The Toxicological Profile of Active Pharmaceutical Ingredients–Containing Nanoparticles: Classification, Mechanistic Pathways, and Health Implications
Abstract
1. Introduction
2. Types of Toxic Nanoparticles
2.1. Metal-Based Nanoparticles
2.2. Carbon-Based Nanoparticles
2.3. Lipid-Based Nanoparticles
2.4. Protein-Based Nanoparticles
2.5. Polymeric Nanoparticles
2.6. Silica Nanoparticles
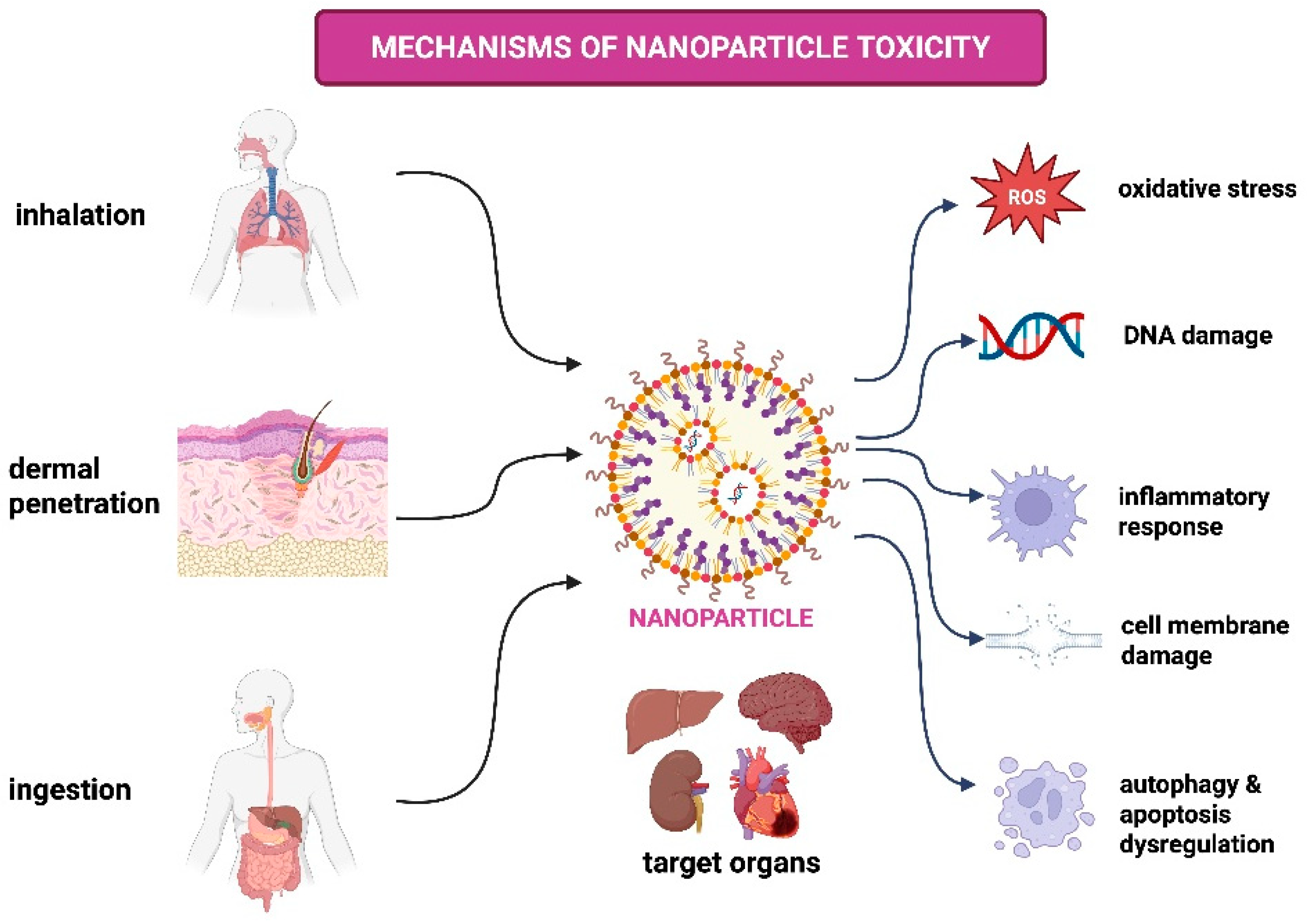
3. Mechanisms of NP Toxicity
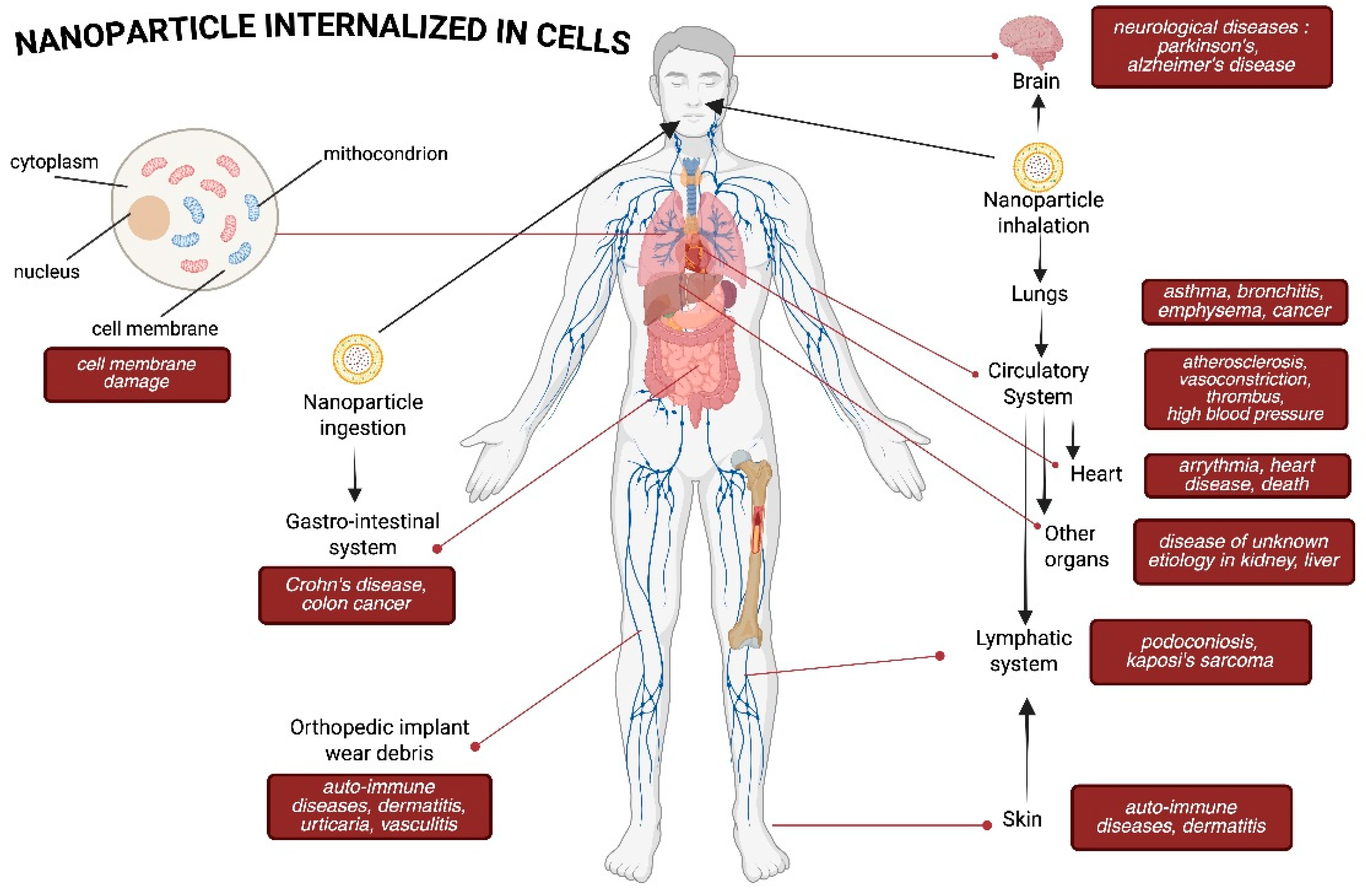
4. Health Impacts of Nanoparticle Exposure
4.1. Respiratory System
4.2. Nervous System
4.3. Immune System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Jayaraman, S.; Muthukaliannan, G.K. A Comprehensive Review on Corn Starch-Based Nanomaterials: Properties, Simulations, and Applications. Polymers 2020, 12, 2161. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical Applications and Therapeutic Potentials of Advanced Nanoparticles: A Comprehensive Review on Completed Human Clinical Trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Pasparakis, G. Recent Developments in the Use of Gold and Silver Nanoparticles in Biomedicine. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1817. [Google Scholar] [CrossRef] [PubMed]
- Muhaimin, M.; Chaerunisaa, A.Y.; Bodmeier, R. Preparation and evaluation of various formulation effects of the second emulsion on the shape and release profile of propranolol HCl from ethyl cellulose microparticle blends. Polym. Int. 2022, 72, 383–391. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and Unknown Health Risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Wang, S.Z.; Karpinski, E.A. Engineered Nanoparticles: Health and Safety Considerations; Employment and Social Development Canada, Labour Program: Gatineau, QC, Canada, 2016; ISBN 9780660067483. [Google Scholar]
- Kumar, K.; Singh, R.K.; Tyagi, P.K.; Gore, D. Assessment of Toxicity and Safety Profiles of Nanoparticles. Lett. Appl. NanoBioScience 2021, 10, 1877–1888. [Google Scholar]
- Yah, C.; Simate, G.S.; Iyuke, S.E. Nanoparticles Toxicity and Their Routes of Exposures. Pak. J. Pharm. Sci. 2012, 25, 477–491. [Google Scholar]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef]
- Tiwari, H.; Rai, N.; Singh, S.; Gupta, P.; Verma, A.; Singh, A.K.; Kajal; Salvi, P.; Singh, S.K.; Gautam, V. Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 2023, 10, 760. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Niculescu, A.-G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery—An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Das Kurmi, B.; Singh, D.; Mehan, S.; Khanna, K.; Karwasra, R.; Kumar, S.; Chaudhary, A.; Jakhmola, V.; Sharma, A.; et al. Nanoparticles Toxicity: An Overview of Its Mechanism and Plausible Mitigation Strategies. J. Drug Target. 2024, 32, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-Induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and Environmental Impacts of Nanoparticles: A Scoping Review of the Current Literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Jadhav, V.; Roy, A.; Kaur, K.; Rai, A.K.; Rustagi, S. Recent Advances in Nanomaterial-Based Drug Delivery Systems. Nano-Struct. Nano-Objects 2024, 37, 101103. [Google Scholar] [CrossRef]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef]
- Yagublu, V.; Karimova, A.; Hajibabazadeh, J.; Reissfelder, C.; Muradov, M.; Bellucci, S.; Allahverdiyev, A. Overview of Physicochemical Properties of Nanoparticles as Drug Carriers for Targeted Cancer Therapy. J. Funct. Biomater. 2022, 13, 196. [Google Scholar] [CrossRef]
- Duan, X.P.; Li, Y.P. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle Adhesion to the Cell Membrane and Its Effect on Nanoparticle Uptake Efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K. Routes of Exposures and Toxicity of Nanoparticles. In Model Organisms to Study Biological Activities and Toxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chhikara, B.S.; Zeybekler, S.E.; Gupta, D.S.; Kaur, G.; Chhillar, M.; Aggarwal, A.K.; Rahdar, A. Nanotoxicity of Multifunctional Stoichiometric Cobalt Oxide Nanoparticles (SCoONPs) with Repercussions Toward Apoptosis, Necrosis, and Cancer Necrosis Factor (TNF-α) at Nano-Biointerfaces. Toxicol. Res. 2023, 12, 716–740. [Google Scholar] [CrossRef]
- Garcés, M.; Cáceres, L.; Chiappetta, D.; Magnani, N.; Evelson, P. Current Understanding of Nanoparticle Toxicity Mechanisms and Interactions with Biological Systems. New J. Chem. 2021, 45, 14328–14344. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the Promise: Exploring the Complex Interactions of Nanoparticles Within Biological Systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef]
- Gehr, P. Interaction of Nanoparticles with Biological Systems. Colloids Surf. B Biointerfaces 2018, 172, 395–399. [Google Scholar] [CrossRef]
- Raftis, J.B.; Miller, M.R. Nanoparticle Translocation and Multi-Organ Toxicity: A Particularly Small Problem. Nano Today 2019, 26, 8–12. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle Exposure and Hormetic Dose–Responses: An Update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef]
- Lujan, H.; Sayes, C.M. Cytotoxicological Pathways Induced after Nanoparticle Exposure: Studies of Oxidative Stress at the “nano-Bio” Interface. Toxicol. Res. 2017, 6, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, M.G.; Anchi, P.; Raja, S.; Rachamalla, M.; Godugu, C. Novel Methods and Approaches for Safety Evaluation of Nanoparticle Formulations: A Focus Towards In Vitro Models and Adverse Outcome Pathways. Front. Pharmacol. 2021, 12, 612659. [Google Scholar] [CrossRef] [PubMed]
- Portugal, J.; Bedia, C.; Amato, F.; Juárez-Facio, A.T.; Stamatiou, R.; Lazou, A.; Campiglio, C.E.; Elihn, K.; Piña, B. Toxicity of Airborne Nanoparticles: Facts and Challenges. Environ. Int. 2024, 190, 108889. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.N.; Prakash, U.; Kannaian, N.; Bhuvaneswari, S.; Sripriya, N.; Prakash, N.K.U. Evaluation of toxicity of nanoparticles. In Proceedings of the AICTE Sponsored FDP on Application of Nanotechnology in Diagnostic and Therapeutic Procedure Evaluation of Toxicity of Nanoparticles, Chennai, India, 15-29 November 2013. [Google Scholar]
- Marquis, B.J.; Love, S.A.; Braun, K.L.; Haynes, C.L. Analytical Methods to Assess Nanoparticle Toxicity. Analyst 2009, 134, 425–439. [Google Scholar] [CrossRef]
- Omoregie, I.P. Regulations and Policy Considerations for Nanoparticle Safety. In Environmental Nanotoxicology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 295–316. [Google Scholar]
- Kübra, A.; Çevik, S. Regulatory Policies for Safety of Nanomaterials. Open J. Nano 2020, 5, 1–16. [Google Scholar]
- Drlickova, M.; Smolkova, B.; Runden-Pran, E.; Dusinska, M. Health Hazard and Risk Assessment of Nanoparticles Applied in Biomedicine. J. Nanomed. Nanotechnol. 2015, 6, 340. [Google Scholar] [CrossRef]
- Qamar, W.; Gulia, S.; Athar, M.; Ahmad, R.; Imam, M.T.; Chandra, P.; Singh, B.P.; Haque, R.; Hassan, I.; Rahman, S. An Insight into Impact of Nanomaterials Toxicity on Human Health. PeerJ 2024, 12, e17807. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The Toxicity of Nanoparticles and Their Interaction with Cells: An In Vitro Metabolomic Perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting their Toxicity for Biomedical Applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A. Toxicity of Nanoparticles_Challenges and Opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Kalaiarasi, M.; Elumalai, M.; Malarkodi, T.; Venkatesh, A.; Prakash, V. Antimicrobial Activity of Metal Oxide Nanoparticles. Biomed. Pharmacol. J. 2024, 17, 1757–1767. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Mohammadian, Y.; Rasoulzadeh, Y.; Azari, M.R.; Khodagholi, F. Toxicity of Carbon-Based Nanomaterials in the Human Lung: A Comparative In-Vitro Study. Tanaffos 2022, 21, 391–400. [Google Scholar]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Xue, H.Y.; Guo, P.; Wen, W.-C.; Wong, H.L. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Muhaimin, M.; Lestari, U.; Hirzan, R.; Chaerunisaa, A.Y. The potential of medicinal plants in tuberculosis treatment: Indigenous plants used by the Anak Dalam tribe of Jambi, Indonesia. S. Afr. J. Botany 2025, 180, 688–709. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Cao, T.G.N.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Dong, L.; Joseph, K.L.; Witkowski, C.M.; Craig, M.M. Cytotoxicity of Single-Walled Carbon Nanotubes Suspended in Various Surfactants. Nanotechnology 2008, 19, 255702. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Ameta, R.K.; Shankar, R.K.; Mehetre, S.S. Protein-Based Nanoparticles as Drug Delivery Nanocarriers. In Protein-Based Biopolymers: From Source to Biomedical Applications; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, H.; Cheng, L.; Wang, Y.; Liu, F.; Wang, S. The Application of Mesoporous Silica Nanoparticles as a Drug Delivery Vehicle in oral Disease Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1124411. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs. Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The Nanosilica Hazard: Another Variable Entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Ihtisham, M.; Noori, A.; Yadav, S.; Sarraf, M.; Kumari, P.; Brestic, M.; Imran, M.; Jiang, F.; Yan, X.; Rastogi, A. Silver Nano-particle’s Toxicological Effects and Phytoremediation. Nanomaterials 2021, 11, 2164. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The Toxic Effect of Silver Ions and Silver Nanoparticles Towards Bacteria and Human Cells Occurs in the Same Concentration Range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Waktole, G. Toxicity and Molecular Mechanisms of Actions of Silver Nanoparticles. J. Biomater. Nanobiotechnol. 2023, 14, 53–70. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef]
- Chaicherd, S.; Killingsworth, M.C.; Pissuwan, D. Toxicity of Gold Nanoparticles in a Commercial Dietary Supplement Drink on Connective Tissue Fibroblast Cells. SN Appl. Sci. 2019, 1, 336. [Google Scholar] [CrossRef]
- Abudayyak, M.; Guzel, E.; Özhan, G. Cupric Oxide Nanoparticles Induce Cellular Toxicity in Liver and Intestine Cell Lines. Adv. Pharm. Bull. 2020, 10, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and Genotoxicity of Copper Oxide Nanoparticles in Human Skin Keratinocytes Cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Fan, X.; Zhu, C.; Xia, W. Copper Nanoparticles Induce Oxidative Stress via the Heme Oxygenase 1 Signaling Pathway in vitro Studies. Int. J. Nanomed. 2021, 16, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, A.; Kabil, S.L.; Jarrar, B.M.; Sabry, M.; Morsy, M. Lycium barbarum Polysaccharide Attenuates Acute Toxicity Caused by Titanium Dioxide Nanoparticles in Splenic and Pulmonary Tissues. Pharm. Sci. 2024, 30, 339–354. [Google Scholar] [CrossRef]
- Lim, J.-O.; Kim, W.-I.; Pak, S.-W.; Lee, S.-J.; Moon, C.; Shin, I.-S.; Kim, S.-H.; Kim, J.-C. Pycnogenol-Assisted Alleviation of Titanium Dioxide Nanoparticle-Induced Lung Inflammation via Thioredoxin-Interacting Protein Downregulation. Antioxidants 2024, 13, 972. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Rojanasakul, Y. The Effects of Carbon Nanotubes on Lung and Dermal Cellular Behaviors. Nanomedicine 2014, 9, 895–912. [Google Scholar] [CrossRef]
- Jafar, A.; Alshatti, Y.; Ahmad, A. Carbon nanotube toxicity: The Smallest Biggest Debate in Medical Care. Cogent Med. 2016, 3, 1217970. [Google Scholar] [CrossRef]
- Nurhasanah, S.; Iskandar, Y.; Muhaimin, M.; Hazrina, A.; Hirzan, R.; Syahri, W. Polymer Type Effect on Premna serratifolia Extract-Loaded Microparticles Preparation by Solvent Evaporation Method with Single Emulsion System. Int. J. Appl. Pharm. 2024, 16, 250–256. [Google Scholar] [CrossRef]
- Fresegna, A.M.; Ciervo, A.; Ursini, C.L.; Maiello, R.; Tombolini, F.; Del Frate, V.; Gentile, M.; Cavallo, D. Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells. Int. J. Mol. Sci. 2024, 25, 13515. [Google Scholar] [CrossRef]
- de Luna, L.A.V.; Loret, T.; Fordham, A.; Arshad, A.; Drummond, M.; Dodd, A.; Lozano, N.; Kostarelos, K.; Bussy, C. Lung Recovery from DNA Damage Induced by Graphene Oxide is Dependent on Size, Dose and Inflammation Profile. Part. Fibre Toxicol. 2022, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Rodríguez-Garraus, A.; de Cerain, A.L.; Azqueta, A.; Catalán, J. Genotoxicity of Graphene-Based Materials. Nanomaterials 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Carbon Black Particle Exhibits Size Dependent Toxicity in Human Monocytes. Int. J. Inflamm. 2014, 2014, 827019. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Olson, F.; Mayhew, E.; Maslow, D.; Rustum, Y.; Szoka, F. Characterization, Toxicity and Therapeutic Efficacy of Adrïamycin Encapsulated in Liposomes. Eur. J. Cancer Clin. Oncol. 1982, 18, 167–176. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In Vivo Toxicity of Cationic Micelles and Liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2024, 19, 6945–6980. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Shen, W.; Li, M.; Wang, W.; Jin, X. Trend of Albumin Nanoparticles in Oncology: A Bibliometric Analysis of Research Progress and Prospects. Front. Pharmacol. 2024, 15, 1409163. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-Based Nanoparticles: A Promising Strategy to Overcome Cancer Drug Resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Saif, A.; Anjum, L.; Faisal, Z.; Akram, N.; Shah, Y.A.; Irfan, R.; Saeed, F.; Afzaal, M.; Asif Shah, M. Recent Advances in Protein-Based Nanoparticles and Their Applications in the Delivery of Bioactive Compounds. Int. J. Food Prop. 2023, 26, 2866–2880. [Google Scholar] [CrossRef]
- Dewi, M.K.; Muhaimin, M.; Joni, I.M.; Hermanto, F.; Chaerunisaa, A.Y. Fabrication of Phytosome with Enhanced Activity of Sonneratia alba: Formulation Modeling and In Vivo Antimalarial Study. Int. J. Nanomed. 2024, 19, 9411–9435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, H.; Tian, J.; Wu, A.; Wang, J.; Ge, C. Self-Assembled Silk Fibroin Nanoparticles Loaded with Binary Drugs in the Treatment of Breast Carcinoma. Int. J. Nanomed. 2016, 11, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Q.; Wang, Q.; Qian, H.; Yu, L.; Liu, B.; Li, R. Novel Silk Fibroin Nanoparticles Incorporated Silk Fibroin Hydrogel for Inhibition of Cancer Stem Cells and Tumor Growth. Int. J. Nanomed. 2018, 13, 5405–5418. [Google Scholar] [CrossRef] [PubMed]
- Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Formulation Development of Natural Polymeric Nanoparticles, In Vitro Antiaging Evaluation, and Metabolite Profiling of Toona sinensis Leaf Extracts. Pharmaceuticals 2025, 18, 288. [Google Scholar] [CrossRef]
- Muhaimin, M.; Chaerunisaa, A.Y.; Bodmeier, R. Real-time particle size analysis using focused beam reflectance measurement as a process analytical technology tool for continuous microencapsulation process. Sci. Rep. 2021, 11, 19390. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials. JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in Translation: Animal Models and Clinical Trials in Cancer Treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Vanivska, K.; Dianová, L.; Halo, M., Jr.; Štefunková, N.; Lenický, M.; Slanina, T.; Tirpák, F.; Ivanič, P.; Stawarz, R.; Massányi, P. Toxicity of Nanoparticles on Animal and Human Organism: Cell Response. J. Microbiol. Biotechnol. Food Sci. 2024, 14, e10844. [Google Scholar] [CrossRef]
- Rostinawati, T.; Muhaimin, M.; Chaerunisaa, A.Y.; Hazrina, A. Development of casticin-loaded ethyl cellulose microparticles by solvent evaporation method with single emulsion system. Int. J. Appl. Pharm. 2023, 15, 235–240. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Hao, Z.; Yao, P.; Bai, J.; Chen, H.; Wu, X.; Zhong, Y.; Xue, D. Synthetic Nanoparticles Functionalized with Cell Membrane-Mimicking, Bone-Targeting, and ROS-Controlled Release Agents for Osteoporosis Treatment. J. Control. Release 2025, 378, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Kladko, D.V.; Falchevskaya, A.S.; Serov, N.S.; Prilepskii, A.Y. Nanomaterial Shape Influence on Cell Behavior. Int. J. Mol. Sci. 2021, 22, 5266. [Google Scholar] [CrossRef]
- Ali, S.; Bahadur, A.; Hassan, A.; Ahmad, S.; Shah, W.; Iqbal, S. Optimized Silver Nanostructures for Enhanced Antibacterial Potential: Recent Trends and Challenges in the Development of Metallo-Antimicrobials. Chem. Eng. J. 2025, 507. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; KashtiAray, A.; Zarei-Shokat, S.; Tian, Y.; Maleki, A. Effects of Morphology and Size of Nanoscale Drug Carriers on Cellular Uptake and Internalization Process: A review. RSC Adv. 2022, 13, 80–114. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Gagné, F. Shape-Dependent Toxicity of Silver Nanoparticles on Freshwater Cnidarians. Nanomaterials 2022, 12, 3107. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, H.; Krithikadatta, J.; Doble, M. Local and Systemic Adverse Effects of Nanoparticles Incorporated in Dental Materials—A Critical Review. Saudi Dent. J. 2024, 36, 158–167. [Google Scholar] [CrossRef]
- Muhaimin, M.; Chaerunisaa, A.Y.; Bodmeier, R. Impact of Dispersion time Interval and Particle Size on Release Profiles of Propranolol HCl and Carbamazepines from Microparticle Blends System. Sci. Rep. 2022, 12, 10360. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Li, T.; He, X.; Wang, Z. Surface Charge effect on the Cellular Interaction and Cytotoxicity of NaYF4:Yb3+, Er3+@SiO2 Nanoparticles. RSC Adv. 2015, 5, 7773–7780. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Z. Negatively Charged Cu1.33S Nanochains: Endocytic Pathway, Photothermal Therapy and Toxic Effect In Vivo. Nanoscale Adv. 2023, 5, 1706–1713. [Google Scholar] [CrossRef]
- Balog, S.; de Almeida, M.S.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. Does the Surface Charge of the Nanoparticles Drive Nanoparticle–Cell Membrane Interactions? Curr. Opin. Biotechnol. 2024, 87, 103128. [Google Scholar] [CrossRef]
- Muhaimin, M.; Chaerunisaa, A.Y.; Hazrina, A. Preparation and evaluation of propranolol HCl and carbamazepine release profiles from poly(є-caprolactone) microparticle blends system. Int. J. Appl. Pharm. 2023, 15, 117–122. [Google Scholar] [CrossRef]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef]
- Das, S.K.; Sen, K.; Ghosh, B.; Ghosh, N.; Sinha, K.; Sil, P.C. Molecular Mechanism of Nanomaterials Induced Liver Injury: A Review. World J. Hepatol. 2024, 16, 566–600. [Google Scholar] [CrossRef]
- Kumar, R. Nanotherapeutics to Cure Inflammation-Induced Cancer. Curr. Cancer Rep. 2024, 6, 193–204. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifica-tions in DNA. Front. Genet. 2021, 12, 728250. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Cordani, M.; Pashootan, P.; Moosavi, M.A.; Zarrabi, A.; Chandrasekaran, N. The Impact of Nanomaterials on Autophagy across Health and Disease Conditions. Cell. Mol. Life Sci. 2024, 81, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical−Chemical Aspects of Protein Corona: Relevance to In Vitro and In Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Qayoomian, M.; Beigoli, S.; Boskabady, M.H. Recent Advances in Nanoparticle Applications in Respiratory Disorders: A review. Front. Pharmacol. 2023, 14, 1059343. [Google Scholar] [CrossRef]
- Byrne, J.D.; A Baugh, J. The Significance of Nanoparticles in Particle-Induced Pulmonary Fibrosis. McGill J. Med. 2008, 11, 43–50. [Google Scholar] [CrossRef]
- Sun, Q.; Tan, D.; Ze, Y.; Sang, X.; Liu, X.; Gui, S.; Cheng, Z.; Cheng, J.; Hu, R.; Gao, G.; et al. Pulmotoxicological Effects Caused by Long-Term Titanium Dioxide Nanoparticles Exposure in Mice. J. Hazard. Mater. 2012, 235–236, 47–53. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In Vivo Acute Toxicity of Titanium Dioxide Nanoparticles to Mice after Intraperitioneal Injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Tomonaga, T.; Izumi, H.; Oyabu, T.; Lee, B.-W.; Kubo, M.; Shimada, M.; Noguchi, S.; Nishida, C.; Yatera, K.; Morimoto, Y. Assessment of Cytokine-Induced Neutrophil Chemoattractants as Biomarkers for Prediction of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 1563. [Google Scholar] [CrossRef]
- Cojocaru, E.; Petriș, O.R.; Cojocaru, C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals 2024, 17, 1059. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Dante, S.; Petrelli, A.; Petrini, E.M.; Marotta, R.; Maccione, A.; Alabastri, A.; Quarta, A.; De Donato, F.; Ravasenga, T.; Sathya, A.; et al. Selective Targeting of Neurons with Inorganic Nanoparticles: Revealing the Crucial Role of Nanoparticle Surface Charge. ACS Nano 2017, 11, 6630–6640. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, V.; Manickam, V.; Perumal, E. Neurobehavioural Toxicity of Iron Oxide Nanoparticles in Mice. Neurotox. Res. 2017, 32, 187–203. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kopaeva, M.; Kashkarov, P. Effects of Prolonged Silver Nanoparticle Exposure on the Contextual Cognition and Behavior of Mammals. Materials 2018, 11, 558. [Google Scholar] [CrossRef]
- Tandon, A.; Singh, S.J.; Chaturvedi, R.K. Nanomedicine against Alzheimer’s and Parkinson’s Disease. Curr. Pharm. Des. 2020, 27, 1507–1545. [Google Scholar] [CrossRef]
- Li, A.; Tyson, J.; Patel, S.; Patel, M.; Katakam, S.; Mao, X.; He, W. Emerging Nanotechnology for Treatment of Alzheimer’s and Parkinson’s Disease. Front. Bioeng. Biotechnol. 2021, 9, 672594. [Google Scholar] [CrossRef]
- Vio, V.; Marchant, M.J.; Araya, E.; Kogan, M.K. Metal Nanoparticles for the Treatment and Diagnosis of Neurodegenerative Brain Diseases. Curr. Pharm. Des. 2017, 23, 1916–1926. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Nanomedicine for Neurodegenerative Disorders: Focus on Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2021, 22, 9082. [Google Scholar] [CrossRef]
- Bhattacharya, T.; e Soares, G.A.B.; Chopra, H.; Rahman, M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Gaubert, A.; Latxague, L.; Dehay, B. PLGA-Based Nanoparticles for Neuroprotective Drug Delivery in Neuro-degenerative Diseases. Pharmaceutics 2021, 13, 1042. [Google Scholar] [CrossRef] [PubMed]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-Induced Immune Response: Health Risk Versus Treatment Opportunity? Immunobiology 2023, 228, 152317. [Google Scholar] [CrossRef]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of Engineered Nanoparticles on the Innate Immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main Trends of Immune Effects Triggered by Nanomedicines in Preclinical Studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef]
- Benne, N.; ter Braake, D.; Stoppelenburg, A.J.; Broere, F. Nanoparticles for Inducing Antigen-Specific T Cell Tolerance in Autoimmune Diseases. Front. Immunol. 2022, 13, 864403. [Google Scholar] [CrossRef]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016, 7, 97. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef]
| Type of Nanoparticle | Description | Examples | Toxicity Effects | Ref |
|---|---|---|---|---|
| Metal-Based NPs | Composed of metals or metal oxides, known for their antimicrobial properties. | Silver (Ag), Gold (Au), Copper (Cu), Titanium Dioxide (TiO2) | Cytotoxicity, oxidative stress, genotoxicity, and potential organ damage (liver, kidney) due to reactive ions. | [15,43,44] |
| Carbon-Based NPs | Include carbon nanotubes, graphene, and fullerenes, which are widely used in various applications. | Carbon Nanotubes (CNTs), Graphene, Carbon Black | Neurotoxicity, pulmonary inflammation, and cytotoxicity; size-dependent effects observed in different studies. | [15,45,46] |
| Lipid-Based NPs | Composed of lipids; often used in drug delivery systems. | Liposomes, Solid Lipid Nanoparticles (SLNs) | Potential for immunotoxicity and cytotoxicity; may induce inflammatory responses depending on lipid composition. | [47] |
| Protein-Based NPs | Made from proteins; used in drug delivery and vaccine development. | Albumin-based NPs, Silk Fibroin NPs | Generally biocompatible but can induce immune responses; toxicity may arise from protein denaturation or aggregation. | [45,47] |
| Polymeric NPs | Composed of synthetic or natural polymers, they are versatile in drug delivery applications. | Poly(lactic-co-glycolic acid) (PLGA), Chitosan NPs | Cytotoxicity is related to polymer degradation products; there is potential for inflammatory responses depending on the polymer type. | [15,44] |
| Silica NPs | Made of silica, and commonly used in biomedical applications and as drug carriers. | Mesoporous Silica NPs | It can induce oxidative stress and inflammation; there is potential for cytotoxic effects depending on particle size and surface modification. | [43,46] |
| Example Nanoparticle | Toxic Effects | Organism/Cell Tested | Concentration | Condition | Ref |
|---|---|---|---|---|---|
| Metal-Based | |||||
| Silver (AgNPs) | Induces cell death, DNA damage, oxidative stress, and inflammation | Human mesenchymal stem cells (hMSCs), E. coli | 0.5 to 5 ppm | Various exposure times | [71,72,73] |
| Gold (AuNPs) | Cytotoxicity, potential genotoxic effects, and inflammation | Human lung adenocarcinoma cells (A-549) | 1 to 100 μg/mL | Short-term and long-term exposure | [74,75] |
| Copper (CuNPs) | Induces oxidative stress, cytotoxicity, and genotoxicity | Various mammalian cell lines | 10 to 100 μg/mL | Varies by study | [76,77,78] |
| Titanium Dioxide (TiO2) | Causes oxidative stress, inflammation, and potential lung toxicity | Human lung epithelial cells | 0.1 to 10 mg/mL | In vitro exposure | [79,80] |
| Carbon-Based | |||||
| Carbon Nanotubes (CNTs) | Induces oxidative stress, DNA damage, lysosomal damage, mitochondrial dysfunction, and apoptosis | Human lung epithelial cells (A549), macrophages | 1 to 100 μg/mL | Various exposure times | [81,82,83,84] |
| Graphene | Causes oxidative stress, inflammatory responses, and induces TNF-α and IL-6 secretion in macrophages | Human bronchial epithelial cells (BEAS-2B) | 1 to 50 μg/mL | In vitro exposure | [85,86,87] |
| Carbon Black (CB) | Induces pyroptosis, inflammation, and cytotoxicity | THP-1 Monocyte Cells | 50–800 μg/mL | In vitro exposure | [88] |
| Lipid-Based | |||||
| Liposomes | Generally low toxicity; potential for hemolysis, cytotoxicity at high concentrations | Human red blood cells, various cancer cell lines | 0.1 to 10 mg/mL | In vitro studies | [89,90,91] |
| Solid Lipid Nanoparticles (SLNs) | Low cytotoxicity; potential for skin irritation, reduced toxicity from essential fatty acids | Various cell lines, human skin fibroblasts | 0.1 to 5 mg/mL | In vitro and in vivo studies | [89,90,91] |
| Protein-Based | |||||
| Albumin-based Nanoparticles | Generally low toxicity; minimal immune response; potential for cytotoxicity at high concentrations | Human cancer cell lines, animal models | 0.1 to 10 mg/mL | In vitro and in vivo studies | [92,93,94,95] |
| Silk Fibroin Nanoparticles | Low toxicity; biocompatible; potential for mild inflammatory response in some cases | Human fibroblasts, mouse models | 1 to 5 mg/mL | In vitro and in vivo studies | [96,97,98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhaimin, M.; Chaerunisaa, A.Y.; Dewi, M.K.; Khatib, A.; Hazrina, A. The Toxicological Profile of Active Pharmaceutical Ingredients–Containing Nanoparticles: Classification, Mechanistic Pathways, and Health Implications. Pharmaceuticals 2025, 18, 703. https://doi.org/10.3390/ph18050703
Muhaimin M, Chaerunisaa AY, Dewi MK, Khatib A, Hazrina A. The Toxicological Profile of Active Pharmaceutical Ingredients–Containing Nanoparticles: Classification, Mechanistic Pathways, and Health Implications. Pharmaceuticals. 2025; 18(5):703. https://doi.org/10.3390/ph18050703
Chicago/Turabian StyleMuhaimin, Muhaimin, Anis Yohana Chaerunisaa, Mayang Kusuma Dewi, Alfi Khatib, and Aghnia Hazrina. 2025. "The Toxicological Profile of Active Pharmaceutical Ingredients–Containing Nanoparticles: Classification, Mechanistic Pathways, and Health Implications" Pharmaceuticals 18, no. 5: 703. https://doi.org/10.3390/ph18050703
APA StyleMuhaimin, M., Chaerunisaa, A. Y., Dewi, M. K., Khatib, A., & Hazrina, A. (2025). The Toxicological Profile of Active Pharmaceutical Ingredients–Containing Nanoparticles: Classification, Mechanistic Pathways, and Health Implications. Pharmaceuticals, 18(5), 703. https://doi.org/10.3390/ph18050703






