Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of SMEE
2.2. Evaluation of the Pharmacological Properties
2.3. In Silico Prediction of Biological Activity of SMEE Compounds
2.4. Safety Assessment of Chronic Exposure to SMEE
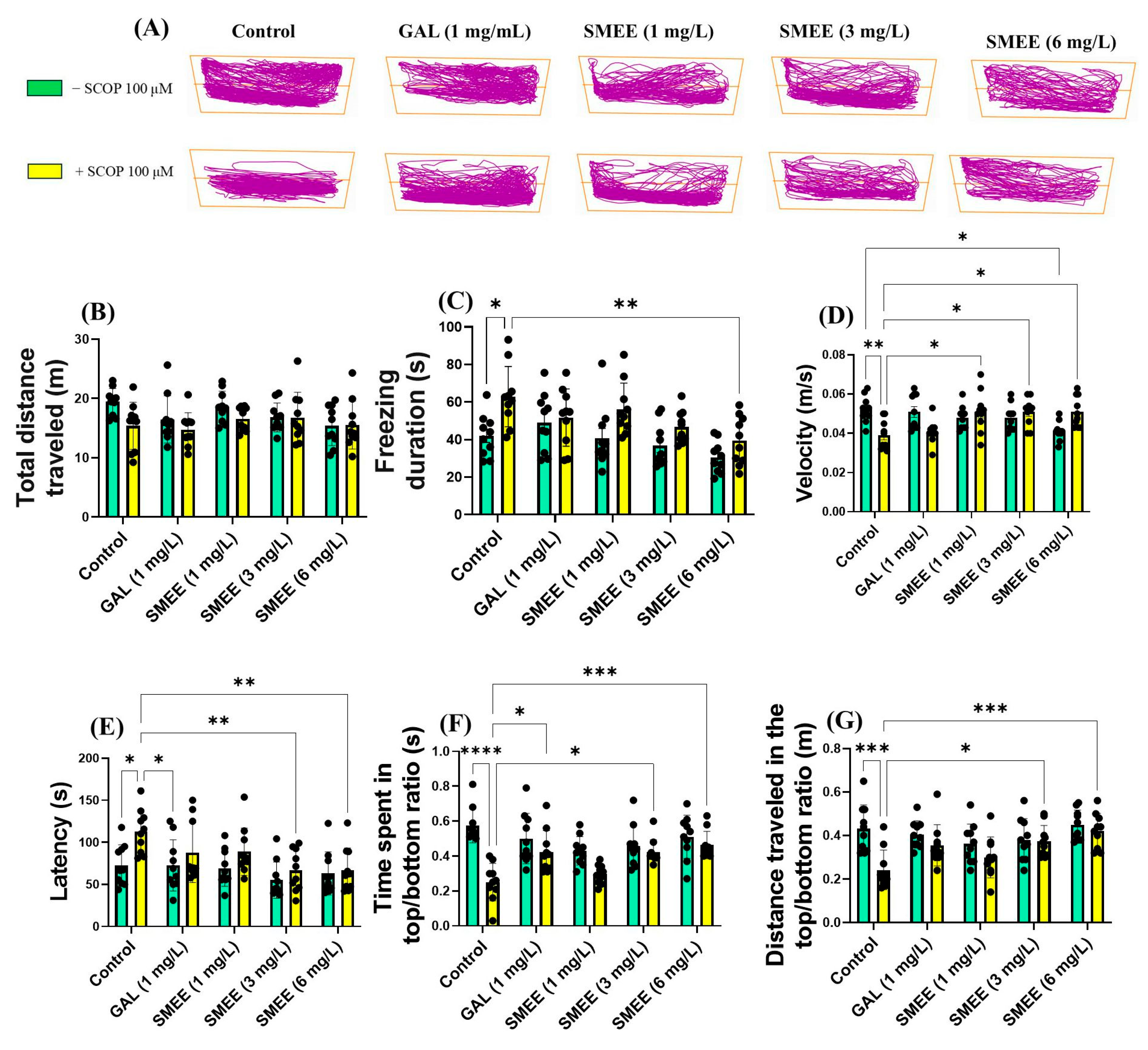
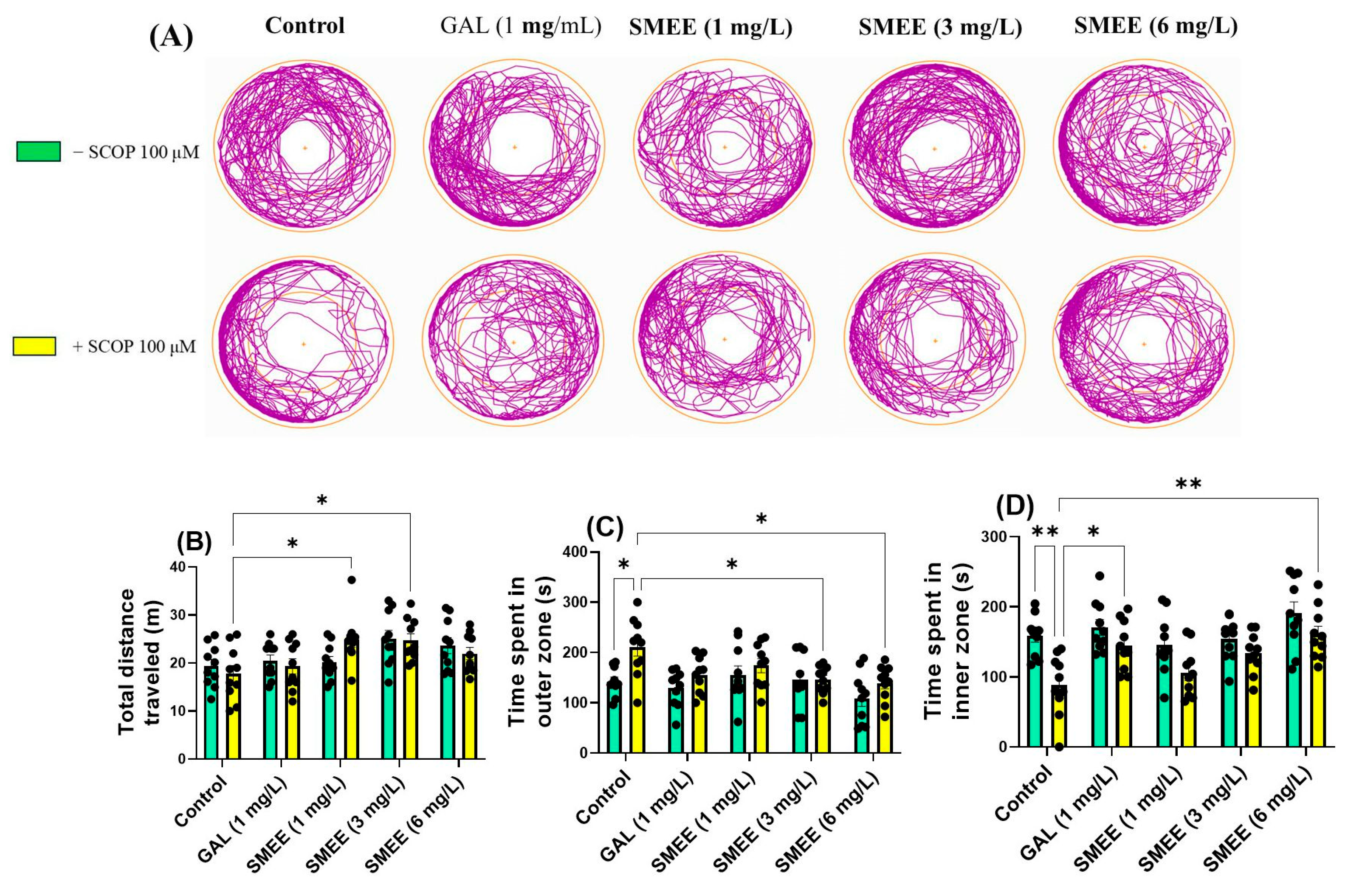
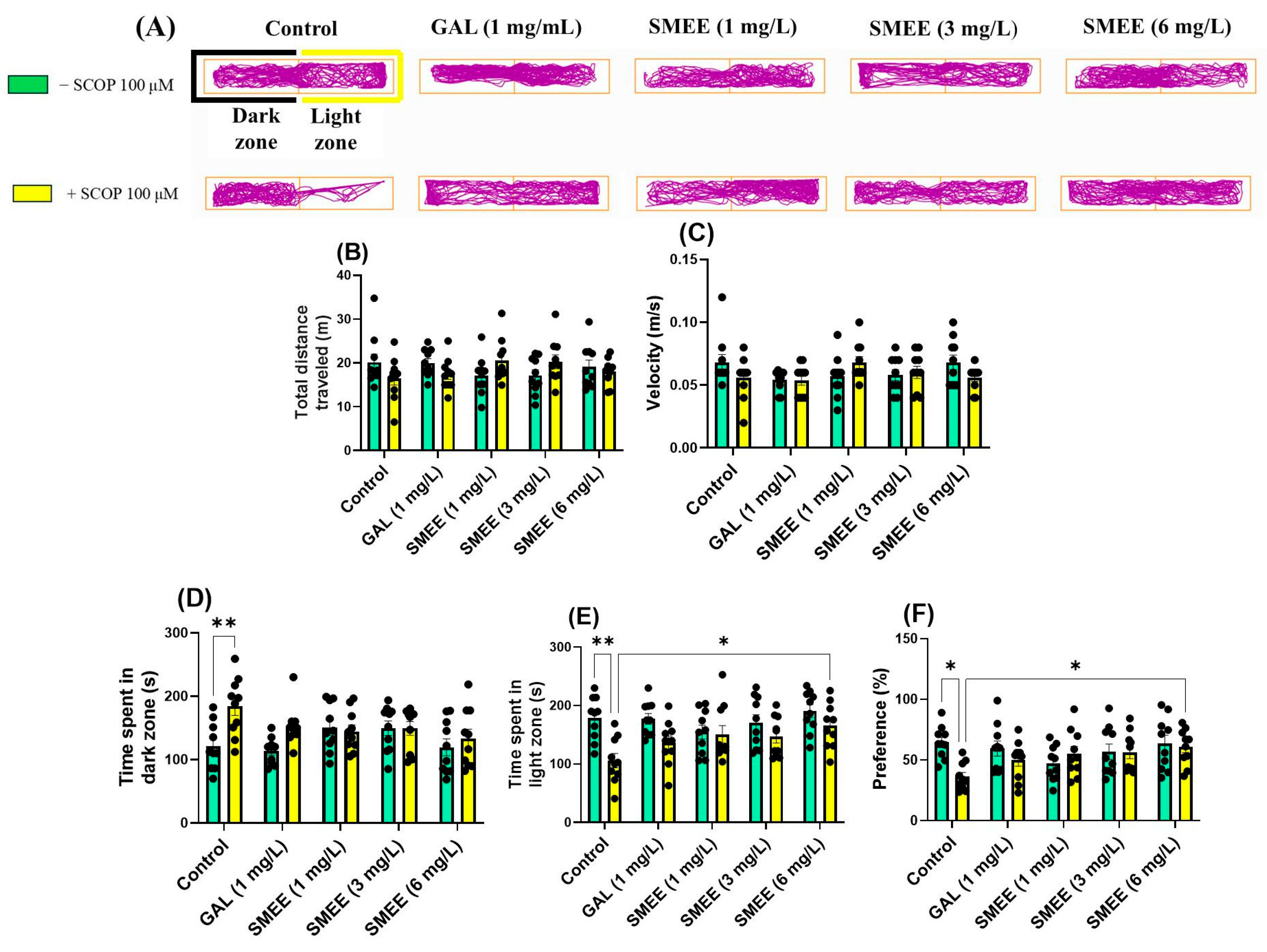
2.5. Effects on Anxiety-like Behavior in NTT, NAT, and LDT
2.6. Effects on Spatial Memory in Y-Maze
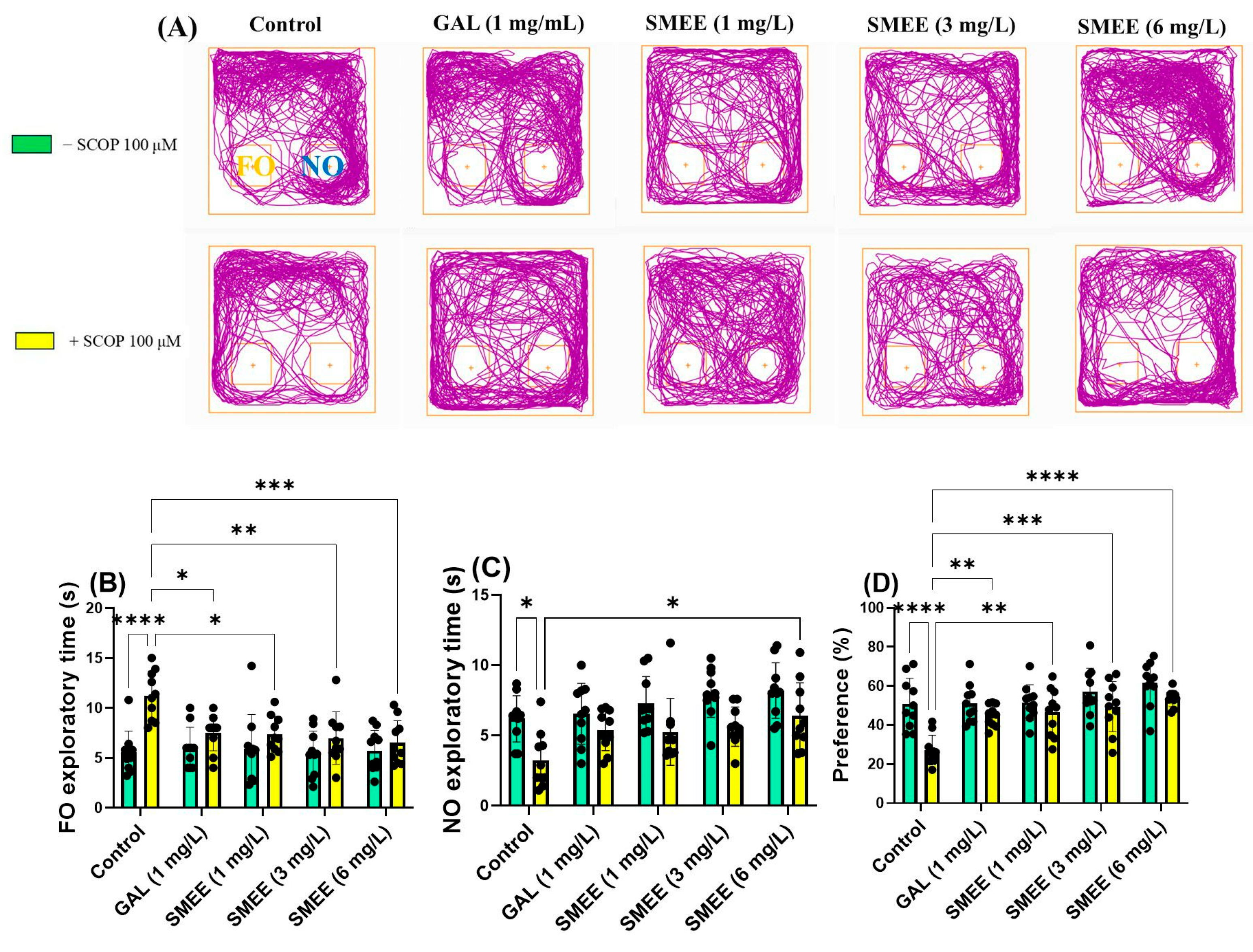
2.7. Effects on Recognition Memory in NOR
2.8. Effects on the Brain AChE Activity
2.9. Effects on Brain Oxidative Status
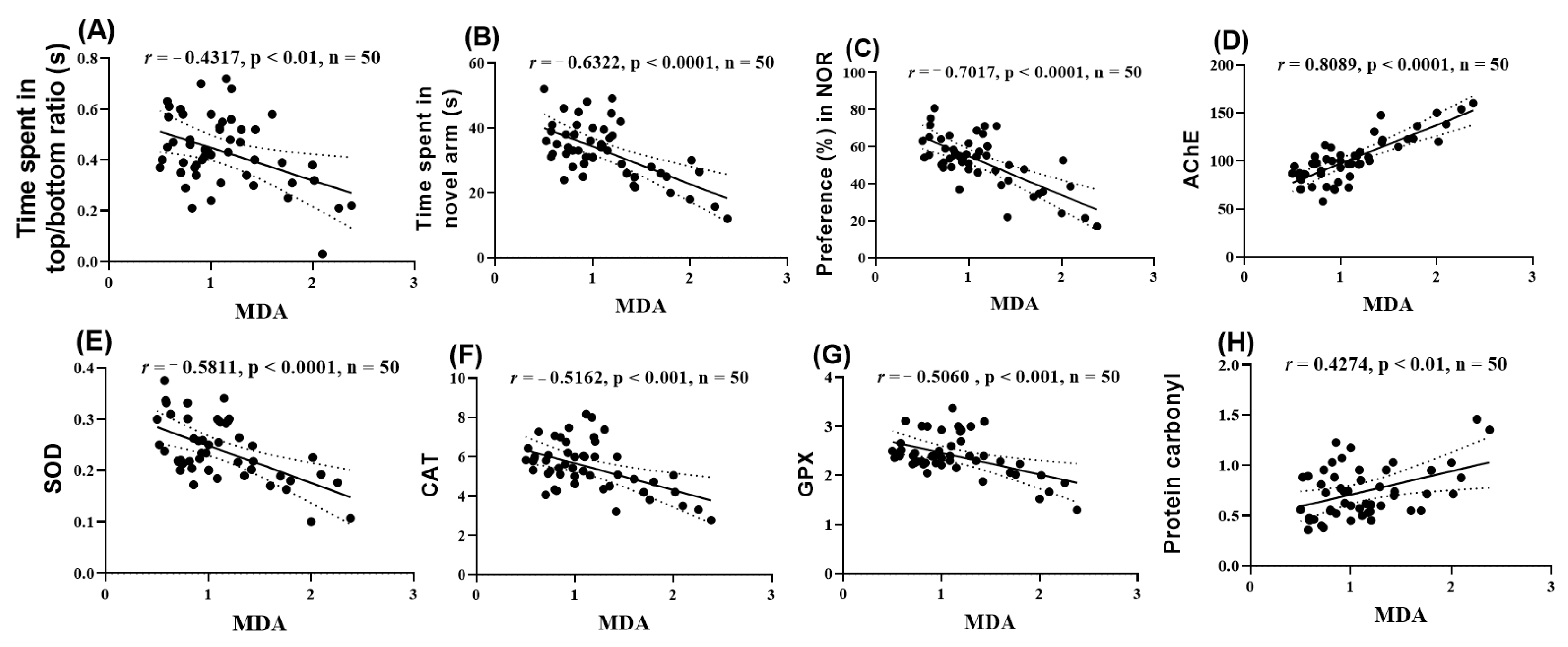
2.10. Pearson Correlations Between Behavioral and Biochemical Variables
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. High-Performance Liquid Chromatograph (HPLC-PDA)
4.3. Computational Estimation of the Pharmacokinetic Profile
4.4. Prediction of Biological Activity and Protein Target Identification
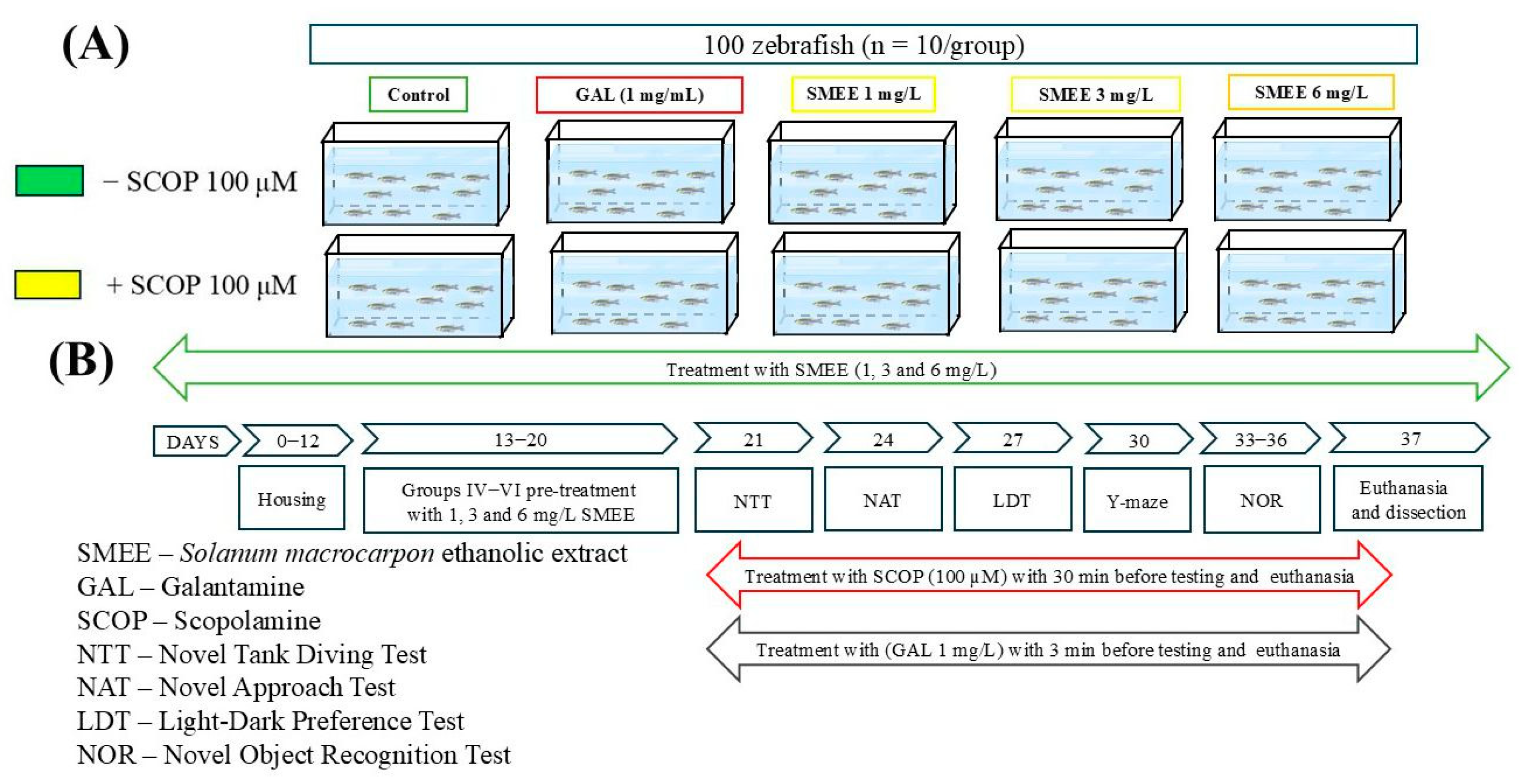
4.5. Study Design and Animal Care
4.6. Behavioral Tasks
4.6.1. Novel Tank Diving Test (NTT)
4.6.2. Novel Approach Test (NAT)
4.6.3. Light–Dark Test (LDT)
4.6.4. Y-Maze
4.6.5. Novel Object Recognition (NOR)
4.7. Biochemical Parameter Testing
4.7.1. Determining Acetylcholinesterase (AChE) Activity
4.7.2. Determining Superoxide Dismutase (SOD) Activity
4.7.3. Determining Catalase (CAT) Activity
4.7.4. Determining Glutathione Peroxidase (GPX) Activity
4.7.5. Determining Carbonylated Protein Contents
4.7.6. Malondialdehyde (MDA) Level
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Ghaffari Jolfayi, A.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Daei Sorkhabi, A.; Golabi, B.; Aletaha, R.; Motlagh Asghari, K.; Hamidi, S.; et al. Alzheimer’s Disease: A Comprehensive Review of Epidemiology, Risk Factors, Symptoms Diagnosis, Management, Caregiving, Advanced Treatments and Associated Challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef] [PubMed]
- Xu Lou, I.; Ali, K.; Chen, Q. Effect of Nutrition in Alzheimer’s Disease: A Systematic Review. Front. Neurosci. 2023, 17, 1147177. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease, and Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis-An Updated Review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Madnani, R.S. Alzheimer’s Disease: A Mini-Review for the Clinician. Front. Neurol. 2023, 14, 1178588. [Google Scholar] [CrossRef] [PubMed]
- Popovici, L.F.; Brinza, I.; Gatea, F.; Badea, G.I.; Vamanu, E.; Oancea, S.; Hritcu, L. Enhancement of Cognitive Benefits and Anti-Anxiety Effects of Phytolacca Americana Fruits in a Zebrafish (Danio Rerio) Model of Scopolamine-Induced Memory Impairment. Antioxidants 2025, 14, 97. [Google Scholar] [CrossRef]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish Models of Neurodevelopmental Disorders: Past, Present, and Future. Front. Mol. Neurosci. 2018, 11, 396075. [Google Scholar] [CrossRef]
- Adamson, K.I.; Sheridan, E.; Grierson, A.J.; Neuroscience, T.; Andrew, D.; Grierson, J. Use of Zebrafish Models to Investigate Rare Human Disease. J. Med. Genet. 2018, 55, 641–649. [Google Scholar] [CrossRef]
- Don, D.W.; Choi, T.-I.; Kim, T.-Y.; Lee, K.-H.; Lee, Y.; Kim, C.-H. Using Zebrafish as an Animal Model for Studying Rare Neurological Disorders: A Human Genetics Perspective. J. Genet. Med. 2024, 21, 6–13. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Genario, R.; Giacomini, A.C.V.V.; Demin, K.A.; Lakstygal, A.M.; Amstislavskaya, T.G.; Fontana, B.D.; Parker, M.O.; Kalueff, A.V. Zebrafish as a Model of Neurodevelopmental Disorders. Neuroscience 2020, 445, 3–11. [Google Scholar] [CrossRef]
- Ezechukwu, C.S.; Mbegbu, E.C.; Nwani, C.D.; Onoja, S.O.; Orji, E.A.; Ugwu, G.C.; Nnamonu, E.I.; Ugwu, G.N. Spermicidal and Antioxidant Potency of Solanum macrocarpon L. (African Eggplant) Leaf Ethanol Extract in Albino Rats. Comp. Clin. Path 2024, 33, 367–377. [Google Scholar] [CrossRef]
- Dougnon, T.V.; Bankolé, H.S.; Johnson, R.C.; Klotoé, J.R.; Dougnon, G.; Gbaguidi, F.; Assogba, F.; Gbénou, J.; Sahidou, S.; Atègbo, J.-M.; et al. Phytochemical Screening, Nutritional and Toxicological Analyses of Leaves and Fruits of Solanum Macrocarpon Linn (Solanaceae) in Cotonou (Benin). Food Nutr. Sci. 2012, 3, 1595–1603. [Google Scholar] [CrossRef]
- Majesty, D.; Amadike, U.; Benjamin, A. Effect of Solanum Macrocarpon Fruit on Haematology, Hepatic and Renal Function. Adv. Biochem. 2013, 1, 28–32. [Google Scholar] [CrossRef][Green Version]
- Chidiebere, M.E.; Samuel, E.C.; Chikwendu, E.V.; Ikechukwu, N.E.; Chigozie, U.G. Phytochemistry, Acute Toxicity and Blood Profile of Albino Rats Treated with Fruit Extract of Solanum Macrocarpon. J. Pharmacogn. Phytother. 2019, 11, 43–51. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Brinza, I.; Boiangiu, R.S.; Ibrahim, M.H.; Honceriu, I.; Al-Maktoum, A.; Cioanca, O.; Hancianu, M.; Amin, A.; Ben-Attia, M.; et al. The Effect of a Tribulus-Based Formulation in Alleviating Cholinergic System Impairment and Scopolamine-Induced Memory Loss in Zebrafish (Danio Rerio): Insights from Molecular Docking and In Vitro/In Vivo Approaches. Pharmaceuticals 2024, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Betanin Mitigates Scopolamine-Induced Cognitive Impairment by Restoring Cholinergic Function, Boosting Brain Antioxidative Status, and Increasing BDNF Level in the Zebrafish Model. Fish. Physiol. Biochem. 2023, 49, 335–349. [Google Scholar] [CrossRef]
- Okesola, M.A.; Ajiboye, B.O.; Oyinloye, B.E.; Osukoya, O.A.; Owero-ozeze, O.S.; Ekakitie, L.I.; Kappo, A.P. Effect of Solanum Macrocarpon Linn Leaf Aqueous Extract on the Brain of an Alloxan-Induced Rat Model of Diabetes. J. Int. Med. Res. 2020, 48, 300060520922649. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Ademiluyi, A.O.; Oboh, G. Solanum Leaves Extracts Exhibit Antioxidant Properties and Inhibit Monoamine Oxidase and Acetylcholinesterase Activities (In Vitro) in Drosophila Melanogaster. J. Basic. Clin. Physiol. Pharmacol. 2020, 31, 20190256. [Google Scholar] [CrossRef]
- Salawu, S.; Akindahunsi, A.; Ibukun, E.; Duodu, K. Antioxidant Activities and Inhibitory Action of Solanum Macrocarpon and Hibiscus Esculentus Phenolic Containing Leaf Extracts against Lipid Oxidation. Int. J. Med. Plants Res. 2013, 2, 190–197. [Google Scholar]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr. Med. Chem. 2022, 30, 3905–3926. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin Ameliorates Inflammation and Improves Metabolic Function: A Comprehensive Analysis of Scientific Literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The Anticancer Potential of the Dietary Polyphenol Rutin: Current Status, Challenges, and Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Madkour, D.A.; Ahmed, M.M.; Elkirdasy, A.F.; Orabi, S.H.; Mousa, A.A.; Madkour, D.A. Rutin: Chemical Properties, Pharmacokinetic Properties and Biological Activities. Matrouh J. Vet. Med. 2024, 4, 26–34. [Google Scholar] [CrossRef]
- Brinza, I.; Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, A.B.; Abd-Alkhalek, A.; El-Nashar, H.; Ayoub, I.; Mostafa, N.; Eldahshan, O.; et al. Rhoifolin, Baicalein 5,6-Dimethyl Ether and Agathisflavone Prevent Amnesia Induced in Scopolamine Zebrafish (Danio Rerio) Model by Increasing the MRNA Expression of Bdnf, Npy, Egr-1, Nfr2α, and Creb1 Genes. Eur. J. Pharmacol. 2024, 984, 177013. [Google Scholar] [CrossRef]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine Effects on Functional Brain Connectivity: A Pharmacological Model of Alzheimer’s Disease. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing Zebrafish as a Model to Study the Anxiolytic Effects of Scopolamine. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Hagels, H.; Kayser, O. Scopolamine: A Journey from the Field to Clinics. Phytochem. Rev. 2016, 16, 333–353. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Omage, F.B.; Olagoke, O.C.; Oboh, G.; Rocha, J.B.T. Phytochemicals from African Eggplants (Solanum macrocarpon L.) and Black Nightshade (Solanum nigrum L.) Leaves as Acetylcholinesterase Inhibitors: An in-Silico Study. J. Biomol. Struct. Dyn. 2023, 41, 7725–7734. [Google Scholar] [CrossRef]
- Idowu, G.P.; Obuotor, E.M.; Onajobi, F.D. In Vitro and in Silico Investigation of Cholinesterase Inhibition and Anti-Radical Properties of Solanum Macrocarpon Leaf Extracts: A Preliminary Anti-Alzheimer’s Study. Alzheimer’s Dement. 2021, 17, e049605. [Google Scholar] [CrossRef]
- Etuk, I.C.; Udobang, J.A.; Ebong, N.O.; Okokon, J.E. Solanum Anomalum Leaf Extract and Fractions Attenuate Oxidative Stress and Liver Injuries in Alloxan-Induced Diabetic Rats. Biol. Med. Nat. Prod. Chem. 2023, 12, 33–44. [Google Scholar] [CrossRef]
- Osukoya, O.A.; Ajiboye, B.O.; Oyinloye, B.E.; Owero-ozeze, O.S.; Ojo, O.A.; Kappo, P.A. Aqueous Extract of Solanum Macrocapon Linn Leaf Abate Diabetic Cardiomyopathy by Attenuating Oxidative Stress and Inflammation in Rats. J. Food Biochem. 2022, 46, e14172. [Google Scholar] [CrossRef] [PubMed]
- Ekin, S.; Kiziltas, H.; Bayramoglu Akkoyun, M.; Ekin, H.N.; Yildirim, S.; Oto, G.; Akbas, E.; Deliorman Orhan, D.; Ozgokce, F. Nephroprotective Effect of Ferulago Angulata Flowers on N-Nitrosodimethylamine-Induced Nephrotoxicity in Rats and Its Phytochemical Profile. J. Food Biochem. 2019, 43, e13030. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An Updated Comprehensive Online ADMET Prediction Platform Enhanced with Broader Coverage, Improved Performance, API Functionality and Decision Support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Bate, S.T.; Clark, R.A. The Design and Statistical Analysis of Animal Experiments; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139344319. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. In Neuromethods; Springer: Berlin/Heidelberg, Germany, 2011; Volume 51, pp. 1–14. [Google Scholar]
- Facciol, A.; Iqbal, M.; Eada, A.; Tran, S.; Gerlai, R. The Light-Dark Task in Zebrafish Confuses Two Distinct Factors: Interaction between Background Shade and Illumination Level Preference. Pharmacol. Biochem. Behav. 2019, 179, 9–21. [Google Scholar] [CrossRef]
- de P Cognato, G.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze Memory Task in Zebrafish (Danio Rerio): The Role of Glutamatergic and Cholinergic Systems on the Acquisition and Consolidation Periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- Faillace, M.; Pisera-Fuster, A.; Medrano, M.; Bejarano, A.; Bernabeu, R. Short- and Long-Term Effects of Nicotine and the Histone Deacetylase Inhibitor Phenylbutyrate on Novel Object Recognition in Zebrafish. Psychopharmacology 2017, 234, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Artenie, V.; Ungureanu, E.; Negură, A.M. Metode de Investigare a Metabolismului Glucidic Și Lipidic; Editura Pim: Iași, Rumania, 2008. [Google Scholar]
- Sinha, A.K. Colorimetric Assay of Catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione Peroxidase Activity in Tissues of Vitamin E-Deficient Mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B. Protein Carbonylation: Avoiding Pitfalls in the 2,4-Dinitrophenylhydrazine Assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
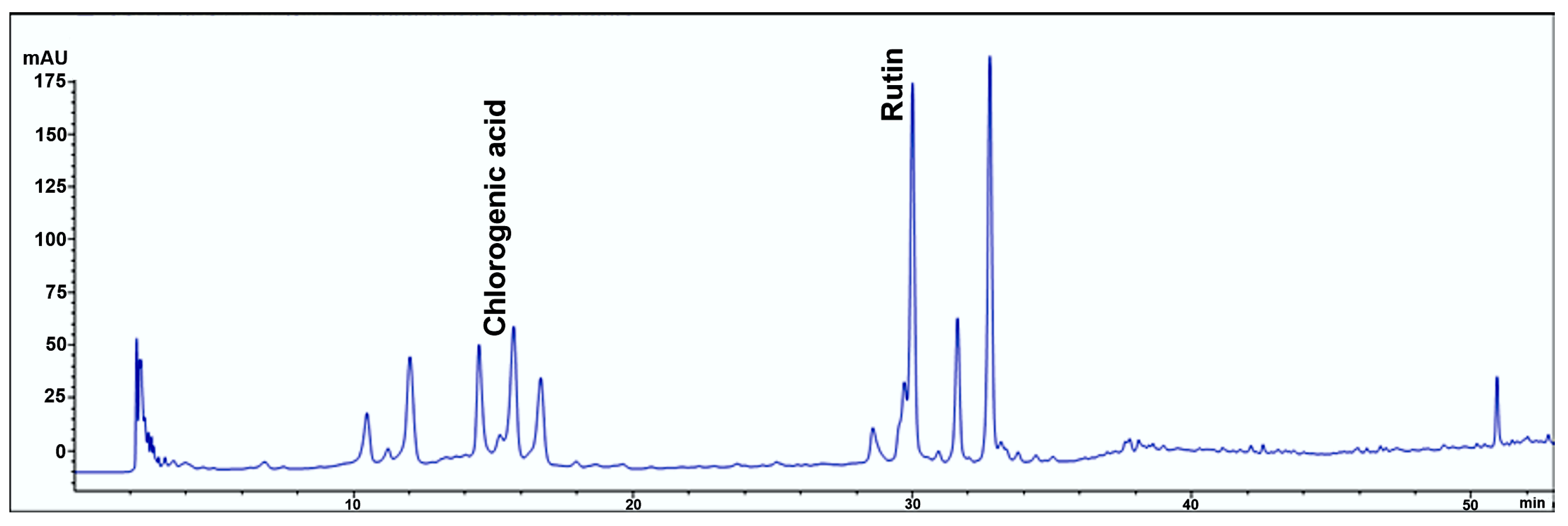




| Sample | Compounds | Retention Time (min) | Maximum Absorbance (nm) | Amount (mg/g, Mean ± SD) | Standard Curve | R2 |
|---|---|---|---|---|---|---|
| SMEE | Chlorogenic acid | 15.595 | 320 | 25.46 ± 0.10 | y = 28.919x + 54.92 | 0.9974 |
| Rutin | 30.000 | 260 | 7.55 ± 0.10 | y = 36.127x + 150.42 | 0.9993 |
| Property | Compound Model Name | SCO | GAL | Chlorogenic Acid | Rutin | Unit |
|---|---|---|---|---|---|---|
| Absorption | Intestinal absorption (human) (low < 30%, high > 30%) | 72.626 | 94.994 | 36.377 | 23.446 | Numeric (% Absorbed) |
| Skin permeability (low logKp > −2.5, high logKp < −2.5) | −4.097 | −3.75 | −2.735 | −2.735 | Numeric (log Kp) | |
| P-glycoprotein substrate | Yes | No | Yes | Yes | Categorical (Yes/No) | |
| P-glycoprotein I inhibitor | No | No | No | No | Categorical (Yes/No) | |
| P-glycoprotein II inhibitor | No | No | No | No | Categorical (Yes/No) | |
| Distribution | VDss (human) (low log VDss < −0.15, high VDss > 0.45) | 0.583 | 0.89 | 0.581 | 1.633 | Numeric (log L/kg) |
| Unbound fraction (human) | 0.414 | 0.36 | 0.658 | 0.187 | Numeric (Fu) | |
| BBB permeability (log BB > 0.3 cross BB, log BB < 0.1 do not cross BB) | −0.043 | 0.081 | −1.407 | −1.899 | Numeric (BB log) | |
| CNS permeability (log PS > −2 penetrates CNS, log PS < −3 does not penetrate) | −3.031 | −2.511 | −3.856 | −5.178 | Numeric (PS log) | |
| Metabolism | CYP3A4 substrate | Yes | Yes | No | No | Categorical (Yes/No) |
| CYP1A2 inhibitor | No | No | No | No | Categorical (Yes/No) | |
| Excretion | Total clearance | 1.096 | 0.991 | 0.307 | −0.369 | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | Yes | No | No | Categorical (Yes/No) | |
| Toxicity | hERG blockers | 0.19 | 0.458 | 0.025 | 0.008 | Numeric |
| hERG blockers (10 µm) | 0.418 | 0.625 | 0.093 | 0.263 | Numeric | |
| DILI | 0.104 | 0.215 | 0.291 | 0.937 | Numeric | |
| AMES toxicity | 0.158 | 0.559 | 0.386 | 0.756 | Numeric | |
| Carcinogenicity | 0.015 | 0.726 | 0.225 | 0.047 | Numeric | |
| Human hepatotoxicity | 0.895 | 0.795 | 0.543 | 0.406 | Numeric | |
| Drug-induced nephrotoxicity | 0.403 | 0.802 | 0.441 | 0.148 | Numeric | |
| Drug-induced neurotoxicity | 0.753 | 0.744 | 0.009 | 0.0 | Numeric | |
| Hematotoxicity | 0.16 | 0.505 | 0.028 | 0.023 | Numeric | |
| Genotoxicity | 0.677 | 0.739 | 0.243 | 0.868 | Numeric | |
| RPMI-8226 immunotoxicity | 0.043 | 0.097 | 0.016 | 0.098 | Numeric | |
| A549 cytotoxicity | 0.058 | 0.069 | 0.203 | 0.86 | Numeric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, I.; Guliev, C.; Oresanya, I.O.; Gok, H.N.; Orhan, I.E.; Hritcu, L. Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model. Pharmaceuticals 2025, 18, 706. https://doi.org/10.3390/ph18050706
Brinza I, Guliev C, Oresanya IO, Gok HN, Orhan IE, Hritcu L. Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model. Pharmaceuticals. 2025; 18(5):706. https://doi.org/10.3390/ph18050706
Chicago/Turabian StyleBrinza, Ion, Corina Guliev, Ibukun Oluwabukola Oresanya, Hasya Nazli Gok, Ilkay Erdogan Orhan, and Lucian Hritcu. 2025. "Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model" Pharmaceuticals 18, no. 5: 706. https://doi.org/10.3390/ph18050706
APA StyleBrinza, I., Guliev, C., Oresanya, I. O., Gok, H. N., Orhan, I. E., & Hritcu, L. (2025). Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model. Pharmaceuticals, 18(5), 706. https://doi.org/10.3390/ph18050706










