Snake Venom Peptide Fractions from Bothrops jararaca and Daboia siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models
Abstract
1. Introduction
2. Results
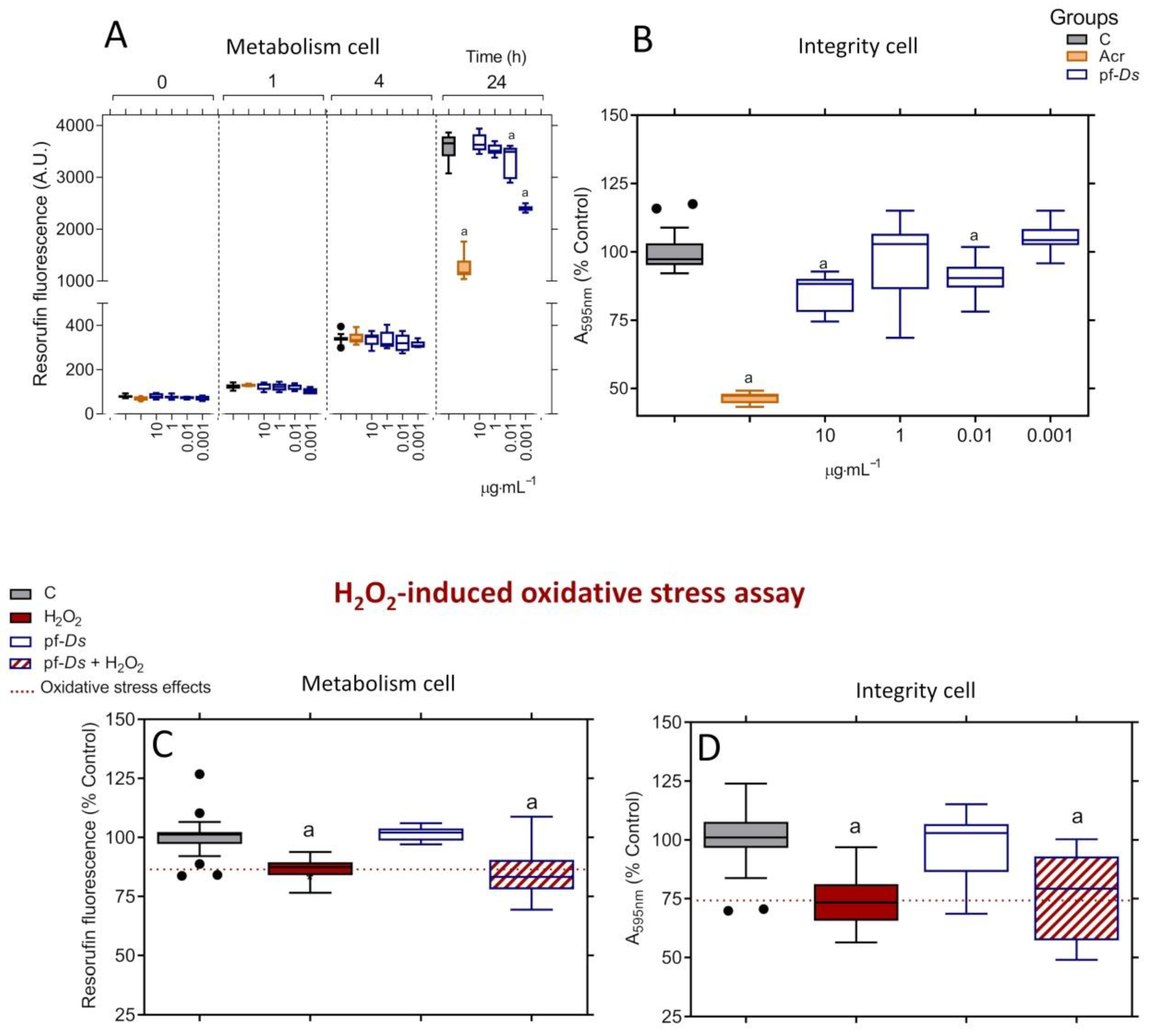
2.1. Toxicological and Neuroprotective Effects of pf-Ds in PC12 Cells
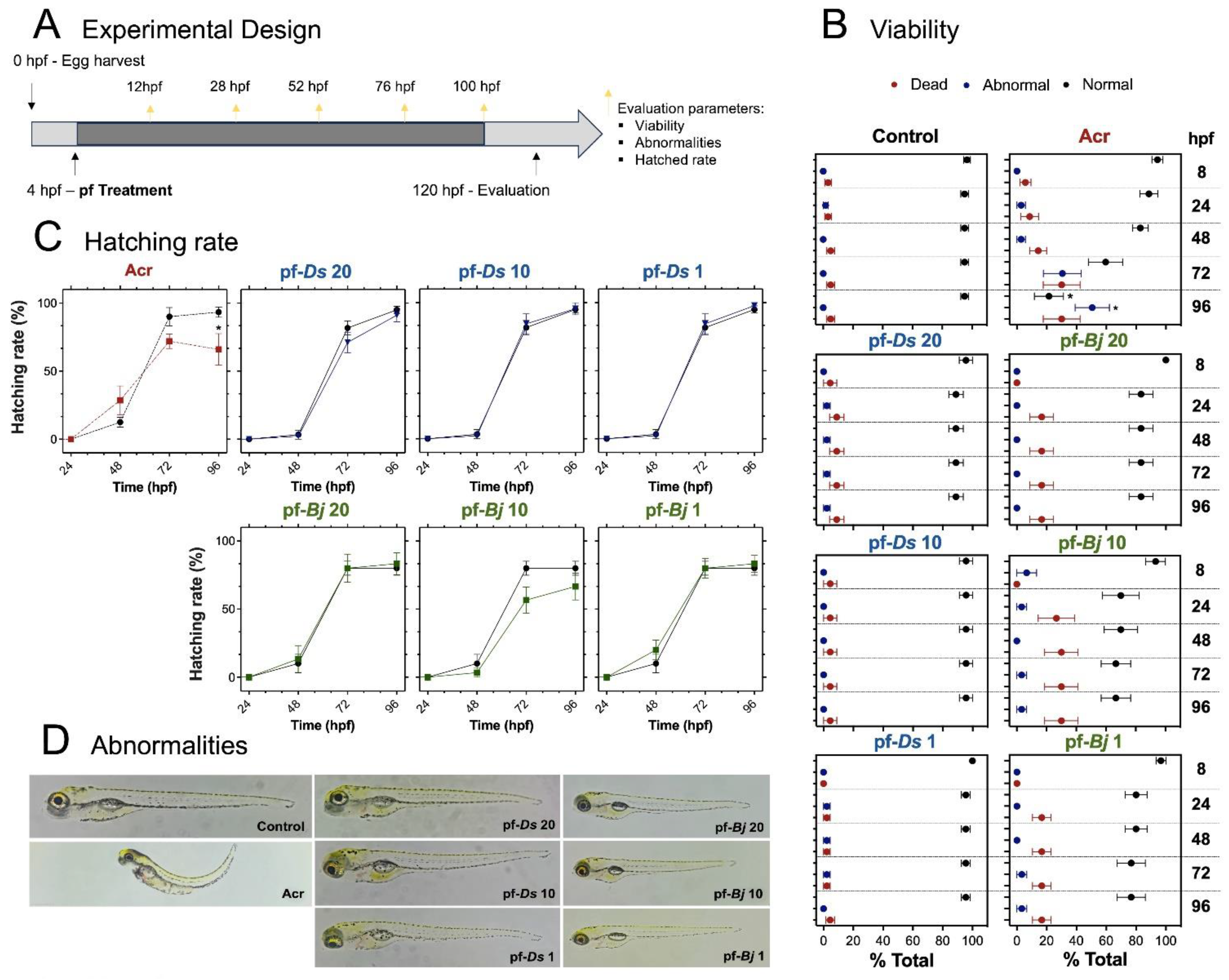
2.2. Toxicological Effects of pf-Ds and pf-Bj on Zebrafish Development
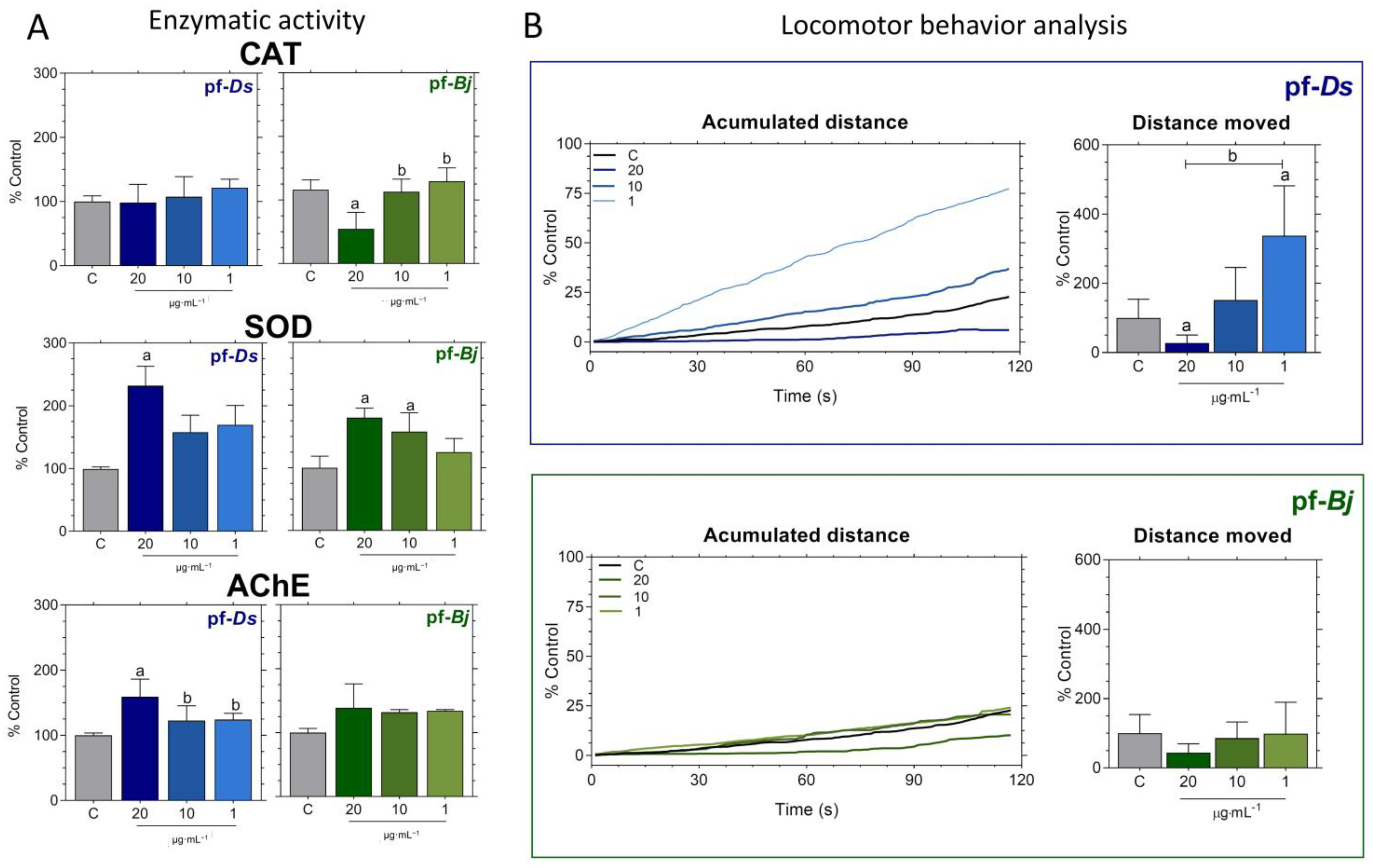
2.3. Neuroprotective Effects of pf-Ds and pf-Bj Against H2O2-Induced Oxidative Stress in Zebrafish Embryos in 4–20 h Model
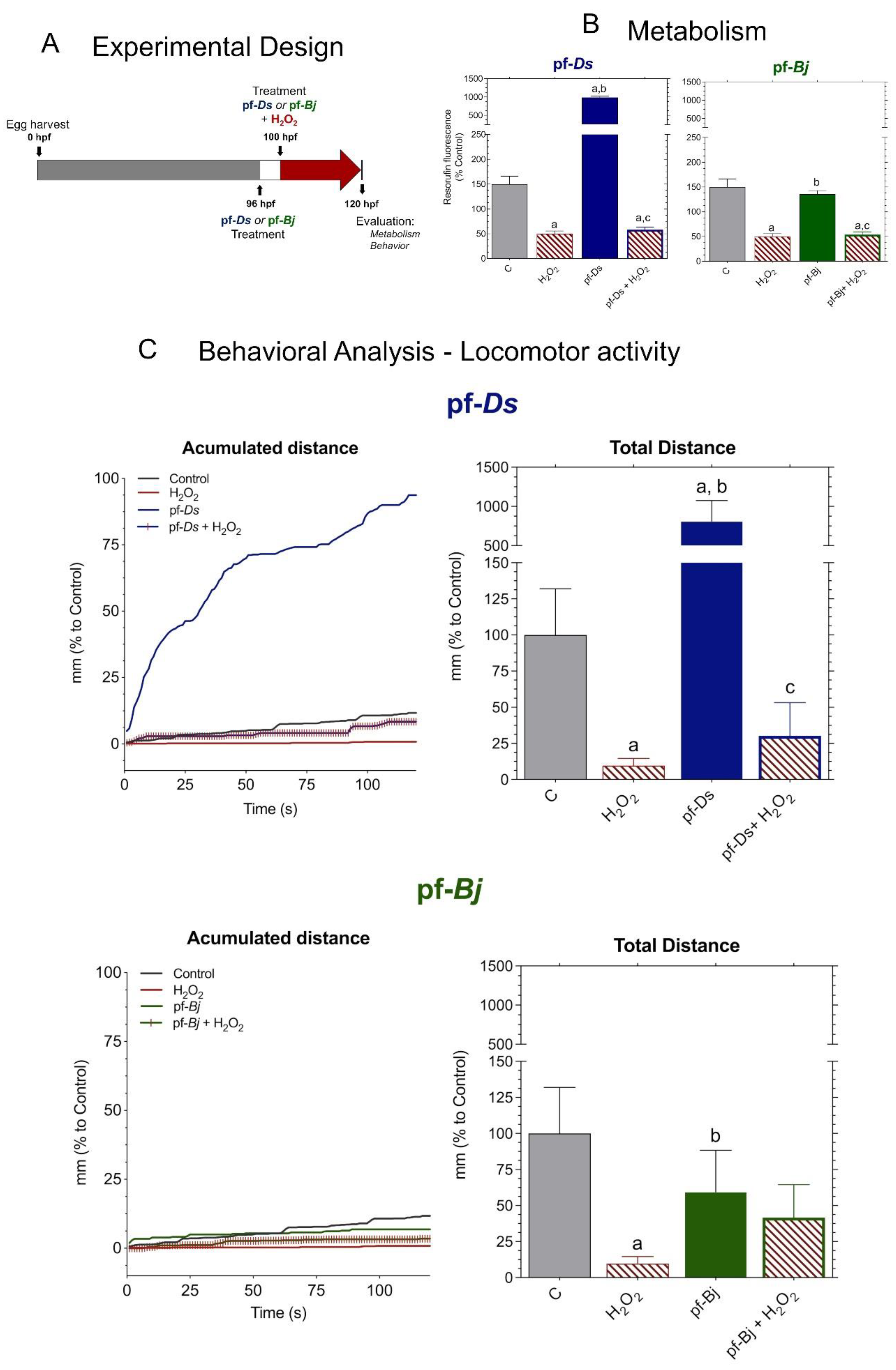
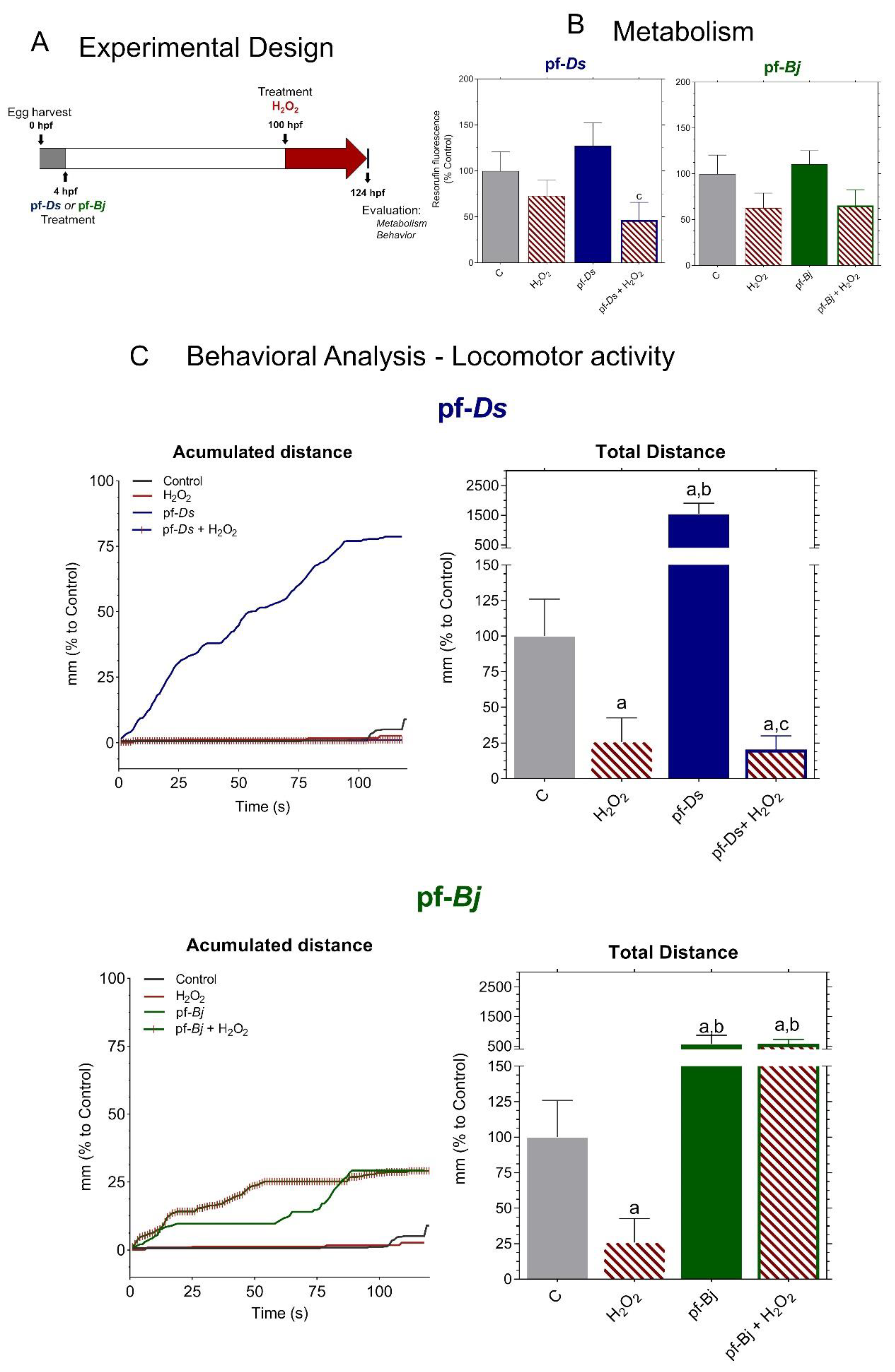
2.4. Neuroprotective Effects of f-Ds and pf-Bj Against H2O2-Induced Oxidative Stress in Zebrafish Embryos in 96–120 h Model
3. Discussion
4. Material and Methods
4.1. Reagents and Peptide Fraction of D. siamensis and B. jararaca Venom
4.2. Cell Lines and Maintenance
4.2.1. Toxicity Studies in Neuronal Cell Lines
4.2.2. H2O2-Induced Oxidative Stress Assay
4.3. Zebrafish Maintenance, Husbandry, and Egg Collection
4.3.1. Monitoring of Zebrafish Development
4.3.2. Neuroprotection Assay Against Oxidative Stress
4.3.3. Behavior Analysis
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Abbate, F.; Maugeri, A.; Laurà, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germanà, A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants 2021, 10, 668. [Google Scholar] [CrossRef]
- Hussain, S.; Slikker, W.; Ali, S.F. Age-Related Changes in Antioxidant Enzymes, Superoxide Dismutase, Catalase, Glutathione Peroxidase and Glutathione in Different Regions of Mouse Brain. Int. J. Dev. Neurosci. 1995, 13, 811–817. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Hu, J.; Tan, B.K. Bioactive Peptides from Marine Organisms. Protein Pept. Lett. 2024, 31, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Alberto-Silva, C.; da Silva, B.R.; da Silva, J.C.A.; Cunha e Silva, F.A.; da Kodama, R.T.; da Silva, W.D.; Costa, M.S.; Portaro, F.C.V. Small Structural Differences in Proline-Rich Decapeptides Have Specific Effects on Oxidative Stress-Induced Neurotoxicity and L-Arginine Generation by Arginosuccinate Synthase. Pharmaceuticals 2024, 17, 931. [Google Scholar] [CrossRef]
- Lima, C.; Disner, G.R.; Falcão, M.A.P.; Seni-Silva, A.C.; Maleski, A.L.A.; Souza, M.M.; Tonello, M.C.R.; Lopes-Ferreira, M. The Natterin Proteins Diversity: A Review on Phylogeny, Structure, and Immune Function. Toxins 2021, 13, 538. [Google Scholar] [CrossRef]
- Boix, N.; Teixido, E.; Pique, E.; Llobet, J.M.; Gomez-Catalan, J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants 2020, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Alberto-Silva, C.; Pantaleão, H.Q.; da Silva, B.R.; da Silva, J.C.A.; Echeverry, M.B. Activation of M1 Muscarinic Acetylcholine Receptors by Proline-Rich Oligopeptide 7a (<EDGPIPP) from Bothrops jararaca Snake Venom Rescues Oxidative Stress-Induced Neurotoxicity in PC12 Cells. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230043. [Google Scholar]
- Pantaleão, H.Q.; da Araujo Silva, J.C.; da Rufino Silva, B.; Echeverry, M.B.; Alberto-Silva, C. Peptide Fraction from B. jararaca Snake Venom Protects against Oxidative Stress-Induced Changes in Neuronal PC12 Cell but Not in Astro-cyte-like C6 Cell. Toxicon 2023, 231, 107178. [Google Scholar] [CrossRef]
- Querobino, S.M.; Carrettiero, D.C.; Costa, M.S.; Alberto-Silva, C. Neuroprotective Property of Low Molecular Weight Fraction from B. jararaca Snake Venom in H2O2-Induced Cytotoxicity in Cultured Hippocampal Cells. Toxicon 2017, 129, 134–143. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute Neuroactive Drug Exposures Alter Locomotor Activity in Larval Zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Tox-icity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Maleski, A.L.A.; Rosa, J.G.S.; Bernardo, J.T.G.; Astray, R.M.; Walker, C.I.B.; Lopes-Ferreira, M.; Lima, C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells 2022, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative Stress Induced Antioxidant and Neurotoxicity Demonstrated in Vivo Zebrafish Embryo or Larval Model and Their Normalization Due to Morin Showing Therapeutic Implications. Life Sci. 2021, 283, 119864. [Google Scholar]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- Alberto-Silva, C.; da Silva, B.R. Molecular and Cellular Mechanisms of Neuroprotection by Oligopeptides from Snake Venoms. Biocell 2024, 48, 897–904. [Google Scholar] [CrossRef]
- Alberto-Silva, C.; Portaro, F.C.V. Neuroprotection Mediated by Snake Venom. In Natural Molecules in Neuroprotection and Neurotoxicity; Elsevier: Amsterdam, The Netherlands, 2024; pp. 437–451. [Google Scholar]
- Silva, B.R.; Mendes, L.C.; Echeverry, M.B.; Juliano, M.A.; Beraldo-Neto, E.; Alberto-Silva, C. Peptide Fraction from Naja Mandalayensis Snake Venom Showed Neuroprotection Against Oxidative Stress in Hippocampal MHippoE-18 Cells but Not in Neuronal PC12 Cells. Antioxidants 2025, 14, 277. [Google Scholar] [CrossRef]
- Cervelli, M.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Fiorucci, C.; Rossi, M.N.; Maura, G.; Mariottini, P.; Cervetto, C.; et al. The Involvement of Polyamines Catabolism in the Crosstalk between Neurons and Astrocytes in Neurodegeneration. Biomedicines 2022, 10, 1756. [Google Scholar] [CrossRef]
- Gogoi, M.; Datey, A.; Wilson, K.T.; Chakravortty, D. Dual Role of Arginine Metabolism in Establishing Pathogenesis. Curr. Opin. Microbiol. 2016, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Spermidine Ameliorates 3-Nitropropionic Acid (3-NP)-Induced Striatal Toxicity: Possible Role of Oxidative Stress, Neuroinflammation, and Neurotransmitters. Physiol. Behav. 2016, 155, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kotagale, N.R.; Taksande, B.G.; Inamdar, N.N. Neuroprotective Offerings by Agmatine. Neurotoxicology 2019, 73, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in Health and Disease. Science 2018, 359, 2788. [Google Scholar] [CrossRef]
- Haas, J.; Storch-Hagenlocher, B.; Biessmann, A.; Wildemann, B. Inducible nitric oxide synthase and argininosuccinate synthetase: Co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with beta-amyloid 1–42 in vitro. Neurosci. Lett. 2002, 322, 121–125. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Amorim, A.M.B.; Piochi, L.F.; Gaspar, A.T.; Preto, A.J.; Rosário-Ferreira, N.; Moreira, I.S. Advancing Drug Safety in Drug Development: Bridging Computational Predictions for Enhanced Toxicity Prediction. Chem. Res. Toxicol. 2024, 37, 827–849. [Google Scholar] [CrossRef]
- Barros, T.P.; Alderton, W.K.; Reynolds, H.M.; Roach, A.G.; Berghmans, S. Zebrafish: An Emerging Technology for in Vivo Pharmacological Assessment to Identify Potential Safety Liabilities in Early Drug Discovery. Br. J. Pharmacol. 2008, 154, 1400–1413. [Google Scholar]
- McGrath, P.; Li, C.Q. Zebrafish: A Predictive Model for Assessing Drug-Induced Toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef]
- Naik, R.A.; Mir, M.N.; Malik, I.A.; Bhardwaj, R.; Alshabrmi, F.M.; Mahmoud, M.A.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Hjazi, A.; et al. The Potential Mechanism and the Role of Antioxidants in Mitigating Oxidative Stress in Alzheimer’s Disease. Front. Biosci. (Landmark Ed.) 2025, 30, 25551. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bertrand, C.; Chatonnet, A.; Takke, C.; Yan, Y.L.; Postlethwait, J.; Toutant, J.P.; Cousin, X. Zebrafish Acetylcholinesterase Is Encoded by a Single Gene Localized on Linkage Group 7. Gene Structure and Polymorphism; Molecular Forms and Expression Pattern during Development. J. Biol. Chem. 2001, 276, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase Activity and Antioxidant Capacity of Zebrafish Brain Is Altered by Heavy Metal Exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef]
- Sant’Anna, M.C.B.; de Soares, V.M.; Seibt, K.J.; Ghisleni, G.; Rico, E.P.; Rosemberg, D.B.; de Oliveira, J.R.; Schrö-der, N.; Bonan, C.D.; Bogo, M.R. Iron Exposure Modifies Acetylcholinesterase Activity in Zebrafish (Danio rerio) Tissues: Distinct Susceptibility of Tissues to Iron Overload. Fish Physiol. Biochem. 2011, 37, 573–581. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Shaw, P.J. Oxidative Stress in ALS: Key Role in Motor Neuron Injury and Therapeutic Target. Free. Radic. Biol. Med. 2010, 48, 629–641. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Slotkin, T.A. Cholinergic Systems in Brain Development and Disruption by Neurotoxicants: Nicotine, Environmental Tobacco Smoke, Organophosphates. Toxicol. Appl. Pharmacol. 2004, 198, 132–151. [Google Scholar] [CrossRef]
- Nordberg, A.; Svensson, A.-L. Cholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. Drug Saf. 1998, 19, 465–480. [Google Scholar] [CrossRef]
- Khademi, N.; Mehrnia, N.; Roshan, M.E. Bioactive Peptides: A Complementary Approach for Cancer Therapy. Asian Pac. J. Cancer Care 2024, 9, 801–811. [Google Scholar]
- Zhang, D.L.; Hu, C.X.; Li, D.H.; Liu, Y.D. Lipid Peroxidation and Antioxidant Responses in Zebrafish Brain Induced by Aphanizomenon Flos-Aquae DC-1 Aphantoxins. Aquat. Toxicol. 2013, 144-145, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hou, Y.; Maeng, J.H.; Shah, N.M.; Chen, Y.; Lawson, H.A.; Yang, H.; Yue, F.; Wang, T. Epigenomic Analysis Reveals Prevalent Contribution of Transposable Elements to Cis -Regulatory Elements, Tissue-Specific Expression, and Alternative Promoters in Zebrafish. Genome Res. 2022, 32, 1424–1436. [Google Scholar] [CrossRef]
- Dyker, A.G.; Lees, K.R. Duration of Neuroprotective Treatment for Ischemic Stroke. Stroke 1998, 29, 535–542. [Google Scholar] [CrossRef]
- Lee, B.; Butcher, G.Q.; Hoyt, K.R.; Impey, S.; Obrietan, K. Activity-Dependent Neuroprotection and CAMP Response Element-Binding Protein (CREB): Kinase Coupling, Stimulus Intensity, and Temporal Regulation of CREB Phosphorylation at Serine 133. J. Neurosci. 2005, 25, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.M.; Santos, N.A.G.; Sartim, M.A.; Cintra, A.C.O.; Sampaio, S.V.; Santos, A.C. A Tripeptide Isolated from Bothrops Atrox Venom Has Neuroprotective and Neurotrophic Effects on a Cellular Model of Parkinson’s Disease. Chem. Biol. Interact. 2015, 235, 10–16. [Google Scholar] [CrossRef]
- Salem, F.E.; Yehia, H.M.; Korany, S.M.; Alarjani, K.M.; Al-Masoud, A.H.; Elkhadragy, M.F. Neurotherapeutic Effects of Prodigiosin Conjugated with Silver-Nanoparticles in Rats Exposed to Cadmium Chloride-Induced Neurotoxicity. Food Sci. Technol. (Campinas) 2022, 42, 97322. [Google Scholar] [CrossRef]
- Hahn, M.E.; Timme-Laragy, A.R.; Karchner, S.I.; Stegeman, J.J. Nrf2 and Nrf2-Related Proteins in Development and Developmental Toxicity: Insights from Studies in Zebrafish (Danio rerio). Free Radic. Biol. Med. 2015, 88, 275–289. [Google Scholar] [CrossRef]
- Marques, E.S.; Severance, E.G.; Arsenault, P.; Zahn, S.M.; Timme-Laragy, A.R. Activation of Nrf2 at Critical Windows of Development Alters Tissue-Specific Protein S-Glutathionylation in the Zebrafish (Danio rerio) Embryo. Antioxidants 2024, 13, 1006. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Badaro-Garcia, S.; Hohmann, M.S.N.; Manchope, M.F.; Zaninelli, T.H.; Casagrande, R.; Verri, W.A. Contribution of Nrf2 Modulation to the Mechanism of Action of Analgesic and Anti-Inflammatory Drugs in Pre-Clinical and Clinical Stages. Front. Pharmacol. 2019, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.G.; Gelain, F. Structure-Activity Relationships of Antibacterial Peptides. Microb. Biotechnol. 2023, 16, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cui, J.Y.; Liu, J.; Lu, H.; Zhong, X.; Klaassen, C.D. RNA-Seq Provides New Insights on the Relative MRNA Abundance of Antioxidant Components during Mouse Liver Development. Free. Radic. Biol. Med. 2019, 134, 335–342. [Google Scholar] [CrossRef]
- Mills, M.G.; Gallagher, E.P. A Targeted Gene Expression Platform Allows for Rapid Analysis of Chemical-Induced Antioxidant MRNA Expression in Zebrafish Larvae. PLoS ONE 2017, 12, e0171025. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; de Barros, F.M.; Andrade, P.M. A Simple Method to Measure Cell Viability in Proliferation and Cytotoxicity Assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, prot087379. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Brussels, Belgium, 2010; pp. 33–79. [Google Scholar]
- Conselho Nacional de Controle de Experimentação Animal (CONCEA). Capítulo 5—Lambari, tilápia e zebrafish. In Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica; Ministério da Ciência, Tecnologia e Inovações: Brasília, Brazil, 2023; pp. 330–483. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Silva, R.F.O.; Pinho, B.R.; Santos, M.M.; Oliveira, J.M.A. Disruptions of Circadian Rhythms, Sleep, and Stress Responses in Zebrafish: New Infrared-Based Activity Monitoring Assays for Toxicity Assessment. Chemosphere 2022, 305, 135449. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Lassiter, T.L.; Hunter, D.L. Biochemical Measurement of Cholinesterase Activity. In Neurodegeneration Methods and Protocols; Harry, G.J., Tilson, H.A., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 237–245. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cunha e Silva, F.A.; Silva, B.R.d.; Barros, L.R.d.; Beraldo-Neto, E.; Maleski, A.L.A.; Alberto-Silva, C. Snake Venom Peptide Fractions from Bothrops jararaca and Daboia siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models. Pharmaceuticals 2025, 18, 678. https://doi.org/10.3390/ph18050678
da Cunha e Silva FA, Silva BRd, Barros LRd, Beraldo-Neto E, Maleski ALA, Alberto-Silva C. Snake Venom Peptide Fractions from Bothrops jararaca and Daboia siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models. Pharmaceuticals. 2025; 18(5):678. https://doi.org/10.3390/ph18050678
Chicago/Turabian Styleda Cunha e Silva, Felipe Assumpção, Brenda Rufino da Silva, Leticia Ribeiro de Barros, Emidio Beraldo-Neto, Adolfo Luis Almeida Maleski, and Carlos Alberto-Silva. 2025. "Snake Venom Peptide Fractions from Bothrops jararaca and Daboia siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models" Pharmaceuticals 18, no. 5: 678. https://doi.org/10.3390/ph18050678
APA Styleda Cunha e Silva, F. A., Silva, B. R. d., Barros, L. R. d., Beraldo-Neto, E., Maleski, A. L. A., & Alberto-Silva, C. (2025). Snake Venom Peptide Fractions from Bothrops jararaca and Daboia siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models. Pharmaceuticals, 18(5), 678. https://doi.org/10.3390/ph18050678








