Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations
Abstract
1. Introduction
2. Results
2.1. Evidence-Gap Mapping
2.2. Risk of Bias of Animal Studies with Syrcle Tool
3. Discussion
3.1. Formulation Strategies for Enhancing Wound Healing via Skin Microbiome Modulation
3.1.1. Probiotics Formulations
Gels
Ointments
Powder Microparticles
Microbeads
Films
Scaffolds
Other Formulations
Formulation Stability
3.1.2. Synbiotic Formulations (Live Microorganisms with Prebiotics)
3.1.3. Postbiotic Formulations (Nonviable Components or Metabolites)
3.2. Clinical Evidence of Postbiotic and Probiotics Formulations for Wound-Healing Therapy
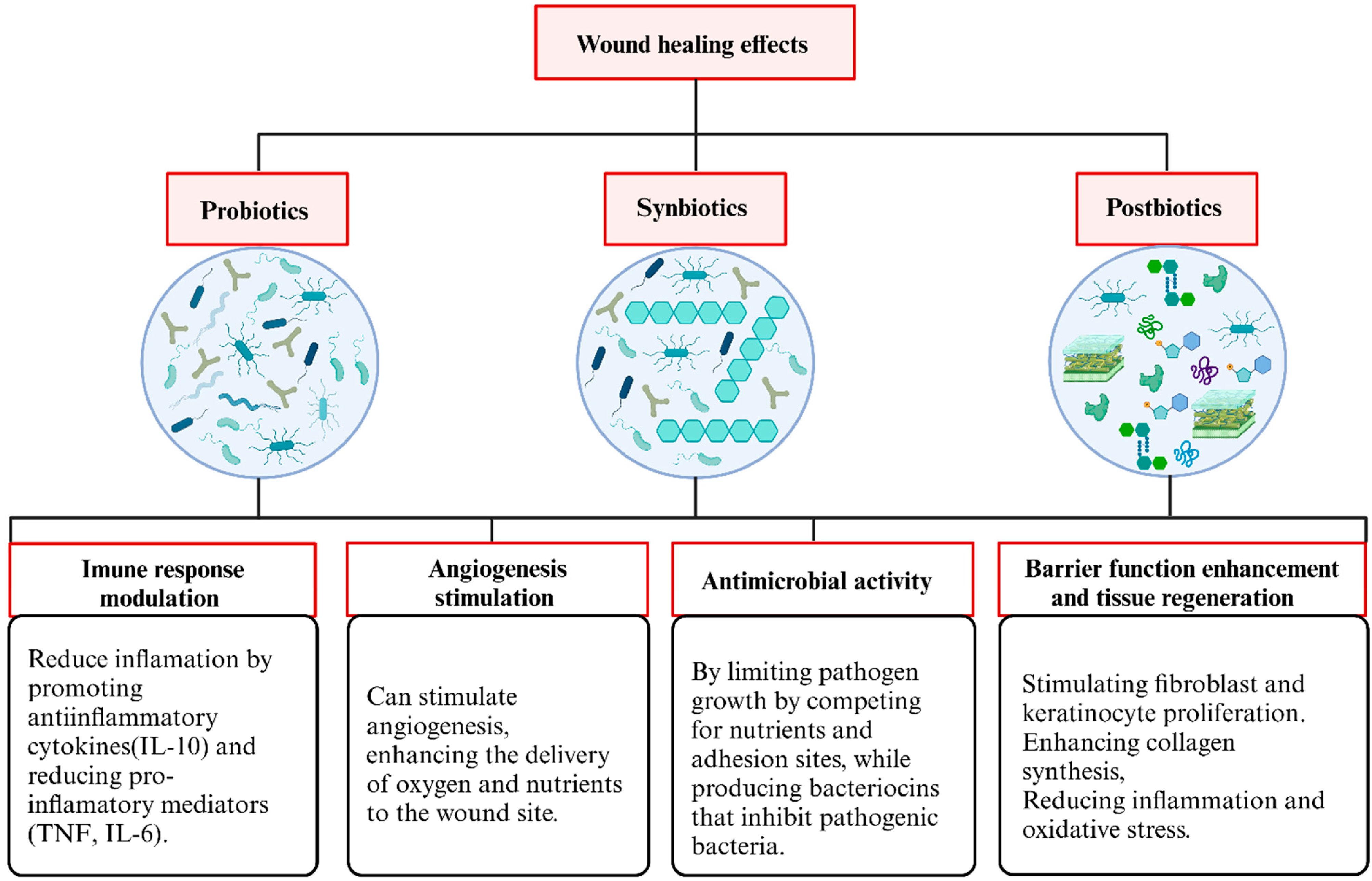
3.3. Overview of the Functions of Probiotics, Synbiotics, and Postbiotics Identified in This Review
4. Expert Opinion and Considerations for Probiotic-, Synbiotic-, and Postbiotic-Based Wound Therapies
5. Materials and Methods
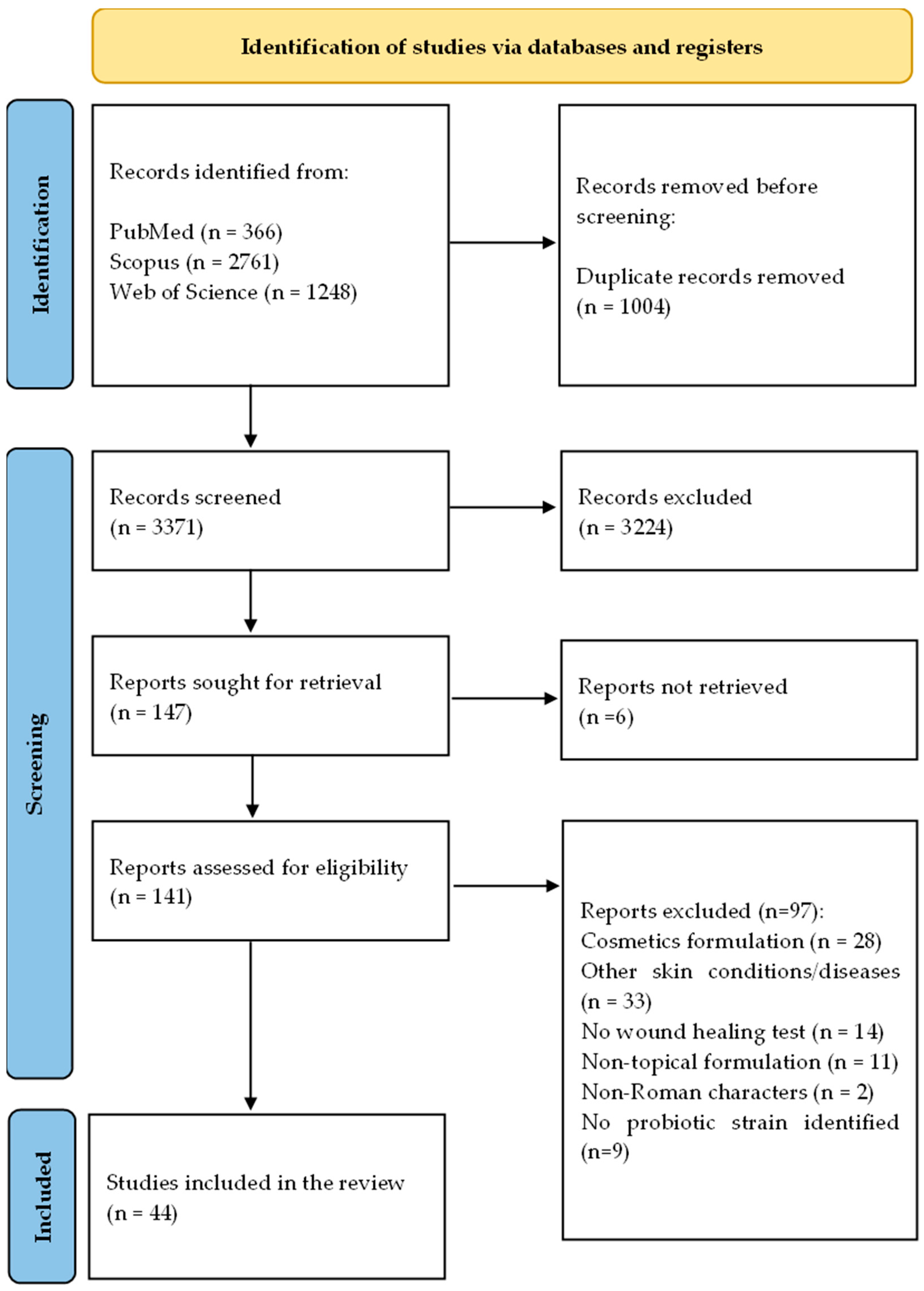
5.1. Search Strategy and Eligibility Criteria
5.2. Study Selection
5.3. Data Extraction and Synthesis
5.4. Quality Assessment
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trøstrup, H.; Bjarnsholt, T.; Kirketerp-Møller, K.; Høiby, N.; Moser, C. What is New in the Understanding of Non Healing Wounds Epidemiology, Pathophysiology, and Therapies. Ulcers 2013, 2013, 625934. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic Wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound Management for the 21st Century: Combining Effectiveness and Efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef]
- Sangha, M.S.; Deroide, F.; Meys, R. Wound Healing, Scarring and Management. Clin. Exp. Dermatol. 2024, 49, 325–336. [Google Scholar] [CrossRef]
- Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical Challenges of Chronic Wounds: Searching for an Optimal Animal Model to Recapitulate Their Complexity. Dis. Model. Mech. 2014, 7, 1205. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Zeng, A.; Long, X.; Yu, N.; Wang, X. The Role of the Skin Microbiome in Wound Healing. Burns Trauma 2024, 12, tkad059. [Google Scholar] [CrossRef]
- Karimi, F.; Montazeri-Najafabady, N.; Mohammadi, F.; Azadi, A.; Koohpeyma, F.; Gholami, A. A Potential Therapeutic Strategy of an Innovative Probiotic Formulation toward Topical Treatment of Diabetic Ulcer: An In Vivo Study. Nutr. Diabetes 2024, 14, 66. [Google Scholar] [CrossRef]
- Bruni, E.; Scaglione, G.L.; Tampone, D.; Primerano, A.; Bartolini, B.; Tenoglio, C.A.; Di Campli, C.; Collina, M.C.; Odorisio, T.; Failla, C.M. The Healing Process of Diabetic Ulcers Correlates with Changes in the Cutaneous Microbiota. Sci. Rep. 2024, 14, 27628. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Pawłowska, A.; Orzeł, A.; Sulej, L.; Muzyka-Placzyńska, K.; Baran, A.; Filipecka-Tyczka, D.; Pawłowska, P.; Nowińska, A.; Bogusławska, J.; et al. Wound Microbiota and Its Impact on Wound Healing. Int. J. Mol. Sci. 2023, 24, 17318. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Dissemond, J.; Romanelli, M. Inflammatory Skin Diseases and Wounds. Br. J. Dermatol. 2022, 187, 167–177. [Google Scholar] [CrossRef]
- Bădăluță, V.A.; Curuțiu, C.; Dițu, L.M.; Holban, A.M.; Lazăr, V. Probiotics in Wound Healing. Int. J. Mol. Sci. 2024, 25, 5723. [Google Scholar] [CrossRef]
- Ashoori, Y.; Mohkam, M.; Heidari, R.; Abootalebi, S.N.; Mousavi, S.M.; Hashemi, S.A.; Golkar, N.; Gholami, A. Development and In Vivo Characterization of Probiotic Lysate-Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing. BioMed Res. Int. 2020, 2020, 8868618. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, Y.; Xu, R.; Han, X.; Xiang, Z.; Guo, H.; Li, X.; Liang, J.; Zhang, X.; Fan, Y.; et al. Metal-Phenolic Self-Assembly Shielded Probiotics in Hydrogel Reinforced Wound Healing with Antibiotic Treatment. Mater. Horiz. 2023, 10, 3114–3123. [Google Scholar] [CrossRef]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S.; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight into the Beneficial Role of Lactiplantibacillus plantarum Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front. Pharmacol. 2021, 12, 728614. [Google Scholar] [CrossRef]
- Bazjoo, A.; Jafari, P.; Marjani, A.; Akbari, N. Effect of Cell-Free Supernatant of Bifidobacterium bifidum Combined with Chitosan Biodegradable Film on Full Thickness Wound Healing in Rats. Physiol. Pharmacol. 2022, 26, 468–479. [Google Scholar] [CrossRef]
- Farahani, F.H.; Moraffah, F.; Samadi, N.; Sharifzadeh, M.; Motasadizadeh, H.; Vatanara, A. Improved Infectious Burn Wound Healing by Applying Lyophilized Particles Containing Probiotics and Prebiotics. Int. J. Pharm. 2023, 636, 122800. [Google Scholar] [CrossRef] [PubMed]
- Mehdi-Alamdarloo, S.; Ameri, A.; Moghimipour, E.; Gholipour, S.; Saadatzadeh, A. Formulation Development of a Topical Probiotic Gel for Antidermatophytosis Effect. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e35893. [Google Scholar] [CrossRef]
- Sousa, M.A.d.S.d.; Ferreira, A.F.; da Silva, C.C.; Silva, M.A.; Bazan, T.A.X.N.; Monteiro, C.A.; Monteiro, A.d.S.; Sousa, J.C.d.S.; da Silva, L.C.N.; Zagmignan, A. Development and Characterization of Hydroxyethyl Cellulose-Based Gels Containing Lactobacilli Strains: Evaluation of Antimicrobial Effects in In Vitro and Ex Vivo Models. Pharmaceuticals 2023, 16, 468. [Google Scholar] [CrossRef]
- Brachkova, M.I.; Duarte, A.; Pinto, J.F. Alginate Films Containing Viable Lactobacillus plantarum: Preparation and In Vitro Evaluation. AAPS PharmSciTech 2012, 13, 357–363. [Google Scholar] [CrossRef]
- Jamaran, S.; Jafari, P.; Marjani, A.; Akbari, N.; Feizabad, M.M. Novel Wound Dressing Based on Postbiotic/Chitosan Film Accelerates Cutaneous Wound Healing. Jundishapur J. Microbiol. 2022, 14, e120806. [Google Scholar] [CrossRef]
- Horikawa, Y. Effects of Lactobacillus casei-Containing Ointment on the Healing and Protection against Opportunistic Infection of Thermal Injury Wounds in Mice. Hiroshima J. Med. Sci. 1986, 35, 1–14. [Google Scholar]
- Brachkova, M.I.; Marques, P.; Rocha, J.; Sepodes, B.; Duarte, M.A.; Pinto, J.F. Alginate Films Containing Lactobacillus plantarum as Wound Dressing for Prevention of Burn Infection. J. Hosp. Infect. 2011, 79, 375–377. [Google Scholar] [CrossRef]
- Jones, M.; Ganopolsky, J.G.; Labbé, A.; Gilardino, M.; Wahl, C.; Martoni, C.; Prakash, S. Novel Nitric Oxide Producing Probiotic Wound Healing Patch: Preparation and In Vivo Analysis in a New Zealand White Rabbit Model of Ischaemic and Infected Wounds. Int. Wound J. 2012, 9, 330–343. [Google Scholar] [CrossRef]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The Concurrent Use of Probiotic Microorganism and Collagen Hydrogel/Scaffold Enhances Burn Wound Healing: An In Vivo Evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, Z.; Ali, S.; Qamar, Z.; Imran, M.; Hafeez, F.Y. Fabrication of Electrospun Probiotic Functionalized Nanocomposite Scaffolds for Infection Control and Dermal Burn Healing in a Mice Model. ACS Biomater. Sci. Eng. 2019, 5, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Khodaii, Z.; Afrasiabi, S.; Hashemi, S.A.; Ardeshirylajimi, A.; Natanzi, M.M. Accelerated Wound Healing Process in Rat by Probiotic Lactobacillus reuteri Derived Ointment. J. Basic. Clin. Physiol. Pharmacol. 2019, 30, 20180150. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.T.; Luti, K.J.; Alaubydi, M.A. A probiotic application of Lactobacillus acidophilus HT1 for the treatment of some skin pathogens. Iraqi J. Agric. Sci. 2020, 51, 1559–1571. [Google Scholar] [CrossRef]
- Ben David, N.; Mafi, M.; Nyska, A.; Gross, A.; Greiner, A.; Mizrahi, B. Bacillus subtilis in PVA Microparticles for Treating Open Wounds. ACS Omega 2021, 6, 13647–13653. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory Effects of Lactobacillus plantarum-GMNL6 on Human Skin Health by Improving Skin Microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Kazemi, A.; Parisa, A.E.; Saeedi, P.; Halabian, R. Evaluation of Antioxidant and Antibacterial Effects of Lactobacilli Metabolites-Preconditioned Bone Marrow Mesenchymal Stem Cells in Skin Lesions Amelioration. Bioorg. Chem. 2022, 124, 105797. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.; Lee, S.M.; Woo, M.R.; Kim, D.W.; Kim, J.O.; Choi, H.G.; Jin, S.G. Development of Guar Gum-Based Dual-Layer Wound Dressing Containing Lactobacillus plantarum: Rapid Recovery and Mechanically Flexibility. Int. J. Biol. Macromol. 2022, 221, 1572–1579. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, D.; Shao, H.; Hao, Y.; Zhang, T.; Zheng, W.; Ji, Y.; Ling, P.; Lu, Y.; Zhou, Q. Injectable and Self-Healing Probiotics-Loaded Hydrogel for Promoting Superbacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550. [Google Scholar] [CrossRef]
- Hua, C.; Yang, F.; Jia, X.; Lu, Y.; Li, X.; Zhao, P.; Xing, M.; Lyu, G. Multi-Comparted Microgels Delivering Human Derived Probiotics and Deferoxamine for Multidrug-Resistant Infection and Healing. Chem. Eng. J. 2024, 483, 148432. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Wang, J.; Yao, M.; Peng, Y.; Liu, T.; Li, Z.; Luo, G.; Deng, J. Engineering Bacteria-Activated Multifunctionalized Hydrogel for Promoting Diabetic Wound Healing. Adv. Funct. Mater. 2021, 31, 2105749. [Google Scholar] [CrossRef]
- Yang, L.; Han, Z.; Chen, C.; Li, Z.; Yu, S.; Qu, Y.; Zeng, R. Novel Probiotic-Bound Oxidized Bletilla Striata Polysaccharide-Chitosan Composite Hydrogel. Mater. Sci. Eng. C 2020, 117, 111265. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, F.; Chen, Z.; Hu, T.; Qiu, R.; Wang, W.; You, W.; Su, X.; Hu, W.; Wang, Z. Coaxial Electrospun Nanofiber Accelerates Infected Wound Healing via Engineered Probiotic Biofilm. Int. J. Biol. Macromol. 2024, 279, 135100. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Y.; Jiang, X.; Wang, M.; Yuan, Y.; Zeng, Y.; Guo, L.; Li, W. Accelerated Infected Wound Healing by Probiotic-Based Living Microneedles with Long-Acting Antibacterial Effect. Bioact. Mater. 2024, 38, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Giordani, B.; Melgoza, L.M.; Prata, C.; Parolin, C.; Dalena, F.; Abruzzo, A.; Bigucci, F.; Luppi, B.; Vitali, B. New Spanish Broom Dressings Based on Vitamin E and Lactobacillus plantarum for Superficial Skin Wounds. J. Drug Deliv. Sci. Technol. 2020, 56, 101499. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K.; Krishnan, S. Antibacterial and Wound Healing Potential of Topical Formulation of Marine Symbiotic Bacillus. Arch. Microbiol. 2022, 204, 648. [Google Scholar] [CrossRef]
- Guan, W.; Gong, C.; Wu, S.; Cui, Z.; Zheng, Y.; Li, Z.; Zhu, S.; Liu, X. Instant Protection Spray for Anti—Infection and Accelerated Healing of Empyrosis. Adv. Mater. 2024, 36, 2306589. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Raut, J.; Kumar, S.; Singh, M.; Ahmed, B.; Singh, J.; Rana, V.; Rishi, P.; Ganesh, N.; Dua, K.; et al. Nanocurcumin and Viable Lactobacillus plantarum Based Sponge Dressing for skin Wound Healing. Int. J. Pharm. 2023, 643, 123187. [Google Scholar] [CrossRef]
- Yang, X.; Che, T.; Tian, S.; Zhang, Y.; Zheng, Y.; Zhang, Y.; Zhang, X.; Wu, Z. A Living Microecological Hydrogel with Microbiota Remodeling and Immune Reinstatement for Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, 2400856. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Song, J.; Zhou, L.; Wu, K.; Lu, X.; Zhai, X.; Wan, Z.; Gao, J. Highly Active Probiotic Hydrogels Matrixed on Bacterial EPS Accelerate Wound Healing via Maintaining Stable Skin Microbiota and Reducing Inflammation. Bioact. Mater. 2024, 35, 31–44. [Google Scholar] [CrossRef]
- Zouari, R.; Moalla-Rekik, D.; Sahnoun, Z.; Rebai, T.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Evaluation of Dermal Wound Healing and In Vitro Antioxidant Efficiency of Bacillus subtilis SPB1 Biosurfactant. Biomed. Pharmacother. 2016, 84, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Kalenova, L.F.; Melnikov, V.P.; Besedin, I.M.; Bazhin, A.S.; Gabdulin, M.A.; Kolyvanova, S.S. Reparation and Immunomodulating Properties of Bacillus sp. Metabolites from Permafrost. Bull. Exp. Biol. Med. 2017, 163, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.S.; Shabestari, T.M.; Heidari, F.; Koohi, S.R.; Afshar, M.; Omranian, R.; Nobakht, M. Macroscopic and Microscopic Survey of the Comparative Effects of Lactobacillus plantarum 299v, Its Supernatant, and Imipenem on Infectious Burn Wound Healing in Rats. Infect. Epidemiol. Microbiol. 2020, 6, 85–93. [Google Scholar] [CrossRef]
- Halper, J.; Leshin, L.S.; Lewis, S.J.; Li, W.I. Wound Healing and Angiogenic Properties of Supernatants from Lactobacillus Cultures. Exp. Biol. Med. 2003, 228, 1329–1337. [Google Scholar] [CrossRef]
- Sinha, A.; Sagar, S.; Madhumathy, M.; Osborne, W. Probiotic Bacteria in Wound Healing; An In-Vivo Study. Iran. J. Biotechnol. 2019, 17, e2188. [Google Scholar] [CrossRef]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A Novel Effective Formulation of Bioactive Compounds for Wound Healing: Preparation, In Vivo Characterization, and Comparison of Various Postbiotics Cold Creams in a Rat Model. Evid.-Based Complement. Altern. Med. 2021, 2021, 8577116. [Google Scholar] [CrossRef]
- Shokatayeva, D.; Savitskaya, I.; Kistaubayeva, A. Wound-Healing Activity of Immobilized Postbiotics from Bacillus subtilis Exometabolites. BIO Web Conf. 2021, 40, 01002. [Google Scholar] [CrossRef]
- Nazari, N.; Imani, R.; Nasiraie, L.R. Fiber/Hydrogel Hybrid Wound Dressing Based on Eggshell Membrane Containing Postbiotic Ingredients. Biomater. Adv. 2024, 165, 214004. [Google Scholar] [CrossRef]
- Ekrami, E.; Mahmoudifard, M.; Khodabandeh Shahraky, M. Lactosporin Loaded Electrospun Nanofibrous Membrane: Novel Antibacterial and Wound Dressing Patch. J. Drug Deliv. Sci. Technol. 2024, 96, 105635. [Google Scholar] [CrossRef]
- Kuhn, T.; Aljohmani, A.; Frank, N.; Zielke, L.; Mehanny, M.; Laschke, M.W.; Koch, M.; Hoppstädter, J.; Kiemer, A.K.; Yildiz, D.; et al. A Cell-Free, Biomimetic Hydrogel Based on Probiotic Membrane Vesicles Ameliorates Wound Healing. J. Control. Release 2024, 365, 969–980. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sharma, M.; Parul; Raut, S.; Gupta, P.; Khatri, N. Healing Wounds, Defeating Biofilms: Lactiplantibacillus plantarum in Tackling MRSA Infections. Front. Microbiol. 2023, 14, 1284195. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Xu, Y.; Zheng, B.; Li, Y.; Zhang, J.; Liu, Z.; Wang, X.; Zhou, Z.; Zeng, D.; Lu, F.; et al. The Core-Shell Microneedle with Probiotic Extracellular Vesicles for Infected Wound Healing and Microbial Homeostasis Restoration. Small 2024, 20, 2401551. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, C.; Hertz-Kleptow, D.; Zoschke, C.; Wanjiku, B.; Wentzien-Odenthal, A.; Kerscher, M.; Schäfer-Korting, M. Reconstructed Human Epidermis Predicts Barrier-Improving Effects of Lactococcus lactis Emulsion in Humans. Skin Pharmacol. Physiol. 2019, 32, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the Skin Microbiota in Health and Disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between Skin Microbiota and Immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-Țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Bianconi, I.; Aschbacher, R.; Pagani, E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics 2023, 12, 1580. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S RRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Regina de Souza, C.; Luna Lazo, R.E.; Mainka, F.; Zinco, A.G.; Mengarda, M.; de Fátima Bonetti, A.; Murakami, F.S. Efficacy and Safety of Probiotics in the Treatment of Depression and Anxiety: An Umbrella Review of Systematic Reviews of Randomized Clinical Trials. PharmaNutrition 2023, 26, 100362. [Google Scholar] [CrossRef]
- Tsiouris, C.G.; Tsiouri, M.G. Human Microflora, Probiotics and Wound Healing. Wound Med. 2017, 19, 33–38. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Cheng, G. Human Skin Bacterial Microbiota Homeostasis: A Delicate Balance between Health and Disease. mLife 2023, 2, 107–120. [Google Scholar] [CrossRef]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in Burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef]
- Gudadappanavar, A.; Hombal, P.; Timashetti, S.; Javali, S. Influence of Lactobacillus acidophilus and Lactobacillus plantarum on Wound Healing in Male Wistar Rats—An Experimental Study. Int. J. Appl. Basic Med. Res. 2017, 7, 233. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Butler, É.; Lundqvist, C.; Axelsson, J. Lactobacillus reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms 2020, 8, 1026. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Duranti, S.; Bottacini, F.; Guglielmetti, S.; Van Sinderen, D.; Ventura, M. Bifidobacterium bifidum as an Example of a Specialized Human Gut Commensal. Front. Microbiol. 2014, 5, 437. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut–Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Van Staden, A.D.P.; Heunis, T.; Smith, C.; Deane, S.; Dicks, L.M.T. Efficacy of Lantibiotic Treatment of Staphylococcus aureus-Induced Skin Infections, Monitored by In Vivo Bioluminescent Imaging. Antimicrob. Agents Chemother. 2016, 60, 3948–3955. [Google Scholar] [CrossRef]
- Pavlou, P.; Tiligada, Z.; Xagorari, V.; Papageorgiou, S.; Varvaresou, A. Microbiome Technology in Strengthening the Skin’s Natural Barrier. Ep. Klin. Farmakol. Farmakokinet. 2023, 41, 49–54. [Google Scholar]
- Canchy, L.; Kerob, D.; Demessant, A.; Amici, J. Wound Healing and Microbiome, an Unexpected Relationship. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 7–15. [Google Scholar] [CrossRef]
- Peral, M.C.; Rachid, M.M.; Gobbato, N.M.; Huaman Martinez, M.A.; Valdez, J.C. Interleukin-8 Production by Polymorphonuclear Leukocytes from Patients with Chronic Infected Leg Ulcers Treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010, 16, 281–286. [Google Scholar] [CrossRef]
- Jebur, M.S. Therapeutic Efficacy of Lactobacillus acidophilus against Bacterial Isolates from Burn Wounds. N. Am. J. Med. Sci. 2010, 2, 586–591. [Google Scholar] [CrossRef]
- Rasheed, H.T.; Luti, K.J.K.; Alaubydi, M.A. Purification and characterization of bacteriocin from Lactobacillus Acidophilus HT1 and its application in a cream formula for the treatment of some skin pathoges. Iraqi J. Agric. Sci. 2020, 51, 1381–1393. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An Injectable Self-Healing Coordinative Hydrogel with Antibacterial and Angiogenic Properties for Diabetic Skin Wound Repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Huang, T.Y.; Wang, H.L.; Chien, P.J.; Chang, W.W.; Lee, H.T. Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds. Cells 2021, 10, 3264. [Google Scholar] [CrossRef] [PubMed]
- Kasatkin, M.; Smirnova, L.; Babaskin, D. Therapeutic Effects of Probiotic Ointment for Atopic Dermatitis. Res. J. Pharm. Technol. 2021, 14, 6041–6048. [Google Scholar] [CrossRef]
- Falholt Elvebakken, H.; Bruntse, A.B.; Vedel, C.; Kjærulff, S. Topical Lactiplantibacillus plantarum LB244R® Ointment Alleviates Skin Aging: An Exploratory Trial. J. Cosmet. Dermatol. 2023, 22, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Azadi, A.; Omidifar, N.; Najafabady, N.M.; Mohammadi, F.; Kazemi, R.; Gholami, A. Pharmacotechnical Aspects of a Stable Probiotic Formulation toward Multidrug-Resistance Antibacterial Activity: Design and Quality Control. BMC Complement. Med. Ther. 2023, 23, 391. [Google Scholar] [CrossRef]
- Xu, X.; Al-Ghabeish, M.; Krishnaiah, Y.S.R.; Rahman, Z.; Khan, M.A. Kinetics of Drug Release from Ointments: Role of Transient-Boundary Layer. Int. J. Pharm. 2015, 494, 31–39. [Google Scholar] [CrossRef]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible Films and Coatings Functionalization by Probiotic Incorporation: A Review. Polymers 2019, 12, 12. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The Role of Biomaterials-Based Scaffolds in Advancing Skin Tissue Construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.d.S.; Spier, M.R.; Medeiros, A.P.B.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Nualkaekul, S.; Deepika, G.; Charalampopoulos, D. Survival of Freeze Dried Lactobacillus plantarum in Instant Fruit Powders and Reconstituted Fruit Juices. Food Res. Int. 2012, 48, 627–633. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Mousavi Khaneghah, A. Alginate and Derivatives Hydrogels in Encapsulation of Probiotic Bacteria: An Updated Review. Food Biosci. 2023, 52, 102433. [Google Scholar] [CrossRef]
- Yin, Z.; Qiu, Y.; Han, Y.; Li, K. Topical Probiotics in Wound Care: A Review of Effects, Mechanisms, and Applications. Interdiscip. Nurs. Res. 2024, 3, 63–71. [Google Scholar] [CrossRef]
- Chen, H.-J.; Lin, D.-A.; Liu, F.; Zhou, L.; Liu, D.; Lin, Z.; Yang, C.; Jin, Q.; Hang, T.; He, G.; et al. Transdermal Delivery of Living and Biofunctional Probiotics through Dissolvable Microneedle Patches. ACS Appl. Bio Mater. 2018, 1, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, H.; Abbas, Z.; Zhang, J.; Wang, J.; Cheng, Q.; Peng, S.; Yang, T.; Bai, T.; Zhou, Y.; et al. Optimizing Postbiotic Production through Solid-State Fermentation with Bacillus amyloliquefaciens J and Lactiplantibacillus plantarum SN4 Enhances Antibacterial, Antioxidant, and Anti-Inflammatory Activities. Front. Microbiol. 2023, 14, 1229952. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Dinić, M.; Burgess, J.L.; Lukić, J.; Catanuto, P.; Radojević, D.; Marjanović, J.; Verpile, R.; Thaller, S.R.; Gonzalez, T.; Golić, N.; et al. Postbiotic Lactobacilli Induce Cutaneous Antimicrobial Response and Restore the Barrier to Inhibit the Intracellular Invasion of Staphylococcus aureus In Vitro and Ex Vivo. FASEB J. 2024, 38, e23801. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2023, 13, 89. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.; Baek, J.; Kim, W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus Chungangensis CAU 1447 on Diabetic Mice. Nutrients 2021, 13, 2666. [Google Scholar] [CrossRef]
- Myo, N.Z.; Kamwa, R.; Khurajog, B.; Pupa, P.; Sirichokchatchawan, W.; Hampson, D.J.; Prapasarakul, N. Industrial Production and Functional Profiling of Probiotic Pediococcus acidilactici 72 N for Potential Use as a Swine Feed Additive. Sci. Rep. 2025, 15, 14940. [Google Scholar] [CrossRef]
- Venkatesh, G.P.; Kuruvalli, G.; Syed, K.; Reddy, V.D. An Updated Review on Probiotic Production and Applications. Gastroenterol. Insights 2024, 15, 221–236. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid.-Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]

| Author/Year | Formulation | Probiotic Strain | In Vitro Activity | Type of Wound; In Vivo Model | Main Outcomes |
|---|---|---|---|---|---|
| Horikawa Y. (1986) [27] | Ointment | Lactobacillus casei | Not found | Burned skin; mice | The L. casei ointment improved healing of thermal injury wounds, eliminated bacteria, and enhanced collagen formation in mice. Its effects were superior to standard treatments, including the ointments Eksalb and Azunol and the creams mafenide acetate and silver sulfadiazine. |
| Brachkova, MI. et al. (2011) [28] | Film | Lactobacillus plantarum | Not found | Burned skin; Wistar rats | Films incorporating L. plantarum at cell concentrations of 108 CFU/mL caused a 5–6 log10 reduction in P. aeruginosa in the model burn wounds. L. plantarum immobilized in freeze-dried calcium alginate films remained viable during six months of storage at 4 °C. |
| Jones M. et al. (2012) [29] | Adhesive patches | Lactobacillus fermentum | Antibacterial activity; Trichophyton rubrum, Trichophyton mentagrophytes, E. coli, S. aureus MRSA, P. aeruginosa, Acinetobacter baumannii | Excisional wound; New Zealand white rabbits | The study demonstrated the efficacy and safety of a probiotic patch containing lyophilized alginate microbeads with L. fermentum 7230, capable of producing gNO, for healing ischemic and infected wounds. |
| Oryan, A. et al. (2018) [30] | Scaffolds | Saccharomyces cerevisiae | Not found | Burned skin; Sprague Dawley rats | The CH-S biological dressing combined with the probiotic microorganism S. cerevisiae significantly increased collagen content and improved the biomechanical properties of healing burned wounds in rats. |
| Khan MA. et al. (2019) [31] | Scaffolds | Enterococcus mundtii | S. aureus | Burned skin; BALB/c mice | A comparative wound closure, histopathology, and wound microbial evaluation demonstrated that the bioscaffolds accelerate epithelialization, collagen deposition, and hair follicle formation, inhibit harmful bacteria, and provide interference benefits. |
| Khodaii Z. et al. (2019) [32] | Ointment | Lactobacillus reuteri | Not found | Excisional wound; Sprague Dawley rats | The probiotic ointment was effective for wound healing, including reducing inflammation, increasing collagen synthesis, decreasing lipid peroxidation and the activity of the MPO, speeding epithelialization, and increasing the percentage of wound contraction. |
| Rasheed, HT. et al. (2020) [33] | Emulgel | Lactobacillus acidophilus | Antibacterial activity: P. aeruginosa, S. aureus, and Staphylococcus epidermidis | Excisional wound; Albino mice | In vivo, L. acidophilus HT1 biomass effectively treated wounds infected with various bacterial pathogens within seven days, outperforming the control groups. |
| Ben David N. et al. (2021) [34] | Microparticles | Bacillus subtilis | Antibacterial activity; MRSA, S. aureus. Cytotoxicity; NIH 3T3 fibroblast. | Excisional wound; C57BL mice | B. subtilis in PVA microparticles showed strong antibacterial activity against MRSA and S. aureus. In in vivo experiments, both B. subtilis and empty PVA microparticles reduced healing time, with B. subtilis microparticles being more effective in the first week. No skin irritation, infection, or adverse effects were observed during the 15-day postoperative period. |
| Dubey AK. et al. (2021) [20] | Gel | Lactiplantibacillus plantarum | Human lung carcinoma | Excisional wound; BALB/c mice | Topical application of Lp2621 to infected and uninfected wounds promoted rapid healing by enhancing angiogenesis, fibroblast proliferation, re-epithelialization, and recruitment of PMNLs. |
| Ming Z. et al. (2021) [35] | Hydrogel microspheres | Lactobacillus reuteri | Antibacterial activity; E. coli, S. aureus, and Salmonella spp. Cytocompatibility; Mouse fibroblasts L929 | Excisional wound; BALB/c mice | This hydrogel, containing live bacteria, significantly reduced bacterial growth in infected skin, exhibited anti-inflammatory properties and effectively promoted wound healing and tissue regeneration. |
| Tsai, WH. et al. H. et al. (2021) [36] | Gel | L. plantarum GMNL-6, Lacticaseibacillus paracasei GMNL-653 | Skin wound repair; Human foreskin fibroblasts | Excisional wound; BALB/c mice | Gels containing heat-killed GMNL-6 or GMNL-653 applied to experimental wounds on mouse tails promoted healing. Lipoteichoic acid provided anti-fibrogenic benefits similar to the heat-killed bacteria in the TGF-β-stimulated Hs68 fibroblast cell model. |
| Kazemi A. et al. (2022) [37] | Ointment | Lactobacillus plantarum, Lactobacillus casei | Antibacterial activity; P. aeruginosa. Cell viability; Bone marrow MSCs | Excisional wound; BALB/c mice | Probiotic metabolites and MSCs independently promote wound healing and, when administered together, exhibit a synergistic effect, leading to faster wound area reduction. |
| Kim, JS et al. (2022) [38] | Hydrogel, wound dressing | Lactobacillus plantarum | Not found | Excisional wound; Sprague Dawley rats | The guar-gum-based dual-layer wound dressing with L. plantarum demonstrated superior swelling capacity, mechanical properties, and promoted rapid wound recovery with complete re-epithelialization. |
| Mei L. et al. (2022) [39] | Hydrogel | Lactobacillus rhamnosus | Antibacterial activity; P. aeruginosa. Cytotoxicity assay; Mouse fibroblasts L929 | Infected wound healing; Sprague Dawley rats | Hydrogel significantly suppressed bacteria-induced infection, increased the formation of re-epithelialization and collagen, and promoted wound healing, comparable to the commercial Prontosan gel. |
| Sousa MADS. et al. (2023) [24] | Gel | Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, Limosilactobacillus fermentum, | Antibacterial activity; S. aureus, Klebisiella pneumoniae, Enterococcus faecalis, P. aeruginosa. Wound infection using porcine skin (ex vivo) | Not found | Lactobacilli strains incorporated into hydroxyethyl cellulose-based gels (Natrosol) showed antimicrobial effects. In the ex vivo assay using porcine skin, the LP-G18-A11 gel (5%) significantly reduced the skin loads of S. aureus and P. aeruginosa after 24 h. In contrast, only P. aeruginosa was reduced after 72 h. |
| Zhou C. et al. (2023) [19] | Hydrogel | Lactobacillus reuteri | Cytotoxicity; Mouse fibroblasts L929. Angiogenesis. Human umbilical vein endothelial | Excisional wound; BALB/c mice | The Gel/L@FeTA hydrogels presented a better performance than the Gel/L in inflammatory regulation, angiogenesis, and tissue regeneration both in in vitro and in vivo models in the presence of antibiotics. |
| Hua, C. et al. (2024) [40] | Microgels | Lactobacillus fermentum | Antibacterial activity; P. aeruginosa; Mouse fibroblasts L929 cells, Human umbilical venous endothelial cells (HUVECs) Angiogenesis. | Excisional wound; Sprague Dawley rats | The microgel system incorporating Lactobacillus fermentum and deferoxamine effectively managed multidrug-resistant P. aeruginosa and promoted wound healing. The system showed good biocompatibility and hemocompatibility. |
| Lu, Y. (2021) [41] | Hydrogel | Lactococcus lactis | Human umbilical vein endothelial (HUVECs), bone marrow-derived macrophages, S. aureus | Diabetic wound | This study introduces a thermoresponsive hydrogel with living Lactococcus and heparin-poloxamer to bioengineer the wound microenvironment and promote angiogenesis. The system enhances VEGF production, endothelial cell activity, and macrophage anti-inflammatory shifts, facilitating diabetic wound healing while minimizing systemic toxicity risks. |
| Yang, L. et al. (2020) [42] | Hydrogel | Lactobacillus plantarum | Antibacterial activity; S. aureus, P. aeruginosa, E coli. Cytotoxicity; Mouse fibroblasts L929. | Excisional wound; Kunming mice | The hydrogel with Lactobacillus plantarum exhibited potent antibacterial activity, excellent biocompatibility, and promoted L929 cell proliferation. In a full-thickness skin defect model, it accelerated wound healing by maintaining moisture, enhancing VEGF expression, reducing inflammation, boosting collagen deposition, and minimizing scarring. |
| Huang, B. et al. (2024) [43] | Nanofiber films | Lactobacillus paracasei | Antibacterial activity; E. coli, S. aureus | Infected wound healing; SD rats | L. paracasei biofilms demonstrated superior antibacterial activity against pathogenic bacteria, including S. aureus, given their ability to activate M2 macrophages, which are key participants in the immune response and tissue repair processes. |
| Jin, Y. et al. (2024) [44] | Microneedles patch | Lactobacillus reuteri | Antibacterial activity; S. aureus, P. aeruginosa, and E. coli. NIH-3T3 cells, Human umbilical venous endothelial cells (HUVECs) | Excisional wound; SPF BALB/c female mice, | In a mouse model of Staphylococcus aureus-infected wounds, a single administration of the microneedle patch exhibited superior antimicrobial efficiency and wound healing performance compared with control groups. |
| Author/Year | Formulation | Probiotic Strain | Prebiotic | In Vitro Activity | Type of Wound; In Vivo Model | Main Outcomes |
|---|---|---|---|---|---|---|
| Cerchiara et al. (2020) [45] | Freeze-dried dressings | Lactobacillus plantarum | Vitamin E | Biocompatibility in human fibroblast; Antibacterial activity against S. aureus and P. aeruginosa | Not found | These formulations were not toxic to human fibroblast cells and assured a sustained release of Vitamin E, preserving its antioxidant property, and showing good antibacterial activity against S. aureus and P. aeruginosa. |
| Kizhakkekalam et al. (2022) [46] | Gel | Bacillus amyloliquefaciens | Macroalgal polysaccharide | Cell migration studies on L929 cell lines; Antibacterial activity against P. aeruginosa MDR, S. pyogenes, E. coli, S. aureus MRSA, K. pneumoniae | Not found | The topical formulation containing the organic extract of marine synbiotic B. amyloliquefaciens MTCC 12716 stimulated epithelial wound healing and improved wound closure. Promising antibacterial properties against clinical wound isolates were also reported |
| Guan et al. (2023) [47] | Spray/hydrogel film | Lactobacillus casei | Flavones | Biosecurity using fibroblast NIH-3T3 cells and red blood cells, and chorioallantoic membrane (CAM) test; Antibacterial assessment against MRSA and E. coli | Infected burn wound; Wister rats | The instant protection spray formed a protective barrier for burns within 30 s, sterilizing 100% of MRSA in vitro and 96.14% in vivo. |
| Farahani et al. (2023) [22] | Powder particles | Lactiplantibacillus plantarum | FOS | Not found | Infected burn wound; Wistar rats | Chitosan-alginate particles showed antibacterial activity and accelerated wound healing. FOS * enhanced L. plantarum stability and survival. |
| Sandhu et al. (2023) [48] | Film | Lactobacillus plantarum UBLP-40 | Curcumin | Antimicrobial activity against S. aureus | Excisional wound; Lacca mice | Curcumin-loaded SLNs with probiotics boosted antimicrobial effects against S. aureus by 560%, accelerated wound closure, reduced bioburden and inflammation, and enhanced healing through growth factors and antioxidants. |
| Yang et al. (2024) [49] | Hydrogel | Lactobacillus plantarum | FOS | Cytotoxicity in fibroblasts NIH/3T3 and human umbilical venous endothelial cells; Cell migration assay in fibroblasts NIH/3T3; Antibacterial activity against S. aureus and P. aeruginosa | Diabetic infectious wounds; Sprague Dawley rats | A living microecological hydrogel containing L. plantarum and FOS * (LP/FOS@Gel) remodeled dysregulated skin microbiota, promoted the proliferation of beneficial bacteria, eliminated pathogenic colonization, and modulated immune responses. |
| Xu, H. et al. (2024) [50] | Hydrogel | Lactobacillus paracasei, Bacillus velezensis | Extracellular polysaccharide EPS-M76 | Antibacterial activity; E. coli, S. aureus; L929 cells | Excisional wound; SD rats | Live probiotic hydrogels reduced the incidence of inflammation during wound healing by promoting angiogenesis and increasing collagen deposition. |
| Author/Year | Formulation | Probiotic Strain | Postbiotic | In Vitro Activity | Type of Wound; In Vivo Model | Main Outcomes |
|---|---|---|---|---|---|---|
| Zouari et al. (2016) [51] | Gel | Bacillus subtilis SPB1 | Crude lipopeptide biosurfactant from cell-free supernatant | Not found | Excisional wound; Wistar rats | The gel containing biosurfactant accelerated wound healing, with lipopeptides showing strong antioxidant, antimicrobial, and antifungal properties. |
| Kalenova et al. (2017) [52] | Ointment | Bacillus sp. | Cell-free metabolites | Not found | Excisional wound; BALB/c mice | Bacillus sp. metabolites promoted 30% faster epithelialization, enhanced immunity, reduced scarring, and supported hair recovery, outperforming Solcoseryl. |
| Moghadam, S.S. et al. (2020) [53] | Ointment | Lactobacillus plantarum | Cell-free supernatant | Not found | Burned skin; Wistar rats | Ointment containing the L. plantarum supernatant had a significantly smaller wound size than the imipenem group. Histological analysis revealed better skin repair in the probiotic cell pellet group. |
| Halper, J. et al. (2003) [54] | Gel | Lactobacillus acidophilus | Cell-free supernatant | Mouse embryonal kidney fibroblastic AKR-2B; Murine macrophage J774.A1; Porcine kidney LLC-PK1 | Excisional wound; Swiss NIH mice; Sprague Dawley rats | The study demonstrates the potential of Lactobacillus strains (ATCC 4356 and 43121) as stimulators of the inflammatory stage of tissue repair, TNF-alpha production, and angiogenesis. |
| Sinha, A. et al. (2019) [55] | Gel | Lactobacillus (VITSAMJ1) | Cell-free supernatant | Antibacterial activity; S. aureus | Excisional wound; Wistar rats | Animals treated with the probiotic gel showed better wound healing compared to the control groups. |
| Ashoori, Y. et al. (2020) [18] | Nanogel | Bacillus subtilis sp. natto, Lactobacillus fermentum, Lactobacillus reuteri. | Cell-free supernatant | Not found | Excisional wound; Sprague- Dawley rats | B. subtilis sp. natto has a better wound healing efficacy, as demonstrated in pathology examination. Favorable effects of probiotic lysate nanogels, including the reasonable wound closing rate, good wound appearance, and good histological observation, were confirmed in vivo. |
| Golkar et al. (2021) [56] | Cream | Bacillus subtilis sp. natto, Lactobacillus reuteri, Lactobacillus fermentum | Cell-free supernatant | Not found | Excisional wound; Sprague Dawley rats | Postbiotic formulations accelerated wound healing. B. subtilis natto cold cream showed the best results |
| Jamaran et al. (2021) [26] | Film | Lactobacillus reuteri | Cell-free supernatant | Antibacterial activity against P. aeruginosa, S. aureus, and E. coli | Excisional wound; Wistar rats | The postbiotic/CS/PEG treatment accelerated wound healing, enhanced cytokine and chemokine expression, promoted immune cell activity, and improved collagen and elastin deposition, enhancing wound integrity. |
| Shokatayeva et al. (2021) [57] | Film | Bacillus subtilis P-2 | Cell-free supernatant | Antibacterial activity against E. coli, P. aeruginosa, S. aureus, and S. epidermidis | Excisional wound; Mongrel rats | Postbiotic integrated into a biocomposite of bacterial cellulose and chitosan reduced wound healing time by 20% in animals, being also effective against Gram-positive and Gram-negative bacteria. |
| Bazjoo, A. et al. (2022) [21] | Film | Bifidobacterium bifidum | Cell-free supernatant | Not found | Excisional wound; Wistar rats | The biodegradable film based on chitosan and CFS of B. bifidum improved the wound healing process. |
| Nazari et al. (2024) [58] | Fiber/ Hydrogel (hybrid wound dressing) | Lactobacillus plantarum | Exopolysaccharide | Cell viability, proliferation, and attachment using human dermal fibroblast (HDF) | Not found | A fiber–hydrogel dressing using eggshell membrane enriched with postbiotic compounds (EPS) from L. plantarum (10 mg/mL) enhanced cell proliferation within five days. |
| Ekrami et al. (2024) [59] | Nanofibrous membrane | Bacillus coagulans | Lactosporin | Cell toxicity in L929 mouse fibroblast; Antibacterial activity against M. luteus, E. coli, Pseudomonas, S. aureus, S. epidermis, and K. pneumoniae | Excisional wound; Sprague Dawley male rats | The hyaluronic acid-based nanofibers loaded with Lactosporin demonstrated antimicrobial efficacy, which was favorable for the wound healing process. |
| Kuhn, T. et al. (2024) [60] | Hydrogel on membrane | Lactobacillus casei, Lactobacillus plantarum | Cell-free extracellular vesicles | Viability assay; Human immortal keratinocyte HaCaT; Monocyte-like THP-1; Peripheral blood mononuclear | Excisional wound; Mice | Hydrogels containing cell-free extracellular vesicles derived from L. casei and L. plantarum improved healing in an in vivo mouse full-thickness wound model. |
| Dubey, AK. et al. (2023) [61] | Gel | Lactiplantibacillus plantarum | Cell-free supernatant | Immune respond; Human leukemia monocyte cell line T-helper; Antibacterial activity; MRSA | Excisional wound; BALB/c mice | Lp2621, a probiotic cell-free supernatant (CFS), had potent antibacterial and antioxidant properties. It also exhibited in vitro biofilm inhibition and eradication activity and anti-MRSA activity. |
| Qi, F. et al. (2024) [62] | Microneedle patch | Lactobacillus druckerii | Extracellular vesicles | Antibacterial activity; Staphylococcus aureus, Escherichia coli; HaCaT; Murine fibroblasts | Excisional wound; Balb/c mice | Core–shell microneedle with sequential delivery of tannic acid–magnesium (TA-Mg) complexes and Lactobacillus druckerii extracellular vesicles (LDEVs). CSMN@TA-Mg/LDEV increased microbial diversity at wound sites. |
| Hausmann, C. et al. (2019) [63] | Emulsion | Lactococcus lactis | Lactococcus lactis lysate | Reconstructed human epidermis | A clinical trial was conducted with 21 women (aged 35–59 years) with Fitzpatrick skin type II–IV, and it was a randomized controlled double-blind trial. | L. lactis formulations enhance the skin barrier by increasing filaggrin and β-defensin-2 expression, reducing TEWL by 18%, and lowering permeability to caffeine. They also improve hydration and surface pH without causing irritation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, P.; Ribeiro, F.N.; Giublin, F.C.W.; Mieres, N.G.; Tonin, F.S.; Pontarolo, R.; Sari, M.H.M.; Lazo, R.E.L.; Ferreira, L.M. Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations. Pharmaceuticals 2025, 18, 704. https://doi.org/10.3390/ph18050704
Machado P, Ribeiro FN, Giublin FCW, Mieres NG, Tonin FS, Pontarolo R, Sari MHM, Lazo REL, Ferreira LM. Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations. Pharmaceuticals. 2025; 18(5):704. https://doi.org/10.3390/ph18050704
Chicago/Turabian StyleMachado, Patrícia, Felipe Neme Ribeiro, Fernanda Cristina Wroblevski Giublin, Naomi Gerzvolf Mieres, Fernanda Stumpf Tonin, Roberto Pontarolo, Marcel Henrique Marcondes Sari, Raul Edison Luna Lazo, and Luana Mota Ferreira. 2025. "Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations" Pharmaceuticals 18, no. 5: 704. https://doi.org/10.3390/ph18050704
APA StyleMachado, P., Ribeiro, F. N., Giublin, F. C. W., Mieres, N. G., Tonin, F. S., Pontarolo, R., Sari, M. H. M., Lazo, R. E. L., & Ferreira, L. M. (2025). Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations. Pharmaceuticals, 18(5), 704. https://doi.org/10.3390/ph18050704









