Development of Rifampicin Eye Drops for the Treatment of Exudative Age-Related Macular Degeneration
Abstract
1. Introduction
2. Results
2.1. Topical Application of Rifampicin Reaches the Retina
2.2. Pharmacokinetic Studies Using a 0.25% Rifampicin Eye Drop Formulation
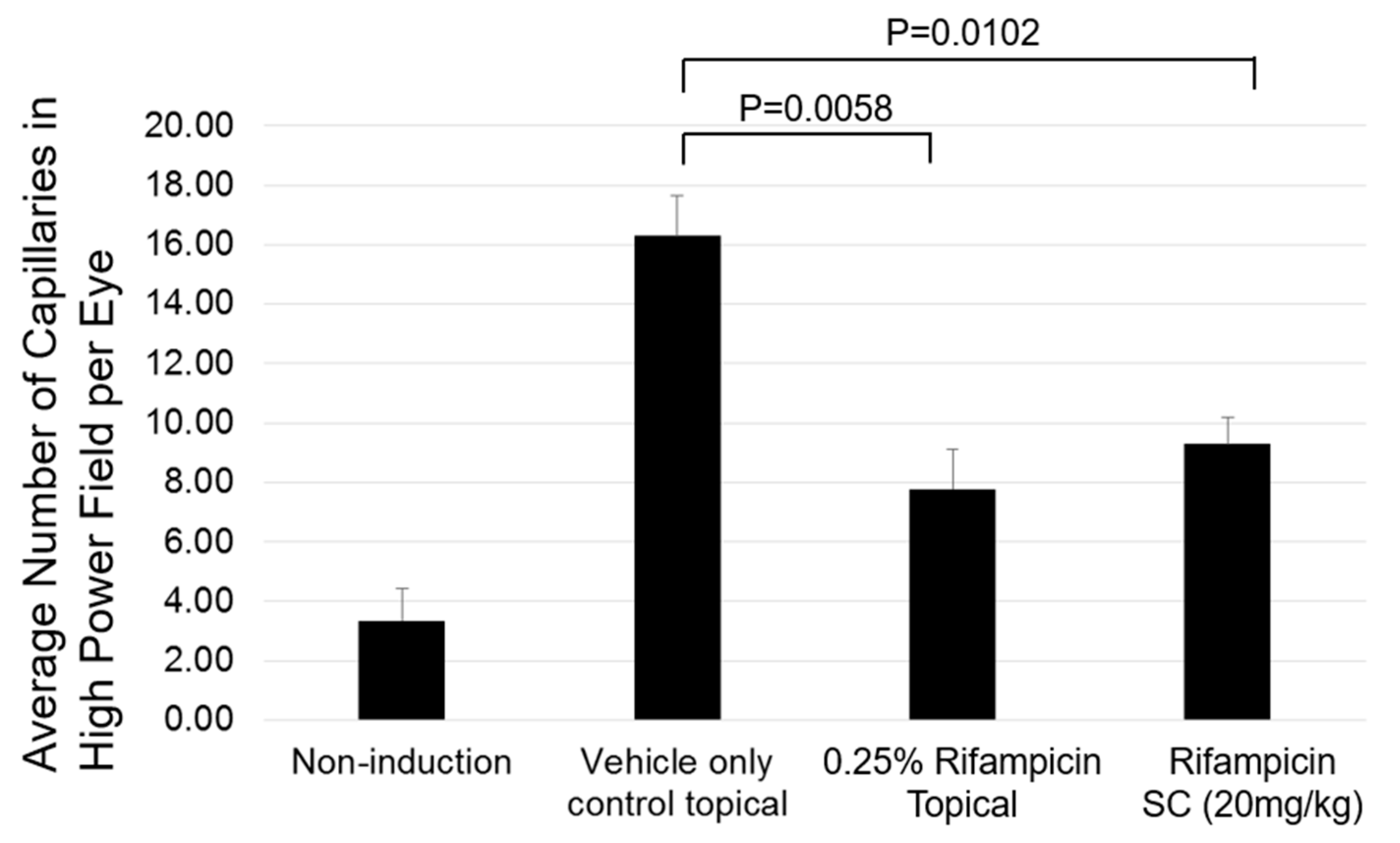
2.3. Preclinical Efficacy
2.4. Determination of Effective Dose of Rifampicin in Mouse Laser-Induced CNV Models
2.5. Ocular Tissue Delivery of Rifampicin via Oil-Based, Water-Based Suspension, and Water-Solubilized Formulations by Topical Applications
2.6. Oil-Based Formulation Plasma Exposure
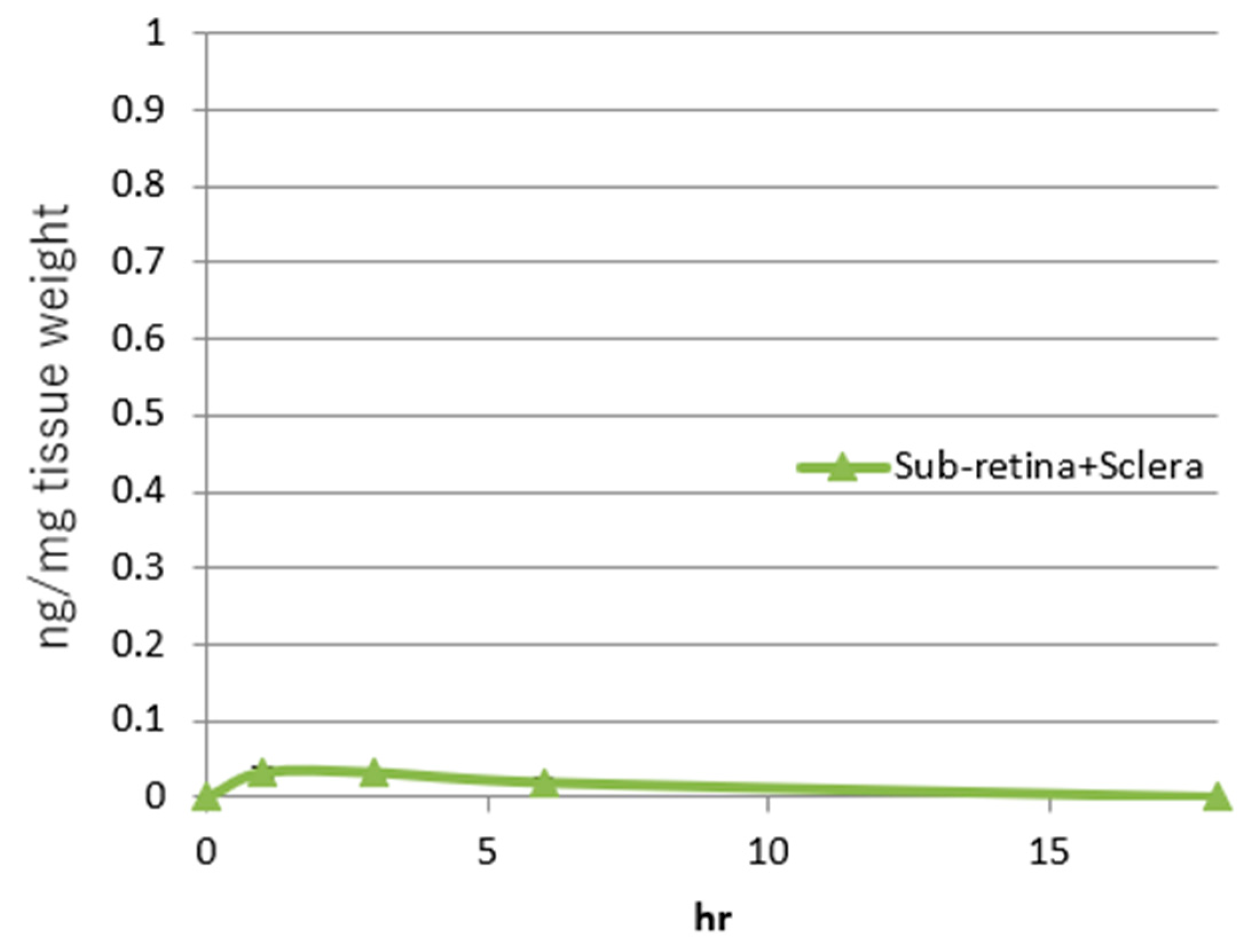
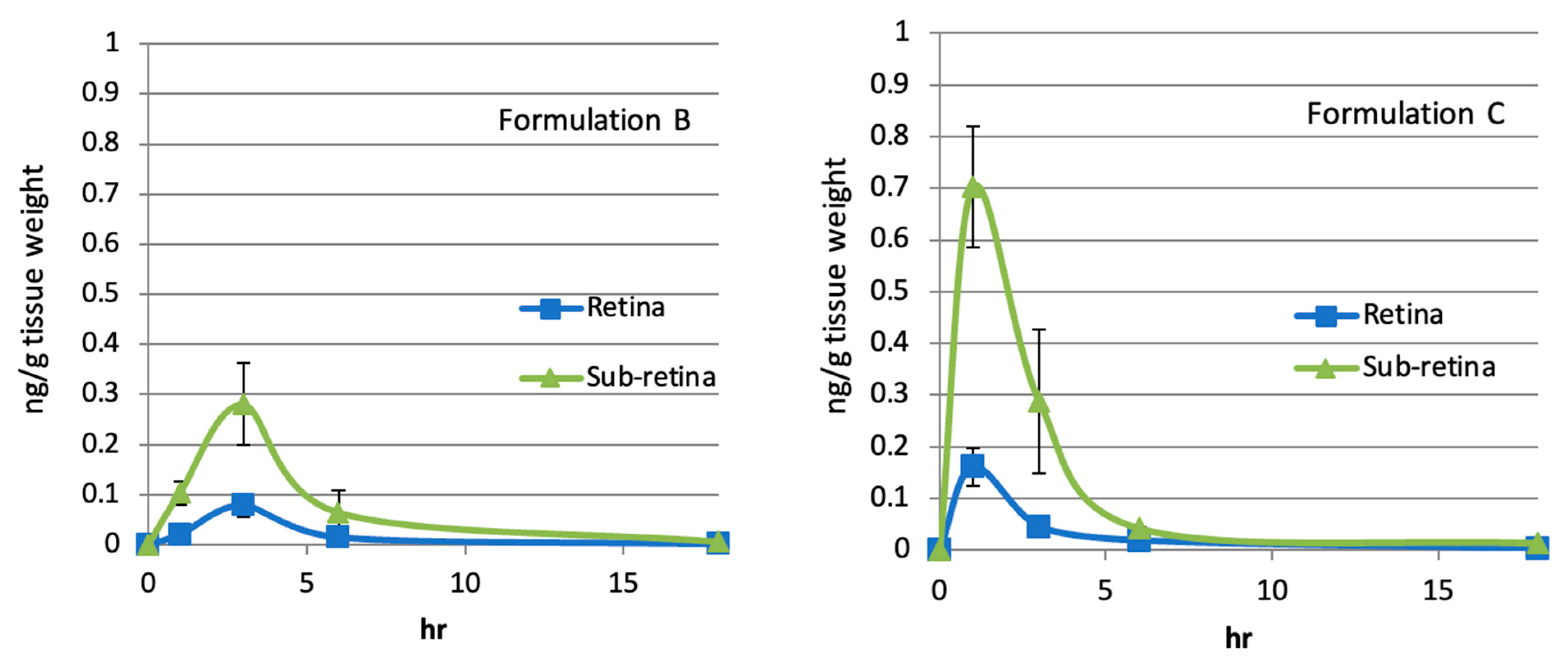
2.7. Sub-Retinal and Retinal Delivery of Rifampicin via Oil-Based Formulations
| Mouse effective AUC (h·ng/mg tissue) | Sub-retinal AUC by oil-based formulation C 0.01% rifampicin (h·ng/mg tissue) | Sub-retinal AUC by oil-based formulation C 0.001% rifampicin (h·ng/mg tissue) | |
| AUC values | 0.27 | 0.01 | 0.001 |
| Folds Greater Than Effective AUC | X1 | X0.04 | X0.004 |
| Mouse effective AUC (h·ng/mg tissue) | Sub-retinal AUC by oil-based formulation E 0.1% rifampicin (h·ng/mg tissue) | Sub-retinal AUC by oil-based formulation E 0.01% rifampicin (h·ng/mg tissue) | |
| AUC values | 0.27 | 0.27 | 0.18 |
| Folds Greater Than Effective AUC | X1 | X1 | X0.7 |
3. Discussion
4. Materials and Methods
4.1. 0.25% and 0.5% Rifampicin Water-Based Solubilized Formulations
4.2. Topical Application of Rifampicin Reaches the Retina in Rat
4.3. Pharmacokinetic Studies Using a 0.25% Rifampicin Eye Drop Formulation
4.4. Preclinical Efficacy
4.5. Determination of Effective Dose of Rifampicin in Mouse Laser-Induced CNV Models
4.6. Oil- or Water-Based Formulations
4.7. Ocular Tissue Delivery of Rifampicin via Oil-Based, Water-Based Suspension, and Water-Solubilized Formulations by Topical Applications
4.8. Oil-Based Formulation Plasma Exposure
5. Conclusions
6. Patents and Patent Applications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Review of Age-Related Macular Degeneration. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y.; Fleckenstein, M.; Keenan, T.D.L.; et al. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Chong, D.D.; Das, N.; Singh, R.P. Diabetic retinopathy: Screening, prevention, and treatment. Clevel. Clin. J. Med. 2024, 91, 503–510. [Google Scholar] [CrossRef]
- Romano, F.; Lamanna, F.; Gabrielle, P.H.; Teo, K.Y.C.; Parodi, M.B.; Iacono, P.; Fraser-Bell, S.; Cornish, E.E.; Nassisi, M.; Viola, F.; et al. Update on Retinal Vein Occlusion. Asia-Pac. J. Ophthalmol. 2023, 12, 196–210. [Google Scholar] [CrossRef]
- Davis, S.A.; Sleath, B.; Carpenter, D.M.; Blalock, S.J.; Muir, K.W.; Budenz, D.L. Drop instillation and glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 171–177. [Google Scholar] [CrossRef]
- Löscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Fung, T.A.; Tran, T.; Lim, L.L.; Samarawickrama, C.; Arnold, J.; Gillies, M.; Catt, C.; Mitchell, L.; Symons, A.; Buttery, R.; et al. Local delivery of corticosteroids in clinical ophthalmology: A review. Clin. Exp. Ophthalmol. 2020, 48, 366–401. [Google Scholar] [CrossRef]
- Joussen, M.A.; Wolf, S.; Kaiser, K.P.; Boyer, D.; Schmelter, T.; Sandbrink, R.; Zeitz, O.; Deeg, G.; Richter, A.; Zimmermann, T.; et al. The Developing Regorafenib Eye drops for neovascular Age-related Macular degeneration (DREAM) study: An open-label phase II trial. Br. J. Clin. Pharmacol. 2019, 85, 347–355. [Google Scholar] [CrossRef]
- Samanta, A.; Aziz, A.A.; Jhingan, M.; Singh, S.R.; Khanani, A.M.; Chhablani, J. Emerging Therapies in Neovascular Age-Related Macular Degeneration in 2020. Asia-Pac. J. Ophthalmol. 2020, 9, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Poor, S.H.; Weissgerber, G.; Adams, C.M.; Bhatt, H.; Browning, D.J.; Chastain, J.; Ciulla, T.A.; Ferriere, M.; Gedif, K.; Glazer, L.C.; et al. A Randomized, Double-Masked, Multicenter Trial of Topical Acrizanib (LHA510), a Tyrosine Kinase VEGF-Receptor Inhibitor, in Treatment-Experienced Subjects With Neovascular Age-Related Macular Degeneration-PubMed. Am. J. Ophthalmol. 2022, 239, 180–189. [Google Scholar] [CrossRef]
- Kumar, R.; Knick, V.B.; Rudolph, S.K.; Johnson, J.H.; Crosby, R.M.; Crouthamel, M.-C.; Hopper, T.M.; Miller, C.G.; Harrington, L.E.; Onori, J.A.; et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 2007, 6, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.; McLaughlin, M.M.; Tolentino, M.; Staurenghi, G.; Ye, L.; Xu, C.F.; Kim, R.Y.; Johnson, M.W.; Pazopanib Eye Drops Study Group. Pazopanib eye drops: A randomised trial in neovascular age-related macular degeneration. Br. J. Ophthalmol. 2014, 98, 172–178. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.M.; Paglione, M.G.; Slakter, J.; Tolentino, M.; Ye, L.; Xu, C.F.; Suttle, A.B.; Kim, R.Y. Initial exploration of oral pazopanib in healthy participants and patients with age-related macular degeneration. JAMA Ophthalmol. 2013, 131, 1595–1601. [Google Scholar] [CrossRef]
- Roshanshad, A.; Moosavi, S.A.; Arevalo, J.F. Association of the Complement Factor H Y402H Polymorphism and Response to Anti-Vascular Endothelial Growth Factor Treatment in Age-Related Macular Degeneration: An Updated Meta-Analysis-PubMed. Ophthalmic Res. 2024, 67, 358–386. [Google Scholar] [CrossRef]
- Csaky, K.G.; Dugel, P.U.; Pierce, A.J.; Fries, M.A.; Kelly, D.S.; Danis, R.P.; Wurzelmann, J.I.; Xu, C.F.; Hossain, M.; Trivedi, T. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age-related macular degeneration. Ophthalmology 2015, 122, 579–588. [Google Scholar] [CrossRef]
- Exonate. Exonate First-in-Class Eye Drop Phase Ib/IIa Trial Data Demonstrate Safety and Biological Activity in Treatment of Diabetic Retinopathy and Diabetic Macular Oedema. 2024. Available online: https://www.exonate.com/updates/exonate-first-class-eye-drop-phase-ibiia-trial-dat/ (accessed on 27 March 2025).
- Francisco, S.G.; Rowan, S. Drugs for Treatment of Age-Related Macular Degeneration-PubMed. Adv. Exp. Med. Biol. 2023, 1415, 73–77. [Google Scholar] [CrossRef]
- Keech, A.; Mitchell, P.; Summanen, P.; O’Day, J.; Davis, T.; Moffitt, M.; Taskinen, M.-R.; Simes, R.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Stefansson, E.; Loftsson, T.; Larsen, M.; Papp, A.; Kaarniranta, K.; Munk, M.R.; Dugel, P.; Tadayoni, R. Topical treatment of diabetic macular edema using dexamethasone ophthalmic suspension: A randomized, double-masked, vehicle-controlled study. Acta Ophthalmol. 2023, 101, 22–33. [Google Scholar] [CrossRef]
- Margalith, P.; Beretta, G.; Margalith, P.; Beretta, G. Rifomycin. XI. taxonomic study on Streptomyces mediterranei nov. sp. Mycopathol. Mycol. Appl. 1960, 13, 321–330. [Google Scholar] [CrossRef]
- Hartmann, G.; Honikel, K.O.; Knüsel, F.; Nüesch, J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim. Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1967, 145, 843–844. [Google Scholar] [CrossRef]
- Artsimovitch, I.; Vassylyeva, M.N.; Svetlov, D.; Svetlov, V.; Perederina, A.; Igarashi, N.; Matsugaki, N.; Wakatsuki, S.; Tahirov, T.H.; Vassylyev, D.G. Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell 2005, 122, 351–363. [Google Scholar] [CrossRef]
- Hartmann, G.; Behr, W.; Beissner, K.-A.; Honikel, K.; Sippel, A. Antibiotics as Inhibitors of Nucleic Acid and Protein Synthesis. Angew. Chem. Int. Ed. Engl. 1968, 7, 693–701. [Google Scholar] [CrossRef]
- Calvori, C.; Frontali, L.; Leoni, L.; Tecce, G. Effect of rifamycin on protein synthesis-PubMed. Nature 1965, 207, 417–418. [Google Scholar] [CrossRef]
- Betzler, B.K.; Gupta, V.; Agrawal, R. Clinics of ocular tuberculosis: A review. Clin. Exp. Ophthalmol. 2021, 49, 146–160. [Google Scholar] [CrossRef]
- Shichiri, M.; Tanaka, Y. Inhibition of cancer progression by rifampicin: Involvement of antiangiogenic and anti-tumor effects. Cell Cycle 2010, 9, 64–68. [Google Scholar] [CrossRef]
- Shichiri, M.; Fukai, N.; Kono, Y.; Tanaka, Y. Rifampicin as an oral angiogenesis inhibitor targeting hepatic cancers. Cancer Res 2009, 69, 4760–4768. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Matsunaga, N.; Shimazawa, M.; Hara, H. Rifampicin inhibits the retinal neovascularization in vitro and in vivo. Exp. Eye. Res. 2008, 86, 131–137. [Google Scholar] [CrossRef]
- Tostmann, A.; Boeree, M.J.; Aarnoutse, R.E.; de Lange, W.C.; van der Ven, A.J.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef]
- Grosset, J.; Leventis, S. Adverse Effects of Rifampin. Rev. Infect. Dis. 1983, 5, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K.; Chen, J.; Raymond, K. Roles of rifampicin in drug-drug interactions: Underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Guengerich, F.P. CYTOCHROME P-450 3A4: Regulation and Role in Drug Metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Human Cytochrome P450 Enzymes; Springer: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Weiner, M.; Peloquin, C.; Burman, W.; Luo, C.-C.; Engle, M.; Prihoda, T.J.; Kenzie, W.R.M.; Bliven-Sizemore, E.; Johnson, J.L.; Vernon, A. Effects of Tuberculosis, Race, and Human Gene SLCO1B1 Polymorphisms on Rifampin Concentrations. Antimicrob. Agents Chemother. 2010, 54, 4192–4200. [Google Scholar] [CrossRef]
- Darougar, S.; Viswalingam, M.; Treharne, J.D.; Kinnison, J.R.; Jones, B.R. Treatment of TRIC infection of the eye with rifampicin or chloramphenicol-PubMed. Br. J. Ophthalmol. 1977, 61, 255. [Google Scholar] [CrossRef]
- Darougar, S.; Jones, B.R.; Viswalingam, N.; Allami, J.; Minassian, D.; Farahmandian, M.A.; Houshmand, A. Topical therapy of hyperendemic trachoma with rifampicin, oxytetracycline, or spiramycin eye ointments. Br. J. Ophthalmol. 1980, 64, 37. [Google Scholar] [CrossRef]
- Osborne, N.N.; DeSantis, L.; Bae, J.H.; Ugarte, M.; Wood, J.P.; Nash, M.S.; Chidlow, G. Topically applied betaxolol attenuates NMDA-induced toxicity to ganglion cells and the effects of ischaemia to the retina. Exp. Eye. Res. 1999, 69, 331–342. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Konraethsdottir, F.; Loftsson, T.; Stefansson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef]
- Sanofi-Aventis. Package Insert of RIFADIN®(Rifampin Capsules USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050420s073,050627s012lbl.pdf (accessed on 27 March 2025).
- Khan, M.S.; Sameen, M.; Lodhi, A.A.; Ahmed, M.; Ahmed, N.; Kamal, M.; Junejo, S.A. Effect of half adult dose of oral Rifampicin (300 mg) in patients with idiopathic central serous chorioretinopathy-PubMed. Pak. J. Med. Sci. 2016, 32, 1158. [Google Scholar] [CrossRef]
- Khorram, D. Rifampin for Central Serous Chorioretinopathy. Available online: https://retinablog.wordpress.com/2010/08/30/rifampin-for-central-serous-chorioretinopathy/ (accessed on 27 March 2025).
- Tanihara, H.; Kakuda, T.; Sano, T.; Kanno, T.; Kurihara, Y. Long-Term Intraocular Pressure-Lowering Effects and Adverse Events of Ripasudil in Patients with Glaucoma or Ocular Hypertension over 24 Months. Adv Ther 2022, 39, 1659–1677. [Google Scholar] [CrossRef]
- Rishi, P.; Majumder, P.D.; Biswas, J. Anterior Chamber Migration of Fluocinolone Acetonide Intravitreal Implant. JAMA Ophthalmol. 2019, 137, e185931. [Google Scholar] [CrossRef] [PubMed]
- Shughoury, A.; Bhatwadekar, A.; Jusufbegovic, D.; Hajrasouliha, A.; Ciulla, T.A. The evolving therapeutic landscape of diabetic retinopathy. Expert Opin. Biol. Ther. 2023, 23, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Parachuri, N.; Kumar, N.; Kuppermann, B.D.; Bandello, F. The Port Delivery System with ranibizumab-journey of mitigating vitreous hemorrhage. Eye 2022, 36, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ciulla, T.A. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin. Emerg. Drugs 2017, 22, 235–246. [Google Scholar] [CrossRef]
- Tolentino, M.J.; Tolentino, A.J. Investigational drugs in clinical trials for macular degeneration. Expert Opin. Investig. Drugs 2022, 31, 1067–1085. [Google Scholar] [CrossRef]
- Shirian, J.D.; Shukla, P.; Singh, R.P. Exploring new horizons in neovascular age-related macular degeneration: Novel mechanisms of action and future therapeutic avenues. Eye 2025, 39, 40–44. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Brown, D.M.; Reed, K.; Berliner, A.J.; Gerstenblith, A.T.; Breazna, A.; Abraham, P.; Fein, J.G.; Chu, K.W.; Clark, W.L.; et al. Effect of High-Dose Intravitreal Aflibercept, 8 mg, in Patients With Neovascular Age-Related Macular Degeneration: The Phase 2 CANDELA Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 834–842. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Traverso, C.E.; Nicolo, M.; Munk, M.R. Faricimab for the Treatment of Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration. Pharmaceutics 2023, 15, 1413. [Google Scholar] [CrossRef]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.-S.; Hagstrom, S.A.; Winter, K.; et al. Risk of scar in the comparison of age-related macular degeneration treatments trials-PubMed. Ophthalmology 2014, 121, 656–666. [Google Scholar] [CrossRef]
- Eng, V.A.; Rayess, N.; Nguyen, H.V.; Leng, T. Complete RPE and outer retinal atrophy in patients receiving anti-VEGF treatment for neovascular age-related macular degeneration-PubMed. PLoS ONE 2020, 15, e0232353. [Google Scholar] [CrossRef]
- Yanni, S.E.; Clark, M.L.; Yang, R.; Bingaman, D.P.; Penn, J.S. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res. Bull. 2010, 81, 310–319. [Google Scholar] [CrossRef][Green Version]
- Ministry of Health, Labour and Welfare, Japan. THE JAPANESE PHARMACOPOEIA, XVII. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-11120000-Iyakushokuhinkyoku/JP17_REV.pdf (accessed on 27 March 2025).
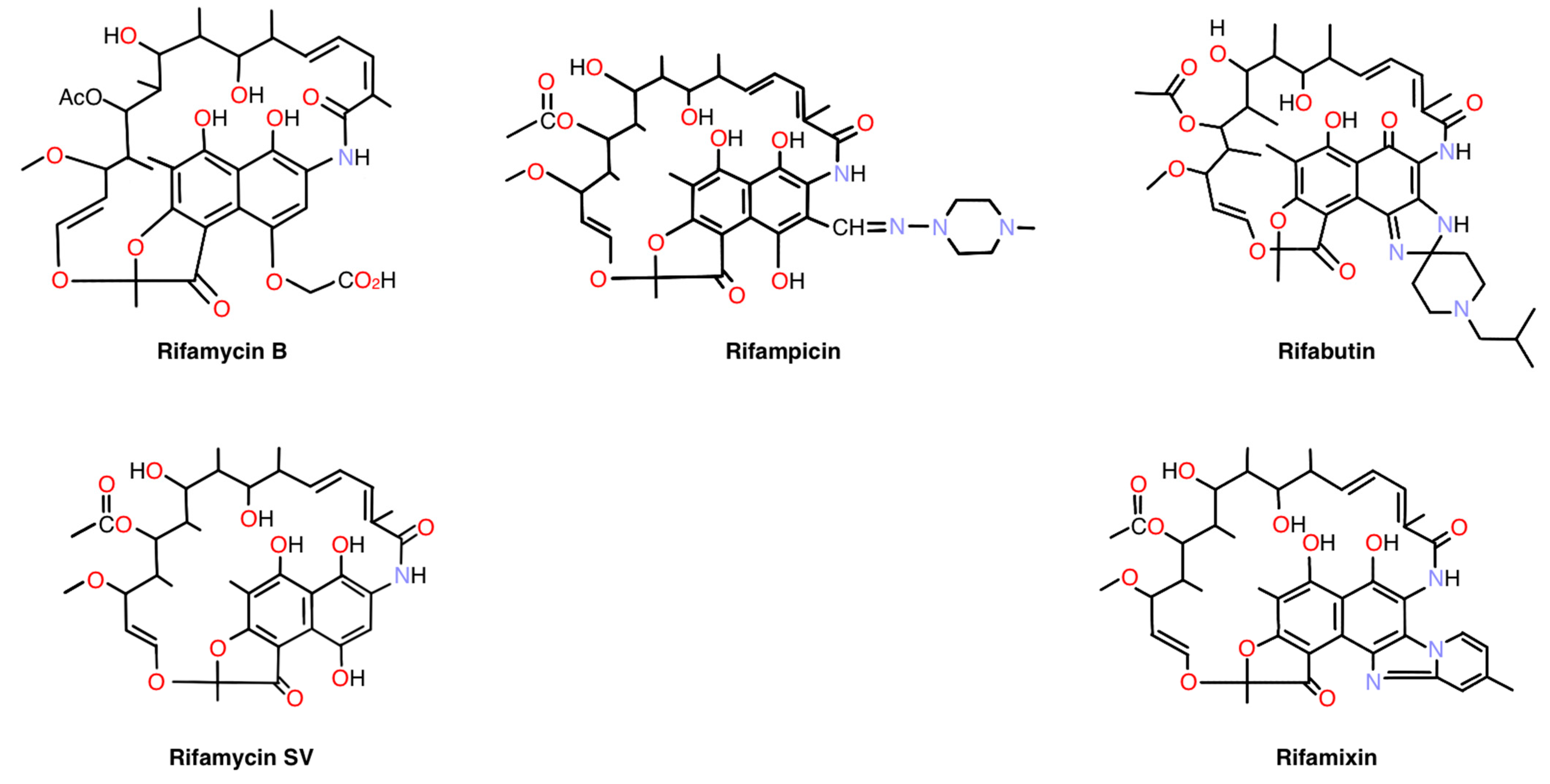



| Compound (Brand Name) Mechanism of Action for Indication or Usage | Drug Amount Administered | Drug Amount Detected in Retina |
|---|---|---|
| Betoptic 0.5% (MW: 307) Beta 1 receptor blocker for glaucoma [39] | 0.5% 4 drops (120–200 μL, 600–1000 μg) | 300 ng/ 1 g of retinal tissues (Rabbit, average of n = 5) |
| 0.5% 8 drops (240–400 μL, 1200–2000 μg) | 480 ng/ 1 g of retinal tissues (Rabbit, average of n = 7) | |
| Dexamethasone (MW: 392) Glucocorticoid for inflammation [40] | 0.5% 50 μL, 250 μg | 33 ng/ 1 g of retinal tissues (Rabbit, average of n = 6) |
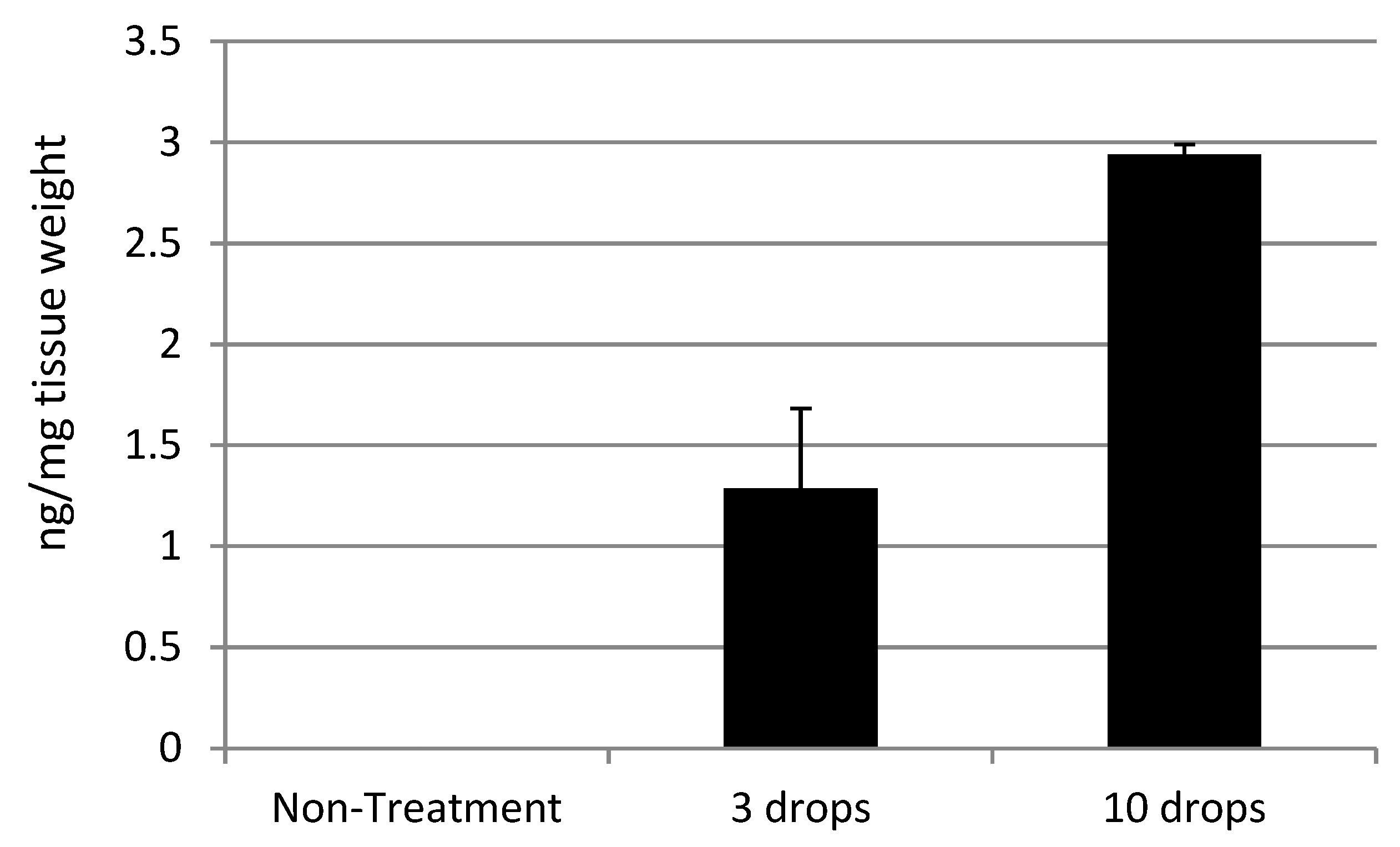
| Rifampicin | 0.25% 3 drops (15 μL, 37.5 μg) | 1280 ng/ 1 g of retinal tissues (Rat) SE: 394.0348 |
| 0.25% 10 drops (50 μL, 125 μg) | 2939 ng/ 1 g of retinal tissues (Rat) SE: 50.54041 |
| (n = 3, Average) Mouse effective AUC (h·ng/mg tissue) | AUC by oil- based formulation A (h·ng/mg tissue) | AUC by oil- based formulation B (h·ng/mg tissue) | AUC by oil- based formulation C (h·ng/mg tissue) | AUC by oil- based formulation D (h·ng/mg tissue) | |
| AUC values | 0.27 | 0.96 | 1.37–2.11 | 2.18–2.68 | 4.40 |
| Folds Greater Than Effective AUC | X1 | X4 | X5–X8 | X8–X10 | X16 |
| AUC by oil- based formulation E (h·ng/mg tissue) | AUC by oil-based suspension formulation F (h·ng/mg tissue) | AUC by water-based suspension formulation G (h·ng/mg tissue) | AUC by water-based solubilized formulation H (h·ng/mg tissue) | AUC by water-based solubilized formulation RK32 (h·ng/mg tissue) | |
| AUC values | 8.77 | 0.62 | 1.82 | 1.33 | 2.05 |
| Folds Greater Than Effective AUC | X32 | X2 | X7 | X5 | X8 |
| (n = 3, Average) Mouse effective AUC (h·ng/mg tissue) | AUC by oil- based formulation A (h·ng/mg tissue) | AUC by oil- based formulation B (h·ng/mg tissue) | AUC by oil- based formulation C (h·ng/mg tissue) | AUC by oil- based formulation D (h·ng/mg tissue) | |
| AUC values | 0.27 | 0.27 | 0.31–0.46 | 0.74 | 1.31 |
| Folds Greater Than Effective AUC | X1 | X1 | X1–X2 | X3 | X5 |
| AUC by oil- based formulation E (h·ng/mg tissue) | AUC by oil- based formulation F (h·ng/mg tissue) | AUC by water-based suspension formulation G (h·ng/mg tissue) | AUC by water-based suspension formulation H (h·ng/mg tissue) | AUC by water-based solubilized formulation RK32 (h·ng/mg tissue) | |
| AUC values | 1.06 | 0.17 | 0.52 | 0.46 | 0.22 |
| Folds Greater Than Effective AUC | X4 | X1 | X2 | X2 | X1 |
| A | B | C | D | RK32 | |
|---|---|---|---|---|---|
| Rifampicin (ng/mL plasma) | 0.70 | 0.47 | 1.59 | 1.96 | 10.24 |
| SE | 0.2144 | 0.0982 | 0.2764 | 0.3129 | 0.9431 |
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Rifampicin | 1% | 1% | 1% | 1% | 1% | 1% |
| White Petroleum | 99% | 79.2% | 49.5% | |||
| Liquid Paraffin | 19.8% | 49.5% | 99% | |||
| Sesame Oil | 99% | |||||
| Light Liquid Paraffin | 99% | |||||
| Viscosity (mPaS) | 3815 | 2145 | 867 | 161 | 63 | 6 |
| Shear Velocity (s−1) | 200 | 200 | 200 | 200 | 200 | 200 |
| G | H | RK32 | |
|---|---|---|---|
| Rifampicin | 1% | 1% | 1% |
| NaCl | 0.67% | 0.4% | |
| Polyoxyethylene castor oil | 5% | ||
| Ethylene glycol monostearate | 3% | ||
| Cellulose polymers | 0.8% | 0.5% | |
| Na2HPO4 | 0.3% | 1.5% | 1.5% |
| EDTA | 0.1% | 0.1% | |
| Anti-oxidants | 0.3% | 0.3% | |
| pH | 7.1 | 7.0 | 8.3 |
| Viscosity (mPaS) | 101 | 1.3 | 39.7 |
| Shear Velocity (s−1) | 200 | 200 | 200 |
| C (0.01% Rifampicin) | C (0.001% Rifampicin) | E (0.1% Rifampicin) | E (0.01% Rifampicin) | |
|---|---|---|---|---|
| Viscosity (mPaS) | 801 | 781 | 62 | 65 |
| Shear Velocity (s−1) | 200 | 200 | 200 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vailoces, V.A.S.; Tolentino, A.J.; Arevalo, J.F.; Adelman, R.A.; Bhisitkul, R.; Do, D.V.; Nguyen, Q.D.; Tolentino, M.J.; Tanito, M.; Serizawa, H. Development of Rifampicin Eye Drops for the Treatment of Exudative Age-Related Macular Degeneration. Pharmaceuticals 2025, 18, 655. https://doi.org/10.3390/ph18050655
Vailoces VAS, Tolentino AJ, Arevalo JF, Adelman RA, Bhisitkul R, Do DV, Nguyen QD, Tolentino MJ, Tanito M, Serizawa H. Development of Rifampicin Eye Drops for the Treatment of Exudative Age-Related Macular Degeneration. Pharmaceuticals. 2025; 18(5):655. https://doi.org/10.3390/ph18050655
Chicago/Turabian StyleVailoces, Valory Anne S., Andrew J. Tolentino, Jose Fernando Arevalo, Ron A. Adelman, Robert Bhisitkul, Diana V. Do, Quan Dong Nguyen, Michael J. Tolentino, Masaki Tanito, and Hiroaki Serizawa. 2025. "Development of Rifampicin Eye Drops for the Treatment of Exudative Age-Related Macular Degeneration" Pharmaceuticals 18, no. 5: 655. https://doi.org/10.3390/ph18050655
APA StyleVailoces, V. A. S., Tolentino, A. J., Arevalo, J. F., Adelman, R. A., Bhisitkul, R., Do, D. V., Nguyen, Q. D., Tolentino, M. J., Tanito, M., & Serizawa, H. (2025). Development of Rifampicin Eye Drops for the Treatment of Exudative Age-Related Macular Degeneration. Pharmaceuticals, 18(5), 655. https://doi.org/10.3390/ph18050655








