Development of Smart pH-Sensitive Collagen-Hydroxyethylcellulose Films with Naproxen for Burn Wound Healing
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Evaluation of pH-Sensitive Films
2.2. Mechanical Characterization
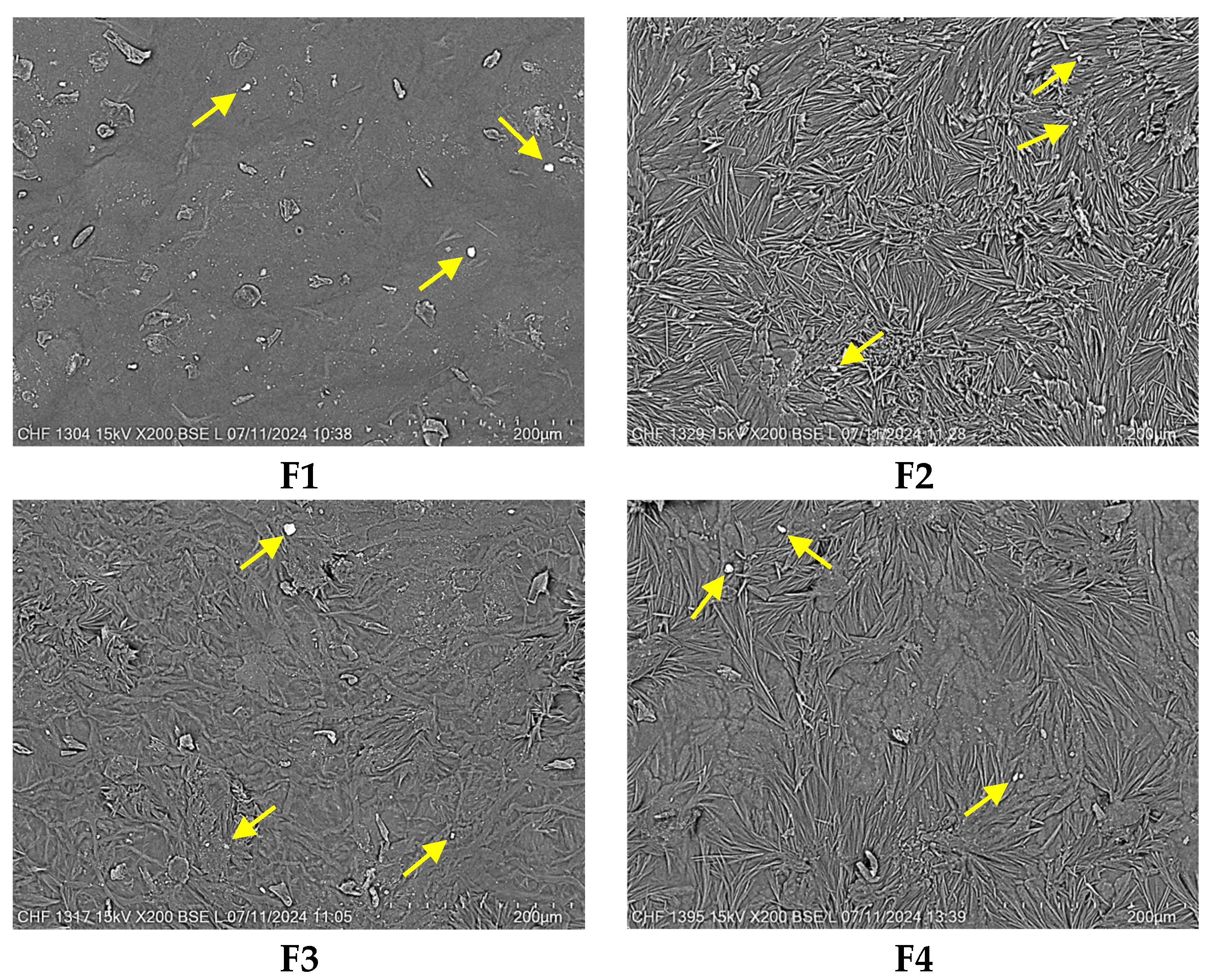
2.3. Scanning Electron Microscopy (SEM)
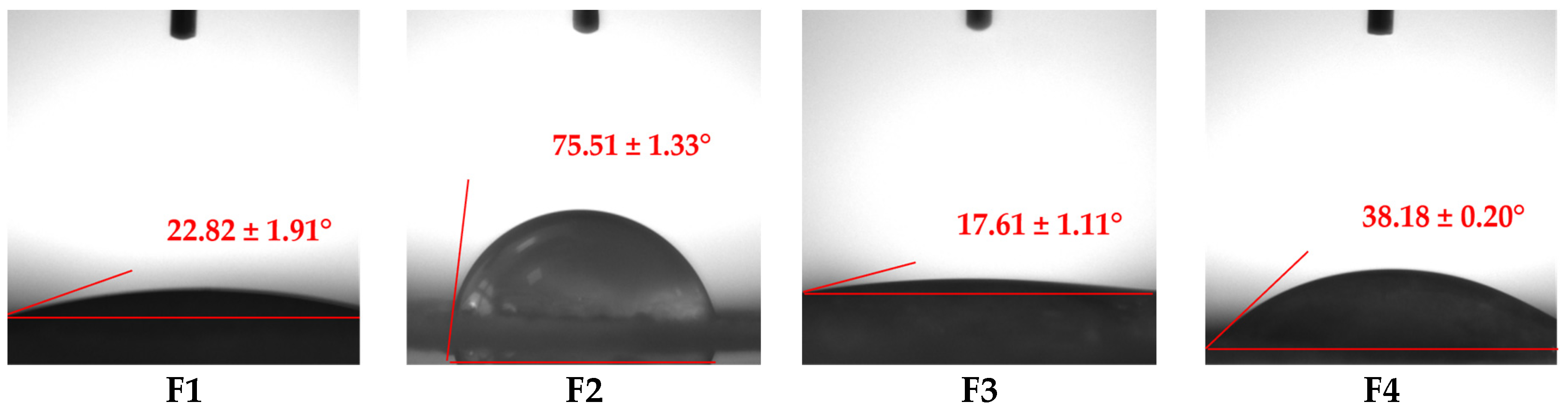
2.4. Wettability Analysis
2.5. Swelling Behavior
2.6. In Vitro Enzymatic Biodegradation
2.7. In Vitro Drug Release Assay
2.8. Colorimetric Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Biopolymeric Films
3.2.2. Physical Evaluation of pH-Sensitive Films
3.2.3. Mechanical Characterization
3.2.4. Scanning Electron Microscopy (SEM)
3.2.5. Wettability Analysis
3.2.6. Swelling Behavior
3.2.7. In Vitro Enzymatic Biodegradation
3.2.8. In Vitro Drug Release Assay
3.2.9. Colorimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose composite wound dressings for real-time pH wound monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.G.; Haalboom, M.; Bowler, P.G.; Gamerith, C.; Sigl, E.; Heinzle, A.; Burnet, M.W.M. Elevated wound fluid pH correlates with increased risk of wound infection. Wound Med. 2019, 26, 100166. [Google Scholar] [CrossRef]
- Gamerith, C.; Luschnig, D.; Ortner, A.; Pietrzik, N.; Guse, J.-H.; Burnet, M.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; et al. pH-responsive materials for optical monitoring of wound status. Sens. Actuators B Chem. 2019, 301, 126966. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Richards, H.; Falder, S. pH of a burn wound. Burns 2018, 44, 2104–2105. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Osti, E. Skin ph variations from the acute phase to re-epithelialization in burn patients treated with new materials (burnshield®, semipermeable adhesive film, dermasilk®, and hyalomatrix®). Non-invasive preliminary experimental clinical trial. Ann. Burn. Fire Disasters 2008, 21, 73–77. [Google Scholar]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Sim, P.; Song, Y.; Yang, G.N.; Cowin, A.J.; Garg, S. In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. Int. J. Mol. Sci. 2022, 23, 7188. [Google Scholar] [CrossRef]
- Pourali, P.; Razavianzadeh, N.; Khojasteh, L.; Yahyaei, B. Assessment of the cutaneous wound healing efficiency of acidic, neutral and alkaline bacterial cellulose membrane in rat. J. Mater. Sci. Mater. Med. 2018, 29, 90. [Google Scholar] [CrossRef]
- Bazbouz, M.B.; Tronci, G. Two-layer Electrospun System Enabling Wound Exudate Management and Visual Infection Response. Sensors 2019, 19, 991. [Google Scholar] [CrossRef] [PubMed]
- Kiti, K.; Thanomsilp, C.; Suwantong, O. The potential use of colorimetric pH sensor from Clitoria ternatea flower for indicating bacterial infection in wound dressing application. Microchem. J. 2022, 177, 107277. [Google Scholar] [CrossRef]
- Petkovska, J.; Geskovski, N.; Marković, D.; Dimova, V.; Mirakovski, D.; Radetić, M.; Jordanov, I. Chitosan-pectin multilayer coating with anthocyanin grape dye as pH indicating wound dressing: Synthesis and characterization. Carbohydr. Polym. Technol. Appl. 2024, 7, 100438. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.-J. A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Moisescu, S. Response surface methodology and Taguchi approach to assess the combined effect of formulation factors on minocycline delivery from collagen sponges. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 340–348. [Google Scholar] [CrossRef]
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ghica, M.V. Collagen-based biomaterials for ibuprofen delivery. Comptes Rendus. Chim. 2016, 19, 390–394. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Marin, Ș.; Albu Kaya, M.G.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Udeanu, D.I.; Mihai, G.; Enachescu, M. Collagen-Polyvinyl Alcohol-Indomethacin Biohybrid Matrices as Wound Dressings. Pharmaceutics 2018, 10, 224. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Silva, C.N.S.; Cruz, M.V.; Fernandes, K.F.; Batista, K.A. Production of anti-inflammatory films based on cashew gum polysaccharide and polyvinyl alcohol for wound dressing applications. 3 Biotech 2023, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine Gellan Gum–Collagen Interpenetrating Network Hydrogels as Mechanically Enhanced Anti-inflammatory Biologic Wound Dressings for Burn Wound Therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef]
- Halarnekar, D.; Ayyanar, M.; Gangapriya, P.; Kalaskar, M.; Redasani, V.; Gurav, N.; Nadaf, S.; Saoji, S.; Rarokar, N.; Gurav, S. Eco synthesized chitosan/zinc oxide nanocomposites as the next generation of nano-delivery for antibacterial, antioxidant, antidiabetic potential, and chronic wound repair. Int. J. Biol. Macromol. 2023, 242, 124764. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Qiao, L.; Huang, Y.; Yang, Y.; Chu, D.; Guo, B. Multifunctional On-Demand Removability Hydrogel Dressing Based on in Situ Formed AgNPs, Silk Microfibers and Hydrazide Hyaluronic Acid for Burn Wound Healing. Adv. Healthc. Mater. 2024, 13, 2303157. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Mazumder, B.; Rajkonwar, J.; Pathak, M.P.; Patowary, P.; Chattopadhyay, P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci. Rep. 2021, 11, 3357. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Albu Kaya, M.G.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C.; et al. Design and evaluation of new wound dressings based on collagen-cellulose derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Al-Khoury, H.; Espinosa-Cano, E.; Aguilar, M.R.; Román, J.S.; Syrowatka, F.; Schmidt, G.; Groth, T. Anti-inflammatory Surface Coatings Based on Polyelectrolyte Multilayers of Heparin and Polycationic Nanoparticles of Naproxen-Bearing Polymeric Drugs. Biomacromolecules 2019, 20, 4015–4025. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Pharm. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef]
- Su, W.-H.; Cheng, M.-H.; Lee, W.-L.; Tsou, T.-S.; Chang, W.-H.; Chen, C.-S.; Wang, P.-H. Nonsteroidal Anti-Inflammatory Drugs for Wounds: Pain Relief or Excessive Scar Formation? Mediat. Inflamm. 2010, 2010, 413238. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Jacob, S.; Bharadia, P.; Haroun, M. Proniosomal vesicles as an effective strategy to optimize naproxen transdermal delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102479. [Google Scholar] [CrossRef]
- Van den Ouweland, F.A.; Eenhoorn, P.C.; Tan, Y.; Gribnau, F.W.J. Transcutaneous absorption of naproxen gel. Eur. J. Clin. Pharmacol. 1989, 36, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.S. Evaluation of Glycofurol-Based Gel as a New Vehicle for Topical Application of Naproxen. AAPS PharmSciTech 2010, 11, 1138–1146. [Google Scholar] [CrossRef]
- Yurdasiper, A.; Ertan, G.; Heard, C.M. Enhanced delivery of naproxen to the viable epidermis from an activated poly N-isopropylacrylamide (PNIPAM) Nanogel: Skin penetration, modulation of COX-2 expression and rat paw oedema. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, X.; Luo, J.; Wen, Q.; Wu, Z.; Ding, Q.; Zhao, L.; Yang, L.; Wang, B.; Fu, S. Naproxen Nanoparticle-Loaded Thermosensitive Chitosan Hydrogel for Prevention of Postoperative Adhesions. ACS Biomater. Sci. Eng. 2019, 5, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Kianipour, A.; Mortazavi, S.A.R. The Analgesic Efficacy of 5% Naproxen Gel for Pain Associated with Orthodontic Separator Placement: A Randomized Double-Blind Controlled Trial. Anesthesiol. Pain Med. 2017, 7, e42708. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Pasquini, D.; Mattoso, L.H.C. Enhanced antibacterial activity of wound dressings based on alginate/hydroxyapatite modified with copper and naproxen. J. Mater. Res. 2024, 39, 762–773. [Google Scholar] [CrossRef]
- Mishra, S.K.; Mary, D.S.; Kannan, S. Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int. J. Biol. Macromol. 2017, 95, 928–937. [Google Scholar] [CrossRef]
- Purnamasari, W.; Budiastanti, T.A.; Aminatun, A.; Rahmah, U.; Sumarsih, S.; Chang, J.-Y.; Fahmi, M.Z. Naproxen release behaviour from graphene oxide/cellulose acetate composite nanofibers. RSC Adv. 2022, 12, 8019–8029. [Google Scholar] [CrossRef]
- Jiao, W.; Kiang, J.G.; Cary, L.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. COX-2 Inhibitors Are Contraindicated for Treatment of Combined Injury. Radiat. Res. 2009, 172, 686–697. [Google Scholar] [CrossRef]
- Fairweather, M.; Heit, Y.I.; Buie, J.; Rosenberg, L.M.; Briggs, A.; Orgill, D.P.; Bertagnolli, M.M. Celecoxib inhibits early cutaneous wound healing. J. Surg. Res. 2015, 194, 717–724. [Google Scholar] [CrossRef]
- Goder, D.; Eshkol-Yogev, I.; Matsliah, L.; Lemberger, M.; Harlev, M.; Furer, A.; Zilberman, M.; Egozi, D. In vivo study of the efficacy of bupivacaine-eluting novel soy protein wound dressings in a rat burn model. Burns 2022, 48, 623–632. [Google Scholar] [CrossRef]
- Morgan, A.; Babu, D.; Reiz, B.; Whittal, R.; Suh, L.Y.K.; Siraki, A.G. Caution for the routine use of phenol red—It is more than just a pH indicator. Chem. Biol. Interact. 2019, 310, 108739. [Google Scholar] [CrossRef] [PubMed]
- Chalitangkoon, J.; Ronte, A.; Sintoppun, T.; Manapradit, N.; Monvisade, P. Dual Cross-Linked Chitosan-Based Films with pH-Sensitive Coloration and Drug Release Kinetics for Smart Wound Dressings. ACS Omega 2025, 10, 7770–7782. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; K. Alruwaili, N.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Sajkiewicz, P.; Zahran, M.K.; Hakeim, O.A. Preparation and characterization of smart therapeutic pH-sensitive wound dressing from red cabbage extract and chitosan hydrogel. Int. J. Biol. Macromol. 2021, 182, 1820–1831. [Google Scholar] [CrossRef]
- Aycan, D.; Selmi, B.; Kelel, E.; Yildirim, T.; Alemdar, N. Conductive polymeric film loaded with ibuprofen as a wound dressing material. Eur. Polym. J. 2019, 121, 109308. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Kirkness, M.W.H.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: Structure and Mechanics, an Introduction. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar]
- Ahmed, M.; Bhat, A.R.; Verma, A.K.; Patel, R. Collagen–PVA Films Plasticized with Choline Acetate Ionic Liquid for Sustained Drug Release: UV Shielding, Mechanical, Antioxidant, and Antibacterial Properties. ACS Appl. Bio Mater. 2023, 6, 663–673. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wu, J.; Zhang, T.; Chen, C.; Ma, Y.; Liu, H.; Chiou, B.-S.; Liu, F.; Li, J. Improving the crosslinking of collagen casing and glutaraldehyde by facilitating the formation of conjugate structure via pH. Collagen Leather 2024, 6, 29. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.X.; Ma, C.P.; Li, H.; Qiu, Z.M. Hydroxyethyl cellulose hydrogel modified with tannic acid as methylene blue adsorbent. J. Appl. Polym. Sci. 2021, 138, 17. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of antibacterial, degradable and ph-responsive chitosan/guar gum/polyvinyl alcohol blended hydrogels for wound dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef] [PubMed]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Khan, M.U.; Razaq, S.I.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible pH-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Tu, C.-X.; Gao, C.-Y. Recent Advances on Surface-modified Biomaterials Promoting Selective Adhesion and Directional Migration of Cells. Chin. J. Polym. Sci. 2021, 39, 815–823. [Google Scholar] [CrossRef]
- Singh, D.; Dilip Saoji, S. The Role of Surface Energy and Wettability in Polymer-Based Drug Delivery Systems: Enhancing Bioadhesion and Drug Release Efficiency. J. Macromol. Sci. Part B 2024, 1–8. [Google Scholar] [CrossRef]
- Liu, T.; Dan, W.; Dan, N.; Liu, X.; Liu, X.; Peng, X. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng. C 2017, 77, 202–211. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y. Tailoring Materials with Specific Wettability in Biomedical Engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.; Zhang, X.; Zou, J.; Chai, W.; Lou, Y. Rapid adsorption for oil using superhydrophobic and superoleophilic polyurethane sponge. J. Chem. Technol. Biotechnol. 2015, 90, 2106–2112. [Google Scholar] [CrossRef]
- Rodrigues, A.P.H.; Pereira, I.M.; de Souza, S.D.; Gil, C.S.B.; Machado, G.; Carvalho, S.M.; Pereira, F.V.; Paiva, P.R.P.; de Oliveira, L.C.A.; de O. Patricio, P.S. Control of properties of nanocomposites bio-based collagen and cellulose nanocrystals. Cellulose 2017, 24, 1731–1744. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Romero, A.; Guerrero, A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A 2016, 104, 1462–1468. [Google Scholar] [CrossRef]
- Ghataty, D.S.; Amer, R.I.; Wasfi, R.; Shamma, R.N. Novel linezolid loaded bio-composite films as dressings for effective wound healing: Experimental design, development, optimization, and antimicrobial activity. Drug Deliv. 2022, 29, 3168–3185. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Zahran, M.K.; Hakeim, O.A. Greener therapeutic pH-sensing wound dressing based on Curcuma Longa and cellulose hydrogel. Eur. Polym. J. 2021, 159, 110744. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.-S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Marin, M.M.; Ianchis, R.; Leu Alexa, R.; Gifu, I.C.; Kaya, M.G.; Savu, D.I.; Popescu, R.C.; Alexandrescu, E.; Ninciuleanu, C.M.; Preda, S.; et al. Development of New Collagen/Clay Composite Biomaterials. Int. J. Mol. Sci. 2022, 23, 401. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Leau, S.-A.; Marin, S.; Holban, A.M.; Vasile, B.-S.; Nicoara, A.-I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Drannikov, A.; Surgutskaia, N.S.; Ozaltin, K.; Postnikov, P.S.; Marina, T.E.; Sedlarik, V. Chitosan-collagen based film for controlled delivery of a combination of short life anesthetics. Int. J. Biol. Macromol. 2019, 140, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf. B Biointerfaces 2013, 102, 102–110. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Mostegel, F.; Griesser, T.; Spirk, S.; Smrke, D.M.; Stana-Kleinschek, K. Cellulose based thin films as a platform for drug release studies to mimick wound dressing materials. Cellulose 2015, 22, 749–761. [Google Scholar] [CrossRef]
- Thu, H.-E.; Zulfakar, M.H.; Ng, S.-F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.J.; Kharrazi, H.; Chang, H.-Y.; Bodycombe, D.; Lemke, K.; Weiner, J.P. Exploring the use of machine learning for risk adjustment: A comparison of standard and penalized linear regression models in predicting health care costs in older adults. PLoS ONE 2019, 14, e0213258. [Google Scholar] [CrossRef]
- Saffron, C.M.; Park, J.-H.; Dale, B.E.; Voice, T.C. Kinetics of Contaminant Desorption from Soil: Comparison of Model Formulations Using the Akaike Information Criterion. Environ. Sci. Technol. 2006, 40, 7662–7667. [Google Scholar] [CrossRef]
- Romero, A.I.; Villegas, M.; Cid, A.G.; Parentis, M.L.; Gonzo, E.E.; Bermúdez, J.M. Validation of kinetic modeling of progesterone release from polymeric membranes. Asian J. Pharm. Sci. 2018, 13, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Arafat, M.T.; Mahmud, M.M.; Wong, S.Y.; Li, X. PVA/PAA based electrospun nanofibers with pH-responsive color change using bromothymol blue and on-demand ciprofloxacin release properties. J. Drug Deliv. Sci. Technol. 2021, 61, 102297. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2011; pp. 23–24. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- SR EN ISO 3376:2020; Leather—Physical and Mechanical Tests—Determination of Tensile Strength and Percentage Elongation. International Organization for Standardization. ASRO: Bucharest, Romania, 2020.
- Güngör, Z.; Ozay, H. Ultra-fast pH determination with a new colorimetric pH-sensing hydrogel for biomedical and environmental applications. React. Funct. Polym. 2022, 180, 105398. [Google Scholar] [CrossRef]
- Vinklárková, L.; Masteiková, R.; Vetchý, D.; Doležel, P.; Bernatonienė, J. Formulation of Novel Layered Sodium Carboxymethylcellulose Film Wound Dressings with Ibuprofen for Alleviating Wound Pain. BioMed Res. Int. 2015, 2015, 892671. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]





| Film Code | Transparency | Air Bubbles | Flexibility | Peelability |
|---|---|---|---|---|
| F1 | ✓ | ✓ | ✓ | |
| F2 | ✓ | ✓ | ✓ | |
| F3 | ✓ | ✓ | ✓ | |
| F4 | ✓ | ✓ | ✓ |
| Film Code | Thickness (mm) | Tensile Strength (N/mm2) |
|---|---|---|
| F1 | 0.19 | 0.61 |
| F2 | 0.16 | 3.33 |
| F3 | 0.12 | 1.56 |
| F4 | 0.16 | 0.62 |
| Films Code | Higuchi Model | Power Law Model | Kinetic Constant, k (1/minn) | Diffusional Exponent, n | Drug Released (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | Adj R2 | AICc | R | Adj R2 | AICc | ||||
| F1 | 0.8502 | 0.6999 | 81.30 | 0.9675 | 0.9310 | 60.73 | 0.228 | 0.22 | 84.59 |
| F2 | 0.8870 | 0.7692 | 78.79 | 0.9601 | 0.9154 | 64.74 | 0.147 | 0.26 | 80.68 |
| F3 | 0.8527 | 0.7050 | 84.82 | 0.9508 | 0.8915 | 70.81 | 0.196 | 0.23 | 88.19 |
| F4 | 0.9028 | 0.7998 | 75.83 | 0.9616 | 0.9186 | 63.23 | 0.120 | 0.28 | 76.60 |
| F1 | F2 | F3 | F4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | L* | a* | b* | ΔE | L* | a* | b* | ΔE | L* | a* | b* | ΔE | L* | a* | b* | ΔE |
| 3 | 92.03 | 0.15 | −8.20 | 1.60 | 92.52 | −0.31 | −7.93 | 2.82 | 91.92 | −0.32 | −7.39 | 0.55 | 92.47 | −0.72 | −7.12 | 1.42 |
| 4 | 92.04 | 1.91 | −11.29 | 4.41 | 91.80 | 1.32 | −9.83 | 3.09 | 91.63 | 1.64 | −10.45 | 3.82 | 92.16 | 1.13 | −9.59 | 2.95 |
| 5 | 91.82 | 2.05 | −11.14 | 3.25 | 91.83 | 1.71 | −10.20 | 3.13 | 91.65 | 1.97 | −10.74 | 3.35 | 92.42 | 1.65 | −10.35 | 3.33 |
| 6 | 91.76 | 2.39 | −12.11 | 2.31 | 92.34 | 2.25 | −10.90 | 1.83 | 91.81 | 2.37 | −11.19 | 1.55 | 91.62 | 1.78 | −8.61 | 1.42 |
| 7 | 91.96 | 2.64 | −12.46 | 2.18 | 92.24 | 2.64 | −10.71 | 0.92 | 91.84 | 3.04 | −11.66 | 1.18 | 91.95 | 2.29 | −9.79 | 1.19 |
| 8 | 92.60 | 3.07 | −12.89 | 2.68 | 91.75 | 3.26 | −13.17 | 1.97 | 91.56 | 3.38 | −12.07 | 2.68 | 91.57 | 3.12 | −12.67 | 2.56 |
| 9 | 91.54 | 3.53 | −13.92 | 2.02 | 91.56 | 4.16 | −14.18 | 2.34 | 91.72 | 3.96 | −13.24 | 2.90 | 91.67 | 3.30 | −14.02 | 2.08 |
| 10 | 91.42 | 3.94 | −14.40 | 2.41 | 90.71 | 3.70 | −14.19 | 1.68 | 90.73 | 4.44 | −14.75 | 1.24 | 91.54 | 4.09 | −14.05 | 1.54 |
| Film Code | Coll/HEC 1,2, (g%/g%) | NPX 2 (g%) | GA 2 (g%) |
|---|---|---|---|
| F1 | 70:30 | 1.00 | 0.005 |
| F2 | 100:0 | 0.50 | 0.010 |
| F3 | 70:30 | 1.00 | 0.000 |
| F4 | 85:15 | 1.00 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudoroiu, E.-E.; Albu Kaya, M.G.; Dinu-Pîrvu, C.E.; Popa, L.; Anuța, V.; Ignat, M.; Visileanu, E.; Kaya, D.A.; Prisada, R.M.; Ghica, M.V. Development of Smart pH-Sensitive Collagen-Hydroxyethylcellulose Films with Naproxen for Burn Wound Healing. Pharmaceuticals 2025, 18, 689. https://doi.org/10.3390/ph18050689
Tudoroiu E-E, Albu Kaya MG, Dinu-Pîrvu CE, Popa L, Anuța V, Ignat M, Visileanu E, Kaya DA, Prisada RM, Ghica MV. Development of Smart pH-Sensitive Collagen-Hydroxyethylcellulose Films with Naproxen for Burn Wound Healing. Pharmaceuticals. 2025; 18(5):689. https://doi.org/10.3390/ph18050689
Chicago/Turabian StyleTudoroiu, Elena-Emilia, Mădălina Georgiana Albu Kaya, Cristina Elena Dinu-Pîrvu, Lăcrămioara Popa, Valentina Anuța, Mădălina Ignat, Emilia Visileanu, Durmuș Alpaslan Kaya, Răzvan Mihai Prisada, and Mihaela Violeta Ghica. 2025. "Development of Smart pH-Sensitive Collagen-Hydroxyethylcellulose Films with Naproxen for Burn Wound Healing" Pharmaceuticals 18, no. 5: 689. https://doi.org/10.3390/ph18050689
APA StyleTudoroiu, E.-E., Albu Kaya, M. G., Dinu-Pîrvu, C. E., Popa, L., Anuța, V., Ignat, M., Visileanu, E., Kaya, D. A., Prisada, R. M., & Ghica, M. V. (2025). Development of Smart pH-Sensitive Collagen-Hydroxyethylcellulose Films with Naproxen for Burn Wound Healing. Pharmaceuticals, 18(5), 689. https://doi.org/10.3390/ph18050689









