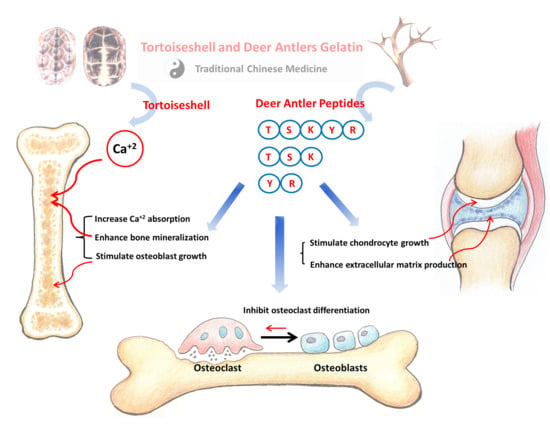
Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism
Abstract
1. Introduction
2. Results and Discussion
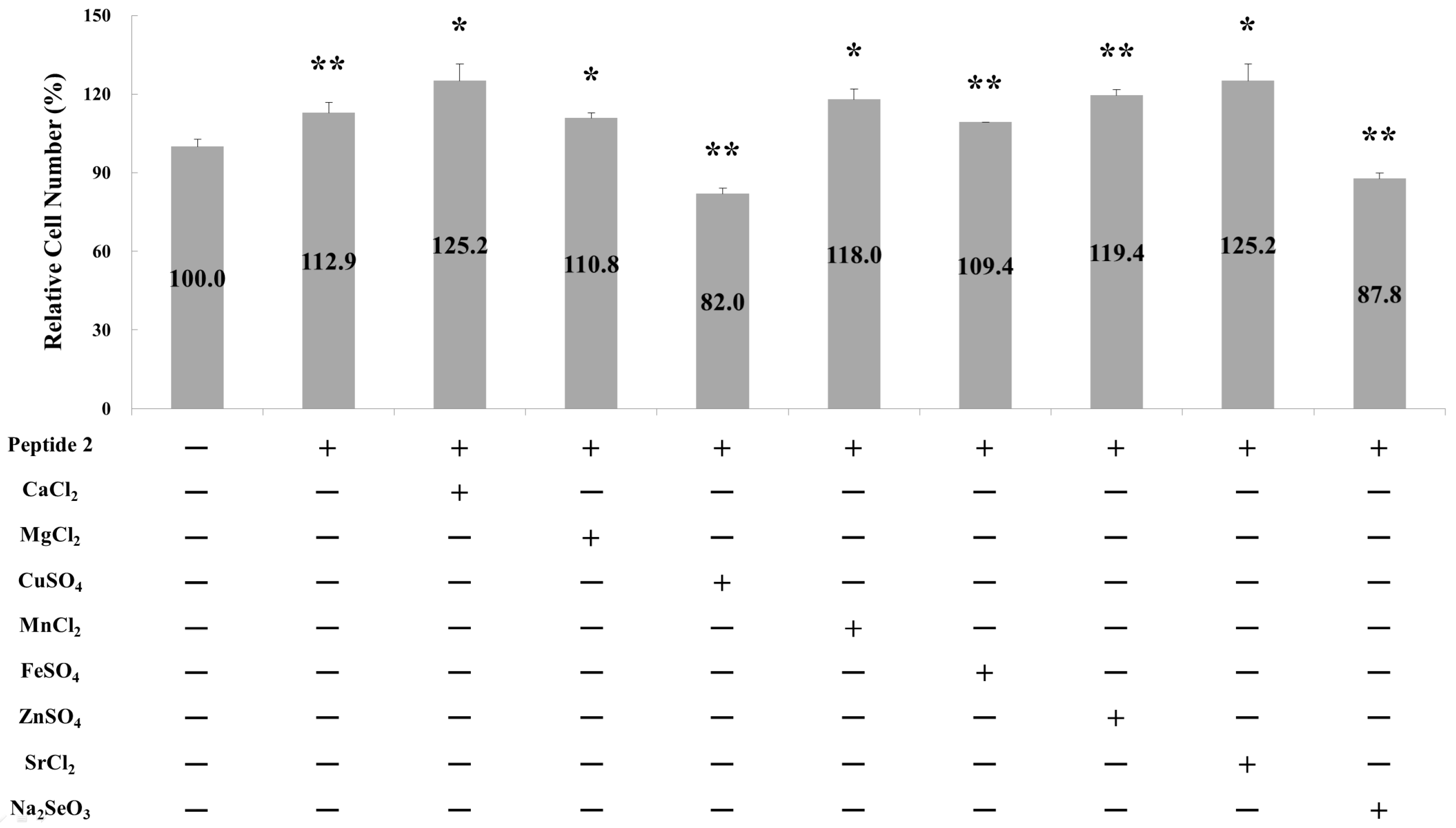
2.1. Effect of Metal Ions on Osteoblast Cell Proliferation
2.2. Effect of Deer Antler Peptides on Skeletal Muscle Cell Proliferation
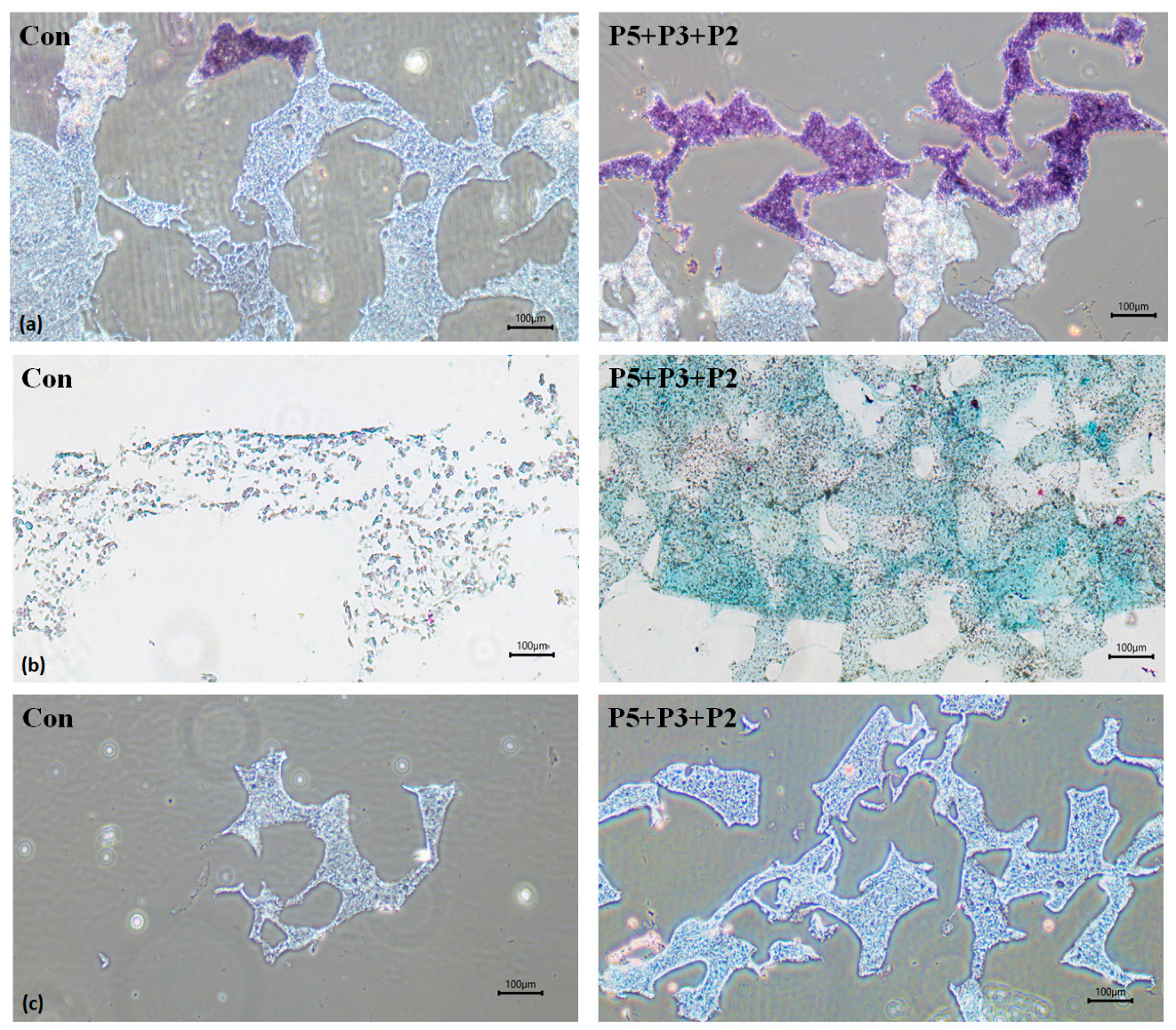
2.3. Biological Activities of Deer Antler Peptides on Chondrocyte Growth
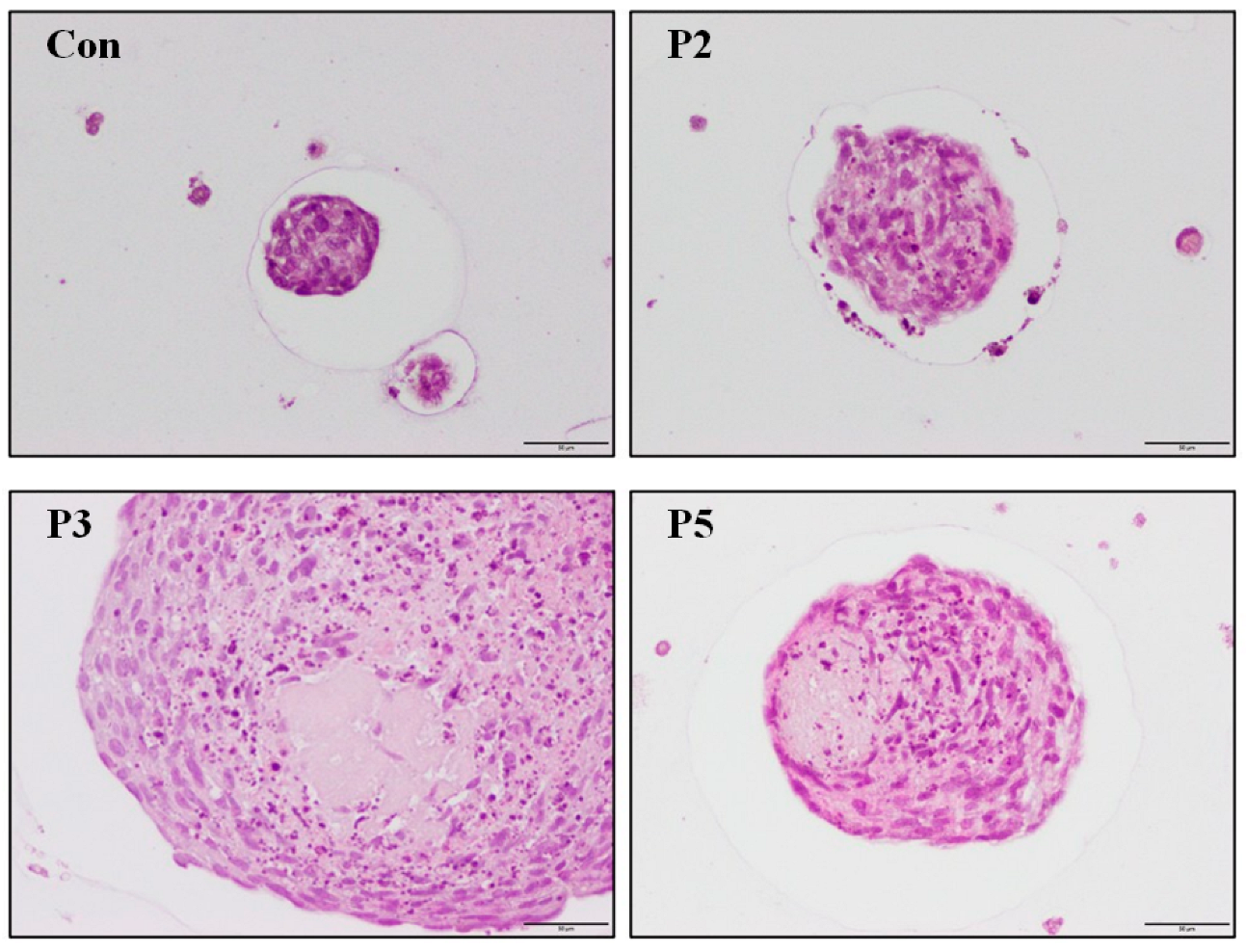
2.4. Chondrocyte Growth in 3D Cell Encapsulation
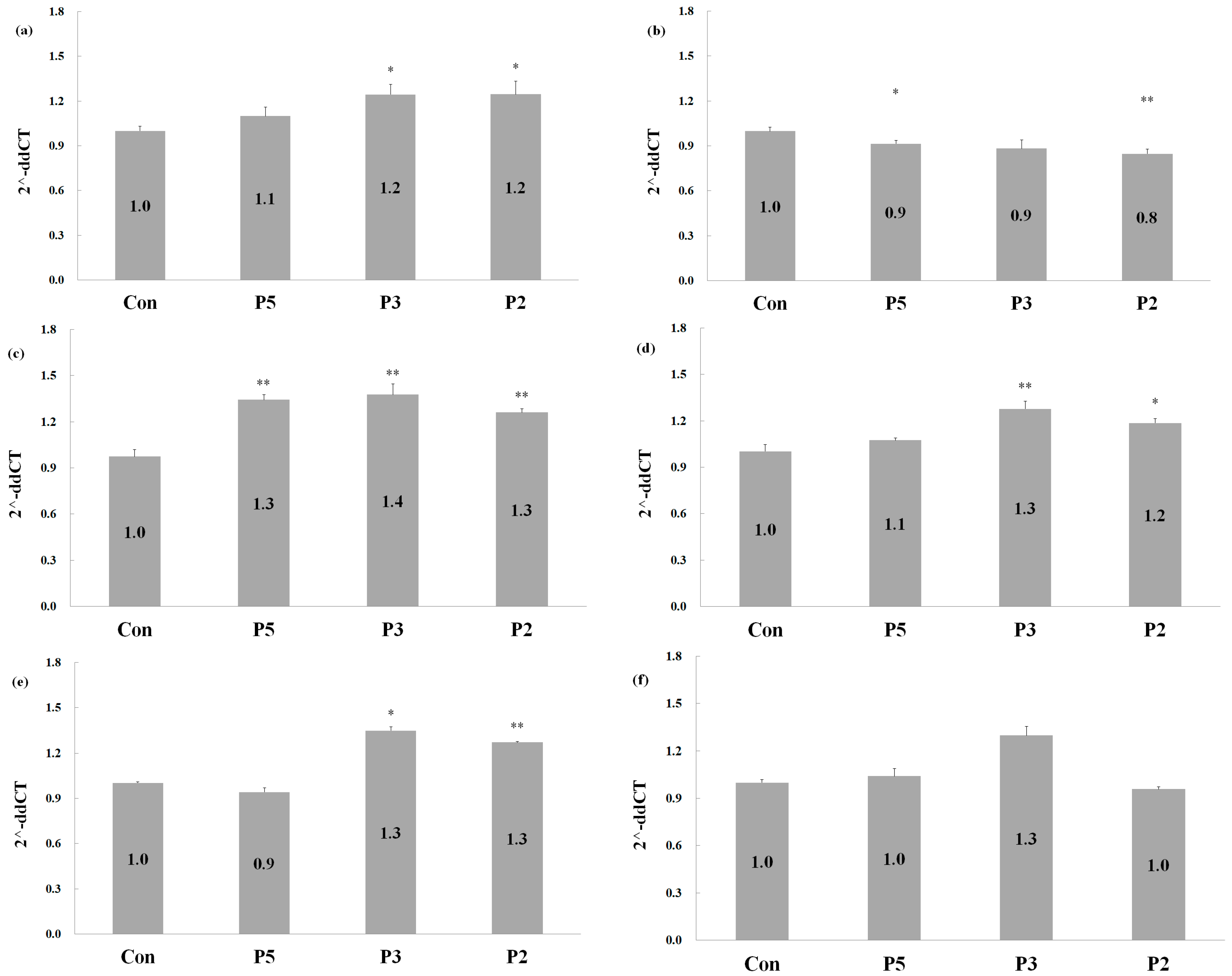
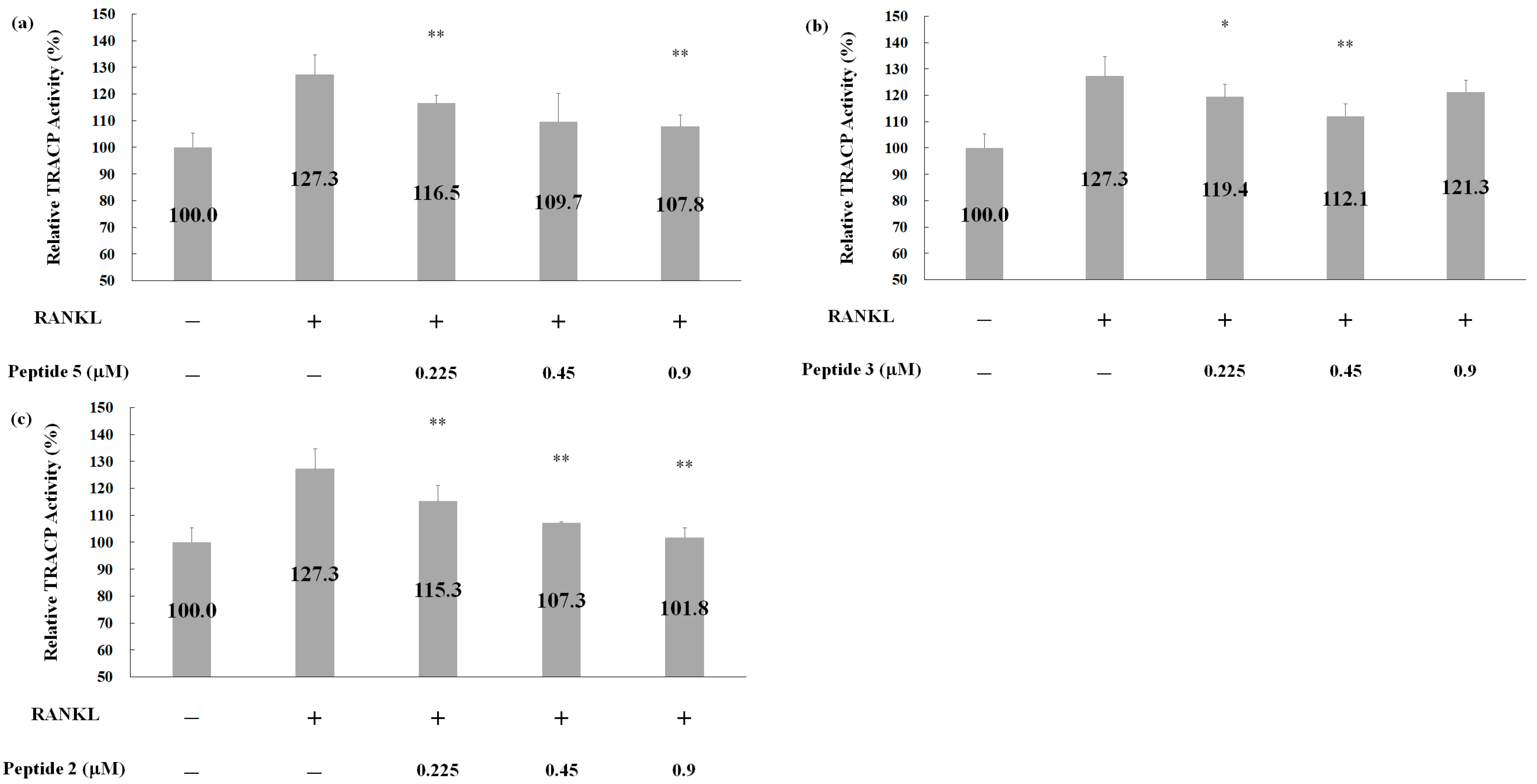
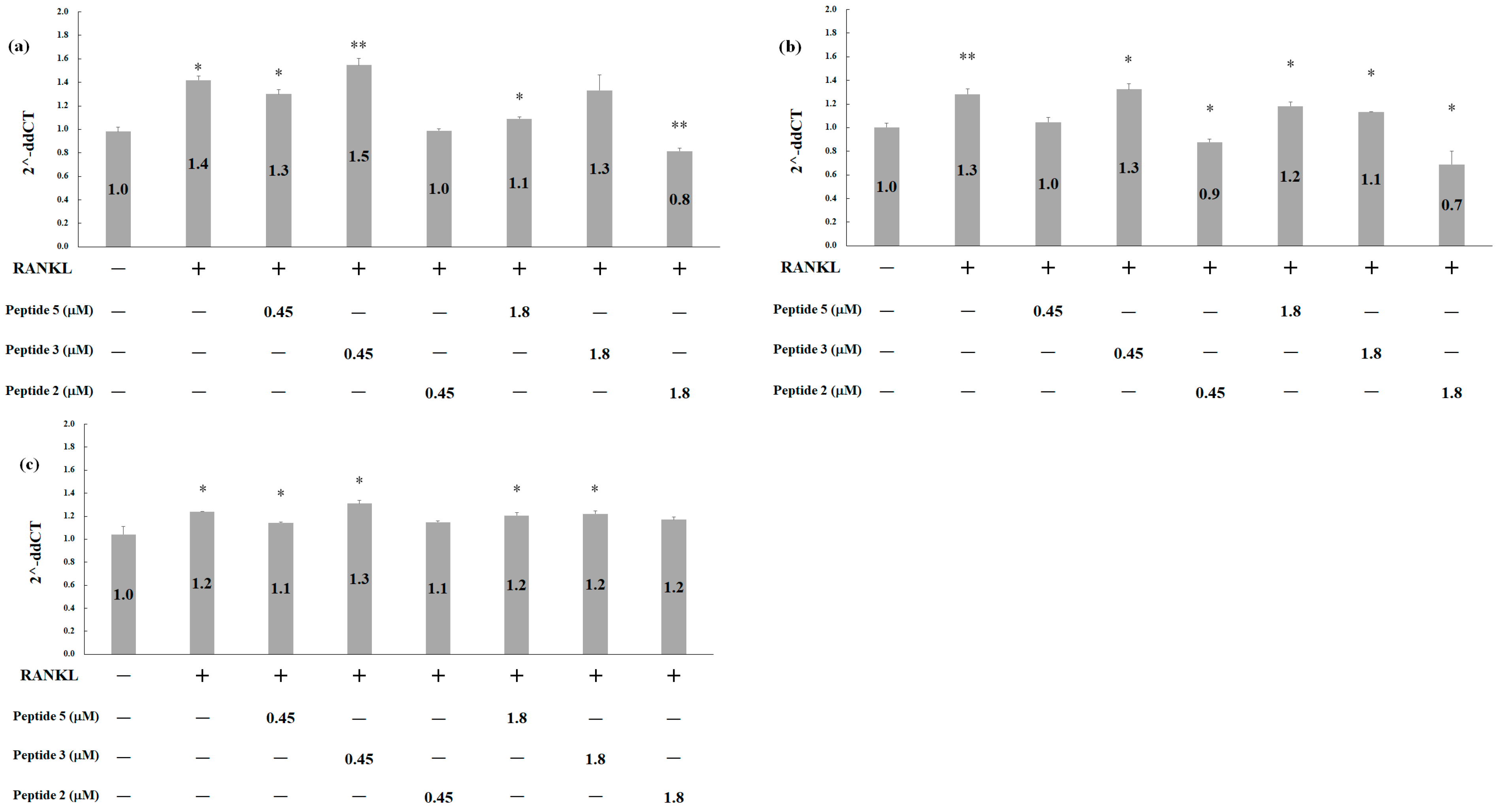
2.5. Effects of Deer Antler Peptides on Homeostasis between Osteoclasts and Osteoblasts
3. Materials and Methods
3.1. Materials and Cell Culture
3.2. Cell Proliferation Assessment
3.3. Cell Encapsulation
3.4. Reverse Transcription and Quantitative PCR
3.5. Detection of ROS Generation
3.6. TRACP (Tartrate-Resistant Acid Phosphatase) Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2023 Global Glucosamine Market Size and Leading Players: 2030 Outlook. In Latest Market Research Updates. Available online: https://www.linkedin.com/pulse/2023-global-glucosamine-market-size-leading-players (accessed on 25 March 2024).
- Julie, S.; Jurenka, M. Anti-inflammatory properties of curcumin, a major constituent. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Liu, S.; Matsuo, T.; Matsuo, C.; Abe, T. Traditional Chinese medicines and prescriptions brought from China to Japan by a monk (Jianzhen, Japanese: Ganjin): A historical review. Compounds 2022, 2, 267–284. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Chou, H.-N.; Hsu, C.-K.; Chou, H.-S.; Sung, P.-J.; Chen, F.-G. Stratum Corneum Hydration and Skin Surface pH Variation Indicate that Organ Blood Flow Is Regulated by Meridian Activity at Certain Hours. Med. Sci. 2014, 2, 161–172. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Ma, Y.; Guo, Y.; Yin, H. Effectiveness and safety of Guilu Erxian Glue (a traditional Chinese medicinal product) for the treatment of postmenopausal osteoporosis: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e20773. [Google Scholar] [CrossRef]
- Liao, J.-A.; Yeh, Y.-C.; Chang, Z.-Y. The efficacy and safety of traditional Chinese medicine Guilu Erxian Jiao in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2022, 46, 101515. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chou, Y.-Y.; Chen, Y.-M.; Tang, Y.-J.; Ho, H.-C.; Chen, D.-Y. Effect of the herbal drug guilu erxian jiao on muscle strength, articular pain, and disability in elderly men with knee osteoarthritis. Evid.-Based Complement. Altern. Med. 2014, 2014, 297458. [Google Scholar] [CrossRef]
- Ho, T.-J.; Lin, J.-H.; Lin, S.Z.; Tsai, W.-T.; Wu, J.-R.; Chen, H.-P. Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. Int. J. Mol. Sci. 2023, 24, 1759. [Google Scholar] [CrossRef]
- Takagi, H.; Shiomi, H.; Ueda, H.; Amano, H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature 1979, 282, 410–412. [Google Scholar] [CrossRef]
- Kiso, Y.; Kitagawa, K.; Nobuyuki, K.; Akita, T.; Takagi, H.; Amano, H.; Fukui, K. Neo-kyotorphin (Thr—Ser—Lys—Tyr—Arg), a new analgesic peptide. FEBS Lett. 1983, 155, 281–284. [Google Scholar] [CrossRef]
- Ueda, H.; Yoshihara, Y.; Misawa, H.; Fukushima, N.; Katada, T.; Ui, M.; Takagi, H.; Satoh, M. The kyotorphin (tyrosine-arginine) receptor and a selective reconstitution with purified Gi, measured with GTPase and phospholipase C assays. J. Biol. Chem. 1989, 264, 3732–3741. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Ueda, H.; Fujii, N.; Shide, A.; Yajima, H.; Satoh, M. Purification of a novel type of calcium-activated neutral protease from rat brain. Possible involvement in production of the neuropeptide kyotorphin from calpastatin fragments. J. Biol. Chem. 1990, 265, 5809–5815. [Google Scholar] [CrossRef]
- O’Connor, S.; Solazzo, C.; Collins, M. Advances in identifying archaeological traces of horn and other keratinous hard tissues. Stud. Conserv. 2015, 60, 393–417. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tian, S.; Zhou, X.; Zhao, T.; Shi, Y. Determination of Ca, Mg, Fe, Zn and Other Eight Trace Elements in Tortoise-Shell Glue and Antler Glue by ICP-MS. Res. Pract. Chin. Med. 2018, 32, 42–45. [Google Scholar]
- Zaichick, V.; Zaichick, S.; Karandashev, V.; Nosenko, S. The effect of age and gender on Al, B, Ba, Ca, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Sr, V, and Zn contents in rib bone of healthy humans. Biol. Trace Elem. Res. 2009, 129, 107–115. [Google Scholar] [CrossRef]
- Zaichick, S.; Zaichick, V. The effect of age and gender on 38 chemical element contents in human iliac crest investigated by instrumental neutron activation analysis. J. Trace Elem. Med. Biol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Zaichick, S.; Zaichick, V.; Karandashev, V.K.; Moskvina, I.R. The effect of age and gender on 59 trace-element contents in human rib bone investigated by inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res. 2011, 143, 41–57. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Niu, X.; Cao, P.; Zhang, Y.; Liu, Y.; Yang, H.; Qiao, A. Multifarious roles of metal elements in bone mineralization. Appl. Mater. Today 2023, 32, 101810. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, H.-W. Chondrogenic potential of dedifferentiated rat chondrocytes reevaluated in two-and three-dimensional culture conditions. Tissue Eng. Regen. Med. 2018, 15, 163–172. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-osteoclast communication and bone homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Xian, Y.; Su, Y.; Liang, J.; Long, F.; Feng, X.; Xiao, Y.; Lian, H.; Xu, J.; Zhao, J.; Liu, Q. Oroxylin A reduces osteoclast formation and bone resorption via suppressing RANKL-induced ROS and NFATc1 activation. Biochem. Pharmacol. 2021, 193, 114761. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics 2019, 9, 4648. [Google Scholar] [CrossRef]
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264. 7 for osteoclastogensis study: Phenotype and stimuli. J. Cell. Mol. Med. 2019, 23, 3077–3087. [Google Scholar] [CrossRef]
- Ethiraj, L.P.; Fong, E.L.S.; Liu, R.; Chan, M.; Winkler, C.; Carney, T.J. Colorimetric and fluorescent TRAP assays for visualising and quantifying fish osteoclast activity. Eur. J. Histochem. EJH 2022, 66, 3369. [Google Scholar] [CrossRef]
- Thomas, A.; South, S.; Vijayagopal, P.; Juma, S. Effect of tart cherry polyphenols on osteoclast differentiation and activity. J. Med. Food 2020, 23, 56–64. [Google Scholar] [CrossRef]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef]
- Montonen, M.; Li, T.F.; Lukinmaa, P.L.; Sakai, E.; Hukkanen, M.; Sukura, A.; Konttinen, Y.T. RANKL and cathepsin K in diffuse sclerosing osteomyelitis of the mandible. J. Oral Pathol. Med. 2006, 35, 620–625. [Google Scholar] [CrossRef]
- Kukita, T.; Wada, N.; Kukita, A.; Kakimoto, T.; Sandra, F.; Toh, K.; Nagata, K.; Iijima, T.; Horiuchi, M.; Matsusaki, H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 2004, 200, 941–946. [Google Scholar] [CrossRef]
- Tong, S.; Xue, L.; Xu, D.P.; Liu, Z.M.; Du, Y.; Wang, X.K. In vitro culture of hFOB1. 19 osteoblast cells on TGF-β1-SF-CS three-dimensional scaffolds. Mol. Med. Rep. 2016, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Subedar, O.D.; Chiu, L.L.; Waldman, S.D. Cell cycle synchronization of primary articular chondrocytes enhances chondrogenesis. Cartilage 2021, 12, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Stachurska, A.; Dorosz, J.; Zurawski, M.; Wegrzyn, J.; Kozakowska, M.; Jozkowicz, A.; Dulak, J. HIF-1 attenuates Ref-1 expression in endothelial cells: Reversal by siRNA and inhibition of geranylgeranylation. Vasc. Pharmacol. 2009, 51, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gourronc, F.; Buckwalter, J.A.; Martin, J.A. Nanog maintains human chondrocyte phenotype and function in vitro. J. Orthop. Res. 2010, 28, 516–521. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Qin, D.; Zheng, X.-X.; Jiang, Y.-R. Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol. Vis. 2013, 19, 2227. [Google Scholar] [PubMed]
- Sanchez, C.; Deberg, M.; Piccardi, N.; Msika, P.; Reginster, J.-Y.; Henrotin, Y. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2005, 13, 988–997. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Wang, Y.; Hu, Q. Role of CD14 and TLR4 in type I, type III collagen expression, synthesis and secretion in LPS-induced normal human skin fibroblasts. Int. J. Clin. Exp. Med. 2015, 8, 2429. [Google Scholar]
- Chen, F.; Zhang, G.; Yu, L.; Feng, Y.; Li, X.; Zhang, Z.; Wang, Y.; Sun, D.; Pradhan, S. High-efficiency generation of induced pluripotent mesenchymal stem cells from human dermal fibroblasts using recombinant proteins. Stem Cell Res. Ther. 2016, 7, 99. [Google Scholar] [CrossRef][Green Version]
- He, F.; Luo, S.; Liu, S.; Wan, S.; Li, J.; Chen, J.; Zuo, H.; Pei, X. Zanthoxylum bungeanum seed oil inhibits RANKL-induced osteoclastogenesis by suppressing ERK/c-JUN/NFATc1 pathway and regulating cell cycle arrest in RAW264. 7 cells. J. Ethnopharmacol. 2022, 289, 115094. [Google Scholar] [CrossRef]
- Sohn, H.; Ko, Y.; Park, M.; Kim, D.; Moon, Y.L.; Jeong, Y.J.; Lee, H.; Moon, Y.; Jeong, B.C.; Kim, O. Effects of light-emitting diode irradiation on RANKL-induced osteoclastogenesis. Lasers Surg. Med. 2015, 47, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Burstone, M. Histochemical demonstration of acid phosphatase activity in osteoclasts. J. Histochem. Cytochem. 1959, 7, 39–41. [Google Scholar] [CrossRef] [PubMed]


| Metal | Human Bone (mg/Kg) | Tortoiseshell (mg/Kg) | Deer Antler (mg/Kg) | Fold * |
|---|---|---|---|---|
| Ca | 171,400 ± 4050 | 1841.6 ± 33.1 | 1902.1 ± 33.3 | approximately 90 |
| Mg | 2139 ± 38 | 429.7 ± 9.0 | 268.4 ± 8.9 | approximately 5–8 |
| Sr | 291 ± 20 | 10.5 ± 0.4 | 8.0 ± 0.4 | approximately 28–36 |
| Fe | 140 ± 11 | 19.1 ± 0.8 | 23.7 ± 0.9 | approximately 6–7 |
| Zn | 92.8 ± 1.5 | 22.8 ± 0.5 | 23.9 ± 1.1 | approximately 4 |
| Cu | 1.35 ± 0.22 | 0.39 ± 0.02 | 0.3 ± 0.02 | approximately 4 |
| Mn | 0.354 ± 0.004 | 0.83 ± 0.03 | 0.29 ± 0.02 | approximately 0.4–1 |
| Se | <0.06 | 0.075 ± 0.004 | 0.018 ± 0.001 | approximately 1–3 |
| Gene | Primer Sequence (5′→3′) | Reference |
|---|---|---|
| HIF1A | FORWARD: TGCTTGGTGCTGATTTGTGA REVERSE: GGTCAGATGATCAGAGTCCA | [34] |
| SOX9 | FORWARD: CCCCAACAGATCGCCTACAG REVERSE: GAGTTCTGGTGGTCGGTGTAGTC | [35] |
| ACAN | FORWARD: CACCTCCCCAACAGATGCTT REVERSE: GGTACTTGTTCCAGCCCTCC | [36] |
| Collagen I | FORWARD: TGGTGGTTATGACTTTGGTTACGAT REVERSE: TGTGCGAGCTGGGTTCTTTCTA | [37] |
| Collagen II | FORWARD: TGCTGCCCAGATGGCTGGAGGA REVERSE: TGCCTTGAAATCCTTGAGGCCC | [38] |
| Collagen III | FORWARD: ATGGTTGCACGAAACACACT REVERSE: CTTGATCAGGACCACCAATG | [39] |
| β-actin | FORWARD: AGAGCTACGAGCTGCCTGAC REVERSE: AGCACTGTGTTGGCGTACAG | [40] |
| MMP-9 | FORWARD: AGTTTGGTGTCGCGGAGCAC REVERSE: TACATGAGCGCTTCCGGCAC | [41] |
| CTSK | FORWARD: GGCCAACTCAAGAAGAAAAC REVERSE: GTGCTTGCTTCCCTTCTGG | [41] |
| DC-STAMP | FORWARD: TCCTCCATGAACAAACAGTTCCAA REVERSE: AGACGTGGTTTAGGAATGCAGCTC | [41] |
| GAPDH | FORWARD: AACTTTGGCATTGTGGAAGG REVERSE: ACACATTGGGGGTAGGAACA | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, T.-J.; Tsai, W.-T.; Wu, J.-R.; Chen, H.-P. Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism. Pharmaceuticals 2024, 17, 434. https://doi.org/10.3390/ph17040434
Ho T-J, Tsai W-T, Wu J-R, Chen H-P. Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism. Pharmaceuticals. 2024; 17(4):434. https://doi.org/10.3390/ph17040434
Chicago/Turabian StyleHo, Tsung-Jung, Wan-Ting Tsai, Jia-Ru Wu, and Hao-Ping Chen. 2024. "Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism" Pharmaceuticals 17, no. 4: 434. https://doi.org/10.3390/ph17040434
APA StyleHo, T.-J., Tsai, W.-T., Wu, J.-R., & Chen, H.-P. (2024). Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism. Pharmaceuticals, 17(4), 434. https://doi.org/10.3390/ph17040434