S.; Li, M.; Day, B.W.; Grabowski, P.J.; Moore, M.J.; et al. Meayamycin inhibits pre–messenger RNA splicing and exhibits picomolar activity against multidrug-resistant cells. Mol. Cancer Ther. 2009, 8, 2308–2318. [Google Scholar] [CrossRef]
- Convertini, P.; Shen, M.; Potter, P.M.; Palacios, G.; Lagisetti, C.; De La Grange, P.; Horbinski, C.; Fondufe-Mittendorf, Y.N.; Webb, T.R.; Stamm, S. Sudemycin E influences alternative splicing and changes chromatin modifications. Nucleic Acids Res. 2014, 42, 4947–4961. [Google Scholar] [CrossRef] [PubMed]
- Lagisetti, C.; Palacios, G.; Goronga, T.; Freeman, B.; Caufield, W.; Webb, T.R. Optimization of Antitumor Modulators of Pre-mRNA Splicing. J. Med. Chem. 2013, 56, 10033–10044. [Google Scholar] [CrossRef] [PubMed]
- Makowski, K.; Vigevani, L.; Albericio, F.; Valcárcel, J.; Álvarez, M. Sudemycin K: A Synthetic Antitumor Splicing Inhibitor Variant with Improved Activity and Versatile Chemistry. ACS Chem. Biol. 2017, 12, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.K.; Kumar, D.; Jones, H.J.; Schwaederlé, M.; Ghia, E.M.; Villa, R.; La Clair, J.J.; Burkart, M.D.; Kipps, T.J.; Castro, J.E. Fd-895 and Pladienolide B Inhibit mRNA Splicing and Induce Apoptosis in Chronic Lymphocytic Leukemia. Blood 2012, 120, 3890. [Google Scholar] [CrossRef]
- Pokhrel, A.R.; Dhakal, D.; Jha, A.K.; Sohng, J.K. Herboxidiene biosynthesis, production, and structural modifications: Prospect for hybrids with related polyketide. Appl. Microbiol. Biotechnol. 2015, 99, 8351–8362. [Google Scholar] [CrossRef]
- Sakai, Y.; Yoshida, T.; Ochiai, K.; Uosaki, Y.; Saitoh, Y.; Tanaka, F.; Akiyama, T.; Akinaga, S.; Mizukami, T. GEX1 Compounds, Novel Antitumor Antibiotics Related to Herboxidiene, Produced by Streptomyces sp. I. Taxonomy, Production, Isolation, Physicochemical Properties and Biological Activities. J. Antibiot. 2002, 55, 855–862. [Google Scholar] [CrossRef]
- Lee, S.C.-W.; Dvinge, H.; Kim, E.; Cho, H.; Micol, J.-B.; Chung, Y.R.; Durham, B.H.; Yoshimi, A.; Kim, Y.J.; Thomas, M.; et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat. Med. 2016, 22, 672–678. [Google Scholar] [CrossRef]
- Li, D.; Yu, W.; Lai, M. Targeting serine- and arginine-rich splicing factors to rectify aberrant alternative splicing. Drug Discov. Today 2023, 28, 103691. [Google Scholar] [CrossRef]
- Wan, L.; Yu, W.; Shen, E.; Sun, W.; Liu, Y.; Kong, J.; Wu, Y.; Han, F.; Zhang, L.; Yu, T.; et al. SRSF6-regulated alternative splicing that promotes tumour progression offers a therapy target for colorectal cancer. Gut 2019, 68, 118–129. [Google Scholar] [CrossRef]
- Tam, B.Y.; Chiu, K.; Chung, H.; Bossard, C.; Nguyen, J.D.; Creger, E.; Eastman, B.W.; Mak, C.C.; Ibanez, M.; Ghias, A.; et al. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020, 473, 186–197. [Google Scholar] [CrossRef]
- Kim, H.; Choi, K.; Kang, H.; Lee, S.-Y.; Chi, S.-W.; Lee, M.-S.; Song, J.; Im, D.; Choi, Y.; Cho, S. Identification of a Novel Function of CX-4945 as a Splicing Regulator. PLoS ONE 2014, 9, e94978. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Bae, K.J.; Lee, Y.; Kim, J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front. Pharmacol. 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Masłyk, M.; Janeczko, M.; Martyna, A.; Kubiński, K. CX-4945: The protein kinase CK2 inhibitor and anti-cancer drug shows anti-fungal activity. Mol. Cell. Biochem. 2017, 435, 193–196. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chang, W.-H.; Chang, Y.-S.; Liu, T.-Y.; Chen, Y.-C.; Wu, Y.-C.; Chang, J.-G. 4β-Hydroxywithanolide E Modulates Alternative Splicing of Apoptotic Genes in Human Hepatocellular Carcinoma Huh-7 Cells. Sci. Rep. 2017, 7, 7290. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Chiu, C.-C.; Chang, F.-R.; Chen, J.Y.-F.; Hwang, C.-C.; Hseu, Y.-C.; Yang, H.-L.; Lee, A.Y.-L.; Tsai, M.-T.; Guo, Z.-L.; et al. 4β-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC Cancer 2010, 10, 46. [Google Scholar] [CrossRef]
- Batson, J.; Toop, H.; Liddell, S.; Daubney, J.; Stewart, E.A.; Habgood, A.; Murphy, A.; McKechnie, K.; Morris, J.; Bates, D.O. EXN407, a novel topical therapeutic candidate with high retinal bioavailability for the treatment of diabetic macular oedema, inhibits ocular neovascularization. Investig. Ophthalmol. Vis. Sci. 2019, 60, 26. [Google Scholar]
- Diken, M.; Kranz, L.M.; Kreiter, S.; Sahin, U. mRNA: A Versatile Molecule for Cancer Vaccines. Curr. Issues Mol. Biol. 2017, 22, 113–128. [Google Scholar] [CrossRef]
- Pastor, F.; Berraondo, P.; Etxeberria, I.; Frederick, J.; Sahin, U.; Gilboa, E.; Melero, I. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 2018, 17, 751–767. [Google Scholar] [CrossRef]

| Target | Drug | Structure | Treatment | Phase | Reference/NCT no. |
|---|---|---|---|---|---|
| HDAC | Vorinostat | 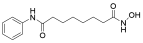 | CTCL | FDA approved | [65] |
| Romidepsin | 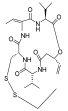 | CTCL, PTCL | FDA approved | [66] | |
| Belinostat |  | PTCL; HCC, Burkitt lymphoma, DLBCL, thymic carcinoma, MDS | FDA approved | [67] | |
| Panobinostat | 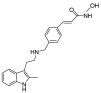 | MM; thyroid carcinoma, RCC, breast cancer, AML | FDA approved | [68] | |
| Chidamide | 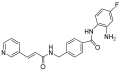 | PTCL | FDA approved | [69] | |
| Valproic acid | 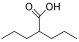 | MDA, AML | II | [70] | |
| Abexinostat | 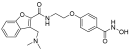 | Lymphoma | I & II | [57] | |
| CUDC-101 | 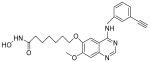 | Solid tumor | I | [71] | |
| Pracinostat | 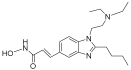 | MLD | II | [59] | |
| Givinostat | 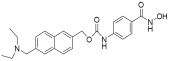 | Polycythemia vera | I & II | NCT01901432 | |
| Mocetinostat |  | Metastatic leiomyosarcoma | II | NCT02303262 | |
| DNMT | Azacytidine |  | MDS, AML | FDA approved | [72] |
| Decitabine | 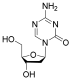 | MDS, AML | FDA approved | [73] | |
| Guadecitabine sodium | 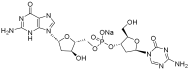 | MDS, AML | II | [74] | |
| Aza-TdC | 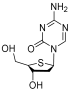 | Solid tumor | I | NCT03366116 | |
| RX-3117 | 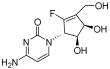 | Solid tumor | I | [75] | |
| Epigallocatechol Gallate | 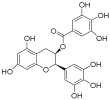 | PC | II | NCT00666562 | |
| Hydralazine | 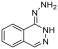 | Refractory solid tumor | II | [76] | |
| MG98 | / | Solid tumor | I | [77] | |
| BET | Molibresib | 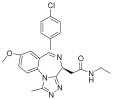 | Solid tumor | I | NCT03925428 |
| Birabresib | 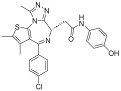 | AML, DLBCL | I | NCT02698189 | |
| Pelabresib | 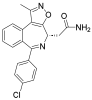 | Progressive lymphoma | I | NCT01949883 | |
| ADZ5153 | 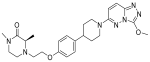 | NHL, DLBCL, non-Hodgkin’s lymphoma | I | NCT03527147 | |
| ABBV-744 |  | CRPC, AML | I | NCT03360006 | |
| BMS-986158 |  | Solid tumor, lymphoma, brain tumor (pediatric) | I | NCT03936465 | |
| PLX51107 | 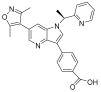 | AML, myelodysplastic syndrome, MDS/MPN | I | NCT04022785 | |
| BI 894999 | 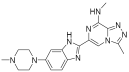 | Neoplasms | I | NCT02516553 | |
| ZEN-3694 | / | MCRPC | II | NCT02705469 | |
| GS-5829 | 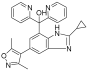 | Solid tumors, lymphomas | I | NCT02392611 | |
| FT-1101 | 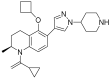 | AML, MDS | I | NCT02543879 |
| Target | Structure | ASO Drug | Dose | Treatment | Reference |
|---|---|---|---|---|---|
| Survival motor neuron-2 (SMN2) mRNA | RNA, [2′-O-(2-methoxyethyl)] (P-thio) (m5U-m5C-A-m5C-m5U-m5U-m5U-m5C-A-m5U-A-A-m5U-G-m5C-m5U-G-G) | Nusinersen | 12 mg once every 4 months (IT) | Spinal muscular atrophy | [132] |
| Exon 51 dystrophinpre-mRNA | RNA, [P-deoxy-P-(dimethylamino)](2′,3′-dideoxy-2′,3′-imino-2′,3′-seco)(2′a ⟶ 5′)(C-m5U-C-C-A-A-C-A-m5U-C-A-A-G-G-A-A-G-A-m5U-G-G-C-A-m5U-m5U-m5U-C-m5U-A-G), 5′-[P-[4-[[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]carbonyl]-1-piperazinyl]-N,N-dimethylphosphonamidate] | Eteplirsen | 30 mg/kg once weekly (IV) | Duchenne muscular dystrophy | [133] |
| Transthyretin (TTR) mRNA | DNA, d(P-thio)([2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]rG-G-T-T-A-m5C-A-T-G-A-A-[2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rC) nonadeca | Inotersen | 300 mg once weekly (SC) | Polyneuropathy of amyloidosis | [134] |
| Apolipoprotein C3 | DNA, d(P-thio)([2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]rG-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-m5C-T-T-G-T-m5C-m5C-A-G-m5C-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]m5rU-[2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]m5rU) | Volanesorsen | 300 mg once weekly (SC) | Familial chylomicronemia syndrome | [135] |
| Dystrophin exon 53 | RNA, [P-deoxy-P-(dimethylamino)](2′,3′-dideoxy-2′,3′-imino-2′,3′-seco)(2′a ⟶ 5′)(G-m5U-m5U-G-C-C-m5U-C-C-G-G-m5U-m5U-C-m5U-G-A-A-GG-m5U-G-m5U-m5U-C), 5′-[P-[4-[[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]carbonyl]-1-piperazinyl]-N,Ndimethylphosphonamidate] | Golodirsen | 30 mg/kg once weekly (IV) | Duchenne muscular dystrophy | [136] |
| Dystrophin exon 53 | RNA, [P-deoxy-P-(dimethylamino)](2′,3′-dideoxy-2′,3′-imino-2′,3′-seco)(2′a ⟶ 5′)(C-C-m5U-C-C-G-G-m5U-m5U-C-m5U-G-A-A-G-G-m5U-Gm5U-m5U-C) | Viltolarsen | 80 mg/kg once weekly (IV) | Duchenne muscular dystrophy | [137] |
| Dystrophin exon 45 | RNA, [P-deoxy-P-(dimethylamino)](2′,3′-dideoxy-2′,3′-imino-2′,3′-seco)(2′a ⟶ 5′)(C-A-A-m5U-G-C-C-A-m5U-C-C-m5U-G-G-A-G-m5U-m5U-C-Cm5U-G), 5′-[P-[4-[[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]carbonyl]-1-piperazinyl]-N,N-dimethylphosphonamidate] | Casimersen | 30 mg/kg once weekly (IV) | Duchenne muscular dystrophy | [128] |
| Superoxide dismutase type 1 | DNA, d([2′-O-(2-methoxyethyl)]m5rC-sp-[2′-O-(2-methoxyethyl)]rA-[2′-O-(2-methoxyethyl)]rG-sp-[2′-O-(2-methoxyethyl)]rG-[2′-O-(2-methoxyethyl)]rA-sp-Tsp-A-sp-m5C-sp-A-sp-T-sp-T-sp-T-sp-m5C-sp-T-sp-A-sp-[2′-O-(2-methoxyethyl)]m5rC-[2′-O-(2-methoxyethyl)]rA-sp-[2′-O-(2-methoxyethyl)]rG-[2′-O-(2-methoxyethyl)]m5rC-sp-[2′-O-(2-methoxyethyl)]m5rU) | Tofersen | 100 mg/kg once every 3 week3(IT) | Amyotrophic lateral sclerosis | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Lai, M. Epigenetic Regulation and Therapeutic Targeting of Alternative Splicing Dysregulation in Cancer. Pharmaceuticals 2025, 18, 713. https://doi.org/10.3390/ph18050713
Lei Y, Lai M. Epigenetic Regulation and Therapeutic Targeting of Alternative Splicing Dysregulation in Cancer. Pharmaceuticals. 2025; 18(5):713. https://doi.org/10.3390/ph18050713
Chicago/Turabian StyleLei, Yan, and Maode Lai. 2025. "Epigenetic Regulation and Therapeutic Targeting of Alternative Splicing Dysregulation in Cancer" Pharmaceuticals 18, no. 5: 713. https://doi.org/10.3390/ph18050713
APA StyleLei, Y., & Lai, M. (2025). Epigenetic Regulation and Therapeutic Targeting of Alternative Splicing Dysregulation in Cancer. Pharmaceuticals, 18(5), 713. https://doi.org/10.3390/ph18050713




